#United States Liquid Biopsy Market
Explore tagged Tumblr posts
Text
The United States liquid biopsy market size was valued at USD 563.6 Million in 2024. Looking forward, IMARC Group estimates the market to reach USD 1,775.8 Million by 2033, exhibiting a CAGR of 13.6% from 2025-2033. The market is primarily driven by the increasing preference for non-invasive diagnostic methods, the widespread adoption of personalized medicine and targeted treatments, and the expanding use of liquid biopsy in areas beyond cancer, including cardiovascular, neurodegenerative, and infectious diseases, driving broader clinical usage.
United States Liquid Biopsy Market Size
#United States Liquid Biopsy Market#United States Liquid Biopsy Market size#United States Liquid Biopsy Market 2025
0 notes
Text
North America Clinical Laboratory Test Market Size, Growth Outlook 2035
North America Clinical Laboratory Test Market Size was valued at USD 19.54 Billion in 2023. The North America Clinical Laboratory Test market industry is projected to grow from USD 20.98 Billion in 2024 to USD 34.6 Billion by 2032

Summary
The North America clinical laboratory test market is experiencing significant growth due to the rising prevalence of chronic diseases, increasing demand for early disease diagnosis, and advancements in laboratory automation and digital pathology. Clinical laboratory tests play a crucial role in disease prevention, diagnosis, and treatment monitoring, contributing to the overall efficiency of healthcare systems. The growing adoption of point-of-care (PoC) testing, molecular diagnostics, and AI-powered laboratory solutions is further revolutionizing the industry. However, challenges such as high operational costs, regulatory compliance burdens, and workforce shortages continue to impact the market. The market is expected to witness steady growth as laboratories continue to expand their service portfolios and integrate technological advancements such as AI, automation, and digitalization.
Market Overview
Clinical laboratory tests involve various diagnostic procedures performed on blood, urine, and tissue samples to detect diseases and monitor patient health. The demand for rapid, accurate, and cost-effective diagnostic solutions has led to increased investment in automated laboratory systems and decentralized testing. The COVID-19 pandemic significantly accelerated the adoption of molecular and immunodiagnostic tests, further driving market expansion. Additionally, the increasing use of genetic and genomic testing for personalized medicine is transforming the clinical laboratory landscape in North America.
Market Size and Growth Analysis
North America Clinical Laboratory Test Market Size was valued at USD 19.54 Billion in 2023. The North America Clinical Laboratory Test market industry is projected to grow from USD 20.98 Billion in 2024 to USD 34.6 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.45% during the forecast period (2024 - 2032). The rising geriatric population, increasing healthcare spending, and technological advancements are key factors driving this growth.
Market Dynamics
Growth Drivers
Rising Prevalence of Chronic Diseases: The increasing incidence of diabetes, cardiovascular diseases, cancer, and infectious diseases is boosting demand for diagnostic tests.
Technological Advancements in Laboratory Automation: AI, robotics, and lab-on-a-chip technologies are enhancing efficiency, accuracy, and turnaround time.
Growing Demand for Personalized Medicine and Genetic Testing: Advancements in next-generation sequencing (NGS) and molecular diagnostics are enabling tailored treatment plans.
Expansion of Point-of-Care (PoC) Testing: The shift toward rapid and decentralized testing is reducing hospital visits and improving patient outcomes.
Challenges and Restraints
High Costs of Advanced Testing Technologies: Cutting-edge diagnostic tests, such as genetic sequencing and liquid biopsy, remain expensive, limiting accessibility.
Stringent Regulatory Frameworks: Compliance with FDA, CLIA (Clinical Laboratory Improvement Amendments), and CAP (College of American Pathologists) standards increases operational complexities.
Shortage of Skilled Laboratory Technicians: The growing demand for diagnostic testing is outpacing the availability of trained professionals.
Regional Analysis
United States
The U.S. dominates the North American clinical laboratory test market, accounting for over 80% of the regional share. The country has a well-established healthcare infrastructure, high adoption of advanced laboratory technologies, and strong government initiatives supporting diagnostic innovations. The rise in direct-to-consumer genetic testing and increased funding for cancer diagnostics and infectious disease research further drive market growth.
Canada
Canada’s market is growing steadily, fueled by expanding healthcare access, increasing investment in laboratory automation, and government initiatives supporting preventive healthcare. The adoption of telehealth-integrated diagnostics and digital pathology is also contributing to market expansion.
Market Segmentation
By Test Type:
Routine Chemistry Tests – Blood glucose, cholesterol, kidney function tests
Molecular Diagnostics Tests – Genetic testing, infectious disease testing
Hematology Tests – Complete blood count (CBC), coagulation tests
Microbiology Tests – Bacterial and viral culture tests
Immunology Tests – Allergy testing, autoimmune disease diagnostics
By Provider Type:
Hospital-Based Laboratories – Conduct large volumes of inpatient and outpatient tests
Standalone Diagnostic Labs – Specialize in molecular and genomic testing
Academic and Research Institutions – Focus on cancer diagnostics and precision medicine research
By Technology:
Automated Laboratory Systems
AI-Powered Diagnostic Platforms
Lab-on-a-Chip and Microfluidics
Key Market Players
The key North America Clinical Laboratory Test companies are as follows
Aurora Diagnostics
Laboratory Corporation of America
LifeLabs Medical Laboratories
Quest Diagnostics
Recent Developments
Expansion of At-Home Testing Services: Companies are launching direct-to-consumer lab testing platforms for genetic and preventive health screening.
AI Integration in Pathology and Radiology: AI-driven automated image analysis is enhancing diagnostic accuracy.
Strategic Collaborations in Oncology and Infectious Disease Testing: Mergers and partnerships are driving innovation in cancer and COVID-19 diagnostics.
Future Outlook and Opportunities
The future of the North America clinical laboratory test market looks promising with continued advancements in AI-driven diagnostics, automation, and personalized medicine. The integration of digital pathology, blockchain for secure lab data management, and telemedicine-based laboratory services will drive further expansion. However, addressing regulatory challenges and the high cost of advanced diagnostics will be key to ensuring sustained market growth and accessibility.
For more information please visit @marketresearchfuture
#North America Clinical Laboratory Test Market Size#North America Clinical Laboratory Test Market Share#North America Clinical Laboratory Test Market Growth#North America Clinical Laboratory Test Market Analysis#North America Clinical Laboratory Test Market Trends#North America Clinical Laboratory Test Market Forecast#North America Clinical Laboratory Test Market Segments
0 notes
Text
Precision Diagnostics Market Surge: $57.5B in 2023 to $157.2B by 2033 (10.5% CAGR)
Precision Diagnostics Market focuses on advanced technologies and methodologies designed to enhance the accuracy of disease detection and support personalized healthcare solutions. This includes molecular diagnostics, imaging technologies, and bioinformatics tools that enable early, precise diagnosis, tailored treatment planning, and ongoing monitoring. The market plays a vital role in the shift toward personalized medicine, which improves patient outcomes and optimizes healthcare resources by offering individualized diagnostic solutions.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS22118 &utm_source=SnehaPatil&utm_medium=Article
Market Growth and Trends
The Precision Diagnostics Market is experiencing robust growth, driven by advancements in molecular diagnostics and imaging technologies. Among the sub-segments, molecular diagnostics lead the market due to their pivotal role in personalized healthcare and early disease detection. Next-generation sequencing (NGS) is the top performer within this segment, thanks to its precision, growing accessibility, and declining costs. Imaging diagnostics, particularly MRI and CT scans, also represent a significant segment, benefitting from ongoing technological advancements and rising healthcare expenditure.
Regional Insights
North America dominates the market, fueled by a strong healthcare infrastructure, substantial R&D investments, and a high adoption rate of innovative diagnostic technologies. The United States is the leading country, driven by advanced healthcare facilities and widespread implementation of precision diagnostics.
Europe ranks second, with Germany and the United Kingdom emerging as key contributors, spurred by increasing demand for early disease diagnosis and precise medical interventions.
Asia-Pacific is rapidly growing, with China and India seeing significant market expansion. The region’s growth is driven by increasing healthcare awareness, rising income levels, and improved access to diagnostic technologies.
Market Segmentation
By Type: Genetic Testing, Molecular Diagnostics, Companion Diagnostics, Point-of-Care Testing, Liquid Biopsy By Product: Reagents & Kits, Instruments, Software & Services, Consumables By Technology: Next-Generation Sequencing, Polymerase Chain Reaction, Fluorescence In Situ Hybridization, Immunohistochemistry, Microarray By Application: Oncology, Cardiology, Infectious Diseases, Neurology, Endocrinology By End User: Hospitals, Diagnostic Laboratories, Research Institutes, Academic Institutes By Component: Hardware, Software, Services By Device: Benchtop, Portable, Handheld, Wearable By Process: Sample Preparation, Data Analysis, Validation By Deployment: On-Premise, Cloud-Based, Hybrid By Solutions: Clinical Decision Support, Data Management, Patient Engagement
Market Volume & Projections
In 2023, the market demonstrated a strong volume of 320 million diagnostic tests globally, with projections indicating a rise to 520 million tests by 2033. The molecular diagnostics segment commands a substantial 45% market share, followed by genetic testing at 30%, and imaging diagnostics at 25%. This market dominance is driven by advancements in genomics and a growing demand for personalized medicine.
Key Market Players
Leading players in the Precision Diagnostics Market include Roche Diagnostics, Abbott Laboratories, and Thermo Fisher Scientific, which continue to influence the market through cutting-edge technology, strategic partnerships, and ongoing innovation to maintain their competitive edge.
#PrecisionDiagnostics #PersonalizedHealthcare #MolecularDiagnostics #GeneticTesting #NextGenerationSequencing #Oncology #Cardiology #EarlyDiagnosis #HealthcareInnovation #ImagingTechnologies #LiquidBiopsy #Bioinformatics #MedicalDevices #HealthcareSolutions #DiagnosticReagents
0 notes
Text
Urothelial Carcinoma Treatment Market : Technology Advancements, Industry Insights, Trends And Forecast 2033
The urothelial carcinoma treatment global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Urothelial Carcinoma Treatment Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.

Market Size - The urothelial carcinoma treatment market size has grown rapidly in recent years. It will grow from $2.41 billion in 2023 to $2.86 billion in 2024 at a compound annual growth rate (CAGR) of 18.8%. The growth in the historic period can be attributed to rising prevalence of urothelial carcinoma, growing awareness of urothelial carcinoma, increasing access to healthcare, rising disposable incomes.
The urothelial carcinoma treatment market size is expected to see rapid growth in the next few years. It will grow to $5.33 billion in 2028 at a compound annual growth rate (CAGR) of 16.9%. The growth in the forecast period can be attributed to aging population, demand for personalized medicine, rising government support for urothelial carcinoma research and treatment, growing investments on urothelial carcinoma treatment. Major trends in the forecast period include biomarker-driven therapies, neoadjuvant and adjuvant approaches, liquid biopsies, minimally invasive surgeries, chemotherapy development, digital health technologies.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/urothelial-carcinoma-treatment-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers - The increase in bladder cancer is expected to propel the growth of the urothelial carcinoma treatment market going forward. Bladder cancer is a prevalent type of cancer that affects the cells lining the urinary bladder and can lead to a range of treatment options being sought by patients and healthcare providers. Bladder cancer research informs targeted therapies and provides insights into urothelial carcinoma, fostering cross-disciplinary approaches for improved treatment outcomes. For instance, in January 2022, according to the report published by the American Cancer Society Inc., a US-based voluntary organization for cancer awareness, the estimated cases of urinary bladder cancer increased to 83,730 in 2021, an increase of 2.8% from 81,400 in 2020 in the United States. Therefore, the increase in bladder cancer will drive the urothelial carcinoma treatment market.
Market Trends - Major companies operating in the urothelial carcinoma treatment market are concentrating on creating innovative products to advance their competitive edge further and address evolving customer needs. For instance, in March 2023, Nonacus Limited, a UK-based medical products manufacturing company, launched GALEAS Bladder, a novel test for the non-invasive detection of bladder cancer. GALEAS Bladder introduces a breakthrough in bladder cancer diagnostics, offering a sample-to-report molecular triage solution for patients that employs a molecular biomarker with remarkable sensitivity, swiftly and accurately identifying bladder cancer. Consequently, the diagnostic process is streamlined, reducing the need for invasive procedures such as cystoscopies. Developed in collaboration with the University of Birmingham, UK, GALEAS Bladder has been rigorously validated with over 600 patient samples from three clinical cohorts in the UK. This comprehensive analysis showcased GALEAS Bladder's outstanding performance, demonstrating high diagnostic accuracy (sensitivity exceeding 90%, specificity over 85%), effectively catering to various bladder cancer grades and stages.
The urothelial carcinoma treatment market covered in this report is segmented –
1) By Type: Non-Invasive Urothelial Carcinoma Treatment; Invasive Urothelial Carcinoma Treatment 2) By Treatment: Immunotherapy; Radiotherapy; Chemotherapy 3) By Cancer Type: Bladder Cancer; Urethral Cancer; Ureteric And Renal Pelvic Cancer 4) By End-Users: Hospitals; Homecare; Specialty Centers; Other End-Users
Get an inside scoop of the urothelial carcinoma treatment market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=12947&type=smp
Regional Insights - North America was the largest region in the urothelial carcinoma treatment market in 2023. The regions covered in urothelial carcinoma treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Key Companies - Major players in the urothelial carcinoma treatment market are Pfizer Inc., Johnson & Johnson Services Inc., F. Hoffmann-La Roche Ltd., Merck & Co. Inc., AbbVie Inc., Bayer AG, Sanofi S.A., Bristol-Myers Squibb Company, AstraZeneca PLC, Abbott Laboratories Inc., GlaxoSmithKline PLC, Astellas Pharma Inc., Agilent Technologies Inc., Eisai Co. Ltd., Incyte Corporation, Hikma Pharmaceuticals PLC, Amneal Pharmaceuticals LLC, Seagen Inc., Lupin Limited, Genentech Inc., UroGen Pharma Inc., Acerta Pharma B.V., Asieris Pharmaceuticals Co. Ltd., Pacific Edge Limited, Protara Therapeutics Inc., Hamlet Pharma AB, CG Oncology Inc., AroCell AB, ImmunityBio Inc., IDL Biotech AB.
Table of Contents 1. Executive Summary 2. Urothelial Carcinoma Treatment Market Report Structure 3. Urothelial Carcinoma Treatment Market Trends And Strategies 4. Urothelial Carcinoma Treatment Market – Macro Economic Scenario 5. Urothelial Carcinoma Treatment Market Size And Growth ….. 27. Urothelial Carcinoma Treatment Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected]
Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Liquid Biopsy Market: Trends, Growth Opportunities, and Future Insights
The liquid biopsy market is expanding rapidly, driven by advancements in precision medicine, non-invasive diagnostic methods, and growing demand for early cancer detection. This technology, which allows doctors to detect cancer and other diseases through blood samples instead of invasive tissue biopsies, is revolutionizing patient care by offering faster, less painful, and more accessible testing options. In this blog, we’ll explore the key trends, market drivers, growth opportunities, and challenges shaping the liquid biopsy industry.

Download PDF Brochure
What is a Liquid Biopsy?
A liquid biopsy is a non-invasive diagnostic test that detects genetic mutations, cancer markers, or other biomarkers in blood, urine, saliva, or other bodily fluids. Unlike traditional biopsies, which require tissue samples through surgery, liquid biopsies provide valuable information about a patient’s condition using small samples, significantly reducing discomfort and recovery time. Liquid biopsies can track a tumor’s progress, identify mutations, and monitor response to treatments, which is particularly useful for conditions that are difficult to monitor through tissue biopsies alone.
Key Drivers of Growth in the Liquid Biopsy Market
The liquid biopsy market is experiencing a surge in growth due to several factors:
1. Rising Incidence of Cancer and Demand for Non-Invasive Diagnostics
Cancer remains one of the leading causes of death worldwide, driving demand for diagnostic tools that can detect it early and with minimal invasiveness. Liquid biopsies provide a way to diagnose and monitor cancer by identifying specific biomarkers in the blood, offering a safer and less invasive alternative to surgical biopsies. This demand is particularly high in regions where the aging population and incidence of cancer are on the rise, such as North America and Europe.
2. Advances in Genomics and Precision Medicine
Advances in genomics and the trend toward precision medicine are pushing the liquid biopsy market forward. As genomic research reveals more about the genetic markers associated with diseases, liquid biopsy technologies are becoming more capable of identifying and monitoring these markers. This enables doctors to tailor treatments to individual patients based on their unique genetic profiles, resulting in more effective and personalized treatment plans.
3. Government Funding and Research Investments
Governments and research institutions are investing in cancer research and diagnostics, increasing funding for technologies that can advance early cancer detection. Many health agencies worldwide, such as the National Institutes of Health (NIH) in the United States, are allocating funds to support the development of non-invasive diagnostic tools, fostering innovation and accelerating the growth of the liquid biopsy market.
4. Growth in Companion Diagnostics
Companion diagnostics are tests that help assess a patient’s likelihood of responding to a specific treatment. These diagnostics are particularly important for cancer patients, as liquid biopsies can identify biomarkers that reveal how a patient might respond to certain therapies. This insight is invaluable in oncology, as it can guide treatment decisions and improve patient outcomes, further driving demand for liquid biopsy solutions.
Emerging Applications and Technologies
The scope of liquid biopsy applications is expanding beyond cancer diagnosis. New technologies are unlocking additional uses in areas such as:
1. Infectious Disease Monitoring
Liquid biopsies can detect circulating pathogens in the bloodstream, opening the possibility of diagnosing and monitoring infectious diseases like HIV, tuberculosis, and hepatitis. This potential is particularly valuable in areas with high rates of these infections, where liquid biopsies could improve diagnosis and treatment tracking in real time.
2. Cardiovascular Disease Detection
Research is exploring the use of liquid biopsies to identify cardiovascular biomarkers, which could assist in early diagnosis and monitoring of heart disease. By detecting certain genetic markers and proteins in the blood, liquid biopsy technology could help predict cardiovascular events, enabling preventive measures and more targeted therapies.
3. Neurological Conditions
Although still in early stages, liquid biopsy applications in neurology could revolutionize the diagnosis and monitoring of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. By detecting biomarkers associated with brain conditions, liquid biopsies offer a non-invasive way to diagnose neurological conditions earlier than traditional imaging techniques allow.
Competitive Landscape and Key Players
The liquid biopsy market is highly competitive, with several established companies and new entrants working to advance the technology. Key players include:
Guardant Health: Known for its Guardant360 test, which screens for cancer mutations in the blood. Guardant Health focuses on developing liquid biopsy tests for advanced-stage cancers and companion diagnostics.
Biocept: Specializes in liquid biopsy tests for both solid tumor cancers and brain metastases, with a focus on molecular diagnostics.
Foundation Medicine: Known for its FoundationOne Liquid test, which provides genomic profiling to inform cancer treatment decisions.
Natera: A leader in reproductive health diagnostics, Natera also offers liquid biopsy tests for oncology, including its Signatera test, which is used for minimal residual disease detection and monitoring.
GRAIL: Recently acquired by Illumina, GRAIL is focused on developing multi-cancer early detection tests using its proprietary liquid biopsy technology.
These companies are investing heavily in R&D, aiming to improve the accuracy, affordability, and applicability of liquid biopsies across a range of diseases.
Market Challenges
Despite its advantages, the liquid biopsy market faces several challenges:
1. Technical Limitations
Detecting circulating tumor DNA (ctDNA) and other biomarkers in blood samples is complex and requires advanced technology. While some cancers shed more ctDNA, making detection easier, others do not, which can limit the effectiveness of liquid biopsies in certain cases.
2. Regulatory and Reimbursement Hurdles
In many regions, liquid biopsy tests face stringent regulatory requirements and approval processes. Additionally, the cost of these tests and limited reimbursement options can restrict their accessibility for patients, particularly in lower-income regions or for those without sufficient healthcare coverage.
3. Data Interpretation and False Positives
Interpreting data from liquid biopsies is complex, and there is a risk of false positives, where benign mutations or low levels of ctDNA are detected as indicators of cancer. This challenge emphasizes the need for high accuracy and specificity in liquid biopsy technology to ensure reliable results.
Future Outlook and Growth Opportunities
The liquid biopsy market is expected to continue its upward trajectory, driven by technological advancements and broader applications. Key growth areas include:
1. Artificial Intelligence Integration
AI and machine learning algorithms are being integrated into liquid biopsy analysis, enhancing the accuracy of biomarker detection and interpretation. By automating complex data analysis, AI could reduce false positives and help refine liquid biopsy results, making them more reliable and accessible.
2. Expanding Beyond Oncology
While cancer detection remains the primary application, there is substantial potential for liquid biopsies in infectious disease, cardiovascular health, and neurology. The expansion into these areas could open new revenue streams for companies and significantly broaden the market’s scope.
3. Global Market Expansion
Emerging markets, particularly in Asia-Pacific and Latin America, offer high growth potential for the liquid biopsy market. Government initiatives, growing healthcare infrastructure, and a rising prevalence of cancer and other diseases are driving demand in these regions, presenting opportunities for companies to expand their global footprint.
Conclusion
The liquid biopsy market is at the forefront of a healthcare revolution, offering a less invasive, faster, and more precise way to detect and monitor diseases. With applications expanding beyond oncology, new technologies like AI integration, and increasing demand for non-invasive diagnostics, the liquid biopsy market holds immense potential for growth. As the industry continues to innovate, companies that leverage these advancements will not only drive the market forward but also play a pivotal role in shaping the future of personalized and preventive healthcare.
0 notes
Text
Meeting the Needs of a Growing Population: The Evolving Global Liquid Biopsy Market
The global liquid biopsy market is positioned for rapid expansion, with an estimated revenue of USD 1,538.3 million in 2023. According to insights from healthcare domain experts, providers in the liquid biopsy sector can anticipate a robust Compound Annual Growth Rate (CAGR) of 21.7% through 2033. By the end of the forecast period, the market is projected to achieve a valuation of USD 10,938.6 million, reflecting significant growth opportunities within the healthcare industry.
Liquid biopsy, a non-invasive diagnostic technique that detects biomarkers and genetic material in bodily fluids, is revolutionizing cancer diagnosis, monitoring, and treatment. This innovative approach offers several advantages over traditional tissue biopsy, including real-time monitoring, early detection of treatment response or resistance, and the ability to capture tumor heterogeneity
Tumor tissue is currently the gold standard for determining the kind and stage of cancer. Important players are attempting to reduce the current obstacles to its utilization in order to increase its potential applications in the future. The feasibility of liquid biopsy techniques for identifying cancer patients’ genetic profiles has also been shown by a number of research. The studies carefully take into account the reactions in order to track the course of treatment and identify any early warning symptoms of therapeutic resistance.
Request Your Detailed Report Sample With Your Work Email : https://www.futuremarketinsights.com/reports/sample/rep-gb-1396
Liquid biopsies have become more common in recent years due to the desire for more personalized treatment options. Since liquid biopsy is widely available and reasonably priced in many labs, particularly in developing countries, its use is growing in popularity. For instance, several labs offer liquid biopsy tests.
“Key players are bringing cost reductions in liquid biopsy tests and partnering with local product distributors to strengthen their network base in target markets. Currently, a trend toward emerging economies has been spotted, as cancer cases are particularly on the rise, and a large population base provides significant opportunities in these markets,” says an FMI analyst.
Key Takeaways from the Liquid Biopsy Market Report
The North America liquid biopsy market is expected to account for a leading share of 50.84%. The United States is predicted to account for 43.8%, enjoying a dominant share in the global and regional markets.
The Europe market is anticipated to acquire a market share of 19.6% in 2023. Germany holds a prominent share in the region. In 2023, the country is predicted to amass a total of 6.6% share in the global market.
In Europe, the United Kingdom is predicted to expand at a robust CAGR of 26.4% through 2033.
In Asia Pacific, China and India display a remarkable percentage of growth, i.e., 29.7% and 24.4%, respectively, through 2033.
CTC (Circulating Tumor Cells) is projected to obtain a significant market share of 56.9% by biomarker type in 2023.
By sample type, blood sample type holds prominence in the liquid biopsy market.
Key Developments by Liquid Biopsy Market Players
QIAGEN N.V. (the Netherlands), in May 2022, introduced a therascreen EGFR Plus RGQ PCR Kit, which is a new in vitro diagnostic test for the analysis of sensitive EGFR mutation.
Guardant Health, Inc., in June 2021, introduced Guardant360 Response test that finds variations in circulating tumor DNA (ctDNA) levels.
Hoffmann-La Roche Ltd. (Switzerland), in October 2020, gained United States FDA approval for the extended claims for cobas EGFR Mutation Test v2 to be deployed as a companion diagnostic for an extensive range of therapies to manage non-small cell lung cancer (NSCLC).
Biocept, Inc., in March 2020, agreed with a California-based Independent Physician Association (IPA) to offer its Target Selector liquid assay services to patients and physicians in the network.
Key Companies Profiled:
BIOCEPT, INC.
Qiagen N.V.
Trovagene, Inc
Janssen Global Services, LLC
MDxHealth SA
Natera, Inc
F. Hoffmann-La Roche Ltd
Silicon Biosystems
Pathway Genomics Corporation
Sysmex Corporation
Others
Key Segments Profiled in the Liquid Biopsy Industry Survey:
By Biomarker Type:
CTCs (Circulating Tumour Cells)
ctNA (Circulating tumor Nucleic Acids)
Exosomes
By Sample Type:
Blood Liquid Biopsy
Urine Liquid Biopsy
Other (Plasma, Saliva, CSF) Liquid Biopsy
By Application Type:
Liquid Biopsy for Lung Cancer
Liquid Biopsy for Gastrointestinal Cancer
Liquid Biopsy for Prostate Cancer
Liquid Biopsy for Breast Cancer
Liquid Biopsy for Colorectal Cancer
Liquid Biopsy for Leukemia
By Region:
North America
Latin America
Western Europe
Eastern Europe
Asia Pacific Excluding Japan
Japan
Middle East and Africa
0 notes
Text
Advancements and Market Trends in Colorectal Cancer Treatment
Colorectal cancer, a type of cancer that starts in the colon or rectum, is one of the most prevalent and deadly cancers worldwide. Despite significant progress in early detection and treatment options, colorectal cancer remains a significant health challenge, driving ongoing research, innovation, and investment in the market. This article explores the current landscape of the colorectal cancer market, including recent advancements, emerging trends, and future prospects.
Market Overview: The global colorectal cancer market has experienced steady growth in recent years, driven by factors such as the rising incidence of colorectal cancer, an aging population, and advancements in treatment options. According to the American Cancer Society, colorectal cancer is the third most common cancer diagnosed in both men and women in the United States, highlighting the significant market potential for colorectal cancer treatments.
Key Players: Several pharmaceutical companies and biotech firms are actively involved in the development and commercialization of colorectal cancer therapies. Key players in the market include:
Roche Holding AG: Roche's blockbuster drug, Avastin (bevacizumab), is widely used in the treatment of colorectal cancer, particularly in combination with chemotherapy.
Merck & Co., Inc.: Merck's immunotherapy drug, Keytruda (pembrolizumab), has shown promising results in treating advanced colorectal cancer with specific genetic mutations.
Bayer AG: Bayer's Stivarga (regorafenib) is approved for the treatment of metastatic colorectal cancer that has progressed after standard therapies.
Bristol Myers Squibb: Bristol Myers Squibb's Opdivo (nivolumab) is another immunotherapy option being explored for the treatment of colorectal cancer.
Pfizer Inc.: Pfizer's Xalkori (crizotinib) and Lorbrena (lorlatinib) are among the targeted therapies being investigated for colorectal cancer treatment.
Recent Advancements: The colorectal cancer market has witnessed several significant advancements in recent years, driving improvements in patient outcomes and treatment efficacy. These advancements include:
Immunotherapy: Immunotherapy has emerged as a promising approach for colorectal cancer treatment, particularly in patients with specific genetic mutations or microsatellite instability-high (MSI-H) tumors. Drugs like Keytruda and Opdivo harness the body's immune system to target and destroy cancer cells.
Targeted Therapies: Targeted therapies, which specifically target cancer cells based on their genetic makeup, have shown efficacy in colorectal cancer treatment. Drugs like Stivarga and Xalkori inhibit specific molecular pathways involved in cancer growth and progression.
Biomarker Testing: Advances in biomarker testing have enabled oncologists to personalize treatment approaches based on the unique genetic profile of each patient's tumor. Biomarker testing helps identify patients who are most likely to benefit from targeted therapies or immunotherapy.
Minimally Invasive Surgery: Minimally invasive surgical techniques, such as laparoscopic and robotic-assisted surgeries, have become standard in the treatment of colorectal cancer. These techniques offer faster recovery times, reduced postoperative pain, and improved cosmetic outcomes compared to traditional open surgery.
Emerging Trends: Several emerging trends are shaping the future of the colorectal cancer market:
Combination Therapies: Researchers are exploring the potential benefits of combining immunotherapy with other treatment modalities, such as chemotherapy, targeted therapy, and radiation therapy, to enhance treatment efficacy and overcome resistance mechanisms.
Liquid Biopsies: Liquid biopsy techniques, which involve analyzing tumor-derived genetic material in blood samples, hold promise for early detection, monitoring treatment response, and detecting the emergence of resistance mutations in colorectal cancer patients.
Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms are being increasingly utilized to analyze complex genomic and imaging data, predict treatment outcomes, and identify novel therapeutic targets in colorectal cancer.
Patient-Centric Care: There is a growing emphasis on patient-centered care in the management of colorectal cancer, with healthcare providers focusing on improving patient education, support services, and survivorship care to enhance overall quality of life.
For more insights into the Colorectal Cancer market forecast, download a free report sample
0 notes
Text
Multi Cancer Early Detection Market Size, In-Depth Assessment, CAGR, Demand, and Opportunity Analysis 2032
The Multi Cancer Early Detection Market market size study shifts its attention to in-depth business challenges, defined investment opportunities, market share coupled with product type and applications, key companies responsible for the production, and upcoming market opportunities.
According to our latest research, the global Multi Cancer Early Detection Market size is estimated to be 2.44 Billion by 2032 from USD 0.83 Billion in 2022, with a change of 14.15% between 2023 and 2032.
The report aims to offer a comprehensive analysis on the global Multi Cancer Early Detection market. It concentrates on the market dynamics, technological inclinations, and understandings on different end-user industries and product types. Additionally, it examines the key players and the competitive landscape in the global Multi Cancer Early Detection market.
Get Free Exclusive Sample of this Premium Report at: https://isayresearch.com/sample/2295
The Global Multi Cancer Early Detection Market analysis report is the outcome of incessant efforts guided by knowledgeable forecasters, innovative analysts and brilliant researchers. With the specific and state-of-the-art information provided in this report, businesses can get idea about the types of consumers, consumer’s demands and preferences, their perspectives about the product, their buying intentions, their response to particular product, and their varying tastes about the specific product which is already present in the market. By providing an absolute overview of the market, Multi Cancer Early Detection Market report covers various aspects of market analysis, product definition, market segmentation, key developments, and the existing vendor landscape.
List of Prominent Players:
Grail, LLC (Illumina, Inc.)
Exact Sciences Corporation.
Foundation Medicine, Inc.
AnchorDx
Guardant Health, Inc.
Burning Rock Biotech Limited
GENECAST
Laboratory for Advanced Medicine, Inc.
Singlera Genomics Inc.
Others
Segmentation of Global Multi Cancer Early Detection Market:
By Type:
Liquid Biopsy
Gene Panel, LDT & Others
By End-Use:
Hopsitals
Diagnostic Laboratories
Others
Market segment by Region, regional analysis covers
North America (U.S., Canada, Mexico)
Europe (Germany, France, UK, Italy, Spain, Rest of Europe)
Asia Pacific (China, Japan, India, Southeast Asia, Rest of APAC)
Latin America (Brazil, Argentina, Rest of Latin America)
Middle East & Africa (GCC Countries, UAE, Rest of MEA)
Report Target Clients:
Investors and Private Equity Firms
Multi Cancer Early Detection Providers
Suppliers as well as Distributors
Government and Regulatory Agencies
Read Summary Of the report @ https://isayresearch.com/report/2295/multi-cancer-early-detection-market/
Read Insights For Your Business
Company Profile
iSay Research is the leading research company offering both tactical and strategic support to all our customers. Customer satisfaction is our goal and that is why, we have a team of skilled and experienced specialist with the ability to do data mining, information management, and revenue enhancement solutions to ensure that our clients make informed decisions when coming to investing in the market.
Contact
iSay Solutions LLC
166 Geary St. 15th Floor Suite #212,
San Francisco, California 94108,
United States
Tel: +14156709191
Email: [email protected]
0 notes
Text
ReVitalMed: Pioneering Single-Use Medical Device Regeneration
Introduction to Single Use Devices Single-use medical devices (SUDs) are designed and labeled by their manufacturers to be discarded after one use on a single patient. Common types of SUDs include biopsy forceps, cardiac catheters, drug-eluting stents, and surgical instruments. SUDs offer convenience for healthcare facilities by eliminating cleaning, disinfection, sterilization, and repair costs associated with reusable devices. However, SUDs also generate a significant amount of medical waste.
The Process of Reprocessing SUDs Some healthcare facilities have begun reprocessing select SUDs to reduce costs and waste. The reprocessing of SUDs involves cleaning, disinfection, sterilization, inspection, and repackaging to prepare a used device for another procedure. The main steps in reprocessing SUDs are:
Cleaning Thorough cleaning is the first and most important step to remove contaminants from the last clinical use. Robotic, ultrasonic, and manual cleaning methods are used with soap solutions to flush out all residues from lumens and crevices of devices.
Disinfection After cleaning, high-level disinfection using liquid chemicals like peracetic acid or hydrogen peroxide vapor helps eliminate infectious pathogens and microbes. Strict adherence to disinfectant contact times is necessary for effectiveness.
Inspection and Function Testing Reprocessed devices then undergo 100% visual, optical, and electronic inspection using magnifying tools. Functionality of moving parts, electrical components, and material integrity are evaluated to prevent failures.
Sterilization Steam sterilization in an autoclave is the standard method to sterilize heat-stable reprocessed SUDs. Other techniques like ethylene oxide gas or plasma sterilization can be used as well if validated.
Repackaging and Labeling Once sterilized, devices are individually wrapped or placed in dedicated trays with internal sterilization indicators. Clear labeling denotes the device as reprocessed. Quality control records trace each device back to its original manufacturer.
Regulatory Considerations around SUD Reprocessing The reprocessing of SUDs raises concerns from a regulatory perspective:
Lack of Manufacturer Validation Original equipment manufacturers did not design or validate SUDs to withstand multiple uses, high-temperature sterilization, or cleaning chemicals. Significant post-market validation is required.
Liability and Safety Issues If a reprocessed device malfunctions or causes harm, liability is difficult to determine between the reprocessor and original manufacturer. Safety and performance could be compromised without oversight.
Regulatory Approval Requirements In many countries like the United States, reprocessors must obtain 510(k) marketing clearance from regulators like the FDA before commercially distributing reprocessed SUDs. However, regulations are still evolving.
Cost-Effectiveness Analysis While initial reprocessing costs can be high, long-term savings from avoiding purchase of new SUDs can offset cleaning and sterilization fees—especially for high-cost devices. Reliability and remaining useful life need evaluation.
Adoption Challenges in Healthcare Systems Changing standards of care and ingrained clinical preferences favor single-use over reprocessed devices. Reprocessors must demonstrate comparable quality and safety to gain broader acceptance.
The Future of SUD Reprocessing As healthcare costs continue rising, SUD reprocessing offers a means to improve sustainability and access to care. However, unresolved concerns around validation, quality assurance, and regulator oversight could slow further development. Standardized best practices, comprehensive regulations, and robust post-market surveillance may encourage more healthcare providers and patients to embrace this option in the future. With appropriate safeguards and diligence by all stakeholders, reprocessing select SUDs shows promise as a practical solution to reduce medical waste and contain costs from expensive disposable technologies.
0 notes
Text
Liquid Biopsy Market Latest Innovations, Drivers and Industry Status 2023 to 2030
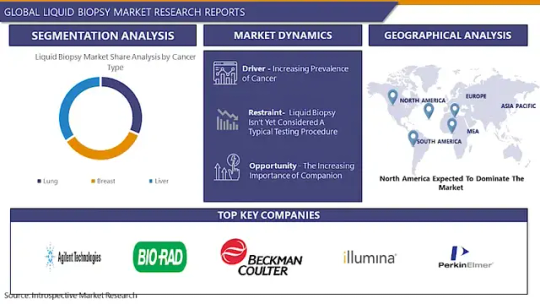
The Global Liquid Biopsy Market size is expected to grow from USD 1.59 billion in 2022 to USD 7.02 billion by 2030, at a CAGR of 20.4% during the forecast period (2023-2030).
A liquid biopsy is a minimally invasive diagnostic procedure that involves analyzing various biomarkers (such as circulating tumor cells, cell-free DNA, exosomes, and other nucleic acids) found in bodily fluids like blood, urine, or saliva. It's primarily used for the detection and monitoring of diseases, particularly cancer.
Advances in technologies such as next-generation sequencing (NGS), polymerase chain reaction (PCR), and digital PCR have greatly enhanced the sensitivity and specificity of liquid biopsy tests, enabling more accurate detection and monitoring of diseases.
Liquid biopsies have gained particular attention in the field of oncology for early cancer detection, monitoring treatment response, and detecting resistance mutations. They offer a non-invasive alternative to tissue biopsies and can provide real-time information on tumor dynamics.
While oncology remains the primary focus of liquid biopsy applications, researchers and companies have been exploring its potential in other areas such as prenatal testing, infectious disease diagnostics, transplant monitoring, and autoimmune disease detection.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/16300
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Leading players involved in the Liquid Biopsy Market include:
Agilent Technologies (United States), Bio-Rad Laboratories, Inc. (United States), Beckman Coulter(United States), Illumina (United States), PerkinElmer (United States), Becton, Dickinson and Company (BD) (United States), Johnson & Johnson (United States), Abbott Laboratories (United States), Cancer Genetics, Inc. (United States), Myriad Genetics, Inc. (United States)
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
If You Have Any Query Liquid Biopsy Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/16300
Segmentation of Liquid Biopsy Market:
By Cancer Type
Lung
Breast
Liver
By Circulating Biomarker
Circulating Tumor DNA
Circulating Tumor Cells
By End-User
Hospitals
Laboratories
Government Research Centre
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
Key Reasons To Invest In Liquid Biopsy Market Report:
To provide a complete structure and a basic overview of the Liquid Biopsy market.
To provide insights into important Liquid Biopsy aspects such as growth trajectory, CAGR value, market share, and revenue analysis.
Assess growth opportunities, threats, market drivers, and associated risks.
To understand the Liquid Biopsy market competition by analysing the top business people along with market profiles, import/export details, revenue, profit, and market shares.
Indicate pricing structure, import/export details, supply chain analysis, SWOT analysis to facilitate key decision-making process.
Analysing emerging Liquid Biopsy market segments and sub-segments to drive ultimate growth, investment analysis, and future growth opportunities.
Understand sources of knowledge, intended research methodology, and important conclusions.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=16300
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#Liquid Biopsy#Liquid Biopsy Market#Liquid Biopsy Market Size#Liquid Biopsy Market Share#Liquid Biopsy Market Growth#Liquid Biopsy Market Trend#Liquid Biopsy Market segment#Liquid Biopsy Market Opportunity#Liquid Biopsy Market Analysis 2023
0 notes
Text

North America Cancer Biopsy Market size at USD 10.34 Billion in 2023. During the forecast period between 2024 and 2030, BlueWeave expects the North America Cancer Biopsy Market size to expand at a CAGR of 9.77% reaching a value of USD 22.87 Billion by 2030. The market is expected to grow significantly due to the high burden of cancer among the population, with a large number of breast, colon, and rectal cancer cases, particularly in the United States and Canada. Initiatives undertaken by the government for the early diagnosis of cancer are also driving market growth such as The National Breast and Cervical Cancer Early Detection Program (NBCCEDP) provides free and low-cost breast and cervical cancer screening services for people who can’t afford them. Moreover, the market is being driven by technological advancements and product launches by key players. For example, the launch of new oncology liquid biopsy products is contributing to market growth.
Opportunity: Collaborations and Partnerships
Collaborations and partnerships represent a pivotal opportunity in the North America Cancer Biopsy Market. The complex landscape of cancer diagnostics and biopsy technologies necessitates strategic alliances between key players, research institutions, and healthcare organizations. By fostering collaborations, stakeholders can leverage combined expertise, resources, and innovative technologies to accelerate advancements in cancer biopsy methodologies. These partnerships facilitate the development of cutting-edge diagnostic tools, enhance research capabilities, and promote the efficient implementation of novel biopsy techniques. For instance, SOPHiA GENETICS, a cloud-native software firm in the healthcare market, has entered into an agreement with Memorial Sloan Kettering Cancer Centre (MSK) to give physicians and researchers new tools to improve their testing and analytical capabilities. This includes using patented complete Genomic Panel (CGP) sequencing assays like MSK-ACCESS®, which will be commercialized by SOPHiA GENETICS as the first complete ctDNA liquid biopsy test powered by the SOPHiA DDMTM Platform. Furthermore, collaborative efforts create synergies that can streamline regulatory processes, ensuring quicker market access for innovative biopsy solutions. Overall, collaborations and partnerships emerge as a key avenue for fostering innovation, addressing unmet needs, and driving the growth of the North America Cancer Biopsy Market.
Impact of Geopolitical Tensions on the North America Cancer Biopsy Market
Geopolitical tensions can impact the North America cancer biopsy market in various ways. The increasing conflicts may disrupt the overall market dynamics, leading to uncertainties in trade and investment. Cross-border capital outflows triggered by rising geopolitical tensions could pose a threat to macro-financial stability, affecting the financial landscape within the region. These uncertainties may influence decision-making processes, leading to delays or fluctuations in healthcare investments, research, and technology adoption in the field of cancer diagnostics. As geopolitical challenges evolve, businesses operating in the North America Cancer Diagnostics Market need to navigate through this landscape strategically to ensure sustained growth and stability.
Sample Request @ https://www.blueweaveconsulting.com/report/north-america-cancer-biopsy-market/report-sample
0 notes
Text
United States liquid biopsy market size reached USD 563.6 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 1,775.8 Million by 2033, exhibiting a growth rate (CAGR) of 13.6% during 2025-2033. The rising need for personalized treatments among individuals across the country is propelling the adoption of this method to analyze the genetic mutations in an efficient manner, which is primarily driving the market growth.
#United States Liquid Biopsy Market#United States Liquid Biopsy Market size#United States Liquid Biopsy Market share#United States Liquid Biopsy Market forecast
0 notes
Text
Revolutionizing the Ovarian Cancer Market: Strategies for Success | DLI
Ovarian Cancer Overview:
Ovarian Cancer is a formidable health challenge affecting women globally. It is characterized by the abnormal growth of cells in the ovaries, which are vital components of the female reproductive system. Often diagnosed at an advanced stage, ovarian cancer poses significant clinical and therapeutic complexities.
Ovarian cancer silently appears as a powerful foe that demands undivided attention. It is unyielding and resilient. It affects women of all ages and socioeconomic backgrounds and spares no one. This sneaky illness hides its presence, frequently coming to light only when the fight grows more difficult.
The Market Competitors Listed Below are Revolutionizing Healthcare with Innovative Diagnostic Inventions:
Price & Market Access
Diagnostic Analysis:
Early Detection Challenges:
Early diagnosis of ovarian cancer remains a critical issue due to its asymptomatic nature in the initial stages. Lack of specific symptoms and effective screening tools makes timely detection a formidable task.
Screening Methods:
Currently, screening methods primarily include pelvic examinations, transvaginal ultrasounds, and blood tests measuring CA-125 levels. However, these methods often lack the sensitivity and specificity needed for reliable early detection.
Treatment Analysis:
Standard Treatment Modalities:
Treatment approaches for ovarian cancer encompass a multidisciplinary approach, including surgery, chemotherapy, radiation therapy, and targeted therapy. The extent of surgical intervention and choice of chemotherapeutic agents are determined by factors like stage, histology, and patient's overall health.
Emerging Therapies:
Immunotherapy and personalized medicine are emerging as promising avenues in ovarian cancer treatment. These approaches aim to enhance the body's natural immune response and tailor treatments based on the genetic profile of the tumor.
Regulatory Framework:
FDA and EMA Guidelines:
Regulatory bodies like the FDA (Food and Drug Administration) in the United States and EMA (European Medicines Agency) in Europe play a pivotal role in approving and regulating treatments for ovarian cancer. Rigorous clinical trials and safety assessments are mandatory before a therapy can be marketed.
Therapeutics:
Targeted Therapies:
Targeted therapies like PARP inhibitors and angiogenesis inhibitors have shown promise in treating specific types of ovarian cancer. They work by interfering with the specific molecular pathways that cancer cells rely on for growth.
Immunotherapies:
Immunotherapies, including checkpoint inhibitors, are being investigated for their potential to enhance the body's immune system to recognize and attack ovarian cancer cells.
Diagnostic Techniques:
Genomic Profiling:
Advancements in genomic profiling have revolutionized the understanding of ovarian cancer. DNA sequencing and molecular profiling enable a more precise characterization of the tumor, guiding treatment decisions.
Liquid Biopsies:
Liquid biopsies, analyzing blood or other bodily fluids, offer a non-invasive method to monitor tumor genetics and detect minimal residual disease.
Treatment Techniques:
Minimally Invasive Surgery:
Minimally invasive techniques, such as laparoscopy, have gained prominence in ovarian cancer surgery. They offer shorter recovery times and reduced post-operative complications.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC):
HIPEC is an innovative technique that delivers heated chemotherapy directly to the abdominal cavity during surgery. This approach targets residual cancer cells and has shown promise in improving survival rates.
Browse More Information:
Clinical Assessment:
Multidisciplinary Teams:
Ovarian cancer treatment requires collaboration among various specialists, including gynecologic oncologists, surgeons, medical oncologists, and radiologists, to create personalized treatment plans.
Survivorship Care:
Long-term survivorship care plans are essential to address the physical, emotional, and psychological aspects of recovery after ovarian cancer treatment.
Expanding Therapeutic Landscape:
The ovarian cancer therapeutics market is witnessing a surge in research and development efforts, with a growing number of targeted and immunotherapeutic agents in the pipeline.
Regional Research Initiatives:
Different regions are contributing to ovarian cancer research with unique perspectives and approaches, fostering a global network of knowledge-sharing and collaboration.
Conclusion:
Ovarian cancer poses significant clinical challenges, but with advances in diagnostics, treatment modalities, and regulatory frameworks, there is hope for improved outcomes and a brighter future for those affected by this disease.
Browse Through More Oncology Diseases Research Reports.
Related Reports:
Diverticulitis: A Complete Guide to the Symptoms, Causes, and Treatments
When it comes to strategic Diabetes Disease consultancy, some of the areas we assist you understand include Regulatory Insights, Disease Landscape, and Market Access Expertise.
Find treatments for HPV illness using specialized market entry strategies. FDA/EMA insights and clinical trial advice. Examine right away!
Learn about specific approaches to Pancreatic Cancer Disease market access, pricing, and reimbursement. Learn about market research and clinical trials. Change your business immediately.
Learn about KOLs, the FDA, epidemiology, price reimbursement, and more in this market report on Anemia Disease. Your trustworthy source for info on anemia. Examine right away!
#clinical assessment#diagnostic analysis#market trends analysis#regnal insights#treatment analysis#disease#lung cancer#branding#economics#editorial design
1 note
·
View note
Text
Global Cancer Diagnostics Market Is Estimated To Witness High Growth Owing To Increasing Demand for Early Cancer Detection

The global Cancer Diagnostics market is estimated to be valued at US$ 59.1 billion in 2023 and is expected to exhibit a CAGR of 9.6% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights. A) Market Overview: The Cancer Diagnostics market includes various diagnostic tools and techniques used for the early detection, diagnosis, and management of cancer. These diagnostics play a crucial role in determining the type, stage, and treatment plan for cancer patients. The market offers a wide range of products such as antibodies, biomarkers, imaging modalities, and molecular tests. The increasing prevalence of cancer and the need for early detection are driving the demand for cancer diagnostics. These diagnostics provide numerous advantages, including precise diagnosis, targeted therapies, and personalized treatment plans, ultimately improving patient outcomes. B) Market key trends: One key trend shaping the Cancer Diagnostics market is the growing emphasis on liquid biopsies. Liquid biopsies refer to the non-invasive testing of tumor biomarkers from bodily fluids such as blood or urine. They offer several advantages over traditional tissue biopsies, including the ability to monitor cancer progression and treatment response in real-time. Liquid biopsies also enable early detection of cancer recurrence and minimal invasiveness for patients. For example, companies like Natera, Inc. have developed liquid biopsy tests, such as Signatera, which can detect minimal residual disease in colorectal cancer patients with high sensitivity. C) PEST Analysis: - Political: Governments worldwide are focusing on implementing cancer control programs and increasing funding for cancer research. This political support is driving the growth of the Cancer Diagnostics market. For instance, the National Cancer Moonshot Initiative in the United States aims to accelerate progress in cancer prevention, diagnosis, and treatment. - Economic: The rising healthcare expenditure and growing disposable income of individuals are fueling the demand for advanced cancer diagnostics. Additionally, the cost-effectiveness of these diagnostics in terms of improved patient outcomes and reduced treatment costs is contributing to market growth. - Social: The increasing awareness about the importance of early cancer detection and the proactive approach towards healthcare are positively influencing the market. Moreover, factors such as lifestyle changes, tobacco consumption, and unhealthy diets are contributing to the rising incidence of cancer. - Technological: Technological advancements in diagnostic techniques, such as next-generation sequencing, liquid biopsies, and companion diagnostics, are driving market growth. These advancements enable earlier detection, better accuracy, and personalized treatment approaches. D) Key Takeaways: Paragraph 1: The Global Cancer Diagnostics Market Size is expected to witness high growth, exhibiting a CAGR of 9.6% over the forecast period. This growth is primarily attributed to increasing demand for early cancer detection. Early diagnosis plays a critical role in improving patient outcomes and reducing the overall burden of cancer. Paragraph 2: Regionally, North America dominates the Cancer Diagnostics market due to factors such as a high prevalence of cancer, well-established healthcare infrastructure, and government initiatives towards cancer control. However, Asia Pacific is expected to witness the fastest growth during the forecast period. The increasing healthcare expenditure, growing awareness about cancer, and improving access to healthcare facilities are driving market growth in this region.
#Cancer Diagnostics Market#Cancer Diagnostics Market demand#Cancer Diagnostics Market growth#Cancer Diagnostics Market analysis#Cancer Diagnostics
0 notes
Text
Cancer Biopsy Market : Technology Advancements, Industry Insights, Trends And Forecast 2033
The cancer biopsy global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Cancer Biopsy Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.

Market Size - The cancer biopsy market size has grown rapidly in recent years. It will grow from $22.12 billion in 2023 to $25.08 billion in 2024 at a compound annual growth rate (CAGR) of 13.4%. The growth in the historic period can be attributed to increasing awareness of breast cancer liquid biopsy procedures, rising awareness of inherited oncology disorders and genetic testing, increasing adoption of liquid biopsies, increasing demand of for minimally invasive biopsy treatments, increasing demand for tissue biopsies. The cancer biopsy market size is expected to see rapid growth in the next few years. It will grow to $41.65 billion in 2028 at a compound annual growth rate (CAGR) of 13.5%. The growth in the forecast period can be attributed to an increase in the number of patients, the growing elderly population, the growing number of patients suspected of having breast cancer, the rise in cancer cases, an increase in the diagnosis and biopsies testing, increase in the number of patients. Major trends in the forecast period include technological advancements, artificial intelligence and machine learning, integration of multi-omics, product innovations, and point-of-care biopsy devices.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/cancer-biopsy-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers - The increasing prevalence of cancer is expected to propel the growth of the cancer biopsy market going forward. Cancer is a complex group of diseases characterized by the abnormal growth and spread of cells. The increasing prevalence of cancer is due to several factors, such as lifestyle changes and urbanization, environmental pollution, tobacco and alcohol consumption, healthcare access, and awareness. A cancer biopsy is used to diagnose and assess cancer type, stage, and spread by extracting and analyzing tissue or cells from a suspicious area. For instance, in January 2023, according to the National Center for Biotechnology Information, a US-based intergovernmental organization, in the United States there were 1,958,310 new cancer cases and 609,820 cancer deaths. Furthermore, in May 2021, according to the National Library of Medicine, a US-based medicine library, the global cancer burden is expected to be 28.4 million cases in 2040, a 47% rise from 2020, with a large number of deaths. Therefore, the increasing prevalence of cancer drives the cancer biopsy market.
Market Trends - Major companies operating in the cancer biopsy market are developing liquid biopsy techniques to enable non-invasive, early detection and monitoring of cancer, improve patient outcomes, and allow for more personalized treatment approaches. Liquid biopsy is an advanced, non-invasive diagnostic technique that detects and analyzes genetic material from a blood sample, such as circulating tumor DNA (ctDNA), RNA, exosomes, and other biomarkers. For instance, in May 2023, Labcorp, a US-based company specializing in the manufacturing of cancer biopsies, launched Labcorp Plasma Focus, a plasma-focused liquid biopsy to detect cancer-related biomarkers. The Plasma Focus test analyzes circulating cell-free DNA (cfDNA) released from tumor cells into the bloodstream and can construct a genomic profile of the patient's tumor, identifying mutations across 33 genes associated with various cancers, including non-small cell lung cancer, colorectal cancer, breast cancer, esophageal cancer, and melanoma.
The cancer biopsy market covered in this report is segmented –
1) By Type: Tissue Biopsies, Liquid Biopsies, Other Types 2) By Application: Breast Cancer, Colorectal Cancer, Cervical Cancers, Lung Cancers, Prostate Cancers, Skin Cancers, Blood Cancers, Kidney Cancers, Other Applications 3) By End User: Hospitals, Diagnostic Laboratories, Other End Users
Get an inside scoop of the cancer biopsy market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=19355&type=smp
Regional Insights - North America was the largest region in the cancer biopsy market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the cancer biopsy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies - Major companies operating in the cancer biopsy market are Thermo Fisher Scientific Inc., Danaher Corporation, Becton Dickinson and Company, Roche Diagnostics GmbH, Hologic Inc., Illumina Inc., Bio-Rad Laboratories Inc., QIAGEN N.V., Exact Sciences Corporation, Myriad Genetics Inc., Guardant Health Inc., Veracyte Inc., GRAIL Inc., Freenome Holdings Inc., Biodesix Inc., Chronix Biomedical Inc., Biocept Inc., Personal Genome Diagnostics Inc., Agena Bioscience Inc., Oncimmune Holdings plc, Exosome Diagnostics Inc., Epigenomics AG, Genesystems Inc., Lucence Diagnostics Pte. Ltd., ANGLE plc
Table of Contents 1. Executive Summary 2. Cancer Biopsy Market Report Structure 3. Cancer Biopsy Market Trends And Strategies 4. Cancer Biopsy Market – Macro Economic Scenario 5. Cancer Biopsy Market Size And Growth ….. 27. Cancer Biopsy Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected]
Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
0 notes