#Pharma Pricing & Market Access Congress 2019
Explore tagged Tumblr posts
Link
Pharma Pricing & Market Access Congress 2019
1 note
·
View note
Link
And Just What Fuckery Be This!? - Phroyd
LESS THAN A month after Democrats — many of them running on “Medicare for All” — won back control of the House of Representatives in November, the top health policy aide to then-prospective House Speaker Nancy Pelosi met with Blue Cross Blue Shield executives and assured them that party leadership had strong reservations about single-payer health care and was more focused on lowering drug prices, according to sources familiar with the meeting.
Pelosi adviser Wendell Primus detailed five objections to Medicare for All and said that Democrats would be allies to the insurance industry in the fight against single-payer health care. Primus pitched the insurers on supporting Democrats on efforts to shrink drug prices, specifically by backing a number of measures that the pharmaceutical lobby is opposing.
Primus, in a slide presentation obtained by The Intercept, criticized single payer on the basis of cost (“Monies are needed for other priorities”), opposition (“Stakeholders are against; Creates winners and losers”), and “implementation challenges.” We have recreated the slides for source protection purposes.
Democrats, Primus said, are united around the concept of universal coverage, but see strengthening the Affordable Care Act as the means to that end. He made his presentation to the Blue Cross executives on December 4. “We don’t discuss private meetings, if there was such a meeting,” said a BCBS spokesperson. Primus said that he did not discuss any kind of deal with the insurers. Henry Connelly, a spokesperson for Pelosi, said that the assessment of single payer was not related to any dealmaking with the industry. “We’re not going to barter lower prescription drug costs for inaction in the rest of the health care industry. The presentation was a broad look at the health care environment and some of House Democrats’ legislative priorities over the next two years in a period of GOP control of the Senate and White House,” Connelly said.
The debate over Medicare for All is playing out on a number of different levels, with no clear consensus over how the government-run, single-payer health plan ought to take shape. Presidential candidates are arguing over whose plan is stronger and gets to full Medicare for All faster, with a debate raging over whether private insurance should be banned outright or operate in addition to universal Medicare coverage.
In the House, even as the idea has picked up momentum with voters and members of the Democratic caucus, Democratic leadership has remained deeply skeptical. Pelosi’s consistent messaging, instead, has been around protecting the Affordable Care Act and lowering prescription drug prices.
“Speaker Pelosi has ensured that Medicare for All will have hearings in the House and tapped Congressman Brian Higgins to take the lead on Medicare buy-in legislation. For the first time, House committees will be seriously examining and tackling some of the questions and possible solutions raised by Medicare for All legislation,” said Connelly.
“The biggest obstacles facing Medicare for All right now are Mitch McConnell and Donald Trump,” he added. “But in the near term, there is a window for Democrats to press Trump to help pass aggressive legislation to negotiate down the skyrocketing price of prescription drugs.”
Primus concluded his presentation with a bullet point that summarized Pelosi’s mission on health care: “Lower your health care costs and prescription drug prices.”
The “your” refers to insurers, who bear costs for medical expenses covered under their plans. That puts insurers and Pelosi, at least in one sense, in alignment, as both have an interest in lower costs. Indeed, insurers regularly negotiate to lower their health care costs, but in practice their efforts have had little effect on the general trend in costs. Drug company patents give pharmaceutical giants outsized power to set prices, and hospital consolidation has also given providers more power in those negotiations. Even where insurers have been able to negotiate lower prices for their own customers, that has done little to shrink the list price of drugs for the public.
At the briefing, Primus mentioned three avenues that Pelosi, a California Democrat, sees toward lower drug prices, sources said. The first, the CREATES Act, is bipartisan legislation, strongly opposed by Big Pharma, that would make it easier for generic drug companies to get access to a sufficient quantity of medications needed to produce generics.
The second measure addresses what’s known as “pay for delay,” in which a drug company pays a generic manufacturer to not produce a generic version of an expensive drug. Democratic leadership wants to ban that practice. The third revolves around the issue of “evergreening,” which is a pharmaceutical industry practice of extending patent protection for a particular drug through a variety of practices. Democrats want to restrict evergreening to encourage cheaper generics make it to the market faster.
Primus’s approach has a strong political logic to it, as taking on every health care stakeholder at once is arguably more difficult than singling out one industry and hammering away, even if the effort is out of step with where progressive energy is at the moment.
PRIMUS IS KNOWN in Congress as one of the staunchest foes of Big Pharma, while Pelosi’s posture toward Medicare for All is more complicated. Publicly, she has long said that she supports it aspirationally. “I was carrying around single-payer signs probably before you were born, so I, you know, I understand that aspiration,” she said in 2017 during an interview with TV host Joy Reid.
“This is an idea that if we had a tabula rasa, if we were just starting clean, would be the most cost-effective way to go forward. We don’t have that,” she said. “Over 120 or 150 million people in our country have employer-based access to their health coverage and insurance.”
At the time, her objection to Medicare for All was that it distracted from the fight to defend the ACA, which Republicans were trying to gut. “So right now, I’m going to be crude. Now we’re in my living room, so I can be crude. It isn’t helpful to tinkle all over the ACA right now,” Pelosi said. “Right now, we need to support the Affordable Care Act and defeat what the Republicans are doing.”
At other moments, she has said that single payer isn’t popular, arguing, also in 2017, that “the comfort level with a broader base of the American people is not there yet.”
The Democratic Congressional Campaign Committee, which operates under Pelosi, in 2017 presented House Democrats with survey data, claiming that it showed that single-payer was a political loser, and that Democrats should focus their messaging on lowering drug prices and protecting the ACA.
Yet a significant number of Democrats who flipped Republican districts blue in 2018 were publicly supportive of Medicare for All, suggesting that it isn’t necessarily the albatross Pelosi and the DCCC believe it to be. A poll from October found that more than half of Republicans support the concept.
Pelosi’s agreement to hold House hearings on Medicare for All came after pressure from the Congressional Progressive Caucus. Yet the hearings will be held by the Budget Committee, which, unlike the powerful Ways and Means and Energy and Commerce committees, would not have final jurisdiction over Medicare for All in the event of a genuine attempt to pass it.
Primus, like Pelosi, is well-known to be a deficit hawk, and both subscribe to the argument put forward by the late Pete Peterson that the debt and deficit are among the gravest threats facing the country. When Peterson, a billionaire who spent hundreds of millions of dollars to push Washington policymakers toward austerity, died in 2018, Pelosi delivered a floor speech that praised him and his vision effusively, speaking of the man as if he’d dedicated his life to eradicating child malnutrition or curing cancer, rather than as a Wall Street tycoon who spent millions pushing for major cuts to Social Security and Medicare. “Pete was a clarion voice for fiscal responsibility, and a strong moral conscience in Washington,” Pelosi said in her House floor eulogy of Peterson, who, by 2012, had already spent half a billion dollars targeting Social Security, Medicare, and other spending programs.
“Pete’s prophetic voice on the importance of fiscal sustainability brought together generations of policymakers, no matter their political background,” Pelosi said. “His legacy will endure in many ways, but especially through the work of the Peterson Foundation, which continues to focus on solutions to America’s fiscal and economic challenges.”
TWO OF PRIMUS’S five objections to single payer before the Blue Cross audience related to such alleged fiscal challenges. That argument, though, runs headlong into a surge of new interest among Democrats in Modern Monetary Theory, the idea that policymakers are still constrained by a mindset that was justifiable when the U.S. was on the gold standard, but is no longer defensible. Now that the U.S. issues a currency independent of its gold reserves, the obstacle to government spending is inflation, not the debt or deficit, proponents of MMT say. “This zero-sum mentality has no place in a post-Bretton Woods world,” said economist Stephanie Kelton in reaction to Primus’s argument that spending on Medicare for All would foreclose other priorities. (Post-Bretton refers to the global agreement that the dollar will be the global economy’s reserve currency, ultimately decoupled from gold.)
“The U.S. dollar is no longer tethered to gold, which means the federal government is not constrained in its spending by the need to raise revenue. The federal government cannot run out of dollars. This should be painfully obvious, but the gold-standard mentality continues to grip many lawmakers,” Kelton said.
As long as inflation remains low, the government can continue to authorize additional spending. That’s not so much an argument as it is simply an observation of the post-gold-standard reality that austerity advocates like Peterson have spent billions to distort. “The government can afford any new program it chooses to fund. The limits are in the real economy — if producers can’t keep up with the additional demand, inflation will result,” said Kelton, a former adviser to the Senate Budget Committee when it was chaired by Sen. Bernie Sanders. “The federal government — as the issuer of the U.S. dollar — can create all the money that is needed to guarantee health care for all of its people. It’s the rest of us — who merely use the dollar — who have to worry about costs and where to come up with the money to pay a huge medical bill when our private insurer refuses to cover the cost of care.”
This reality has been recognized by former Federal Reserve Chairs Alan Greenspan and Ben Bernanke, as well. “The United States can pay any debt it has because we can always print money to do that. So, there is zero probability of default,” Greenspan once said.
Ryan Grim is the author of the forthcoming book “We’ve Got People: The End of Big Money and the Rise of a Movement.” Sign up here to get an email when it’s released.
Phroyd
17 notes
·
View notes
Text
Pfizer Court Fight Could Legalize Medicare Copays and Unleash ‘Gold Rush’ in Sales
Three years ago, pharma giant Pfizer paid $24 million to settle federal allegations that it was paying kickbacks and inflating sales by reimbursing Medicare patients for out-of-pocket medication costs.
By making prohibitively expensive medicine essentially free for patients, the company induced them to use Pfizer drugs even as the price of one of those medicines, covered by Medicare and Medicaid, soared 44% to $225,000 a year, the Justice Department alleged.
Now Pfizer is suing Uncle Sam to legalize essentially the same practice it was accused of three years ago — a fighting response to a federal crackdown that has resulted in a dozen drug companies being accused of similar practices.
A Pfizer win could cost taxpayers billions of dollars and erase an important control on pharma marketing after decades of regulatory erosion and soaring drug prices, say health policy analysts. A federal judge’s ruling is expected any day.
“If this is legal for Pfizer, Pfizer will not be the only pharmaceutical company to use this, and there will effectively be a gold rush,” government lawyer Jacob Lillywhite said in oral arguments last month.
Pfizer’s legal argument “is aggressive,” said Chris Robertson, a professor of health law at Boston University. “But I think they’ve got such a political tailwind behind them” because of pocketbook pain over prescription medicine — even though it’s caused by pharma manufacturers. Pfizer’s message, “‘We’re just trying to help people afford their drugs,’ is pretty attractive,” he said.
That’s not all that’s working in Pfizer’s favor. Courts and regulations have been moving pharma’s way since the Food and Drug Administration allowed limited TV drug ads in the 1980s. Other companies of all kinds also have gained free speech rights allowing aggressive marketing and political influence that would have been unthinkable decades ago, legal scholars say.
Among other court arguments, Pfizer initially claimed that current regulation violates its speech protections under the First Amendment, essentially saying it should be allowed to communicate freely with third-party charities to direct patient assistance.
“It’s infuriating to realize that, as outlandish as they seem, these types of claims are finding a good deal of traction before many courts,” said Michelle Mello, a professor of law and medicine at Stanford University. “Drug companies are surely aware that the judicial trend has been toward more expansive recognition of commercial speech rights.”
Pfizer’s lawsuit, in the Southern District of New York, seeks a judge’s permission to directly reimburse patient expenses for two of its heart-failure drugs each costing $225,000 a year. An outside administrator would use Pfizer contributions to cover Medicare copays, deductibles and coinsurance for those drugs, which otherwise would cost patients about $13,000 a year.
Letting pharma companies put money directly into patients’ pockets to pay for their own expensive medicines “does induce people to get a specific product” instead of shopping for a cheaper or more effective alternative, said Stacie Dusetzina, an associate professor of health policy at Vanderbilt University. “It’s kind of the definition of a kickback.”
Government rule-makers have warned against such payments since the launch of Medicare’s Part D drug benefit in 2006. Drug companies routinely help privately insured patients with cost sharing through coupons and other means, but private carriers can negotiate the overall price.
Because Congress gave Medicare no control over prescription drug prices, having patients share at least part of the cost is the only economic force guarding against unlimited price hikes and industry profits at taxpayer expense.
At the same time, however, regulators have allowed the industry to help patients with copays by routing money through outside charities — but only as long as the charities are “bona fide, independent” organizations that don’t match drugmaker money with specific drugs.
Several charities have blatantly violated that rule in recent years by colluding with pharma companies to subsidize particular drugs, the Justice Department has alleged. A dozen companies have paid more than $1 billion to settle allegations of kickback violations.
Pfizer set up an internal fund at one of the charities, the Patient Access Network Foundation, to cover patient costs for a heart arrhythmia drug at exactly the same time it was raising the wholesale cost from $220 to $317 for a package of 40 capsules, the Justice Department said. Pfizer referred Medicare patients who needed the drug to the PAN Foundation, the government said.
Under such arrangements, every $1 million channeled through a charity “has the potential to generate up to $21 m[illion] for the sponsor company, funded by the U.S. government,” Andrew Baum, a Citi pharma stock analyst, wrote in 2017.
Pfizer settled the case, saying it was not an admission of wrongdoing but resulted from its “desire to put this legal matter behind us.”
The PAN Foundation and three other charities also made deals to resolve allegations that they functioned as disallowed conduits for patient assistance for multiple pharma companies. One organization, the Virginia-based Caring Voice Coalition, shut down after government scrutiny.
PAN’s settlement did not mention the alleged Pfizer transactions. Those were described in the separate government deal with Pfizer.
The 2019 PAN agreement related to “legacy matters” and “did not involve any of PAN’s current operations or disease funds,” organization CEO Dan Klein said via a spokesperson. “Nonprofit patient assistance programs like PAN are necessary to help people access the critical medications they need to stay healthy.”
But legal troubles have hardly slowed the pharma-funded patient assistance business.
Four penalized nonprofits agreed to stop directing money to specific drugs, but they continue to accept hundreds of millions of dollars in pharma donations to indirectly cover copays and other patient drug costs, organization reports and IRS filings show. HHS regulators allow the practice because the drug companies are not involved in deciding which patients and which drugs are subsidized.
Donations to six pharma-funded patient assistance charities reached $1.8 billion in 2019, only slightly less than the year before, a KHN analysis of their IRS filings shows. That was nearly 50% higher than the amount from five years previously, before the Justice Department started cracking down.
Last year Pfizer donated $39.7 million to PAN and five other charities helping patients with out-of-pocket drug costs, company disclosures show.
If Pfizer’s lawsuit seeking to earmark such donations for its tafamidis heart-failure drugs opens the way for similar practices industrywide, it would drive up Medicare costs through rising prices and numbers of prescriptions, said Gerard Anderson, an economist and health policy professor at Johns Hopkins University’s Bloomberg School of Public Health. Such a program for tafamidis alone would increase Medicare costs by $30 billion, the Health and Human Services Department’s inspector general estimated.
Pharma companies can “learn which patients are using the drug, and they can market [and offer financial assistance] directly to that patient,” Anderson said. “You get a huge return.”
Pfizer argues that its proposal, which the HHS inspector general called “highly suspect” in an advisory opinion before the company filed its lawsuit, is legal and sensible.
“Providing copay assistance to middle-income patients who have been prescribed tafamidis is an efficient and equitable way to lower their out-of-pocket costs,” company spokesperson Steven Danehy said.
But the real affordability problem for patients is that tafamidis is too expensive, federal attorney Lillywhite said in court arguments last month. (HHS’ Office of Inspector General declined to comment.)
Pfizer has “priced itself out of the market,” he said. The company is seeking to “do something that’s unprecedented, to upend decades of settled law and agency guidance” to boost sales of “what is the most expensive cardiovascular drug ever launched in the United States.”
After the oral arguments, Pfizer dropped claims that HHS rules violate its free speech rights. Judge Mary Kay Vyskocil is considering only the company’s contention that a dedicated fund for tafamidis would not violate kickback prohibitions because, among other arguments, it is the doctor who decides to prescribe the drug and create revenue for Pfizer, not the patient getting the financial assistance.
But legal analysts still see the case as part of a broad movement toward deregulation and corporate rights.
A 1970s Supreme Court case, viewed as paving the way for an explosion of drug, lawyer and liquor ads as well as corporate campaign donations, was about speech rights for prescription drug sellers in Virginia. In 2011 the court found that the First Amendment allows data miners to buy and sell prescription records from pharmacies, provided the patients aren’t identified.
A year later, a federal appeals court cited speech protections when it overturned the conviction of a pharma sales rep who had been promoting a drug for uses not approved by the FDA.
Even if Pfizer loses its case, the climate may be ripe for similar challenges by other drugmakers, especially after the appointment of more than 200 federal judges by business-friendly President Donald Trump, legal scholars said.
The federal kickback law doesn’t mention copay assistance charities “and wasn’t designed with these programs in mind,” said Mello, of Stanford. Pfizer’s lawsuit “should be a loud, clanging call to Congress” to explicitly define drug assistance subsidies as illegal kickbacks, she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
USE OUR CONTENT
This story can be republished for free (details).
Pfizer Court Fight Could Legalize Medicare Copays and Unleash ‘Gold Rush’ in Sales published first on https://smartdrinkingweb.weebly.com/
0 notes
Text
HEALTH CARE BRIEFING: Biden Plans Order Amid Health-Care Mergers
New Post has been published on https://tattlepress.com/health/health-care-briefing-biden-plans-order-amid-health-care-mergers/
HEALTH CARE BRIEFING: Biden Plans Order Amid Health-Care Mergers

The Biden administration is preparing a government-wide plan to encourage competition in markets across the economy, according to people familiar with the process, a move that could have wide implications for industries including technology, pharmaceuticals and agriculture.
The White House plans to issue an executive order as soon as next week that would require federal agencies to take steps to promote competition in the industries they oversee, said the people, who asked not to be named because the initiative isn’t yet public. Biden is “committed to increasing competition in the American economy, including by banning noncompete agreements,” said White House spokeswoman Emilie Simons.
The move would give Biden a way to focus on the decade-plus consolidation of key consumer-facing industries in the U.S., including health care.
Health-care and life sciences transactions continued at a strong pace in May, with 231 deals announced or closed, the third month in 2021 with at least 225 transactions. The total volume of deals is up almost 70% from 2020, said Larry Kocot of KPMG, Christopher Brown reported earlier this week.
But tax and health-care cost offset provisions in legislative packages favored by Democrats and Biden could create headwinds for the health-care and life sciences sector. Chances for passage of key bills in a narrowly divided Congress remain unclear, but incremental health reforms appear possible, said Kocot, Brown reported in a May 28 story.

Biden’s upcoming order will echo an Obama administration order in 2016 that said government agencies beyond those responsible for antitrust enforcement had a key role to play in protecting consumers, workers and business from being harmed by instances of market power in the economy.
That order built off a report by the White House Council of Economic Advisers outlining concern about evidence indicating that industries across the U.S. economy suffer from rising consolidation and declining competition. It recommended other agencies use regulations to tackle the issue in addition to traditional antitrust enforcement by the Federal Trade Commission and the Justice Department.
Since then, attention on the power of dominant companies has only grown as economists and policy makers raise concerns that rising concentration is ailing large sections of the economy and contributing to problems including income inequality and wage stagnation. Anna Edgerton and David McLaughlin have more.
On Lawmakers’ Radars
Vaccine Hesitancy: The House Select Coronavirus Crisis Subcommittee holds a hearing today on vaccine hesitancy.
Drug Pricing Group Targets Four Finance Panel Senators: A consumer group that wants Congress to empower the government to negotiate the price of drugs will launch an ad campaign aimed at four key senators this week. Patients for Affordable Drugs Now, an advocacy group launched with the support of the Arnold Foundation, will start a six-figure ad campaign targeting four Senate Finance Committee Democrats: Michael Bennet (Colo.), Tom Carper (Del.), Bob Casey (Pa.) and Bob Menendez (N.J.).
Advocates for drug-price negotiation legislation are turning their attention to the Finance Committee, where Chairman Ron Wyden (D-Ore.) says he wants the federal government to be able to negotiate lower prices on some drugs. “Patients are depending on these senators to join with other supporters of meaningful reform,” David Mitchell, founder of Patients For Affordable Drugs Now, said in a statement.
Menendez voted against a measure to allow Medicare to negotiate with drugmakers brought before the Finance Committee in 2019. Carper said earlier this year he wants to revive a bipartisan drug pricing package from Wyden and Sen. Chuck Grassley (R-Iowa), which didn’t include negotiation language, Alex Ruoff reports.
Democrats Want DOJ to Oppose Purdue Plan: House Oversight and Reform Chair Carolyn Maloney (D-N.Y.) and Rep. Mark DeSaulnier (D-Calif.) sent a letter to Attorney General Merrick Garland urging the Justice Department to oppose Purdue Pharma’s Chapter 11 reorganization plan. Under the proposal, “members of the Sackler family would contribute $4.2 billion, less than half of the fortune they amassed from the company, to resolve all legal claims related to their role in the opioid epidemic,” they wrote.
House Panel Approves Agriculture-FDA Spending Bill: The House Appropriations Committee advanced the proposed Agriculture-FDA appropriations bill yesterday after adopting three amendments offered by both Democrats and Republicans. The legislation, which would allocate $26.6 billion in fiscal 2022, includes an amendment by Rep. Barbara Lee (D-Calif.) to revoke certain meat and poultry plant line speed waivers issued during the coronavirus pandemic and another by Rep. Dan Newhouse (R-Wash.) to prohibit companies owned by China from purchasing farmland and to block their participation in Agriculture Department programs. A manager’s amendment by Rep. Sanford Bishop (D-Ga.) made technical changes, Megan Boyanton and Jack Fitzpatrick report.
The Coronavirus Pandemic
Johnson & Johnson to Study Shot in Teens: Johnson & Johnson expects to start studying its one-dose vaccine in children 12-17 years old this fall, a company official said at a Johns Hopkins University event. The drugmaker plans to sign up at least 4,500 adolescents and will assess their progress a year later, J&J’s head of clinical development, Macaya Douoguih, said. J&J is planning four studies in minors, she said. Read more from Jeannie Baumann.
Hospitals Ask OSHA for Halt of Covid-19 Standard: The American Hospital Association is asking OSHA to delay the compliance schedule of its Covid-19 emergency temporary standard for health care, asserting that providers need more time to navigate “complex��� requirements. The organization called for the six-month delay in a letter to Occupational Safety and Health Administration official James Frederick. Fatima Hussein has more.
More Pandemic Headlines:
From the GAO:
Industry & Regulation
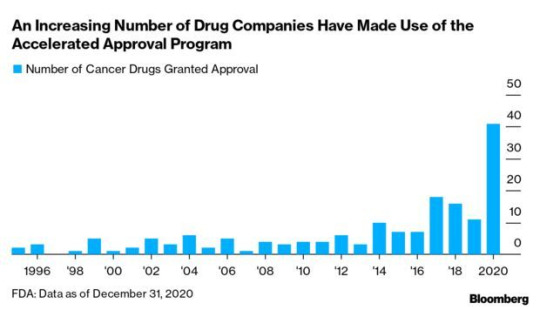
Alzheimer’s Drug Fight Puts FDA Under Scrutiny: An accelerated approval program for U.S. drugs that’s been around for nearly three decades is under fire for the criteria used by regulators to decide which therapies should be greenlighted, and for letting ineffective treatments linger on the market. The Food and Drug Administration’s accelerated process has been hailed for novel treatments and unmet medical needs. But critics argue changes are needed to make it more transparent and to better measure efficacy.
Approval of Biogen’s drug Aduhelm to treat Alzheimer’s has revved up debate on the program. Rather than being cleared based on its effectiveness, Aduhelm won approval by showing it can reduce amyloid plaques in the brain, a physical biomarker, or surrogate, linked to the disease. Meanwhile, Biogen has nine years to finish a trial on its efficacy. Read more from Fiona Rutherford.
An influential nonprofit that evaluates drugs said Biogen’s $56,000-a-year Adulhelm is priced at an order of magnitude more than it should, and said there isn’t enough evidence it works. An appropriate price for the therapy would be in the range of $3,000 to $8,400 annually, an 85% to 95% discount from Biogen’s list price, if it is effective, the Institute for Clinical and Economic Review said. Read more from John Tozzi.
Organ Transplant Push Seen Endangered by Plan to Rein in Costs: Prominent medical groups fear a federal push to increase organ transplants could face a significant slowdown under a proposed rule by the Biden administration that would reduce Medicare payment for certain costs related to the organ acquisition process.
One payment revision in the proposed rule would save an estimated $4.1 billion over 10 years and help cut wasteful and duplicative program spending—sometimes for organs not used by Medicare beneficiaries. Transplant hospitals, doctors, and organ procurement organizations (OPOs) say the proposals would hurt the acquisition of organs from deceased donors—and access to those organs—while increasing the number of patients who die while awaiting a transplant. Read more from Tony Pugh.
More Headlines:
From the Courts
Internet Data-for-Insurance Partners Defend Plan: A novel arrangement providing health benefits to 50,000 people who agreed to allow their internet data to be tracked is an invaluable lifeline for the self-employed, middle-class workers “left behind” by Obamacare, three people covered by the partnership told the Fifth Circuit. Adam Rochester and two other partners of Data Marketing Partnership want the court to bless their health insurance arrangement over objections from the Labor Department. Read more from Jacklyn Wille.
Indiana Abortion Pill Reversal Message Blocked: Indiana is blocked for now from requiring abortion providers to give patients a state-mandated message that medication abortions can be reversed because it hasn’t shown a likelihood of proving the message is true, a federal trial court in the state said. All-Options Inc. and other abortion providers have shown they are likely to win a lawsuit alleging the state’s specific required disclosure violates their free speech rights, the court ruled. Read more from Mary Anne Pazanowski.
Related:
More Headlines:
With assistance from Megan U. Boyanton and Jack Fitzpatrick
To contact the reporters on this story: Brandon Lee in Washington at [email protected]; Alex Ruoff in Washington at [email protected]
To contact the editors responsible for this story: Zachary Sherwood at [email protected]; Giuseppe Macri at [email protected]; Michaela Ross at [email protected]
Source link
0 notes
Text
Meds And Money: The Rising Price For Medications And Insulin
By Henry Jacobson, Florida State University Class of 2020
July 29, 2020

Big pharma has a less than positive public image in America. The negative public perception is largely because of the rising cost of lifesaving medications in the US. This is interpreted as blatant greed by the American people. And perhaps it is. Many medications that have been around for decades are increasing in price at a rate that is 2.5 times higher than inflation. This problem seems to be unique to America as well. Americans “spend twice as much on health care as the average for wealthy industrialized countries while having the lowest life expectancy and the highest infant mortality rates” [1]. “U.S. drug prices are, on average, 3.7 times higher than the combined average of 11 other similar countries, according to a just-released study of 79 single-source brand-name drugs by the House Ways & Means Committee. In some cases, individual drug prices were an astounding 67 times higher” [1]. This is frightful information. The US is one of the leading innovators when it comes to medicine, but the American people are paying the cost for such innovation.
In 2020 over 500 medications have risen in price. “According to GoodRx, a startup that tracks prescription drug prices and helps consumers find discounts, the prices of more than 560 medications have gone up since Dec. 31.On average, the increase has been about 5 percent”. One medication, meant to treat depression, managed to increase in price by 14 percent [2]. Some argue that these price increases will only affect insurers. However, I highly doubt that insurance companies would not be willing to pass on the burden of these costs to the American people. As hundreds of various medications rise in price, one lifesaving medicine outpaces all others.
The rise in the price of insulin is mind-boggling. Insulin is used to help people with stage one and stage two diabetes. This lifesaving medicine helps regulate blood sugar levels to avoid damaging, and sometimes life-threatening, health complications. “In 2009, the list price for a 10-milliliter vial of Humalog, a fast-acting insulin made by Eli Lilly, was about $93. Today it costs closer to $275. Similarly, Novo Nordisk's fast-acting insulin Novolog cost almost $93 for a 10-milliliter vial in 2009. Today, it costs about $290” [3]. The price increase for insulin medications has forced some diabetics to ration their insulin. Rationing insulin has led to death in the US [3]. Many blame the pharmaceutical companies for the steep incline in prices. Pharmaceutical companies are engaging in a process called “ever-greening”, making small adjustments to patent-protected medicine to receive a new patent that will keep the medicine from being offered in a cheaper, generic form [4]. By denying the production of generic insulin and suing any other companies that try to create a generic form of insulin, the big pharmaceutical companies can remain in control of pricing and profits. The price also stems from a lack of competition. 90 percent of the insulin market is controlled by three pharmaceutical giants: Eli Lilly, Novo Nordisk, and Sanofi[5]. The complete lack of competition is bad for the American consumer. These companies could communicate with another to discuss price increase schemes and non-competing agreements.
However, the three companies argue that they are not at fault for the rising prices. The big three blame much of the rising costs on industry middlemen. These pharmaceutical companies must appease government regulatory administrations, pharmacy benefits managers, and insurance companies [3][6]. These companies argue that the complexity of regulations and other market factors as a whole can drastically affect out-of-pocket costs. The companies also state that the list price for the medication is often not what the consumer pays.Greg Kueterman, senior director of communications, for Eli Lilly, said that “people using Medicaid, for example, can access insulin for nearly free because we provide 100% rebates to the government” [3]. These companies also offer a variety of programs for people to access insulin at a discounted price if they are eligible. No one wants to take responsibility for increasing the difficulty of receiving lifesaving medicine.
Regardless of who to blame, what can be done? An article from Harvard Business Review offered three suggestions. First, “link innovation-friendly policies to price concessions”. By offering companies tax credits for innovation and removing regulatory clutter, companies will fear failure less and save millions of dollars. Second, “revamp how long and how thoroughly new drugs enjoy monopoly protection”. This addresses the “ever-greening” issue mentioned previously. By adjusting the number of patents that a pharma company has access to for a given medicine will prevent the misuse of patents and allow for generics to be made by other companies. Third, “remove obstacles to competition from generics”. The creation and implementation of anti-trust laws would benefit the US greatly [7]. As stated previously, three companies produce and sell the vast majority of insulin. Breaking these companies up or encouraging competitors will create more competitive pricing models.
The government can do many things to improve the life of the people, but as always, the best bet to improve one’s life is to improve oneself. A variety of lifestyle changes can benefit people that are in need of insulin. Better diets and more exercise can reduce the need for insulin in type 2 diabetics and may remove the need for insulin in type 2 diabetics completely [6]. However, Type 1 diabetics will still need to rely on the government to enact the proper change to lower the cost of insulin.
________________________________________________________________
[1]https://www.healthaffairs.org/do/10.1377/hblog20191003.118206/full/
[2]https://www.healthline.com/health-news/prescription-drug-prices-have-gone-up-this-year#What-action-may-lie-ahead
[3]https://www.businessinsider.com/insulin-price-increased-last-decade-chart-2019-9
[4]https://www.ontrackdiabetes.com/type-1-diabetes/insulin-prices-still-high
[5]https://www.t1international.com/blog/2019/01/20/why-insulin-so-expensive/
[6]https://www.medicaleconomics.com/view/coronavirus-feds-extend-public-health-emergency-declaration
[7]https://hbr.org/2020/02/3-actions-congress-can-take-to-reduce-drug-prices?ab=hero-main-text
0 notes
Text
What science issues President Trump did—and did not—address in this year’s State of the Union
New Post has been published on https://nexcraft.co/what-science-issues-president-trump-did-and-did-not-address-in-this-years-state-of-the-union/
What science issues President Trump did—and did not—address in this year’s State of the Union


Science got a nod early on in Tuesday’s 2019 State of the Union address. “In the 20th century, America transformed science,” President Donald Trump said, emphasizing the Apollo 11 mission that landed the first humans on the moon. Here are the other science and health topics he commented on during his second SOTU, in which he was tasked with reporting to Congress “such measures as he shall judge necessary and expedient.”
“We have unleashed a revelation in American energy. The United States is now the number one producer of oil and natural gas anywhere in the world.”
The country’s production and consumption of energy stood at the core of Trump’s 2016 campaign, when he vowed to “end the war on clean coal.” In Tuesday’s address, the president instead focused on other fossil fuels, stating that for the first time in 65 years, the United States is a net exporter of energy––oil and natural gas.
President Trump recently faced criticism for his failure to attend COP24, a key United Nations climate change conference held in Katowice, Poland, after months of cutting down on environmental protections and regulations. The only event hosted by the U.S. at the conference was one promoting the use of fossil fuels.
Carbon dioxide accounts for 65 percent of all greenhouse gas emissions. Fossil fuel use is the primary source of CO2 emissions, which heat the planet and exacerbate wildfires, rising sea levels, and hurricanes.
Cities including Minneapolis, Minnesota have recently made plans to move to 100 percent reliance on clean energy in the next decade, and not a moment too soon. According to Environmental Protection Agency data, electricity and heat contribute to one-quarter of global greenhouse gas emissions. Energy associated with fuel extraction, refining, processing, and transportation makes up another 10 percent.
“In 2018 drug prices experienced their biggest decline in 46 years. But we must do more. It’s unacceptable that Americans pay vastly more for the exact same drugs.”
The Trump Administration wants to hold pharmaceutical companies accountable for rising prescription drug prices, an initiative that draws support from both sides of the aisle.
Last week Health and Human Services proposed a plan to eliminate rebates to Pharmacy Benefit Managers, who act as the middlemen between pharmaceutical manufacturers and patients. Pharmaceutical manufacturers set a price for each drug and PBMs negotiate rebates for patients, which make the drug cheaper. But patients don’t actually benefit: PMBs take a cut of these rebates, which are typically 20-30 percent of the original cost set by the manufacturer. The pressure to offer larger and larger rebates, drug manufacturers say, is the main reason they raise prices. Since rebates are negotiated as a percentage, both PMBs and pharmaceutical companies benefit from these hikes.
Under the new proposal, such rebates would work differently under Medicaid and Medicare. Drug manufacturers would pass discounts usually negotiated by PBMs directly to patients. According to MarketWatch, analysts don’t all believe this action will hurt PBMs. In August, STAT reported on the general sense of confusion around the administration’s PBM policies.
The proposal is the latest addition to the president’s American Patients First plan. Blueprints of the plan also include requiring drug manufacturers to disclose cost in television ads. The president stated on Tuesday that drug companies should be forced to openly list their prices—driving a competitive pharmaceutical market and keeping drug prices in check.
“Scientific breakthroughs have brought a once distant dream within reach. My budget will ask democrats and republicans to make the needed commitment to eliminate the HIV epidemic in the United States within 10 years.”
About 1.1 million Americans live with HIV, a virus that attacks the immune system. Taking medication regularly can keep the virus at bay, enough for it to become undetectable in the blood and therefore not transmittable. The CDC reports that already, HIV is virally suppressed in 51 of every 100 people in the U.S. living with the disease.
“Together we will defeat AIDS in America,” said the president. Improvements in medication access, better healthcare for all, and the decrease of HIV stigma could make that a reality in our lifetimes, but it’s important to note that HIV does not always lead to AIDS. Acquired immunodeficiency syndrome is the final and most serious stage of HIV, and is very preventable even in individuals who have contracted the virus.
“Most childhood cancers have not seen new therapies in decades.”
Trump announced that his new budget would ask for $500 million to support childhood cancer research. The National Cancer Institute estimates that over 15,500 adolescents were diagnosed with cancer last year.
“Lawmakers in New York cheered with delight upon the passage of legislation that would allow a baby to be ripped from the mother’s womb moments before birth.”
On January 22, New York Gov. Andrew M. Cuomo signed the Reproductive Health Act, legally ensuring the right to abortion in the state of New York. This marked the 46th anniversary of the landmark Roe v. Wade Supreme Court decision, which legalized abortion on the federal level (though it left much room for interpretation in how states should weigh a person’s right to abortion with its own policies).
The Reproductive Health Act allows abortions after 24 weeks if the fetus is not viable or if the mother’s health is at risk. The bill replaced a law which only permitted abortion after 24 weeks if a woman’s life was threatened. These terminations accounted for just 1.3 percent of all abortions in the U.S. in 2015, according to CDC data.
But not all the science and health issues you’ve seen in the news lately made it into the 2019 SOTU. Here are some topics the president skipped:
The Shutdown
The State of the Union was postponed because of the recent government shutdown, but Trump’s speech didn’t mention this event.
At 35 weeks long, it was was the longest U.S. government shutdown in history. It cost the U.S. an estimated $11 billion. It left unmanned National Parks vulnerable to destruction. It slowed Superfund site mitigation, which cleans up the most polluted places in the country. It furloughed 40 percent of the people who usually monitor the safety of our food system.
It also slowed down science.
The National Science Foundation funds nearly $8 billion in research grants each year, driving progress in everything from weather data to the tracking endangered species. The foundation funds nearly one-quarter of all federally-supported basic research conducted by U.S. colleges and universities.
NPR reported Friday that 111 panels of scientists and engineers had planned to review more than 2,000 proposals but were not able to meet during the shutdown. Some grants that were approved before the shutdown were put on hold.
“This year astronauts will go back to space on American rockets,” the president said after introducing Buzz Aldrin, one of the first two humans to walk on the moon. NSF provides virtually all of U.S. federal funding for ground-based astronomy and works closely with NASA.
Infrastructure
Some speculated the president would touch on the nation’s problematic infrastructure––54,259 of the nation’s 612,677 bridges are rated “structurally deficient” and the system of locks and dams that make waterway transportation possible are deteriorating.
Drinking water is a part of infrastructure, too.
Residents of Flint, Michigan have been dealing with a water crisis since 2014. The city identified more than 18,000 lead and galvanized steel water lines that may have contributed to their infamous water contamination. The plan is to replace them all by the end of this year, but Flint isn’t the only water system at risk.
Drinking water flows through 1 million miles of pipes throughout the U.S., and according to the American Society of Civil Engineers, a lot of these pipes are near the end of their lifespan.
In November, elevated lead levels were found in Newark, New Jersey where around 15,000 lead pipes deliver water throughout the city. Chicago, Detroit, Baltimore, Milwaukee, and New York City Public Schools also struggle with lead-laden water. Any level of lead can cause permanent damage in developing children.
Bacteria such as E. coli and Legionella pneumophila, which causes Legionnaires’ disease, as well as parasites, also plague water systems in the U.S.—especially in rural areas.
Opioids
The president did mention America’s growing drug epidemic: “Tens of thousands of innocent Americans are killed by lethal drugs that cross over our borders and flood into our cities including meth, heroine, cocaine, and fentanyl.” But that’s as far as he went on opioids.
The Centers for Disease Control and Prevention estimates that misuse of prescription opioids alone costs the U.S. $78.5 billion every year. The rate of opioid-related deaths among people in the U.S. was five times higher in 2016 than in 1999, and Americans are now more likely to die due to opioid use than from a car crash. One recent study suggested that for teen users, routine procedures like wisdom tooth extraction could encourage and enable eventual drug abuse.
Massachusetts Attorney General Maura Healey filed a lawsuit last week against Purdue Pharma, the pharmaceutical company behind OxyContin, on behalf of 670 Massachusetts residents who were prescribed the drug, became addicted to opiods, and died of an overdose.
Purdue Pharma hired a consulting firm to target “high-prescribing doctors” after OxyContin sales took a hit in 2013.
Written By Kaitlin Sullivan
0 notes
Text
Gottlieb skewers US drug pricing: “It’s time to stop the shell games”

FDA Commissioner Scott Gottlieb has spoken out against the US drug pricing system, attacking the practices of PBMs, insurers and pharma company as anti-competitive and rigged against patients.
In a wide ranging and detailed speech on Wednesday at an America’s Health Insurance Plans’ (AHIP) national conference, Gottlieb criticised anti-competitive practices, and in particular urged rebate-based contracts between pharma companies, PBMs and insurers to end.
Through these pharma companies offer discounts on their drugs’ list prices to PBMs and health insurers – but these price cuts are not passed on to patients, and are instead typically skimmed off as profits.
A similar rebate system is also being used by pharma companies to prevent biosimilars taking more market share.
Gottlieb has already broken with FDA Commissioner convention by speaking about prices and competition in previous remarks, but went much further than ever before in his criticism of the market practices.
Speaking about how biosimilars are being blocked from broader uptake in a ‘rigged’ system Gottlieb said:
“Everybody wins. The health plans get the big rebates. The PBMs get paid on these spreads. And branded sponsors hold onto market share.
“Everyone that is, but the patients, who in the long run, don’t benefit from the full value of increased competition Congress intended.”
He concluded: “It’s time to stop the shell games over drug pricing, and start competing on delivering better health outcomes.”
Gottlieb has the strong backing of President Trump, and the newly appointed Secretary of Health and Human Services, Alex Azar, who say tackling drug prices is one of their main priorities.
While many are sceptical that Azar and Trump will deliver on their promises, Gottlieb does have the power to streamline the process around bringing new biosimilars to market.
In his speech he revealed that a new ‘Biosimilar Access Plan’ would be launched to help speed the path to market and lower costs for biosimilar developers.
The speech came just one day after a potentially game-changing development, with major health insurer United Healthcare announcing it would pass on all drug rebates to more than 7 million people in its fully insured plans from 2019.
“This is a potentially disruptive step,” commented Gottlieb, who said he had already been briefed on the change by United’s CEO, Dan Schumacher.
Gottlieb said this change could bring patients savings of hundreds, or even thousands of dollars, particularly those in high deductible health plans.
The AHIP has responded by saying that greater competition will come via the launch of more biosimilars. It points out that the US passed its Biologics Price Competition and Innovation Act of 2010, but that since then just nine biosimilar products have been approved, and only four are currently available.
In legislative terms, the CREATES Act is currently the most likely vehicle for major change. Already being debated in Washington, the bill has bipartisan support, including from conservative Republicans, who often protect pharma industry interests. However the appeal of the CREATES act is in simply allowing a free market to operate unhindered – something which chimes with Republican instincts – and the potential new law would introduce various measures to bring generic products to market faster.
New HHS Secretary Alex Azar has also just given a major speech on his vision for health reform, and this included greater transparency in the system, new experimental models for Medicare and Medicaid and a move towards value-based healthcare.
Azar stopped short of identifying anti-competitive practices like Gottlieb however – something which critics say is hardly surprising, as Azar has spent his career as a pharma industry leader.
Some advocates for major healthcare reform are now looking to November’s mid-term elections, which could see Democrats seize control of Congress, and potentially opening the door for new legislation.
Read Scott Gottlieb’s speech in full here
The post Gottlieb skewers US drug pricing: “It’s time to stop the shell games” appeared first on Pharmaphorum.
from Pharmaphorum https://pharmaphorum.com/news/gottliebs-skewers-us-drug-pricing-time-stop-shell-games/
0 notes
Text
China Scar Treatment Market To Surpass US$ 5,317.7 Million By 2027

Burn injury or other trauma condition, including surgery can lead to a scar. Several factors impact the appearance and treatment of scar after an injury. Topical Scar Treatment, Laser Treatment, and Invasive Surgical Treatment are some of the options for scar tenement.
China Scar Treatment Market is estimated to account for US$ 2,422.5 Mn in terms of value by the end of 2019.
China Scar Treatment Market: Drivers
Increasing aesthetic appeal among the populace in China is expected to boost growth of China scar treatment market. Moreover, increasing product launch and assessment is also expected to boost growth of the market over the forecast period. For instance, in June 2019, AVITA Medical, a regenerative medicine company, announced new preliminary RECELL Autologous Cell Harvesting Device (RECELL System) data at the 24th annual World Congress of Dermatology Meeting. The RECELL system is used in the treatment of vitiligo and facial acne scars.
China Scar Treatment Market: Opportunities
Increasing R&D in skin defect reconstruction is expected to offer lucrative growth opportunities for market players over the forecast period. For instance, in August 2019, researchers from Naval Medical University, China, reported that use of minced split-thickness skin grafts with Pelnac as an overlay can be effective in reconstruction of full-thickness skin defects.
China Scar Treatment Market: Restraints
Stringent regulations in China are expected to hamper growth of the market. China has complicated and stringent regulatory requirements. It requires over 18 months to gain market access in the country.
Key Takeaways:
The Aesthetic Lasers segment in the China scar treatment market was valued at US$ 1,119.8 Mn in 2018 and is expected to reach US$ 2,717.3 Mn by 2027 at a CAGR of 10.4% during the forecast period. The growth of the segment is attributed to increasing number of aesthetic surgical procedures in the country
The laser treatment segment held dominant position in China scar treatment market in 2018, accounting for 45% share in terms of volume, followed by topical treatment and invasive surgical treatment, respectively. The growth of the segment is attributed to increasing demand for non-invasive surgical procedures.
* The sample copy includes: Report Summary, Table of Contents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/3154
Market Trends
The demand for over-the-counter cosmetic laser and light devices has increased in the recent past. These home use devices use various technologies such as intense pulsed light (IPL) and light-emitting diodes (LED).
Cosmetic or plastic surgery and other scar treatment and related products are available at a very affordable price in China. This can be attributed to high competitiveness among market players.
Regulations
The market is regulated by China’s Good Manufacturing Practices and FDA.
Manufacturer needs to establish a quality management system as China’s FDA guidelines to maintain product consistency and quality throughout
Manufacturer are required to strictly implement GMP with integrity without any falsification
Measurability of quality, consistency and efficacy needs to be well documented to ensure pharmacovigilance
Browse Research Report: https://www.coherentmarketinsights.com/market-insight/china-scar-treatment-market-3154
Sampling, manufacturing, and development of drugs should only be carried out by trained personnel
Location, design, layout, construction, adaption and maintenance of premises should suit the drug production requirements and minimize risk of contamination
China Scar Treatment Market: Competitive Landscape
Major players operating in China scar treatment market include, Cynosure, Inc., Syneron Medical Ltd., Mölnlycke Health Care, Ostar Beauty Sci-Tech Co., Ltd., Shanghai Fosun Pharmaceutical Co. Ltd., Beijing Toplaser Technology Co., Ltd., Merz Pharma, and Luca Pharmaceuticals.
Key Developments
Key players in the market are focused on adopting inorganic growth strategies to expand their product portfolio. For instance, in November 2019, AVITA Medical collaborated with the Gates Center for Regenerative Medicine at the University Of Colorado School Of Medicine to establish proof-of-concept and explore further development of a spray-on treatment of genetically modified cells for patients with epidermolysis bullosa (EB) and other genetic skin disorders.
In December 2019, Janssen Biotech, Inc. entered a definitive agreement to acquire all rights to the investigational compound bermekimab – an anti-IL-1alpha monoclonal antibody for the treatment of atopic dermatitis and hidradenitis suppurativa— from XBiotech Inc.
Buy-Now this research report: https://www.coherentmarketinsights.com/insight/buy-now/3154
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
mailto:[email protected]
U.S. Office:
Name: Mr. Shah
Coherent Market Insights 1001 4th Ave,
# 3200 Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737
0 notes
Link
Americans spend $300 billion a year in prescription drugs and that number is climbing. As is the number of citizens who struggle to pay for the drugs they need to live; 1 in 4 in 2019. It is no wonder that many have turned to the internet for access to pharmacies in countries that boast low drug costs.
This is a double-edged sword. While there are many legitimate and verified companies from which to order, there are just as many if not more looking to scam, or worse, harm you. Find out which Canadian pharmacy is right for you and why!
1. Not All Online Pharmacies Are Created Equal
Online pharmacies are cheaper because they host a much larger clientele than your local pharmacy. Any family struggling to keep up with drug treatments will almost certainly find cheaper answers online. There are even some online options for prescriptions based in the U.S.
Unfortunately, drug costs are much higher in the U.S. than in other countries, especially Canada. Our current administration is even seeking to import drugs directly from Canada, undermining U.S. pharmaceutical companies’ monopoly of the market.
The flaws in this solution are evident in this study that found if even 20% of Americans were able to fill their prescriptions in Canada, the country’s supply of drugs would deplete in just 180 days. Definitely not a long term solution and certainly not going to ease your drug-related financial worries right now.
Therefore, we look to our Northern neighbor as opposed to any in the South or the far West because their drug regulations process and testing are very similar to the standards of the FDA.
This is why Canadian online pharmacies are the first we look to for drug answers.
2. What Does the FDA Have to Say?
According to the FDA in 2015, only 3% of online pharmacies were legitimate. And the WHO stated that 50% of the drugs sold online were counterfeit.
The BeSafeRx campaign was created to help inform the public of the dangers surrounding the world of online drug purchasing. Sadly, many of the links that should be loaded with information for consumers, are broken or missing.
Basically, it is not the Canadian drugs that are risky to purchase. It is the fraudulent websites that lie about where your drugs are coming from, what they are, and even the dosage amounts.
3. You Know It’s Suspicious When:
Here are some serious red flags to look out for when beginning your online pharmacy inquiries:
You don’t need a prescription, especially for controlled substances.
Legitimate drug imports require your physician’s information.
The discount is so amazing it doesn’t seem real.
You can’t reach customer service, or there is none listed.
You found the website by clicking on an ad.
There is an online Canadian pharmacy that wants nothing more than to help you. But help yourself first by avoiding the warning signals listed above.
4. Is It Really That Much Cheaper?
The cholesterol-lowering prescription drug, Crestor, would cost a Canadian $40 a month whereas an American would pay up to $300 a month for the same rx.
In the last decade, Americans have seen Congress go toe-to-toe with pharmaceutical companies whose leaders and CEO’s show no remorse and little responsibility for how their price increases affect the public.
In 2009, Mylan increased the prices of Epipen’s 500%. With the rise in food allergies among children who depend on the swift delivery system of the patented Epipen, this move by Mylan seems unfathomable.
Martin Shkreli of Turing Pharmaceuticals increased the price of an anti-parasitic drug, Daraprim by 5,000%.
According to a Kaiser poll, 3 out of 4 Americans believe that pharma companies put profit before humans. With annual profit margins of 20%, the pharma industry is extremely lucrative.
The FDA can sue, but it does not hold the power to regulate drug prices and prevent such blatant mercenaries from trying to gauge prices unreasonably.
5. The Canadian International Pharmacy Assoc.
Or CIPA for short. These are licensed and accredited Canadian pharmacies. Their website boasts that pharmacies with their seal of approval have served over 10 million U.S. patients without harm since 2002.
At prices up to 80% less than brand-name drugs sold in the U.S.
Check out the full list of CIPA certified online pharmacies here.
6. Guidelines for Safe Purchasing
In addition to looking for the CIPA seal when seeking an online pharmacy, it will also help to look for the National Association of Boards of Pharmacy seal as well.
If you cannot find either of these then you can copy and paste the website into LegitScript and it will tell you whether or not it is approved. It does not give specifics when a website isn’t approved, unfortunately.
If you are still in doubt, call the phone number. One sure way to get your questions answered is to talk to a human.
7. Finding the Best Online Pharmacies
Alongside the CIPA list of approved online pharmacies is the RX Pharmacy Reviews website. This site offers honest and unbiased reviews of hundreds of online pharmacies.
As previously stated, you are not alone in your search for cheaper drugs. While the internet is home to plenty of scandals and scams, it is also a magical place where people come together to help one another.
The Right Online Canadian Pharmacy for You
Drugs are a hot topic for debate and will be for some time. Until the demand for drugs lessens, especially opioids, then the potential for cartels and internet scammers to take advantage will continue to be a real threat.
No one doubts that you are doing the best you can as a U.S. citizen. Unfortunately, that does not always mean you can afford your prescriptions. Leaning on our friends to the North can help ease your financial woes while still achieving the health and life that you deserve.
There are many legitimate Canadian pharmacies that offer affordable prescriptions.
Find the right online Canadian pharmacy for you, put in the work and research now…your wallet will thank you later!
The post 7 Must-Know Facts About Ordering Meds Online From a Canadian Pharmacy appeared first on Biid.org.
0 notes
Photo

Pharma Pricing & Market Access Congress 2019
0 notes
Text
Report: Americans cutting back on meds due to cost, Congress looking into ‘product hopping’
WASHINGTON, D.C. — A new report finds that a growing number of Americans are cutting back on their prescription medications because they can’t afford them.
The report comes out as a Congressional committee is looking at ways to bring the high cost of prescription medications down.
A report this week in the Journal of the American Heart Association said one in eight Americans cut back on prescription heart medication because of the cost.
“Prices are skyrocketing, and people are going bankrupt and even dying because they can’t afford their prescription medication,” Rep. David Cicilline (D-RI) said.
Cicilline has sponsored a bill that targets a drug industry practice known as “product hopping.”
That’s where a drug maker slightly changes a drug’s formula, creating a new 20-year patent and preventing its competitors from selling a cheaper, generic version due to state laws, according to the AMA Journal of Ethics. State laws often then prevent pharmacists from substituting generic versions for the newly-altered drugs.
“Doctors and patients, therefore, have essentially no choice to switch to the new, but not improved, drug, for which the drug company can continue to charge monopoly prices,” said Rep. Jerry Nadler (D-NY).
Nadler said the Congressional committee’s efforts would help Americans physically and financially.
“This legislation will save American taxpayers more than half a billion dollars over a 10-year period,” he said.
The pharmaceutical industry has pushed back on efforts to control drug prices, saying this would end the current market-based system and restrict patient access to medications.
“This conduct focuses on the delivery of profits to big pharma rather than meaningful innovation for sick patients,” Nadler said.
Pharmaceutical industry trade group Pharma said it is, “…committed to working with members of Congress of both sides of the aisle to ensure legislation doesn’t hurt U.S. innovation…”
from FOX 4 Kansas City WDAF-TV | News, Weather, Sports https://fox4kc.com/2019/11/30/report-americans-cutting-back-on-meds-due-to-cost-congress-looking-into-product-hopping/
from Kansas City Happenings https://kansascityhappenings.wordpress.com/2019/11/30/report-americans-cutting-back-on-meds-due-to-cost-congress-looking-into-product-hopping/
0 notes
Text
Democrats Eye Medicare Negotiations to Lower Drug Prices
Democrats, newly in control of Congress and the White House, are united behind an idea that Republican lawmakers and major drugmakers fiercely oppose: empowering the Department of Health and Human Services to negotiate the prices of brand-name drugs covered by Medicare.

This story also ran on Fortune. It can be republished for free.
But they do not have enough votes without Republican support in the Senate for the legislation they hope will lower the price consumers pay for prescription drugs. That raises the possibility that Democrats will use a legislative tactic called reconciliation, as they did to pass President Joe Biden’s covid relief package, or even eliminate the Senate filibuster to keep their promise to voters.
Regardless, Democrats hope to authorize Medicare negotiations on payments for at least some of the most expensive brand-name drugs and to base those prices on the drugs’ clinical benefits. Such a measure could put Republicans in the uncomfortable position of opposing an idea that most voters from both parties generally support.
As chairman of a health and retirement subcommittee, Sen. Bernie Sanders (I-Vt.) on Tuesday was set to hold one of this Congress’ first hearings on drug prices, seen as a way for Sanders and his allies to highlight that drug prices in the United States are among the highest in the world.
Dr. Aaron Kesselheim, a Harvard Medical School professor who researches the drug industry and will testify at the hearing, said there is no practical reason the federal government cannot negotiate a price based on independent assessments of a drug’s clinical benefits — as every other industrialized nation, and even some state Medicaid programs, do.
“The real reason is the drug industry’s lobbying power,” he said.
Negotiating Medicare drug prices has ebbed and flowed as a political issue for years, repeatedly defeated in Congress under pressure from the pharmaceutical industry. The government has been banned from negotiating Medicare drug prices since the creation of the Part D prescription drug benefit in 2006. Instead, the optional private plans through which Americans get Medicare drug benefits negotiate with drugmakers.
It has been two years since Congress summoned executives from Big Pharma companies and pharmacy benefit plans to Capitol Hill for a scolding over skyrocketing prices and the loopholes and secretive contracts they use to block competitors and secure profits.
Despite then-President Donald Trump’s keen interest in lowering drug prices, most proposals by both Democrats and Republicans on Capitol Hill went nowhere under Republican leaders, who argue government intrusion in the free market would hamper future innovation. They point to an estimate from the Congressional Budget Office suggesting the cuts to drugmakers’ revenue under Medicare negotiations could lead to nearly 40 fewer new drugs being developed in the next 20 years.
The government currently approves about 30 drugs per year.
The drug industry, bolstered by its quick efforts to develop a vaccine, has seen public opinion turn in its favor after several years of sharp declines. In early 2020, before the pandemic shut down much of the United States, only about one-third of Americans rated the industry positively, according to a Harris public opinion poll. In February, as vaccination efforts ramped up, about 62% rated it positively — a larger turnaround than any other industry in the past year.
PhRMA, the lobbying organization that represents brand-name drugmakers, came out strong this month against the administration’s first drug-pricing action, a measure in Biden’s sprawling covid relief package that is expected to result in drugmakers paying higher rebates to state Medicaid programs for their drugs.
Brian Newell, a PhRMA spokesperson, suggested the fight is just beginning for Democrats. “The American people reject government price setting when they realize it will lead to fewer new cures and treatments and less access to medicines,” Newell said in a statement. “Our industry has partnered closely with policymakers in fighting the pandemic, and we hope they will partner with us to develop solutions that will lower drug costs for patients, protect access to life-saving medicines and preserve future innovation.”
The Power of Negotiation
Though they disagree on some of the details, such as how far penalties should go, Democrats are united on the need to address drug pricing. Biden, progressives like Sanders and moderates such as Sen. Joe Manchin (D-W.Va.) support proposals that would generally allow the government to set restrictions on brand-name drugs. Researchers say these drugs, initially priced without any competition or regulation, are a leading factor driving up costs for Americans, their employers and the government.
In 2019, the Democratic-controlled House passed legislation that would allow the secretary of Health and Human Services to negotiate the prices for at least 25 of the most expensive drugs marketed in the United States that lack at least one competitor — prices that could be available to people insured by private plans as well. Senate Republicans refused to consider the bill, arguing the policy would discourage drug development.
Top Democrats, including Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee, say that is likely to be incorporated into drug-pricing reform this year.
Under the 2019 House bill, the negotiated price could not exceed 120% of the highest price in one of six other industrialized nations. Drugmakers would face escalating penalties for not complying.
Sanders and some Democrats took a slightly different path in the previous Congress, sponsoring a package that would enable Medicare negotiations, as well as allow the importation of drugs and broadly tie drug prices to median drug prices in Canada, the United Kingdom, France, Germany and Japan.
But party leaders prefer the House proposal for negotiating prices as a model for this year’s efforts.
In addition to allowing negotiated payments for drugs, Democrats also want to cap prices so they could not rise faster than inflation and limit how much Medicare beneficiaries pay out-of-pocket each year.
Democrats say there are more savings to be gained through giving negotiating power to the government, which would have more heft than any individual plan. In 2017, Medicare accounted for about 30% of the nation’s total retail spending on prescription drugs, according to KFF.
Advocates of Medicare negotiation often cite the Veterans Health Administration as a possible model, noting the government already negotiates with drugmakers on behalf of retired service members and often secures drug prices that are about 35% lower than those paid by Medicare beneficiaries.
Flashback to 2019
Fresh off the campaign trail and invigorated by polls showing about 8 in 10 Americans believe drug prices are unreasonable, senior lawmakers from both parties called the leaders of brand-name drugmakers and pharmacy benefit managers to testify about rising drug costs in early 2019.
That year saw a wave of bills introduced, the most ambitious of which constrained the cost of brand-name drugs through direct price controls. Trump, who bucked his party and supported Medicare negotiation and other price-setting measures, offered a series of changes that mostly fell apart under court challenges.
Sen. Chuck Grassley (R-Iowa) and Wyden, then the chairman and top Democrat on the Finance Committee, respectively, unveiled a proposal that, among other measures, would cap the price Medicare pays for brand-name drugs to the pace of inflation and trigger rebates if prices rise too quickly.
Medicaid already uses a similar inflation cap — and tends to pay lower prices on drugs than Medicare. The HHS inspector general has said Medicare could collect billions of dollars from the drug industry if it followed Medicaid’s lead.
But other Republicans refused to support Grassley on the bill, saying inflation caps amount to government intrusion in the free market, and Republican leaders never brought it up for a vote. Even Wyden said he was not sure he could vote for the proposal unless he was afforded an opportunity to offer a broader cost-containment measure, including price negotiation.
“We’re not going to sit by while opportunities for seniors to use their bargaining power in Medicare are frittered away,” Wyden said at the time.
The former legislative partners are still pushing the issue. Grassley has continued to press lawmakers to consider the earlier bill. Wyden has said he intends to “build off the bipartisan work” he did with Grassley and work with the House-passed Medicare negotiation bill as Democrats consider a reform package this year.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
USE OUR CONTENT
This story can be republished for free (details).
Democrats Eye Medicare Negotiations to Lower Drug Prices published first on https://nootropicspowdersupplier.tumblr.com/
0 notes
Text
Democrats Eye Medicare Negotiations to Lower Drug Prices
Democrats, newly in control of Congress and the White House, are united behind an idea that Republican lawmakers and major drugmakers fiercely oppose: empowering the Department of Health and Human Services to negotiate the prices of brand-name drugs covered by Medicare.

This story also ran on Fortune. It can be republished for free.
But they do not have enough votes without Republican support in the Senate for the legislation they hope will lower the price consumers pay for prescription drugs. That raises the possibility that Democrats will use a legislative tactic called reconciliation, as they did to pass President Joe Biden’s covid relief package, or even eliminate the Senate filibuster to keep their promise to voters.
Regardless, Democrats hope to authorize Medicare negotiations on payments for at least some of the most expensive brand-name drugs and to base those prices on the drugs’ clinical benefits. Such a measure could put Republicans in the uncomfortable position of opposing an idea that most voters from both parties generally support.
As chairman of a health and retirement subcommittee, Sen. Bernie Sanders (I-Vt.) on Tuesday was set to hold one of this Congress’ first hearings on drug prices, seen as a way for Sanders and his allies to highlight that drug prices in the United States are among the highest in the world.
Dr. Aaron Kesselheim, a Harvard Medical School professor who researches the drug industry and will testify at the hearing, said there is no practical reason the federal government cannot negotiate a price based on independent assessments of a drug’s clinical benefits — as every other industrialized nation, and even some state Medicaid programs, do.
“The real reason is the drug industry’s lobbying power,” he said.
Negotiating Medicare drug prices has ebbed and flowed as a political issue for years, repeatedly defeated in Congress under pressure from the pharmaceutical industry. The government has been banned from negotiating Medicare drug prices since the creation of the Part D prescription drug benefit in 2006. Instead, the optional private plans through which Americans get Medicare drug benefits negotiate with drugmakers.
It has been two years since Congress summoned executives from Big Pharma companies and pharmacy benefit plans to Capitol Hill for a scolding over skyrocketing prices and the loopholes and secretive contracts they use to block competitors and secure profits.
Despite then-President Donald Trump’s keen interest in lowering drug prices, most proposals by both Democrats and Republicans on Capitol Hill went nowhere under Republican leaders, who argue government intrusion in the free market would hamper future innovation. They point to an estimate from the Congressional Budget Office suggesting the cuts to drugmakers’ revenue under Medicare negotiations could lead to nearly 40 fewer new drugs being developed in the next 20 years.
The government currently approves about 30 drugs per year.
The drug industry, bolstered by its quick efforts to develop a vaccine, has seen public opinion turn in its favor after several years of sharp declines. In early 2020, before the pandemic shut down much of the United States, only about one-third of Americans rated the industry positively, according to a Harris public opinion poll. In February, as vaccination efforts ramped up, about 62% rated it positively — a larger turnaround than any other industry in the past year.
PhRMA, the lobbying organization that represents brand-name drugmakers, came out strong this month against the administration’s first drug-pricing action, a measure in Biden’s sprawling covid relief package that is expected to result in drugmakers paying higher rebates to state Medicaid programs for their drugs.
Brian Newell, a PhRMA spokesperson, suggested the fight is just beginning for Democrats. “The American people reject government price setting when they realize it will lead to fewer new cures and treatments and less access to medicines,” Newell said in a statement. “Our industry has partnered closely with policymakers in fighting the pandemic, and we hope they will partner with us to develop solutions that will lower drug costs for patients, protect access to life-saving medicines and preserve future innovation.”
The Power of Negotiation
Though they disagree on some of the details, such as how far penalties should go, Democrats are united on the need to address drug pricing. Biden, progressives like Sanders and moderates such as Sen. Joe Manchin (D-W.Va.) support proposals that would generally allow the government to set restrictions on brand-name drugs. Researchers say these drugs, initially priced without any competition or regulation, are a leading factor driving up costs for Americans, their employers and the government.
In 2019, the Democratic-controlled House passed legislation that would allow the secretary of Health and Human Services to negotiate the prices for at least 25 of the most expensive drugs marketed in the United States that lack at least one competitor — prices that could be available to people insured by private plans as well. Senate Republicans refused to consider the bill, arguing the policy would discourage drug development.
Top Democrats, including Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee, say that is likely to be incorporated into drug-pricing reform this year.
Under the 2019 House bill, the negotiated price could not exceed 120% of the highest price in one of six other industrialized nations. Drugmakers would face escalating penalties for not complying.
Sanders and some Democrats took a slightly different path in the previous Congress, sponsoring a package that would enable Medicare negotiations, as well as allow the importation of drugs and broadly tie drug prices to median drug prices in Canada, the United Kingdom, France, Germany and Japan.
But party leaders prefer the House proposal for negotiating prices as a model for this year’s efforts.
In addition to allowing negotiated payments for drugs, Democrats also want to cap prices so they could not rise faster than inflation and limit how much Medicare beneficiaries pay out-of-pocket each year.
Democrats say there are more savings to be gained through giving negotiating power to the government, which would have more heft than any individual plan. In 2017, Medicare accounted for about 30% of the nation’s total retail spending on prescription drugs, according to KFF.
Advocates of Medicare negotiation often cite the Veterans Health Administration as a possible model, noting the government already negotiates with drugmakers on behalf of retired service members and often secures drug prices that are about 35% lower than those paid by Medicare beneficiaries.
Flashback to 2019
Fresh off the campaign trail and invigorated by polls showing about 8 in 10 Americans believe drug prices are unreasonable, senior lawmakers from both parties called the leaders of brand-name drugmakers and pharmacy benefit managers to testify about rising drug costs in early 2019.
That year saw a wave of bills introduced, the most ambitious of which constrained the cost of brand-name drugs through direct price controls. Trump, who bucked his party and supported Medicare negotiation and other price-setting measures, offered a series of changes that mostly fell apart under court challenges.
Sen. Chuck Grassley (R-Iowa) and Wyden, then the chairman and top Democrat on the Finance Committee, respectively, unveiled a proposal that, among other measures, would cap the price Medicare pays for brand-name drugs to the pace of inflation and trigger rebates if prices rise too quickly.
Medicaid already uses a similar inflation cap — and tends to pay lower prices on drugs than Medicare. The HHS inspector general has said Medicare could collect billions of dollars from the drug industry if it followed Medicaid’s lead.
But other Republicans refused to support Grassley on the bill, saying inflation caps amount to government intrusion in the free market, and Republican leaders never brought it up for a vote. Even Wyden said he was not sure he could vote for the proposal unless he was afforded an opportunity to offer a broader cost-containment measure, including price negotiation.
“We’re not going to sit by while opportunities for seniors to use their bargaining power in Medicare are frittered away,” Wyden said at the time.
The former legislative partners are still pushing the issue. Grassley has continued to press lawmakers to consider the earlier bill. Wyden has said he intends to “build off the bipartisan work” he did with Grassley and work with the House-passed Medicare negotiation bill as Democrats consider a reform package this year.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
USE OUR CONTENT
This story can be republished for free (details).
Democrats Eye Medicare Negotiations to Lower Drug Prices published first on https://smartdrinkingweb.weebly.com/
0 notes
Text
Cheaper drug prices in the U.S. shouldn’t involve raiding Canada’s medicine cabinet
ANDRÉ PICARD, The Globe and Mail
JULY 29, 2019
On Sunday, U.S. Senator Bernie Sanders travelled to Windsor, Ont., accompanying a group of American patients with Type 1 diabetes who crossed the border to purchase insulin.
The presidential hopeful used the occasion to denounce the “greed” of Big Pharma and promote the idea that Americans should import “cheap” drugs from Canada, an idea he has been touting for two decades.
Since Mr. Sanders first took a busload of Vermont seniors to Montreal in 1999 to fill their prescriptions at a fraction of the price they would pay at home, this cross-border idea has gained momentum.
There are now 27 different legislative initiatives in the U.S. Congress and state legislatures that would allow the importation of prescription drugs from Canada. At least 10 states, as well as U.S. President Donald Trump, have embraced the approach.
But let’s be clear. It’s a ridiculously simplistic idea that is untenable both politically and practically; it needs to be killed and buried, and quickly.
There is one reason that Canada’s drug prices are lower than those in the U.S.: Canada (like almost every developed country) regulates and caps prices, while the United States has a free market free-for-all that encourages price gouging.
If Americans want lower drug prices, their politicians need to put on their big boy pants and regulate, not try to shamelessly raid and pillage the neighbour’s medicine cabinet.
The politicians and advocates who have embraced the idea that Canada is an easy source of cheap drugs are fooling themselves. They clearly do not understand either the complexity of the global drug market, or U.S. or Canadian law.
Very few prescription drugs are actually manufactured in Canada. Our drug supply is largely imported from the United States, Europe and Asia.
Prices of brand name drugs here are set by the Patented Medicine Prices Review Board; they examine prices in seven comparator countries – Italy, France, Germany, Sweden, Switzerland, Britain and the U.S. to ensure the Canadian list price of drugs are not “excessive.” (The PMPRB does not regulate the price of generics.)
The result is that our prescription drug prices are the fourth highest in the world, after Switzerland, Germany and, of course, the U.S.
Manufacturers whose drugs are approved for sale in Canada have a “duty to supply” the market. The quid pro quo is that we forbid re-export, though perhaps the law is not as explicit as it should be on this point.
The U.S. also bans the importation of medications, a rule the proposed laws would like to circumvent.
Both Canada and the U.S., however, tolerate the export-import of small quantities of drugs for personal use. Technically, Americans need to purchase these drugs in person, and can carry a 90-day supply, except for narcotics and biologics.
In recent months, caravans of parents of children with Type 1 diabetes have been advertising their trip to Canada to purchase insulin, largely to pressure U.S. manufacturers to lower prices. But they are sensible enough to realize buying lifesaving medication in Canada is not a long-term solution.
But let’s not forget that insulin is not a prescription drug in Canada.
For Americans to purchase prescription drugs in Canada, they would require a prescription from a Canadian physician.
There are currently a small number of physicians in Canada who hold dual provincial-state licences to cater to cross-border shoppers.
But what the U.S. legislative initiatives seem to suggest is that Americans should be able to buy Canadian drugs online, presumably with a U.S. prescription, or no prescription.
If you know your history, you know that’s not going to happen. (And, as an aside, if the U.S. truly wants cheap drugs, why doesn’t it import from Mexico or India?)
The U.S. Food and Drug Administration has spent more than a decade prosecuting and shutting down online pharmacies, including those based in Canada (or pretending to be based in Canada), because they are a haven for counterfeit and unsafe drugs.
Finally, exporting prescription drugs from Canada to the U.S. would harm Canadian patients because it would almost certainly result in more drug shortages. (However, the causes of drug shortages are complex.)
As a coalition of pharmacists, physicians, nurses and patient groups said in a letterto federal Health Minister Ginette Petitpas Taylor, pharmacies in Canada “are not equipped to support the needs of a country 10 times its size without creating important access and quality issues.”
As self-deluding excitement builds in the U.S. around plans to import cheap Canadian drugs, Ottawa has to stand up and unequivocally throw cold water on this half-baked idea.
0 notes
Text
A Senator From Arizona Emerges As A Pharma Favorite
Sen. Kyrsten Sinema formed a congressional caucus to raise “awareness of the benefits of personalized medicine” in February. Soon after that, employees of pharmaceutical companies donated $35,000 to her campaign committee.
Amgen gave $5,000. So did Genentech and Merck. Sanofi, Pfizer and Eli Lilly all gave $2,500. Each of those companies has invested heavily in personalized medicine, which promises individually tailored drugs that can cost a patient hundreds of thousands of dollars.
Explore The Database
Campaign Contributions Tracker
Pharma Cash To Congress
By Elizabeth Lucas and Sydney Lupkin May 22
A Kaiser Health News database tracks campaign donations from drugmakers over the past 10 years.
Sinema is a first-term Democrat from Arizona but has nonetheless emerged as a pharma favorite in Congress as the industry steers through a new political and economic landscape formed by the coronavirus.
She is a leading recipient of pharma campaign cash even though she’s not up for reelection until 2024 and lacks major committee or subcommittee leadership posts. For the 2019-20 election cycle through March, political action committees run by employees of drug companies and their trade groups gave her $98,500 in campaign funds, Kaiser Health News’ Pharma Cash to Congress database shows.
That stands out in a Congress in which a third of the members got no pharma cash for the period and half of those who did got $10,000 or less. The contributions give companies a chance to cultivate Sinema as she restocks from a brutal 2018 election victory that cost nearly $25 million. Altogether, pharma PACs have so far given $9.2 million to congressional campaign chests in this cycle, compared with $9.4 million at this point in the 2017-18 period, a sustained surge as the industry has responded to complaints about soaring prices.
Sinema’s pharma haul was twice that of Sen. Susan Collins of Maine, considered one of the most vulnerable Republicans in November, and approached that of fellow Democrat Steny Hoyer, the powerful House majority leader from Maryland.
It all adds up to a bet by drug companies that the 43-year-old Sinema, first elected to the Senate in 2018, will gain influence in coming years and serve as an industry ally in a party that also includes many lawmakers harshly critical of high drug prices and the companies that sell them.
Email Sign-Up
Subscribe to KHN’s free Morning Briefing.
Sign Up
Please confirm your email address below:
Sign Up
“This is a long-term play,” said Steven Billet, a former AT&T lobbyist who teaches PAC management at George Washington University. “She’s more of a moderate than people are giving her credit for. If I’m a pharmaceutical guy, I’m saying, ‘You know what? Maybe this is somebody we can work with down the road.’”
The industry’s pivot to Sinema comes as powerful favorites such as former Sen. Orrin Hatch of Utah and retiring Rep. Greg Walden of Oregon, both Republicans, fade from the scene.
Bisexual, an LGBTQ rights advocate and a former member of the Arizona Green Party, Sinema said in 2006 that she was the most liberal member of the Arizona State Legislature, according to HuffPost. These days, representing traditionally conservative Arizona statewide, she portrays herself as a moderate. She favors better medical coverage by improving the insurance company-friendly Affordable Care Act, for example, not by scrapping it in exchange for “Medicare for All.”
“Sinema is a talented politician who knows where she needs to be politically and will get there,” said Nathan Gonzales, editor of Inside Elections, a nonpartisan newsletter.
Sinema’s spokesperson did not respond to queries from KHN.
First elected to the U.S. House in 2012, she has a history of supporting pharmaceutical and biotech firms, dozens of which have operations in Arizona. Her acceptance of drug industry campaign contributions sets her apart from Democrats such as Sen. Cory Booker of New Jersey who have pledged to reject pharma money, not to mention those who spurn all corporate cash.
“The Republican Party tends to be more receptive to pharma cash,” said Paul Jorgensen, a political science professor at the University of Texas Rio Grande Valley, who analyzes campaign finance. “You’re going to see divisions within the party on pharma on the Democratic side.”
In 2017 Sinema introduced a House bill, strongly supported by the Biotechnology Innovation Organization trade group, that would have eased financial regulation on publicly traded biotech firms with little revenue. The measure has not become law, but two weeks later BIO named Sinema “Legislator of the Year,” calling her a “stalwart advocate” for life sciences jobs.
“We welcome the opportunity to work with any policymaker who understands the value of science, the risks, costs and challenges of developing new medicines, and the need to ensure patients have access to medicines with out-of-pocket costs they can afford,” BIO spokesperson Brian Newell said.
Sinema portrayed her backing of a 2016 measure to accelerate the introduction of scarce generic drugs as a blow against high drug costs. A version became law the next year. But support for the bill by the Pharmaceutical Research and Manufacturers of America, the main brand-drug lobby, prompted some to question its potential to bring down overall drug prices.
Sinema was a strong advocate of the biggest overhaul of over-the-counter drug regulation in almost half a century. The measure became law in March with little public notice as part of the CARES Act to rescue the economy and fight the coronavirus. It gives the Food and Drug Administration new leeway to move against possibly dangerous drugs, sets up industry fees to pay for accelerated reviews and creates incentives to bring new medicines to market.
The changes drew widespread, bipartisan support. The old OTC regulation “wasn’t good for anyone,” said Joshua Sharfstein, who was deputy FDA commissioner in the Obama administration. “It wasn’t good for consumers. It wasn’t good for industry.”
The new system resembles the user-fee financing of regulation for prescription drugs. But making the FDA dependent on drug company money for OTC oversight — subject to periodic negotiation with industry — makes the agency beholden to the companies it oversees, said David Hilzenrath, chief investigative reporter for the Project on Government Oversight, a watchdog nonprofit.
Accelerating review of OTC medicines “may be a double-edged sword,” he said. “It could speed decisions that benefit the public and it could speed decisions that put the public at risk.”
Personalized medicine — also known as precision medicine — promises to use genetic characteristics and other traits to identify which treatments are best for a particular patient.
Sinema co-chairs the Personalized Medicine Caucus along with Republican Sen. Tim Scott of South Carolina and two House members. The lawmakers introduced the group in coordination with a pharma industry group, the Personalized Medicine Coalition.
“Raising awareness of the benefits of personalized medicine helps detect and prevent diseases, while making health care more affordable and accessible for Arizona families” was Sinema’s quote in the press release.
But affordability has not been a hallmark of personalized medicine so far. Like other recent pharma products, genetically targeted medicines and tests can come with extremely high prices while sometimes delivering mediocre benefits, health policy analysts say.
One of the best-known precision medicines is Merck’s Keytruda, used against a variety of cancer tumors with certain genetic profiles. It costs more than $100,000 a year.
“It’s a good drug,” said Vinay Prasad, an associate professor at the University of California-San Francisco who studies health policy and cancer drugs. “But behind it is a marketing machine that is trying to maximize its use.”
In any case, personalized medicine generally “has been a mixed bag,” with prices for cancer drugs that are “universally horrendous,” he said. Industry enthusiasm may be “motivated by the fact that when something is called precision or personalized, the regulatory bar needed to approve it is lower,” he added. “And that is often good for profits.”
from Updates By Dina https://khn.org/news/a-senator-from-arizona-emerges-as-a-pharma-favorite/
0 notes
Text
Americans’ Trust in U.S. Healthcare Lags Tech — and Women Are Particularly Cynical
The 2019 Edelman Trust Barometer measured the biggest gap in trust for the healthcare industry between the U.S. “informed public” and the mass population.
Fewer American women, too, trust the healthcare industry than men do.
“This inequality of trust may be reflective of the mass population continuing to feel left behind as compared to others, even as they recognize the advances that are being made that could benefit them. Given tone and tenor of the day, and particularly among mass population, healthcare may continue to see increasing demands for change and regulation,” Susan Isenberg, Edelman’s head of healthcare, notes in her observations of the 2019 Edelman Trust Barometer.
This week, Edelman released more granular healthcare insights from the annual omnibus survey that was published during the World Economic Forum in Davos in January. I analyzed those general results with health/care implications here in Health Populi.
In this study, the healthcare industry is comprised of five segments: hospitals and clinics; pharma; biotech (separate from pharma); consumer health (e.g., over-the-counter medicines); and, health insurance.
Trust in the overall healthcare industry grew by 4 percentage points on a global basis, with a net trust score of 67 points in the Edelman Trust index. This falls behind the top-trusted industry, tech, garnering 78 points, followed by manufacturing, education and automotive at 70, and retail at 69. Healthcare’s score is marginally greater than fashion, energy, consumer packaged goods, and financial services at the bottom at 57 points.
It’s important to note differences in trust across the five healthcare industry segments — and there are big differences. Pharmaceuticals garner the lowest score at 57, compared with biotech and life sciences at 64. While hospitals and clinics have the highest index across the five sectors, there was a decline in trust for providers — whereas the other four segments enjoyed trust increases.
What might account for this drop in trust for healthcare providers? My hypothesis would include the growing high-deductible world where patients facing high-cost hospital bills, sometimes surprise bills due to lack of transparency of costs and/or out-of-network “gotchas.” In addition, there is a growing risk of cybersecurity hacks of patients’ personal health information, with stories reaching local news media on a regular basis.
Compared with other countries, Americans’ trust in hospitals/clinics is on the lower end of the spectrum, just ahead of South Africa, Germany, Turkey, Colombia and Russia. The high index marks for hospitals were found in China at the top end (at 88), Indonesia, UAE, The Netherlands, and Malaysia.
The trust-gap between women and men is striking in the U.S. Not only do fewer women trust American healthcare, but that trust gap is the largest in the world across all countries Edelman polled, shown in the second graph.
As we have noted for several years here on Health Populi, roughly equal proportions of Americans would trust digital companies and large retailers to help them manage personal health as trust health care providers.
Edelman’s Trust Barometer measured consumers’ health tech sentiment, shown in the third graphic which captures peoples’ forecast on how tech will impact healthcare in the next five years. Leading to better outcomes, and empowering clinicians with useful information, could positively shape healthcare in the near term. However, tech could also make healthcare more expensive and create unforeseen issues we’re not prepared to address, consumers also expect.
Health consumers in America in the post Facebook/Cambridge Analytica era are much more attuned to privacy and cybersecurity risks of personal health data. Edelman identified three practices that would help U.S. health citizens in feeling more comfortable sharing their personal data:
The company would place data in the most secure data protection system available
The privacy of personal data would be guaranteed by the government, and
The government or company or provider would guarantee that no one would ever link personal data back to “me.”
Ultimately, to earn and sustain trust with people, healthcare organizations — the five segments — should be transparent about the costs of products and services, provide balanced information about both benefits and side effects, and provide tools and support to help people manage their health.
Health Populi’s Hot Points: There is much to mine for health/care industry stakeholders in Edelman’s 2019 Trust poll at this particular moment-in-time for U.S. health consumers. The first layer concerns how trust between what Edelman segments as the “informed public” versus the mass population. There is a 10 percentage point gap between the informed public’s trust in U.S. healthcare compared with trust among the mass population.
Edelman defines the informed public as U.S. adults ages 25-64, college educated, in the top 25% of household income, who are avid, engaged consumers of media for business and public policy news.
By that definition, the informed public represents a higher socioeconomic stratum than the mass population, are more news-informed, and would likely be employed with access to employer-sponsored health insurance.
It’s no surprise, then, that trust among this U.S. population segment would run substantially higher than that among mainstream Americans.
Secondly, we see a significant gap in healthcare trust among U.S. women compared with men — the highest chasm in the world across all nations Edelman studied.
Susan Isenberg rightly pointed out in her analysis that women tend to be the “Chief Medical Officers” of their households.
I would expand that to be the “Chief Household Officers,” beyond medical and health/care issues. Women determine most health/care spending in their homes. At the same time, women have borne a “pink tax” for household goods and, indeed, healthcare prices, a topic I covered here in the Huffington Post a while ago in an essay I titled, “The Gender Disparity of Taxes: Toys, Tech and Tampons.”
Cost continues to be top-of-mind for healthcare consumers, who largely blame pharma first in the Edelman research. This data point gives further impetus to prescription drug price hearings in Congress and various proposals promulgated on Capitol Hill to address the cost of medicines.
I write this as I received an announcement that Amazon has begun to more visibly market PillPack, the subscription mail service for prescription drugs in convenient medication-adherence packaging.
Amazon is also piloting selling health insurance in India. As health consumers in the U.S. continue to embrace new options for sourcing, consuming and paying for health care services, from “care” to supplies like prescription drugs and food-as-medicine, we can expect people to seek trusted channels for health engagement beyond the legacy system of hospitals, traditional health plans, and pharma.
The post Americans’ Trust in U.S. Healthcare Lags Tech — and Women Are Particularly Cynical appeared first on HealthPopuli.com.
Americans’ Trust in U.S. Healthcare Lags Tech — and Women Are Particularly Cynical posted first on https://carilloncitydental.blogspot.com
0 notes