#Respiratory Protection Market
Explore tagged Tumblr posts
Text
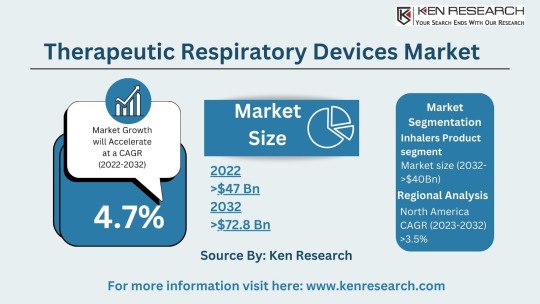
The $64.2 billion Respiratory Market & Its Future Trends, Segmentation and Forecast
The global respiratory market size reached a staggering USD 42.3 billion in 2023. This impressive figure highlights the significant need for respiratory devices and treatments to address a wide range of respiratory conditions. The market is projected to grow at a Compound Annual Growth Rate (CAGR) of approximately 7.4%, reaching an estimated USD 64.2 billion by 2030. This growth can be attributed to several factors:
Rising Prevalence of Chronic Respiratory Diseases: Conditions like asthma, chronic obstructive pulmonary disease (COPD), and sleep apnea are on the rise due to factors like air pollution, smoking, and an aging population.
Increased Life Expectancy: With an aging population, the demand for respiratory support devices for chronic conditions is expected to rise.
Technological Advancements: The development of innovative respiratory devices, such as portable nebulizers and advanced ventilators, offers improved treatment options.
Growing Focus on Homecare: The increasing emphasis on home-based care for respiratory patients fuels the demand for user-friendly respiratory devices.
Respiratory Market Segmentation: Catering to Diverse Needs
The respiratory market segmentation reflects the vast array of products and technologies available to address different respiratory needs:
By Application:
Therapeutic Respiratory Devices Market: This segment includes devices used for treatment, such as nebulizers, metered-dose inhalers (MDIs), and continuous positive airway pressure (CPAP) machines used for sleep apnea. The respiratory inhalers market is a significant sub-segment due to the widespread use of inhalers for asthma and COPD.
Anesthesia & Respiratory Devices: Specialized equipment used in surgical settings to deliver oxygen and maintain proper ventilation during anesthesia. The anesthesia and respiratory devices market caters to the specific needs of hospitals and surgical centers.
Respiratory Gas Analysis: This technology analyzes the composition of respiratory gases to assess lung function and identify potential respiratory issues.
By Device Type:
Respiratory Care Devices: This broad category encompasses various devices used for diagnosis, treatment, and monitoring of respiratory conditions. Examples include nebulizers, inhalers, ventilators, and CPAP (continuous positive airway pressure) machines.
Respiratory Monitoring Devices: These devices track vital signs such as blood oxygen levels and respiratory rate, allowing for continuous monitoring of patients with respiratory difficulties. The respiratory monitoring devices market is experiencing significant growth due to the increasing focus on patient safety and remote monitoring.
Respiratory Measurement Devices: These devices measure lung function and capacity, providing vital diagnostic information for respiratory conditions. The respiratory disease testing market relies heavily on these devices for accurate diagnosis.
Respiratory Protective Equipment (RPE): This equipment protects users from inhaling harmful substances, including masks and respirators. The respiratory protective equipment market is expected to witness growth due to rising concerns about air pollution and pandemics.
Take a look at: Forecasting the Respiratory Market, Size, Segmentation and Future Trends
Top Players in Respiratory Market: Breathing Innovation
Several established medical device manufacturers and specialty respiratory companies dominate the respiratory market:
Some of the top players in the respiratory market include:

Philips Healthcare
ResMed
Medtronic
GE Healthcare
Fisher & Paykel
Emerging Markets: A Rising Demand for Respiratory Solutions
Developing nations with growing populations and increasing healthcare expenditure present a significant opportunity. For instance, the bovine respiratory disease treatment market highlights the growing demand for respiratory solutions in the animal health sector.
Respiratory Market Trends: Shaping the Future of Respiratory Care
Exciting trends are shaping the respiratory market and transforming how we manage respiratory conditions:
Focus on Homecare Solutions: The emphasis on providing effective respiratory care solutions for patients in a home setting is driving innovation in portable and user-friendly devices.
Telemedicine Integration: Telehealth platforms allow remote monitoring and consultations with healthcare professionals, improving respiratory care management.
Connected Devices and Data Analytics: The integration of Internet of Things (IoT) technology allows for real-time data collection and analysis of respiratory parameters, leading to personalized treatment plans.
Emphasis on Early Detection and Prevention: The trend towards early detection and prevention of respiratory diseases through screening programs and lifestyle modifications is gaining momentum.
Challenges and Opportunities: Navigating the Respiratory Landscape
While the respiratory market offers promising opportunities, challenges also exist:
Challenges:
Cost Concerns: The high cost of some respiratory devices, particularly advanced equipment, can be a barrier to access for some patients.
Counterfeit Products: The presence of counterfeit respiratory products poses a safety risk and necessitates stringent quality control measures.
Compliance with Regulations: Navigating evolving regulatory requirements for medical devices can be complex and requires ongoing compliance efforts.
Opportunities:
Focus on Homecare: The trend towards homecare for respiratory patients creates a demand for portable and user-friendly respiratory devices.
Telemedicine Integration: Integrating respiratory monitoring devices with telemedicine platforms allows for remote patient monitoring and improved care coordination.
Emerging Technologies: The potential of new technologies like artificial intelligence and wearable devices can revolutionize respiratory care and diagnosis.
Respiratory Market Future Outlook: A Collaborative Approach
The respiratory market future outlook is promising, with a projected market size of USD 64.2 billion by 2030. And this suggests a market driven by innovation, collaboration, and a focus on improving patient outcomes. Here's what we can expect:
Collaboration between Medical Device Manufacturers and Healthcare Providers: Collaboration between these entities will be crucial for developing and implementing effective respiratory care solutions that address real-world clinical needs.
Increased Focus on Patient Education and Self-Management: Empowering patients with respiratory conditions to manage their health through education and user-friendly technology will be a key focus.
Conclusion:
The respiratory market plays a vital role in supporting lung health and improving the lives of millions suffering from respiratory illnesses. As the market continues to evolve, driven by innovation, collaboration, and a focus on patient-centric care, we can expect a future where managing respiratory conditions becomes more effective, accessible, and empowering for individuals and healthcare professionals alike.You can also read about: Future Forecast and Trends in the $35.58 Billion Respiratory Market
#Respiratory Market#Respiratory Industry#Respiratory Sector#Respiratory Market Size#Respiratory Market Segmentation#Respiratory Care Devices Market#Respiratory Devices Market#Therapeutic Respiratory Devices Market#Respiratory measurement devices market#respiratory gas analysis#anesthesia and respiratory devices market#respiratory disease testing market#bovine respiratory disease treatment market#respiratory inhalers market#respiratory monitoring devices market#respiratory protective equipment market#Top Players in Respiratory Market#Respiratory Market Trends#Respiratory Market Future Outlook
0 notes
Text
#Respiratory Protective Equipment Market#Respiratory Protective Equipment Market Trends#Respiratory Protective Equipment Market Growth#Respiratory Protective Equipment Market Industry#Respiratory Protective Equipment Market Research#Respiratory Protective Equipment Market Report
0 notes
Text
Also preserved on our archive (Daily updates!)
By Stephani Sutherland
Gentle nasal spray vaccines against COVID, the flu and RSV are coming. They may work better than shots in the arm
Alyson Velasquez hates needles. She never liked getting shots as a kid, and her anxiety only grew as she got older. “It really ballooned in my teens and early 20s,” she says. “It became a full-blown phobia.” She would panic at the sight of a needle being brought into an exam room; more than once she passed out. Velasquez says that she took an antianxiety medication before one appointment yet still ran around the room screaming inconsolably “like I was a small child; I was 22.” After that episode Velasquez, now a 34-year-old financial planner in southern California, quit needles completely. “No vaccinations, no bloodwork. For all of my 20s it was a no-go for me,” she says.
Then COVID showed up. “It finally hit a point where it wasn’t just about me,” Velasquez says. “It felt so selfish not to do this for the greater public health and the safety of our global community.” So she got vaccinated against the SARS-CoV-2 virus in 2021, although she had to sit on her husband’s lap while he held her arms. “It was a spectacle. The poor guy at CVS ... he did ask me, ‘Are you sure you want to do this?’” She very much did. “I’m very pro-vaccine. I am a rational human. I understand the necessity of [getting] them,” she insists. But today she still struggles with each injection.
Those struggles would end, however, if all her future vaccinations could be delivered by a nasal spray. “Oh, my God, amazing!” Velasquez says.
The amazing appears to be well on its way. Vaccines delivered through the nose are now being tested for several diseases. In the U.S., early clinical trials are showing success. Two of these vaccines have generated multiple immune system responses against the COVID-causing virus in people who received them through a puff up the nose; earlier this year their makers received nearly $20 million from Project NextGen, the Biden-Harris administration’s COVID medical initiative. Researchers are optimistic that a nasal spray delivering a COVID vaccine could be ready for the U.S. as soon as 2027. Although recent efforts have focused on inoculations against SARS-CoV-2, nasal vaccines could also protect us against the flu, respiratory syncytial virus (RSV), and more.
A few nasal vaccines have been introduced in the past, but they’ve been beset by problems. The flu inoculation FluMist has not gained popularity because of debates about its effectiveness, and a different vaccine was pulled from the market decades ago because some people had serious side effects. In China and India, nasal vaccines for COVID have been approved because those countries prioritized their development during the pandemic, whereas the U.S. and other wealthy nations opted to stick with arm injections. But this new crop of vaccines takes advantage of technology that produces stronger immune responses and is safer than preparations used in the past.
In fact, immunologists say these spritzes up the nose—or inhaled puffs through the mouth—can provide faster, stronger protection against respiratory viruses than a shot in the arm. That is because the new vaccines activate a branch of the immune system that has evolved for robust, rapid responses against airborne germs. “It may be more likely to really prevent infection from getting established,” says Fiona Smaill, an infectious disease researcher at McMaster University in Ontario. Such inoculations may also help reduce the enormous inequities in vaccine access revealed by the pandemic. These formulations should be cheaper and easier to transport to poor regions than current shots.
But nasal vaccines still face technical hurdles, such as how best to deliver them into the body. And unlike injected vaccines, which scientists can measure immune responses to with blood tests alone, testing for immunity that starts in nose cells is more challenging. But researchers working in this field agree that despite the hurdles, nasal formulations are the next step in vaccine evolution.
Traditional vaccines injected through the skin and into an arm muscle provide excellent protection against viruses. They coax immune cells into making widely circulated antibodies—special proteins that recognize specific structural features on viruses or other invading pathogens, glom on to them and mark them for destruction. Other immune cells retain a “memory” of that pathogen for future encounters.
Intramuscular injection vaccines are good at preventing a disease from spreading, but they do not stop the initial infection. A nasal spray does a much better job. That’s because sprays are aimed directly at the spot where many viruses first enter the body: the nose and the tissue that lines it, called the mucosa.
Mucosa makes up much of our bodies’ internal surfaces, stretching from the nose, mouth and throat down the respiratory tract to the lungs, through the gastrointestinal tract to the anus, and into the urogenital tract. Mucosa is where our bodies encounter the vast majority of pathogenic threats, Smaill says, be it flu, COVID, or bacterial infections that attack the gut. This tough, triple-layered tissue is specialized to fight off invaders with its thick coating of secretory goo—mucus—and with a cadre of resident immune cells waiting to attack. “Mucosa is really the first line of defense against any infection we’re exposed to,” Smaill says.
Mucosal immunity not only prepares the immune system for the fight where it occurs but also offers three different types of protection—at least one more than a shot does. Nasal vaccines and shots both mobilize immune messenger cells, which gather the interlopers’ proteins and display them on their surfaces. These cells head to the lymph nodes, where they show off their captured prize to B and T cells, which are members of another part of the immune system called the adaptive arm. B cells, in turn, produce antibodies, molecules that home in on the foreign proteins and flag their owners—the invading microbes—for destruction. Killer T cells directly attack infected cells, eliminating them and the microbes inside. This provides broad protection, but it takes time, during which the virus continues to replicate and spread.
That’s why a second type of protection, offered only by the mucosal tissue, is so important. The mucosa holds cells of the innate immune system, which are the body’s “first responders.” Some of these cells, called macrophages, recognize invasive microbes as foreign and swallow them up. They also trigger inflammation—an alarm sounded to recruit more immune cells.
Another part of this localized response is called tissue-resident immunity. These cells don’t have to detect telltale signs of a pathogen and make a long journey to the infected tissue. They are more like a Special Forces unit dropped behind enemy lines where a skirmish is occurring rather than waiting for the proverbial cavalry to arrive. This localized reaction can be quite potent. Its activation is notoriously difficult to demonstrate, however, so historically it’s been hard for vaccine makers to show they’ve hit the mark. But it turns out that one type of antibody, called IgA, is a good indicator of mucosal immunity because IgAs tend to predominate in the mucosa rather than other parts of the body. In an early trial of CoviLiv, a nasal COVID vaccine produced by Codagenix, about half of participants had detectable IgA responses within several weeks after receiving two doses. That trial also showed the vaccine was safe and led to NextGen funding for a larger trial of the vaccine’s efficacy.
It’s possible an inhaled vaccine may provide yet one more layer of protection, called trained innate immunity. This reaction is a bit of a mystery: although immunologists know it exists and appears also to be produced by intramuscular injections, they can’t quite explain how it works. Immune cells associated with trained innate immunity seem to have memorylike responses, reacting quickly against subsequent infections. They also have been found to respond against pathogens entirely unrelated to the intended vaccine target. Smaill and her colleagues found that when they immunized mice with an inhaled tuberculosis vaccine and then challenged them with pneumococcal bacteria, the mice were protected. In children, there is some evidence that a tuberculosis vaccine, in the arm, generates this type of broad response against other diseases.
Akiko Iwasaki, an immunologist at Yale University who is working to develop a nasal vaccination for COVID, sees two major potential benefits to nasal immunity in addition to better, faster, more localized protection. First, attacking the virus in the nose could prevent the disease from being transmitted to others by reducing the amount of virus that people breathe out. And second, Iwasaki says, the spray may limit how deeply the infection moves into the body, so “we believe that it will also prevent long COVID.” That debilitating postinfection condition, sometimes marked by signs of entrenched viral particles, disables people with extreme fatigue, chronic pain, a variety of cognitive difficulties, and other symptoms.
Making a new vaccine is hard, regardless of how you administer it. It needs to raise an immune response that’s strong enough to protect against future invasions but not so strong that the components of that response—such as inflammation and fever—harm the host.
The lining of the nose puts up its own barriers—literal, physical ones. Because the nasal mucosa is exposed to so many irritants from the air, ranging from pet hair to pollen, the nose has multiple lines of defense against invading pathogens. Nostril hair, mucus, and features called cilia that sweep the nasal surface all aim to trap small foreign objects before they can get deeper into the body—and that includes tiny droplets of vaccine.
And lots of small foreign particles—often harmless—still make it through those defenses. So the nose has developed a way to become less reactive to harmless objects. This dampened reactivity is called immunological tolerance, and it may be the biggest hurdle to successful development of a nasal vaccine. When foreign particles show up in the bloodstream, a space that is ostensibly sterile, immune cells immediately recognize them as invaders. But mucosal surfaces are constantly bombarded by both pathogens and harmless materials. The immune system uses tolerance—a complex series of decisions carried out by specialized cells—to determine whether a substance is harmful. “This is very important because we can’t have our lungs or gastrointestinal tract always responding to nonharmful foreign entities that they encounter,” says Yale infectious disease researcher Benjamin Goldman-Israelow. For example, inflammation in the lungs would make it hard to breathe; in the gut, it would prevent the absorption of water and nutrients.

These barriers may hamper the effectiveness of a nasal flu vaccine that’s been around for a while, called FluMist in the U.S. and Fluenz in Europe. The inoculation is safe, says infectious disease scientist Michael Diamond of Washington University in St. Louis, but it faces a similar problem as do injected flu vaccines: it isn’t very effective at warding off new seasonal flu strains. This might be because flu strains are so common, and people are frequently infected by the time they are adults. Their immune systems are already primed to recognize and destroy familiar flu particles. FluMist is built from a live flu virus, so immune cells probably treat the vaccine as an invader and demolish it as soon as it shows up in the nose, before it has a chance to do any good. This preexisting immunity isn’t such an issue in children, who are less likely to have had multiple flu infections. Nasal flu vaccines are routinely used to inoculate kids in Europe.
In other vaccines, researchers often use adjuvants, special agents that attract the attention of immune cells, to boost a response. Some nasal vaccines use adjuvants to overcome tolerance, but in the nose, adjuvants can pose unique dangers. In at least one case, a nasal adjuvant led to disastrous consequences. An intranasal vaccine for influenza, licensed in Switzerland for the 2000–2001 season, used a toxin isolated from Escherichia coli bacteria as an adjuvant to provoke a reaction to the inactivated virus. No serious side effects were reported during the trial period, but once the vaccine was released, Swiss officials saw a concerning uptick in cases of Bell’s palsy, a disease that causes weakness or paralysis of the facial muscles, often leading to a drooping or disfigured face. Researchers at the University of Zurich estimated that the adjuvanted flu vaccine had increased the risk of contracting Bell’s palsy by about 20 times, and the vaccine was discontinued. “We need to be cautious about using adjuvants like that from known pathogens,” says pharmaceutical formulations scientist Vicky Kett of Queen’s University Belfast in Northern Ireland.
To get around the challenges posed by the nose, some researchers are exploring vaccines inhaled through the mouth. Smaill is working on one of them. She and her McMaster colleagues aerosolized their vaccine for COVID into a fine mist delivered by a nebulizer, from which it rapidly reaches the lungs. Experiments in mice have shown promising results, with mucosal immunity established after administration of the vaccine.
Another vaccine strategy is to use a harmless virus to carry viral genes or proteins. Researchers at the Icahn School of Medicine at Mount Sinai in New York City selected a bird pathogen, Newcastle disease virus (NDV). “It’s naturally a respiratory pathogen,” so it infects nasal cells, says Michael Egan, CEO and chief scientific officer of CastleVax, a company that formed to develop the NDV vaccine for COVID. A small early clinical trial showed the CastleVax vaccine was safe and caused robust immune responses in people. “Those results were very promising,” Egan says. People who received the vaccine also produced antibodies that indicated multitiered mucosal immunity, not simply the adaptive immunity from a shot in the arm.
Following that trial, the CastleVax project received NextGen funding, and results from a trial of 10,000 people are expected in 2026. Half of those people will receive a messenger RNA (mRNA) injection, and half will get the new NDV nasal spray. The data should show whether the new nasal vaccine can do a better job of preventing infection than the mRNA injections. Egan has high hopes. “We’re expecting to see a lot fewer breakthrough infections in people who got the vaccine up the nose by virtue of having those mucosal immune responses,” he says.
Florian Krammer, one of the Mount Sinai researchers behind the vaccine, engineered NDV particles to display a stabilized version of the spike protein that’s so prominent in SARS-CoV-2. “You end up with a particle that’s covered with spike,” he says. Spike protein in the bloodstream can raise an immune response. But the NDV vaccine works in another way, too. The virus particle can also get into cells, where it can replicate enough times to cause virus particles to emerge from the cells, provoking another immune reaction. Before moving into human trials, however, researchers had to complete clinical trials to establish that the Newcastle virus is truly harmless because the nose is close to the central nervous system—it has neurons that connect to the olfactory bulb, which is part of the brain. Those trials confirmed that it is safe for this use.
Nasal sprays aim directly at the spot where most viruses first enter the body: the nose. This type of caution is one reason a COVID nasal vaccine approved in India hasn’t been adopted by the U.S. or other countries. The inoculation, called iNCOVACC, uses a harmless simian adenovirus to carry the spike protein into the airway. The research originated in the laboratories of Diamond and some of his colleagues at Washington University at the start of the pandemic, when they tested the formulation on rodents and nonhuman primates. “The preclinical data were outstanding,” Diamond says. Around the time he and his colleagues published initial animal results in Cell in 2020, Bharat Biotech in India licensed the idea from the university. In a 2023 phase 3 clinical trial in India, the nasal vaccine produced superior systemic immunity compared with a shot.
Diamond says American drug companies didn’t pursue this approach, because “they wanted to use known quantities,” such as the mRNA vaccines, which were already proving themselves in clinical trials in 2020. As the pandemic took hold, there was little appetite to develop nasal vaccine technology to stimulate mucosal immunity while the tried-and-true route of shots in the arm was available and working. But now, four years later, an inhaled vaccine using technology similar to iNCOVACC’s is being developed for approval in the U.S. by biotech company Ocugen. Both inhaled and nasal forms of the vaccine are set to undergo clinical trials as part of Project NextGen. These new vaccines are using classical vaccine methods based on the virus rather than using new, mRNA-based technology. The mRNA preparations were developed specifically for intramuscular injections and would have to be significantly modified.
Codagenix, which is developing CoviLiv, sidestepped the need for a new viral vector or an adjuvant by disabling a live SARS-CoV-2 virus. To make it safe, scientists engineered a version of the virus with 283 mutations, alterations to its genetic code that make it hard for the virus to replicate and harm the body. Without all these genetic changes, there would be a chance the virus could revert to a dangerous, pathogenic form. But with hundreds of key mutations, “statistically, it’s basically impossible that this will revert back to a live virus in the population,” says Johanna Kaufmann, who helped to develop the vaccine before leaving Codagenix for another company earlier this year.
Because most people on the planet have now been exposed to SARS-CoV-2—in the same way they’re regularly exposed to the flu—some nasal vaccines are being designed as boosters for a preexisting immune response that is starting to wane. For example, Yale researchers Iwasaki and Goldman-Israelow are pursuing a strategy in animals deemed “prime and spike.”
The idea is to start with a vaccine injection—the “prime” that stimulates adaptive immunity—then follow it a few weeks later with a nasal puff that “spikes” the system with more viral protein, leading to mucosal immunity. In a study published in 2022 in Science, Iwasaki and her colleagues reported that they primed rodents with the mRNA vaccine developed by Pfizer and BioNTech, the same shot so many of us have received. Two weeks later some of the mice received an intranasal puff of saline containing a fragment of the SARS-CoV-2 spike protein. Because the animals had some preexisting immunity from the shot, the researchers didn’t add any adjuvants to heighten the effects of the nasal puff. Two weeks later researchers detected stronger signs of mucosal immunity in mice that had received this treatment compared with mice that got only the shot.
“Not only can we establish tissue-resident memory T cells” to fight off the virus in the nose, Iwasaki says, but the prime-and-spike method also produces those vigorous IgA antibodies in the mucosal layer. “And that’s much more advantageous because we can prevent the virus from ever infecting the host,” she notes. The study suggests that this approach might also lessen the chances of transmitting the disease to others because of the lower overall viral load. Experiments in hamsters demonstrated that vaccinated animals shed less virus, and they were less likely to contract COVID from infected cage mates that had not been vaccinated themselves.
Although most of the new vaccine strategies are aimed at COVID, nasal vaccines for other diseases are already being planned. Kaufmann, formerly of Codagenix, says the company currently has clinical trials underway for nasal vaccines against flu and RSV. CastleVax’s Egan says “we have plans to address other pathogens” such as RSV and human metapneumovirus, another leading cause of respiratory disease in kids.
Vaccines that don’t need to be injected could clear many barriers to vaccine access worldwide. “We saw with COVID there was no vaccine equity,” Smaill says. Many people in low-income countries never received a shot; they are still going without one four years after the vaccines debuted.
In part, this inequity is a consequence of the high cost of delivering a vaccine that needs to stay frozen on a long journey from manufacturing facilities in wealthy countries. Some of the nasal sprays in development don’t need deep-cold storage, so they might be easier to store and transport. And a nasal spray or an inhaled puff would be much easier to administer than a shot. No health professional is required, so people could spray it into their noses or mouths at home.
For these reasons, needle-free delivery matters to the World Health Organization. The WHO is using the Codagenix nasal spray in its Solidarity Trial Vaccines program to improve vaccine equity. The CoviLiv spray is now in phase 3 clinical trials around the world as part of this effort. “The fact that the WHO was still interested in a primary vaccination trial in the geographies it’s passionate about—that’s indicative that there is still a gap,” Kaufmann says. CoviLiv was co-developed with the Serum Institute of India, the world’s largest maker of vaccines by dose. The partnership enabled production at the high volume required for Solidarity.
The CastleVax vaccine with the NDV vector provides another layer of equity because the facilities required to make it already exist in many low- and middle-income countries. “The cool thing is that NDV is a chicken virus, so it grows very well in embryonated eggs—that’s exactly the system used for making flu vaccines,” Krammer says. For example, for a clinical trial in Thailand, “we just shipped them the seed virus, and then they produced the vaccine and ran the clinical trials,” he says. Many countries around the world have similar facilities, so they will not need to depend on pharma companies based in richer places.
Even high-income countries face barriers to vaccination, although they may be more personal than systemic. For very many people, the needle itself is the problem. Extreme phobia such as Velasquez’s is uncommon, but many people have a general fear of needles that makes vaccinations stressful or even impossible for them. For about one in 10 people needle-related fear or pain is a barrier to vaccinations, says C. Meghan McMurtry, a psychologist at the University of Guelph in Ontario. Needle fear “is present in most young kids and in about half of adolescents. And 20 to 30 percent of adults have some level of fear.” A review of studies of children showed that “concern around pain and needle fear are barriers to vaccination in about 8 percent of the general population and about 18 percent in the vaccine-hesitant population,” McMurtry adds.
Some people are wary of injected vaccines even if they’re not afraid of needles, Kett says; they see injections as too invasive even if the needle doesn’t bother them. “We’re hopeful that something administered by the nasal route would be less likely to come across some of those issues,” Kett says.
In the U.S., however, sprays and puffs won’t be available until they are approved by the Food and Drug Administration, which requires clear evidence of disease protection. As Diamond points out, standards for such evidence are well established for injections, and vaccine makers can follow the rule book: regulations point to particular antibodies and specific ways to measure them with a simple blood test. But for nasal vaccines, Iwasaki says, “we don’t have a standard way to collect nasal mucus or measure antibody titers. All these practical issues have not been worked out.”
Iwasaki is also frustrated with a restriction by the U.S. Centers for Disease Control and Prevention that stops researchers from using existing COVID vaccines in basic research to develop new nasal sprays. The rule is a holdover from 2020, when COVID injections had just been developed and were in short supply; people had to wait to get vaccinated until they were eligible based on factors such as age and preexisting conditions. “That made sense back then, but those concerns are years old; things are different now,” Iwasaki says. “Now we have excess vaccine being thrown out, and we cannot even get access to the waste, the expired vaccine.”
Today scientists want to contrast the effectiveness of nasal formulations with injections already in use. “Those comparisons are really important for convincing the FDA that this is a worthy vaccine to pursue,” Iwasaki says. But the restriction has held up studies by her company, Xanadu, slowing down work. (The CDC did not respond to a request for comment.)
Despite the bureaucratic and scientific hurdles, the sheer number of nasal vaccines now in clinical trials encourages Iwasaki and other scientists pursuing the needle-free route. They say it seems like only a matter of time before getting vaccinated will be as simple as a spritz up the nose.
Velasquez, for one, can’t wait for that day to arrive. The circumstances that finally forced her to reckon with her fear of needles (a global pandemic, the prospect of parenthood and the numerous blood tests that accompanied her pregnancy) were so much bigger than her. If not for them, she might still be avoiding shots. “So having vaccines without needles—I would get every vaccine any doctor wanted me to get, ever. It would be a complete game changer for me.”
#vaccination#mask up#covid#pandemic#public health#wear a mask#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#get vaccinated#vaccinate your kids
43 notes
·
View notes
Text
F1 and Biometrics
Biometric gloves came into play in F1 in 2018 to give medical teams immediate access to important information regarding driver conditions after an incident. It allows for vitals to be measured before, at the time of an incident and after the crash until they are rescued and more advanced monitoring is able to be applied. It is also a big help if a driver is involved in a crash that means they are not accessible straight away or cannot be visualized for monitoring so this allows teams to get immediate access to this information.
According to the FIA website, this was a difficulty by medical teams when Carlos Sainz crashed in the 2015 Russian GP where he hit the barrier head-on at 153km/h (roughly 95mph). The first row of the barrier was resting on top of him, so medical teams had to wait for this to be removed before they had access to him. Thankfully he wasn’t hurt during this crash, but medical teams didn’t know this initially as this technology wasn’t available and being used.
The sensors were basically made custom to F1 drivers. Regular sensors had not been fire tested and were not comfortable enough for the drivers to wear for long races. They use Bluetooth technology and can send data within a 500m and are powered by a small battery that drivers charge before races.
Drivers gloves have a 3mm sensor that is stitched into the palm of the fabric and monitors their vital signs during races. They measure pulse oximetry which measures the amount of oxygen being carried in the blood as well as drivers pulse rates. Obviously if a driver has an injury that is affecting their breathing, this will show in the saturations that would decline rapidly. Having this technology allows physiological readings and biometrics to be continuously monitored throughout the race from start to finish. Data from the sensor transmits to an iPhone app and gives medical crews remote and advance information on the driver’s condition. The small biometric readers are flexible and fire resistant up to 1,800 degrees Celsius (3,272 degrees Fahrenheit) for 22 seconds.


In the future there are even plans to implement sensors for respiratory rate and temperature to further monitor drivers which will not only enhance safety features but allow teams and drivers to monitor performance better.
OMP, an equipment supplier, has been developing wearable biometric monitoring systems since the introduction of the biometric sensors in 2019. An undershirt equipped with sensors and a measurement unit would transmit and record biometric data allowing for real-time monitoring of health through ECG and thoracic expansion. This would allow monitoring of drivers heart rhythms and breathing rate which would not only benefit medical teams in the case of an incident but also help identify stress, fatigue and any alteration in conditions. This would be useful considering the amount of stress drivers have been put through in the past in hot countries for example Saudi Arabia GP 2023 when many drivers retired, had to be taken to medical, threw up in their helmets or passed out after the race.


TV crews can also display biometric data during broadcasts to show the physical condition of a driver as they battle on track. However, there have been questions about the ethics and use of biometrics and why can’t we as an audience see drivers heart rates etc on screen if this data is being constantly collected. The FIA has strict guidelines about the use of raw biometric data. Section 2.4 of the FIA Guidelines for the Collection and Usage of Biometric Data in Motorsport, states that the use of biometric data can be used for more than just medical and performance monitoring and can be used for entertainment and marketing purposes but only if it is changed from raw data into a variable to protect the private health information of the driver. The FIA won’t allow the use of biometric data to be publicly available in the original form/ measurement unless the driver provides informed consent.
Essentially, driver onboard vitals are likely not to be available live due to strict laws on data protection and sharing health related information about drivers as it is protected health information. In the case of an accident or emergency, data is not allowed to be used even if the information is changed to protect the driver unless it is for medical and rescue use and post-accident information.
Sources: (x) (x) (x) (x) (x)
This also led me down a rabbit hole and found another study (it was anonymous but if you’re a sleuth you can probably take a guess at what F1 driver it was) where they monitored his heart rate during qualifying to see what his average was throughout the race to test the cardiovascular strain F1 drivers are put under!
52 notes
·
View notes
Text
Robert F. Kennedy Jr. warns against taking the flu shot due to limited protection against circulating flu strains and increased risk of non-flu viral infections.
Research from the British Medical Journal indicates that the flu shot may prime the immune system for non-flu viral upper-respiratory infections.
A Pentagon study found that individuals who received the flu shot were 36% more likely to catch coronavirus.
Flu shots contain toxic mercury, with levels exceeding EPA standards, posing a risk of neurological damage as mercury can cross the blood-brain barrier.
Despite high mercury content, flu vaccines are marketed as risk-free without scientific clinical trials to prove efficacy, highlighting the need for transparency and accountability in the pharmaceutical industry.
“I would not take the flu shot in a million years, and I’ll tell you why,” says Robert F. Kennedy Jr., the newly appointed head of Health and Human Resources for the United States of America. Basically, when you get a flu shot, you are only protected against a few (sometimes only one) particular strains of flu, out of about a dozen or more possible that could be circulating that flu season, which lasts from October through May. Those injected folks are FOUR TIMES more likely to get a non-flu viral infection. Ever notice how many people who get the flu shot get sick right away?
15 notes
·
View notes
Note
☕+Herbs!
Herbs? I'd love to talk about herbs! I'll tell you about an herb I've been cultivating for use in the palace lately. It's called horehound. It looks like this:


Horehound is a member of the mint family. A perennial plant, horehound grows in abundance and looks a lot like its sister, mint. It's covered in tiny hairs, and the leaves of this plant have a cloudy or hoary appearance, giving it its name.
For centuries, horehound has been valued for its ability to treat cough and remove excess phlegm.
Horehound is an anti-inflammatory herb. Those suffering from arthritis may find relief from painful, inflamed joints when using horehound. The anti-inflammatory effect even extends itself to improving circulation by reducing inflammation of the blood vessels.
This greatly benefits the whole body, making it easier for blood and oxygen to get to all organs and greatly reducing the pressure placed on the heart. When pressure is taken off the heart, instances of strokes, heart attacks, and heart disease are greatly reduced.
Horehound may also reduce inflammation in the respiratory system and therefore help soothe sore throats and irritation.
There has been some indication that white horehound extract may help to reduce levels of LDL cholesterol. LDL cholesterol is cholesterol that clogs the arteries with plaque and often leads to the development of atherosclerosis, heart attacks, and strokes.
Horehound is also considered to be an antispasmodic, meaning it helps to alleviate cramps and spasms. By drinking a tea made of horehound leaves, you can relax the nervous system and body, greatly reducing spasms.
Horehound’s antispasmodic abilities also lends itself to alleviating menstrual cramps. Horehound may not only help soothe cramps, but it also may help to get hormones under control and improve mood.
Some research indicates that horehound may help reduce blood sugar levels. Horehound helps the body handle sugar by improving the body’s response to it, as opposed to going haywire when large doses of sugar are consumed. This can help diabetics get a better handle on their glucose levels.
Horehound is considered a medicinal plant due largely in part to its many healing compounds, such as its antimicrobial and antibiotic properties. These properties help protect the body and help your immune system fend off illness and disease.
The antiseptic properties contained in horehound are why it’s commonly used in toothpastes and mouthwashes currently on the market.
Yet another benefit of horehound is that it has the ability to induce sweating, helping the body to eliminate toxins, as well as excess sweat, fat, and water. Horehound’s ability to facilitate sweating is also an effective way to help break a fever and cool the body down.
The flavor of horehound is described as having a menthol and minty taste, often used to flavor cough drops and syrup. Horehound is available on the market as capsules, tea, cut leaves, liquid extract, and candy.
Horehound can also be made into an extract, infusion, syrup, or tincture. When combined with other herbs such as ginger root, marshmallow root, and licorice root, horehound can make an effective cough syrup.
...
...Sorry. I've been rambling on for quite a while, haven't I? It's a really useful herb that I don't think a lot of people know about, unlike mint or sage.
Um, I hope I gave you some useful information in all that...
Source
Tags: @mxrmaid-poet @veryinactice @rou-luxe @5mary5 @welp-back-on-my-bs @keithsandwich @lostsnowflake3 @a11-mynames-r-taken @sh0jun @floydsteeth
#sorry if something sounds ooc#mod is not sure how to make it sound better 😔#ikemen prince#keith howell#ask keith
13 notes
·
View notes
Text
65,000 non - human primates are used in laboratory experiments every year in the united states
Each year, more than 110 million animals - including mice, rats, dogs, cats, rabbits, hamsters, fish and birds - are killed in U.S. laboratories for chemical, drug, food, and cosmetics testing. In order for a drug to be approved in the United States, the FDA typically requires toxicity tests on one rodent species such as a mouse or rat and one nonrodent species such as a monkey or dog.
Around 65,000 non - human primates (NHP) are used every year in the United States, and around 7,000 across the European Union. No new biomedical research projects have been approved on chimpanzees in the US since 2015.
Macaques are now the most commonly used NHP - most are imported from China and Cambodia.
The huge demand for research monkeys and their rising costs have created a market for monkey smugglers.
While most macaques imported by the US are identified as captive-bred on paper, some experts believe that many of those in US labs have been trafficked from the wild as the illegal trade in wild-caught macaques is widespread. Sources state that prices vary from $5 000 - $20 000 per monkey.
NHPs are used because of their similarities to humans with respect to genetic makeup, anatomy, physiology, and behavior which make it possible to approximate the human condition.
NHPs are used in research into HIV, neurology, behavior, cognition, reproduction, Parkinson's disease, stroke, malaria, respiratory viruses, infectious disease, genetics, xenotransplantation, drug abuse, and also in vaccine and drug testing.
The NIH is the largest public source of funding for biomedical research in the United States.
Last year new U.S. law eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. It allows the U.S. Food and Drug Administration (FDA) to approve new drugs without requiring animal data.
Signed in December, the law doesn't ban the testing of new drugs on animals outright. Instead it simply lifts the requirement that pharmaceutical companies use animals to test new drugs before human trials. Companies can still test drugs on animals if they choose to.
And pro-research groups are downplaying the law, saying it signals a slow turning of the tide. Jim Newman, communications director at Americans for Medical Progress, which advocates for animal research, argues non-animal technologies are still “in their infancy” and won’t be able to replace animal models for “many, many years.” The FDA still retains tremendous discretion to require animal tests, he says.
- National Institutes of Health ( https://www.ncbi.nlm.nih.gov), Science Direct, World Animal Protection, science.org, National Anti - Vivisection Society and HSUS.
Image with kind permission from The Ethic Whisper.
@theethicwhisper

#vegan#veganism#animal rights#animal experiments#animal experimentation#animals in laboratories#ban animal experimentation
12 notes
·
View notes
Text

Children still mining cobalt for gadget batteries in Congo
A CBS News investigation of child labor in cobalt mines in the Democratic Republic of Congo has revealed that tens of thousands of children are growing up without a childhood today – two years after a damning Amnesty report about human rights abuses in the cobalt trade was published. The Amnesty report first revealed that cobalt mined by children was ending up in products from prominent tech companies including Apple, Microsoft, Tesla and Samsung.
There's such sensitivity around cobalt mining in the DRC that a CBS News team traveling there recently was stopped every few hundred feet while moving along dirt roads and seeing children digging for cobalt. From as young as 4 years old, children can pick cobalt out of a pile, and even those too young to work spend much of the day breathing in toxic fumes.
What's life like for kids mining cobalt for our gadgets?
So, what exactly is cobalt, and what are the health risks for those who work in the DRC's cobalt mining industry?
What is cobalt?
Cobalt – a naturally occurring element – is a critical component in lithium-ion, rechargeable batteries. In recent years, the growing global market for portable electronic devices and rechargeable batteries has fueled demand for its extraction, Amnesty said in its 2016 report. In fact, many top electronic and electric vehicle companies need cobalt to help power their products.
The element is found in other products as well.
"Cobalt-containing products include corrosion and heat-resistant alloys, hard metal (cobalt-tungsten-carbide alloy), magnets, grinding and cutting tools, pigments, paints, colored glass, surgical implants, catalysts, batteries, and cobalt-coated metal (from electroplating)," says the U.S. Centers for Disease Control and Prevention.
More than half of the world's supply of cobalt comes from the DRC, and 20 percent of that is mined by hand, according to Darton Commodities Ltd., a London-based research company that specializes in cobalt.

Health risks of chronic exposure
According to the CDC, "chronic exposure to cobalt-containing hard metal (dust or fume) can result in a serious lung disease called 'hard metal lung disease'" – a kind of pneumoconiosis, meaning a lung disease caused by inhaling dust particles. Inhalation of cobalt particles can cause respiratory sensitization, asthma, decreased pulmonary function and shortness of breath, the CDC says.
The health agency says skin contact is also a significant health concern "because dermal exposures to hard metal and cobalt salts can result in significant systemic uptake."
"Sustained exposures can cause skin sensitization, which may result in eruptions of contact dermatitis," a red, itchy skin rash, the CDC says.
Despite the health risks, researchers with Amnesty International found that most cobalt miners in Congo lack basic protective equipment like face masks, work clothing and gloves. Many of the miners the organization spoke with for its 2016 report – 90 people in total who work, or worked, in the mines – complained of frequent coughing or lung problems. Cobalt mining's dangerous impact on workers and the environment
Some women complained about the physical nature of the work, with one describing hauling 110-pound sacks of cobalt ore. "We all have problems with our lungs, and pain all over our bodies," the woman said, according to Amnesty.
Moreover, miners said unsupported mining tunnels frequently give way, and that accidents are common.
Miners know their work is dangerous, Todd C. Frankel wrote late last month in The Washington Post.
"But what's less understood are the environmental health risks posed by the extensive mining," he reported. "Southern Congo holds not only vast deposits of cobalt and copper but also uranium. Scientists have recorded alarming radioactivity levels in some mining regions. Mining waste often pollutes rivers and drinking water. The dust from the pulverized rock is known to cause breathing problems. The mining industry's toxic fallout is only now being studied by researchers, mostly in Lubumbashi, the country's mining capital."

"These job are really desired"
Despite the dangers and risks of working as miners in the cobalt industry, at least of the some miners in the Congo "love their jobs," according to Frankel.
"When I talked to the miners there, none of them want to lose their jobs or give up their jobs. They love their jobs," Frankel said Tuesday, speaking on CBSN. "In a country like Congo, mining is one of the few decently paying jobs to be had there, and so they want to hold onto these jobs."
They also want fair treatment, decent pay, and some safety, "and they would love for their kids to not work in the mines," he said.
"It's a poverty problem," Frankel said. "These parents I talked to – they don't want their kids working in these mines. The problem is that their school fees – schools cost money, and you know, food costs money, and they sort of need their kids to work in there."
Poverty also drives children into the mines instead of school – an estimated 40,000 of them work in brutal conditions starting at very young ages.
The thousands of miners who work in tunnels searching for cobalt in the country "do it because they live in one of the poorest countries in the world, and cobalt is valuable," Frankel wrote in the Washington Post article.
"Not doing enough"
CBS News spoke with some of the companies that use cobalt in their lithium-ion batteries. All of the companies acknowledged problems with the supply chain, but said they require suppliers to follow responsible sourcing guidelines. Apple, an industry leader in the fight for responsible sourcing, said walking away from the DRC "would do nothing to improve conditions for the people or the environment."
Read company responses here
Amnesty said in November, however, that "major electronics and electric vehicle companies are still not doing enough to stop human rights abuses entering their cobalt supply chains."
"As demand for rechargeable batteries grows, companies have a responsibility to prove that they are not profiting from the misery of miners working in terrible conditions in the DRC," the organization said. "The energy solutions of the future must not be built on human rights abuses."
An estimated two-thirds of children in the region of the DRC that CBS News visited recently are not in school. They're working in mines instead.
CBS News' Debora Patta spoke with an 11-year-old boy, Ziki Swaze, who has no idea how to read or write but is an expert in washing cobalt. Every evening, he returns home with a dollar or two to provide for his family.
"I have to go and work there," he told Patta, "because my grandma has a bad leg and she can't."
He said he dreams of going to school, but has always had to work instead.
"I feel very bad because I can see my friends going to school, and I am struggling," he said.
Amnesty says "it is widely recognized internationally that the involvement of children in mining constitutes one of the worst forms of child labour, which governments are required to prohibit and eliminate."
#cobalt#PD Congo#PDR Congo#cobalt mining by children#amnesty university#The toll of the cobalt mining industry on health and the environment#Congo Economic Theft#minerals#rare earth minerals#tesla#iphones#cellphone batteries#ev batteries#lithium batteries#child labour#forced child labor#poverty#systemic racism
53 notes
·
View notes
Text
Moderna on Monday said its combination vaccine that targets both Covid-19 and the flu was more effective than existing standalone shots for those viruses in a late-stage trial. The biotech company is the first to release positive phase three data on a Covid and flu combination shot, giving it a potential lead over rival vaccine makers Pfizer and Novavax. Moderna plans to file for regulatory approval for its combination jab this summer in the U.S. and hopes it can enter the market in 2025, the company’s CEO Stephane Bancel said in an interview. Moderna, Pfizer and Novavax have said that combination shots will simplify how people can protect themselves against respiratory viruses that typically surge around the same time of the year. The added convenience is critical as fewer Americans roll up their sleeves to get vaccinated against Covid.
13 notes
·
View notes
Text
The skin of a siren is protected by a miniscule layer of scales that form a smooth surface. These scales are so small that they regularly fall off and regrow like flakes of dead skin on a human.
Though they are small they still hold value, especially if they are preserved.
The top of a siren's head contains keratinous fibres, much like human hair, that poke through the gaps in the thin layer of scales. Colours vary, though black is most common.
Their faces harbour traits that contrast a humans'; They have no eyebrows nor outer ear structure, but instead grow fins in their place. They have no eyelashes, relying on a set of inner and outer eyelids to block bothersome sediment and sunlight both in and out of the water. They can see a great distance farther underwater than they can above water.
A siren's eyes are always yellow, but will fade to grey upon death.
Sirens are equipped with a small set of lungs, allowing them to survive without using their gills for a short period of time. Their respiratory tracts are very similar to that of a human's, though they have a smaller air capacity.
The mouth of a siren is extremely dangerous. Their eyeteeth typically grow long and pointy as they age, over time developing a toxin inside that can paralyze a target if it penetrates their flesh. The older the siren, the stronger the toxin. Because of this, all sirens that are kept in captivity have both eyeteeth surgically removed and replaced with false ones for safety reasons.
Sirens are known to be bloodthirsty. Most, if not all, adapt an otherworldly ability to bend the will of any living creature through song. Anything that hears their melody will feel compelled to do as the siren commands. Anyone who happens across a siren is advised to wear hearing protection at all costs. Your life may depend on it.
The first set of gills is on each side of a siren's neck. These are small and are not the main source of oxygen filtering. Instead they are typically adorned with objects that display power, status, strength, wealth etc. Many sirens have been seen with hooks in these gills, a popular "piercing" gained at a certain rites of passage.
The back of the shoulders, neck, and forearms of a siren may be adorned with extra fins to assist with gliding in the water. This varies per individual.
Sirens are not mammals, therefore they do not have breasts or nipples. All sirens have bare chests, except for any markings that might be present.
There is another set of gills that sits just below the bottom ribs. These ones are much larger than the ones on the neck, and are responsible for the majority of oxygen filtering throughout the body.
The hands of a siren contain thin translucent webbing between the fingers, which are tipped with sharp keratinous claws for tearing prey apart. Ripping flesh is easy for a siren, and caution is advised near any unrestrained ocean predator.
The beginning of the tail on a siren varies per individual. The scales of the skin gradually start getting bigger near their lower midsection, gaining extra colour in the process. The more bright and full a siren's tail is, the better chance of finding a mate. These larger and more colourful scales are highly sought after for their beauty, often fetching a high price in any market.
Unfortunately, unlike their skin scales, these do not grow back once ripped out.
The tail of a siren may contain extra fins on the front, back, or sides. These serve many purposes like precision swimming and diematic displays. They are also used in courting, various body languages, and social gestures.
All sirens have a large fin at the end of their tail, which varies widely per individual. Some of them are wide and stiff, some of them are long and flexible, but all are necessary for survival and all are considered a culinary delicacy.
Sirens live to be around one hundred years old, and can lay many eggs throughout their lives. The egg of a siren initially has a soft exterior, and is cared for and watched over for an entire moon cycle before it begins to harden. At that point the parent begins to leave the egg alone, and it is often left to hatch on its own. Because of this many sirens do not know their parents, and many lack any sort of deep connection to them.
Once they break through their shell, a siren immediately knows how to swim and is able to start finding food for themselves in the sand and the crevices of the surrounding rocks. Because it can take many years for them to develop any offensive features, young sirens will usually stick to hunting small fish and crustaceans to survive.
9 notes
·
View notes
Link
North America held the highest revenue share of over 34% in 2019 and will continue to do so during the projected period...
0 notes
Text

Bad Pharma by Pam Lazos and Abraham Johns
Onward Pharmaceutical Labs (OPL), one of the world’s largest pharma companies, is completing the development of a new vaccine, RSVIX, to protect children from a respiratory virus — the respiratory syncytial virus, or RSV — that endangers the lives of infants and young children. OPL expects RSVIX to be their next blockbuster and hopes to quickly capture most of the $7B U.S. market. The final clinical study before licensure is a head-to-head comparison with RESPIRWELL, the currently licensed vaccine produced by OPL's rival, Beamer Labs. To succeed in the trial, OPL must prove equal protection with the four common serotypes their vaccine shares with Beamer’s licensed vaccine while adding coverage for five additional strains of the virus that RESPIRWELL does not have.
When Siddhartha Kumar, OPL’s lead medical monitor assigned to the trial, discovers that RSVIX is not performing as planned, he notifies his superiors, recommending they stop the trial and offer a dose of RESPIRWELL to all the study participants to ensure their protection. When the company refuses to inoculate the trial population with the licensed vaccine, Sid questions the ethics behind this decision while continuing to advocate for the safety of the children. Sid’s insistence leads to his dismissal, leaving like-minded others in the company scrambling to fill the void.
Inspired by a true story, Bad Pharma delves into the consequential issues surrounding drug development, which often puts science and business at odds, and asks the ultimate question: when is the risk not worth the reward?
"A deep dive into company greed....... their narrative asks salient questions about corporate accountability, profit and ethics in drug development" KIRKUS REVIEWS 2024
Order YOUR Copy NOW: amzn.to/41AGznV
3 notes
·
View notes
Text
A Sustainable Solution for Lebanon’s Plastic Waste Crisis


1. Introduction: Why CleanLeb?
Lebanon faces a severe plastic waste crisis, with pollution damaging its environment, health, and economy. CleanLeb is a pioneering initiative designed to tackle this issue by transforming plastic waste into sustainable building materials. Our approach not only reduces plastic pollution but also contributes to economic growth and innovation in the construction industry. By creating a circular economy for plastic waste, CleanLeb aims to drive sustainable development and a cleaner future for Lebanon.
2. The Problem: Lebanon’s Plastic Waste Crisis
Excessive Plastic Waste: Lebanon generates thousands of tons of plastic waste annually, with little to no recycling infrastructure.
Environmental Damage: Plastic pollution harms soil, water, and marine life, affecting biodiversity and agricultural productivity.
Health Hazards: Microplastics and toxic waste seep into food and water sources, causing long-term health risks such as respiratory diseases and cancer.
Economic Loss: Inefficient waste management leads to high government spending on waste removal, while missed opportunities in recycling and sustainable industries limit economic growth.
Urban and Rural Impact: Overflowing landfills and illegal dumping sites worsen living conditions, particularly in underserved rural areas, impacting public health and tourism.
3. The Solution: CleanLeb’s Innovative Model
CleanLeb transforms plastic waste into valuable construction materials, reducing environmental harm while providing a sustainable alternative to traditional building resources. Our model is built on three main pillars:
A. Collection & Recycling
Establishing plastic collection points in urban and rural areas, including schools, community centers, and businesses.
Partnering with municipalities, NGOs, and local businesses to streamline waste sorting and collection processes.
Introducing incentive-based programs where citizens and businesses receive benefits for contributing plastic waste.
Raising awareness through public campaigns to promote waste segregation at the source.
B. Processing & Production
Implementing cutting-edge technology to convert plastic waste into durable and high-quality construction materials such as bricks, paving stones, roofing tiles, and insulation panels.
Ensuring that our products meet rigorous safety and environmental standards for sustainable building practices.
Innovating in research and development to continuously enhance product quality, reduce costs, and explore additional applications for recycled plastic materials.
Reducing reliance on imported building materials, strengthening Lebanon’s self-sufficiency in the construction sector.
C. Market Implementation
Supplying government infrastructure projects, private construction companies, and housing developers with eco-friendly building materials.
Advocating for the adoption of green building regulations and sustainable procurement policies at the national level.
Supporting small and medium-sized enterprises (SMEs) by providing cost-effective, durable materials that align with Lebanon’s economic and environmental goals.
Expanding CleanLeb’s reach through partnerships with international organizations working on environmental sustainability.
4. The Benefits of CleanLeb
A. Environmental Impact
Drastically reduces plastic waste in landfills, rivers, and coastal areas.
Decreases pollution levels, protecting soil, groundwater, and marine ecosystems.
Reduces carbon emissions associated with plastic production and disposal.
Promotes responsible waste management practices nationwide.
B. Economic & Social Impact
Creates thousands of green jobs in waste collection, recycling, and sustainable construction industries.
Encourages local entrepreneurship and innovation in the recycling sector.
Reduces municipal waste management costs by diverting plastic from landfills.
Lowers construction costs through the availability of affordable, locally-produced materials.
Improves urban and rural infrastructure by utilizing eco-friendly, durable construction materials.
C. Innovation & Sustainability
Pioneering Lebanon’s transition towards a circular economy.
Fostering collaboration between research institutions, universities, and industry experts to develop advanced recycling technologies.
Aligning with the UN Sustainable Development Goals (SDGs) by promoting responsible consumption and production.
Setting an example for other developing countries on how to efficiently manage plastic waste.
5. How the Government Can Support CleanLeb
A. Policy & Regulation
Enacting strict waste management laws that mandate plastic recycling and penalize illegal dumping.
Introducing tax incentives for companies using recycled materials in their products.
Supporting a national standard for eco-friendly building materials in government-funded infrastructure projects.
Establishing a dedicated regulatory body to oversee and promote sustainable waste management initiatives.
B. Funding & Investment
Providing financial grants and subsidies for research and development in plastic recycling technologies.
Encouraging public-private partnerships (PPPs) to expand CleanLeb’s recycling and production facilities.
Offering low-interest loans and incentives to startups and businesses engaged in green initiatives.
Launching government-backed investment funds to drive sustainable infrastructure development.
C. Infrastructure & Logistics
Allocating land and facilities for CleanLeb’s recycling and production plants.
Enhancing nationwide waste collection infrastructure, including smart bins and sorting stations.
Implementing smart waste management systems to optimize recycling efficiency.
Establishing regional recycling hubs to decentralize waste management and reduce transportation costs.
6. CleanLeb’s Vision for a Greener Lebanon
CleanLeb envisions a future where Lebanon thrives as a leader in sustainable waste management, transforming environmental challenges into economic opportunities. Through technological innovation, strategic partnerships, and government collaboration, we aim to:
Build a national circular economy where plastic waste is repurposed into valuable materials.
Promote environmental awareness and instill a culture of responsible consumption and recycling.
Strengthen Lebanon’s infrastructure with eco-friendly building solutions.
Position Lebanon as a regional pioneer in green construction and sustainable urban development.
7. Call to Action
We urge the Lebanese government to take immediate action by:
Collaborating with CleanLeb to implement an efficient and scalable waste management system.
Investing in sustainable recycling infrastructure and incentivizing the private sector’s involvement.
Adopting eco-friendly building materials in all government-led construction projects.
Educating the public through national awareness campaigns on the importance of recycling and sustainability.
Implementing smart policies that make plastic recycling a mandatory practice for businesses and municipalities.
With strong government support, CleanLeb can drive Lebanon’s transition toward a cleaner, greener, and more sustainable future. Together, let’s transform Lebanon’s plastic waste crisis into a national success story.
CleanLeb – Turning Waste into Opportunity
#climate change#donate#lebanon#lebanese#recycling#recycledmaterials#sustainability#reduce#reuse#reduce reuse recycle#plastic waste#encouragement#government
2 notes
·
View notes