#Respiratory diagnostics market
Explore tagged Tumblr posts
Text
The respiratory diagnostics market involves a wide range of novel technologies and devices that assist healthcare professionals in assessing and managing lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and respiratory infections.
#Respiratory Diagnostics Market#Respiratory Diagnostics Report#Respiratory Diagnostics Industry#Healthcare#BIS Research
1 note
·
View note
Text
Breathing New Life: Exploring the $43.2 Billion Respiratory Market
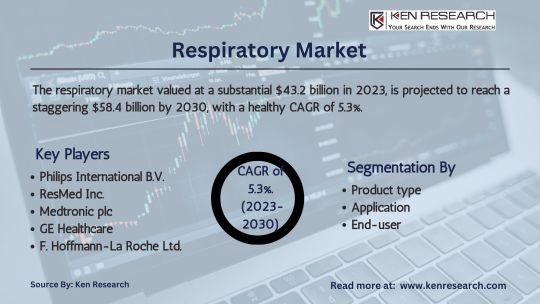
Discover the $43.2 billion respiratory market, analyzing its revenue, growth rate, size, and segmentation. Explore therapeutic devices, care equipment, and accessories driving growth in this vital healthcare sector.
#Respiratory Market#Respiratory Industry#Respiratory Sector#Respiratory Market Analysis#Respiratory Market Revenue#Respiratory Market Growth Rate#Respiratory Market Size#Respiratory Market Segmentation#Respiratory Care Devices Market#Respiratory Devices Market#Therapeutic Respiratory Devices Market#Respiratory device accessories market#Respiratory Care Equipment Market#Respiratory diagnostics market#Respiratory measurement devices market#growth of Respiratory industry#Respiratory Industry Competitors#Opportunities in Respiratory Industry
0 notes
Text
Respiratory syncytial virus (RSV) is a viral infection of the lungs and respiratory tract, which can cause asthma, hospitalization, pneumonia, repeated infections, middle ear infection, and death. It can be diagnosed using blood tests, chest x-rays, a swab of secretions, and pulse oximetry by checking for signs like lung inflammation, white blood cell (WBC) count, and oxygen levels in the blood. Some of the commonly available treatment options for RSV are over-the-counter (OTC) medications, intravenous (IV) fluids, humidified oxygen, mechanical ventilation, and inhaled bronchodilators.
0 notes
Text
The latest report by Precision Business Insights, titled Respiratory Molecular Diagnostics Market covers complete information on market size, share, growth, trends, segment analysis, key players, drivers, and restraints.
0 notes
Text
#Respiratory Diagnostic Market#Respiratory Diagnostic#Respiratory#Diagnostic#Diagnostic Market#Respiratory Diagnostic Market Size#Respiratory Diagnostic Market Share#Respiratory Diagnostic Market Forecast
0 notes
Text
Infectious Respiratory Disease Diagnostics Market Segmented On The Basis Of Product Type, Sample Type, Application, Technology, End-Use, Region And Forecast 2030: Grand View Research Inc.
San Francisco, 2 Aug 2023: The Report Infectious Respiratory Disease Diagnostics Market Size, Share & Trends Analysis By Product Type (Instruments, Services), By Sample Type (Blood, NPS), By Application, By Technology, And Segment Forecasts, 2022 – 2030 The global infectious respiratory disease diagnostics market size is expected to reach USD 43.6 billion by 2030, registering a CAGR of -1.8%…

View On WordPress
#Infectious Respiratory Disease Diagnostics Industry#Infectious Respiratory Disease Diagnostics Market#Infectious Respiratory Disease Diagnostics Market 2022#Infectious Respiratory Disease Diagnostics Market 2030#Infectious Respiratory Disease Diagnostics Market Share#Infectious Respiratory Disease Diagnostics Market Size
0 notes
Text
Philippines Pharmacy Retail Market is anticipated to grow by USD 6Th Mn by 2025- How will the market gain traction to be able to reach at its targeted number?
The government distribution of medicines will improve in future due to the initiatives such as Botika Ng Bayan, Distribution through Local Health Units and Rural Health Units, says a report by Ken Research
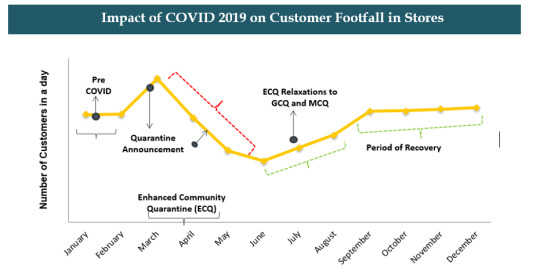
1.Corona Virus effect on pharmacy sales in Philippines Pharmacy Retail Market.
Other Challenges in Philippines Pharmacy Retail Market Outlook to 2025
Demand for OTC products like Alcohol Based Cleaners, Vitamin C and D Tablets, Immunity Boosters and Health Supplements was boosted during quarantine. Maintenance Medicines were also in high demand. During the starting of the Quarantine, Pharmacies witnessed increase in sales due to bulk purchase, later the sales declined. Pharmacies faced supply chain issues especially in the ECQ zones. This has affected the sales of the pharmacies. Further, Philippines witnessed lockdown for a long duration.
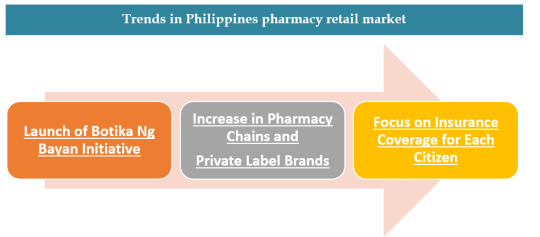
2.Trends and Development that the Pharmacy retail market of Philippines will experience.
Download Sample Report
In July 2018, DOH relaunched Botika Ng Bayan initiative to provide free essential medicines for common diseases focusing on rural and poor people. The chain stores have increased tremendously in the Philippines. Especially TGP and Generika as they have adopted franchise model for store expansion. This increase in chained pharmacies has led to the growth of house brands or private labels. In starting of 2019, President Rodrigo signed Universal Health Care (UHC) Bill into law (Republic Act No. 11223) which automatically enrolls all Filipino citizens in the National Health Insurance Program. This will increase the healthcare affordability in the region.
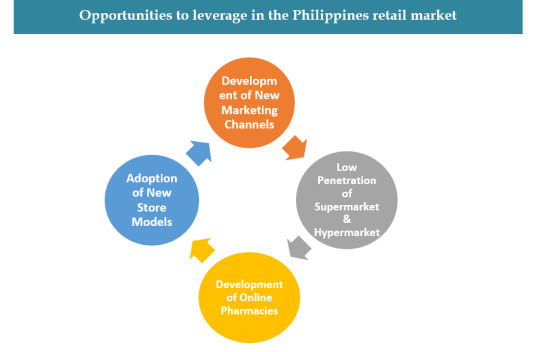
3. Assessing the Possibilities in the Philippines Pharmacy Retail Market
Ask For Customization
Penetration of internet, growth of social media has opened more connectivity options for the companies.
Drive Through Stores, self-service stores are increasing customer convenience.
This has helped in increasing the footfall in pharmacies for purchasing non pharmaceutical products.
Chain stores can serve regional customers where they don’t have strong presence.
Key Segments Covered
By Market Structure (In USD Billion)
Organized Market
Unorganized Market
Type of Store Location (In USD Billion)
Standalone Pharmacy
Hospital Based Pharmacy
By Region (In USD Billion)
North
Central
South
By Type of Sales (In USD Billion)
Prescribed Medicines
OTC Products
Non Pharmaceutical Products
Medical Equipment
By Type of Drug (In USD Billion)
Generic
Patented
By Therapeutic Class (In USD Million)
Cardiovascular
Anti-Infectives
Pain/Analgesics
Anti Diabetic
Vitamins/Minerals /Nutrients
Dermatology
Respiratory
Gastro Intestinal
Neuro
Oncology
Contraceptive
Gynecological
Ophthalmological
Others
Companies Covered
Mercury Drug Corporation
Watsons Personal Care Stores
SouthStar Drug Inc.
The Generics Pharmacy
Rose Pharmacy
Generika Drugstores
Several Regional Players & Others
Key Target Audience
Pharmaceutical Manufacturer Companies
Pharmaceutical Importing Companies
Pharmaceutical Distributing Companies
Major Retail Companies
E-Commerce Pharmaceutical Retailers
Consultancy Companies
Industry Associations
Regulation Bodies
Time Period Captured in the Report:
Historical Period: 2014-2019
Forecast Period: 2020-2025
Key Topics Covered in the Report
Healthcare System in the Philippines
Major Hospitals & Doctors in the Women Healthcare
Usage of Contraception & Family Planning in the Philippines
Health Insurance in the Philippines
Supply Structure of the Philippines Pharmacy Retail Market
Trends & Development in the Philippines Pharmacy Retail Market
Issues & Challenges in the Philippines Pharmacy Retail Market
SWOT Analysis & BCG Matrix in the Philippines Pharmacy Retail Market
Philippines Pharmacy Retail Market Size & Segmentation, 2014-2019
Online Pharmacy Regulations in the Philippines
Telemedicine & Growth of Online Medical Consultation in the Philippines
Major Telemedicine Providers in the Philippines
Operating Model of Hybrid Pharmacies
Competition Scenario, Market Share, Cross Comparison of Major Players (Online & Offline) and Company Profile
Assessing Regional Pharmacies in the Philippines
Corona Virus Impact on the Consumer Behavior & Pharmacy Sales
Future Market Size and Segmentations, 2019-2025F
Covid 19 Impact on the Philippines Pharmacy Retail Market
Analysts’ Recommendations
Contact us:
Ankur Gupta, Head of Marketing and Communications
+91-9015378249
Follow Us
Facebook | Twitter | LinkedIn | Instagram
#Philippines Pharmacy Retail Industry#Philippines Retail Drug Market Report#Philippines Retail Pharmaceutical Sector#Philippines Retail Healthcare Market#Philippines Retail Wellness Market#Philippines Retail Medical Industry#Number of Intravenous Device Philippines#Number of Hospital Beds Philippines#Number of Test Centers Philippines#Number of Imported Medical Device Philippines#Number of Local Medical Device Philippines#Number of Diagnostic Labs Philippines#Number of Respiratory Devices Philippines#Organized Pharmacy Retail Market Philippines#Unorganized Pharmacy Retail Market Philippines#Standalone Retail Drug Market Philippines#Hospital Based Pharmacy Industry Philippines#North Retail Healthcare Market Philippines#Central Retail Pharmaceutical Market Philippines#Prescribed Medicines Market Philippines#OTC Products Industry Philippines#Non-Pharmaceutical Products Industry Philippines#Generic Drug Sector Philippines#Philippines Pharmacy Retail Market Opportunities#Philippines Retail Drug Market Trends#Philippines Retail Wellness Industry Challenges#Major Retail Healthcare Philippines#Leading Retail Pharmaceutical Partners Philippines#Emerging Retail Drug Market Philippines#Top Captive Players Philippines Retail Medical Sector
0 notes
Text
0 notes
Text
Chinese Hospitals Are Housing Another Deadly Outbreak
In Beijing and other megacities in China, hospitals are overflowing with children suffering pneumonia or similar severe ailments. However, the Chinese government claims that no new pathogen has been found and that the surge in chest infections is due simply to the usual winter coughs and colds, aggravated by the lifting of stringent COVID-19 restrictions in December 2022. The World Health Organization (WHO) has dutifully repeated this reassurance, as if it learned nothing from Beijing’s disastrous cover-up of the COVID-19 outbreak.
There is an element of truth in Beijing’s assertion, but it is only part of the story. The general acceptance that China is not covering up a novel pathogen this time appears reassuring. In fact, however, China could be incubating an even greater threat: the cultivation of antibiotic-resistant strains of a common, and potentially deadly, bacteria.
Fears of another novel respiratory pathogen emerging from China are understandable after the SARS and COVID-19 pandemics, both of which Beijing covered up. Concerns are amplified by Beijing’s ongoing obstruction of any independent investigation into the origins of SARS-CoV-2, the virus that causes COVID-19—whether it accidentally leaked from the Wuhan lab performing dangerous gain-of-function research or derived from the illegal trade in racoon dogs and other wildlife at the now-infamous Wuhan wet-market.
Four years ago, during the early weeks of the COVID-19 outbreak, Beijing failed to report the new virus and then denied airborne spread. At pains to maintain their fiction, Chinese authorities punished doctors who raised concerns and prohibited doctors from speaking even to Chinese colleagues, let alone international counterparts. Chinese medical statistics remain deeply unreliable; the country still claims that total COVID-19 deaths sit at just over 120,000, whereas independent estimates suggest the number may have been over 2 million in just the initial outbreak alone. Now, Chinese doctors are once again being silenced and not communicating with their counterparts abroad, which suggests another potentially dangerous cover-up may be underway.
We don’t know exactly what is happening, but we can offer some informed guesses.
The microbe causing the surge in hospitalization of children is Mycoplasma pneumoniae, which causes M. pneumoniae pneumonia, or MPP. First discovered in 1938, the microbe was believed for decades to be a virus because of its lack of a cell membrane and tiny size, although in fact it is an atypical bacterium. These unusual characteristics makes it invulnerable to most antibiotics (which typically work by destroying the cell membrane). The few attempts to make a vaccine in the 1970s failed, and low mortality has provided little incentive for renewed efforts. Although MPP surges are seen every few years around the world, the combination of low mortality and difficult diagnostics has meant there is no routine surveillance.
Although MPP is the most common cause of community-acquired pneumonia in school children and teenagers, pediatricians such as myself refer to it as “walking pneumonia” because symptoms are relatively mild. Respiratory Syncytial Virus (RSV), influenza, adenoviruses, and rhinoviruses (also known as the common cold) all cause severe inflammation of the lungs and are far more common causes of emergency-room visits, hospitalization, and death in infants and young children. Why should MPP be acting differently now?
One contributing factor to the severity of this outbreak may be “immunity debt.” Around the globe, COVID-19 lockdowns and other non-pharmaceutical measures meant that children were less exposed to the usual range of pathogens, including MPP, for several years. Many countries have since seen rebound surges in RSV. Several experts agree with Beijing’s explanation that the combination of winter’s arrival, the end of COVID-19 restrictions, and a lack of prior immunity in children are likely behind the surging infections. Some even speculate that that substantial lockdown may have particularly compromised young children’s immunity, because exposure to germs in infancy is essential for immune systems to develop.
In China, MPP infections began in early summer and accelerated. By mid-October, the National Health Commission had taken the unusual step of adding MPP to its surveillance system. That was just after Golden Week, the biggest tourism week in China.
Infection by two diseases at the same time can make things worse. The usual candidates for coinfection in children—RSV and flu—have not previously caused comparable surges in pneumonia. One difference this time is COVID-19. It is possible that the combination of COVID-19 and MPP is particularly dangerous. Although adults are less susceptible to MPP due to years of exposure, adults hospitalized for COVID-19 who were simultaneously or recently coinfected by MPP had a significantly higher mortality rate, according to a 2020 study.
Infants and toddlers are immunologically naive to MPP, and unlike COVID-19, RSV, and influenza, there is no vaccine against MPP. It seems implausible that no child (or adult) has died from MPP, yet China has not released any data on mortality, or on extrapulmonary complications such as meningitis.
Most disturbing, and a fact being downplayed by Beijing, is that M. pneumoniae in China has mutated to a strain resistant to macrolides, the only class of antibiotics that are safe for children less than eight years of age. Beyond discouraging parents to start ad hoc treatment with azithromycin, the most common macrolide and the usual first-line antibiotic for MPP, Beijing has barely mentioned this fact. Even more worrying is that WHO has assessed the risk of the current outbreak as low on the basis that MPP is readily treated with antibiotics. Broader azithromycin resistance in MPP is common across the world, and China’s resistant strain rates in particular are exceptionally high. Beijing’s Centers for Disease Control and Prevention reported macrolide resistance rates for MPP in the Beijing population between 90 and 98.4 percent from 2009 to 2012. This means there is no treatment for MPP in children under age eight.
Fears over a novel pathogen are already abating. After all, MPP is rarely lethal. But antimicrobial resistance (AMR) is. Responsible for 1.3 million deaths a year, AMR kills more people than COVID-19. No country is immune to this growing threat. Since China, where antibiotics are regularly available over the counter, leads the world in AMR, it is inconceivable that this issue hasn’t yet come up, particularly during WHO’s World AMR Awareness week, from Nov. 18 to Nov. 24.
Any infectious disease physician would want to know: Did WHO asked China the obvious question—what is the level of azithromycin resistance of M. pneumonia in the current outbreak—and include the answer in its risk assessment? Or did it ask about resistance to doxycycline and quinolones, antibiotics that can be used to treat MPP in adults? Even if WHO did ask, China isn’t telling, and WHO isn’t talking.
China’s silence isn’t surprising. Its antibiotic consumption per person is ten times that of the United States, and policies for AMR stewardship are predominantly cosmetic. While surveillance is China’s strong point, reporting is not.
Despite Spring Festival, the Chinese celebration of the Lunar New Year and another peak travel period, approaching in February 2024, WHO hasn’t advised any travel restrictions. It should have learned the danger of accepting Beijing’s statements at face value. Four years ago, Beijing’s delay enabled more than 200 million people to travel from and through Wuhan for Spring Festival. That helped COVID-19 go global. Since China’s AMR rates are already so high, importing AMR from other countries isn’t a major concern for China. Export is the issue, and China’s track record in protecting other countries is abysmal.
Rather than repeating the self-serving whitewashing coming from Beijing, WHO should be publicly pressing China about the threat of mutant microbes. Halting AMR is essential. Before antisepsis and antibiotics, surgery was a treatment of last resort. Without antibiotics, we lose 150 years of clinical and surgical advances. Within ten years, we are at risk of few antibiotics being effective. It may not be the novel virus that people were expecting, but the next pandemic is already here.
13 notes
·
View notes
Text
Home PCR Tests: A Closer Look at the PCR Test At Home Dubai Option
The COVID-19 pandemic sparked major growth in the development and usage of diagnostic and antibody tests that patients can self-administer from home. Home PCR tests in particular enable private, convenient detection of active coronavirus infections. For those wondering whether accurate PCR Test At Home Dubai kits are available, exploring the leading options provides helpful guidance.

How Do Home PCR Tests for COVID-19 Work?
The PCR (polymerase chain reaction) technique is the gold standard for directly detecting the presence of the COVID-19 virus from respiratory samples. Home PCR test kits allow patients to collect their own nasal or saliva samples and perform the PCR assay without visiting a clinic.
PCR tests work by identifying the specific genetic material of the COVID-19 virus. Users collect a sample, mix it with chemical reagents, and insert the solution into the test kit for analysis. Results are displayed indicating whether viral genetic material was detected based on any color change reaction on the test strips.
Kits include step-by-step instructions to ensure patients perform the easy, quick tests properly using non-invasive nasal swabs or saliva collection. Many provide results within 10-30 minutes.
Here is a video from MedCram Youtube Channel about At Home Rapid COVID 19 Tests and False Positives (Coronavirus Antigen Tests). Watch the video
youtube
Benefits of At-Home PCR Testing
Here are some of the major advantages of having access to accurate home PCR tests for COVID-19:
Convenience: Test from the privacy of your residence without traveling to clinics.
Speed: Get results rapidly within minutes rather than waiting days for lab tests.
Self-Administered: Users can collect their own sample comfortably rather than relying on technicians.
Affordability: Individual kits are very competitively priced.
Detection Reliability: PCR technology directly identifies viral presence with high accuracy.
Ease of Use: Tests have simple, straightforward instructions for patients of all ages.
Infection Verification: Confirms active infections unlike antibody tests.
Having the option to privately, quickly, and accurately test for possible COVID-19 infections at home provides significant peace of mind during the pandemic.
How Reliable Are Home PCR Tests?
Many people reasonably wonder whether DIY home PCR test kits can match the reliability of lab-based PCR tests. The good news is that leading home PCR kits on the market have very high accuracy.
Most kits have published sensitivity and specificity above 90% when compared to lab PCR tests. High quality home tests analyze samples using comparable PCR methodology and match labs in detecting positives and negatives.
Furthermore, unlike Rapid PCR Test At Home kits some vendors offer, full home PCR tests analyze the sample through many amplification cycles to maximize accuracy. With good sampling collection, top home PCR kits offer laboratory-grade results conveniently at home.
Leading Home PCR Test Kit Options
For those exploring PCR Test At Home Dubai choices, here are some of the top-rated home PCR kits to consider:
Cue Health PCR Test: Cue offers an FDA-authorized home PCR test delivering highly accurate results in 20 minutes with nasal swab samples.
Lucira Check It PCR Test: This is a single-use PCR kit with 98% validated accuracy that provides molecular-level detection from nasal samples in 30 minutes or less.
Ellume COVID-19 Home Test: This over-the-counter home kit uses a mid-turbinate nasal sample and provides an amplified PCR digital reading of positive or negative in 15 minutes on a connected analyzer.
Pixel by LabCorp PCR Test: Pixel is a monitored at-home nasal PCR test analyzed through LabCorp with over 98% accuracy returning results within 1-2 days.
Doximity's Covid-19 PCR Test: Doximity partners with qualified labs for monitored video-observed PCR testing with 97%+ accuracy and results in 24 hours.
All these options allow for convenient, accurate at-home COVID-19 testing using PCR with trusted partners. Kits can be purchased online and shipped directly to your home in Dubai.
When Are Home PCR Tests Recommended?
The CDC recommends utilizing home PCR tests in situations such as:
If you have any symptoms of COVID-19. Home testing allows quick confirmation.
After exposure events to quickly check for possible infection.
Before visiting individuals at higher risk for severe illness.
Before travel or group events for added assurance.
For frequent screening in schools or workplaces.
Even fully vaccinated individuals should test if they experience COVID-like symptoms or have a known exposure. Home PCR tests make quick detection fast and easy.
Home PCR Tests Offer Accuracy and Convenience
High quality Home PCR Tests have become an important tool in the fight against COVID by making reliable diagnostic testing accessible outside of clinics. There are excellent PCR Test At Home Dubai options available matching the standards of lab PCR sensitivity and specificity. Home PCR kits allow people to conveniently and confidently check themselves for possible COVID-19 infections from the privacy of home. As the technology continues advancing, home collection PCR will likely take on an increasingly vital role supporting public health and safety.
2 notes
·
View notes
Text
Medical Disposables Market to be worth US$ 326 Billion by 2033, Reveals Future Market Insights
The Medical Disposables Market revenues were estimated at US$ 153.5 Billion in 2022 and is anticipated to grow at a CAGR of 7.1% from 2023-2033, according to a recently published Future Market Insights report. By the end of 2033, the market is expected to reach US$ 326 Billion. Bandages and Wound Dressings commanded the largest revenue share in 2022 and is expected to register a CAGR of 6.8% from 2023 to 2033.
The rising incidence of Hospital Acquired Infections, an increasing number of surgical procedures, and the growing prevalence of chronic diseases leading to longer hospital admission have been the key factors driving the market.
The subsequent spike in the number of chronic illness cases and a rise in the rate of hospitalizations has fueled the field of emergency medical disposables growth. The expansion of the medical disposables market is being fueled by an increase in the prevalence of hospital-acquired illnesses and disorders, as well as a greater focus on infection prevention. For example, the prevalence of healthcare-associated infection in high-income countries ranges from 3.5% to 12%, whereas it ranges from 5.7% to 19.1% in low and medium-income countries.
A growing geriatric population, an increase in the incidence of incontinence issues, mandatory guidelines that must be followed for patient safety at healthcare institutions, and an increase in demand for sophisticated healthcare facilities is driving the medical disposables market.
The market in North America is expected to reach a valuation of US$ 131 Billion by 2033 from US$ 61.7 Billion in 2022. In August 2000, the Food and Drug Administration (FDA) issued guidance concerning healthcare single-use items reprocessed by third parties or hospitals. In this guidance, FDA stated that hospitals or third-party reprocessors would be considered manufacturers and regulated in the exact same manner. A newly used single-use device still has to fulfill the criteria for device activation required by its flagship when it was originally manufactured. Such regulations have been creating a positive impact on the medical disposables market in the U.S. market in specific and the North American market in general
Competitive Landscape
The key companies in the market are engaged in mergers, acquisitions and partnerships.
The key players in the market include 3M, Johnson & Johnson Services, Inc., Abbott, Becton, Dickinson & Company, Medtronic, B. Braun Melsungen AG, Bayer AG, Smith and Nephew, Medline Industries, Inc., and Cardinal Health.
Some of the recent developments of key Medical Disposables providers are as follows:
In April 2019, Smith & Nephew PLC purchased Osiris Therapeutics, Inc. with the goal of expanding its advanced wound management product range.
In May 2019, 3M announced the acquisition of Acelity Inc., with the goal of strengthening wound treatment products.
For More Information: https://www.futuremarketinsights.com/reports/medication-dispenser-market
More Insights Available
Future Market Insights, in its new offering, presents an unbiased analysis of the Medical Disposables Market, presenting historical market data (2018-2022) and forecast statistics for the period of 2023-2033.
The study reveals essential insights by Product (Surgical Instruments & Supplies, Infusion, and Hypodermic Devices, Diagnostic & Laboratory Disposables, Bandages and Would Dressings, Sterilization Supplies, Respiratory Devices, Dialysis Disposables, Medical & Laboratory Gloves), by Raw Material (Plastic Resin, Nonwoven Material, Rubber, Metal, Glass, Others), by End-use (Hospitals, Home Healthcare, Outpatient/Primary Care Facilities, Other End-use) across five regions (North America, Latin America, Europe, Asia Pacific and Middle East & Africa).
Market Segments Covered in Medical Disposables Industry Analysis
By Product Type:
Surgical Instruments & Supplies
Would Closures
Procedural Kits & Trays
Surgical Catheters
Surgical Instruments
Plastic Surgical Drapes
By Raw Material:
Plastic Resin
Nonwoven Material
Rubber
Metals
Glass
Other Raw Materials
By End-use:
Hospitals
Home Healthcare
Outpatient/Primary Care Facilities
Other End-uses
2 notes
·
View notes
Text
0 notes
Text
Infectious Disease Molecular Diagnostics Market: Advancements, Challenges, and Investment Opportunities
The global infectious disease molecular diagnostics market size is expected to reach USD 59.0 billion by 2030, according to a new report by Grand View Research, Inc. The market is estimated to expand at a CAGR of 3.9% from 2024 to 2030. The market is estimated to expand at a CAGR of 3.0% from 2020 to 2028. The market is driven by the introduction of technologically advanced products and an increase in demand for molecular diagnostics in PoC settings.
Rapid technological advancements with portability, accurate results, and cost-effectiveness are anticipated to serve as crucial drivers of the market for infectious disease molecular diagnostics. Companies are upgrading their products by implementing new techniques to gain specific and accurate results. Key players are updating their product portfolio for PCR instruments with increased R&D initiatives for developing novel kits to target emerging diseases or by entering into agreements with other kit manufacturing companies.
Technologies such as INAAT, mass spectroscopy, and ISH are advanced and have a low false-positive rate as compared to other traditional diagnostic tests. Advantages such as the cost-effectiveness and user-friendliness of this technology and the accuracy offered are estimated to increase the adoption of this technology.
The use of molecular diagnostics in research institutes is increasing. The use of techniques such as PCR, western blotting, and southern blotting is becoming common. Moreover, the spread of SARS-CoV-2 infection globally has led to an increase in research funding by governments as well as diagnostic companies to search for innovative molecular diagnostics.
Molecular diagnostics deliver effective and accurate results. Moreover, these tests enable the early detection of diseases, maintaining a low threat of substitutes. However, the high prices of these tests are expected to encourage patients to shift to external substitutes. Moreover, for the detection of newer infections such as SARS-CoV-2, the rate of internal substitution is high, which boosts competitive rivalry.
Infectious Disease Molecular Diagnostics Market Report Highlights
The reagent segment is expected to exhibit the fastest growth rate attributable to increased adoption
The growing geriatric population in Asian countries, such as Japan and China, with high untapped opportunities is expected to drive the market for infectious disease molecular diagnostics in the region during the forecast period
The In Situ Hybridization (ISH) segment is anticipated to witness the fastest growth rate owing to high sensitivity and lower complexity
The high growth rate of the market for infectious disease molecular diagnostics in the Asia Pacific can be attributed to increased access to healthcare in the developing nations
Infectious Disease Molecular Diagnostics Market Segmentation
Grand View Research has segmented the global infectious disease molecular diagnostics market report based on product, technology, application, end use, and region.
Infectious Disease Molecular Diagnostics Product Outlook (Revenue, USD Million, 2018 - 2030)
Instruments
Reagents
Services
Infectious Disease Molecular Diagnostics Technology Outlook (Revenue, USD Million, 2018 - 2030)
Polymerase Chain Reaction (PCR)
Multiplex PCR
Instruments
Reagents
Others
Other PCR
In Situ Hybridization
Instruments
Reagents
Services
Isothermal Nucleic Acid Amplification Technology (INAAT)
Instruments
Reagents
Services
Chips and Microarrays
Instruments
Reagents
Services
Mass Spectrometry
Instruments
Reagents
Services
Transcription Mediated Amplification
Instruments
Reagents
Services
Others
Instruments
Reagents
Services
Infectious Disease Molecular Diagnostics Application Outlook (Revenue, USD Million, 2018 - 2030)
Respiratory Diseases
Tuberculosis
Meningitis
Gastrointestinal Tract Infections
HPV
Sexually Transmitted Infections
Sepsis
Drug Resistance Diseases
Others
Infectious Disease Molecular Diagnostics End Use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Clinics
Diagnostics Laboratories
Research Institutes
Infectious Disease Molecular Diagnostics Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
Japan
India
Australia
South Korea
Thailand
Latin America
Brazil
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players
Abbott
Danaher Corporation
Bio-Rad Laboratories, Inc.
bioMérieux SA
Hoffmann-La Roche Ltd
Agilent Technologies, Inc.
Becton, Dickinson and Company
Hologic, Inc. (Gen-Probe)
Illumina, Inc.
Grifols S.A.
Qiagen
Siemens Healthineers AG
Sysmex Corporation
Order a free sample PDF of the Infectious Disease Molecular Diagnostics Market Intelligence Study, published by Grand View Research.
0 notes
Text
Current Trends and Market Projections for Mucopolysaccharidosis Type I
Mucopolysaccharidosis Type I (MPS I) is a rare inherited disorder that results in the inability to break down glycosaminoglycans (GAGs), leading to their harmful accumulation in organs and tissues. This accumulation causes progressive organ dysfunction and a variety of symptoms, such as developmental delays and skeletal defects. Although the market for MPS I therapeutics is niche due to the rarity of the condition, it is crucial for enhancing the quality of life of affected patients.
Overview of Mucopolysaccharidosis Type I Drugs Market
MPS I is one of several mucopolysaccharidoses (MPS), a group of disorders caused by enzyme deficiencies that disrupt GAG breakdown. Specifically, MPS I is marked by a deficiency in the enzyme alpha-L-iduronidase (IDUA), leading to an accumulation of heparan sulfate and dermatan sulfate. This buildup can result in systemic issues such as cardiomyopathy, respiratory challenges, hearing loss, and, in severe cases, cognitive decline.
The severity of MPS I varies, with the most severe form, Hurler syndrome, resulting in a drastically reduced life expectancy if untreated, whereas milder forms like Hurler-Scheie and Scheie syndrome have better prognoses.
Current Mucopolysaccharidosis Type I Treatment Market Landscape
The management of MPS I remains complex due to the rarity and severity of the disease. Several treatment options are currently available for patients, although these therapies do not offer a complete cure for the disease.
Enzyme Replacement Therapy (ERT): The primary treatment for MPS I, enzyme replacement therapy with laronidase (Aldurazyme), replaces the missing IDUA enzyme, reducing GAG accumulation and alleviating some symptoms. However, it doesn’t address neurological damage caused by GAG buildup in the brain.
Hematopoietic Stem Cell Transplantation (HSCT): Used in severe cases like Hurler syndrome, HSCT can slow disease progression if performed early. However, the procedure carries significant risks, such as complications and graft failure, and is less effective for neurological symptoms.
Gene Therapy (Emerging): Gene therapy has shown promise in recent years. This treatment aims to introduce a functional IDUA gene into the patient’s cells, allowing for long-term enzyme production. Ongoing clinical trials could potentially offer a one-time treatment that addresses both physical and neurological symptoms.
Key Drivers in the MPS I Drug Market
Several factors are driving the growth of the Mucopolysaccharidosis Type I Therapeutics Market. These include:
Increasing Awareness: Rising awareness of rare diseases, including MPS I, is improving diagnosis and creating more treatment opportunities. Enhanced funding for rare disease research and advances in diagnostics are also expanding knowledge of MPS I and its management.
Ongoing Research and Development: Research efforts are accelerating, especially in gene therapy. The possibility of curative treatments is attracting significant investment, encouraging pharmaceutical companies to explore new therapeutic options.
Government Initiatives: Regulatory incentives, such as orphan drug status, are being introduced by governments globally. These incentives provide financial support for developing rare disease treatments, including extended market exclusivity and research tax credits, attracting more companies to the MPS I market.
Expanding Treatment Access: Efforts to increase access to therapies like laronidase, particularly in developing regions, will expand the market and enable more patients to receive timely treatment.
Challenges in the MPS I Drug Market
Despite growth potential, several challenges remain:
High Treatment Costs: The costs of treatment for MPS I are a major barrier, particularly enzyme replacement therapy, which is costly. Stem cell transplants also have high expenses, limiting access to care, particularly in low- and middle-income countries.
Limited Treatment Options: While ERT has proven effective for some symptoms, there is still no cure for MPS I. Treatments mainly address physical symptoms, leaving neurological issues largely untreated. The market needs innovative therapies that can address both physical and cognitive symptoms.
Early Diagnosis Challenges: The rare nature of MPS I means its symptoms may be confused with those of other diseases, causing diagnostic delays. Early diagnosis is crucial for optimizing treatment outcomes, but lack of widespread screening and low awareness can hinder timely intervention.
Competitive Landscape in the MPS I Drug Market
The MPS I drugs market is primarily driven by a few major players, such as Sanofi Genzyme, the manufacturer of laronidase (Aldurazyme). In addition, pharmaceutical companies involved in gene therapy development, like Alexion Pharmaceuticals, are expected to shape the future of MPS I treatment.
Emerging companies are focusing on innovative approaches, such as gene editing and biologics, which may complement or replace current therapies. This increased competition will likely foster innovation and improve treatment options for MPS I patients.
Future Outlook for the MPS I Market
The future of the MPS I market is promising, with significant advancements expected in both treatments and market expansion. Gene therapy and gene editing technologies hold the potential for curative treatments that could transform how MPS I is managed. With increased research into alternative therapies, better early diagnosis, and rising awareness, the market for MPS I therapeutics is poised for growth.
The introduction of new therapies, including breakthrough treatments, will likely bring substantial benefits to patients and fuel market growth. Ongoing government support and investment in R&D will be crucial in addressing current market challenges.
Conclusion
While still in its early stages, the MPS I drugs market is set to expand, with the increasing availability of effective therapies, advancements in gene therapy, and a growing focus on rare disease treatments paving the way for improved patient outcomes and market growth.
Other Key Market Reports
ADHD Market | Atherosclerosis Market | Biopsy Devices Market | Blood Purification Devices Market | Brucellosis Market | Chronic Heart Failure Market | Endoscopic Ultrasound Market | Joint Reconstruction Devices Market | Meibomian Gland Dysfunction Market | Ornithine Transcarbamylase Deficiency Market | Psoriasis Vulgaris Market | Pulmonary Emphysema Market | Scoliosis Market | Skin Grafting Devices Market | Temporomandibular Disorders Market
About DelveInsight DelveInsight is a leading market research and consulting firm specializing in life sciences and healthcare. The firm offers actionable insights that empower pharmaceutical, biotech, and medical device companies to make informed decisions in competitive and dynamic markets.
Contact Information Kanishk [email protected]
0 notes
Text
Medical Device is expected to expand at 7% CAGR by 2023
The medical devices market is projected to grow at a CAGR of 7% from 2025 to 2030, driven by technological advancements, a rapidly growing aging population, and the rising prevalence of chronic diseases. Key factors fueling growth include increased healthcare expenditure, expansion of minimally invasive procedures, and rising adoption of digital health solutions like wearable devices. However, the market faces challenges such as stringent regulatory requirements, and high development costs which could impede growth.
The medical devices market encompasses a wide range of instruments, equipment, and implants used to prevent, diagnose, treat, and manage diseases or injuries. These devices include simple tools like thermometers to advanced technologies such as robotic surgical systems and implantable cardioverter defibrillators (ICDs). Medical devices are regulated for safety and efficacy and play a vital role in improving patient outcomes and enhancing healthcare delivery. The market caters to hospitals, clinics, home care settings, and diagnostic centers, driving continuous innovation to meet diverse medical needs.
Click here to get our sample PDF report for Medical Devices market: https://meditechinsights.com/medical-devices-market/request-sample/
Chronic Disease Management Driving Demand for Advanced Medical Technologies
The surging prevalence of chronic diseases, including diabetes, cardiovascular conditions, respiratory disorders, and cancer, serves as a critical driver for the medical devices market. These conditions necessitate advanced diagnostic, monitoring, and therapeutic solutions for effective management and improved patient outcomes. For instance, the global rise in diabetes cases has fueled demand for continuous glucose monitoring (CGM) systems, insulin pumps, and advanced lancet devices. Similarly, the increasing incidence of cardiovascular diseases has heightened the need for pacemakers, ICDs, and stents. Chronic respiratory disorders like Chronic Obstructive Pulmonary Disease (COPD) have driven the adoption of portable oxygen concentrators and nebulizers. Additionally, cancer management relies heavily on devices such as infusion pumps for chemotherapy and advanced imaging systems for precise tumor localization. The growing burden of these diseases is driving healthcare providers to adopt innovative and patient-centric technologies, fostering market growth and encouraging continuous advancements in device design and functionality.
Advancements in 3D Printing Technology
The adoption of 3D printing technology is revolutionizing the medical device market by enabling the customization of implants, prosthetics, and surgical instruments. These advancements allow for tailored solutions that fit the specific anatomical needs of individual patients, improving outcomes and patient satisfaction. For example, 3D-printed orthopaedic implants and dental devices have gained traction for their precision and affordability. Additionally, the technology facilitates faster prototyping and production cycles, reducing development timelines and costs. Innovations in biocompatible materials for 3D printing are further enhancing the utility of this technology in complex applications, including tissue engineering. The ability to personalize devices while maintaining efficiency positions 3D printing as a transformative trend in the medical devices sector.
Competitive Landscape Analysis
The global medical devices market is highly competitive and fragmented. Some of the key and emerging players in this market include Medtronic; Johnson & Johnson Services, Inc.; Koninklijke Philips N.V.; GE Healthcare; Siemens Healthineers AG; Stryker; Abbott; Becton, Dickinson and Company and Cardinal Health among others.
Get PDF Report for Competitive Analysis: https://meditechinsights.com/medical-devices-market/request-sample/
Global Medical Devices Market Segmentation
This report by Medi-Tech Insights provides the size of the global medical devices market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product type, application, and end-user.
Market Size & Forecast (2023-2030), By Product Type, USD Billion
Diagnostic Devices
Therapeutic Devices
Surgical Devices
Monitoring Devices
Others
Market Size & Forecast (2023-2030), By Application, USD Billion
Cardiovascular Diseases
Orthopedic Disorders
Neurology Diseases
Respiratory Diseases
Diabetes
General Surgery
Oncology
Others
Market Size & Forecast (2023-2030), By End-user, USD Billion
Hospitals
Ambulatory Surgical Centers
Diagnostic Centers
Others
Market Size & Forecast (2023-2030), By Region, USD Billion
North America
US
Canada
Europe
UK
Germany
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
1 note
·
View note