#Plaque Capture Devices Market
Explore tagged Tumblr posts
Text
Embolic Protection Devices Market – Industry Trends and Forecast to 2029 Graph: Growth, Share, Value, Size, and Insights
"Embolic Protection Devices Market Size And Forecast by 2029
According to Data Bridge Market Research Data Bridge Market Research analyses that the embolic protection devices market to be grow at a CAGR of 8.65% in the forecast period of 2022-2029 and is likely to reach the USD 964.58 million by 2029.
Embolic Protection Devices Market is making waves in the industry with its latest advancements and market-driven strategies. As a leading player, Stroke Prevention Devices Market continues to push boundaries by offering cutting-edge solutions that cater to evolving consumer demands. With a strong focus on innovation, Embolic Protection Devices Market has successfully expanded its global footprint, providing businesses with high-quality services and products. The rapid growth of Vascular Embolic Filters Market is fueled by technological advancements, customer-centric approaches, and strategic partnerships. As Embolic Protection Devices Market strengthens its position, it remains committed to delivering value-driven solutions that enhance market efficiency and growth.
Embolic Capture Device Market's impact on the industry is undeniable, with continuous efforts to enhance product offerings and service quality. By leveraging data-driven insights and advanced technologies, Embolic Protection Devices Market ensures it stays ahead of market trends. The adaptability of Blood Clot Protection Device Market has allowed it to address diverse industry challenges while maintaining a competitive edge. Companies relying on Embolic Protection Devices Market benefit from its expertise, robust infrastructure, and commitment to excellence. With increasing global demand, Embolic Shielding Market is poised for sustained growth, driving innovation and transformation across various sectors.
Our comprehensive Embolic Protection Devices Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-embolic-protection-devices-market
**Segments**
- By Type: Distal Filter Devices, Proximal Occlusion Devices, Distal Occlusion Devices, Dual Filter Devices - By Material: Nitinol, Polymers - By Application: Cardiovascular Diseases, Neurovascular Diseases, Peripheral Diseases - By End User: Hospitals, Ambulatory Surgical Centers, Specialty Clinics
The global embolic protection devices market is segmented based on type, material, application, and end user. In terms of type, the market is categorized into distal filter devices, proximal occlusion devices, distal occlusion devices, and dual filter devices. Distal filter devices are commonly used in cardiovascular procedures to capture debris, while proximal occlusion devices provide protection by blocking blood flow to prevent emboli. The materials used in these devices include nitinol and polymers, each offering unique advantages in terms of flexibility and durability. In terms of application, embolic protection devices are utilized in cardiovascular diseases, neurovascular diseases, and peripheral diseases. Hospitals, ambulatory surgical centers, and specialty clinics are the major end users of these devices, where they are integral in ensuring patient safety during interventional procedures.
**Market Players**
- Boston Scientific Corporation - Medtronic - Abbott - Cordis (A Cardinal Health Company) - Terumo Corporation - Silk Road Medical - Contego Medical - Allium Medical Solutions - AngioDynamics - Cardinal Health - Claret Medical - Metactive Medical - Gore - Emboline, Inc.
Several key players operate in the global embolic protection devices market. Boston Scientific Corporation, Medtronic, Abbott, Cordis (A Cardinal Health Company), and Terumo Corporation are some of the prominent companies leading the market with their innovative product offerings. Other notable players include Silk Road Medical, Contego Medical, Allium Medical Solutions, AngioDynamics, Cardinal Health, Claret Medical, Metactive Medical, Gore, Emboline, Inc., among others. These market players engage in strategic initiatives such as product launches, collaborations, acquisitions, and partnerships to enhance their market presence and cater to the evolving needs of healthcare professionals and patients.
https://www.databridgemarketresearch.com/reports/global-embolic-protection-devices-Market The global embolic protection devices market is experiencing significant growth due to the increasing prevalence of cardiovascular diseases, neurovascular diseases, and peripheral diseases. The demand for these devices is driven by the rising number of interventional procedures being performed worldwide. Technological advancements in materials such as nitinol and polymers have led to the development of more efficient and durable embolic protection devices, thereby improving patient outcomes and reducing the risk of complications during procedures. Hospitals, ambulatory surgical centers, and specialty clinics are investing in these devices to ensure the safety of patients undergoing interventional treatments, further fueling market growth.
Market players in the global embolic protection devices market are focusing on innovation to gain a competitive edge and expand their market share. Companies like Boston Scientific Corporation, Medtronic, and Abbott are investing in research and development to launch advanced products that address the specific needs of healthcare professionals and patients. Collaborations and partnerships with key stakeholders in the healthcare industry are also being leveraged to enhance product offerings and expand market reach. Additionally, mergers and acquisitions are enabling market players to strengthen their presence in key regions and tap into new market segments.
The evolving regulatory landscape and increasing emphasis on patient safety are shaping the growth trajectory of the embolic protection devices market. Stringent regulations governing the approval and commercialization of medical devices are influencing market players to adhere to quality standards and ensure product efficacy. Moreover, healthcare providers are becoming more aware of the benefits of using embolic protection devices in reducing procedural complications and improving patient outcomes. This growing awareness is driving the adoption of these devices across different healthcare settings and creating opportunities for market players to broaden their customer base.
As the global healthcare industry continues to witness technological advancements and innovations, the embolic protection devices market is expected to witness continuous growth. Market players will need to adapt to changing market dynamics, customer preferences, and regulatory requirements to stay ahead in this competitive landscape. Collaborative efforts between industry stakeholders, healthcare professionals, and regulatory bodies will be crucial in driving innovation and ensuring the safe and effective use of embolic protection devices in diverse clinical settings. The future of the embolic protection devices market looks promising, with potential for further expansion and innovation to meet the evolving needs of patients and healthcare providers worldwide.**Segments**
Global Embolic Protection Devices Market, By Product: - Distal Filter Devices - Distal Occlusion Devices - Proximal Occlusion Devices
Material: - Nitinol - Polyurethane
Usage: - Disposable Devices - Re-Usable Devices
Application: - Cardiovascular Diseases - Neurovascular Diseases - Peripheral Vascular Diseases
Indication: - Percutaneous Coronary - Carotid Artery - Saphenous Vein Graft Diseases - Transcather Aortic Valve Replacement - Others
End Users: - Hospital - Ambulatory Surgical Centers - Others
Country: - U.S. - Canada - Mexico - Germany - Italy - U.K. - France - Spain - Netherland - Belgium - Switzerland - Turkey - Russia - Rest of Europe - Japan - China - India - South Korea - Australia - Singapore - Malaysia - Thailand - Indonesia - Philippines - Rest of Asia-Pacific - Brazil - Argentina - Rest of South America - South Africa - Saudi Arabia - UAE - Egypt - Israel - Rest of Middle East and Africa
Industry Trends and Forecast to 2029.
**Market Players**
- Siemens Healthcare GmbH - Hologic, Inc. - Sinduri Biotec. - SEKISUI MEDICAL CO., LTD. - NIPRO - Danaher - F. Hoffman-La Roche Ltd - Abbott - Thermo Fischer Scientific Inc. - Abaxis - Bio-Rad Laboratories Inc. - BD - Enzo Biochem Inc. - Trivitron Healthcare - Shenzhen New Industries Biomedical Engineering Co., Ltd. - Shenzhen Mindray Bio-Medical Electronics Co., Ltd. - Medtronic - Boston Scientific Corporation - Allium Medical Solutions - Cardinal Health - Others
The global embolic protection devices market is witnessing significant growth with the increasing prevalence of cardiovascular, neurovascular, and peripheral diseases worldwide. The market segmentation based on product, material, usage, application, indication, end users, and country provides a comprehensive outlook of the industry landscape. With advancements in materials like nitinol and polyurethane, the development of more efficient and durable embolic protection devices is enhancing patient safety and procedure outcomes. The market players are investing in research and development initiatives focusing on innovation, partnerships, and product launches to cater to the evolving needs of healthcare professionals and patients.
Market players such as Siemens Healthcare GmbH, Medtronic, Boston Scientific Corporation, and Abbott, among others, are driving market growth through strategic collaborations and technological advancements. The emphasis on regulatory compliance and patient safety is influencing market dynamics, urging companies to adhere to rigorous quality standards. Adoption of embolic protection devices is increasing across healthcare settings, driven by the awareness of procedural benefits and improved patient outcomes. As the industry evolves, collaborative efforts between stakeholders will play a pivotal role in driving innovation and ensuring the widespread adoption of these devices globally.
The future of the embolic protection devices market holds promise for further expansion and innovation to meet the evolving demands of patients and healthcare providers worldwide. Market players need to adapt to changing trends, preferences, and regulations to stay competitive in this dynamic landscape. Continuous research, technological upgrades, and collaborations will be key drivers for sustained market growth and enhanced patient care outcomes. The global embolic protection devices market is poised for continued advancement, offering opportunities for market players to capitalize on the growing demand for these essential medical devices.
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Embolic Protection Devices Market : https://www.databridgemarketresearch.com/reports/global-embolic-protection-devices-market/companies
Key Questions Answered by the Global Embolic Protection Devices Market Report:
What is the current state of the Embolic Protection Devices Market, and how has it evolved?
What are the key drivers behind the growth of the Embolic Protection Devices Market?
What challenges and barriers do businesses in the Embolic Protection Devices Market face?
How are technological innovations impacting the Embolic Protection Devices Market?
What emerging trends and opportunities should businesses be aware of in the Embolic Protection Devices Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-monoclonal-antibodies-markethttps://www.databridgemarketresearch.com/reports/global-volumetric-display-markethttps://www.databridgemarketresearch.com/reports/global-hidradenitis-suppurativa-markethttps://www.databridgemarketresearch.com/reports/global-chronic-bronchitis-markethttps://www.databridgemarketresearch.com/reports/global-infrared-lamps-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1001
✉ Email: [email protected]"
#Stroke Prevention Devices Market#Vascular Embolic Filters Market#Embolic Capture Device Market#Blood Clot Protection Device Market#Embolic Shielding Market#Thrombosis Prevention Device Market#Plaque Capture Devices Market#Distal Protection Devices Market#Endovascular Embolic Protection Market#Interventional Cardiology Protection Device Market
0 notes
Text
Electric Toothbrush Market Competition: How Key Players Innovate to Secure Leadership Positions Globally
The electric toothbrush market is thriving, driven by an increasing focus on oral hygiene and advancements in technology. As consumers seek effective solutions to improve their dental health, competition among electric toothbrush manufacturers has intensified. Market players are focusing on differentiation strategies and leveraging innovations to gain a competitive edge in this rapidly growing sector. This blog explores the competitive landscape, key players, product differentiation, and emerging trends shaping the future of the electric toothbrush market.

Key Players in the Electric Toothbrush Market
The electric toothbrush market features a mix of global giants, regional players, and niche innovators. Prominent companies include Oral-B (Procter & Gamble), Philips Sonicare, Colgate-Palmolive, and Panasonic. These leading brands invest significantly in R&D and marketing to maintain their dominance. Additionally, smaller brands like Burst Oral Care and Goby focus on direct-to-consumer models, offering affordability and subscription services that attract younger demographics.
Factors Driving Competition
Technological Advancements: Smart toothbrushes equipped with AI, Bluetooth connectivity, and real-time feedback are setting new industry standards. These features cater to tech-savvy consumers, creating a competitive battleground for innovation.
Product Differentiation: Brands are tailoring their offerings based on design, battery life, and specialized brush heads. For example, sensitive teeth and gum health-specific variants are capturing niche markets.
Pricing Strategies: Price remains a crucial factor. While premium brands dominate the high-end market, affordable alternatives attract budget-conscious consumers, creating a price-versus-value competition.
Sustainability: Growing consumer demand for eco-friendly products has prompted brands to introduce biodegradable or recyclable brush heads. Sustainability initiatives not only meet environmental concerns but also foster brand loyalty.
Marketing and Endorsements: Partnerships with dental professionals and engaging advertising campaigns strengthen brand trust. Companies also use social media influencers to target specific consumer segments effectively.
Emerging Trends in the Electric Toothbrush Market
Personalization: Customization features such as intensity settings and personalized brushing recommendations are gaining traction. AI-driven analysis of brushing habits allows tailored dental care suggestions.
Subscription Services: Subscription-based models for replacement brush heads and other accessories have become a strong trend, improving consumer convenience and fostering recurring revenue.
Regional Expansion: While North America and Europe dominate the market, significant growth opportunities exist in Asia-Pacific and Latin America due to rising disposable incomes and awareness of oral hygiene.
Health Integration: Electric toothbrushes integrating health metrics, such as plaque removal efficiency or gum health scores, position themselves as essential health devices rather than just oral hygiene tools.
Challenges and Opportunities
While the market offers immense growth opportunities, it also presents challenges. Rising competition compresses profit margins, while stringent regulatory requirements and certification processes increase operational costs. However, brands that invest in innovation, sustainability, and effective consumer engagement are likely to secure substantial growth.
Conclusion
The electric toothbrush market is marked by fierce competition, where success depends on constant innovation, strategic pricing, and meeting evolving consumer preferences. As health awareness continues to grow worldwide, the market’s potential remains vast. Companies that adapt to trends, harness technology, and focus on consumer satisfaction will lead the way in this dynamic and promising industry.
0 notes
Text
Dental Equipment Market to Witness a Robust Growth in the Upcoming Years | Major players: Danaher, Dentsply Sirona, Institut Straumann AG
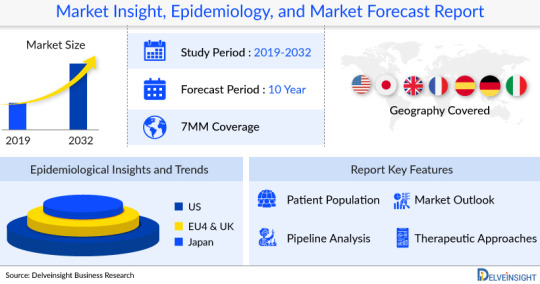
DelveInsight's "Dental Equipment Market Insight & Forecast 2030" report will offer an in-depth understanding of the Dental Equipment market, further benefiting the competitors or stakeholders operating in the Dental Equipment arena.
Read more about Dental Equipment Market Report Offerings @
The Dental Equipment market report provides an overview of Dental Equipment, applications of Dental Equipment as well as its advantages and limitations. Additionally, the report provides insight on the Dental Equipment market share by segments, along with an assessment of market share by regions, the qualitative and quantitative data will be provided for all the segments in the scope.
The dental equipment market report also covers the market drivers, market challenges and restraints, and opportunities, along with the impact COVID-19 has had on this market. Assessment of Key 12-15 Players operating in the Dental Equipment market will be covered in-depth in the report comprising company overview, financial overview, product overview and company share analysis of key 3-5 players.
Find a sample copy of the Dental Equipment Market report @ https://www.delveinsight.com/sample-request/dental-equipment-market
Dental Equipment Overview
Dental equipment comprises various devices and instruments used by dental professionals to diagnose, treat, and prevent oral health issues. These tools are essential for maintaining oral hygiene, diagnosing dental conditions, and performing various dental procedures.
Dental Equipment Uses:
Diagnosis: Dental equipment is used for diagnosing various oral health conditions, including cavities, gum disease, and oral cancer.
Treatment: Dental instruments are employed to treat dental problems such as tooth decay, gum disease, and dental trauma. They facilitate procedures like fillings, root canals, extractions, and dental implants.
Preventive Care: Dental equipment is utilized for preventive measures such as dental cleanings, fluoride treatments, sealants, and oral hygiene education to maintain optimal oral health.
Cosmetic Dentistry: Some dental equipment is used for cosmetic procedures like teeth whitening, veneers, and orthodontic treatments to enhance the appearance of the teeth and smile.
Dental Equipment Types:
Diagnostic Equipment:
X-ray Machines: Used for capturing images of the teeth, jaw, and surrounding structures to diagnose dental issues.
Intraoral Cameras: Small cameras used to capture images of the inside of the mouth for diagnosis and patient education.
Treatment Equipment:
Dental Chairs: Adjustable chairs where patients sit during dental procedures, equipped with various controls and attachments for dental instruments.
Handpieces: Dental drills and rotary instruments used for removing decayed tooth structure, shaping teeth, and preparing them for restorations.
Ultrasonic Scalers: Devices that use high-frequency vibrations to remove tartar and plaque from teeth during dental cleanings.
Sterilization and Infection Control Equipment:
Autoclaves: Machines used for sterilizing dental instruments and equipment by subjecting them to high-pressure steam.
Disinfectants and Sterilizing Solutions: Chemical agents used to disinfect surfaces, dental instruments, and impression materials.
Auxiliary Equipment:
Dental Loupes: Magnifying glasses worn by dental professionals to enhance visibility during procedures.
Dental Lights: Illumination devices mounted on dental chairs or overhead to provide adequate lighting during dental procedures.
Dental Equipment Working:
The working principle of dental equipment varies depending on the type and purpose of the device. However, most dental equipment operates based on established dental procedures and techniques. For example:
X-ray machines emit radiation to produce images of the teeth and jaws.
Dental handpieces use rotary motion to remove decayed tooth structure or shape teeth.
Ultrasonic scalers generate high-frequency vibrations to remove tartar and plaque from teeth.
Autoclaves use steam under high pressure to sterilize dental instruments and equipment.
Intraoral cameras capture digital images of the inside of the mouth for diagnostic purposes.
Overall, dental equipment plays a crucial role in enabling dental professionals to provide comprehensive oral health care and treatment to patients.
Read more about the operations & working of Dental Equipment @ https://www.delveinsight.com/sample-request/dental-equipment-market
Dental Equipment Market Analysis
The dental equipment market segment encompasses a comprehensive overview of the global dental equipment market, including detailed market segmentation. Additionally, the dental equipment market report offers quantitative data at a regional level, providing cross-segmentation insights. Moreover, the report assesses the anticipated market growth throughout the study period from 2019 to 2027, projecting a significant compound annual growth rate (CAGR).
To know more about the Dental Equipment market growth, get a snapshot of the report: https://www.delveinsight.com/report-store/dental-equipment-market
Table of Contents
1. Dental Equipment Market Report Introduction
2. Dental Equipment Market Executive Summary
3. Regulatory and Patent Analysis
4. Dental Equipment Market Key Factors Analysis
5. Dental Equipment Market Porter’s Five Forces Analysis
6. COVID-19 Impact Analysis on Dental Equipment Market
7. Dental Equipment Market Layout
8. Dental Equipment Market Global Company Share Analysis – Key 3-5 Companies
9. Dental Equipment Market Company and Product Profiles
10. KOL Views
11. Project Approach
12. About DelveInsight
13. Disclaimer & Contact Us
Request the TOC of the Dental Equipment Market report here https://www.delveinsight.com/sample-request/dental-equipment-market
Trending Reports by DelveInsight:
Adalimumab Biosimilar Market | Arbovirus Infection Market | Artificial Pancreas Device System Market | Dental Equipment Market | Gluten Sensitivity Market | Hypothyroidism Market | Inflammatory Bowel Disease Market | Mayus Kinase Jak Inhibitors Market | Mild Dry Eye Market | Mucopolysaccharidosis Market | Oncolytic Virus Cancer Therapy Market | Pyoderma Gangrenosum Market | Transdermal Drug Delivery Devices Market | Intrathecal Pumps Market | Hedgehog Pathway Inhibitors Market | Yellow Fever Market | Laryngeal Cancer Market | Female Infertility Market | Gender Dysphoria Market | Chronic Brain Damage Market | Spain Healthcare Outlook Market | Malignant Fibrous Histiocytoma Market | Asthma Diagnostic Devices Market | Chronic Obstructive Pulmonary Disease Treatment Devices Market | Airway Management Devices Market | Cough Assist Devices Market | Pulse Oximeters Market | Hemodialysis Catheter Devices Market | Chronic Spontaneous Urticaria Market | Gender Dysphoria Market | Germany Healthcare Outlook | Biopsy Devices Pipeline Insight | Bacterial Conjunctivitis Market | Infliximab Biosimilar Insight | Eosinophilic Asthma Market | Cushing Syndrome Market | Functional Dyspepsia Market | Peripherally Inserted Central Catheters (PICC) Devices Market
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
Contact Us:
Kritika Rehani
+91-9650213330
www.delveinsight.com
0 notes
Text
Embolic Protection Devices Market: Technologies, Innovations, Dynamics, Growth Prospects and Industry Forecast 2023-2032
In the intricate landscape of cardiovascular health, where the flow of blood is the lifeline, the Embolic Protection Devices market emerges as a stalwart defender against potential threats. This article delves into the global trends shaping this market, exploring the innovations and strategies that contribute to the safeguarding of vascular health and the prevention of embolic events.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐑𝐞𝐩𝐨𝐫𝐭 : https://www.alliedmarketresearch.com/request-toc-and-sample/11497
Importance of Embolic Protection Devices Post-Surgery:
An embolism, often arising as a blood clot, fat globule, air bubble, or foreign material, poses a significant risk when it lodges inside blood vessels, potentially causing blockages. Post-surgery, the risk of embolism increases due to potential damage to vessel walls, leading to clot formation. To mitigate this risk, the application of embolic protection devices becomes crucial, as they aid in capturing and retrieving plaques, debris, and other friable constituents released during interventional procedures.
Preserving Vascular Health: Embolic Protection Devices as Safeguards:
The significance of embolic protection devices lies in their ability to remove lipid-rich plaques and debris before they can lodge in the distal microcirculation. This not only prevents potential blockages but also plays a vital role in preserving vascular health post-surgery. By allowing for the capture and retrieval of embolic materials, these devices contribute to the overall safety and efficacy of interventional procedures, highlighting their importance in maintaining vascular integrity and preventing adverse outcomes.
Understanding the Significance of Embolic Protection:
Embolic events, characterized by the release of emboli (clots or debris) in blood vessels, pose a significant risk to vascular health. These events can lead to serious complications, including stroke, heart attack, or peripheral vascular diseases. The Embolic Protection Devices market plays a crucial role in preventing such occurrences by providing specialized tools and solutions designed to trap or filter embolic debris during various cardiovascular procedures.
𝐏𝐫𝐞-𝐛𝐨𝐨𝐤 𝐭𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭 𝐍𝐨𝐰 : https://www.alliedmarketresearch.com/embolic-protection-devices-market/purchase-options
Technological Advancements:
The market is witnessing a surge in technological innovations aimed at enhancing the efficacy of embolic protection devices. Advanced materials, intricate designs, and improved deployment mechanisms are driving the development of devices that offer higher levels of protection with minimal interference in the natural flow of blood.
Expanding Applications:
Originally associated with carotid artery stenting and coronary interventions, embolic protection devices are finding new applications across a spectrum of vascular procedures. From peripheral interventions to transcatheter aortic valve replacement (TAVR), the versatility of these devices is expanding, contributing to a broader impact on vascular health.
Integration with Interventional Procedures:
The seamless integration of embolic protection devices with various interventional procedures is a notable trend. As physicians strive for comprehensive vascular care, these devices become integral components of procedures such as angioplasty and stent placement, ensuring that embolic events are proactively addressed during interventions.
Rising Cardiovascular Disease Burden:
The increasing prevalence of cardiovascular diseases globally has elevated the demand for effective embolic protection. As the burden of conditions such as atherosclerosis grows, so does the need for advanced technologies that can mitigate the risks associated with embolic events.
Emphasis on Minimally Invasive Techniques:
The shift towards minimally invasive techniques in cardiovascular interventions is influencing the adoption of embolic protection devices. Patients and healthcare providers alike are embracing procedures that offer effective protection without the need for extensive surgical interventions, driving the market's growth.
𝐈𝐧𝐭𝐞𝐫𝐞𝐬𝐭𝐞𝐝 𝐭𝐨 𝐏𝐫𝐨𝐜𝐮𝐫𝐞 𝐭𝐡𝐞 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐑𝐞𝐩𝐨𝐫𝐭? 𝐈𝐧𝐪𝐮𝐢𝐫𝐞 𝐁𝐞𝐟𝐨𝐫𝐞 𝐁𝐮𝐲𝐢𝐧𝐠 : https://www.alliedmarketresearch.com/purchase-enquiry/11497
Future Trajectories:
The future of the Embolic Protection Devices market holds promising avenues for growth. As technology continues to advance, and the understanding of vascular health deepens, these devices are likely to become even more sophisticated and tailored to specific patient needs. The integration of artificial intelligence and data analytics may further enhance the precision and predictive capabilities of embolic protection systems.
Conclusion:
The Embolic Protection Devices market stands at the forefront of cardiovascular care, offering a shield against the silent threats that lurk within blood vessels. As global trends evolve, the commitment to safeguarding vascular health remains unwavering, ensuring that these devices play a pivotal role in the ongoing quest for cardiovascular well-being on a global scale.
About Us
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
Pawan Kumar, the CEO of Allied Market Research, is leading the organization toward providing high-quality data and insights. We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
0 notes
Text
Intravascular Ultrasound IVUS Devices Market Analysis with Size, Revenue, Growth Drivers and Forecast to 2030
The latest market report published by Credence Research, Inc. “Global Intravascular Ultrasound IVUS Devices Market: Growth, Future Prospects, and Competitive Analysis, 2022 – 2030. The global demand for Intravascular ultrasound IVUS devices was valued at USD 0.9 Billion in 2022 and is expected to reach USD 1.47 Billion in 2030, growing at a CAGR of 7.30% between 2023 and 2030.
The intersection of precision, diagnostic accuracy, and patient care, IVUS devices are making significant strides in improving the way medical professionals assess and treat cardiovascular diseases. In this comprehensive article, we delve into the intricacies of IVUS technology, its applications, benefits, and the transformative impact it is having on the healthcare industry.
Understanding Intravascular Ultrasound (IVUS) Devices-
IVUS Devices
IVUS devices, short for Intravascular Ultrasound devices, are cutting-edge medical tools used to visualize the interior of blood vessels with remarkable precision. These devices employ high-frequency sound waves to create detailed cross-sectional images of blood vessels from the inside. Unlike conventional imaging methods, such as X-rays or angiography, IVUS provides clinicians with a unique, real-time view of arterial walls, plaque buildup, and other structural details, enabling more accurate diagnoses and treatment decisions.
The Technology Behind IVUS
At the heart of IVUS technology is a tiny ultrasound transducer mounted on the tip of a catheter. This catheter is carefully guided into the blood vessel of interest, usually through an artery in the wrist or groin. Once inside, the transducer emits sound waves that bounce off the vessel walls and are then captured, processed, and converted into high-resolution images by a computer. The result is a live, 360-degree view of the vessel's interior, allowing physicians to assess the extent of blockages, vessel diameter, and the composition of arterial plaque.
Browse 245 pages report Intravascular Ultrasound IVUS Devices Market By Modality (Virtual Histology IVUS, iMap IVUS, Integrated Backscatter IVUS) By Product (Consoles, Accessories, Catheters, Guidewires, Others) By End-use (Hospitals, Diagnostic Imaging Centers, Academic & Research Institutes) - Growth, Future Prospects & Competitive Analysis, 2016 – 2030) https://www.credenceresearch.com/report/intravascular-ultrasound-ivus-device-market
Applications of IVUS Devices
Cardiovascular Disease Diagnosis
IVUS devices have become an invaluable tool in diagnosing and managing cardiovascular diseases. By providing unparalleled clarity of the arterial structures, IVUS enables healthcare professionals to detect early signs of atherosclerosis, assess stent placement, and determine the most suitable treatment strategies. This level of precision has significantly improved patient outcomes and reduced the risk of complications during cardiac interventions.
Guiding Interventional Procedures
In addition to diagnostics, IVUS plays a pivotal role in guiding interventional procedures. Whether it's angioplasty, stent placement, or atherectomy, IVUS provides real-time feedback to interventional cardiologists, ensuring optimal device positioning and deployment. This not only enhances the success rates of these procedures but also reduces the risk of complications, ultimately leading to faster patient recovery.
Advantages of IVUS Technology
Unprecedented Clarity
The clarity and detail offered by IVUS images are unmatched by any other imaging modality. This level of precision empowers healthcare providers to make informed decisions, leading to better patient outcomes and a higher standard of care.
Minimized Radiation Exposure
Unlike X-rays and CT scans, IVUS relies on ultrasound waves, eliminating the need for ionizing radiation. This reduces the risk to both patients and healthcare providers and makes IVUS a safer option, especially for those who require repeated imaging.
Enhanced Research Capabilities
IVUS devices have opened new avenues for medical research. Researchers can use IVUS to study disease progression, evaluate the effectiveness of novel therapies, and gain insights into the complex world of vascular biology.
The Future of IVUS Technology
As technology continues to advance, so does the potential of IVUS devices. Future developments may include even higher image resolution, improved catheter design for enhanced maneuverability, and integration with artificial intelligence for automated analysis of IVUS images. These advancements will further solidify IVUS as a cornerstone of modern medical practice.
Conclusion
Intravascular Ultrasound (IVUS) devices have revolutionized the way we diagnose and treat cardiovascular diseases. Their ability to provide unparalleled clarity and precision has elevated the standard of care in the medical field. As we look to the future, it's clear that IVUS technology will continue to play a pivotal role in improving patient outcomes, guiding interventions, and advancing medical research. Embracing IVUS is not just a step forward; it's a leap towards a healthier future.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global Intravascular Ultrasound IVUS Devices Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global Intravascular Ultrasound IVUS Devices Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major player analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/intravascular-ultrasound-ivus-device-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/microfluidic-modulation-spectroscopy-market
Related Report: https://www.credenceresearch.com/report/mesoporous-silica-nanoparticles-drugs-market
For More Information Browse Our Blog: https://www.linkedin.com/pulse/country-level-analysis-intravascular-ultrasound-ivus-device-dean/
Browse Our Blog: https://www.linkedin.com/pulse/intravascular-ultrasound-ivus-devices-market-analysis-priyanshi-singh
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Reverse Gum Recession Without Surgery
Your gums are made of powerful pink tissue. The gums are actually also named the gingivae. Puffy gums are actually often a sign that something is wrong which you need to carry out something about it. You may have unbelievably inflamed, distressing gums which are going to probably hemorrhage. Tender gums are actually making it bothersome to eat.
Can You Reverse Gum Recession?
You can easily conquer gum disease whenever you're readied to optimize your dental hygiene as well as produce some adjustments to your diet plan. The innovative assortments of gum disease may certainly not be managed, however could be kept along with the help of dental specialists. Knowing what is actually creating your gum disease is very important to choose the proper cure to deal with the gums. It might be actually avoided by avoiding the development of germs in the mouth. The gum disease is the effect of the many unwanted micro-organisms dwelling in human mouth. Avoiding gum disease is actually very easy.
There are actually a range of therapies readily on call, based on the cruelty of tissue reduction. In the stretch of menopause along with post-menopausal phase, dental treatment is actually truly required to have still. Indeed there are lots of organic therapies and cure you may attempt to turn around receding gums. Read Here Reverse Gum Recession Naturally
Treatment To Reverse Gum Recession
Gum disease treatment is vital, yet it's far better if we may stop it. Definitely there are in reality an assortment of natural treatments and additionally cure it is straightforward to make an initiative to turn around receding gums. They are in fact some of the best famous kind of traditional medication, as well as also are really highly rewarding in the international market. Recently, numerous natural treatments have in fact been created for Receding Gums at the particular same instant. Treatment and also the amount of symptoms are going to contrast, relying on the factor responsible for light gums.
Gums decline for a stable of distinctive aspects. Receding gums may be actually caused by a team of points, in addition to the issue isn't truly usually an outcome of poor dental tidiness. Medical diagnosis Diagnosis Receding gums and various other varieties of gum disease are diagnosed along with a dentist.
Remedies For Reverse Gum Recession
If you truly need to stop receding gums as well as desire to get a manner in which functions If you're frustrated with slow-moving or non-existent remodeling in the wellness of your gums. Receded gums activates space in between cells in addition to tooth beginning. Without a doubt, it's you to decide regarding what you do to your raw gums. Raw gums could be tremendously bothersome. Raw gums are normally reddish and sorewhich makes your smile appear humiliating.
You could make receding gums if you're taking a treatment which impacts your mouth to be drier in evaluation to standard. In the unlikely event you have receding gums that are due to gum disease at that point I honestly suggested that you think about using a training course of Dental Pro 7. Receding gums could be somewhat startling. Despite the fact that they will certainly certainly not improve back, there are actually still several traits you can possibly do to prevent each one of all of them off receding a great deal extra. Whatever is actually the source of your receding gums, it is actually incredibly noticeable that getting rid of principal trouble is actually required for receiving any level of succeeding.
How Do You Reverse Gum Recession?
Gums are brought in to guard your teeth. Receding gums could be extremely worrying. If you understand the stable of people have concerns with receding gums then it's opportunity to take some precautions. Receding Gums can be come by hindering the development of micro-organisms in the mouth. Record low pipe Receding gums reside in truth a regular affliction.
As your teeth are actually uncovered as well as found progressively even more, whenever your teeth roots are beginning to show as well as when your leader gum tissues are actually pulling back, you could be in for what is frequently called receding gums. Then, they will definitely certainly not just be very vulnerable however will certainly also be at risk of creating a Dental caries. In the contemporary society, brightening your teeth is actually a very preferred cosmetic technique that is actually non-invasive and also produces a wonderful white colored smile that is actually pretty eye-catching. Laser teeth brightening, which is often described as power brightening, is amongst the absolute most regular approaches to address the problem of blemished teeth.
Any Way To Reverse Gum Recession
If your tooth is actually infected you'll require prescription antibiotics in addition to dental caries treatment. Therefore the advancements in oral wellness innovation, you might not need to accept delicate teeth as a reality of lifestyle. If you've acquired vulnerable teeth, it's tough to enjoy all your dishes since you're aware that even the slightest adjustment in temperature will deliver a considerable amount of pain. Phone Our Office Today If you've obtained sensitive teeth, it may be an indicator of gum recession. Just because you've obtained a delicate tooth doesn't symbolize that you've come to skip the possibility for a whiter, brighter smile. Possessing delicate teeth is a typical concern amongst individuals nowadays.
In the event the gums are actually extremely harmed, the only treatment choice to recover receded gums is the utilization of a soft tissue graft coming from one more area of the mouth. Receding gums may be a repercussion of lots of traits. If you do not find a specialist regarding receding gums, the teeth are going to possess nothing to stay in the mouth along with.

Can I Reverse Gum Recession?
If you've found your gums started to recede, You perhaps are actually experiencing some Periodontal disease. Receding gums is actually an obvious sign of gum disease that lots of individuals may dismiss. There are actually many illustrations for why a guy or girl may possess receding gums.
Gums might start to form pockets under the gumline, capturing food items as well as oral plaque buildup. In the event you possess receding gums, cells grafts can easily assist safeguard your teeth roots and minimize sensitivity. Receding gums are actually an evident evidence of gum disease, which several males and females could disregard. Usually, the very best approach to treat receding gums is actually to accomplish a gum graft.
Can You Reverse Gum Recession Naturally?
There are actually a number of kinds of treatment available, and each dentist has her or even his faves they are actually probably to recommend, depending on to Culotta-Norton. Laser device treatment is the ideal option for lots of folks as it permits you to prevent surgical procedure when going through the efficient treatment you will certainly need. At that point you are actually going to be actually sought to continue treatment to bleach your teeth in the house false teeths well-maintained, check out a house remedy to lighten all of them.
Immediate treatment is actually actually significant, as it not only reduces sensitivity yet has the prospective to help in improving gum tissue health as well as decrease the probability of getting gum disease. Based on the cause for your gum recession, you might need to get surgical therapy. There are a bunch of feasible treatments. Effective dental treatment as well as strengthened property treatment can easily often aid stop more damages.
1 note
·
View note
Text
The novel cardiovascular drug delivery devices market is projected to grow at a CAGR of 9.3%, till 2035
Given the growing number of patients suffering from cardiovascular disorders and the high associated financial burden, cardiovascular drug eluting stents have emerged as a safe and efficient novel drug delivery device to assist in providing effectual treatment.
Roots Analysis has announced the addition of “Novel Cardiovascular Drug Delivery Devices Market, 2022-2035” report to its list of offerings.
In order to prevent the critical effects of vessel narrowing and plaque buildup in arteries, various coronary interventional devices are being used; cardiovascular stents have emerged as one of the most preferred solutions to mitigate the aforementioned concerns. Traditionally, bare-metal stents were used extensively to open the blocked passages and restore adequate blood flow to the heart. However, in recent years, a shift in preference has been observed towards the use of drug-eluting stents, which are coated with a polymer carrying an antiproliferative drug.
Key Market Insights
Over 90 cardiovascular drug eluting stents are presently available in this market space
About 65% of these devices are coated with sirolimus drug, followed by those coated with everolimus and paclitaxel. Further, more than 70% of the stents are manufactured by using cobalt alloy. It is interesting to mention that stent length of more than 85% of the devices is in the range of 31 mm to 50 mm.
~40 players are currently be engaged in the production of cardiovascular drug eluting stents
Majority of the players engaged in this domain are large firms (501-10,000 employees, 41%), followed by mid-sized players (51-500 employees, 26%), very large players (10,000+ employees, 18%) and small (2-50 employees, 15%) companies. Additionally, most of the service providers (38%) are based in Asia, followed by those having headquarters in Europe and North America.
Over 70 novel therapeutics are being developed to target a wide range of cardiovascular diseases
Majority (50%) of the therapies targeting cardiovascular disorder are currently in early stages of development (discovery and preclinical). It is also worth mentioning that over 65% of the drug candidates targeting cardiovascular diseases are gene therapies.
More than 50 players are engaged in offering novel cardiovascular-based services / solutions
Over 60% of the players offering such services / solutions are based in North America. It is interesting to mention that this domain is dominated (more than 40%) by the players offering digital therapeutics services.
Till date, 900+ patents have been filed related to novel cardiovascular drug delivery devices
In terms of the intellectual property developed across the world, the R&D activity is currently concentrated in North America and Europe. Further, most patented inventions are focused on the development of bioresorbable and biodegradable drug eluting stents.
Global demand for novel cardiovascular drug delivery devices is anticipated to grow at a CAGR of over 13%, during 2022-2035
In 2035, sirolimus eluting stents are expected to contribute over 50% of the total demand for novel cardiovascular drug delivery devices, majority (~70%) of such devices are manufactured using cobalt chromium as a stent material.
Players based in North America are anticipated to capture over 80% of the market share by 2035
The overall market of novel cardiovascular drug delivery devices is expected to be primarily driven by the rising demand for therapeutic interventions and recent technology advancements in the field. The estimates in our report suggest that stents manufactured using cobalt chromium are expected to hold more than 70% of the market share in 2035. It is interesting to mention that the market for stents manufactured using platinum chromium are expected to grow at a relatively faster pace (11.5%), during the forecast period.
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/novel-cardiovascular-drug-delivery-devices-market/request-sample.html
Key Questions Answered
§ Who are the leading players engaged in offering cardiovascular drug eluting stents?
§ Which type of drug eluting stent is most commonly offered by developers engaged in this market space?
§ What is the relative competitiveness of players engaged in the development / manufacturing of cardiovascular drug eluting stents?
§ How has the patent landscape in this domain evolved over the last two decades?
§ What is the present and likely future demand for cardiovascular drug eluting stents in the overall healthcare sector?
§ What are the anticipated future trends related to cardiovascular drug eluting stents market?
§ How is the current and future opportunity likely to be distributed across key market segments?
The financial opportunity within the novel cardiovascular drug delivery devices market has been analyzed across the following segments:
§ Type of Drug Eluted
§ Sirolimus
§ Paclitaxel
§ Everolimus
§ Zotarolimus
§ Others
§ Type of Stent Material
§ Cobalt Chromium
§ Platinum Chromium
§ Stainless Steel
§ Others
§ Key Geographical Regions
§ North America
§ Europe
§ Asia
§ Middle East and North Africa
§ Latin America
§ Rest of the World
The research includes profiles of key players (listed below); each profile features a brief overview of the company, details related to its recent developments (including funding and collaborations) and an informed future outlook.
§ Alvimedica
§ B. Braun
§ Boston Scientific
§ Cook Medical
§ Eurocor Scientific
§ Lepu Medical
§ Medtronic
§ Meril
§ Relisys
§ Sahajanand Medical Technologies
For additional details, please visit https://www.rootsanalysis.com/reports/novel-cardiovascular-drug-delivery-devices-market.html or email [email protected]
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
0 notes
Text
Strong as Stone -Part Twenty-Two
WELCOME BACK!
Last week we got a lovely, fluffy, light-hearted vacation/one year anniversary celebration with Okoye and M’Baku.
This week marks the conclusion of the “Klaue’s Associate” plot line! Yes, babes, this is it! Part Four of ‘Cat and Mouse,’ as I’ve been calling it on Ao3, is here!
Rating: T.
Warnings: Language, suspense, mentions of cancer, and mentions of death.
Pairings: Okoye x M’Baku and T’Challa x Nakia.
@the-last-hair-bender, @skysynclair19
See everything you can through to the end. You won’t be able to see everything through --and there will be things that you shouldn’t see through--but what you can, do.
Try to keep your heads out of the clouds though, my dears. We have the tendency to get caught up in our ideas, our fantasies, of how things ought to conclude. We often craft out the perfect ending, or anticipate things down to the most minute detail.
Stay away from it, if you can help it. The results will often disappoint your preconceptions.
It’s been a lot of effort. A lot of patience. A lot of consecutive missions that required rotating the Dora Milaje and War Dogs to keep everyone from growing over tired. A lot of Shuri going in tearing out the associate’s invisible hand in their system.
Which kept growing back. Every two to three days it would register on the security scans, almost full size and intact despite Shuri continually increasing the protective measures in every way she could conceive of.
Okoye had decided, after the third time that Shuri had to tear our the associate’s tap strand by strand of code, that Klaue’s associate was a great deal smarter than he had been.
But, in the end, it paid off. Keeping the associate moving on a near constant basis meant that Okoye’s teams kept recovering more and more materials from the safe houses --and that Shuri had time to track the associate back to the main base of operations.
A two bedroom apartment Central London. Shuri had traced the root of all the associate’s coding back to there.
When Okoye had heard the news, she’d let out a victory cry of relief.
She’d spent idle moments over the past few months imagining it, imagining what it would feel like when it came, and it was finally here.
They’d found the associate and were ready to capture them.
The first thing she did, candidly, was call M’Baku.
He answered on the third ring. “‘Koye? Is everything alright?”
“We found Klaue’s associate! They’re in London. We’re moving in tonight to capture them!”
M’Baku grinned. “Well done, my love. Go kick their ass.”
Okoye smiled and blew him a kiss before she ended the call. She took a deep breath, then pumped her fist as satisfaction flowed through her. It’s all coming to an end.
“We need to assume that Klaue’s associate will be armed and heavily dangerous,” Okoye said as she manipulated a three dimensional holographic display of the apartment building they were heading to. “We’re going in fast. Take out the associate and whatever technology they’re using to hold us back as quickly as possible. We want to minimize the damage caused and our mission time. I don’t think I need to explain why a serious mess would not be in our best interests.”
They were flying over the Atlantic ocean, headed straight for the United Kingdom. T’Challa was already wearing his Black Panther suit --sans mask--and Nakia stood at his side in her usual War Dog gear. Four other Dora Milaje stood at the other side of the table, dressed in their armor. At the end opposite where Okoye stood, Shuri watched the display spin and shift.
The King, albeit reluctantly, had deemed it necessary that his sister accompany them on their mission. As Wakanda’s foremost mind and innovator with all things vibranium, they needed Shuri present to make sure that they collected and dismantled all the weapons properly.
Admittedly, he’d demanded that Shuri make herself a suit of vibranium armor as well, which was a choice Okoye wasn’t complaining about. Shuri was smart, yes, but she was incredibly new to the outside world --to say nothing of missions like these.
“All due respect, General Okoye,” Shuri said with a nervous, pinched expression, “but I doubt we’ll encounter that much resistance. If Klaue’s associate was making weapons, wouldn’t we have seen them hit the market by now --or seen a greater demonstration of their capabilities than the detonators they’ve used at various warehouses?”
“You could be right,” Okoye said, using the same calm, non-condescending voice she used with new trainees and recruits. “But it’s wiser to prepare for the worse.”
“We’ve talked about this before, Shuri,” T’Challa said in slightly exasperated tone. “This is not a social call. Klaue’s associate is a danger to Wakanda and the rest of the world; they need to be taken down before they amass too many weapons.”
“Yes, except they aren’t because they haven’t been making weapons,” Shuri retorted. “None of what we recovered matches any weaponry designs I’ve ever seen.”
“You can’t know for certain, Shuri.”
“Actually, yes, I can!”
“We have to assume Klaue’s associate is dangerous for our own safety and the safety of any innocent bystanders,” Nakia interjected before the two siblings could start arguing. “He only worked with certain kinds of people --thugs, ex-convicts, the morally gray or black. We have good reason to assume that the associate is dangerous because all of Klaue’s other friends and associates have been.”
Shuri let out a huff, looked away, and crossed her arms over her chest. “Well, I still think you’re wrong.”
Night had fallen over the city of London by the time they’d found a good place to hover over the roof of the associate’s apartment building.
Once everyone was off, Shuri cued the ship to cloak itself. “What now?”
“We could kick down the door,” Djabi said with a smirk.
“Or just pick the lock,” Nakia fired back as she knelt in front of the door to do just that.
Okoye took the lead as she treaded down the flights of concrete stairs towards the twelfth floor.
“Are you ready, Okoye?” T’Challa asked, right on her heels, in a teasing tone that belied the seriousness of the situation.
“You have no idea, my King.” She checked to make sure the hall just off the stairs was clear, then stepped out of the stairwell.
The hall was lined with doors stained to a dark walnut color. It was eerily silent; not even a shred of noise emanated from behind the closed doors. No footsteps, no music, no conversation, nothing.
Okoye narrowed her eyes as the others stepped into the hall behind her. “I seriously doubt, even though it’s late, that absolutely everyone is already asleep for the night.” She lived in apartment complex, for Bast’s sake; she knew from experience that some people kept odd hours. Having everything be one hundred percent quiet meant something was wrong. Seriously wrong.
“You’re right,” Nakia agreed as she took stock of her surroundings. “It’s not even midnight. People should be returning from evening shifts, getting ready for night shifts, going out to clubs, or just be staying up late. Something’s wrong.”
“Maybe they’re not sleeping,” Djabi suggested with a dark grimace. “Maybe they’re dead.”
T’Challa shook his masked head. “Killing an entire floor of people would be a severe departure from what we’ve seen out of the associate thus far.”
“Or maybe they’ve finally completed whatever they were trying to build,” Aneka offered. “Maybe they’re trying to show us just how powerful they are.”
“I don’t think it’s that,” Shuri interjected, “but I am only picking up one life sign other than ours.”
Shit. “Spears out. Be at the ready for anything.” Okoye walked up the door with the correct number plaque and glanced at T’Challa. “Do we knock or kick it in?”
Before he could answer, the electronic lock on the door made the ‘key accepted’ chirp. There was the snapping sound of the bolt sliding back, then the door swung open to reveal an empty, dimly lit room.
“Well, that’s auspicious,” Djabi said dryly.
“They know we’re here,” Nakia whispered. “They’ve probably been watching us the whole time.”
“We can’t abort the mission now,” T’Challa said as he crept towards the door. “We need to retrieve the stolen vibranium.”
Okoye managed to edge past him, spear in hand, and peered around the edge of the door.
The space was one large room that ran the entire length of the hall. Various desks and tables dotted the room; the desks and tables themselves were littered with machine parts, computer pieces, and a variety of desktop screens, computers, and laptops. A giant display screen interface --like the largest iPad Okoye had ever seen--ran along one wall, showing various calculations, research read outs, and files in almost every language conceivable.
And, in the center of the screen, was a video feed of them crouching by the door.
“We’re on camera,” Okoye muttered as she swept her gaze around the room in search of the recording device. “And I know why there aren’t any other life signs. This is just one giant room. The other doors in the hall are fakes.”
“Any sign of the associate?” Nakia asked.
“Not yet. It looks empty, almost abandoned.”
They all filed in one by one, on full alert and ready to strike whatever flew at them.
Well, except for Shuri, who immediately pulled on a pair of sterile gloves and started rifling through the technology on the tables. “It’s exactly what I suspected...”
“What are we looking at?” T’Challa asked as he surveyed the space.
“Junk. Stripped and harvested for whatever was needed.”
“If it’s junk, then why keep it?” Aneka asked.
“My best guess is that it’d look suspicious to have so much electronic trash coming out of the one building all the time. They probably have a special disposal service remove everything for them to keep a low profile.”
From across the room, in the darkest of the shadows, someone started clapping their hands.
Okoye whirled towards the noise, ready to face down whoever was waiting for them.
The lights flicked on, one by one, until they could see who had been clapping.
Okoye gasped. “You!”
An Indian woman with long, wavy black hair smirked back at her. “Me.”
“You know this woman, General?” T’Challa asked.
“She interviewed me at the dinner after President Trump’s non-apology speech,” Okoye spat out. “And she posed as a scientist during Shuri’s lecture on Wakandan science and technology.”
“That’s right!” Shuri exclaimed. “Dr. Khatri!”
“Not your real name, I take it,” T’Challa added.
She shrugged, expression impeccably unruffled. “It can be, if you want it to.”
“I’m not referring to you by a fake alias.”
“All my aliases are fake. But, if you’d like something for this conversation, you can call me Jhanvi Singh. That’s the name I’ve been using most recently.”
“I assume you know why we’re here, Ms. Singh.”
“What, you mean this isn’t a house warming party?”
“Your little game is done,” T’Challa said flatly. “Surrender yourself and whatever weapons you’ve created.”
Jhanvi chuckled as she turned away from them, walking towards an empty desk at the back of the room. “Mm, I think not.”
“That wasn’t a request.”
“No, I’m not saying I won’t.” She spun and hopped up onto the edge of the desk, sitting there. “I’m saying I can’t.”
“You’ve already sold the weapons,” Nakia concluded. “We’re going to need the list of your buyers.”
“I don’t have one,” Jhanvi said as she tapped at her phone.
“Enough of this,” Okoye growled. “She has no weapons with her. There are eight of us versus her.”
“Oh, I didn’t say I didn’t have any.” Jhanvi looked up, hazel eyes glowing a faint shade of copper.
Two panels in the ceiling opened, allowing two massive artillery style guns to drop down and take aim.
Okoye gritted her teeth as the guns deployed --then gasped when her spear retracted into its storage capsule, seemingly of its own volition.
Next to her, T’Challa’s suit shut off, retracting into the necklace Shuri designed and revealing the shirt and slacks he was wearing underneath. He stared levelly at Jhanvi. “That’s why you were so unconcerned over our presence.”
Jhanvi smirked triumphantly. “Well, would you look at that. The deck was stacked in my favor after all.”
“You have mental control over the technology in the room, right?” Shuri asked. “How do you manipulate everything? Is it an implant?”
Jhanvi shook her head. “I’ve always been like this. No one knows why, but you have to make the most of what you have.”
“And you can mentally control any technology?”
“As long as I’ve had enough time to interface with it or the mother technology it stems from.” She paused, blinked, then raised her eyebrows. “Except your shielded jacket. Holy shit, you actually made something I can’t break into! That’s amazing!”
Shuri grinned. “I’ve had several weeks to get familiar with your capabilities. You’re extremely adept at evading my firewalls.”
“Don’t take it too hard. Security measures are the easiest to get past; you just have to figure out how to climb the wall --by the way, the Pentagon ought to contact you for an upgrade. Their defense measures are feeble compared to your most basic stuff.”
“I’m guessing that when you infiltrated the conference, you had enough time to get into enough of our systems so that you could always regrow whatever I tore out? It’s the only explanation I can think of.”
“Basically. Your coding is gorgeous, by the way. I practically wept every time I crawled through it.”
“Can we save the love fest for a later date?” T’Challa asked with an annoyed expression at his sister. “If you don’t have the weapons, and you don’t have a list, what can you give us to help us track down your buyers?”
“Nothing, I’m afraid. And there won’t be a ‘later date,’ either.”
T’Challa frowned. “I’m not following.”
“She has a brain tumor,” Shuri said softly. “Don’t you? I picked up on some irregularities in your coding, but I wasn’t sure...”
Jhanvi’s smirk turned into a sad smile and she tapped the spot just above her left eyebrow. “Cancerous. Inoperable because of excessive entanglement in the blood vessels. Based on the prognosis I was given, I’ve got... three hours left. At the most.”
Okoye felt disturbed as she processed Jhanvi’s statement. This isn’t a round up. It’s a last good-bye. “If you’re dying, why let us find you?”
Jhanvi shrugged. “Like the King said. The game’s up. I’d like to be recognized for my efforts in evading you, instead of only existing as a faceless figment of your imaginations. I mean, how many of you thought I was a woman?”
Okoye shared a silent glance with Nakia. Even though they’d never assigned a gender to the associate during their efforts to track them down, she’d assumed --they all had assumed--it was a man. It just made sense, given Klaue’s background.
“Exactly. I’d like to be remembered accurately, even in the official ‘pain in the ass’ records of Wakanda’s justice system.”
“Miss Singh, I’m incredibly sorry that you’re in your current predicament, but we need anything you can give us about the weapons you designed,” T’Challa said urgently. “Vibranium weaponry could devastate the world --and undo all our efforts to integrate ourselves with the rest of the world. We’d lose our abilities to help those that need us most.”
“Very compelling --but no.”
“Why not?”
“Because she hasn’t made any weapons,” Shuri said.
Jhanvi grinned and made a clicking noise with her mouth as she pointed double finger guns at Shuri. “Boom. There it is.”
T’Challa let out an annoyed sigh. “If you haven’t made any weaponry, then what have you been doing with the vibranium?”
“She’s been making medical technology.” Shuri fiddled with her kimoyo beads and images of some of their salvages from the safe house and warehouse sites flashed on the screen embedded in the wall. “Prosthetic arms and legs. Implants to reverse Alzheimers and memory loss. Nano technology blood clotting injections to help hemophiliacs. You’ve been using the last of Klaue’s vibranium to help people. Your random jumping around was you going where you were needed.”
“Having a tumor in your head gives you perspective. Trying to spend your life coloring inside the lines only limits how much you can help people.”
“So you teamed up with Klaue to get access to the materials that would let you help people quickly,” Nakia surmised.
“He needed someone to help him fly under the radar after you branded him,” she said. “I needed vibranium so I wouldn’t have to wait for the outside world to catch up with you.”
“You helped a man that killed our people,” Okoye hissed, disgusted. “Men, women, children!”
“You aren’t any better!” Jhanvi snapped. “You’re sitting on a mountain of technology and research that could do so much good for the world, but you refuse to release any means to make your results tangible for everyone else because of your paranoia! Do you have any idea how many people would benefit from having access to medical centers with vibranium technology? Cancer patients. Auto-immune disorder victims. People with cystic fibrosis. Prematurely born babies. Your death count is higher than Klaue’s could ever be!”
“We haven’t always made the right choices,” T’Challa admitted. “But we’re working on fixing that.”
“Yeah, you released your research, but ninety percent of it is fucking useless without vibranium! You gave the world a cart without a horse!”
“Wait.” Nakia held up her hand. “I agree we can do more, but I don’t understand something. Why let us think you were building weapons when you weren’t? We could’ve helped you.”
“Right. Because you’d absolutely let someone have a vibranium powered prosthetic. Sure. Besides, I’m dying in three hours. I’d like to have a little fun before I go.”
“You don’t have to.” Shuri stepped forward. “We have the ability to operate on tumors like yours. We can take you back to Wakanda and perform brain surgery on you.”
Jhanvi smirked bitterly. “Right. Because you’d absolutely perform surgery for a thief and known associate of criminals.”
“I would,” Shuri said. “You could be a major asset to us.”
“Asset!” Jhanvi let out a harsh laugh. “All my life I’ve been asset! Everyone wants to use my abilities! I can disable enemy satellites, guide unmanned crafts to carry out airstrikes, take down an entire country’s communication system! Everyone wants a piece of me without ever considering if I’m okay with what they’re using the pieces for! No, Princess, I would rather die than let you use my abilities for your own ends!”
“Your ability to control technology is not your greatest asset!” Shuri exclaimed. “And it’s not what Wakanda needs most right now.”
“Isn’t it?”
“No. Your humanity is. Your perspective. We’re trying to help the world become a better place, and we don’t even have the awareness to realize that most our medical procedures are unattainable without vibranium technology; it’s such a commonplace thing for us that we forget that we’re the only ones that have it. We need someone like you to help us focus our efforts in the right areas. Otherwise, all our work will be in vain because we’ll never reach the people that need us most.”
Jhanvi hesitated, glancing between Shuri and T’Challa warily. “You’re not the King. You can’t make that kind of decision.”
Okoye watched as T’Challa mulled the idea over. It wasn’t, technically, the safest option, but...
There were a lot of technicalities in Jhanvi’s favor. She hadn’t stolen the vibranium --Klaue had, and she’d actually kept his cache from falling into the wrong hands by using it to make medical technology.
She also hadn’t directly killed anyone. An extremely fine line --not one the council was likely to walk--but a line nonetheless.
Okoye watched as T’Challa looked to Nakia --who nodded--then took a moment to regard Jhanvi when he looked over to her. She hasn’t built any weaponry. She just wants help people.
She pursed her lips together as another thought occurred to her. She’ll die in three hours if we don’t help.
Ayo wasn’t going to like this. At all.
Ayo is an adult. The risks are minimal --at the very least, we can save her from the tumor, then incarcerate her. She looked back at T’Challa and nodded.
“I think we can come to an arrangement,” T’Challa said.
Jhanvi was quiet for a moment --then nodded. “You’ve got a deal.”
Okoye yawned and tried to rub the exhaustion out of her stinging eyes as she wrapped up the mission report. Almost done. Then you can sleep.
Shuri had used a neural stabilizing helmet to medically induce a coma and ‘freeze’ the cancer once they were back on the ship. As soon as they landed in Wakanda she whisked Jhanvi off the ship on a stretcher; three doctors were already waiting on the platform, and Shuri disappeared in the palace with them in seconds.
Ayo had been waiting for them, too. She glared at Jhanvi’s stretcher, her expression a rare picture of unmitigated fury.
Fortunately, Aneka had been able to coax her partner back inside the palace. Okoye trusted that the youngest soldier would be able to talk Ayo down --eventually.
She looked up when the door to her office opened and gasped softly when M’Baku stepped in. “What are you doing here?”
“Emergency council meeting. To talk about the fate of Klaue’s associate.”
“Right.” Okoye yawned again. “I forgot. I should get some sleep before that happens.”
“It’s in the evening. Don’t worry.” M’Baku kissed her forehead. “Dewani said Shuri was performing surgery on the associate?”
“Removing a malignant tumor. It’s a long story.”
“I gathered.” He rubbed his hand up and down the back of her neck. “Are you almost done?”
“Basically.” Okoye filled out the last few requirements, then submitted her mission report. She sighed tiredly and let her head drop to her desk. “I hate night missions. They always take the longest. Always.”
M’Baku chuckled and gently tugged her out of her chair. “Come on, Okoye. Let’s get you back to your quarters.”
“I’d rather sleep in your quarters.”
He raised an eyebrow. “Really?”
She nodded sleepily. “Yes. Sleeping with you sounds like heaven right now.”
He carried her to his room --at her request; the palace was basically dead right now anyway, and walking felt downright impossible--and gently set her on his bed. “I’ll find something for you to change into,” he murmured as he pressed his lips against her brow.
“One of your sleep shirts, please,” Okoye mumbled as he rifled through the dresser. Once she had a shirt, she pushed herself off the bed and shuffled to the bathroom. “I’m changing and washing my face, and then I’m passing out.”
Even having the water one at the coldest temperature the sink cold go did nothing to kick-start her system. I’m getting too old for this.
And, admittedly, that was a reality of her job. Being the General was a highly physical task. Most women retired between the ages of thirty-five and forty to start working as trainers for the Dora program or advisers to the Council, tribes, or War Dogs program.
Right now, all it meant was that she’d start shifting more of the night missions to Ayo, who was set to become General when Okoye stepped down.
Candidly, it wasn’t a choice that Okoye felt any particular grief over. Night duties of any kind had never been her favorite.
She dropped on the bed next to M’Baku and nestled against him as he pulled the blankets up over her. “Good night.”
“Technically, it’s morning.”
“Shut up and let me sleep.”
“Miss Singh.”
“Not anymore. But you can still call me that, if you like.”
Okoye narrowed her eyes at Jhanvi and crossed her arms over her chest. “I’m pleased to see your surgery went well.”
“So am I. Still have the magic touch, which is nice.” She flicked at glance at Ayo, who was glaring stonily at her. “I don’t think she likes me much.”
“You killed two of my friends with your ‘harmless detonators,’” Ayo spat out.
“And your country could’ve prevented the deaths of millions with your technology.”
Ayo clenched her teeth together. “You should be rotting in a prison cell.”
Jhanvi smiled, expression indicating that she was fully aware how badly she was pissing off Ayo. “And yet, here I stand. Free as a bird and an unofficial consultant for the Wakandan outreach program.”
Aneka latched on to Ayo’s wrist. “Come on, babe. Leave her alone. She’s only having fun pissing you off.”
Jhanvi watched the two women leave, edgy smile unfaltering. “Shame. Anger looks good on her.”
“You’d do well to leave the Commander well alone, should your paths ever cross again,” Okoye lectured in a stern, threatening tone.
“Or?”
Okoye took a step towards Jhanvi and leveled her fiercest, most intimidating glare at the woman. “I don’t need technology to kill you. Remember that.”
The edgy smile didn’t even wobble. “Duly noted.”
Okoye watched as two of the Honor guard members escorted Jhanvi to the landing platform. I’ll be watching you, associate. One misstep on your part, and I won’t hesitate to take you out.
#sass writes#black panther fanfiction#okoye x m'baku#t'challa x nakia#suspenseful but not angsty#not too much#more focused on the plot#tw: cancer mention#tw: death mention#this one was fun to write#and we'll be seeing more of the associate in the future#they'll be back!!!#wakanda forever
2 notes
·
View notes
Text
Product Review: TriClip™ Transcatheter Tricuspid Valve Repair System | TechSci Research
Summary: Abbott’s Triclip™ is one-of-its-kind transcatheter valve repair clip device designed for patients with multiple co-morbidities. The non-surgical treatment alternative prevents the probability of high-risk open-heart surgery and improves quality of life for people with severe and symptomatic tricuspid regurgitation.

Cardiovascular diseases are one of the leading causes of death worldwide, but most of them could be prevented with proper medical interventions. However, the introduction of smart applications, remote monitoring, and minimally invasive approaches have been successful in targeting the gaps in vascular health care through technology and saving millions of lives. Many new treatment options are being developed to eliminate the need of open-heart surgery as it carries a high degree of risk. The advent of transcatheter tricuspid valve intervention (TTVI) devices as an alternative treatment option for tricuspid regurgitation (TR) or the leaky tricuspid valve have revolutionized the field of structural cardiology. Designed to reduce TR severity by valve leaflet plication, the percutaneous coronary devices have gained a lot of recognition due to their ability to treat severe TR in a minimally invasive way without posing the risk of severe complications. Currently, 80% of mitral regurgitation treatments are being performed with the MitraClip/TriClip™ system worldwide to address the specific features of the transcupid valve anatomy.
Abbott's TriClip™ is the first-of-its-kind transcatheter tricuspid valve repair clip device to receive CE mark clearance as a non-surgical treatment option for people with TR. The minimally invasive clip-based device is an iteration of the company's highly popular and successful MitraClip used for transcatheter mitral valve. The next-generation clip-based therapy, also known as TriClip™ G4 consists of a leaflet grasping feature and new clip sizes to accommodate and tailor repair of the unique anatomy of each patient. Leaving TR unaddressed for a long period can lead to atrial fibrillation, heart failure, or even death so Abbott's TriClip™ serves as a suitable option for patients with multiple co-morbidities. Thus, the device prevents the high-risk patients undergo the open-heart surgery, which could present a number of complications. The new-generation clip therapy can enhance the cardiologists' ability to repair tricuspid valves safely and effectively, thus dramatically improves the quality of life for people with severe and symptomatic tricuspid regurgitation. Post-implantation of the clip device, the patients can significantly eliminate symptoms such as shortness of breath, fatigue, decline in endurance, ascites, and peripheral edema.
The six-month pivotal TRILUMINATE CE Mark study examining edge-to-edge repair technique using Abbott's new clip therapy demonstrated a significant reduction in the severity of debilitating TR, sustained symptomatic improvements and enhanced functional status of the heart. Therefore, the physicians consider the device to be highly safe, durable, and reliable for the treatment of TR in high-risk patients or people with moderate or severe TR. The TriClip™ device has the potential to repair the tricuspid valve without open-heart surgery only by clipping together a portion of leaflets to reduce the blood flow. The minimally invasive approach allows the heart to pump blood more efficiently and relieve symptoms associated with the tricuspid valve leakage.
Although Abbott's TriClip™ device is built upon the same technology of the company's MitraClip transcatheter mitral valve therapy that is employed to treat leaky mitral valves, the next-gen device differs in terms of the delivery system. The new, steerable guiding catheter system is engineered to adapt the right side of the heart where the transcupid valve is present. To meet the needs of unique anatomies of patients, Abbott's TriClip™ is available in two different sizes (NT & XT). Currently, the TriClip™ is approved for use in Europe and other countries that recognize the CE mark.
The TriClip™ system consists of mainly two parts, steerable guide catheter (SGC) and clip delivery system (CDS). The CDS includes a steerable sleeve, delivery catheter, and wide chrome cobalt clip of 4 mm (NT size) and 7 mm (XT size) with articulated arms. The SGC in the new TriClip™ design contains two knobs for steering maneuvers. Besides, the steerable sleeve includes only one knob intended for deflecting the tip and reducing the radius of the distal curve. The modifications facilitate the bending and guiding of maneuvers into the right atrium. The device is used to clip the two leaflets together at the center and create two smaller openings to form a tighter seal. The upgraded TriClip™ allows clinicians to customize the procedure to each patient's unique valve and independently grasp leaflets before fastening. With 100% implant success rate, the device can help to save time and costs of patients by reducing the need for hospitalization by 40%.
Other Alternative for Minimally Invasive Tricuspid Therapy
Edward LifeSciences-PASCAL The PASCAL device can be successfully adapted to reduce TR as it creates a new surface for coaptation of tricuspid leaflets, which helps in reducing leakage. The medical device consists of a foam-filled spacer that is inserted through axillary vein into the regurgitant orifice. PASCAL has a length of 42 mm and diameter of 12 and 15 mm and advocated for use in moderate-to-severe TR. Its 22F delivery system enable easy manoeuvring in three planes and stabilizers lock handles for convenient procedure. The major benefit of central spacer is that the surgeon does not need to pull the leaflets of valve as one would do during a conventional procedure. Thus, the implantation of the device helps to repair the valve by better distributing the forces of the system throughout valve leaflets and thus help in efficient working of the heart.
The PASCAL repair system offers a minimally invasive approach for patients as the device is inserted through the groin area making an entry point of 0.7-0.8 cm. The independent clasping function of the PASCAL repair system provides some benefits to the physicians. During the surgery, the surgeon can individually control each side of the clasp machine, which could not be performed earlier. The technique allows the physicians to reopen one side of the clasp while keeping the other clasp in the stationary position, which helps to optimize its capture and reclose the system. The independent leaf capture method enhances the efficacy of the PASCAL system and provides optimal results. The patient can be easily extubated post-procedure and sent to normal ward. Unlike with valve surgery, the patient does not require pain medications and can be discharged within two days. After a week, the patient can even return to their daily activities and live a normal life.
The relatively new tricuspid therapy by Edward Lifesciences, known as FORMA, has been designed specifically for the treatment of leakage in tricuspid leaflets, but the medical device is currently under clinical trials and has yet to receive CE approval.
Conclusion TriClip™ Transcatheter Tricuspid Valve Repair System is a promising technique that offers feasibility, safety, and excellent outcomes. In the coming years, the clip-based therapy is set to bring forward a paradigm shift in the management of tricuspid regurgitation (TR). However, the medical device needs to analyzed in more clinical trials to determine its efficacy against the tricuspid therapies. Also, the use of transcatheter tricuspid valve does not provide satisfactory results for patients with previous valve surgery, PPML, and moderate to severe organic or aortic valve diseases. So, new methods and techniques need to be devised to expand the scope of the treatment modalities for more patients with cardiovascular problems.
According to TechSci research report on "Global Interventional Cardiology Devices Market By Product Type (Angioplasty Balloons, Angioplasty Stents, Structural Heart Devices, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices, Others) By End User (Hospitals & Clinics, Ambulatory Surgery Centers, Others) By Region, Competition Forecast & Opportunities, 2026", the global interventional cardiology devices market is anticipated to grow at a significant CAGR during the forecast period. The growth can be attributed to advancements in medical devices and rapid surge in the demand for minimally invasive surgeries. Additionally, high demand for novel products for the treatment of cardiovascular diseases to reduce risks and rising incidences of heart problems are contributing to the growth of global interventional cardiology devices market.
According to another TechSci research report on "United States Heart Valve Devices Market By Type (Mechanical Heart Valves, Biological Heart Valves, Transcatheter Heart Valves), By Product Type (Replacement (Aortic, Mitral, Others), Repair), By Valve Type (Tissue Valve, Mechanical Valve), By Procedure (Surgical, Transcatheter), By Application (Aortic Stenosis, Aortic Regurgitation, Mitral Stenosis, Mitral Regurgitation, Pulmonary Valvular Heart Disease), By Region, Forecast & Opportunities, 2025", United States heart valve devices market is expected to witness significant growth during the forecast period. The growth can be attributed to the increasing geriatric population and rising prevalence of cardiovascular diseases. Moreover, rapid advancements in healthcare infrastructure and easy access to healthcare services are boosting the growth of United States Heart Valve Devices market. In addition, rise in sedentary lifestyle and growing obese population are increasing the number of patients with heart problems, which is contributing to the growth of United States heart valve devices market.
#TechSci#Market Research Reports#United States Heart Valve Devices Market#Global Interventional Cardiology Devices Market#Cardiovascular Diseases#Healthcare#Medical Devices#Clinical Trials#Abbott#Triclip™
0 notes
Text
Product Review: TriClip™ Transcatheter Tricuspid Valve Repair System | TechSci Research
Summary: Abbott’s Triclip™ is one-of-its-kind transcatheter valve repair clip device designed for patients with multiple co-morbidities. The non-surgical treatment alternative prevents the probability of high-risk open-heart surgery and improves quality of life for people with severe and symptomatic tricuspid regurgitation.

Cardiovascular diseases are one of the leading causes of death worldwide, but most of them could be prevented with proper medical interventions. However, the introduction of smart applications, remote monitoring, and minimally invasive approaches have been successful in targeting the gaps in vascular health care through technology and saving millions of lives. Many new treatment options are being developed to eliminate the need of open-heart surgery as it carries a high degree of risk. The advent of transcatheter tricuspid valve intervention (TTVI) devices as an alternative treatment option for tricuspid regurgitation (TR) or the leaky tricuspid valve have revolutionized the field of structural cardiology. Designed to reduce TR severity by valve leaflet plication, the percutaneous coronary devices have gained a lot of recognition due to their ability to treat severe TR in a minimally invasive way without posing the risk of severe complications. Currently, 80% of mitral regurgitation treatments are being performed with the MitraClip/TriClip™ system worldwide to address the specific features of the transcupid valve anatomy.
Abbott's TriClip™ is the first-of-its-kind transcatheter tricuspid valve repair clip device to receive CE mark clearance as a non-surgical treatment option for people with TR. The minimally invasive clip-based device is an iteration of the company's highly popular and successful MitraClip used for transcatheter mitral valve. The next-generation clip-based therapy, also known as TriClip™ G4 consists of a leaflet grasping feature and new clip sizes to accommodate and tailor repair of the unique anatomy of each patient. Leaving TR unaddressed for a long period can lead to atrial fibrillation, heart failure, or even death so Abbott's TriClip™ serves as a suitable option for patients with multiple co-morbidities. Thus, the device prevents the high-risk patients undergo the open-heart surgery, which could present a number of complications. The new-generation clip therapy can enhance the cardiologists' ability to repair tricuspid valves safely and effectively, thus dramatically improves the quality of life for people with severe and symptomatic tricuspid regurgitation. Post-implantation of the clip device, the patients can significantly eliminate symptoms such as shortness of breath, fatigue, decline in endurance, ascites, and peripheral edema.
The six-month pivotal TRILUMINATE CE Mark study examining edge-to-edge repair technique using Abbott's new clip therapy demonstrated a significant reduction in the severity of debilitating TR, sustained symptomatic improvements and enhanced functional status of the heart. Therefore, the physicians consider the device to be highly safe, durable, and reliable for the treatment of TR in high-risk patients or people with moderate or severe TR. The TriClip™ device has the potential to repair the tricuspid valve without open-heart surgery only by clipping together a portion of leaflets to reduce the blood flow. The minimally invasive approach allows the heart to pump blood more efficiently and relieve symptoms associated with the tricuspid valve leakage.
Although Abbott's TriClip™ device is built upon the same technology of the company's MitraClip transcatheter mitral valve therapy that is employed to treat leaky mitral valves, the next-gen device differs in terms of the delivery system. The new, steerable guiding catheter system is engineered to adapt the right side of the heart where the transcupid valve is present. To meet the needs of unique anatomies of patients, Abbott's TriClip™ is available in two different sizes (NT & XT). Currently, the TriClip™ is approved for use in Europe and other countries that recognize the CE mark.
The TriClip™ system consists of mainly two parts, steerable guide catheter (SGC) and clip delivery system (CDS). The CDS includes a steerable sleeve, delivery catheter, and wide chrome cobalt clip of 4 mm (NT size) and 7 mm (XT size) with articulated arms. The SGC in the new TriClip™ design contains two knobs for steering maneuvers. Besides, the steerable sleeve includes only one knob intended for deflecting the tip and reducing the radius of the distal curve. The modifications facilitate the bending and guiding of maneuvers into the right atrium. The device is used to clip the two leaflets together at the center and create two smaller openings to form a tighter seal. The upgraded TriClip™ allows clinicians to customize the procedure to each patient's unique valve and independently grasp leaflets before fastening. With 100% implant success rate, the device can help to save time and costs of patients by reducing the need for hospitalization by 40%.
Other Alternative for Minimally Invasive Tricuspid Therapy
Edward LifeSciences-PASCAL The PASCAL device can be successfully adapted to reduce TR as it creates a new surface for coaptation of tricuspid leaflets, which helps in reducing leakage. The medical device consists of a foam-filled spacer that is inserted through axillary vein into the regurgitant orifice. PASCAL has a length of 42 mm and diameter of 12 and 15 mm and advocated for use in moderate-to-severe TR. Its 22F delivery system enable easy manoeuvring in three planes and stabilizers lock handles for convenient procedure. The major benefit of central spacer is that the surgeon does not need to pull the leaflets of valve as one would do during a conventional procedure. Thus, the implantation of the device helps to repair the valve by better distributing the forces of the system throughout valve leaflets and thus help in efficient working of the heart.
The PASCAL repair system offers a minimally invasive approach for patients as the device is inserted through the groin area making an entry point of 0.7-0.8 cm. The independent clasping function of the PASCAL repair system provides some benefits to the physicians. During the surgery, the surgeon can individually control each side of the clasp machine, which could not be performed earlier. The technique allows the physicians to reopen one side of the clasp while keeping the other clasp in the stationary position, which helps to optimize its capture and reclose the system. The independent leaf capture method enhances the efficacy of the PASCAL system and provides optimal results. The patient can be easily extubated post-procedure and sent to normal ward. Unlike with valve surgery, the patient does not require pain medications and can be discharged within two days. After a week, the patient can even return to their daily activities and live a normal life.
The relatively new tricuspid therapy by Edward Lifesciences, known as FORMA, has been designed specifically for the treatment of leakage in tricuspid leaflets, but the medical device is currently under clinical trials and has yet to receive CE approval.
Conclusion TriClip™ Transcatheter Tricuspid Valve Repair System is a promising technique that offers feasibility, safety, and excellent outcomes. In the coming years, the clip-based therapy is set to bring forward a paradigm shift in the management of tricuspid regurgitation (TR). However, the medical device needs to analyzed in more clinical trials to determine its efficacy against the tricuspid therapies. Also, the use of transcatheter tricuspid valve does not provide satisfactory results for patients with previous valve surgery, PPML, and moderate to severe organic or aortic valve diseases. So, new methods and techniques need to be devised to expand the scope of the treatment modalities for more patients with cardiovascular problems.
According to TechSci research report on "Global Interventional Cardiology Devices Market By Product Type (Angioplasty Balloons, Angioplasty Stents, Structural Heart Devices, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices, Others) By End User (Hospitals & Clinics, Ambulatory Surgery Centers, Others) By Region, Competition Forecast & Opportunities, 2026", the global interventional cardiology devices market is anticipated to grow at a significant CAGR during the forecast period. The growth can be attributed to advancements in medical devices and rapid surge in the demand for minimally invasive surgeries. Additionally, high demand for novel products for the treatment of cardiovascular diseases to reduce risks and rising incidences of heart problems are contributing to the growth of global interventional cardiology devices market.
According to another TechSci research report on "United States Heart Valve Devices Market By Type (Mechanical Heart Valves, Biological Heart Valves, Transcatheter Heart Valves), By Product Type (Replacement (Aortic, Mitral, Others), Repair), By Valve Type (Tissue Valve, Mechanical Valve), By Procedure (Surgical, Transcatheter), By Application (Aortic Stenosis, Aortic Regurgitation, Mitral Stenosis, Mitral Regurgitation, Pulmonary Valvular Heart Disease), By Region, Forecast & Opportunities, 2025", United States heart valve devices market is expected to witness significant growth during the forecast period. The growth can be attributed to the increasing geriatric population and rising prevalence of cardiovascular diseases. Moreover, rapid advancements in healthcare infrastructure and easy access to healthcare services are boosting the growth of United States Heart Valve Devices market. In addition, rise in sedentary lifestyle and growing obese population are increasing the number of patients with heart problems, which is contributing to the growth of United States heart valve devices market.
#TechSci#Market Research Reports#Abbott#Triclip™#Healthcare#Clinical Trials#Medical Devices#United States Heart Valve Devices Market#Global Interventional Cardiology Devices Market#Cardiovascular Diseases
0 notes
Text
Peripheral Embolic Protection Devices - Medical Devices Pipeline Assessment, 2020 published on
https://www.sandlerresearch.org/peripheral-embolic-protection-devices-medical-devices-pipeline-assessment-2020.html
Peripheral Embolic Protection Devices - Medical Devices Pipeline Assessment, 2020
Peripheral Embolic Protection Devices – Medical Devices Pipeline Assessment, 2020
Summary
GlobalData’s Medical Devices sector report, “Peripheral Embolic Protection Devices – Medical Devices Pipeline Assessment, 2020” provides comprehensive information about the Peripheral Embolic Protection Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Embolic Protection Devices (EPDs) are used to capture and remove plaque debris that is dislodged during interventional procedures.
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data in relation to the equipment type.
Scope
– Extensive coverage of the Peripheral Embolic Protection Devices under development – The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities – The report reviews the major players involved in the development of Peripheral Embolic Protection Devices and list all their pipeline projects – The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage – The report provides key clinical trial data of ongoing trials specific to pipeline products – Recent developments in the segment / industry
Reasons to Buy
The report enables you to – – Formulate significant competitor information, analysis, and insights to improve R&D strategies – Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage – Identify and understand important and diverse types of Peripheral Embolic Protection Devices under development – Develop market-entry and market expansion strategies – Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline – In-depth analysis of the product’s current stage of development, territory and estimated launch date
0 notes
Text
Otoplasty Market Projected to Witness Vigorous Expansion by 2030
Otoplasty: Introduction
Otoplasty, also known as cosmetic ear surgery, is a procedure for changing position, shape, or size of the ears. It involves surgical reshaping of the pinna or outer ear in order to improve its appearance or to correct defects, if any.
Report Overview @
https://www.transparencymarketresearch.com/otoplasty-market.html
The procedure is more common during childhood but also can be performed at any age after the ears have reached their full size. It can also be done at the age of three.
Otoplasty can be used to correct he defects that are present in the ear structure at the time of birth. The procedure can treat macrotia, a condition having overly large ears and protruding ears
Scarring, asymmetry in ear placement, changes in skin sensation, problems with stitches, and overcorrection are various risk associated with otoplasty
Planning To Lay Down Future Strategy? Request Brochure Of Otoplasty Market
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=77435
Key Drivers and Restraints of Global Otoplasty Market
Increase in awareness about appearance of the ear among the youth; rise in adoption of cosmetic surgeries; and increase in prevalence of congenital ear deformities such as hemifacial microsomi, Treacher Collins syndrome, and others drive the global otoplasty market
Increase in cases of trauma to the external ear resulting from blasts, puncture, and damage due to blunt objects are projected to drive the global market during the forecast period
Furthermore, increase in influence of cosmetic surgery; psychological impact of appearance, looks, and beauty on young generation; and increase in screening for deformities at birth are projected to boost the growth of the market during the forecast period
To Obtain All-Inclusive Information On Forecast Analysis Of Otoplasty Market , Request A Discount
https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=77435
Ear Augmentation Segment to Account for Major Share of Global Otoplasty Market
In terms of type of fittings, the global otoplasty market can be classified into ear augmentation, ear reduction, and ear pin back
The ear augmentation segment is anticipated to dominate the global market during the forecast period, owing to increase in demand for otoplasty for ear augmentation to improve appearance and rise in number of ear surgeons offering otoplasty under reconstructive surgery
Surgical Segment to Capture Major Share of Global Market
Based on technique, the global otoplasty market can be categorized into surgical and non-surgical. The surgical segment is further divided into anti-helical fold manipulation, conchal alteration, and correction of earlobe prominence. The non-surgical segment is bifurcated into tissue molding and others.
The surgical segment is expected to account for major share of the global otoplasty market by 2027. This can be attributed to growing number of patients preferring cosmetic surgery for ear augmentation and rise in availability of surgical augmentation of ear.
Request For Covid19 Impact Analysis –
https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=77435
North America to Dominate Global Otoplasty Market
In terms of region, the global otoplasty market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is projected to dominate the global otoplasty market during the forecast period.
Increase in prevalence of congenital ear deformity and increase in number of cosmetic surgical procedures is anticipated to drive the market in North America
As per the Plastic Surgery Statistics Report, 22,884 otoplasty procedures were conducted in the U.S. in 2018. Out of these, 13,289 procedures were performed on female patients.
Key Players Operating in Global Otoplasty Market
The global otoplasty market is highly fragmented, with a large number of domestic players accounting for major market share. Key players operating in the global otoplasty market include:
Allergan (AbbVie Inc.)
Invotec International, Inc.
Sklar Surgical Instruments
EarBuddies
Phoenix Medical Systems Pvt. Ltd.
More Trending Reports by Transparency Market Research –
Plaque Modification Devices Market –
http://www.prnewswire.co.uk/news-releases/plaque-modification-devices-market-to-reach-us-2-1-bn-by-2027-tmr-826918739.html
Prosthetics Market –
https://www.biospace.com/article/prosthetics-market-is-driven-by-increase-in-incidence-of-diabetes-among-the-population-leading-to-amputations/
0 notes
Text
Worldwide Lancets Market Ready to Set outstanding Growth from 2019 to 2023
The Global Lancets Market is growing rapidly because of the rising prevalence of diabetes and growing number of blood borne diseases across the globe. The global market of lancets is expected to reach USD 2,882.6 million in 2022 and is expected to grow with a CAGR of approximately 11.3% during the forecast period.
Key Findings:
The Global Lancets Market and is expected to reach USD 2,882.6 million by 2022
Regionally, North America holds the largest market share of global Lancets Market and is expected to grow at a CAGR of 11.2% during the forecast period from 2016-2022
On the basis of type, safety lancets accounts the largest market share in 2015 and is expected to reach USD 2,061.8 million by 2022.
Globally, lancets are being utilized by patients who are affected by endocrine related complications, cardiovascular issues, and many others. Lancets are being used for diabetes/glucose test, tests in infants, heel-stick screening tests, as well as for scarred emergency patients or severely burned patients. The global lancet market is growing at an exponential rate and it is expected to grow at a CAGR of 11.3% during 2016 to 2022.
To Get Sample Report visit https://www.marketresearchfuture.com/sample_request/2078
The increasing prevalence of diabetes and growing incidence number of contagious and non-contagious diseases has been the major factor for influencing the growth of the market. Incidence of contagious diseases is increasing rapidly on a global level. According to the WHO, it has been calculated that, infectious diseases like plaque which is also known as black death has an estimated 50 million deaths in the 14th century, this infectious disease can be a serious disease if not treated, this disease has a case fatality ratio of around 30%-60%, as of 2013 there were 783 cases worldwide which includes 126 deaths.
The global lancet market appears to be oligopolistic owing to the presence of large players active in the regional market. The market is also characterized by a reasonable degree of brand loyalty where establishing a brand name is difficult for newcomers. However, the cost involved in manufacturing setup is low to medium which discreetly comfort the new entrants to enter in the market easily. Developments in the medical device industry are made to simplify the diagnosis, prevention, and treatment of various diseases. One of such device is lancet, which is capturing a huge market share due to its application. Safety lancets and personal lancets being the two major types of the lancets are dominating the global lancet market.
Key Players for Global Lancets Market
Market Research Future (MRFR) recognizes the following companies as the key players in Lancets Market: There are plenty of large and small market players which operate in this market all over the globe.
Some of the Key Players in This Market Are: F. Hoffmann-La Roche AG, Becton, Dickinson and Company, Greiner Bio One International GmbH, Improve Medical Technology Co. Ltd, Terumo Medical Corporation, Bayer Cropscience Limited, HTL-STREFA S.A, Sarstedt AG & Co and Others
Global Lancets Market - Competitive Analysis
Many key players involved in this market are keen in introducing new advanced safety lancets for the treatment of various contagious diseases. HTL-STREFA S.A. accounted for major market share of global lancet market, with more than 35% of market share. The large share of the company is attributed to the high demand for safety lancet globally. Moreover, this company has a strong sales and distribution network and this company also provides after sales services and help which play a major role in the satisfaction of the customers. F. Hoffmann-La Roche AG accounts for approximately 22% which can be attributed to their product the ACCU-CHEK which is one of the safety lancets having high demand in the market.
Industry Updates
June, 2017: F. Hoffmann-La Roche AG received European approval of Avastin Drug in combination with Tarceva for cancer patients with a specific type advance lung cancer.
January, 2017: Catalent Inc., a company involved in biologics announced its research collaboration with Roche on Smartag(TM) technology. Catalent claims Roche will pay Catalent an up-front fee of $1 million. Roche to provide additional research funding during the initial phase of the collaboration between these two companies.
Segments for Global Lancets Market
Global Lancet market has been segmented on the basis of types which comprises of personal, safety and others. On the basis of end users which consists of hospitals & clinics, diagnostic centers and pathology laboratories, home diagnostics and others.
Regional Analysis for Global Lancets Market
Globally North America is the largest market for Lancet. The North American market for Lancet is expected to grow at a CAGR of 11.2% during the forecasted period. This is due to increasing prevalence of diabetic patients. Europe is the second-largest market for Lancet with the market value of USD 592.8 million in 2015. Whereas Asia pacific is expected to be a growing market for Lancet market and expected to grow at a rapid rate.
To Browse Complete Report visit https://www.marketresearchfuture.com/reports/lancet-market-2078
Some Brief Table of Contents of Report
Chapter 1. Report Prologue
Chapter 2. Market Introduction
2.1 Definition
2.2 Scope Of The Study
2.2.1 Research Objective
2.2.2 Assumptions
2.2.3 Limitations
Chapter 3. Research Methodology
3.1 Introduction
3.2 Primary Research
3.3 Secondary Research
3.4 Market Size Estimation
Chapter 4. Market Dynamics
4.1 Drivers
4.2 Restrains
4.3 Opportunities
4.4 Challenges
4.5 Macroeconomic Indicators
4.6 Technology Trends & Assessment
Chapter 5. Market Factor Analysis
5.1 Porters Five Forces Analysis
5.1.1 Bargaining Power Of Suppliers
TOC Continued…
Do You Have Specific Requirement? Ask To Our Experts https://www.marketresearchfuture.com/enquiry/2078
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Statistical Report, Continuous-Feed Research (CFR), and Market Research & Consulting Services.
Contact Us:
Market Research Future
Hadapsar, Pune – 411028
Maharashtra, India
Phone: +1 646 845 9312
Email: [email protected]
0 notes
Text
Peripheral Vascular Stents Market is Expected to Grow at a Impressive CAGR of 5.88%. by 2022
A detailed market research study about, “Peripheral Vascular Stents Market by Product and End User-2022” examine the performance by crystal market research. This report analyzes the potential of Peripheral Vascular Stents Market in the present and the future prospects from various angles in detail. This report focuses on the top manufacturers in North America, Europe, Japan, China, India, Southeast Asia and other regions (Middle East & Africa). Click For to Get Exclusive Sample Copy At: www.crystalmarketresearch.com/report-sample/HC06147
Competitive Insights The key players operating in the peripheral vascular stents market emphasizes on product development in order to introduce improve peripheral vascular stents devices and capture a larger share of the market. Some of the major players in this market are Boston Scientific, Terumo Medical Corporation, Abbott Laboratories, Braun Melsungen, Biotronik,, Cook Medical, C.R. Bard, Intact Vascular, W.L. Gore & Associates, Medtronic, Edward Lifesciences Corporation, and AngioScore. Market Trend Outlook- Peripheral Vascular Stents Market is expected to experience a moderate growth in coming years between 2014 and 2022. The market is expected to reach $9.38 billion by 2022 at a moderately growing CAGR of 5.88%. Peripheral vascular stents devices are used to diagnose peripheral artery disease (PAD). The PAD is characterized by building plaque in the arteries which in turn blocks the arteries and restricts the blood flow to the organs. When this condition is left untreated, it can lead to gangrene and in turn it may end up to amputation of limbs. Sometimes, it may also result into blockage of artery which can even lead to stroke. The increasing incidence of PAD across the globe, rising geriatric population and technological innovation related to peripheral vascular stents, will be the major drivers of the peripheral vascular stents market over the forecast period. Peripheral artery disease (PAD) is a common phenomenon among the geriatric population. For instance, as per CDC, in US, more than 8.3 million people suffered from PAD in 2016. Brief Market Business Overview Advanced technologies, rise in the need for procedures of angioplasty, increasing number of incidents for vascular issues, growing demand for procedures having minimal invasion and increase in the geriatric population have been the market driving factors. The successful implementation and results of these devices have led to the expansion of the market industry for peripheral vascular stents. This indicates increase in demand of peripheral vascular stents devices in the upcoming years. In addition, development of new imaging techniques is expected to improvise the vascular surgeries involving placement of peripheral vascular stents which in turn will increase the demand of stenting procedures. However, high price of peripheral stents and lack of awareness among general population about PAD, will restrain the growth of the peripheral vascular stents market in the future years. Thus, considering the drivers and challenges, peripheral vascular stents market is expected to have a moderate growth over the forecast period. Market Segmentation- By Product Balloon Expanding Stent Self-expanding Stent Drug Eluting Stent Bare Metal Stent By End User Ambulatory Surgical Centers Specialty Clinics Hospitals Market Analysis by Regions Middle East And Africa (Saudi Arabia, UAE, Egypt, Nigeria And South Africa) North America (U.S, Canada, Mexico) Europe (Germany, France, UK, Italy, Spain, Rest Of Europe) Asia-Pacific (Japan, China, Australia, India, South Korea, Rest Of Asia-Pacific) Rest Of The World (Brazil, South Africa, Saudi Arabia, Turkey, United Arab Emirates, Others) Brief Research Study of Peripheral Vascular Stents Market Stents that are balloon-expandable are implemented in treating calcified lesions, ostial lesions and lesions of short segments due to the deployment of precision and enhanced radial force. These provide excellent results for mesenteric, subclavian, iliac and renal lesions. Stents that are self-expandable are used for tortuous vessels, long lesions and regions which carry risk of compression or external forces since these have more track-ability, flexibility and come in long lengths. These are preferred for femoral-popliteal lesions. A number of products applied in intervention by endovascular specialists of which stents and balloons form the core. Placing a metallic stent across an occluded or stenotic blood vessel extends provision of structural support on the inner side and hence serves in maintaining the patency of that vessel along with flow re-establishment through it. Regional Insights North America held a significant share of the peripheral vascular stents market in 2016 due to the increase in geriatric population and occurrence of PAD in various states. In addition, technological innovation of the devices by the key players of the market will also enhance the market growth in this region. The Asia-Pacific market has not experienced any substantial growth due to lack of large scale adoption of peripheral vascular stents but can experience favorable growth in coming years owing to changing lifestyles related to peripheral vascular stents and increasing geriatric population in the region. Major Table of Contents: Chapter 1. Introduction 1.1. Report Description 1.2. Research Methodology 1.2.1. Secondary Research 1.2.2. Primary Research Chapter 2. Executive Summary 2.1. Key Highlights Chapter 3. Market Overview 3.1. Introduction 3.1.1. Market Definition 3.1.2. Market Segmentation 3.2. Market Dynamics 3.2.1. Drivers 3.2.2. Restraints 3.2.3. Opportunities ....CONTINUED FOR TOC For More Information to Enquiry About Report You Can Click On www.crystalmarketresearch.com/send-an-enquiry/HC06147 Reason to Buy – • Highlights key business priorities in order to assist companies to realign their business strategies. • The key findings and recommendations highlight crucial progressive industry trends in the Peripheral Vascular Stents Market, thereby allowing players to develop effective long term strategies. • Develop/modify business expansion plans by using substantial growth offering developed and emerging markets. • Scrutinize in-depth global market trends and outlook coupled with the factors driving the market, as well as those hindering it. • Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to products, segmentation and industry verticals. To Buy This Informative Research Report, Please Click On The Link @ www.crystalmarketresearch.com/checkout/HC06147 About Crystal Market Research: Crystal Offers One Stop Solution For Market Research, Business Intelligence, And Consulting Services To Help Clients Make More Informed Decisions. It Provides Both Syndicated As Well As Customized Research Studies For Its Customers Spread Across The Globe. The Company Offers Market Intelligence Reports Across A Broad Range Of Industries Including Healthcare, Chemicals & Materials, Technology, Automotive, And Energy. Contact Us: Judy S, 304 South Jones Blvd, Suite 1896, Las Vegas NV 89107, United States Toll Free: +1-888-213-4282 Email: [email protected]
0 notes
Text
Peripheral Vascular Devices Market by Type (Angioplasty Balloon, Stent, Catheters (Angiography, IVUS), Plaque Modification (Atherectomy, Thrombectomy), Hemodynamic Flow Alteration, IVC Filters, Guidewires), and Region - Global Forecast to 2025 published on
https://www.sandlerresearch.org/peripheral-vascular-devices-market-by-type-angioplasty-balloon-stent-catheters-angiography-ivus-plaque-modification-atherectomy-thrombectomy-hemodynamic-flow-alteration-ivc-filters-guidew.html
Peripheral Vascular Devices Market by Type (Angioplasty Balloon, Stent, Catheters (Angiography, IVUS), Plaque Modification (Atherectomy, Thrombectomy), Hemodynamic Flow Alteration, IVC Filters, Guidewires), and Region - Global Forecast to 2025
“Peripheral vascular devices market to register a CAGR of 6.5% from 2020 to 2025.”
The global peripheral vascular devices market size is projected to reach USD 12.6 billion by 2025 from USD 9.2 billion in 2020, at a CAGR of 6.5% during the forecast period. The rapid growth in the geriatric population and the associated increase in the prevalence of peripheral vascular diseases, approval of new and advanced products, and the growing preference for minimally invasive surgeries are driving the growth of the peripheral vascular devices.
“Atherectomy devices accounted for the larger share of the plaque modification devices market in 2019.”
Based on type, the plaque modification devices market is segmented into atherectomy and thrombectomy. In 2019, the atherectomy devices segment accounted for the larger market share, mainly due to the increasing incidence of atherosclerosis as a result of the rising global prevalence of obesity.
“Embolic protection devices accounted for the largest share of the peripheral vascular devices market in 2019.”
Based on type, the hemodynamic flow alteration devices market is segmented into embolic protection devices and chronic total occlusion devices. In 2019, the embolic protection devices segment accounted for the larger share of the hemodynamic flow alteration devices market. This can be attributed to the advantages of EPDs over CTO devices, such as the ability to capture embolic debris without interrupting continuous blood flow.
“Asia Pacific estimated to register the highest CAGR during the forecast period.”
In this report, the peripheral vascular devices market is segmented into four major regional segments, namely, North America, Europe, Asia Pacific, and the Rest of the World (RoW). The market in Asia Pacific is projected to grow at the highest growth rate during the forecast period. The growth in this market is primarily driven by the presence of a large pool of aging population, increasing prevalence of diabetes, and rising focus of key players in this region.
Break down of primary supply-side interviews, by company type, designation, and region:
By Company Type: Tier 1 (70%) , Tier 2 (20%), and Tier 3 (10%)
By Designation: C-level (30%), Director-level (20%), and Others (50%)
By Region: North America (35%), Europe (24%), Asia Pacific (25%), and Rest of the World(16%)
List of players profiled in this report:
Medtronic (Ireland)
Boston Scientific Corporation (US)
B. Braun Melsungen AG(Germany)
Abbott (US)
Cardinal Health (US)
BD (US)
Penumbra, Inc. (US)
Terumo Corporation (Japan)
PanMed US (US)
Alvimedica (Turkey)
ENDOCOR GmbH (Germany)
Biosensor International (Singapore)
Koninklijke Philips N.V. (Netherlands)
AMG International GmbH (Germany)
iVascular (Spain)
Cardionovum (Germany)
SMT (India)
Meril Life Sciences Pvt Ltd. (India)
Medinol (Israel)
InSitu Technologies, Inc. (US)
Andramed GmbH (Germany)
Cook (US)
REX Medical (US)
Degania Silicon Ltd. (Israel)
Brosmed Medical (China)
Research Coverage
This report studies the peripheral vascular devices market based on product and region. The report also analyzes factors (such as drivers, restraints, opportunities, and challenges) affecting the market growth. It evaluates the opportunities and challenges in the market for stakeholders and provides details of the competitive landscape for market leaders. The report also studies micro markets with respect to their growth trends, prospects, and contributions to the total peripheral vascular devices market. The report forecasts the revenue of the market segments with respect to four major regions.
Reasons to Buy the Report:
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the peripheral vascular devices offered by the top 10 players in the market. The report analyzes the peripheral vascular devices market by product and region
Market Development: Comprehensive information about lucrative emerging markets. The report analyzes the markets for various peripheral vascular devices across key geographic regions
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the peripheral vascular devices market
Competitive Assessment: In-depth assessment of market shares and strategies of the leading players in the peripheral vascular devices market
0 notes