#Point-of-Care Coagulation Testing
Explore tagged Tumblr posts
Text
Yknow when we want to have some serious injury or illness in hopes of finally getting the care and attention we want so much but think we don't deserve?
I'm in that situation right now, a few days ago I was diagnosed with deep vein thrombosis.
My mom almost died with a double liver thrombosis. I was 8, and from there I knew I could also have it.
All my close friends (4) know about what happened to my mom, and they also know about my diagnosis... I told them when I was at the ER, still in total shock and scared shitless and they didn't bat an eye???
One of them sent "I'm getting ready to go to a clinic to do a knee ultrasound"
The other was like "oh at least you're at the doctor"
And the other didn't say a thing.
I received more messages asking how i was from a class "friend", someone i met a few months ago, then from my close friends.
I have BPD and I was going nuts on the way to the hospital bc they weren't answering me, they weren't worried about me! I was crying so much, my leg was hurting so much but the feeling that i didn't matter was way more painful.
When i got to the hospital, when i finally digested what happened, i simply stopped caring about the thrombosis bc if they didn't care I shouldn't care about it too...
I even told them that my case is a lil serious bc the pieces of coagulated blood could go to my lungs. They just sent exclamation points and emojis.
I'm the friend who always makes time for my friends, i could be completely overflowing with responsibilities and stuff to do but if a friend says they need me, I'll go to that friend.
But I feel disposable, feel like a unimportant person who just happens to be there.
It's been years, I always try to teach and explain how my brain works and what could trigger me, but it never seems to fase them...
I'm so tired of always accommodate others but no one ever tries to accommodate me. I always try to make everyone comfortable and happy, but no one tries to make me comfortable or happy.
All I wanna do is vanish, simply go away, not to test anything I already gave up on trying to be important... I really don't want friends anymore.
#borderline personality disorder#bpd#social anxiety#vent post#bpd fp#actuallybpd#bpd life#being borderline#borderline problems#borderline vent#thrombosis#bpd mood#borderline things#borderline thoughts#friends#health#mental health
2 notes
·
View notes
Text
The Science Behind INR Devices: How They Measure Coagulation
The management of blood coagulation is crucial for individuals taking anticoagulant medications, particularly those prescribed warfarin. Monitoring the International Normalized Ratio (INR) helps ensure that patients maintain the appropriate level of anticoagulation to prevent clotting or bleeding complications. This blog will delve into the science behind INR devices, explaining how they measure coagulation and the technology that powers these essential tools.
Understanding Coagulation and INR
Coagulation is the process through which blood changes from a liquid to a gel, forming a blood clot. This mechanism is vital for stopping bleeding and healing wounds. However, in patients taking anticoagulants like warfarin, managing this process is crucial, as excessive coagulation can lead to dangerous blood clots, while insufficient coagulation can result in uncontrolled bleeding.
The INR is a standardized measure of blood coagulation that allows healthcare providers to assess how long it takes for blood to clot. An INR of 1.0 is considered normal, while patients on anticoagulants typically aim for an INR range of 2.0 to 3.0, depending on their condition.
How INR Devices Work
INR devices, also known as point-of-care (POC) testing devices, utilize advanced technology to provide rapid and accurate measurements of blood coagulation. These devices have revolutionized patient care by enabling individuals to monitor their INR levels at home, reducing the need for frequent lab visits. Here’s how these devices work:
Sample Collection
INR devices typically require a small blood sample, which can be obtained through a finger prick. This minimally invasive method allows patients to perform the test conveniently at home. After the blood sample is collected, it is placed onto a test strip or cartridge designed for the specific INR device.
Measurement Process
Once the blood sample is applied to the test strip, the INR device analyzes the sample to determine the prothrombin time (PT) and calculate the INR. The test strip contains reagents that react with the blood sample, initiating a clotting process.
Most INR devices use one of two methods to measure coagulation:
. Electrochemical Method: This method involves measuring the electrical signals generated during the coagulation process. As the blood clots, the electrical resistance changes, and the device uses this data to calculate the INR.
. Optical Method: In this approach, the device uses light to assess clot formation. The blood sample is illuminated, and the device measures the light absorption or scattering, providing real-time data on coagulation.
Result Display
After analyzing the sample, the INR device displays the results on a screen. This immediate feedback empowers patients to make informed decisions about their medication and lifestyle, enhancing their overall management of anticoagulation therapy.
Advantages of INR Devices
The introduction of INR devices has transformed the landscape of anticoagulation monitoring, offering several advantages:
Convenience: Patients can perform tests at home, saving time and reducing the need for frequent trips to a laboratory.
Immediate Results: With quick results, patients can adjust their anticoagulant dosage promptly if needed, improving therapeutic outcomes.
Increased Compliance: The ease of use encourages more patients to monitor their INR levels regularly, leading to better adherence to treatment plans.
The Role of Technology in INR Devices
Modern INR devices are equipped with advanced technology that enhances their accuracy and usability. Many devices connect to smartphones or computers, allowing patients to track their INR history, receive reminders for testing, and share results with healthcare providers. This integration of technology not only simplifies the monitoring process but also promotes better communication between patients and their healthcare teams.
The science behind INR devices is integral to effective anticoagulation management. By understanding how these devices measure coagulation, patients can take charge of their health, ensuring they maintain their INR levels within the therapeutic range. With the convenience and accuracy provided by these devices, individuals can monitor their coagulation status from the comfort of their homes. At PatientSelfTesting, we are committed to providing high-quality INR devices that empower patients to manage their anticoagulation therapy effectively. Embrace the technology and take control of your health with our reliable INR devices, understanding that they play a crucial role in monitoring your coagulation effectively.
In summary, the science behind INR devices: how they measure coagulation is essential for patients seeking to maintain optimal health while on anticoagulant therapy.
0 notes
Text
Africa IVD Market - Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®, a premier global market research firm, has released its latest report titled, “Africa IVD Market Size, Share, Forecast & Trends Analysis by Offering, Technology (Immunoassay, PoC, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoCT), End User - Forecast to 2031.”
The report projects that the Africa in vitro diagnostics (IVD) market will reach $1.65 billion by 2031, growing at a CAGR of 3.1% from 2024 to 2031. This growth is driven by several factors, including:
Increasing prevalence of chronic and infectious diseases.
Significant investments from IVD market players in Africa.
Rising demand for Point-of-Care (POC) and rapid diagnostic solutions.
Growing geriatric population.
Government initiatives enhancing healthcare infrastructure.
Increased healthcare expenditure and R&D investments.
Download Sample Report Here : https://www.meticulousresearch.com/download-sample-report/cp_id=5415
Despite these opportunities, the market faces challenges such as high costs associated with advanced IVD products, variability in rapid test results, and stringent regulatory requirements for high and moderate-complexity tests.
Emerging Opportunities
The report highlights the growing awareness of early diagnosis, advancements in genomics and proteomics, and the rising adoption of personalized medicine as potential growth drivers for market stakeholders. However, uneven healthcare access and a shortage of trained professionals remain significant hurdles.
Check complete table of contents with list of table and figures: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Key Players in the Africa IVD Market
Leading companies in the Africa IVD market include:
Abbott Laboratories (U.S.)
Becton, Dickinson and Company (U.S.)
bioMérieux SA (France)
Danaher Corporation (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
QIAGEN N.V. (Netherlands)
Siemens Healthineers AG (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
Illumina, Inc. (U.S.)
Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China)
Market Insights and Future Outlook
The report segments the Africa IVD market by offering (reagents & kits, instruments, and software & services), technology (including immunoassay, molecular diagnostics, and others), application (focusing on infectious diseases, diabetes, oncology, etc.), diagnostic approach (laboratory testing, point-of-care testing, and OTC/self-testing), and end user (diagnostic laboratories, hospitals, home healthcare, etc.).
Reagents & Kits Segment: Expected to register the highest CAGR of 3.3% during the forecast period, driven by the increasing incidence of infectious diseases and rising test volumes.
Immunoassay Technologies: Anticipated to dominate with a 34.5% market share in 2024, favored for their efficiency and accuracy in diagnosing prevalent diseases like HIV and malaria.
Infectious Diseases Application: Projected to hold the largest market share due to the high prevalence of diseases such as COVID-19 and malaria.
Quick Buy : https://www.meticulousresearch.com/Checkout/47708335
Geographical Insights
The report provides an extensive analysis of key markets, including South Africa, Nigeria, Egypt, and more. Notably, South Africa is expected to exhibit the highest growth rate of 8.3% during the forecast period, fueled by increasing healthcare investments and improved access to diagnostic services.
Key Questions Addressed in the Report
What is the current revenue generated by IVD products in Africa?
What growth rate is expected for IVD product demand over the next 5-7 years?
What are the major factors impacting the Africa IVD market?
Which segments are experiencing significant traction?
What are the key geographical trends and opportunities?
For a deeper dive into these insights and more, access the full report : https://www.meticulousresearch.com/request-sample-report/cp_id=5415
Contact Information
For more details, please contact:
Meticulous Research® Email: [email protected] Sales Contact: +1-646-781-8004 Connect with us on LinkedIn.
0 notes
Text
In Vitro Diagnostics Market Growth Trends and Strategies 2024 - 2030
The global in vitro diagnostics (IVD) market size was estimated at USD 77.92 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 4.4% from 2024 to 2030.
The growth can be attributed to increasing adoption of IVD owing to a rise in the incidence of infectious and chronic diseases. The development of automated IVD systems for laboratories and hospitals to provide efficient, accurate, and error-free diagnoses is expected to fuel market growth. The rising number of IVD products being launched by key players is also fueling market growth. For instance, in November 2023, ARUP Laboratories received a CE mark from EU-IVDR for AAV5 DetectCDx, a companion diagnostic to select the eligibility of severe hemophilia A-affected patients for BioMarin’s new gene therapy, Roctavian.
Technological advancements in terms of accuracy, portability, and cost-effectiveness are expected to be one of the high-impact rendering drivers of this market. Introduction of novel and highly accurate clinical laboratory tests is boosting the adoption of novel IVD tests worldwide. In June 2023, Toray Industries, Inc. received marketing approval from Japan’s Ministry of Health, Labour and Welfare for its Toray APOA2-iTQ used to diagnose pancreatic cancer. Moreover, in March 2023, Abbott received U.S. FDA clearance for its novel laboratory Traumatic Brain Injury (TBI) blood test in the U.S. Increasing approvals of IVD tests for life-threatening diseases are expected to create new opportunities in the untapped market.
Gather more insights about the market drivers, restrains and growth of the In Vitro Diagnostics Market
In Vitro Diagnostics Market Report Highlights
• Molecular diagnostics is anticipated to grow at the fastest CAGR from 2024 to 2030 owing to the rising adoption and usage rate
• Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
• The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
• North America dominated the global market in 2023 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
• The global hepatitis diagnostic market size was valued at USD 3.82 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030.
• The global hematology diagnostics market size was valued at USD 7.54 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030.
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics (IVD) market report based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Revenue, USD Million, 2018 - 2030)
• Instruments
• Reagents
• Services
IVD Technology Outlook (Revenue, USD Million, 2018 - 2030)
• Immunoassay
o Instruments
o Reagents
o Services
• Hematology
o Instruments
o Reagents
o Services
• Clinical Chemistry
o Instruments
o Reagents
o Services
• Molecular Diagnostics
o Instruments
o Reagents
o Services
• Coagulation
o Instruments
o Reagents
o Services
• Microbiology
o Instruments
o Reagents
o Services
• Others
o Instruments
o Reagents
o Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
• Infectious Diseases
• Diabetes
• Oncology
• Cardiology
• Nephrology
• Autoimmune Diseases
• Drug Testing
• Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
• Point of Care
• Home-care
• Others
IVD End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals
• Laboratory
• Home-care
• Others
IVD Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Spain
o Italy
o Russia
o Denmark
o Sweden
o Norway
• Asia Pacific
o Japan
o China
o India
o South Korea
o Australia
o Thailand
o Singapore
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa (MEA)
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the In Vitro Diagnostics Market Intelligence Study, published by Grand View Research.
#In Vitro Diagnostics Market#In Vitro Diagnostics Market size#In Vitro Diagnostics Market share#In Vitro Diagnostics Market analysis#In Vitro Diagnostics Industry
0 notes
Text
Coagulation Markers Market By Product Type, By Manufacturers, By End-User And Market Trend Analysis Forecast 2033
The Coagulation Markers Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033).

Learn More On The Coagulation Markers Market: https://www.thebusinessresearchcompany.com/report/coagulation-markers-global-market-report
According to The Business Research Company’s Coagulation Markers Global Market Report 2024, The coagulation markers market size is expected to see strong growth in the next few years. It will grow to $1.52 billion in 2028 at a compound annual growth rate (CAGR) of 6.9%. The growth in the forecast period can be attributed to a growing incidence of chronic diseases, rising adoption of personalized medicine, a growing global population, rising healthcare expenditures, and expansion in emerging markets. Major trends in the forecast period include the integration of AI and machine learning, the development of novel biomarkers, enhanced point-of-care technologies, automation in laboratories, wearable health technologies, telehealth and remote monitoring, and sustainable diagnostic practices.
The rise in the prevalence of blood disorders is expected to propel the growth of the coagulation markers market going forward. Blood disorders refer to a wide range of conditions that affect the blood's ability to function correctly. The prevalence of blood disorders is growing due to aging populations, increased awareness, and improved diagnostic capabilities. Coagulation markers help diagnose and manage blood disorders by assessing clotting function, identifying abnormalities, and monitoring treatment effectiveness for conditions like hemophilia and thrombosis. For instance, in May 2024, according to the Leukaemia Foundation, an Australia-based non-profit organization, currently around 135,000 people in Australia are living with a blood cancer or blood disorder, with this number projected to exceed 275,000 by 2035. Therefore, an increasing prevalence of blood disorders is driving the coagulation marker market.
Get A Free Sample Of The Report (Includes Graphs And Tables): https://www.thebusinessresearchcompany.com/sample.aspx?id=17117&type=smp
The coagulation markers market covered in this report is segmented –
1) By Product: D-Dimer Assay, Fibrinogen Assay 2) By Sample Type: Plasma, Whole Blood Sample, Other Sample Types 3) By Technology: Fluorescence Immunoassay, Enzyme-Linked Immuno Sorbent Assay (ELISA), Rapid Test, Particle Enhanced Immunoturbidimetric Assay 4) By Application: Deep Venous Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Trauma, Other Applications 5) By End User: Hospital, Specialty Clinics, Academic And Research Institute, Diagnostics Laboratories
Major companies operating in the coagulation markers market are focusing on developing technologically innovative solutions, such as automated blood coagulation analyzers, to improve diagnostic accuracy, enhance efficiency, and provide faster results for better patient management. An automated blood coagulation analyzer is a medical device that automatically performs various tests to evaluate blood clotting function, providing rapid and accurate results for diagnosing and managing coagulation disorders. For instance, in July 2021, Sysmex Corporation, a Japan-based provider of in-vitro diagnostic solutions, announced the launch of its new automated blood coagulation analyzers named CN-6000 and CS-5100, designed to provide efficient and accurate coagulation testing in clinical laboratories. The CN-6000 is a fully automated coagulation analyzer with a maximum throughput of 240 tests per hour, featuring automatic sample pre-treatment, clot detection, and quality control management. The CS-5100, on the other hand, is a compact and versatile coagulation analyzer suitable for small to medium-sized laboratories, offering a throughput of up to 120 tests per hour and a user-friendly interface. Both analyzers support a wide range of coagulation assays and are designed to streamline coagulation testing workflows, improve turnaround times, and enhance patient care in clinical laboratories.
The coagulation markers market report table of contents includes:
1. Executive Summary
2. Coagulation Markers Market Characteristics
3. Coagulation Markers Market Trends And Strategies
4. Coagulation Markers Market - Macro Economic Scenario
5. Global Coagulation Markers Market Size and Growth ............
32. Global Coagulation Markers Market Competitive Benchmarking
33. Global Coagulation Markers Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Coagulation Markers Market
35. Coagulation Markers Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
0 notes
Text
0 notes
Text
goin post it up a ring binder then

Pre-Analytical Processing, Clinical Biochemistry, Haematology and Coagulation, Immunology, Blood Transfusion, Point of Care Testing, and Phlebotomy.
1 note
·
View note
Text
Hemostasis/ Coagulation Analyzer Market Will Grow at Highest Pace Owing to Point-of-Care Diagnostics
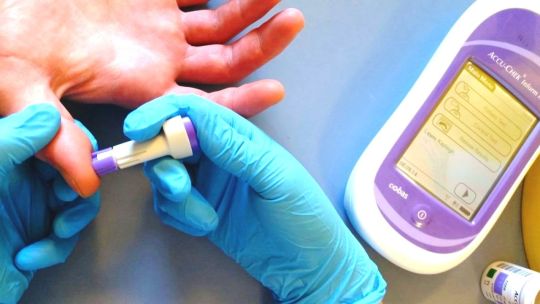
Hemostasis and coagulation analyzers are medical devices used to test coagulation factors in blood plasma. They help in monitoring blood coagulation disorders and management of anticoagulation therapies. Hemostasis and coagulation analyzers detect abnormalities related to hemostasis that may lead to excessive bleeding or thrombosis. Technological advancements have improved portability and results time of these analyzers enabling point-of-care diagnosis. The Global Hemostasis/ Coagulation Analyzer Market is estimated to be valued at US$ 6.47 Bn in 2024 and is expected to exhibit a CAGR of 5.8% over the forecast period 2024 to 2031. Key Takeaways Key players operating in the Hemostasis/ Coagulation Analyzer Market Growth are Horiba Medical, Siemens Healthineers AG, Agappe Diagnostics Ltd, NIHON KOHDEN CORPORATION, Sysmex Corporation, F. Hoffmann-La Roche Ltd, Stago Group, Maccura Biotechnology Co., Ltd., Thermo Fisher Scientific Inc., Haemonetics Corporation, Genrui Biotech Co., Ltd., BIOLABO S.A.S, Erba Mannheim, Sclavo Diagnostics International, and Helena Laboratories Corporation. The growing demand for point-of-care hemostasis testing is driving the market. Portable analyzers provide rapid results at the patient's bedside, ER, OR, and other locations to expedite clinical decision making. Technological advancements like dry chemistry methods, microfluidics, and automated sample-to-result technologies are reducing complex manual tasks. New devices have enhanced throughput, usability, and data management features for busy healthcare settings. Market Trends Point-of-care testing trend continues to grow for decentralized healthcare models. New devices offer miniaturized assaysoutside conventional laboratories directly at the site of patient care. Artificial intelligence and machine learning are being applied for tasks like automatic result interpretation, quality control, predictive maintenance, and remote diagnostics support. This helps maximize analyzer uptime for uninterrupted patient testing. Market Opportunities Emerging markets show strong growth potential driven by increasing access to healthcare and focus on non-communicable diseases. Local product assembly, innovative financingmodels, and regulatory approval strategies can help manufacturers tap opportunities. Integration of hemostasis testingonto general chemistry and coagulation analyzer platformsprovides a one-stop solution for routine and specialized testing. This consolidatestesting workflow while reducing costs per reportable result. Get More Insights On This Topic: Hemostasis/ Coagulation Analyzer Market
0 notes
Text

Top 5 Innovations in Critical Care Technology You Need to Know About
Innovations in critical care technology are continually evolving to enhance patient outcomes and streamline healthcare delivery. Here are five notable advancements:
Telemedicine and Remote Monitoring: Telemedicine platforms and remote monitoring devices enable healthcare providers to monitor patients in critical care settings from a distance. These technologies allow for real-time data collection, including vital signs, ECG readings, and oxygen levels, facilitating early detection of deterioration and timely intervention.
Artificial Intelligence (AI) and Predictive Analytics: AI-powered algorithms analyze large datasets to predict patient deterioration, identify patterns, and recommend personalized treatment plans. These technologies can assist clinicians in making faster and more accurate diagnoses, optimizing resource allocation, and improving patient outcomes.
Advanced Ventilation Strategies: Innovations in mechanical ventilation systems aim to improve patient comfort, minimize ventilator-induced lung injury, and enhance oxygenation. Adaptive ventilation modes, lung protective strategies, and personalized ventilation algorithms are examples of advancements in this field.
Extracorporeal Life Support (ECLS): ECLS, including extracorporeal membrane oxygenation (ECMO) and extracorporeal CO2 removal (ECCO2R), provides temporary support to failing cardiac or respiratory systems. Recent innovations in ECLS technology focus on miniaturization, improved biocompatibility, and simplified circuit design to make these therapies more accessible and effective.
Point-of-Care Testing (POCT): Point-of-care testing devices enable rapid and accurate diagnostic testing at the patient's bedside, reducing turnaround times and facilitating prompt decision-making. These devices cover a wide range of parameters, including blood gases, electrolytes, coagulation factors, and biomarkers, allowing clinicians to monitor patient status more closely and adjust treatment accordingly.
These innovations represent a glimpse into the future of critical care, where technology continues to play a vital role in improving patient outcomes, enhancing workflow efficiency, and advancing medical knowledge.
0 notes
Text
Research Breakthroughs in PT INR Medication: Future Prospects
In the realm of healthcare, breakthroughs and advancements continually shape the landscape, offering hope and progress for patients and practitioners alike. One area witnessing notable strides is the management of PT INR medication, particularly in anticoagulation therapy. Let's delve into the latest research breakthroughs and their future prospects in this critical domain.
Understanding PT INR Medication
Before delving into recent advancements, it's crucial to grasp the significance of PT INR medication. Prothrombin Time (PT) and International Normalized Ratio (INR) are vital metrics in assessing blood coagulation levels. Patients prescribed with anticoagulants, such as Warfarin, rely on regular PT INR tests to ensure their medication is effectively managing their condition, preventing blood clots without inducing excessive bleeding.
Recent Research Breakthroughs
. Novel Drug Formulations: Researchers are exploring innovative drug formulations to enhance the efficacy and safety of PT INR medication. These formulations aim to achieve more stable INR levels, reducing the need for frequent dosage adjustments and mitigating the risk of complications.
. Targeted Therapies: Advancements in personalized medicine have paved the way for targeted anticoagulation therapies. By analyzing genetic factors and individual patient characteristics, clinicians can tailor treatment regimens to optimize outcomes while minimizing adverse reactions.
. Point-of-Care Testing: The emergence of point-of-care testing devices has revolutionized PT INR monitoring. Patients now have access to portable, user-friendly devices that enable convenient self-testing at home, empowering them to take a proactive role in managing their anticoagulation therapy.
. Artificial Intelligence (AI) Integration: AI-powered algorithms are being integrated into PT INR management systems to provide real-time insights and predictive analytics. These systems can identify patterns, predict potential INR fluctuations, and offer personalized dosage recommendations, enhancing treatment precision and patient safety.
Future Prospects
The future of PT INR medication holds promising prospects fueled by ongoing research and technological innovation. Here are some anticipated developments:
. Precision Medicine: With a deeper understanding of genetic variations and biomarkers, the field is moving towards precision medicine approaches tailored to individual patient profiles. This personalized approach aims to optimize therapeutic outcomes while minimizing adverse effects.
. Telehealth Integration: The integration of telehealth platforms into PT INR management enables remote monitoring and virtual consultations, enhancing accessibility and continuity of care for patients, especially those in rural or underserved areas.
. Continuous Monitoring Solutions: Continuous monitoring technologies offer real-time INR measurements, allowing for proactive intervention in response to fluctuations. These solutions provide clinicians with comprehensive data insights to optimize anticoagulation therapy and prevent adverse events.
. Patient Empowerment: Empowering patients through education, self-testing capabilities, and digital health tools fosters active participation in their care journey. By promoting self-management and adherence to treatment plans, patient outcomes can be significantly improved.
As research continues to drive innovation in PT INR medication, the future holds immense potential for improving patient outcomes and enhancing the quality of care. PatientSelfTesting is at the forefront of this evolution, pioneering advancements in anticoagulation management to ensure safer, more effective treatment strategies. Stay informed, stay empowered, and embrace the possibilities of tomorrow with PatientSelfTesting and the evolving landscape of PT INR medication.
0 notes
Text
Meticulous Research® Unveils Comprehensive Analysis of the Africa IVD Market Forecasting Growth to $1.65 Billion by 2031
Meticulous Research®, a premier global market research firm, has released its latest report titled, “Africa IVD Market Size, Share, Forecast & Trends Analysis by Offering, Technology (Immunoassay, PoC, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoCT), End User - Forecast to 2031.”
The report projects that the Africa in vitro diagnostics (IVD) market will reach $1.65 billion by 2031, growing at a CAGR of 3.1% from 2024 to 2031. This growth is driven by several factors, including:
Increasing prevalence of chronic and infectious diseases.
Significant investments from IVD market players in Africa.
Rising demand for Point-of-Care (POC) and rapid diagnostic solutions.
Growing geriatric population.
Government initiatives enhancing healthcare infrastructure.
Increased healthcare expenditure and R&D investments.
Download Sample Report Here : https://www.meticulousresearch.com/download-sample-report/cp_id=5415
Despite these opportunities, the market faces challenges such as high costs associated with advanced IVD products, variability in rapid test results, and stringent regulatory requirements for high and moderate-complexity tests.
Emerging Opportunities
The report highlights the growing awareness of early diagnosis, advancements in genomics and proteomics, and the rising adoption of personalized medicine as potential growth drivers for market stakeholders. However, uneven healthcare access and a shortage of trained professionals remain significant hurdles.
Check complete table of contents with list of table and figures: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Key Players in the Africa IVD Market
Leading companies in the Africa IVD market include:
Abbott Laboratories (U.S.)
Becton, Dickinson and Company (U.S.)
bioMérieux SA (France)
Danaher Corporation (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
QIAGEN N.V. (Netherlands)
Siemens Healthineers AG (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
Illumina, Inc. (U.S.)
Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China)
Market Insights and Future Outlook
The report segments the Africa IVD market by offering (reagents & kits, instruments, and software & services), technology (including immunoassay, molecular diagnostics, and others), application (focusing on infectious diseases, diabetes, oncology, etc.), diagnostic approach (laboratory testing, point-of-care testing, and OTC/self-testing), and end user (diagnostic laboratories, hospitals, home healthcare, etc.).
Reagents & Kits Segment: Expected to register the highest CAGR of 3.3% during the forecast period, driven by the increasing incidence of infectious diseases and rising test volumes.
Immunoassay Technologies: Anticipated to dominate with a 34.5% market share in 2024, favored for their efficiency and accuracy in diagnosing prevalent diseases like HIV and malaria.
Infectious Diseases Application: Projected to hold the largest market share due to the high prevalence of diseases such as COVID-19 and malaria.
Quick Buy : https://www.meticulousresearch.com/Checkout/47708335
Geographical Insights
The report provides an extensive analysis of key markets, including South Africa, Nigeria, Egypt, and more. Notably, South Africa is expected to exhibit the highest growth rate of 8.3% during the forecast period, fueled by increasing healthcare investments and improved access to diagnostic services.
Key Questions Addressed in the Report
What is the current revenue generated by IVD products in Africa?
What growth rate is expected for IVD product demand over the next 5-7 years?
What are the major factors impacting the Africa IVD market?
Which segments are experiencing significant traction?
What are the key geographical trends and opportunities?
For a deeper dive into these insights and more, access the full report : https://www.meticulousresearch.com/request-sample-report/cp_id=5415
Contact Information
For more details, please contact:
Meticulous Research® Email: [email protected] Sales Contact: +1-646-781-8004 Connect with us on LinkedIn.
0 notes
Text
In Vitro Diagnostics Market To Reach $101.58 Billion By 2030
The global in vitro diagnostics market size is expected to reach USD 101.58 billion by 2030, according to a new report by Grand View Research, Inc. It is estimated to register a CAGR of 4.4% over the forecast period driven by the increasing geriatric population, COVID-19 pandemic, and technological advancements in diagnostics that are supporting its adoption. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be one of the high-impact rendering drivers. Technological advancements were further accelerated by the launch of COVID-19 IVD diagnostics and enhanced the adoption of instruments and consumables for technologies, such as PCR. Competitors in the market are increasingly adopting agreement and partnership strategies to maintain a constant flow of business for manufacturers & diagnostics for users.
These agreements are also a result of the harsh price containment strategies for government laboratories, which lowers the price in government settings. For instance, in April 2021, the Italian subsidiary of Seegene, Inc. received a USD 108.25 million tenders for public procurement for the supply of extraction reagents, as well as 7.15 million SARS-CoV-2 diagnostic tests. However, it increases the multiparty nature and complexity of the supply chain. The high prevalence of cancer and Cardiovascular Diseases (CVDs) globally is anticipated to drive diagnostic innovation to facilitate early diagnosis and meet the constantly evolving needs of consumers. Novel technologies, such as plasmonic PCR, are anticipated to commercially enter the market during the forecast period, influencing the business of existing products adversely.
Request a free sample copy or view the report summary: In Vitro Diagnostics Market Report
In Vitro Diagnostics Market Report Highlights
Molecular diagnostics is anticipated to grow at the fastest CAGR from 2024 to 2030 owing to the rising adoption and usage rate
Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
North America dominated the global market in 2023 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics (IVD) market report based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Revenue, USD Million, 2018 - 2030)
Instruments
Reagents
Services
IVD Technology Outlook (Revenue, USD Million, 2018 - 2030)
Immunoassay
Instruments
Reagents
Services
Hematology
Instruments
Reagents
Services
Clinical Chemistry
Instruments
Reagents
Services
Molecular Diagnostics
Instruments
Reagents
Services
Coagulation
Instruments
Reagents
Services
Microbiology
Instruments
Reagents
Services
Others
Instruments
Reagents
Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
Infectious Diseases
Diabetes
Oncology
Cardiology
Nephrology
Autoimmune Diseases
Drug Testing
Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
Point of Care
Home-care
Others
IVD End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Laboratory
Home-care
Others
IVD Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Spain
Italy
Russia
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Singapore
Latin America
Brazil
Mexico
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of In Vitro Diagnostics (IVD) Market
Abbott
bioMérieux SA
QuidelOrtho Corporation
Siemens Healthineers AG
Bio-Rad Laboratories, Inc.
Qiagen
Sysmex Corporation
Charles River Laboratories
Quest Diagnostics Incorporated
Agilent Technologies, Inc.
Danaher Corporation
BD
F. Hoffmann-La Roche Ltd.
0 notes
Text
Unveiling the Dynamics of the Global Hematology Testing Market

Introduction:
The field of hematology plays a crucial role in understanding and diagnosing various blood-related disorders. Hematology testing has evolved significantly over the years, with technological advancements paving the way for more accurate and efficient diagnostic methods. In this blog, we will delve into the dynamics of the Global Hematology Testing Market, exploring the key trends, challenges, and opportunities that define this rapidly growing sector.
Market Overview:
The Global Hematology Testing Market is witnessing unprecedented growth, driven by factors such as the rising prevalence of blood disorders, increasing geriatric population, and the continuous quest for innovative diagnostic solutions. Hematology testing encompasses a broad spectrum of tests, ranging from complete blood count (CBC) to more specialized tests like coagulation testing and blood typing.
Key Market Trends:
Automation and Integration: The demand for automated hematology analyzers is on the rise as laboratories seek to enhance efficiency and reduce turnaround times. Integrated systems that combine multiple testing functions are becoming increasingly popular, allowing for seamless workflow and improved accuracy.
Point-of-Care Testing (POCT): The shift towards decentralized testing is evident in the growing adoption of POCT devices. These compact, user-friendly instruments provide rapid results, enabling healthcare professionals to make quick decisions and initiate timely interventions.
Technological Advancements: The development of advanced technologies, including flow cytometry and molecular diagnostics, is revolutionizing hematology testing. These innovations not only improve the precision of diagnostics but also open doors to a more comprehensive understanding of blood-related disorders.
Experience Exponential Growth: Secure Your Sample Report and Dominate the Global Hematology Testing Market @ https://bisresearch.com/requestsample?id=778&type=download
Challenges in the Market:
Cost Constraints: The initial investment required for state-of-the-art hematology testing equipment can be a deterrent, especially for smaller healthcare facilities. The challenge lies in striking a balance between technological advancement and cost-effectiveness.
Regulatory Compliance: The hematology testing market is subject to stringent regulatory frameworks. Navigating these compliance requirements poses challenges for manufacturers and healthcare providers, necessitating a keen focus on quality control and assurance.
Opportunities on the Horizon:
Emerging Markets: Developing regions present untapped opportunities for growth in the hematology testing market. Increasing awareness, improving healthcare infrastructure, and rising disposable incomes contribute to the expansion of the market in these areas.
Personalized Medicine: The integration of hematology testing into the realm of personalized medicine is an exciting prospect. Tailoring treatment strategies based on individual patient profiles can significantly improve outcomes and redefine the landscape of blood disorder management.
Conclusion:
As the Global Hematology Testing Market continues to evolve, driven by technological advancements and a growing awareness of the importance of early and accurate diagnostics, the future holds immense promise. Overcoming challenges and leveraging emerging opportunities will be crucial for stakeholders in this dynamic and vital sector, ultimately contributing to better patient outcomes and a healthier global population.
#Global Hematology Testing Market#Global Hematology Testing Market Report#Global Hematology Testing Market Industry
0 notes
Text
0 notes
Text
0 notes