#Uterine Fibroid Treatment Drugs Market Revenue
Explore tagged Tumblr posts
Text
Uterine Fibroid Treatment Drugs Market Segment Analysis By Drug Class, Type, End-User, Region And Forecast Till 2030: Grand View Research Inc.
San Francisco, 17 Aug 2023: The Report Uterine Fibroid Treatment Drugs Market Size, Share & Trends Analysis Report By Drug Class (GnRH Agonists, GnRH Antagonists), By Type (Submucosal Fibroids, Intramural Fibroids), By End-user, And Segment Forecasts, 2023 – 2030 The global uterine fibroid treatment drugs market size is expected to reach USD 3.91 billion by 2030, expanding at a CAGR of 10.2%…

View On WordPress
#Uterine Fibroid Treatment Drugs Industry#Uterine Fibroid Treatment Drugs Market#Uterine Fibroid Treatment Drugs Market 2023#Uterine Fibroid Treatment Drugs Market Revenue#Uterine Fibroid Treatment Drugs Market Share#Uterine Fibroid Treatment Drugs Market Size
0 notes
Text
Women’s Health Market Size, Share & Trends Analysis Report, 2030
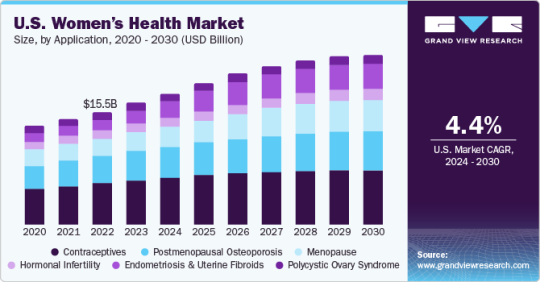
Women's Health Market Growth & Trends
The global women’s health market size is expected to reach USD 67.07 billion by 2030, registering a CAGR of 5.7% from 2024 to 2030, according to a new report by Grand View Research, Inc. The introduction of innovative novel products and the presence of a strong pipeline of women’s health products are prime factors driving the market growth. For instance, in May 2021, the Food & Drug Administration approved Myfembree, developed by Pfizer Inc. in collaboration with Myovant Sciences, for uterine fibroids associated with heavy menstrual bleeding. An increase in the incidence of endometriosis and a rise in support from non-profit organizations are expected to fuel the market growth over the forecast period.
For instance, the Bill & Melinda Gates Foundation pledged USD 280 million every year from 2021 to 2030 for the development of new contraceptive technologies and to support family planning initiatives. According to the WHO report 2021, globally, around 10% (190 million) of reproductive-age girls and women are affected by endometriosis. It is a chronic disease related to severe pain during periods, bowel movements and/or urination, abdominal bloating, nausea, fatigue, etc. The COVID-19 pandemic had a negative impact on the market. Strict measures undertaken by governments to control the spread of the SARS-CoV-2, such as social distancing and community-wide lockdowns, have had a detrimental impact on treatment facilities & gynecological clinics.
For instance, in low- and middle-income countries there has been a decline in the usage of long- and short-acting reversible contraceptives. Strategic initiatives undertaken by key players, such as collaborations, agreements, and partnerships, for the development and commercialization of products are anticipated to drive market growth. For instance, in October 2021, Richter and Hikma signed an exclusive licensing agreement to commercialize denosumab, comprising biosimilar of Xgeva and Prolia in the U.S. Moreover, the growing competition from generic drugs increases the pricing pressure after patent expiration, which is anticipated to impede market growth.
For instance, after the patent expiry, the revenue of Forteo declined by 23% between 2020 and 2021. North America dominated the global market in 2021 owing to favorable reimbursement policies, the presence of key market players, supportive government regulations, approval & commercialization of products, and high usage of contraceptives among women. The Asia Pacific region is expected to grow at the fastest CAGR during the forecast period due to rising government spending on women’s health. For instance, the Australian Government announced an investment of USD 333 million to support health services and support.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/womens-health-market
Women's Health Market Report Highlights
The contraceptives segment held the highest market share of more than 35.25% of the global revenue in 2023 due to increased awareness about family planning and rapid technological advancements in contraception
The University of California Bixby Center released a reimbursement guide to help health providers offer women a full range of contraceptives
The endometriosis segment is expected to witness significant growth over the forecast period due to the launch of products, such as Relugoliz and the impending launch of Linzagolix for the treatment of women with uterine fibroids
Based on age, the 50 years and above segment is expected to register the fastest growth rate of 6.7% over the forecast period as an increase in life expectancy is boosting the overall menopausal population across the globe
According to the International Menopause Society, globally, women aged between 45 and 55 years typically experience menopause, with the average age of onset at 51.5 years
Women's Health Market Segmentation
Grand View Research has segmented the global Women's Health market on the basis age, application, drugs, and region:
Women's Health Application Outlook (Revenue, USD Million, 2018 - 2030)
Hormonal Infertility
Contraceptives
Postmenopausal Osteoporosis
Endometriosis & Uterine Fibroids
Menopause
Polycystic Ovary Syndrome (PCOS)
Women's Health Drug Outlook (Revenue, USD Million, 2018 - 2030)
ACTONEL
YAZ,Yasmin,Yasminelle
FORTEO
Minastrin 24 Fe
Mirena
NuvaRing
ORTHO TRI-CY LO
Premarin
Prolia
Reclast/Aclasta
XGEVA
Zometa
Others
Women's Health Age Outlook (Revenue, USD Million, 2018 - 2030)
50 years and above
Postmenopausal Osteoporosis
Endometriosis & Uterine Fibroids
Menopause
Others
Others
Women's Health Regional Outlook (Revenue, USD Million; 2018 - 2030)
North America
S.
Canada
Europe
Germany
K.
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
India
Japan
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of Women’s Health Market
AbbVie, Inc.
Bayer AG
Merck & Co., Inc.
Pfizer, Inc.
Teva Pharmaceutical Industries Ltd.
Agile Therapeutics
Amgen, Inc.
Apothecus Pharmaceutical Corp.
Blairex Laboratories, Inc.
Ferring B.V.
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/womens-health-market
#Women’s Health Market#Women’s Health Market Size#Women’s Health Market Share#Women’s Health Market Trends
0 notes
Text
Uterine Fibroids Drug Market Size, Business Revenue Forecast, Leading Competitors and Growth Trends 2026
Global uterine fibroids drug market is expected to grow at a substantial CAGR in the forecast period of 2020-2026. Uterine fibroids are also known as leiomyoma or myomas, can be characterized by the formation of abnormal non-cancerous tissue growth inside the wall of the uterus.
Competitive Analysis: Global Uterine Fibroids Drug Market
Global uterine fibroids drug market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of uterine fibroids drug market for Global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.

Download PDF Sample report @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-uterine-fibroids-drug-market
A woman may develop one or many fibroids in uterus and these can vary in sizes depending upon the condition and level. Uterine fibroids symptoms include heavy bleeding between or during the menstrual cycle, pain in pelvis or lower back, increased menstrual cramping and enlargement of the abdomen.
Segmentation: Global Uterine Fibroids Drug Market
· By Types (Subserosal Fibroids, Submucosal Fibroids, Intramural Fibroids and Pedunculated Fibroids)
· By Mechanism of Action (GnRH Agonists, Steroids, Contraceptives, NSAIDs and Vitamins)
· By Drugs Type (Progesterone, Levonorgestrel, Mefenamic, Raloxifene and Others)
· By Diagnosis (Ultrasound, Lab Tests and Imaging Tests)
· By Treatment (Medication, Dietary Supplements and Surgery)
· By Route of Administration (Oral, Intravenous and Others)
· By End-Users (Hospitals, Homecare, Specialty Clinics and Others)
· By Geography (North America, Europe, Asia-Pacific, South America, Middle East and Africa)
Key Market Players: Global Uterine Fibroids Drug Market
The key market players in the global uterine fibroids drug market are GlaxoSmithKline plc, F. Hoffmann-La Roche Ltd, Pfizer Inc, Novartis AG, Bristol-Myers Squibb Company, Sanofi, Teva Pharmaceutical Industries Ltd., Amgen Inc, Medtronic, Endo Pharmaceuticals Inc, Mylan N.V, Sun Pharmaceutical Industries Ltd, Hologic, Inc, Smith & Nephew, Merck & Co., Inc, ALLERGAN, Bayer AG, Koninklijke Philips N.V, IceCure Medical Ltd and others.
Market Drivers
· Increasing prevalence of uterine fibroids is driving the market growth
· Rising female population across the globe is driving the market growth.Growing screening across the world can also acts as a market driver
· Rising awareness amongst people about the uterine fibroids and its treatment is also boosting the market growth
Market Restraints
· Side effects of treatment such as bleeding and infection during surgery can hamper the market growth
· Low healthcare expenditure in developing regions also restricts the market growth
· High cost-containment measures undertaken by governments along with the ongoing healthcare reforms.
· Limited treatment options for uterine fibroids are restricting the market growth.
Inquiry Before Buying @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-uterine-fibroids-drug-market
Reasons to Purchase this Report:
· Current and future of global uterine fibroids drug market outlook in the developed and emerging markets.
· The segment that is expected to dominate the market as well as the segment which holds highest CAGR in the forecast period.
· Regions/Countries that are expected to witness the fastest growth rates during the forecast period.
· The latest developments, market shares, and strategies that are employed by the major market players.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact:
Data Bridge Market Research
US: +1 888 387 2818
Related Reports:
Opioids Drug Market
Monoamine Oxidase (MAO) Inhibitor Drugs Market
#Uterine Fibroids Drug Market#Uterine Fibroids Drug#Uterine Fibroids Drug Market Trends#Uterine Fibroids Drug Market Industry#Uterine Fibroids Drug Market News
0 notes
Text
Allergan abandons women’s health sale plan as profit slumps

Shares in Allergan fell after the company said it had swung to a loss in the last quarter of 2018, and forecast 2019 revenues below analyst expectations.
The Ireland-headquartered specialty drugmaker, best known for its Botox wrinkle treatment, reported a loss of $4.3 billion that reversed a profit of over $3 billion a year ago, even though sales were a little better than expected at $4.08 billion.
The loss resulted from big write-offs to the tune of a whopping $5.4 billion, in part resulting from lower-than-expected sales of double chin treatment Kybella/Belkyra – the main asset in the company’s $2.1 billion acquisition of Kythera – which brought in just $8 million in the quarter.
It also revealed that it had dropped plans to sell off its female health division, which was put on the block last year along with its anti-infectives business. CEO Brent Saunders said on a conference call that it made more sense at the moment to continue “managing and optimising” the women’s health business, but that he still expected anti-infectives to be sold as planned.
It’s possible that the volte-face on women’s health is related to the failure by Allergan to get FDA approval for uterine fibroids therapy Esmya (ulipristal acetate) last year, which had been tipped as a potential blockbuster. Approval would have made the division much more valuable.
Allergan’s cash-cow Botox (onabotulinumtoxinA) had a good quarter, rising 9% to $946m, but its second biggest product – Restasis (cyclosporine) for dry eye disease – fell 18% to $341m thanks to the start of generic competition in some international markets, lower demand and pricing pressure.
For the full year, the company is predicting net revenues of between $15 billion and $15.3 billion, which is a little lower than analysts’ projections.
The fall-off in Restasis sales is likely to gather pace as patent protection is lost in other countries, particularly the US which accounts for 95% of its sales. US exclusivity for the drug is due to be lost in March of this year.
Meanwhile, Botox is also facing the threat of biosimilar competition from US biotech Evolus, which resubmitted a marketing application in the US last August after its first attempt was rejected because of manufacturing issues. It’s hoping for a positive verdict from the FDA second time around in the spring.
In a bid to protect its franchise, Allergan acquired privately-held biotech Bonti last year for an upfront payment of $195 million, gaining control of a new botulinum toxin ingredient with a rapid onset of action (within 24 hours) and a two- to four-week duration of effect.
Shares in Allergan fell almost 9% after the results announcement to their lowest level for almost three years.
Some analysts attributed that in part to what they perceived as downbeat discussion by Allergan’s management on NMDA-targeting antidepressant rapastinel, which is due to report pivotal data later in 2019 and is in a race to market with Johnson & Johnson’s esketamine.
The post Allergan abandons women’s health sale plan as profit slumps appeared first on Pharmaphorum.
from Pharmaphorum https://pharmaphorum.com/news/allergan-abandons-womens-health-sale-plan-as-profit-slumps/
0 notes
Text
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential
New Post has been published on http://khalednaser.com/abbvie-this-dividend-aristocrat-offers-double-digit-total-return-potential/
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential


Source: Abbvie Media Resources
With the financial markets likely to enter 2019 having just had the worst December since the Great Depression, there have been many stocks that have taken a beating the past month.
Abbvie (ABBV) has not been exempt from this market plunge as it has fallen nearly ~27.6% from its all-time high of $125.86 a share in January 2018. Moreover, Abbvie has fallen ~3.3% since the beginning of December, from $94.27 a share to $91.12 as of close on December 28, meaning the company has been hit harder than other pharma stocks this year. In addition to concerns over Abbvie’s concentration risk with Humira, another concern in the past 9 months to the investor community was Abbvie’s announcement this past March that it wouldn’t pursue accelerated approval for Rova T to treat RR SCLC, due to disappointing Phase 2 results. Although the company will move forward with the ongoing Phase 3 studies, investor consensus is that the $10.2 billion StemCentryx acquisition, once thought to be a promising oncology acquisition, will instead prove to be a bust. Consequently, the company’s shares have not rebounded to the $110+ range the shares were at January through most of March.
As such, I believe that Abbvie is currently worthy of a dividend growth investor’s consideration. There are three main reasons to support my opinion that Abbvie is an attractive investment, worthy of further due diligence from a potential investor. The first reason is that although its dividend history as an independent company has been brief, it has been spectacular, and I believe it will continue to deliver solid dividend increases going forward. This leads me into my second point that Abbvie has the appropriate drug pipeline to offset the eventual declines in revenue from Humira, which would lead to continued strong dividend increases. Lastly, the company’s current valuation is appealing.
Reason #1: Abbvie’s Safe And Growing Dividend
Since the formation of the company in 2013 as a spin-off of the biopharmaceuticals division of Abbott Laboratories, Abbvie has raised its dividend each year. Abbvie is technically a dividend aristocrat when we factor in its former parent company’s 46 years of consecutive dividend increases.
The company recently announced an 11.5% dividend increase from $0.96/share quarterly to current quarterly dividend of $1.07. Using the company’s midpoint earnings guidance of adjusted diluted EPS of $7.91/share for FY 2018, we can see that the payout ratio in terms of EPS is 54.1%. This is about where I’d like a pharma dividend payer to be at as it leaves the company plenty of capital to invest in the research and development that will be necessary to develop new blockbuster drugs in the future, not to mention also returning capital to shareholders in the form of share buybacks when the company’s shares are attractively priced.
Moreover, we can examine the company’s free cash flow numbers to arrive at the FCF dividend payout ratio. Per the company’s most recent financial release, Abbvie has generated FCF of $9.5 billion while paying $4.1 billion in dividends during that time. This would mean the payout ratio using FCF is roughly 43%.
Both methods of gauging the company’s payout ratio show us that the dividend is clearly safe and reasonable, allowing raises fairly similar to the most recent of 11.5%, while still heavily investing in the company’s future through R&D.
With a dividend yield of 4.7%, it is wise to question why the yield is so high currently. This leads us into our next point in which we’ll discuss the risks that Abbvie is facing, and why I believe these risks are being more than factored into the company’s stock price.
Reason #2: While Bears Argue Abbvie Will Not Be Able To Offset Humira’s Eventual Revenue Declines, I Believe The Contrary Is True
The primary concern that investors have had with Abbvie for several years is that Abbvie is too reliant on Humira. They conclude that because Humira accounts for 62% of Abbvie’s revenue as of Q3 2018 and 70%+ of its profits, when Humira sales eventually decline, the company’s prospects will also decline.
Fortunately, per company forecasts for the remainder of 2018, sales of Humira are as follows:
International: $6.3 billion (32%)
US: $13.7 billion (68%)
Total Humira sales: $20 billion
The company is currently facing tough biosimilar competition in the European Union as competitors such as Amgen, Biogen, Mylan, and Norvartis releasing biosimilar versions of Humira. With discounting of Humira ranging from as low as 10%, to as high as 80% in Nordic countries, international Humira sales are expected to decline 26-27% in 2019, per Reuters.

Source: Abbvie Investor Presentation
Although this isn’t the most encouraging news, the bulk of Humira’s sales are generated in the United States. Assuming a 27% decline in international Humira sales, the company’s international sales will decline by $1.7 billion. This would equate to a ~5% sales overall sales decline by itself. However, with strong Humira patent protection in the US, Abbvie won’t face any rival biosimilar Humira drugs in the US until 2023. In the meantime, Humira sales will continue to grow at a clip of roughly 10-12% in the US, before peaking at $21 billion in total sales in 2020. This would equate to an increase in Humira sales of nearly $1.4 billion to $1.6 billion. An overall decline in Humira sales of $100 million to $300 million would mean that the company will see a revenue decline of less than 1% in 2019, not accounting for any growth experienced from other drugs on the market or in the pipeline.

Source: Abbvie Investor Presentation
Abbvie’s management recognizes the threats posed by concentration risk from Humira and they have developed a corporate strategy focused on extensive research and development to drive innovation, and deliver results to shareholders in the process.
Even if we assume that Rova T will be a complete bust, producing no revenue for Abbvie, and that Humira’s sales will fall considerably in the next 5 years, Abbvie has several promising drugs at various phases of development and marketing, as shown by the above illustration including:
Venclexta
Imbruvica
Risankizumab
Upadacitinib
Orilissa
In the Oncology division, Venclexta and Imbruvica offer a lot of promise to Abbvie. Venclexta earned FDA approval for the treatment of chronic lymphocytic leukemia (CLL) in April 2016. Upon examining Venclexta, we can see that Fierce Biotech is estimating that Venclexta could generate peak sales of $2 billion if it earns up to three separate breakthrough designations, which could potentially occur. Moreover, Imbruvica has shown tremendous promise and it appears as though the 2022 sales forecast by Fierce Biotech of $8.29 billion could prove to be accurate. According to the Abbvie news release, sales of Imbruvica totaled $972 million, representing a 41.3% YOY growth. It’s easy to see how these two oncology drugs alone could offset quite a bit of revenue decline in Humira over the coming years.
Moving to the immunology segment, the two most promising late-stage assets include Risankizumab and Upadacitinib. After a Phase III sweep by Risankizumab, it is reasonable to conclude that Risankizumab will be able to easily take market share from older drugs, such as Stelara and Abbvie’s own Humira. The only question regarding how much Abbvie is able to monetize this drug lies in how well it can compete with other newer, similar drugs including Eli Lilly’s Taltz, and Novartis’ Cosentyx as both of those drugs also delivered promising results in treating psoriasis. The blockbuster potential is there as Abbvie projects that Risankizumab could mature into a drug with peak sales of $4-5 billion. Regarding Upadacitinib, there is guidance from Abbvie that the drug could mature into $6.3 billion in sales by 2025.
This seems like a reason estimate, given that recent studies have shown the drug’s remission rates are 66%, double that of the standard of care (Methotrexate). The concern with Upadacitinib is the possibility that it may not be able to avoid fate of the black box warning cautioning against thrombosis that Eli Lilly’s Olumiant was forced to comply with when the FDA approved Olumiant with that caveat, in addition to only clearing the use of Olumiant in patients that have already tried drugs, such as Humira and Enbrel. If Upadacitinib is able to avoid the black box warning, it absolutely could become a $6+ billion blockbuster for Abbvie. I believe that the data of one adverse event out of the 631 patients treated with Upadacitinib in the trial is encouraging, but as with all pharma stocks, we won’t know for certain what the future holds for Abbvie’s recent Upadacitinib submission to the FDA until the company receives a decision from the FDA.
Finally, moving into the focused investment segment of Abbvie, we will discuss the most promising drug in that segment. As seen above, that drug is Elagolix aka Orilissa. Orilissa is the first FDA approved oral treatment for moderate to severe endometriosis pain in over a decade. The most encouraging news to come out of the FDA approval is that regulators green-lighted the product at two dosage strengths, without adding a black box warning for bone mineral density changes. This likely de-risks Orilissa and means that it could become the standard of care in treating moderate to severe endometriosis, a largely under-served market. Ultimately, Goldman Sachs analyst Jami Rubin believes that the drug could generate sales of $1 billion a year by 2020, and $2 billion a year by 2025 if industry experts are correct in their prediction of a uterine fibroids OK from the FDA by 2020.
Although the above figures that have been provided on the sales potential of the aforementioned drugs are just estimates, I believe these estimates are reasonable and even if some prove to be incorrect, it only takes one or two blockbuster drugs to be able to offset the eventual revenue declines in Humira, and drive earnings growth for years to come.
One final bearish concern present in the entire pharma industry is the worry that the government will eventually place more stringent pricing regulations on the drugs that Abbvie and other pharma giants produce. Back in July 2018, President Trump criticized and vaguely threatened Pfizer after it announced price hikes on over 40 of its drugs. Although this has been an issue that politicians have talked about for some time, as prescription expenditures rise and threaten to destroy Medicare budgets, it is reasonable to conclude the government will face no choice but to eventually take action to more stringently regulate drug price hikes, unless the billions of lobbying dollars by pharma continue to convince legislators to continue to ignore the issue. If/when this more stringent pricing regulation does happen, this would be a blow to the pharma industry as a whole, causing margins to crater. I don’t believe that this is an immediate threat to Abbvie or other pharma companies because as we all know, the federal government generally defers issues for years at a time. However, I do believe this is a risk that must be carefully monitored by investors in the pharma industry, in order to be proactive rather than reactive to a potential headwind in the industry.

Source: Abbvie Investor Presentation
In summary in regards to Abbvie’s growth story moving forward, I believe that Abbvie’s growth platform pitched in the above slide isn’t just another rosy picture that is portrayed by management. We haven’t even covered promising drugs in Abbvie’s early to mid stage development phase, so the thought that Abbvie could reach $35 billion in non-Humira sales by 2025 seems reasonable. The growth that is likely to occur in Imbruvica of another $4+ billion in sales by 2022, in combination with Venclexta peak sales of $2 billion, $4-5 billion from Risankizumab, $6+ billion from Upadacitinib, and $1-2 billion from Orilissa more than offset the projected $9 billion decline in Humira sales from 2020 to 2025. Even factoring in a couple of disappointing results in Abbvie’s pipeline, Abbvie shouldn’t face the same fate of over-reliance on one drug as Gilead Sciences did when its blockbuster Hep C drug, Harvoni experienced a sudden decline in sales. Having examined various drugs of Abbvie’s, both currently on the market and in development, there is certainly a lot of potential for growth moving forward. Having examined the company’s current dividend yield and the risks/opportunities that Abbvie is presented with, this leads me into my next point.
Reason #3: Abbvie’s Valuation is Currently Attractive
The above analysis would be all for naught if I didn’t believe that Abbvie’s current price is compelling for those willing to tolerate the risks mentioned above.
Though the stock has since rebounded strongly from its 52 week low of $77.50 a share, the December 28 share price of $91.12 represents a solid buying opportunity.
Abbvie’s current dividend yield of 4.7% compares very favorably to the 5 year average yield of 3.5%. If the company’s yield reverts back to its average yield of around 3.5%, this would result in a 34.3% increase in the stock price. This would translate into a current stock price of $122.36, which isn’t far off of what the stock’s 52 week high was in January. Of course, I can’t accurately predict if or when the company’s yield will revert back to 3.5%. It may take several years for Abbvie’s yield to revert to around 3.5% as the market waits for Abbvie’s diversification away from Humira to play out.
Moreover, the company’s earnings midpoint of $7.91 for FY 2018 compared against the current price of $91.12 means that Abbvie is sporting an 11.5 PE ratio. For a large-cap pharma stock with a projected 5 year annual growth rate of nearly 17%, this is an attractive entry point.
Summary:
It is my opinion that Abbvie’s dividend is currently safe and unless Abbvie is unable to offset eventual declines in Humira revenue (which I view as unlikely), the dividend should remain safe and continue to grow going forward.
As with any company, an investor must consider the future of the company, industry/company risks, and valuation, before making an investment. The biotech space faces their own set of risks, including concentration risk, patent expirations, generic competition, pipeline issues, and regulatory risks.
Despite Humira set to face stiff biosimilar competition in the United States starting in 2023, I believe Abbvie has the pipeline necessary to more than offset the eventual revenue declines in Humira, driving future growth.
As far as regulatory risks go, the risks that Abbvie faces are risks that its industry peers are also facing. Increased public and government scrutiny regarding drug price increases is a concern, but without rewarding companies such as Abbvie for serving the unmet health needs of patients, the outcome of a full-fledged regulatory backlash against pharma companies is one the United States and the world in general can’t afford. I don’t view this risk as an immediate risk, but one for investors to monitor coming years.
At the current stock price, I believe that the bearish arguments are being given too much credence, while the bullish arguments are not being given enough. This is the reason for the current disconnect between Abbvie’s stock price and its true valuation, from an objective standpoint. Abbvie’s 4.7% dividend yield and an 8% 5 year earnings growth rate (less than half the projected ~17% and just over a third of the previous 5 year growth rate), offers a 12.7% annual return over the next 5 years, assuming a static valuation multiple. Despite these perhaps overly conservative assumptions, Abbvie still offers one of the more compelling investment opportunities in today’s market for those who can tolerate the risks. I believe this creates an attractive entry point for both those considering initiating a position in Abbvie or those looking to add to their position.
Disclosure: I am/we are long ABBV. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
0 notes
Text
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential
New Post has been published on http://loanstop20.com/2019/01/04/abbvie-this-dividend-aristocrat-offers-double-digit-total-return-potential/
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential


Source: Abbvie Media Resources
With the financial markets likely to enter 2019 having just had the worst December since the Great Depression, there have been many stocks that have taken a beating the past month.
Abbvie (ABBV) has not been exempt from this market plunge as it has fallen nearly ~27.6% from its all-time high of $125.86 a share in January 2018. Moreover, Abbvie has fallen ~3.3% since the beginning of December, from $94.27 a share to $91.12 as of close on December 28, meaning the company has been hit harder than other pharma stocks this year. In addition to concerns over Abbvie’s concentration risk with Humira, another concern in the past 9 months to the investor community was Abbvie’s announcement this past March that it wouldn’t pursue accelerated approval for Rova T to treat RR SCLC, due to disappointing Phase 2 results. Although the company will move forward with the ongoing Phase 3 studies, investor consensus is that the $10.2 billion StemCentryx acquisition, once thought to be a promising oncology acquisition, will instead prove to be a bust. Consequently, the company’s shares have not rebounded to the $110+ range the shares were at January through most of March.
As such, I believe that Abbvie is currently worthy of a dividend growth investor’s consideration. There are three main reasons to support my opinion that Abbvie is an attractive investment, worthy of further due diligence from a potential investor. The first reason is that although its dividend history as an independent company has been brief, it has been spectacular, and I believe it will continue to deliver solid dividend increases going forward. This leads me into my second point that Abbvie has the appropriate drug pipeline to offset the eventual declines in revenue from Humira, which would lead to continued strong dividend increases. Lastly, the company’s current valuation is appealing.
Reason #1: Abbvie’s Safe And Growing Dividend
Since the formation of the company in 2013 as a spin-off of the biopharmaceuticals division of Abbott Laboratories, Abbvie has raised its dividend each year. Abbvie is technically a dividend aristocrat when we factor in its former parent company’s 46 years of consecutive dividend increases.
The company recently announced an 11.5% dividend increase from $0.96/share quarterly to current quarterly dividend of $1.07. Using the company’s midpoint earnings guidance of adjusted diluted EPS of $7.91/share for FY 2018, we can see that the payout ratio in terms of EPS is 54.1%. This is about where I’d like a pharma dividend payer to be at as it leaves the company plenty of capital to invest in the research and development that will be necessary to develop new blockbuster drugs in the future, not to mention also returning capital to shareholders in the form of share buybacks when the company’s shares are attractively priced.
Moreover, we can examine the company’s free cash flow numbers to arrive at the FCF dividend payout ratio. Per the company’s most recent financial release, Abbvie has generated FCF of $9.5 billion while paying $4.1 billion in dividends during that time. This would mean the payout ratio using FCF is roughly 43%.
Both methods of gauging the company’s payout ratio show us that the dividend is clearly safe and reasonable, allowing raises fairly similar to the most recent of 11.5%, while still heavily investing in the company’s future through R&D.
With a dividend yield of 4.7%, it is wise to question why the yield is so high currently. This leads us into our next point in which we’ll discuss the risks that Abbvie is facing, and why I believe these risks are being more than factored into the company’s stock price.
Reason #2: While Bears Argue Abbvie Will Not Be Able To Offset Humira’s Eventual Revenue Declines, I Believe The Contrary Is True
The primary concern that investors have had with Abbvie for several years is that Abbvie is too reliant on Humira. They conclude that because Humira accounts for 62% of Abbvie’s revenue as of Q3 2018 and 70%+ of its profits, when Humira sales eventually decline, the company’s prospects will also decline.
Fortunately, per company forecasts for the remainder of 2018, sales of Humira are as follows:
International: $6.3 billion (32%)
US: $13.7 billion (68%)
Total Humira sales: $20 billion
The company is currently facing tough biosimilar competition in the European Union as competitors such as Amgen, Biogen, Mylan, and Norvartis releasing biosimilar versions of Humira. With discounting of Humira ranging from as low as 10%, to as high as 80% in Nordic countries, international Humira sales are expected to decline 26-27% in 2019, per Reuters.

Source: Abbvie Investor Presentation
Although this isn’t the most encouraging news, the bulk of Humira’s sales are generated in the United States. Assuming a 27% decline in international Humira sales, the company’s international sales will decline by $1.7 billion. This would equate to a ~5% sales overall sales decline by itself. However, with strong Humira patent protection in the US, Abbvie won’t face any rival biosimilar Humira drugs in the US until 2023. In the meantime, Humira sales will continue to grow at a clip of roughly 10-12% in the US, before peaking at $21 billion in total sales in 2020. This would equate to an increase in Humira sales of nearly $1.4 billion to $1.6 billion. An overall decline in Humira sales of $100 million to $300 million would mean that the company will see a revenue decline of less than 1% in 2019, not accounting for any growth experienced from other drugs on the market or in the pipeline.

Source: Abbvie Investor Presentation
Abbvie’s management recognizes the threats posed by concentration risk from Humira and they have developed a corporate strategy focused on extensive research and development to drive innovation, and deliver results to shareholders in the process.
Even if we assume that Rova T will be a complete bust, producing no revenue for Abbvie, and that Humira’s sales will fall considerably in the next 5 years, Abbvie has several promising drugs at various phases of development and marketing, as shown by the above illustration including:
Venclexta
Imbruvica
Risankizumab
Upadacitinib
Orilissa
In the Oncology division, Venclexta and Imbruvica offer a lot of promise to Abbvie. Venclexta earned FDA approval for the treatment of chronic lymphocytic leukemia (CLL) in April 2016. Upon examining Venclexta, we can see that Fierce Biotech is estimating that Venclexta could generate peak sales of $2 billion if it earns up to three separate breakthrough designations, which could potentially occur. Moreover, Imbruvica has shown tremendous promise and it appears as though the 2022 sales forecast by Fierce Biotech of $8.29 billion could prove to be accurate. According to the Abbvie news release, sales of Imbruvica totaled $972 million, representing a 41.3% YOY growth. It’s easy to see how these two oncology drugs alone could offset quite a bit of revenue decline in Humira over the coming years.
Moving to the immunology segment, the two most promising late-stage assets include Risankizumab and Upadacitinib. After a Phase III sweep by Risankizumab, it is reasonable to conclude that Risankizumab will be able to easily take market share from older drugs, such as Stelara and Abbvie’s own Humira. The only question regarding how much Abbvie is able to monetize this drug lies in how well it can compete with other newer, similar drugs including Eli Lilly’s Taltz, and Novartis’ Cosentyx as both of those drugs also delivered promising results in treating psoriasis. The blockbuster potential is there as Abbvie projects that Risankizumab could mature into a drug with peak sales of $4-5 billion. Regarding Upadacitinib, there is guidance from Abbvie that the drug could mature into $6.3 billion in sales by 2025.
This seems like a reason estimate, given that recent studies have shown the drug’s remission rates are 66%, double that of the standard of care (Methotrexate). The concern with Upadacitinib is the possibility that it may not be able to avoid fate of the black box warning cautioning against thrombosis that Eli Lilly’s Olumiant was forced to comply with when the FDA approved Olumiant with that caveat, in addition to only clearing the use of Olumiant in patients that have already tried drugs, such as Humira and Enbrel. If Upadacitinib is able to avoid the black box warning, it absolutely could become a $6+ billion blockbuster for Abbvie. I believe that the data of one adverse event out of the 631 patients treated with Upadacitinib in the trial is encouraging, but as with all pharma stocks, we won’t know for certain what the future holds for Abbvie’s recent Upadacitinib submission to the FDA until the company receives a decision from the FDA.
Finally, moving into the focused investment segment of Abbvie, we will discuss the most promising drug in that segment. As seen above, that drug is Elagolix aka Orilissa. Orilissa is the first FDA approved oral treatment for moderate to severe endometriosis pain in over a decade. The most encouraging news to come out of the FDA approval is that regulators green-lighted the product at two dosage strengths, without adding a black box warning for bone mineral density changes. This likely de-risks Orilissa and means that it could become the standard of care in treating moderate to severe endometriosis, a largely under-served market. Ultimately, Goldman Sachs analyst Jami Rubin believes that the drug could generate sales of $1 billion a year by 2020, and $2 billion a year by 2025 if industry experts are correct in their prediction of a uterine fibroids OK from the FDA by 2020.
Although the above figures that have been provided on the sales potential of the aforementioned drugs are just estimates, I believe these estimates are reasonable and even if some prove to be incorrect, it only takes one or two blockbuster drugs to be able to offset the eventual revenue declines in Humira, and drive earnings growth for years to come.
One final bearish concern present in the entire pharma industry is the worry that the government will eventually place more stringent pricing regulations on the drugs that Abbvie and other pharma giants produce. Back in July 2018, President Trump criticized and vaguely threatened Pfizer after it announced price hikes on over 40 of its drugs. Although this has been an issue that politicians have talked about for some time, as prescription expenditures rise and threaten to destroy Medicare budgets, it is reasonable to conclude the government will face no choice but to eventually take action to more stringently regulate drug price hikes, unless the billions of lobbying dollars by pharma continue to convince legislators to continue to ignore the issue. If/when this more stringent pricing regulation does happen, this would be a blow to the pharma industry as a whole, causing margins to crater. I don’t believe that this is an immediate threat to Abbvie or other pharma companies because as we all know, the federal government generally defers issues for years at a time. However, I do believe this is a risk that must be carefully monitored by investors in the pharma industry, in order to be proactive rather than reactive to a potential headwind in the industry.

Source: Abbvie Investor Presentation
In summary in regards to Abbvie’s growth story moving forward, I believe that Abbvie’s growth platform pitched in the above slide isn’t just another rosy picture that is portrayed by management. We haven’t even covered promising drugs in Abbvie’s early to mid stage development phase, so the thought that Abbvie could reach $35 billion in non-Humira sales by 2025 seems reasonable. The growth that is likely to occur in Imbruvica of another $4+ billion in sales by 2022, in combination with Venclexta peak sales of $2 billion, $4-5 billion from Risankizumab, $6+ billion from Upadacitinib, and $1-2 billion from Orilissa more than offset the projected $9 billion decline in Humira sales from 2020 to 2025. Even factoring in a couple of disappointing results in Abbvie’s pipeline, Abbvie shouldn’t face the same fate of over-reliance on one drug as Gilead Sciences did when its blockbuster Hep C drug, Harvoni experienced a sudden decline in sales. Having examined various drugs of Abbvie’s, both currently on the market and in development, there is certainly a lot of potential for growth moving forward. Having examined the company’s current dividend yield and the risks/opportunities that Abbvie is presented with, this leads me into my next point.
Reason #3: Abbvie’s Valuation is Currently Attractive
The above analysis would be all for naught if I didn’t believe that Abbvie’s current price is compelling for those willing to tolerate the risks mentioned above.
Though the stock has since rebounded strongly from its 52 week low of $77.50 a share, the December 28 share price of $91.12 represents a solid buying opportunity.
Abbvie’s current dividend yield of 4.7% compares very favorably to the 5 year average yield of 3.5%. If the company’s yield reverts back to its average yield of around 3.5%, this would result in a 34.3% increase in the stock price. This would translate into a current stock price of $122.36, which isn’t far off of what the stock’s 52 week high was in January. Of course, I can’t accurately predict if or when the company’s yield will revert back to 3.5%. It may take several years for Abbvie’s yield to revert to around 3.5% as the market waits for Abbvie’s diversification away from Humira to play out.
Moreover, the company’s earnings midpoint of $7.91 for FY 2018 compared against the current price of $91.12 means that Abbvie is sporting an 11.5 PE ratio. For a large-cap pharma stock with a projected 5 year annual growth rate of nearly 17%, this is an attractive entry point.
Summary:
It is my opinion that Abbvie’s dividend is currently safe and unless Abbvie is unable to offset eventual declines in Humira revenue (which I view as unlikely), the dividend should remain safe and continue to grow going forward.
As with any company, an investor must consider the future of the company, industry/company risks, and valuation, before making an investment. The biotech space faces their own set of risks, including concentration risk, patent expirations, generic competition, pipeline issues, and regulatory risks.
Despite Humira set to face stiff biosimilar competition in the United States starting in 2023, I believe Abbvie has the pipeline necessary to more than offset the eventual revenue declines in Humira, driving future growth.
As far as regulatory risks go, the risks that Abbvie faces are risks that its industry peers are also facing. Increased public and government scrutiny regarding drug price increases is a concern, but without rewarding companies such as Abbvie for serving the unmet health needs of patients, the outcome of a full-fledged regulatory backlash against pharma companies is one the United States and the world in general can’t afford. I don’t view this risk as an immediate risk, but one for investors to monitor coming years.
At the current stock price, I believe that the bearish arguments are being given too much credence, while the bullish arguments are not being given enough. This is the reason for the current disconnect between Abbvie’s stock price and its true valuation, from an objective standpoint. Abbvie’s 4.7% dividend yield and an 8% 5 year earnings growth rate (less than half the projected ~17% and just over a third of the previous 5 year growth rate), offers a 12.7% annual return over the next 5 years, assuming a static valuation multiple. Despite these perhaps overly conservative assumptions, Abbvie still offers one of the more compelling investment opportunities in today’s market for those who can tolerate the risks. I believe this creates an attractive entry point for both those considering initiating a position in Abbvie or those looking to add to their position.
Disclosure: I am/we are long ABBV. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
0 notes
Text
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential
New Post has been published on http://brummy80.com/abbvie-this-dividend-aristocrat-offers-double-digit-total-return-potential/
AbbVie: This Dividend Aristocrat Offers Double-Digit Total Return Potential


Source: Abbvie Media Resources
With the financial markets likely to enter 2019 having just had the worst December since the Great Depression, there have been many stocks that have taken a beating the past month.
Abbvie (ABBV) has not been exempt from this market plunge as it has fallen nearly ~27.6% from its all-time high of $125.86 a share in January 2018. Moreover, Abbvie has fallen ~3.3% since the beginning of December, from $94.27 a share to $91.12 as of close on December 28, meaning the company has been hit harder than other pharma stocks this year. In addition to concerns over Abbvie’s concentration risk with Humira, another concern in the past 9 months to the investor community was Abbvie’s announcement this past March that it wouldn’t pursue accelerated approval for Rova T to treat RR SCLC, due to disappointing Phase 2 results. Although the company will move forward with the ongoing Phase 3 studies, investor consensus is that the $10.2 billion StemCentryx acquisition, once thought to be a promising oncology acquisition, will instead prove to be a bust. Consequently, the company’s shares have not rebounded to the $110+ range the shares were at January through most of March.
As such, I believe that Abbvie is currently worthy of a dividend growth investor’s consideration. There are three main reasons to support my opinion that Abbvie is an attractive investment, worthy of further due diligence from a potential investor. The first reason is that although its dividend history as an independent company has been brief, it has been spectacular, and I believe it will continue to deliver solid dividend increases going forward. This leads me into my second point that Abbvie has the appropriate drug pipeline to offset the eventual declines in revenue from Humira, which would lead to continued strong dividend increases. Lastly, the company’s current valuation is appealing.
Reason #1: Abbvie’s Safe And Growing Dividend
Since the formation of the company in 2013 as a spin-off of the biopharmaceuticals division of Abbott Laboratories, Abbvie has raised its dividend each year. Abbvie is technically a dividend aristocrat when we factor in its former parent company’s 46 years of consecutive dividend increases.
The company recently announced an 11.5% dividend increase from $0.96/share quarterly to current quarterly dividend of $1.07. Using the company’s midpoint earnings guidance of adjusted diluted EPS of $7.91/share for FY 2018, we can see that the payout ratio in terms of EPS is 54.1%. This is about where I’d like a pharma dividend payer to be at as it leaves the company plenty of capital to invest in the research and development that will be necessary to develop new blockbuster drugs in the future, not to mention also returning capital to shareholders in the form of share buybacks when the company’s shares are attractively priced.
Moreover, we can examine the company’s free cash flow numbers to arrive at the FCF dividend payout ratio. Per the company’s most recent financial release, Abbvie has generated FCF of $9.5 billion while paying $4.1 billion in dividends during that time. This would mean the payout ratio using FCF is roughly 43%.
Both methods of gauging the company’s payout ratio show us that the dividend is clearly safe and reasonable, allowing raises fairly similar to the most recent of 11.5%, while still heavily investing in the company’s future through R&D.
With a dividend yield of 4.7%, it is wise to question why the yield is so high currently. This leads us into our next point in which we’ll discuss the risks that Abbvie is facing, and why I believe these risks are being more than factored into the company’s stock price.
Reason #2: While Bears Argue Abbvie Will Not Be Able To Offset Humira’s Eventual Revenue Declines, I Believe The Contrary Is True
The primary concern that investors have had with Abbvie for several years is that Abbvie is too reliant on Humira. They conclude that because Humira accounts for 62% of Abbvie’s revenue as of Q3 2018 and 70%+ of its profits, when Humira sales eventually decline, the company’s prospects will also decline.
Fortunately, per company forecasts for the remainder of 2018, sales of Humira are as follows:
International: $6.3 billion (32%)
US: $13.7 billion (68%)
Total Humira sales: $20 billion
The company is currently facing tough biosimilar competition in the European Union as competitors such as Amgen, Biogen, Mylan, and Norvartis releasing biosimilar versions of Humira. With discounting of Humira ranging from as low as 10%, to as high as 80% in Nordic countries, international Humira sales are expected to decline 26-27% in 2019, per Reuters.

Source: Abbvie Investor Presentation
Although this isn’t the most encouraging news, the bulk of Humira’s sales are generated in the United States. Assuming a 27% decline in international Humira sales, the company’s international sales will decline by $1.7 billion. This would equate to a ~5% sales overall sales decline by itself. However, with strong Humira patent protection in the US, Abbvie won’t face any rival biosimilar Humira drugs in the US until 2023. In the meantime, Humira sales will continue to grow at a clip of roughly 10-12% in the US, before peaking at $21 billion in total sales in 2020. This would equate to an increase in Humira sales of nearly $1.4 billion to $1.6 billion. An overall decline in Humira sales of $100 million to $300 million would mean that the company will see a revenue decline of less than 1% in 2019, not accounting for any growth experienced from other drugs on the market or in the pipeline.

Source: Abbvie Investor Presentation
Abbvie’s management recognizes the threats posed by concentration risk from Humira and they have developed a corporate strategy focused on extensive research and development to drive innovation, and deliver results to shareholders in the process.
Even if we assume that Rova T will be a complete bust, producing no revenue for Abbvie, and that Humira’s sales will fall considerably in the next 5 years, Abbvie has several promising drugs at various phases of development and marketing, as shown by the above illustration including:
Venclexta
Imbruvica
Risankizumab
Upadacitinib
Orilissa
In the Oncology division, Venclexta and Imbruvica offer a lot of promise to Abbvie. Venclexta earned FDA approval for the treatment of chronic lymphocytic leukemia (CLL) in April 2016. Upon examining Venclexta, we can see that Fierce Biotech is estimating that Venclexta could generate peak sales of $2 billion if it earns up to three separate breakthrough designations, which could potentially occur. Moreover, Imbruvica has shown tremendous promise and it appears as though the 2022 sales forecast by Fierce Biotech of $8.29 billion could prove to be accurate. According to the Abbvie news release, sales of Imbruvica totaled $972 million, representing a 41.3% YOY growth. It’s easy to see how these two oncology drugs alone could offset quite a bit of revenue decline in Humira over the coming years.
Moving to the immunology segment, the two most promising late-stage assets include Risankizumab and Upadacitinib. After a Phase III sweep by Risankizumab, it is reasonable to conclude that Risankizumab will be able to easily take market share from older drugs, such as Stelara and Abbvie’s own Humira. The only question regarding how much Abbvie is able to monetize this drug lies in how well it can compete with other newer, similar drugs including Eli Lilly’s Taltz, and Novartis’ Cosentyx as both of those drugs also delivered promising results in treating psoriasis. The blockbuster potential is there as Abbvie projects that Risankizumab could mature into a drug with peak sales of $4-5 billion. Regarding Upadacitinib, there is guidance from Abbvie that the drug could mature into $6.3 billion in sales by 2025.
This seems like a reason estimate, given that recent studies have shown the drug’s remission rates are 66%, double that of the standard of care (Methotrexate). The concern with Upadacitinib is the possibility that it may not be able to avoid fate of the black box warning cautioning against thrombosis that Eli Lilly’s Olumiant was forced to comply with when the FDA approved Olumiant with that caveat, in addition to only clearing the use of Olumiant in patients that have already tried drugs, such as Humira and Enbrel. If Upadacitinib is able to avoid the black box warning, it absolutely could become a $6+ billion blockbuster for Abbvie. I believe that the data of one adverse event out of the 631 patients treated with Upadacitinib in the trial is encouraging, but as with all pharma stocks, we won’t know for certain what the future holds for Abbvie’s recent Upadacitinib submission to the FDA until the company receives a decision from the FDA.
Finally, moving into the focused investment segment of Abbvie, we will discuss the most promising drug in that segment. As seen above, that drug is Elagolix aka Orilissa. Orilissa is the first FDA approved oral treatment for moderate to severe endometriosis pain in over a decade. The most encouraging news to come out of the FDA approval is that regulators green-lighted the product at two dosage strengths, without adding a black box warning for bone mineral density changes. This likely de-risks Orilissa and means that it could become the standard of care in treating moderate to severe endometriosis, a largely under-served market. Ultimately, Goldman Sachs analyst Jami Rubin believes that the drug could generate sales of $1 billion a year by 2020, and $2 billion a year by 2025 if industry experts are correct in their prediction of a uterine fibroids OK from the FDA by 2020.
Although the above figures that have been provided on the sales potential of the aforementioned drugs are just estimates, I believe these estimates are reasonable and even if some prove to be incorrect, it only takes one or two blockbuster drugs to be able to offset the eventual revenue declines in Humira, and drive earnings growth for years to come.
One final bearish concern present in the entire pharma industry is the worry that the government will eventually place more stringent pricing regulations on the drugs that Abbvie and other pharma giants produce. Back in July 2018, President Trump criticized and vaguely threatened Pfizer after it announced price hikes on over 40 of its drugs. Although this has been an issue that politicians have talked about for some time, as prescription expenditures rise and threaten to destroy Medicare budgets, it is reasonable to conclude the government will face no choice but to eventually take action to more stringently regulate drug price hikes, unless the billions of lobbying dollars by pharma continue to convince legislators to continue to ignore the issue. If/when this more stringent pricing regulation does happen, this would be a blow to the pharma industry as a whole, causing margins to crater. I don’t believe that this is an immediate threat to Abbvie or other pharma companies because as we all know, the federal government generally defers issues for years at a time. However, I do believe this is a risk that must be carefully monitored by investors in the pharma industry, in order to be proactive rather than reactive to a potential headwind in the industry.

Source: Abbvie Investor Presentation
In summary in regards to Abbvie’s growth story moving forward, I believe that Abbvie’s growth platform pitched in the above slide isn’t just another rosy picture that is portrayed by management. We haven’t even covered promising drugs in Abbvie’s early to mid stage development phase, so the thought that Abbvie could reach $35 billion in non-Humira sales by 2025 seems reasonable. The growth that is likely to occur in Imbruvica of another $4+ billion in sales by 2022, in combination with Venclexta peak sales of $2 billion, $4-5 billion from Risankizumab, $6+ billion from Upadacitinib, and $1-2 billion from Orilissa more than offset the projected $9 billion decline in Humira sales from 2020 to 2025. Even factoring in a couple of disappointing results in Abbvie’s pipeline, Abbvie shouldn’t face the same fate of over-reliance on one drug as Gilead Sciences did when its blockbuster Hep C drug, Harvoni experienced a sudden decline in sales. Having examined various drugs of Abbvie’s, both currently on the market and in development, there is certainly a lot of potential for growth moving forward. Having examined the company’s current dividend yield and the risks/opportunities that Abbvie is presented with, this leads me into my next point.
Reason #3: Abbvie’s Valuation is Currently Attractive
The above analysis would be all for naught if I didn’t believe that Abbvie’s current price is compelling for those willing to tolerate the risks mentioned above.
Though the stock has since rebounded strongly from its 52 week low of $77.50 a share, the December 28 share price of $91.12 represents a solid buying opportunity.
Abbvie’s current dividend yield of 4.7% compares very favorably to the 5 year average yield of 3.5%. If the company’s yield reverts back to its average yield of around 3.5%, this would result in a 34.3% increase in the stock price. This would translate into a current stock price of $122.36, which isn’t far off of what the stock’s 52 week high was in January. Of course, I can’t accurately predict if or when the company’s yield will revert back to 3.5%. It may take several years for Abbvie’s yield to revert to around 3.5% as the market waits for Abbvie’s diversification away from Humira to play out.
Moreover, the company’s earnings midpoint of $7.91 for FY 2018 compared against the current price of $91.12 means that Abbvie is sporting an 11.5 PE ratio. For a large-cap pharma stock with a projected 5 year annual growth rate of nearly 17%, this is an attractive entry point.
Summary:
It is my opinion that Abbvie’s dividend is currently safe and unless Abbvie is unable to offset eventual declines in Humira revenue (which I view as unlikely), the dividend should remain safe and continue to grow going forward.
As with any company, an investor must consider the future of the company, industry/company risks, and valuation, before making an investment. The biotech space faces their own set of risks, including concentration risk, patent expirations, generic competition, pipeline issues, and regulatory risks.
Despite Humira set to face stiff biosimilar competition in the United States starting in 2023, I believe Abbvie has the pipeline necessary to more than offset the eventual revenue declines in Humira, driving future growth.
As far as regulatory risks go, the risks that Abbvie faces are risks that its industry peers are also facing. Increased public and government scrutiny regarding drug price increases is a concern, but without rewarding companies such as Abbvie for serving the unmet health needs of patients, the outcome of a full-fledged regulatory backlash against pharma companies is one the United States and the world in general can’t afford. I don’t view this risk as an immediate risk, but one for investors to monitor coming years.
At the current stock price, I believe that the bearish arguments are being given too much credence, while the bullish arguments are not being given enough. This is the reason for the current disconnect between Abbvie’s stock price and its true valuation, from an objective standpoint. Abbvie’s 4.7% dividend yield and an 8% 5 year earnings growth rate (less than half the projected ~17% and just over a third of the previous 5 year growth rate), offers a 12.7% annual return over the next 5 years, assuming a static valuation multiple. Despite these perhaps overly conservative assumptions, Abbvie still offers one of the more compelling investment opportunities in today’s market for those who can tolerate the risks. I believe this creates an attractive entry point for both those considering initiating a position in Abbvie or those looking to add to their position.
Disclosure: I am/we are long ABBV. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
0 notes
Text
Female Infertility: Opportunity Analysis and Forecasts to 2028 published on
http://www.sandlerresearch.org/female-infertility-opportunity-analysis-and-forecasts-to-2028.html
Female Infertility: Opportunity Analysis and Forecasts to 2028
Female Infertility: Opportunity Analysis and Forecasts to 2028
Summary
Female infertility (FI) is a complex disorder defined as the inability to conceive after at least one year of timed, unprotected intercourse. The etiology of the condition spans a wide range of disorders that can be broadly classified into the following categories: diminished ovarian reserve & ovulation disorders, endometriosis, fallopian tube abnormalities, uterine factors and unexplained infertility. Several factors may be considered before a treatment decision is made, including treatment effectiveness (live birth rate), treatment burden (such as number of required injections and monitoring appointments), safety (such as risk of multiple gestation or ovarian hyperstimulation), and cost of therapy. Overall, the most common treatments include ovulation induction drugs and/or advanced techniques, referred to as Assisted Reproductive Technology (ART), such as in vitro fertilization (IVF).
Key Highlights
The greatest drivers of growth in the global FI market are a growing number of women are postponing pregnancy until later in life and at a reproductively older age when fertility has generally decreased and the launch of nolasiban in the US and 5EU, the first major innovation in this indication in many years. The main barriers to growth in the female infertility market include the sparse pipeline for new female infertility treatments, and the limited number of innovative drugs under development. Among the late-stage pipeline products, the launch of nolasiban early in the forecast period will add significant growth to sales by 2028. The most important unmet needs in FI include further improving IVF success rates, orally and less frequently administered drugs involved in IVF, additional treatment options for reproductively older infertile women and young women with diminished ovarian reserve (DOR), development of novel non-surgical treatment options for the management of uterine fibroid-related infertility and reducing high IVF treatment patient dropout rates.
KEY QUESTIONS ANSWERED
Despite progress made in the management of female infertility since the introduction of ART and other improvements in treating patients, KOLs thought challenges associated with treatment remain, throughout the 7MM. – Which unmet needs are the most pressing in the 7MM? – Where should pharmaceutical companies focus drug development efforts in order to become a significant player in the space? GlobalData expects that pipeline development of nolasiban will contribute significantly to the growth of the FI market going forward. – How much is nolasiban expected to generate over the forecast period? – What other assets are under development and how do KOLs see them competing against current treatment options? – Although female infertility is a prevalent condition, it remains a niche indication on the industry level. – Which have been historically the companies leading the way? – What new companies are emerging in the space?
Scope
– Overview of Female Infertility including epidemiology, etiology and current treatment options, namely ovulation induction and ART – Topline Female Infertility market revenue, annual cost of therapy, and major pipeline product sales in the forecast period. – Key topics covered include current treatment and pipeline therapies, unmet needs and opportunities, and the drivers and barriers affecting Female Infertility therapeutics sales in the 7MM. – Pipeline analysis: Comprehensive data split across different phases, emerging novel trends under development, and detailed analysis of late-stage pipeline drugs. – Analysis of the current and future market competition in the global Female Infertility therapeutics market. Insightful review of the key industry drivers, restraints and challenges. Each trend is independently researched to provide qualitative analysis of its implications.
Reasons to buy
The report will enable you to – – Develop and design your in-licensing and out-licensing strategies, using a detailed overview of current pipeline products and technologies to identify companies with the most robust pipelines. – Develop business strategies by understanding the trends shaping and driving the global Female Infertility therapeutics market. – Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the global Female Infertility market in the future. – Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the performance of various competitors. – Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage. – Track drug sales in the global Female Infertility therapeutics market from 2018-2028. – Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments and strategic partnerships.
0 notes
Text
Women’s Health Market to Expand at a Modest CAGR of 5.7% by 2025
The global women’s health market has been analyzed on the basis of treatment type, disease indication and geography. Major treatment type segments are hormonal treatment and non-hormonal treatment. Hormonal treatment segment is subdivided into estrogen therapy, progestin therapy, combination therapy, thyroid replacement therapy, parathyroid hormone therapy, and others. While non-hormonal treatment segment considers cancer targeted therapy drugs, antibiotics, bisphosphonates, vitamin D treatment, calcitonin, RANK-Ligand, non-steroidal anti-inflammatory drugs and others. The disease indication segment of global women’s health market bifurcated into breast cancer, cervical cancer, ovarian cancer, hypothyroidism, post-menopausal syndrome, osteoporosis, contraceptive, uterine fibroid, urinary tract infection, and other disease indications. Geographically, global women’s health market is divided into major five geographical regions, including North America, Europe, Asia-Pacific, Latin America and Middle East and Africa.
Obtain Report Details @
https://www.transparencymarketresearch.com/women-health-therapeutics-market.html
Non-hormonal Treatments to Remain Popular Segment Under Global Women’s Health Market
The non-hormonal treatment segment accounted for significant share of the global women’s health market in 2016. The segment is projected to be the most lucrative market for global women’s health market during the forecast period owing to the effectiveness of the products, growing adoption of these products, and new product development are the key factors for the segment growth. The decreasing use of hormonal products due to the risk of breast cancer has boosted the demand for non-hormonal pharmaceutical products. According to eHealthMe, about 52.3% of women with menopause uses antidepressant therapy between the age of 50 and 59.
Request for Sample Copy of Report @
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=214
Asia Pacific to Show Keen Interest in Women’s Health as Awareness Increases
The U.S. is estimated to remain the largest market for women’s health therapeutics due to high investments made in cutting-edge drug research resulting in new, sophisticated treatments with specific mechanism of action; rising emphasis on cancer care, and concentration of some of the world’s major pharmaceutical and biotechnology organizations in the region. While Asia pacific market is anticipated to grow with highest CAGR during forecast period. Emerging markets with their populations in billions have become a potent source of revenue. Health care in developing countries is undergoing rapid changes.
Request Report Brochure @
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=214
The BRICS (Brazil, Russia, India, China, and South Africa) countries are among the fastest-growing economies in the world. According to Siemens Healthineers, health care expenditure in emerging economies has increased 11% from 1995 to 2012 and expected to reach 33% in 2022. Rising population in emerging countries will lead to increased demand for pharmaceutical and biopharmaceutical products in the near future. Rising geriatric female population and economic growth are likely to create large opportunity in the women’s health market.
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Contact Us
Transparency Market Research State Tower, 90 State Street, Suite 700 Albany, NY 12207 United States Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Email: [email protected] Website: www.transparencymarketresearch.com
0 notes
Link
Embolization Particle or embolisation refers to the passage and lodging of an embolus within the bloodstream. It may be pathological (in which sense it is also called embolism), for example a pulmonary embolism, or therapeutic, as a hemostatic treatment for bleeding or as a treatment for some types of cancer by deliberately blocking blood vessels to starve the tumor cells. Scope of the Report: This report focuses on the Embolization Particle in Global market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This report categorizes the market based on manufacturers, regions, type and application. Market Segment by Manufacturers, this report covers Sirtex Medical Merit Medical Cook Medical BTG Medical Boston Scientific Corporation Terumo Corporation HENGRUI Medical INterface BIOmaterials B.V. Alicon Market Segment by Regions, regional analysis covers North America (United States, Canada and Mexico) Europe (Germany, France, UK, Russia and Italy) Asia-Pacific (China, Japan, Korea, India and Southeast Asia) South America (Brazil, Argentina, Colombia etc.) Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa) Market Segment by Type, covers Microspheres Particles (e.g. PVA Particles, Gelfoam Particles) Drug-eluting Beads (DEBs) Radio-Embolic Microspheres (e.g. Therasphere and SIRSphere) Market Segment by Applications, can be divided into Uterine Fibroid Embolization Prostatic Artery Embolization (treatment for Benign Prostatic Hyperplasia or BPH) Liver Tumor Embolization Trauma Embolization Other There are 15 Chapters to deeply display the global Embolization Particle market. Chapter 1, to describe Embolization Particle Introduction, product scope, market overview, market opportunities, market risk, market driving force; Chapter 2, to analyze the top manufacturers of Embolization Particle, with sales, revenue, and price of Embolization Particle, in 2016 and 2017; Chapter 3, to display the competitive situation among the top manufacturers, with sales, revenue and market share in 2016 and 2017; Chapter 4, to show the global market by regions, with sales, revenue and market share of Embolization Particle, for each region, from 2013 to 2018; Chapter 5, 6, 7, 8 and 9, to analyze the market by countries, by type, by application and by manufacturers, with sales, revenue and market share by key countries in these regions; Chapter 10 and 11, to show the market by type and application, with sales market share and growth rate by type, application, from 2013 to 2018; Chapter 12, Embolization Particle market forecast, by regions, type and application, with sales and revenue, from 2018 to 2023; Chapter 13, 14 and 15, to describe Embolization Particle sales channel, distributors, traders, dealers, Research Findings and Conclusion, appendix and data source
#(north america#asia-pacific#america#and africa) embolization particle market#and africa) embolization particle market trends#and africa) embolization particle market size#and africa) embolization particle market data#and africa) embolization particle market structure#and africa) embolization particle industry analysis#and africa) embolization particle market research
0 notes
Text
Uterine Fibroid Diagnostics and Therapeutics Market to Garner Brimming Revenues by 2024
Uterine fibroid is a type of tumor that arises from the smooth muscle tissues of uterus. Common non-cancerous growths that develop in the muscular wall of the uterus, they are also known as leiomyoma (benign tumor), fibroleiomyoma, fibromyoma and myoma. They vary in size from tiny to larger than a cantaloupe. Sometimes, they led the uterus to grow to the size of a five-month pregnancy. Fibroids presence usually is multiple is number and if its number is uncountable, it is referred to as uterine leiomyomatosis. Fibroids are amongst the most common form of benign tumors and usually affect the females during the middle of reproductive age and later reproductive age. The malignant form of fibroid, known as leiomyosarcoma, is very uncommon.
Uterine fibroids are usually asymptomatic, however once they start growing, they create various problems such as painful and heavy menstruation, affecting urine urgency and frequency, and painful sexual intercourse. It sometimes, may also interfere with pregnancy and dramatically increase in size during pregnancy. It is thought to be because of increase in levels of estrogen during pregnancy. It does not affect the women post menopause as the level of estrogen that circulates in the blood, reduces drastically. However, women who are taking estrogen supplement in hormone replacement therapy are expected to experience the symptoms disease.
Uterine fibroids are of various types namely subserosal fibroids, intramural fibroids and submucosal fibroids. Subserosal fibroids develop under the uterus outside covering and expand outward through uterine wall and result in knobby appearance of the uterus. Intramural fibroids develop into the lining of the uterus and hence, expand inward resulting in increase in the size of uterus. Amongst all, it is the most common uterine fibroids. Submucosal fibroids develop under the uterus linings and it causes various medical conditions such as heavy bleeding and prolonged menstruation. It is rarest of all uterus fibroids.
Request to View Brochure of Report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=14963
Uterine fibroids can be diagnosed using various imaging modalities such as medical resonance imaging (MRI) scanners, computed tomography (CT) scanners and ultrasound devices. Amongst all, ultrasound devices are used extensively for diagnosing uterine fibroids owing to its efficiency and popularity for gynecological purpose. Also, it does not produce any harmful rays that may harm the fetus (if women suffering from uterine fibroids is pregnant), increasing its preference among radiologist. Other diagnosing procedures include hysterosonography, hysterosalpingography and hysteroscopy.
Various treatment procedures have been adopted by physicians to cure the disease that include nonsurgical uterine fibroid embolization, magnetic resonance guided focused ultrasound, myolysis, endometrial ablation and resection of submucosal fibroids, hysterectomy and myomectomy surgery. Among all the aforementioned treatment procedures, nonsurgical uterine fibroid embolization is the most extensive used method of treatment. Various medications are also advised to the patients suffering from uterine fibroids that include gonadotropin-releasing hormone agonists, progestin-releasing intrauterin, progestins or oral contraceptives and nonsteroidal anti-inflammatory drugs.
The Uterine Fibroid Diagnostics and Therapeutics Market is expected to grow at a significant CAGR during 2014 to 2020. This growth is primarily driven by increasing number of women of reproductive age (WRA) across the globe. According to the Centers for Disease Control and Prevention (CDC), approximately 60 million WRA were reported in 2012 and this number is expected to rise with increasing number of women globally. According to the World Data Book, total population of women was around 3,439.4 million in 2011 and this number is expected to grow due to improving education systems mainly in developing countries such as India and China. Other factors that are also supporting the growth of uterine fibroid diagnostics and therapeutics market are rising prevalence of uterine fibroids and change in lifestyle mainly dietary pattern. Low intake of fruits and green vegetables increases the risk for developing uterine fibroids.
Enquiry for discount on this report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=14963
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: http://www.transparencymarketresearch.com
0 notes
Text
U.S.Uterine Fibroid Market 2018 - Industry Growth, Analysis, Size and Share to 2022
Uterine Fibroids are the abnormal growth that gets developed inside or on the wall of a woman’s uterus. These are also known as leiomyoma or myomas. The U.S. Uterine Fibroids Market is expected to reach USD 39,993.2 million by the end of the forecasted period and is expected to grow at a CAGR of 3.24%.
Uterine fibroids are the noncancerous growths of the uterus, which mostly appear during childbearing years. It is the abnormal growth of that gets developed inside or on the wall of a woman’s uterus. Uterine fibroids is also known as myomas or leiomyomas. The symptom of uterine fibroids are frequent urination, constipation, backache, heavy menstrual bleeding, pelvic pressure, or menstrual periods lasting more than a week. The women with mild symptoms may go for medical treatments such as hormonal drugs, gonadotropin agonists, progestin and oral contraceptives. Women with moderate to severe symptoms may need surgery to treat uterine fibroids. According to the U.S. Department of Health and Human Services office on Women Health, about 20% to 80% of women develop fibroids by the age of 50 and nearly 30% will get them at the age of 35. Uterine fibroids are most common in women in their 40s and early 50s.
U.S. Uterine Fibroid Market - Key Players
Some of the key players in this market are:
· AstraZeneca (England)
· Cook Medical Inc. (US)
· Boston Scientific Corporation (US)
· GE Healthcare (U.K.)
· AbbVie Inc. (U.S.)
· Hologic (U.S.)
U.S. Uterine Fibroid Market - Study Objectives
· To provide detailed analysis of the market structure along with forecast for the next 5 years of the various segments and sub-segments of the U.S. Uterine Fibroid Market
· To provide insights about factors affecting the market growth
· To analyze the market based on various factors - price analysis, supply chain analysis, porters five force analysis etc.
· To provide historical and forecast revenue of the market segments and sub-segments with respect to four main geographies and their countries- Americas, Europe, Asia-Pacific, and Middle East & Africa.
Click to Get Sample Report @ https://www.marketresearchfuture.com/sample_request/2394 .
U.S. Uterine Fibroid Market - Regional Analysis
The market of Uterine Fibroid devices is much higher in the United States region, owing to its high prevalence in African- American race, change in lifestyle. According to one large cohort study of NIS on African-American women in Maryland, uterine fibroids was the cause of 65.4% of total 53,000 women undergoing hysterectomy.
Moreover, most of the major market players belong from this region, thus they introduce the advance technology firstly in the United States in comparison to others region. Additionally the market players of this region also involve in export of Uterine Fibroid devices in the developing regions of Asia Pacific for meeting the unmet needs. Also Government of developing countries are willing to adopt advanced treatment option available in developed countries, in order to improve the quality of life of their citizen, which fuel the market of Uterine Fibroid devices. Countries like India and China are more focused market for United States market players owing to the huge patient population and growing purchasing power as compare to other countries in this region. Thus the players belong from the United States have opportunity of increase the market share and maximize the profit by exporting to these nation.
U.S. Uterine Fibroid Market - Competitive Analysis
Increasing incidence of uterine fibroid United States generate the interest of companies toward the market of uterine fibroid, as they feel there are huge opportunities and gaps between the market demand and supply of quality devices. Thus, companies are showing more interest in research and development activities for introducing the advanced treatment that meets the requirement of market. For instant, in Feb 2017, AstraZeneca has entered into an agreement with TerSera Therapeutics LLC (TerSera) for the commercial rights to Zoladex (goserelin acetate implant) in the US and Canada. Zoladex is an injectable luteinising hormone-releasing hormone agonist, used to treat prostate cancer, breast cancer and certain benign gynaecological disorders. Moreover, in Nov 2016, Boston Scientific Corporation Acquires Resectr Tissue Resection Device from Distal Access, LLC. The portfolio includes the Resectr Tissue Resection Device, a single-use solution designed to effectively remove uterine polyps. The companies are introducing better devices for getting the leadership in the Uterine Fibroid devices market. Additionally company are focusing more into research and development activity in order to launch advanced device for the treatment of uterine fibroid. In this regard, GE today announced the launch of the Optima MR430s, a new specialty scanner with breakthrough technology that delivers precise imaging with exceptional comfort and the 1.5T image quality radiologists require. These invention help company to lead the market of uterine fibroid in United States.
U.S. Uterine Fibroid Market – Segments
U.S. Uterine Fibroid Market has been segmented on the basis of type which comprises of Subserosal Fibroids, Intramural Fibroids, Submucosal Fibroids and others. On the basis of diagnosis the market is segmented into Medical Resonance Imaging (MRI) scanners, Computed tomography (CT), Ultrasounds and others. On the basis of treatment the market is segmented into Uterine Fibroid Embolization, Magnetic Resonance Guided Focused Ultrasound, Myolysis, Endometrial Ablation, Hysterectomy and Myomectomy.
Major Table of Content
Chapter 1. Report Prologue
Chapter 2. Market Introduction
2.1 Definition
2.2 Scope of the Study
2.2.1 Research Objective
2.2.2 Assumptions
2.2.3 Limitations
Chapter 3. Research Methodology
3.1 Introduction
3.2 Primary Research
3.3 Secondary Research
3.4 Market Size Estimation
Chapter 4. Market Dynamics
Continued….
Ask to an Expert @ https://www.marketresearchfuture.com/enquiry/2394 .
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.s
Contact: Market Research Future Office No. 528, Amanora Chambers Magarpatta Road, Hadapsar, Pune – 411028 Maharashtra, India +1 646 845 9312 Email: [email protected]
0 notes
Text
Is Allergan Stock Finally Going to Reward Shareholders?
Allergan Stock has been a painful ride for investors. Starting last year when the proposed merger with Pfizer fell through, Allergan (NYSE: AGN) stock has fallen. As of this writing, shares are trading at $186 and nearing their 52 week low.
But the interesting thing about Allergan stock is that they have a lot going for it and most analysts agree. In this post, I will show you all of the great things going on with this company and what might be some of the things holding the stock back.
Things Going Right For Allergan Stock
As I mentioned, Allergan has a lot going for it. Looking first at earnings, the company reported earnings per share of $4.02 which beast estimates by $0.10. Revenues came in at $4 billion, also beating estimates, this time by $70 million. This was an increase of 9%.
And this growth isn’t expected to slow. Analysts expect earnings growth of 13% over the next 5 years.
What is fueling this growth? First are the drugs Botox and Juvederm. Both are seeing increases in sales and these 2 treatments only account for around 10% market share in their respective segments. In other words, there is a lot of room for future growth.
In addition to these, Allergan has 6 drugs in it pipeline that are getting rave reviews and if they pass through clinical trials, are expected to be revenue machines.
Here are the 6 drugs:
Ubrogepant: treatment for migraines
Atogepant: treatment for migraines
Rapastinel: treatment for depression
ESMYA: treatment for uterine fibroids
Relamorelin: treatment for diabetic gastroparesis
Ceniciviroc: treatment for HIV infection
Abicipar: treatment for macular degeneration
Next up is international sales growth. During its recent earnings report, international sales are up over 16%, with China leading the way with growth of 61%. Most analysts agree that this speed of international growth should continue.
Finally, Allergan has decided to institute a share buyback program of $2 billion and has raised revenue guidance for the remainder of 2017. Add to this some analysts have a price target of $275 per share, which leaves a lot of room for price appreciation.
The Case Against Allergan Stock
With such a great outlook, what are some things that could potentially be holding the stock price back? There are a few things, and here they are in no particular order.
Debt: the company has over $30 billion of debt on the books. I’m not sure why this would be a major factor now as most investors ignored it when merger talks were happening with Pfizer. But since that deal fell through, the focus has been on the large amount of debt. Maybe is it because Allergan seems to have no interest in paying it off quickly?
Slowing sales: some of the drugs the company has on the market are seeing declines in sales. These are outweighed by the great performance of other drugs it is selling. And since drugs in the pipeline are not a guaranteed revenue stream until they actually come to market, investors can’t assume the declining sales will be further hidden by newer, hot selling drugs.
So Should You Invest In Allergan Stock?
Personally, I don’t understand why investors are beating this stock down so much. The company has a great outlook and the negatives that are potentially weighing it down are not the biggest of concerns. At these levels, I would be investing in this stock.
If analysts are right and the stock hits its target of $275, you are making a ton of money. And even if it doesn’t hit that target, there is still tremendous upside potential for this stock.
This author has no positions in any stock mentioned and does not plan to open any positions in any stocks mentioned for at least 72 hours after publication of this article.
Research AGN
Show Tweets Mentioning AGNHide Tweets Mentioning AGNShow Tweets Mentioning AGN
head over to this web-site for additional information about gold investment
from victorparker https://www.modestmoney.com/allergan-stock-finally-going-reward-shareholders/
0 notes
Text
United States Uterine Fibroid Market Analysis- Size, Share, Growth, Industry Demand, Forecast, Application Analysis To 2022

“United States Uterine Fibroid Market Research Report - Forecast To 2022” is a professional and incisive analysis on the market dynamics of the United States Uterine Fibroid Market industry and future growth prospects of the key market players across the global. The study offers detailed quantitative and qualitative analysis of the industry by offering an overview of key market conditions and statistics on market estimations. An in-depth insight into the industry overview is offered in the study in terms of product definition and classification, applications analysis and manufacturing technology. The market participants can going through the study explore the profile of international market players in terms of their capacity utilization, market shares of the major segments, growth opportunities and challenges they need to overcome to gain a foothold in the market.
Click here for sample report @ https://www.researchtrades.com/request-sample/1188290
Introduction
Uterine Fibroids are the abnormal growth that gets developed inside or on the wall of a womans uterus. These are also known as leiomyoma or myomas. These are noncancerous growths that cause symptoms such as heavy bleeding between or during the menstrual cycle, pain in pelvis or lower back, increased menstrual cramping, swelling enlargement of the abdomen. Increasing prevalence due to heredity and rising number of treatments to cure this disease has fuelled the growth of this market.
U.S. Uterine Fibroid Market has been segmented on the basis of type which comprises of Subserosal Fibroids, Intramural Fibroids, Submucosal Fibroids and others. On the basis of diagnosis the market is segmented into Medical Resonance Imaging (MRI) scanners, Computed tomography (CT), Ultrasounds and others. On the basis of treatment the market is segmented into Uterine Fibroid Embolization, Magnetic Resonance Guided Focused Ultrasound, Myolysis, Endometrial Ablation, Hysterectomy and Myomectomy.
The U.S. Market for Uterine Fibroids is expected to reach USD 39,993.2 million by the end of the forecasted period and is expected to grow at a CAGR of 3.24%.
Key Players
Some of the key players in this market are: AstraZeneca (England), Cook Medical Inc. (US), Boston Scientific Corporation (US), GE Healthcare (U.K.), AbbVie Inc. (U.S.), Hologic (U.S.), and others.
Study Objectives:
To provide insights about factors, influencing and affecting the market growth.
To provide historically and forecast revenue of the market segments and sub-segments with respect to regional markets and their countries.
To provide historical and forecast revenue of the market segments based on types, diagnosis, and treatment.
To provide strategic profiling of key players in the market, comprehensively analyzing their market share, core competencies, and drawing a competitive landscape for the market.
Buy a Copy of this report for a single user price of $ 4450 @ https://www.researchtrades.com/checkout/1188290
Key Chapter
Target Audience
Uterine Fibroid Devices and Drug Manufacturers
Uterine Fibroid Devices and Drug Suppliers
Private Research Laboratories
Research and Development (R&D) Companies
Market Research and Consulting Service Providers
Medical Research Laboratories
Key Finding
The U.S. Uterine Fibroid market and is expected to reach USD 39,993.2 million by 2022.
By Type, Intramural Fibroids holds the largest market share of U.S. uterine fibroid market and is expected to reach USD 14,972.5 million by 2022.
By Treatment, hysterectomy holds the largest market share of U.S. uterine fibroid market and is expected to reach USD 8,042.3 million by 2022.
The reports also cover country level analysis:
America
North America
Europe
Western Europe
Germany
France
Italy
Spain
UK
Rest of Western Europe
Eastern Europe
Asia
China
India
Japan
South Korea
Rest of Asia
Middle East & Africa
Continue………….
Browse for report customization @ https://www.researchtrades.com/report-customization
Who we are
Research Trades has team of experts who works on providing exhaustive analysis pertaining to market research on a global basis. This comprehensive analysis is obtained by a thorough research and study of the ongoing trends and provides predictive data regarding the future estimations, which can be utilized by various organizations for growth purposes.
We distribute customized reports that focus on meeting the client’s specific requirement. Our database consists of a large collection of high-quality reports obtained using a customer-centric approach, thus providing valuable research insights. The research encompasses information gathered and examined by subject-matter experts, laying down growth opportunities and developmental strategies for enterprises.
Reach at us:
Email: [email protected]
Call us: +1 6269994607 / +91 7507349866
Skype ID: researchtradescon
Web: http://www.researchtrades.com
#United States Uterine Fibroid Market#Uterine Fibroid Market Analysis#Uterine Fibroid Market Size#Uterine Fibroid Market Share#Research Trades
0 notes
Text
Gynecological Devices Market Size, Industry Analysis Report, Competitive Market Share & Forecast To 2024 | Hexa Research
Growing healthcare awareness and changing lifestyles are key factors that fuel the global Gynecological Devices Market. The industry is poised to surpass USD 21 billion by the end of the forecast period (2016 to 2024). Rising income levels in developing countries, such as Indonesia, Vietnam, and India may significantly contribute to industry demand in the years to come. High incidence of gynecological anomalies, like menorrhagia, uterine fibroids, and cervical cancer could positively influence global demand.
Gynecological devices are an integral part of the medical devices industry. Adoption of specialized gynecological procedures in hospitals and in healthcare centers fuels the need for such devices. Introduction of sophisticated imaging devices, such as 3D endoscopes holds promise for further market progress. High incidence of diseases, such as uterine fibroids and sexually transmitted diseases can propel demand in the years to come.
Browse Details of Report @
https://www.hexaresearch.com/research-report/gynecological-device-market
The global gynecological devices market is segmented as per products and regions. On the basis of products, the industry is trifurcated into surgical devices, hand instruments, and diagnostic imaging systems. Hand instruments are further segmented into biopsy forceps, vaginal speculum, trocars, tenaculum, curettes, and others. Surgical devices comprise gynecological endoscopy devices, endometrial ablation devices, fluid management systems, and female contraceptive/sterilization devices.
Surgical devices hold the highest share in the overall Gynecological Devices Market because of growing demand for female contraceptives & endoscopy devices. Use of endoscopes in gynecology is gradually increasing. Some of their key applications in gynecology are resectoscopes, endoscopic imaging, hysteroscopes, and laparoscopes. Being minimally invasive, procedures, such as hysterectomy, hysterotomy, and laparoscopy may favorably impact demand for endoscopes in the near future.
Awareness about the importance of early detection of gynecological disorders and use of lighting in diagnostics solutions could play a key role in fuelling industry growth in the years to come.
Geographically, the gynecological devices industry is segmented into Latin America, Europe, North America, Asia Pacific, and the Middle East & Africa. North America generates the highest revenues in the overall industry. High concentration of market competitors and widespread adoption of advanced technologies are key regional drivers. Healthcare centers in the region carry out routine check-ups for women to rule out the possibilities of cervical & breast cancers.
Asia Pacific can witness robust growth over the forecast period. Factors that fuel this region are medical tourism and affordable treatment costs. Most manufacturing facilities are increasingly being established in India & China. Reasons that support such a trend are cheap labor costs, availability of skilled healthcare professionals, and advanced manufacturing facilities. This has also lead to low manufacturing costs of medical devices.
Key companies operating in the global gynecological devices market are Medtronic plc; Stryker Corporation; Cooper Surgical Inc.; Boston Scientific Corporation; and Richard Wolf Gmbh among many others. In September 2016, Aqueduct Medical received FDA (Food and Drug Administration) approval for a device that creates cervical dilation within a few minutes. Aqueduct Medical is an Israel based startup dealing in medical devices. The process is carried out without general anesthesia. At present, the company is conducting clinical trials in Europe. The product would soon be available in Israel once it is approved by the Health Ministry.
Browse Related Category Market Reports @
https://www.hexaresearch.com/research-category/medical-devices-industry
About Us:
Hexa Research is a market research and consulting organization, offering industry reports, custom research and consulting services to a host of key industries across the globe. We offer comprehensive business intelligence in the form of industry reports which help our clients obtain clarity about their business environment and enable them to undertake strategic growth initiatives.
Contact Us:
Ryan Shaw
Hexa Research
Felton Office Plaza
6265 Highway 9
Felton, California 95018
United States
Phone: +1-800-489-3075
Email: [email protected]
Website - http://www.hexaresearch.com/
Visit our Blog: https://hexaresearchinc.wordpress.com/
#Gynecological Devices Market#Gynecological Devices Market share#Gynecological Devices Market size#gynecological devices market growth#gynecological devices market analysis#Gynecological Devices Market trends#gynecological devices market forecast#global Gynecological Devices Market#gynecological devices market research#gynecological devices market report
0 notes
Text
Uterine Fibroids Treatment Market Research Report: Forecast up to 2024
U.S. Uterine Fibroids Market: Snapshot
The uterine fibroid treatment market is driven by growing number of hysterectomy surgeries every year in the country. Furthermore, the rising coverage of healthcare and increasing prevalence of non-cancerous tumors in women of childbearing age is expected to be the most vital reason for expansion of the uterine fibroids market growth. Now a days, fibroids are an important public health concern, due of the large number of women affected by the problem and the large number of hysterectomy procedure are done to treat the symptoms.
The National Institute of Health (NIH) conducts research on uterine fibroids, supports studies of fibroids at academic institutions, and sponsors interdisciplinary conferences where researchers share and discuss the results of their studies. Medical therapy is used for many women who have symptoms from fibroids and sometimes is used prior to surgery to shrink the fibroids. Commercially available drugs that shrink fibroids include gonadotropin-releasing hormones, which usually cause symptoms of menopause. Drugs that block the hormone progesterone can slow or stop the growth of fibroids. Some health care providers may use hormonal or over-the-counter medications to control pain and bleeding.
Hysterectomy Gains Preference over Others
Of the various procedures used for treating uterine fibroids, hysterectomy is the most popular one. The hysterectomy segment is further divided into abdominal hysterectomy, laparoscopic hysterectomy, vaginal hysterectomy, robotic hysterectomy, and hysteroscopic morcellation. Analysts anticipate that hysterectomy is expected to be the major segment in the country and the leading revenue generator. The procedure removes the uterus to tackle the symptoms such as chronic pelvic pain, disrupted lifestyle, and uncontrollable menstrual bleeding. The American Congress of Obstetricians and Gynecologists states that about 600,000 hysterectomy procedures are performed each year. Of these surgeries, one in every third case is due indications of fibroids. However, the growth of this segment is being challenged due to the introduction of several alternatives to the life-altering procedure.
The end users of uterine fibroids treatments are hospitals and ambulatory surgical centers. Out of these, the hospitals segment is leading the U.S. market as it is well-equipped to handle emergencies and complicated matters pertaining to uterine fibroids. The growing concerns about uterine fibroids, physical morbidity, poor quality of life, rising disposable incomes, and well-established healthcare infrastructure are contributing towards the rise of the U.S. uterine fibroids market in the coming years.
This exhaustive report includes 6 data tables and 24 figures to give readers a 360° view of the Uterine Fibroids Treatment Market. Browse through this 102-page report to know what factors will shape the market during the period 2016-2024
http://www.transparencymarketresearch.com/us-uterine-fibroid-treatment-market.html
U.S. Uterine Fibroids Market: Trends and Opportunities
According to the U.S. Department of Health and Human Services Office on Women’s Health, about 30 percent of all women will get them by age 35, and around 20-80 percent of women will do so by age 50. For some reason, African American women are more likely to experience fibroids, and to do so at a younger age. As women age, fibroid growth rates decline for most women, but not for African American women. Several treatment options are available now a days, which are driving the overall uterine fibroids market such as self-help techniques, drug therapy, uterine artery embolization and less invasive surgical procedures (myomectomy & hysterectomy).
The report is a combination of primary and secondary research. Primary research formed the bulk of our research efforts, with information collected from telephonic interviews and interactions via e-mail. Secondary research involved study of company websites, annual reports, press releases, stock analysis presentations, and various national and international databases. The report provides market size in terms of US$ Mn for each segment for the period from 2016 to 2024, considering the macro and micro-environmental factors. Growth rates for each segment within the U.S uterine fibroids market have been determined after a thorough analysis of past trends, demographics, future trends, technological developments, vaccination expenditure, and regulatory requirements.
U.S. Uterine Fibroids Market: Drivers and Restraints
The market overview section of the report includes qualitative analysis of the overall uterine fibroids market including the determining factors and market dynamics such as drivers, restraints, market trends and opportunities, along with white space analysis. In addition, market attractiveness analysis by country and end-user along with competitive landscape by key players have been provided which explain the intensity of competition in the market. The competitive scenario between market players has been evaluated through market share analysis. These factors would help the market players take strategic decisions in order to strengthen their positions and increase their shares in the global market. Pricing analysis holds a crucial part of the report, which further describes the cost to end-user and cost to patient.
The market is driven by changing prevalence of cancerous tumors. The market is projected to grow due to increasing investment in healthcare sector. In terms of distribution channel segment orthopedic clinics is projected to expand at a high CAGR on the backdrop of growing investment healthcare during sports injuries. The hospitals and ambulatory surgical center segment are expected to expand at a significant CAGR over the forecast period.
Geographically, the U.S uterine fibroids market is showing a substantial growth due to increasing direct and indirect investments by the government and other private companies. Increasing investments and penetration by key market players globally are expected to drive the uterine fibroids market during the forecast period.
Key Players Mentioned in the Report are:
Key companies profiled in the report include Blue Endo, Richard Wolf Medical Instruments, Boston Scientific Corporation, Cooper Surgical, Halt Medical, Inc., Karl Storz, LiNA Medical USA, Merit Medical Systems, Olympus Corporation, Richard Wolf GmbH and Halt Medical, Inc.
Request a sample of this report to know what opportunities will emerge in the rapidly evolving Uterine Fibroids Treatment Market during 2016- 2024
http://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=16775
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector – such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: http://www.transparencymarketresearch.com
0 notes