#Revlimid
Explore tagged Tumblr posts
Text
Where can I buy Revlimid online?: Looking to buy Revlimid online?
PsychedelicsPharmacySale offers high-quality, pharmaceutical-grade Revlimid (Lenalidomide) at competitive prices. Used for treating multiple myeloma and other blood disorders, Revlimid is a trusted medication prescribed by doctors worldwide. Our reliable online pharmacy ensures secure transactions, fast delivery, and discreet packaging for your convenience. At PsychedelicsPharmacySale, we prioritize product authenticity, customer satisfaction, and seamless service. Order today and get your Revlimid delivered safely and hassle-free to your doorstep with a trusted supplier!
0 notes
Text
Big Pharma Faces Revenue Loss: Cheaper Drugs Threaten Merck, Bristol-Myers Squibb, and Johnson & Johnson #biosimilars #BristolMyersSquibb #genericdrugs #JohnsonJohnson #Keytruda #Merck #patentcliff #pharmaceuticalindustry #Remicade #Revlimid #Stelara
#Business#biosimilars#BristolMyersSquibb#genericdrugs#JohnsonJohnson#Keytruda#Merck#patentcliff#pharmaceuticalindustry#Remicade#Revlimid#Stelara
0 notes
Text
Understanding the Generic Revlimid Cost: What Patients Need to Know
As pharmaceutical patents expire, generic versions of brand-name drugs become available, potentially offering more affordable options for patients. This is particularly relevant in the case of Revlimid, a medication commonly prescribed for various blood cancers, including multiple myeloma and myelodysplastic syndromes. Understanding the generic Revlimid cost is crucial for patients and healthcare providers alike, as it can significantly impact access to treatment and healthcare budgets.
What is Revlimid?
Revlimid, whose active ingredient is lenalidomide, is a medication primarily used to treat multiple myeloma, a cancer that forms in plasma cells. It works by slowing or stopping the growth of cancer cells. Additionally, Revlimid is prescribed for certain types of myelodysplastic syndromes, a group of disorders in which the bone marrow does not produce enough healthy blood cells.
The Cost of Brand-name Revlimid:
Before the availability of generic alternatives, brand-name Revlimid was considerably expensive, often placing a significant financial burden on patients and healthcare systems. The high cost of brand-name medications can be a barrier to access for many patients, particularly those without adequate insurance coverage or financial resources.
Introduction of Generic Revlimid:
With the expiration of Revlimid’s patent, generic versions of the drug have entered the market. Generic drugs contain the same active ingredients as their brand-name counterparts and are approved by regulatory authorities as safe and effective. Generic Revlimid offers a more affordable alternative to the brand-name medication, potentially reducing healthcare costs and improving access to treatment for patients.

Factors Affecting Generic Revlimid Cost:
Several factors influence the cost of generic Revlimid, including competition among manufacturers, production costs, and healthcare policies. As more manufacturers produce generic versions of the drug, competition typically drives prices down, making it more affordable for patients. Additionally, healthcare policies, such as insurance coverage and government subsidies, can further impact the out-of-pocket cost of generic Revlimid for patients.
Accessibility and Availability:
While the availability of generic Revlimid may vary depending on the region and healthcare system, efforts are often made to ensure widespread accessibility. Healthcare providers and pharmacies may work closely with patients to facilitate access to generic medications, including Revlimid, through various channels such as prescription assistance programs and formulary optimization.

Consultation with Healthcare Providers:
Patients considering switching to generic Revlimid should consult with their healthcare providers to ensure that it is a suitable option for their condition. Healthcare providers can provide valuable guidance regarding medication options, potential cost savings, and any concerns or considerations specific to the individual patient.
Conclusion:
The availability of generic Revlimid offers a promising opportunity to improve access to essential cancer treatment while potentially reducing healthcare costs for patients and healthcare systems. Understanding the factors influencing the generic Revlimid cost is essential for patients and healthcare providers to make informed decisions regarding treatment options. By working collaboratively, patients and healthcare providers can navigate the landscape of generic medications and ensure access to quality care for individuals affected by blood cancers.
0 notes
Text
Katie Porter contre-interroge un fa$$derat de big pharma (qui chie dans son froc mais ne donne pas un phoque) à savoir la raison du pourquoi comment qui et coudon un seul comprimé de Revlimid coûte 763 piastres aujourd'hui.
Demandez à votre pousheur médecin.cine de vous en ordonner lorsque vous irez vous faire piquouzer une 5e fois cet automne.
Combien notre bon curé Dubé de la religion sanitariste caquisse les paie en notre nom, lui ?

#le monde à bicyclette#pédale jamais autant que ce moron patine#big pharma est toujours en bonne santé#big pharmla#phoque ton capitalisme
3 notes
·
View notes
Text
What Medications Usually Require Prior Authorization?
If you’ve ever been told that your prescription needs prior authorization before you can pick it up, you’re not alone. Prior authorization (PA) is a process used by insurance companies to determine if they will cover certain medications. It helps manage costs and ensure that prescriptions are medically necessary. But what medications typically require prior authorization services? Let’s break it down!
1️⃣ High-Cost Medications
Expensive drugs often need PA to ensure they are necessary before approval. Examples include: 💰 Biologics – Humira, Enbrel, Stelara 💰 Specialty drugs – Revlimid, Ibrance, Ocrevus 💰 Gene therapies – Zolgensma, Luxturna
2️⃣ Brand-Name Medications with Generic Alternatives
If a generic version exists, insurers may require PA for the brand-name drug unless there’s a medical reason to avoid the generic. Examples: 🔹 Brand: Crestor | Generic: Rosuvastatin 🔹 Brand: Nexium | Generic: Esomeprazole 🔹 Brand: Advair Diskus | Generic: Fluticasone/Salmeterol
3️⃣ Medications for Chronic Conditions
Some long-term treatments need PA to ensure proper monitoring and cost control. These include: 💖 Cardiology drugs – Entresto, Repatha, Xarelto 🌿 Rheumatoid arthritis drugs – Xeljanz, Rinvoq 🧠 Neurology drugs – Aimovig, Botox (for migraines)
4️⃣ Controlled Substances & Opioids
To prevent misuse or overuse, many controlled substances need PA. Examples include: ⚠️ OxyContin (Oxycodone ER) ⚠️ Adderall (Amphetamine/Dextroamphetamine) ⚠️ Suboxone (Buprenorphine/Naloxone)
5️⃣ Weight Loss & Lifestyle Medications
Drugs that aren’t deemed medically necessary often need extra approval. Examples: ⚖️ Wegovy, Saxenda (Weight loss) 🔬 Viagra, Cialis (Erectile dysfunction) 💆♀️ Botox (for cosmetic use)
How to Handle a Prior Authorization?
If your medication requires prior authorization, here’s what you can do: ✔️ Talk to your doctor – They can submit the necessary paperwork. ✔️ Check with your insurance – Understand coverage options. ✔️ Ask about alternatives – A different medication might not need PA.
Final Thoughts
Prior authorization may feel like an inconvenience, but it exists to balance cost, safety, and necessity. If you ever run into issues, be proactive—your doctor or pharmacist can help you navigate the process!
#PriorAuthorization#Medications#Pharmacy#Healthcare#Insurance#Cardiology#Rheumatology#Neurology#SpecialtyDrugs#PrescriptionApproval#PatientCare#HealthTips
0 notes
Text
Oncology Drugs Market Report 2024-2032: Industry Analysis

The Oncology Drugs Market size was USD 221.38 billion in 2023 and is expected to reach USD 525.08 billion by 2031, growing at a CAGR of 11.4% over the forecast period of 2024-2031.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3241
Regional Analysis
The oncology drugs market exhibits significant growth across various regions:
North America: Dominates the market due to advanced healthcare infrastructure, substantial R&D investments, and high cancer prevalence.
Europe: Experiences growth driven by increasing cancer awareness, supportive government initiatives, and the presence of key pharmaceutical companies.
Asia-Pacific: Anticipated to witness the fastest growth, attributed to a rising patient population, improving healthcare facilities, and growing adoption of advanced therapies.
Market Segmentation
The oncology drugs market is segmented based on:
Drug Class:
Targeted Drugs
Cytotoxic Drugs
Hormonal Drugs
Others
Therapy:
Chemotherapy
Targeted Therapy
Immunotherapy
Indication:
Lung Cancer
Breast Cancer
Colorectal Cancer
Prostate Cancer
Others
Dosage Form:
Oral
Injectable
Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Key Players and Their Oncology Products
F. Hoffmann-La Roche Ltd. – Avastin, Herceptin, Tecentriq, Perjeta, Kadcyla, Alecensa, Rozlytrek
AbbVie Inc. – Venclexta, Imbruvica, Empliciti
Novartis AG – Kymriah, Kisqali, Tabrecta, Scemblix, Jakavi, Tafinlar, Mekinist
Pfizer Inc. – Ibrance, Lorbrena, Talzenna, Inlyta, Xtandi, Besponsa, Daurismo
Bristol Myers Squibb Company – Opdivo, Yervoy, Revlimid, Pomalyst, Breyanzi, Abecma, Reblozyl
GlaxoSmithKline plc. – Zejula, Jemperli, Blenrep
Eli Lilly and Company – Verzenio, Cyramza, Retevmo, Jaypirca
AstraZeneca – Tagrisso, Imfinzi, Lynparza, Enhertu, Calquence
Sanofi – Sarclisa, Libtayo, Jevtana
Bayer AG – Stivarga, Nubeqa, Xofigo, Vitrakvi
Merck & Co., Inc. – Keytruda, Welireg, Tepmetko
Key Points
Increasing global cancer prevalence is a primary driver of the oncology drugs market.
Advancements in medical research and technology have led to innovative and targeted therapies.
High costs and complexities associated with drug development pose challenges to market growth.
Emerging economies present lucrative opportunities for market expansion.
Future Scope
The oncology drugs market is poised for substantial growth, driven by continuous research and development leading to novel therapies with improved efficacy and safety profiles. Personalized medicine, focusing on treatments tailored to individual genetic profiles, is expected to revolutionize cancer therapy. Additionally, collaborations between pharmaceutical companies and research institutions are likely to expedite the development of cutting-edge treatments, addressing unmet medical needs and enhancing patient outcomes.
Conclusion
The oncology drugs market is on a robust growth trajectory, propelled by rising cancer incidence and significant advancements in therapeutic options. Despite challenges such as high development costs and complex regulatory landscapes, the market offers promising opportunities, particularly in emerging economies. Ongoing innovations and strategic collaborations are set to transform cancer treatment paradigms, offering hope for improved patient survival and quality of life.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Smart Healthcare Market
Digital Therapeutics Market
Post Traumatic Stress Disorder Treatment Market
#Oncology Drugs Market#Oncology Drugs Market Share#Oncology Drugs Market Size#Oncology Drugs Market Trends
0 notes
Text
CELMoDs: A Next-Generation Approach in Multiple Myeloma Treatment
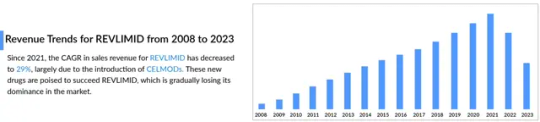
The landscape of multiple myeloma treatment is rapidly evolving, with CELMoDs (Cereblon E3 Ligase Modulators) emerging as a promising next-generation therapy. While Revlimid has long been a cornerstone in managing multiple myeloma, challenges such as drug resistance and adverse effects have driven the need for innovative alternatives. CELMoDs offer a compelling solution by providing enhanced efficacy and improved safety profiles.
These novel drugs function by modulating the cereblon protein, leading to the degradation of key proteins essential for tumor cell survival. While their mechanism is similar to immunomodulatory drugs (IMiDs) like Revlimid, CELMoDs exhibit greater potency and selectivity, positioning them as a breakthrough treatment option.
The multiple myeloma treatment market stands to gain significantly from CELMoDs, as they introduce a novel approach to combating this complex and often relapsing disease. Clinical trials have demonstrated that CELMoDs, particularly in combination therapies, outperform traditional treatments in efficacy. As resistance to established therapies like Revlimid continues to grow, CELMoDs are shaping up to be key players in the multiple myeloma drug market.
Beyond efficacy, CELMoDs are designed to improve safety profiles, minimizing side effects that often hinder current therapies. With the multiple myeloma treatment landscape expanding, these next-generation drugs have the potential to improve patient outcomes, extend survival rates, and offer new hope to those with limited treatment options.
As advancements in multiple myeloma treatment continue, CELMoDs are paving the way for a new era of therapeutic innovation. Their potential to succeed Revlimid and redefine treatment standards highlights their importance in the future of multiple myeloma care.
Top Reports Offered By Delveinsight
radicava mechanism of action | how many people have cidp | therapy light benefits | internet of things in medical | health monitoring apps | cardiac monitoring devices | immunomodulatory drugs | izervay reviews | adenovirus stages | what is hemoglobinuria | peripheral artery disease prevalence | arteriotomy | orthopedic implant companies | ropressa | oceanic af | cervical dysplasia stages | tev-48574 | welireg fda approval | nevro hfx programs | axillary hidrosis | lentiginous definition | voquezna gerd | visiox pharma | fluid management systems | cln1 disease | can achondroplasia be treated
About DelveInsight
DelveInsight is a premier market research and consulting firm specializing in life sciences and healthcare. We provide in-depth insights to help pharmaceutical, biotechnology, and medical device companies navigate a dynamic and competitive industry
Contact Information
Kanishk
0 notes
Text
Global Lenalidomide Market Size,Growth Rate,Industry Opportunities 2025-2031
According to our (Global Info Research) latest study, the global Lenalidomide market size was valued at US$ million in 2024 and is forecast to a readjusted size of USD million by 2031 with a CAGR of %during review period.
Lenalidomide, sold under the trade name Revlimid among others, is a medication used to treat multiple myeloma (MM) and myelodysplastic syndromes (MDS). For MM it is used after at least one other treatment and generally together with dexamethasone.
In China, Lenalidomide key players include Celgene, SL Pharma. In terms of product, 25 mg Capsules is the largest segment, with a share over 40%. And in terms of application, the largest application is Multiple myeloma (MM), followed by Myelodysplastic syndromes (MDS).
Our Lenalidomide Market report is a comprehensive study of the current state of the industry. It provides a thorough overview of the market landscape, covering factors such as market size, competitive landscape, key market trends, and opportunities for future growth. It also pinpoints the key players in the market, their strategies, and offerings.
The report offers an in-depth look into the current and future trends in Lenalidomide, making it an invaluable resource for businesses involved in the sector. This data will help companies make informed decisions on research and development, product design, and marketing strategies. It also provides insights into Lenalidomide’ cost structure, raw material sources, and production processes. Additionally, it offers an understanding of the regulations and policies that are likely to shape the future of the industry. In essence, our report can help you stay ahead of the curve and better capitalize on industry trends.
The research report encompasses the prevailing trends embraced by major manufacturers in the Lenalidomide Market, such as the adoption of innovative technologies, government investments in research and development, and a growing emphasis on sustainability. Moreover, our research team has furnished essential data to illuminate the manufacturer's role within the regional and global markets.
The research study includes profiles of leading companies operating in the Lenalidomide Market:
The report is structured into chapters, with an introductory executive summary providing historical and estimated global market figures. This section also highlights the segments and reasons behind their progression or decline during the forecast period. Our insightful Lenalidomide Market report incorporates Porter's five forces analysis and SWOT analysis to decipher the factors influencing consumer and supplier behavior.
Segmenting the Lenalidomide Market by application, type, service, technology, and region, each chapter offers an in-depth exploration of market nuances. This segment-based analysis provides readers with a closer look at market opportunities and threats while considering the political dynamics that may impact the market. Additionally, the report scrutinizes evolving regulatory scenarios to make precise investment projections, assesses the risks for new entrants, and gauges the intensity of competitive rivalry.
Lenalidomide Market by Type: 5 mg Capsules、10 mg Capsules、15 mg Capsules、25 mg Capsules
Lenalidomide Market by Application: Multiple myeloma (MM)、Myelodysplastic syndromes (MDS)
Key Profits for Industry Members and Stakeholders:
The report includes a plethora of information such as market dynamics scenario and opportunities during the forecast period. Which regulatory trends at corporate-level, business-level, and functional-level strategies. Which are the End-User technologies being used to capture new revenue streams in the near future. The competitive landscape comprises share of key players, new developments, and strategies in the last three years. One can increase a thorough grasp of market dynamics by looking at prices as well as the actions of producers and users. Comprehensive companies offering products, relevant financial information, recent developments, SWOT analysis, and strategies by these players. The content of the study subjects, includes a total of 15 chapters: Chapter 1, to describe Lenalidomide product scope, market overview, market estimation caveats and base year. Chapter 2, to profile the top manufacturers of Lenalidomide, with price, sales, revenue and global market share of Lenalidomide from 2020 to 2025. Chapter 3, the Lenalidomide competitive situation, sales quantity, revenue and global market share of top manufacturers are analyzed emphatically by landscape contrast. Chapter 4, the Lenalidomide breakdown data are shown at the regional level, to show the sales quantity, consumption value and growth by regions, from 2020 to 2031. Chapter 5 and 6, to segment the sales by Type and application, with sales market share and growth rate by type, application, from 2020 to 2031. Chapter 7, 8, 9, 10 and 11, to break the sales data at the country level, with sales quantity, consumption value and market share for key countries in the world, from 2020 to 2024.and Lenalidomide market forecast, by regions, type and application, with sales and revenue, from 2025 to 2031. Chapter 12, market dynamics, drivers, restraints, trends and Porters Five Forces analysis. Chapter 13, the key raw materials and key suppliers, and industry chain of Lenalidomide. Chapter 14 and 15, to describe Lenalidomide sales channel, distributors, customers, research findings and conclusion.
Global Info Research is a company that digs deep into global industry information to support enterprises with market strategies and in-depth market development analysis reports. We provides market information consulting services in the global region to support enterprise strategic planning and official information reporting, and focuses on customized research, management consulting, IPO consulting, industry chain research, database and top industry services. At the same time, Global Info Research is also a report publisher, a customer and an interest-based suppliers, and is trusted by more than 30,000 companies around the world. We will always carry out all aspects of our business with excellent expertise and experience.
0 notes
Text
0 notes
Text
0 notes
Text
Trends in the Lymphoma Treatment Market
The global lymphoma treatment market size is expected to reach USD 11.97 billion by 2030, registering a CAGR of 8.3% from 2025 to 2030, according to a new report by Grand View Research, Inc. Rising research activities for development of new treatments and increasing product line extension are likely to expedite growth. Increasing commercialization of novel drugs are further expected to drive the growth.
Availability of wide variety of products is further expected to aid growth. For instance, in January 2019, BeiGene received a breakthrough therapy designation by the U.S. FDA for its product Zanubrutinib for treating mantle cell lymphoma in adults. This drug is currently in late-stage clinical trial and is expected to be launched during the forecast period.
Growing awareness about early diagnosis of lymphoma among healthcare professionals & patients and rising government funding for R&D are some factors expected to propel market growth. For instance, institutes such as National Cancer Institute receives funding from the U.S. Congress to support cancer-related research and associated activities.
Lymphoma Treatment Market Report Highlights
Non-Hodgkin Lymphoma (NHL) dominated the market with the largest revenue share of 86.2% in 2024, driven by the increasing incidence of NHL, with projections indicating a significant rise in cases over the coming years.
Hodgkin Lymphoma (HL) is expected to grow at a CAGR of 15.7% over the forecast period attributed to a rising number of diagnosed cases and advancements in diagnostic technologies that facilitate earlier detection.
Rituxan (MabThera) dominated the market and accounted for the largest revenue share of 28.8% in 2024, attributed to its established efficacy and broad usage across various indications, particularly non-Hodgkin lymphoma (NHL).
Keytruda is expected to grow at the fastest CAGR of 18.9% over the forecast period, owing to its status as a leading immunotherapy option, particularly for advanced or refractory cases.
Hospital pharmacies dominated the market and accounted for the largest revenue share of 36.1% in 2024 attributed to the increasing prevalence of lymphoma cases requiring specialized care.
The lymphoma treatment market in North America dominated the global market and accounted for the largest revenue share of 49.0% in 2024, owing to the high prevalence of lymphoma cases, particularly non-Hodgkin lymphoma, which accounts for a significant portion of cancer diagnoses.
The Asia Pacific lymphoma treatment market is projected to grow rapidly due to rising incidence rates and increasing healthcare spending. Countries in this region are experiencing improvements in healthcare infrastructure and access to innovative treatments
Lymphoma Treatment Market Segmentation
Grand View Research has segmented the global lymphoma treatment market on the basis of on type, drug, distribution channel, and region:
Lymphoma Treatment Type Outlook (Revenue, USD Million, 2018 - 2030)
Hodgkin Lymphoma
Non-Hodgkin Lymphoma
Lymphoma Treatment Drug Outlook (Revenue, USD Million, 2018 - 2030)
Rituxan/MabThera
Revlimid
Imbruvica
Adcetris
Keytruda
Opdivo
Others
Lymphoma Treatment Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
Hospital Pharmacy
Retail Pharmacy
Specialty Pharmacy
Others
Lymphoma Treatment Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
Germany
UK
France
Italy
Spain
Asia Pacific
China
Japan
India
South Korea
Australia
Latin America
Brazil
Argentina
Middle East and Africa (MEA)
South Africa
UAE
Order a free sample PDF of the Lymphoma Treatment Market Intelligence Study, published by Grand View Research.
0 notes
Text
Buy Revlimid online:
Looking for Revlimid for sale online? Our PsychedelicsPharmacySale guarantees quality, trusted, and authentic Revlimid (Lenalidomide), besides a secure and hassle-free purchase procedure. It is a well-known medication in treating multiple myeloma and some other serious conditions, and Revlimid has always remained a safe and much trusted cancer therapy. We guarantee the best prices market has to offer, together with great customer support, discreet packaging, and fast worldwide delivery. Order: safe and secure Revlimid delivery from PsychedelicsPharmacySale-a trusted source!
0 notes
Text
Cereblon E3 Ligase Modulators Market: Size, Target Population, Competitive Landscape, and Forecast to 2034
Cereblon E3 ligase modulators (CELMoDs) are a new frontier in targeted therapies, particularly in the treatment of cancers such as multiple myeloma. This emerging drug class is showing immense potential to surpass conventional immunomodulatory drugs (IMiDs), offering greater efficacy and improved safety profiles. The market for these innovative therapeutics is expected to grow significantly, driven by advancements in clinical research, the increasing prevalence of multiple myeloma, and the need for more effective treatment options.
Cereblon E3 Ligase Modulators Market Size and Growth Potential
The global market for CELMoDs is poised for robust growth through 2034. With leading candidates such as iberdomide and mezigdomide in development, companies are focusing on addressing unmet needs in cancer treatment. Iberdomide has demonstrated enhanced potency and binding affinity to cereblon, leading to better degradation of transcription factors like Ikaros and Aiolos, critical in tumor survival. This specificity makes CELMoDs a superior alternative to earlier drugs like Revlimid, whose market share is declining due to patent expirations and generic competition.
By 2034, the CELMoD market is expected to capture a significant portion of the oncology treatment landscape, particularly within hematological malignancies like multiple myeloma. Key factors contributing to this growth include increasing investments in research, strategic collaborations among pharmaceutical companies, and the expansion of the eligible patient population due to the aging global demographic.
Request for sample pages @ https://www.delveinsight.com/report-store/cereblon-e3-ligase-modulators-market-forecast
Cereblon E3 Ligase Modulators Target Population
Multiple myeloma patients represent the primary target population for CELMoDs. This disease predominantly affects older adults, with the majority of diagnoses occurring in individuals aged 65 and above. The increasing incidence of multiple myeloma, coupled with the rising prevalence of relapsed or refractory cases, underscores the need for more effective treatments. CELMoDs are particularly promising for patients who have shown resistance to traditional therapies, including IMiDs and proteasome inhibitors.
Additionally, the potential expansion of CELMoDs into other cancers and autoimmune diseases may further broaden their target population. Preclinical and early-phase studies are exploring the use of CELMoDs in solid tumors and inflammatory conditions, indicating a wider application of this drug class in the future.
Download sample pages @ https://www.delveinsight.com/sample-request/cereblon-e3-ligase-modulators-market-forecast
Cereblon E3 Ligase Modulators Competitive Landscape
The competitive landscape for CELMoDs is shaped by pharmaceutical giants like Bristol-Myers Squibb (BMS), which has pioneered this space with iberdomide and mezigdomide. These compounds are designed to improve upon the efficacy and safety profiles of earlier IMiDs such as Revlimid and Pomalyst. BMS is investing heavily in clinical trials to establish CELMoDs as the new standard of care for multiple myeloma, aiming to replace Revlimid in earlier lines of treatment.
Other companies are also entering the market, recognizing the potential of CELMoDs. Collaboration and competition in this space are expected to accelerate innovation, leading to the development of next-generation ligase modulators with broader applications and fewer adverse effects.
Read more about the market landscape @ https://www.delveinsight.com/sample-request/cereblon-e3-ligase-modulators-market-forecast
Challenges and Opportunities
One of the main challenges facing the CELMoD market is the high cost of development and clinical trials. Additionally, long-term safety data are still being gathered, and regulatory hurdles may slow the entry of these drugs into broader markets. However, the opportunities outweigh these challenges. The unmet need for effective and well-tolerated treatments in multiple myeloma provides a fertile ground for the success of CELMoDs.
The growing understanding of cereblon biology and protein degradation pathways opens doors for the design of more targeted and efficient therapies. Furthermore, the anticipated approval of CELMoDs in major markets like the U.S., Europe, and Asia will likely drive substantial growth over the forecast period.
Cereblon E3 Ligase Modulators Market Forecast to 2034
The CELMoD market is expected to expand significantly, supported by rising demand for advanced treatments and favorable regulatory environments. By 2034, CELMoDs could become a cornerstone of oncology treatment, particularly for hematologic cancers. Pharmaceutical companies are expected to continue investing in this area, with potential breakthroughs leading to the introduction of new compounds and therapeutic strategies.
The Cereblon E3 Ligase Modulators market represents a transformative development in cancer treatment. With their enhanced efficacy, safety profiles, and potential applications beyond multiple myeloma, CELMoDs are set to redefine therapeutic standards. The coming decade will likely witness rapid growth in this market, driven by innovation, strategic collaborations, and an increasing understanding of the underlying science. By 2034, CELMoDs could emerge as a dominant force in targeted cancer therapy, offering hope to patients worldwide.
For more detailed insights, visit [DelveInsight's report on the Cereblon E3 Ligase Modulators Market](https://www.delveinsight.com/report-store/cereblon-e3-ligase-modulators-market-forecast).
0 notes
Link
#market research future#orphan drugs market#orphan drugs market size#orphan drugs market trends#orphan drugs market growth
0 notes
Text
What Medications Usually Require Prior Authorization?
Prior authorization services is a process in which a healthcare provider must obtain approval from an insurance company before prescribing certain medications. This requirement ensures that the prescribed drug is medically necessary, cost-effective, and appropriate for the patient’s condition.
Practolytics, a trusted leader in healthcare RCM and prior authorization services, helps healthcare providers streamline this process, reducing administrative burdens and improving patient access to essential medications.
Below is a detailed breakdown of the types of medications that usually require prior authorization and how Practolytics can assist providers in navigating the approval process.
Types of Medications That Usually Require Prior Authorization
1. High-Cost Brand-Name Medications
Many brand-name drugs require prior authorization due to their high cost. Insurance providers may require patients to try lower-cost generic alternatives first.
Examples include:
Humira (adalimumab) – Used for autoimmune diseases like rheumatoid arthritis.
Enbrel (etanercept) – Treats rheumatoid arthritis and psoriasis.
Xeljanz (tofacitinib) – Used for rheumatoid arthritis and ulcerative colitis.
Dupixent (dupilumab) – Treats eczema and asthma.
Ozempic (semaglutide) – A diabetes drug also used for weight management.
With Practolytics, providers can reduce PA delays by ensuring all necessary documentation is submitted correctly and promptly.
2. Specialty Medications
Specialty drugs used for chronic or complex conditions are expensive and require extensive insurance review.
Examples include:
Cancer drugs (Keytruda, Opdivo, Revlimid)
Multiple sclerosis drugs (Ocrevus, Gilenya, Tecfidera)
HIV/AIDS treatments (Biktarvy, Descovy)
Cystic fibrosis medications (Trikafta, Kalydeco)
Since specialty drugs often require additional documentation, Practolytics assists in managing these requests efficiently, minimizing treatment delays.
3. Medications With High Potential for Abuse
To prevent misuse and addiction, controlled substances often require prior authorization.
Examples include:
Opioids (OxyContin, fentanyl, hydrocodone)
ADHD medications (Adderall, Ritalin, Vyvanse)
Benzodiazepines (Xanax, Ativan, Valium)
Sleep medications (Ambien, Lunesta)
By using Practolytics’ automated PA solutions, providers can ensure compliance with regulatory guidelines while expediting approvals.
4. Medications for Off-Label Use
Insurance companies often require PA for medications prescribed outside of their FDA-approved use.
Examples include:
Methotrexate – Used off-label for autoimmune diseases.
Ketamine – Used off-label for depression and pain management.
Low-dose naltrexone (LDN) – Prescribed for fibromyalgia and multiple sclerosis.
Practolytics assists providers in submitting the necessary medical justifications to secure approvals for off-label prescriptions.
5. Cosmetic and Lifestyle Medications
Drugs used for non-medically essential purposes often require PA.
Examples include:
Hair loss treatments (Propecia, Rogaine)
Weight loss medications (Wegovy, Saxenda)
Erectile dysfunction drugs (Viagra, Cialis)
Hormone therapy (testosterone replacement)
With Practolytics, healthcare providers can navigate these approvals by ensuring proper documentation is in place.
6. Biologics and Immunotherapy Drugs
Biologic medications are highly specialized and require thorough insurance approval.
Examples include:
Monoclonal antibodies (Rituxan, Herceptin)
Interleukin inhibitors (Cosentyx, Stelara)
Checkpoint inhibitors (Keytruda, Opdivo)
Practolytics’ dedicated team ensures that PA requests for biologics are processed quickly, preventing disruptions in patient care.
7. Preventive Medications With Limited Coverage
Some preventive medications require PA based on insurance policies.
Examples include:
Shingles vaccine (Shingrix)
HPV vaccine (Gardasil)
Malaria prevention drugs (Malarone, doxycycline)
Hepatitis B treatments (Viread)
Using Practolytics, providers can secure approvals by ensuring that preventive medication requests meet insurance guidelines.
8. Fertility and Reproductive Medications
Fertility treatments often require prior authorization due to their high cost.
Examples include:
Clomid (clomiphene citrate) – Induces ovulation.
Follistim and Gonal-F – Injectable fertility drugs.
IVF-related medications (Menopur, Lupron)
Practolytics simplifies the PA process for fertility drugs, ensuring faster approval times for patients undergoing treatment.
9. Combination Medications
Combination medications often require PA because insurers may prefer patients to take individual components separately.
Examples include:
Duexis (ibuprofen + famotidine)
Treximet (sumatriptan + naproxen)
Advair, Symbicort – Combination inhalers for asthma and COPD.
With Practolytics’ expertise, providers can justify the need for combination drugs and reduce approval wait times.
10. Step Therapy or Alternative Treatment Requirements
Many insurance plans require patients to try lower-cost drugs before approving a more expensive medication.
Examples include:
Metformin before Jardiance or Trulicity (for diabetes)
SSRIs before Spravato (esketamine) for depression
Practolytics helps providers navigate step therapy protocols to ensure patients receive the most effective treatment without unnecessary delays.
How Practolytics Helps Providers Navigate the Prior Authorization Process
Prior authorization can be time-consuming, but Practolytics offers streamlined solutions to reduce administrative burdens and speed up approvals. Here’s how:
1. Automated PA Submissions
Using advanced technology, Practolytics automates prior authorization requests, minimizing manual effort.
2. Real-Time Status Tracking
Providers can track the status of PA requests in real time, ensuring timely approvals.
3. Expert Documentation and Appeals
If a request is denied, Practolytics assists with appeals, providing necessary justifications to insurance companies.
4. Compliance and Insurance Coordination
Practolytics ensures all PA requests comply with payer policies, reducing the risk of claim denials.
Final Thoughts
Prior authorization is a necessary but complex process that can delay patient care if not managed efficiently. By partnering with Practolytics, healthcare providers can streamline PA approvals, reduce paperwork, and improve patient access to essential medications.
For more information on how Practolytics can optimize your prior authorization workflow, visit their website or contact their expert team today!
#healthcarercmservices#medical billing services#priorauthorization#medical coding services#ehr#practolytics
1 note
·
View note
Text
Multiple Myeloma: An Incurable B-cell Malignancy and the Challenge of Recurrence
Multiple myeloma is a complex and incurable B-cell malignancy characterized by the uncontrolled proliferation of plasma cells within the bone marrow. Despite significant progress in multiple myeloma treatment, the disease remains chronic, with cycles of remission and relapse.
Understanding Multiple Myeloma Diagnosis and Prognosis
Early multiple myeloma diagnosis is crucial for managing disease progression. Diagnostic methods include blood markers, bone marrow biopsies, and imaging techniques. Despite aggressive treatments, the multiple myeloma cure rate remains low, and relapse occurs due to the persistence of resistant cancer cells.
Current Treatment Options for Multiple Myeloma
The multiple myeloma treatment landscape includes chemotherapy drugs for multiple myeloma, targeted therapies, and immunotherapies. One of the most effective approaches involves stem cell transplantation, which can prolong remission. Key treatments include Revlimid and proteasome inhibitors like Carfilzomib. Immunotherapy is also emerging as a promising approach, with ongoing clinical trials exploring new possibilities.
Challenges in Managing Multiple Myeloma Recurrence
One of the most significant challenges is disease relapse. The continuous evolution of multiple myeloma drugs has led to new therapeutic developments to combat resistance. The newest treatment for multiple myeloma includes monoclonal antibodies, CAR-T cell therapy, and next-generation proteasome inhibitors. However, no therapy has yet provided a definitive cure, emphasizing the need for ongoing research and innovation in multiple myeloma therapeutics.
Nutrition and Future Outlook
A comprehensive approach, including proper nutrition and adherence to multiple myeloma treatment guidelines, can significantly improve patients’ quality of life. As research advances, novel targeted therapies and evolving treatment protocols continue to enhance patient outcomes.
While multiple myeloma remains incurable, continuous advancements in treatments and clinical trials provide hope for better disease management and extended survival.
While multiple myeloma remains incurable, advancements in treatments and ongoing multiple myeloma clinical trials provide hope for better disease management and prolonged survival.
Latest Blogs Offered By DelveInsight:
Insights Into The Cutaneous T-cell Lymphoma Treatment Market
Roche’s HEMLIBRA: A Game Changer in Hemophilia A Treatment Landscape
Emerging Role of Digital Health in the Field of Oncology
How Will Emerging Therapies Drift the Amyotrophic Lateral Sclerosis (ALS) Treatment Landscape
How are Technological Trends and Innovations Reshaping the Dementia Care
Latest Blogs Offered By DelveInsight:
Medtronic Secures FDA Green Light for Affera™ Mapping and Ablation System Alongside Sphere-9™ Catheter; Precision Optics Gets FDA 510(k) Clearance; Abbott Launches New Clinical Trial Aimed at Enhancing Care for Advanced Heart Failure Patients; Fresenius Medical Care’s Study Confirms Efficacy of New Anemia Therapy Software in Enhancing Outcomes for Hemodialysis Patients; Inspira™ Announces New Distribution Center to Support INSPIRA™ ART100’s U.S. Introduction; WellSky Expands Home Care Offerings with Acquisition of Bonafide
7 Key Technologies Pioneering Cybersecurity in the Healthcare Sector
FDA Grants Orphan Status to MDL-101 for LAMA2-CMD; Pfizer’s ABRYSVO Approved for High-Risk Adults (18-59); KIND’s AND017 Gains Orphan Designation for Sickle Cell Disease; HiberCell’s HC-7366 Fast-Tracked for AML; ORLYNVAH Approved for Uncomplicated UTIs
Pfizer’s ABRYSVO Outpaces GSK’s AREXVY with Expanded FDA Approval – But Can It Sustain the Momentum?
CAR-T Cells vs. CAR-Exosome Agents: Exploring the Future of Cancer Immunotherapy
Another Reports Offered By Delveinsight
cmo contract manufacturing organization | keynote-a18 | generic for stelara | mucodyne | uromune usa | drugs for hyperkalemia | electronic devices that help the heart maintain normal rhythm | camoteskimab | artificial intelligence app iphone | msa p disease | gilead adc | what meds are used for ptsd | lack of interoperability in healthcare | d&d pharmatech | emicizumab kxwh | nxstage crrt | neuropathic ocular pain | chemo induced diarrhea | nf type 1 treatment | whats mrd | whats bci | eular 2024 abstract | does pritelivir cure herpes | ml-004 | copd statistics in the united states | bms earnings call transcript | history of breast cancer awareness month | what are some cardiac diseases
About DelveInsight
DelveInsight is a premier market research and consulting firm specializing in life sciences and healthcare. We provide in-depth insights to help pharmaceutical, biotechnology, and medical device companies navigate a dynamic and competitive industry
Contact Information
Kanishk
0 notes