#Point-of-Care Diagnostics Market
Explore tagged Tumblr posts
Text
Point-of-Care Diagnostics Market Trends, Size, Growth, Leading Companies and Industry Report 2023-2028
IMARC Group has recently released a new research study titled “Point-of-Care Diagnostics Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028“, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios. How big is the point-of-care diagnostics…

View On WordPress
#Global Point-of-Care Diagnostics Market#Point-of-Care Diagnostics Market#Point-of-Care Diagnostics Market Report#Point-of-Care Diagnostics Market Size
1 note
·
View note
Text
#Point-Of-Care Diagnostics Market#Point-Of-Care Diagnostics Market Size#Point-Of-Care Diagnostics Market Share#Point-Of-Care Diagnostics Market Trends#Point-Of-Care Diagnostics Market Analysis#Point-Of-Care Diagnostics Market Growth#Point-Of-Care Diagnostics Market Forecast#Point-Of-Care Diagnostics Market Report#Point-Of-Care Diagnostics Industry Report#Point-Of-Care Diagnostics Industry
0 notes
Link
TOP 10 COMPANIES IN POINT OF CARE DIAGNOSTICS MARKET
#point-of-care diagnostics market#Point Of Care Diagnostics#Point Of Care#poc#healthcare#Immunoassay#Microfluidics#health#medical#poc testing#covid testing#cancer hematology
0 notes
Text
The Future of Diagnostics: A Deep Dive into Point of Care Testing
The global point of care diagnostics market size is expected to reach USD 68.5 billion by 2030, registering a CAGR of 6.1% from 2024 to 2030, according to a new report by Grand View Research, Inc. The introduction of advanced technologies such as Telehealth enabled POCT is expected to fuel market growth. Furthermore, the rise in the geriatric population and their need for accessible home-based care are anticipated to drive the market.
The adoption of POCT in emerging economies such as Africa and Latin America is anticipated to be a growth determinant of the global POCT market. The authorities are decentralizing the healthcare system and increasing their investments. For instance, the African regulations in Kenya allotted USD 346.7 to Managed Equipment Services project for the government's initiative toward modernizing facilities by procuring new equipment. The Ministry of Health selected GE Health and Philips to offer equipment across 47 countries. Similarly, Latin America decentralized IVD testing, which encourages private players to take strategic initiatives to meet the region’s existing demand for affordable POCT, especially in the infectious diseases segment.
Currently developed POC devices and tests are employed across different medical diagnostic applications, including cancer, pregnancy, and infectious diseases. Patients and physicians employ POC tests to screen conditions, confirm diagnoses, and design suitable therapeutic approaches based on patient health. However, the enthusiasm displayed by different consumers, such as doctors, patients, and caregivers, varies widely. This dynamic consumption pattern of POC diagnostic products is also attributed to economic scalability, financial interests, and lack of a universal healthcare structure.
In the light of COVID-19, the lockdown imposed across the globe has necessitated virtual visits and rapid diagnostic tests that assist patients to avoid hospital visits. Post-lockdown, the eHealth strategy is the emerging area of priority and investment such as an automated patient appointment system, lab result transmission system, healthcare workers' communication system, and medical products procurement system for the companies and governments. It is anticipated to continue to prioritize home-based healthcare delivery even after the pandemic, especially for patients with pre-existing conditions. For instance, NeuroMetrix, Inc. appointed a team to develop the business of DPNCheck, a POCT for peripheral neuropathies. The team is responsible for expanding the footprint in the healthcare market and focusing on the Medicare Advantage population.
Point Of Care Diagnostics Market Report Highlights
The Infectious disease led the market and accounted for 27.8% of global revenue share in 2023. Growth of the segment is attributed to increasing demand for rapid tests, which has encouraged industry players to deliver point of care solutions to decentralized regions and launch innovative solutions.
Home sector is projected to witness the highest growth rate over the forecast period owing to the comfort level and cost-effectiveness of point of care diagnostics provided to patients at home. POCT devices are easy to use and do not, mandatorily, require any modern lab infrastructure for testing simpler target analytes in a patient's sample
North America dominated the market and accounted for a 42.9% share in 2023 owing to the increasing demand for new technologies, a large pool of key players, and advanced healthcare infrastructure
The Asia Pacific is anticipated to witness significant growth in the market owing to the rising prevalence of cancer, diabetes, cardiovascular diseases, and infectious diseases. The increasing population with limited disposable income is the potential target market for the key players in the region
Point Of Care Diagnostics Market Segmentation
Grand View Research has segmented the global point of care diagnostics market on the basis of on product, end use, and region:
Point Of Care Diagnostics Product Outlook (Revenue, USD Billion, 2018 - 2030)
Glucose Testing
Hb1Ac Testing
Coagulation Testing
Fertility/Pregnancy
Infectious Disease
HIV POC
Clostridium Difficile POC
HBV POC
Pneumonia or Streptococcus Associated Infections
Respiratory Syncytial Virus (RSV) POC
HPV POC
Influenza/Flu POC
HCV POC
MRSA POC
TB and Drug-resistant TB POC
HSV POC
COVID-19
Other Infectious Diseases
Cardiac Markers
Thyroid Stimulating Hormone
Hematology
Primary Care Systems
Decentralized Clinical Chemistry
Feces
Lipid Testing
Cancer Marker
Blood Gas/Electrolytes
Ambulatory Chemistry
Drug of Abuse (DOA) Testing
Autoimmune Diseases
Urinalysis/Nephrology
Point Of Care Diagnostics End-use Outlook (Revenue, USD Billion, 2018 - 2030)
Clinics
Hospitals
Home
Assisted Living Healthcare Facilities
Laboratory
Point Of Care Diagnostics Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
US
Canada
Europe
Germany
UK
France
Italy
Spain
Russia
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of Point Of Care Diagnostics Market
Hoffmann-La Roche Ltd.
Qiagen
Danaher Corporation
Becton Dickinson (BD)
bioMérieux
Abbott
Siemens Healthcare GmbH
Werfen
Nova Biomedical
Trividia Health, Inc.
QuidelOrtho Corporation
Trinity Biotech
Sekisui Diagnostics
Orasure Technologies, Inc.
Spectral Medical, Inc.
EKF Diagnostics Holdings plc.
Anbio Biotechnology Co., Ltd.
AccuBioTech Co., Ltd
ALPHA LABORATORIES.
Order a free sample PDF of the Point Of Care Diagnostics Market Intelligence Study, published by Grand View Research.
0 notes
Text
The United States IVD market is experiencing robust growth, driven by the increasing prevalence of chronic and infectious diseases, including diabetes, cardiovascular disorders, cancer, and infectious diseases such as COVID-19. This, in turn, has heightened the need for accurate and timely diagnostic tools like IVD, thus creating lucrative growth opportunities for the market.
#United States In Vitro Diagnostics Market Report by Test Type (Clinical Chemistry#Molecular Diagnostics#Immunodiagnostics#Hematology#and Others)#Product (Reagent and Kits#Instruments)#Usability (Disposable IVD Devices#Reusable IVD Devices)#Application (Infectious Disease#Diabetes#Cancer/Oncology#Cardiology#Autoimmune Disease#Nephrology#End User (Hospitals Laboratories#Clinical Laboratories#Point-of-care Testing Centers#Academic Institutes#Patients#and Region 2025-2033
1 note
·
View note
Text
Point-of-Care Diagnostics Market: A New Era in Quick Healthcare Solutions

The Point-of-Care (POC) diagnostics market is on the rise, fueled by the growing need for rapid and convenient healthcare solutions. POC testing delivers accurate results for conditions like diabetes and infections directly to patients, minimizing the need for centralized lab testing. This advancement not only enhances patient care but also contributes to the efficiency of global healthcare systems. For deeper insights into this transformative market, visit Wissen Research's analysis here.
#Point of Care testing#point of care diagnostics#Medical devices report#Healthcare market#Wissen Research
0 notes
Text
Point of Care Diagnostics Market to Hit $95.89 Billion by 2032
The global Point of Care Diagnostics Market was valued at USD 45.06 Billion in 2024 and it is estimated to garner USD 95.89 Billion by 2032 with a registered CAGR of 9.9% during the forecast period 2024 to 2032.
Are you looking for the Point of Care Diagnostics Market Research Report? You are at the right place. If you desire to find out more data about the report or want customization, Contact us. If you want any unique requirements, please allow us to customize and we will offer you the report as you want.
The global Point of Care Diagnostics Market can be segmented on the basis of product type, Applications, distribution channel, market value, volume, and region [North America, Europe, Asia Pacific, Latin America, Middle East, and Africa]. The Point of Care Diagnostics Industry 2024 report provides a comprehensive overview of critical elements of the industry including drivers, restraints, and management scenarios.
Download Sample PDF: @ https://www.vantagemarketresearch.com/point-of-care-diagnostics-market-1821/request-sample
Top Players
F. Hoffmann-La Roche Ltd., Qiagen, Danaher Corp., Becton Dickinson (BD), Biomerieux SA, Abbott Laboratories, Siemens Healthcare AG, Zoetis Inc., Instrumentation Laboratory, Nova Biomedical, Trividia Health Inc., Quidel Corp., Trinity Biotech, Sekisui Diagnostics, Orasure Technologies Inc., Nipro Corp., Spectral Medical Inc. and others.
Trending 2024: Point of Care Diagnostics Market Report Highlights:
A comprehensive assessment of the parent Industry
Development of key aspects of the business
A study of industry-wide market segments
Evaluation of market value and volume in past, present, and future years
Evaluation of market share
Tactical approaches of market leaders
Innovative strategies that help companies to improve their position in the market
You Can Buy This Report From Here: https://www.vantagemarketresearch.com/buy-now/point-of-care-diagnostics-market-1821/0
Analysis Of The Top Companies, Product Types, and Applications In The Market Report:
This report provides sales, revenue growth rate, and verified information about the major players. Also includes a regional analysis and a labor cost analysis, tables, and figures. It also highlights characteristics such as technological growth. The product type segment is expected to continue to maintain its leading position in the future and capture a significant market share based on sales. This report provides analysis, discussion, forecast, and debate on key industry trends, market share estimates, Industry size, and other information. This report also discusses drivers, risks, and opportunities.
Global Point of Care Diagnostics Market report contains detailed data and analysis on the Point of Care Diagnostics Market drivers, restraints, and opportunities. Experts with market and industry knowledge as well as research experience from regional experts validate the report. The Point of Care Diagnostics Market report provides forecast, historical and current revenue for each industry, region, and end-user segment.
Regions Included
-North America [United States, Canada, Mexico]
-South America [Brazil, Argentina, Columbia, Chile, Peru]
-Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
-Middle East & Africa [GCC, North Africa, South Africa]
-Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
Global Point of Care Diagnostics Market report data will help you make more informed decisions. For example, in relation to prices, distribution channels are means of marketing or identifying opportunities to introduce a new product or service. These results will also help you make more informed decisions about your existing operations and activities.
Read Full Research Report with [TOC] @ https://www.vantagemarketresearch.com/industry-report/point-of-care-diagnostics-market-1821
You Can Use The Point of Care Diagnostics Market Report To Answer The Following Questions:
What are the growth prospects of the Point of Care Diagnostics Market business?
Who are the key manufacturers in the Point of Care Diagnostics Market space?
What Forecast Period for Global Point of Care Diagnostics Industry Report?
What are the main segments of the global Point of Care Diagnostics Market?
What are the key metrics like opportunities and market drivers?
The Point of Care Diagnostics Market Insights
Product Development/Innovation: Detailed Information On Upcoming Technologies, R&D Activities, And Product Launches In The Market.
Competitive Assessment: In-Depth Assessment Of Market Strategies, Geographic And Business Segments Of Key Market Players.
Market Development: Comprehensive Information On Emerging Markets. This Report Analyzes The Market For Different Segments In Different Regions.
Market Diversification: Comprehensive Information On New Products, Untapped Regions, Latest Developments, And Investments In The Point of Care Diagnostics Market.
Check Out More Reports
Global Hemophilia Market: Report Forecast by 2032
Global Agricultural Fumigants Market: Report Forecast by 2032
Global Collagen Casings Market: Report Forecast by 2032
Global Automotive Test Equipment Market: Report Forecast by 2032
Global Quantum Sensors Market: Report Forecast by 2032
#Point of Care Diagnostics Market#Point of Care Diagnostics Market 2024#Global Point of Care Diagnostics Market#Point of Care Diagnostics Market outlook#Point of Care Diagnostics Market Trend#Point of Care Diagnostics Market Size & Share#Point of Care Diagnostics Market Forecast#Point of Care Diagnostics Market Demand#Point of Care Diagnostics Market sales & price
0 notes
Text

Global Point-of-Care Diagnostics Market Size, Share, Growth and Forecast 2031
Global point-of-care diagnostics market is projected to witness a CAGR of 7.90% during the forecast period 2024-2031, growing from USD 47.21 billion in 2023 to USD 86.74 billion in 2031.
Factors driving the point-of-care diagnostics market include the rising prevalence of infectious diseases, increased investment in technology that includes artificial intelligence, growing demand for decentralized healthcare solutions, and increased corporate investments. Apart from this, improvement in the trend towards home care improves the patient’s experience and lowers the costs of healthcare as well.
Global point-of-care (POC) diagnostics consists of rapid, on-site tests that enable immediate clinical decision-making. These tests are significant as they allow accurate and timely reporting, improving patient care results and reducing the need for centralized lab facilities. Its high ability to deliver fast, reliable diagnostics at the point of patient care positions it in a valuable category in every healthcare setting, initiating a more efficient, accessible healthcare delivery worldwide.
Companies are strengthening their diagnostics segment through R&D investment to gain a solid stand in the advanced point-of-care diagnostics market. Moreover, they obtain funds to develop their capabilities and speed up innovation in diagnostic solutions. Overall, these strategic initiatives enhance the quality and efficiency of POC diagnostics, fueling market growth and steadily increasing demand for faster, more accurate testing. For instance, in July 2024, NOWDiagnostics, Inc. (NOWDx) closed a USD 22.5 million Series B funding aimed at fast-tracking rapid at-home diagnostic tests. NOWDxwill utilize the funding for the launch of innovative diagnostics, and home-based tests and expand the portfolio of tests. NOWDx has a promising clinical research pipeline of around 30 diagnostic tests, and the company is willing to utilize the funding for the approval and commercialization of these tests.
Similarly, in August 2024, Lumos Diagnostics Holdings Limited (Lumos), a rapid POC diagnostic solutions manufacturer, extended its partnership with the Burnet Institute to conduct a clinical trial of a new POC test for monitoring liver health. This kind of development suggests that the market is expanding and increasing demand to innovate liver function monitoring and pave the way for future home-based tests.
Technological Advancements of Point-of-Care Diagnostics
Technological advancement is driving the growth of point-of-care diagnostics as a viable means to ease healthcare burdens. These devices are user-friendly and inexpensive, with rapid turnaround times; thus, they suit patients with financial constraints, social stigma, and mobility issues. Miniaturized biochip devices can detect diseases rapidly using simple samples such as blood or saliva. Integrating AI and microfluidic technologies improves diagnostic precision and efficiency, making it possible for POC devices to detect biomarkers in a wide range of conditions, from infectious diseases to oncology and many more. For instance, in June 2023, Sysmex Corporation launched its first point-of-care testing system for Europe, designed to diagnose antimicrobial susceptibility in 30 minutes rapidly. Using microfluidic technology, the system rapidly detects bacteria, analyzes the effectiveness of antimicrobial drugs using urine samples, and is likely to alter conventional approaches toward the diagnosis of infectious diseases and deal with the imperative urgency of speeding up the detection of UTI infections.
Growing Preference for Home Care Driving Market Growth
The growing preference for healthcare services at home is a key driver for growth in the point-of-care diagnostics segment. Patients are now looking for convenient, cost-effective, and more customized care delivery. There has been a rising demand for diagnostic tests that can be performed outside traditional clinical settings. POC diagnostics allow people to track their health conditions from the comfort of their homes. This reduces the number of hospital visits and decreases healthcare costs. The availability, accessibility, and usability of home-based diagnosis solutions are attractive factors contributing to increased usage and driving the market in the health sector. For instance, in March 2023, Cue Health Inc. launched a portfolio of home-based diagnostic test kits that offer company test-to-treat services to help people with easy health management from their homes. The marketed kits include heart health test kits, sexual health test kits, women’s health test kits, metabolic health test kits, and wellness test kits. These kits are enabled with Cue Care, allowing a simple and private collection of samples for the CLIA-certified independent laboratory analysis. The results are efficiently provided in the Cue Health App, coupled with relevant health information.
Favorable Funding Scenario to Fuel Market Growth
The COVID-19 pandemic has significantly transformed the funding landscape of point-of-care diagnostics. Health disparities caused during the pandemic highlight the need for fast, accurate, and accessible testing. In response, funding for R&D has surged to develop advanced and more point-of-care diagnostic tests. That additional funding helped drive faster the development of new POC diagnostics solutions that could rapidly and effectively diagnose and treat health conditions. The capital inflow has also expanded testing capabilities beyond COVID-19 to a broader set of conditions and applications. For this reason, the market is likely to experience tremendous growth, with ongoing investment remaining in a core position to drive diagnostic innovations in terms of improved access to healthcare for populations.
For instance, in March 2024, BioMérieux SA invested USD 10.76 million (NOK 115 million) to develop a small-scale immunoassay POC analyzer with SpinChip Diagnostics ASA. This advanced platform delivers diagnostic performance from a single blood droplet within minutes and has scalability across the POC market. The first product is a high-sensitivity cardiac troponin I test, which will be a monumental step in the method of emergency triaging and treatment of acute heart attacks.
Infectious Disease Testing Products Dominating Point-of-Care Diagnostics Market
Infectious disease testing products hold the largest share of the POC diagnostics market. They create an utmost need to provide rapid, on-site diagnosis for conditions such as COVID-19, HIV, influenza, and tuberculosis. These tests can be done immediately; thus, results can speed up the decisions about treatment, and infectious diseases will not spread faster as they are being diagnosed promptly. The portability and ease with which they may be used in even clinical settings, as well as remote or resource-limited areas, ensure their effectiveness. The growing epidemics of infectious diseases coupled with the technological advancements in POC testing devices further gave strength to these tools for controlling outbreaks and managing public health challenges effectively.
In August 2023, QuidelOrtho Corporation received De Novo FDA approval for its Sofia 2 SARS Antigen+ FIA. The prescription test is well-suited to point-of-care settings, supporting companies’ efforts to expand their product offerings for infectious disease testing. This approval reflects the ongoing trend of companies looking at upgrading diagnostic offerings by securing regulatory approvals and launching more advanced tests to respond to growing demand.
North America Dominates Point-of-Care Diagnostics Market Share
North America holds the largest share in the point-of-care diagnostics market due to robust healthcare infrastructure and is largely supported by the government, which leads to the encouragement of innovation and significant adoption of more advanced diagnostic technologies. The presence of prominent market players creates competition, and this, in turn, leads to a continued development in product offerings. For instance, in July 2023, Vital Biosciences Inc., a Canada-based company, launched its first point-of-care testing platform, VitalOne, a compact device roughly the size of a desktop computer, at the Annual Meeting of the American Association of Clinical Chemistry. It will revolutionize diagnostics, as the system can provide more than 50 lab-grade results within 20 minutes. Moreover, regional companies are streamlining efforts, helping drive its leadership. In July 2024, Babson Diagnostics, Inc. introduced its new BetterWay blood testing service at Austin retail pharmacies. This BetterWay enables patients to be tested at retail pharmacies. This makes access easier for consumers. This will encourage more people to seek such diagnostic services, further fueling the demand in the market.
Download Free Sample Report
Future Market Scenario (2024-2031F)
This market is expected to hold several growth opportunities in the coming years due to an increasing prevalence of infectious disease, advancement in point-of-care diagnostic technologies, growing advancements in home care diagnostics, and an increase in investments toward research and development activities. In addition, AI-integrated testing holds great promise in the future toward both accuracy and usability, and researchers are working to make the innovation even better. For example, in August 2024, a team at the University of California, Los Angeles (UCLA) managed to design an AI-based test similar to home-based COVID-19 tests that would detect Lyme disease accurately in under 20 minutes.
Report Scope
“Point-of-Care Diagnostics Market Assessment, Opportunities and Forecast, 2017-2031F”, is a comprehensive report by Markets and Data, providing in-depth analysis and qualitative and quantitative assessment of the current state of the global point-of-care diagnostics market, industry dynamics, and challenges. The report includes market size, segmental shares, growth trends, opportunities, and forecast between 2024 and 2031. Additionally, the report profiles the leading players in the industry, mentioning their respective market share, business models, competitive intelligence, etc.
Click here for full report- https://www.marketsandata.com/industry-reports/point-of-care-diagnostics-market
Latest reports-
Contact
Mr. Vivek Gupta 5741 Cleveland street, Suite 120, VA beach, VA, USA 23462 Tel: +1 (757) 343–3258 Email: [email protected] Website: https://www.marketsandata.com
0 notes
Text
Point-of-Care Diagnostics Market Share, Size, Trends and Outlook 2023-2028
IMARC Group has recently released a new research study titled “Point-of-Care Diagnostics Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.How big is the point-of-care diagnostics…

View On WordPress
#Global Point-of-Care Diagnostics Market#Point-of-Care Diagnostics Market#Point-of-Care Diagnostics Market Growth#Point-of-Care Diagnostics Market Report#Point-of-Care Diagnostics Market Size
1 note
·
View note
Text
Point of Care Diagnostics Market worth $77.8 billion by 2028
The global point-of-care diagnostics market, valued at $49.7 billion in 2023, is projected to reach $77.8 billion by 2028, growing at a CAGR of 9.4%. Key drivers include the rising prevalence of infectious diseases like influenza, HIV, and tuberculosis, supportive government policies, and the shift towards healthcare decentralization. Challenges include pricing pressures due to reimbursement cuts and stringent regulatory approvals. Significant growth opportunities exist in emerging markets like China, India, Brazil, and Mexico. In 2022, glucose monitoring products held a significant market share, with lateral flow assays leading by platform. Major players include Abbott Laboratories, F. Hoffman-La Roche Ltd., BD, Danaher Corporation, and Siemens Healthineers. Recent developments feature FDA approvals for new diagnostic panels by bioMérieux and BD, and a partnership between Thermo Fisher Scientific and Project HOPE to expand HIV testing in Sub-Saharan Africa.

Download an Illustrative overview:
Browse in-depth TOC on "Point of Care Diagnostics Market"
191 - Tables
51 - Figures
322 – Pages
The Lateral Flow Assays segment, by platform, is expected to register the largest market share of the global point of care diagnostics industry in 2022.
Based on platform, the point-of-care diagnostics market is segmented into lateral flow assays, immunoassays, microfluidics, dipsticks, and molecular diagnostics. The lateral flow assays segment dominated this market with a share of in 2022.Lateral flow assays are simple in usage, relatively inexpensive, making it more accessible for patients and healthcare provider, LFA technology does not require trained person to operate, allowing them to be used in various healthcare settings, from clinics and pharmacies to remote arears, promoting healthcare decentralization. All these factors are contributing towards the dominance of lateral flow technology in point of care diagnostics market.
The OTC testing product segment, by mode of purchase, to dominate the global point of care diagnostics industry in 2022.
On the basis of mode of purchase OTC testing products segment accounted the largest market share in 2022 attributed to the increasing adoption of OTC products owing to its ease of usage and convenience. The presence of diverse range of OTC point of care devices for diseases screening, personal health monitoring and various other conditions further contribute to the rising demand for OTC PoC testing devices among consumers.
North America to account for the largest share of point of care diagnostics industry during the forecast period.
North America is the expected to be the largest regional market for point of care diagnostics during the forecast period. The presence of a well-established healthcare system, rapid adoption of advanced point of care testing products, increase in the availability of medical reimbursement, favourable government support for the novel product development, and higher user awareness about the presence of point of care testing products are anticipated to support the market growth in the region
Request Sample Pages:
Point of Care Diagnostics Market Dynamics:
Drivers:
Rising incidence of infectious diseases
Increasing prevalence of target conditions
Favorable government initiatives for POC testing
Rising number of CLIA-waived POC tests
Restraints:
Pricing pressure on POC manufacturers
Stringent regulatory approval process for product commercialization
Opportunities:
High growth potential of emerging markets
Decentralization of healthcare
Innovative product development
Challenge:
Inadequate standardization with centralized lab methods
Limited awareness in emerging markets
Premium pricing of novel platforms
Key Market Players of Point of Care Diagnostics Industry:
As of 2022, the point of care diagnostics market was dominated by Abbott Laboratories (US), F. Hoffmann-La Roche Ltd. (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), and Becton, Dickinson and Company (US)
Point of Care Diagnostics Market - Key Benefits of Buying the Report:
The report will enable established firms as well as entrants/smaller firms to gauge the pulse of the market, which, in turn, would help them to garner a larger market share. Firms purchasing the report could use one or a combination of the below-mentioned strategies for strengthening their market presence.
This report provides insights on the following pointers:
Analysis of Key divers (rising prevalence of infectious and chronic disease, growing adoption of self-testing kits, helathacre decentralization, rising number of CLIA-wavier Poc Tests), restraints (rising pricing pressure on PoC manufactures, stringent regulatory approval procedures for new PoC devices), Opportunities (emerging markets, emerging technologies, such s microfluidics) , Challenge (Training & education in low resource countries)
Market Penetration: Comprehensive information on the product portfolios offered by the top players in the point-of-care diagnostics market
Product Development/Innovation: Detailed insights on the upcoming trends, R&D activities, and product launches in the point-of-care diagnostics market
Market Development: Comprehensive information on lucrative emerging regions
Market Diversification: Exhaustive information about new products, growing geographies, and recent developments in the point-of-care diagnostics market
Competitive Assessment: In-depth assessment of market segments, growth strategies, revenue analysis, and products of the leading market players.
Related Links:
1 note
·
View note
Text
Non-Hospital-Based Point-Of-Care Diagnostic Products Market Size, Overview, Share and Forecast 2031
#Non-Hospital-Based Point-Of-Care Diagnostic Products Market#Non-Hospital-Based Point-Of-Care Diagnostic Products Market research
1 note
·
View note
Text
United States point of care diagnostics market size is projected to exhibit a growth rate (CAGR) of 6.90% during 2024-2032. Numerous advancements in portable and handheld diagnostic devices have enhanced the convenience and user-friendliness of testing, which is primarily driving the market growth.
#United States Point of Care Diagnostics Market Report by Product Type (Blood-Glucose Monitoring Kit#Cardio-Metabolic Monitoring Kit#Pregnancy and Fertility Testing Kit#Infectious Disease Testing Kit#Cholesterol Test Strip#Hematology Testing Kit#and Others)#Platform (Lateral Flow Assays#Dipsticks#Microfluidics#Molecular Diagnostics#Immunoassays)#Prescription Mode (Prescription-Based Testing#OTC Testing)#End User (Professional Diagnostic Centers#Home Care#Research Laboratories#and Region 2024-2032
0 notes
Text
#Point of Care Diagnostics Market#Point of Care Diagnostics#Point of Car#Point#care#diagnostics#maket#cancer
0 notes
Text
The Point-Of-Care Diagnostics Devices And Equipment Market in 2023 is US$ 32.34 billion, and is expected to register a CAGR of 13.80% in 2023-2031.
#Point-Of-Care Diagnostics Devices And Equipment Market#Point-Of-Care Diagnostics Devices And Equipment Market Report#Point-Of-Care Diagnostics Devices And Equipment Market Size
0 notes
Text
Veterinary Point of Care Diagnostics Market Size, Share, Growth and Forecast (2021-2027)
The Global Veterinary Point of Care Diagnostics Market is anticipated to grow significantly by 2027, with an estimated CAGR of around 12% during the forecast period from 2021 to 2027. Point-of-care diagnostics (POCD) have become crucial in the healthcare sector for their ability to rapidly diagnose various life-threatening and infectious diseases such as cancer and diabetes, as well as for ongoing patient health monitoring. These devices are seen as essential diagnostic tools that help prevent treatment delays, thereby reducing mortality rates and the spread of infectious agents.
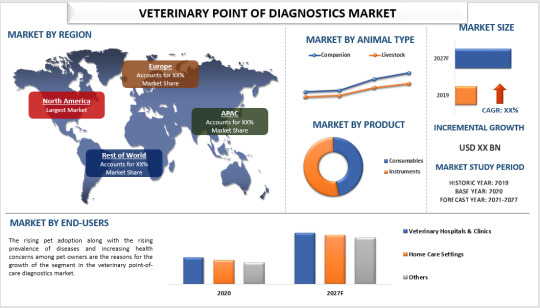
Key Growth Drivers
A major factor driving the veterinary industry's growth is the increasing adoption of pets. Additionally, the rise in diseases affecting animals contributes to the expanding veterinary point-of-care diagnostics market. For instance, according to Vikaspedia (Government of India), the country's total livestock population was 535.78 million in 2019, marking a 4.6% increase since the 2012 Livestock Census. The bovine population (including cattle, buffalo, mithun, and yak) reached 302.79 million in 2019, a 1.0% increase from the previous census. Furthermore, the growing number of zoological parks and heightened efforts in animal conservation significantly influence the demand for point-of-care diagnostics in veterinary medicine.
For a comprehensive analysis of the market drivers, visit: https://univdatos.com/report/veterinary-point-of-care-diagnostics-market/
Market Segmentation
By Animal Type
The market is divided into two main segments: livestock and companion animals. The livestock segment is expected to witness significant growth, driven by the increasing cattle population and the adoption of modern technologies for animal care. For instance, the Department of Animal Husbandry and Dairying (Government of India) reported that the female cattle population reached 145.12 million in 2019, an 18.0% increase since 2012.
By End-Users
The market is segmented into veterinary hospitals & clinics, home care settings, and others. The veterinary hospitals & clinics segment is projected to experience substantial growth due to rising pet adoption, the prevalence of diseases, and increasing health concerns among pet owners. The specialized services offered by these hospitals and clinics further bolster the segment's growth in the veterinary point-of-care diagnostics market.
Regional Insights
The report also provides a detailed regional analysis of the veterinary point-of-care diagnostics market, covering North America (United States, Canada, Rest of North America), Europe (Germany, France, Italy, Spain, United Kingdom, Rest of Europe), Asia-Pacific (China, India, Australia, Japan, Rest of APAC), and the Rest of the World. North America represents a significant market due to the high rate of pet adoption. According to the American Pet Products Association (APPA), 67% of U.S. households owned pets in 2021, with total expenditure on pet care products estimated at $123.6 billion.
For a sample report, visit: https://univdatos.com/get-a-free-sample-form-php/?product_id=22912
Key Market Players
Prominent companies in the global veterinary point-of-care diagnostics market include IDEXX Laboratories, Zoetis, Virbac, Heska Corporation, Thermo Fisher Scientific Inc., Neogen Corporation, Mindray, Esaote SpA, FUJIFILM Corporation, and Woodley Equipment Company Ltd. These players have engaged in numerous mergers and acquisitions, as well as partnerships, to develop various veterinary point-of-care diagnostics solutions.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website -www.univdatos.com
#Veterinary Point of Care Diagnostics Market#Veterinary Point of Care Diagnostics Market Size#Veterinary Point of Care Diagnostics Market Report#Veterinary Point of Care Diagnostics Market Forecast
0 notes