#Next Generation IVD Market Report
Explore tagged Tumblr posts
Text
Next Generation In Vitro Diagnostics Market, Market Size, Market Share, Key Players | BIS Research
In recent years, the field of healthcare has witnessed remarkable advancements, and one of the pivotal areas driving this transformation is the Next Generation In Vitro Diagnostics (NG IVD) market.
The next-generation IVD market was valued at $86.21 billion in 2022 and is expected to reach $152.62 billion by 2033, growing at a CAGR of 5.41% between 2023 and 2033.
Understanding Next Generation In Vitro Diagnostics:
In vitro diagnostics involve the examination of biological samples outside the human body to detect diseases and monitor health conditions. Next Generation In Vitro Diagnostics, as the name suggests, represents the latest wave of diagnostic technologies that go beyond traditional methods.
The market for next-generation in-vitro diagnostics (IVD) holds considerable growth potential, driven by multiple factors. These include advancements in technology used for IVD, rising aging population, a growing prevalence of chronic diseases, increasing demand for personalized medicine, and the need for more efficient diagnostic solutions. Further, the global IVD market has exhibited consistent growth, and the introduction of next-generation IVD technologies is expected to further fuel market expansion. Market is projected to maintain a robust growth trajectory in the years ahead.
Get a free sample page @ next generation In-Vitro-Diagnostics market
Key Market Drivers:
Advancements in Technology:The NG IVD market is fueled by rapid technological advancements, including high-throughput sequencing, advanced imaging techniques, and the integration of artificial intelligence.
Rising Chronic Diseases:The global increase in chronic diseases, such as cancer, diabetes, and cardiovascular disorders, has intensified the demand for precise and early diagnostic solutions.
Personalized Medicine:The era of personalized medicine is upon us, and NG IVD is a key enabler. By analyzing an individual's genetic makeup and molecular profile, healthcare providers can tailor treatments to the unique characteristics of each patient, optimizing therapeutic outcomes and minimizing adverse effects.
Point-of-Care Testing:NG IVD technologies are driving the development of point-of-care testing devices, allowing for rapid and on-the-spot diagnostics.
Market Segmentation
Segmentation 1: by Type
Segmentation 2: by Product
Segmentation 3: by Application
Segmentation 4: by End User
Segmentation 5: by Region
Market Challenges:
Regulatory Hurdles:The rapid evolution of NG IVD technologies poses challenges for regulatory bodies in ensuring the safety and effectiveness of these diagnostic tools.
Cost Implications:Despite their potential benefits, some NG IVD technologies can be expensive.
Key Companies Profiled
Abbott Laboratories Agilent Technologies, Inc. Becton, Dickinson and Company bioMérieux SA (BioFire Diagnostics) Bio-Rad Laboratories, Inc. Danaher Corporation F Hoffmann-La Roche Ltd. (Roche Molecular Systems, Inc.) Guardant Health
Future Outlook
The Next Generation In Vitro Diagnostics market is poised for continued growth and innovation. As technologies mature and become more accessible, we can anticipate:
Expanded Applications: NG IVD technologies are likely to find new applications across various medical specialties, leading to a broader range of diagnostic solutions.
Integration of Big Data and AI:The integration of big data analytics and artificial intelligence will enhance the interpretation of complex diagnostic data, providing healthcare professionals with actionable insights for more informed decision-making.
Global Accessibility:Efforts to address cost challenges and streamline regulatory processes are expected to increase the global accessibility of NG IVD technologies, ensuring that patients worldwide can benefit from these advancements.
Grab a better understanding visit our vertical page @ Precision Market Report
Conclusion
The Next Generation In Vitro Diagnostics market represents a paradigm shift in the field of healthcare diagnostics. With its potential to provide precise, personalized, and timely information, NG IVD technologies are paving the way for a new era of healthcare that is more focused on prevention, early detection, and targeted treatment strategies.
#Next Generation IVD Market#Next Generation IVD Market Industry#Next Generation IVD Market Report#Next Generation IVD Market Trends#Next Generation IVD Key Players
0 notes
Text
Meticulous Research® Forecasts Middle East IVD Market to Reach $2.15 Billion by 2031
The report highlights market dynamics, growth drivers, key challenges, and opportunities across the region.
Meticulous Research®, a globally recognized leader in market intelligence, has released a new report titled, "Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2031." According to the findings, the Middle East in vitro diagnostics (IVD) market is projected to reach $2.15 billion by 2031, growing at a compound annual growth rate (CAGR) of 4.1% from 2024 to 2031.
Key Market Drivers
The growth of the Middle East IVD market is driven by several factors, including the rising prevalence of chronic and infectious diseases, increased demand for point-of-care (PoC) and rapid diagnostic solutions, expanding healthcare expenditures, and an aging population. Moreover, growing healthcare awareness, especially in countries such as the UAE and Saudi Arabia, is also contributing to market expansion. However, the high costs associated with IVD tests and inconsistencies in results from rapid diagnostic tests present challenges to sustained growth.
Download Sample Report Here: https://www.meticulousresearch.com/download-sample-report/cp_id=5764
Emerging Opportunities
Increasing awareness around early disease detection and personalized medicine, particularly in the UAE and Saudi Arabia, is creating opportunities for market players. These regions are witnessing an uptick in the adoption of advanced diagnostic technologies, which is expected to fuel demand in the coming years. Nonetheless, issues such as false positives in immunoassays and PoC tests remain significant hurdles that stakeholders need to address.
Competitive Landscape
The Middle East IVD market is dominated by global healthcare giants such as Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), and Siemens Healthineers AG (Germany). Other key players include QIAGEN N.V. (Netherlands), Thermo Fisher Scientific Inc. (U.S.), and Bio-Rad Laboratories, Inc. (U.S.), among others.
Request Sample Report Here: https://www.meticulousresearch.com/request-sample-report/cp_id=5764
Market Segmentation Insights
The report provides a detailed breakdown of the Middle East IVD market based on various parameters:
Offerings: The kits and reagents segment is expected to register the highest growth rate due to their extensive use in diagnosing chronic and infectious diseases. This is attributed to the increasing volume of tests for diseases such as HIV and influenza, alongside growing consumer awareness of self-testing options.
Technologies: Molecular diagnostics, including PCR and next-generation sequencing (NGS), is expected to capture the largest market share in 2024, driven by the rising prevalence of communicable and non-communicable diseases and the need for high-volume, accurate testing.
Applications: Infectious disease testing is set to dominate the market, with respiratory infections, hepatitis, HIV, and sexually transmitted diseases (STDs) being the primary drivers. The region's aging population and increasing healthcare demand are also contributing to the rise in testing.
Diagnostic Approach: Point-of-care testing is expected to exhibit the highest growth, propelled by the increasing burden of chronic diseases and the launch of innovative PoC diagnostics by market leaders.
End Users: Hospitals and clinics will continue to be the largest segment, performing a wide range of diagnostic tests for various medical conditions, particularly in urban areas where access to healthcare services is more robust.
Access Full Report Details Here : https://www.meticulousresearch.com/request-sample-report/cp_id=5764
Country Insights
Among the Middle Eastern countries, Saudi Arabia is projected to dominate the IVD market in 2024, largely due to its rapidly aging population, the high incidence of chronic and infectious diseases, and the growing adoption of self-testing. The Kingdom’s healthcare sector is also experiencing a boost thanks to its Vision 2030 strategy, which aims to elevate healthcare standards and increase investments in medical technologies. Healthcare expenditure in Saudi Arabia is expected to grow annually by 3.0% from 2022 to 2025, further supporting market growth.
Conclusion
The Middle East IVD market presents lucrative opportunities for growth, driven by evolving healthcare needs, technological advancements, and rising awareness around early disease detection. However, market players must navigate challenges related to pricing and diagnostic accuracy to fully capitalize on the region’s potential.
Quick Buy: https://www.meticulousresearch.com/Checkout/69732912
About Meticulous Research®
Meticulous Research® is a globally recognized market research and consulting firm known for delivering in-depth insights across a variety of industries. We provide high-quality market intelligence and strategic guidance to clients across the globe.
Contact Information Email: [email protected] Sales Inquiry: +1-646-781-8004 Connect with us on LinkedIn: Meticulous Research® LinkedIn
0 notes
Text
The global demand for In-Vitro Diagnostics (IVD) was valued at USD 117545.8 Million in 2023 and is expected to reach USD 182352.1 Million in 2032, growing at a CAGR of 5.00% between 2024 and 2032.In-Vitro Diagnostics (IVD) is a critical segment of the healthcare industry, playing a vital role in the detection, diagnosis, and monitoring of diseases. These tests are conducted on samples such as blood, urine, or tissues, and they provide crucial information for making clinical decisions. The IVD market has witnessed substantial growth in recent years, driven by technological advancements, an aging population, and a rising prevalence of chronic diseases. This article delves into the current state of the IVD market, key trends, opportunities, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/in-vitro-diagnostics-ivd-market
Current Market Landscape
The global IVD market is experiencing robust growth, with projections estimating its value to reach USD 113.1 billion by 2025, growing at a CAGR of 4.7% from 2020 to 2025. North America holds the largest market share, followed by Europe and Asia-Pacific. The high prevalence of chronic and infectious diseases, advanced healthcare infrastructure, and strong R&D capabilities are key factors driving market growth in these regions.
Key Drivers of Growth
1. Technological Advancements: Innovations in molecular diagnostics, next-generation sequencing (NGS), and point-of-care testing (POCT) have revolutionized the IVD market. These technologies offer faster, more accurate, and less invasive diagnostic solutions, enhancing patient outcomes and streamlining clinical workflows.
2. Aging Population: The global population is aging, leading to an increase in age-related diseases such as cardiovascular diseases, diabetes, and cancer. The elderly population is more susceptible to these conditions, necessitating frequent diagnostic tests, thereby driving the demand for IVD products.
3. Rising Prevalence of Chronic Diseases: The increasing incidence of chronic diseases, including diabetes, cancer, and cardiovascular diseases, has significantly boosted the demand for IVD. Early and accurate diagnosis is crucial for effective disease management, making IVD an essential tool in healthcare.
4. Pandemic Impact: The COVID-19 pandemic has underscored the importance of diagnostic testing. The rapid development and deployment of COVID-19 tests have accelerated the growth of the IVD market, highlighting the need for robust diagnostic infrastructure to manage public health crises.
Key Market Segments
The IVD market is segmented based on product type, technology, application, and end-user.
1. Product Type: Reagents and kits dominate the market, followed by instruments and software. The continuous demand for reagents and kits for routine diagnostics and research purposes fuels this segment's growth.
2. Technology: Immunoassays, molecular diagnostics, clinical chemistry, microbiology, and hematology are the leading technologies in the IVD market. Molecular diagnostics, particularly PCR and NGS, are witnessing rapid growth due to their accuracy and speed.
3. Application: The market is categorized into infectious diseases, oncology, cardiology, endocrinology, and others. Infectious diseases hold the largest share, driven by the need for rapid and accurate diagnostic tests for diseases such as COVID-19, HIV, and tuberculosis.
4. End-User: Laboratories, hospitals, academic institutions, and point-of-care settings are the primary end-users of IVD products. Laboratories account for the largest share, attributed to the high volume of diagnostic tests conducted in these settings.
Opportunities and Challenges
The IVD market presents numerous opportunities, including the growing adoption of personalized medicine, the expansion of healthcare infrastructure in emerging economies, and the increasing focus on preventive healthcare. Personalized medicine, which tailors treatment to individual patients based on their genetic makeup, relies heavily on advanced diagnostic tests, offering significant growth potential for the IVD market.
However, the market also faces challenges. Regulatory hurdles, reimbursement issues, and the high cost of advanced diagnostic tests can hinder market growth. Additionally, the need for highly skilled professionals to operate sophisticated diagnostic equipment and interpret results poses a challenge in many regions.
Future Prospects
The future of the IVD market looks promising, with ongoing advancements in technology and a greater emphasis on early disease detection and prevention. Artificial intelligence (AI) and machine learning (ML) are set to play a pivotal role in diagnostics, enabling more accurate and efficient analysis of test results. The integration of digital health technologies and the increasing use of telemedicine are also expected to drive the demand for IVD products.
Key Players
Invitae Corporation
Freenome Holdings, Inc.
Natera, Inc.
F. Hoffmann-La Roche Ltd.
InterVenn Biosciences
Guardant Health
Agilent Technologies, Inc.
Exact Sciences Corporation
Illumina, Inc.
NeoGenomics Laboratories
Thermo Fisher Scientific, Inc.
Segmentation
By Product Type:
Reagents and Kits
Instruments
Data Management Software
By Technology:
Clinical Chemistry
Immunoassay/Immunochemistry
Molecular Diagnostics
Hematology
Coagulation and Hemostasis
Microbiology
Point-of-Care Testing (POCT)
Others
By Application:
Infectious Disease Testing
Oncology/Cancer Testing
Diabetes Testing
Cardiology Testing
Nephrology Testing
Autoimmune Disease Testing
Drug Testing/Pharmacogenomics
Others
By End-User:
Hospitals and Clinics
Diagnostic Laboratories
Academic and Research Institutes
Home Care
Others
By Usability:
Disposable IVD Devices
Reusable IVD Devices
By Type of Sample:
Blood Testing
Urine Testing
Cerebrospinal Fluid (CSF) Testing
Saliva Testing
Others
By Automation:
Fully Automated IVD Systems
Semi-Automated IVD Systems
By Point-of-Care Testing (POCT):
Glucose Monitoring
Pregnancy and Fertility Testing
Infectious Disease POCT
Cardiac Markers POCT
Others
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/in-vitro-diagnostics-ivd-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Cancer Diagnostics Market on Track for Remarkable Year of Growth
The Cancer Diagnostics Market, valued at USD 138.2 Billion in 2022, is poised to reach USD 279.7 Billion by 2032, with a robust CAGR of 7.4%.
This market is undergoing significant growth and evolution driven by various factors. Firstly, advancements in technology, particularly in molecular diagnostics and imaging techniques, have transformed cancer diagnosis, enabling more precise and timely detection of the disease. Molecular diagnostic methods like polymerase chain reaction (PCR) and next-generation sequencing (NGS) have bolstered the capacity to identify specific genetic mutations and biomarkers linked to various cancer types, facilitating tailored treatment approaches.
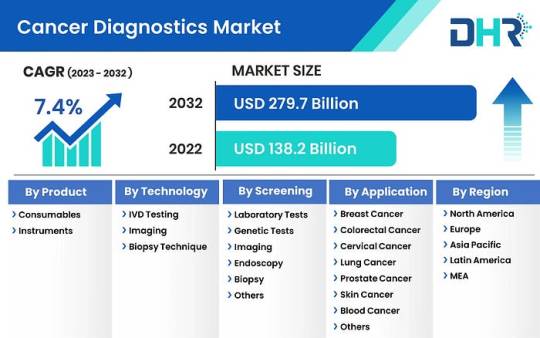
The growth of the cancer diagnostics Market is influenced by several factors, including:
· Technological advancements: Innovations in molecular diagnostics, imaging techniques, and artificial intelligence enhance the accuracy and efficiency of cancer detection.
· Rising cancer prevalence: Increasing incidence rates globally drive the demand for diagnostic tests and screening programs.
· Emphasis on early detection: Healthcare initiatives and awareness campaigns promote early diagnosis, improving patient outcomes and driving market growth.
· Personalized medicine: Tailored treatment approaches based on genetic profiling and biomarker analysis increase the demand for diagnostic tools that enable precision medicine.
· Regulatory landscape: Regulatory approvals and reimbursement policies impact the adoption of new diagnostic technologies and tests.
· Collaboration and partnerships: Collaboration between industry players, research institutions, and healthcare providers accelerates innovation and market expansion.
Top Companies are:
· GE Healthcare
· Abbott
· F. Hoffmann-La Roche Ltd.
· Qiagen N.V.
· BD
· Siemens Healthcare GmbH
· Quest Diagnostics
· Thermo Fisher Scientific, Inc.
· Hologic, Inc.
· Koninklijke Philips N.V. (Philips)
· Illumina, Inc.
Market Segmentations:
By Product- Consumables, Instruments
By Technology- IVD Testing, Imaging, Biopsy Technique
By Screening- Laboratory Tests, Genetic Tests, Petroleum Refining, Endoscopy, Biopsy, Others
By Application- Breast Cancer, Colorectal Cancer, Cervical Cancer, Lung Cancer, Prostate Cancer, Skin Cancer, Blood Cancer, Kidney Cancer, Liver Cancer, Pancreatic Cancer, Ovarian Cancer, Others
For Further Information Regarding this Report: Ask For Discount:
Regional Analysis
North America is poised to maintain its dominance in the cancer diagnostics market, bolstered by its well-established healthcare infrastructure and significant contributions to the sector. The United States stands as the largest contributor, owing to its advanced healthcare system and substantial investments in cancer research and diagnostics. Moreover, the region’s market growth is fueled by the escalating incidence of cancer. For instance, the American Cancer Society estimates approximately 1.9 million new cancer cases and expects around 0.61 million fatalities in 2022.
Following North America, Europe emerges as the second-largest market for cancer diagnostics, with countries like Germany, France, and the United Kingdom holding considerable market shares. The region benefits from robust healthcare systems, government initiatives, and extensive research and development activities. According to research conducted by the University of Milan, the European Union is expected to witness around 1.2 million cancer deaths in 2022, highlighting the significant impact of the disease in the region.
Competitive Landscape:
In the competitive landscape of the cancer diagnostics market, numerous players vie for prominence through a spectrum of innovative solutions, strategic collaborations, and expansive market initiatives. Leading the forefront are established giants like Abbott Laboratories, Roche Diagnostics, and Thermo Fisher Scientific, renowned for their diverse portfolios encompassing molecular diagnostics, imaging technologies, and companion diagnostics. Siemens Healthineers and Bio-Rad Laboratories also wield significant influence with their advanced diagnostic imaging and molecular assay offerings. Additionally, Illumina, Qiagen N.V., and Hologic, Inc. play pivotal roles, leveraging their expertise in genomics, molecular diagnostics, and women’s health diagnostics, respectively, to address the multifaceted challenges of cancer detection and management.
Key Highlights Of the Report:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
The Report Answers Questions Such As:
· How is the cancer diagnostics market expected to grow in the future?
· What is the current market size and projected value for the cancer diagnostics market?
· What factors are fueling demand in the cancer diagnostics market?
· Which segment leads the industry?
· What investment opportunities show potential in the market?
About DataHorizzon Research:
DataHorizzon is a market research and advisory company that assists organizations across the globe in formulating growth strategies for changing business dynamics. Its offerings include consulting services across enterprises and business insights to make actionable decisions. DHR’s comprehensive research methodology for predicting long-term and sustainable trends in the market facilitates complex decisions for organizations.
0 notes
Text
Molecular Diagnostics Market Statistics and Global Analysis Report 2030
The Global Molecular Diagnostics Market was valued at US$ 15,843.6 Million in 2022 and is anticipated to reach US$ 36,766.9 Million by the end of 2030 with a CAGR of 11.1% from 2023 to 2030.
Make The Smart Decision. Download A Free Sample Of Our Report @ https://cognizancemarketresearch.com/request/molecular-diagnostics-market/
Businesses are increasingly conducting high-throughput molecular-level testing for infectious diseases. Companies are now offering molecular diagnostic tests that can identify various pathogens with a single patient sample.
The market’s growth is driven by large-scale bacterial and viral epidemics worldwide, the need for point-of-care diagnostics, advancements in pharmacogenomics, and rapidly evolving technology. According to the India TB Report 2022, the number of new and relapsed TB cases registered in India in 2022 was 1,933,381, compared to 1,628,161 in 2023. This suggests that increasing patient numbers and prevalence will drive demand for molecular diagnostics, boosting the market.
Due to the automation of the research process, molecular diagnostic systems are in high demand. These systems automate DNA extraction, amplification, and data analysis, resulting in improved productivity, accurate data, and faster results, all of which support the industry’s growth.
Molecular Diagnostics Market Amid COVID-19 Pandemic
Covid-19 has had a favorable effect on the global market for molecular diagnostics. The core of the COVID-19 reaction during the coronavirus disease pandemic is diagnostic. It helps with the containment efforts made to lessen the outbreak. Also, the number of businesses expanding their regional reach as a result of COVID-19 IVD test approval is anticipated to support market expansion.
Globally, the disturbance in the distribution networks around the globe has a significant impact on the seamless supply chains that guarantee higher quality, safety, and new techniques for transmission across the healthcare industry. With the quick development of diagnostics, expedited regulatory clearances, and increased dissemination in multiple locations to help slow the virus’ spread, COVID-19 has shone a spotlight on the global molecular diagnostics sector.
Due primarily to rising demand for polymerase chain reaction (PCR) tests, serology-based rapid-test products, next-generation sequencing (NGS), and a substantial increase in the target patient population, the demand for diagnostic products as a result of COVID-19 is anticipated to expand. By increasing the COVID-19 PCR catalog on the Cobas 5800 System in February 2022, for instance, Roche continued to give more individuals access to precise, quick, and efficient treatments in the battle against COVID-19. Because of this, more COVID-19 molecular diagnostic tests may be used, which would fuel the post-pandemic market’s expansion.
Increasing Prevalence of contagious disease, rapid technological advancement, growing R & D investment to Boost in Molecular Diagnostics Market
Molecular diagnostics is the method of identifying diseases by examining molecules such as proteins, DNA, and RNA in a tissue or liquid form. It is a group of techniques that apply molecular science to laboratory trials to dissect natural markers in the genome and proteome, as well as how cells express their genetics as proteins. In medicine, the technique is used to assess and track sickness, identify risks, and determine which therapies will work best for specific individuals. Agricultural biosecurity also includes crop and animal infection monitoring, risk assessment, and the selection of appropriate isolation measures. The most effective method for locating and describing a microbe is thought to be molecular diagnostics.
The rising prevalence of infectious disorders is the main factor propelling the growth of the global molecular diagnostics market. The main causes of morbidity and mortality in catastrophes, according to the WHO, are acute respiratory infections, measles, diarrheal diseases, and vector-borne diseases. The effects of seasonal influenza epidemics in impoverished countries are unknown, however, research has shown that 99% of children under the age of 5 who have lower respiratory tract illnesses connected to influenza die in these countries.
Rapid technical improvements are also accelerating the market’s expansion. For instance, molecular diagnostics is crucial in the diagnosis of infectious diseases because it provides quick and effective results. It functions with accuracy, economy, and portability. Moreover, advances in molecular diagnostics have helped in disease identification and are particularly useful when dealing with challenging differential diagnoses. Many organizations are enhancing their items by utilizing new strategies to obtain precise results that are also anticipated to support the market’s expansion. For the examination of malignancies, companies like QIAGEN and Sigma Aldrich Corporation are developing novel techniques like Transcription-Mediated Amplification & Loop-Mediated Isothermal Amplification (LAMP).
Yet, rising diagnostic costs and a lack of uniform and clear regulatory frameworks could restrain industry expansion. Despite this, increasing R&D spending may present more chances for the molecular diagnostics industry to expand.
North America to spearhead the Molecular Diagnostics market
The molecular diagnostics market is poised to lead the global market’s development in North America, driven by several key factors. Firstly, rising consumer awareness and an increase in disease occurrence contribute to the region’s growth. Additionally, technological advancements and a robust healthcare system in North America further facilitate the accessibility and availability of modern technologies such as molecular diagnostics.
Collaboration and association between research hospitals and academic institutions in the region play a pivotal role in driving market expansion. These organizations actively engage in discussions to enhance the molecular diagnosis process. Notably, the United States dominates this market, holding the largest income share due to its substantial existing market and advanced adoption of molecular diagnostics.
Moreover, the increasing incidence of contagious diseases in North America has prompted both public and private institutions to make significant investments in healthcare infrastructure and research and development to address the growing patient population.
Furthermore, the regulatory environment in North America is becoming more favorable, simplifying the launch of new drugs as regulatory agencies establish clearer policies and procedures for their approval.
Lastly, the region’s growing geriatric population, coupled with an emphasis on advanced technologies by leading players, contributes to the continuous development of the molecular diagnostics market in North America
Make The Smart Decision. Download A Free Sample Of Our Report @ https://cognizancemarketresearch.com/request/molecular-diagnostics-market/
Numerous key players in the global molecular diagnostics market, such as BD, bioMérieux SA, Bio-Rad Laboratories, Inc., Abbott, Agilent Technologies, Inc., Danaher, Hologic Inc. (Gen Probe), Illumina, Inc., Johnson & Johnson Services, Inc., Grifols, S.A., QIAGEN, F. Hoffmann-La Roche, Ltd., Siemens Healthineers AG, and Sysmex Corporation, are actively employing a range of strategies to enhance their market presence and revenue generation.
0 notes
Text
Global IVD Raw Materials Market Is Estimated To Witness High Growth Owing To Increasing Demand for In vitro Diagnostic Products

The global IVD raw materials market is estimated to be valued at US$ 24,340.0 Mn in 2022 and is expected to exhibit a CAGR of 5.8% over the forecast period 2022-2028, as highlighted in a new report published by Coherent Market Insights. Market Overview: The IVD raw materials market refers to the market for materials that are used in the production of in vitro diagnostic products. In vitro diagnostic products are medical devices that are used for the diagnosis of diseases or monitoring of health conditions using samples taken from the human body. These products play a crucial role in clinical decision making and improving patient outcomes. The market for IVD raw materials is driven by the increasing demand for in vitro diagnostic products, especially with the rise in chronic diseases and the aging population worldwide. The advancements in technology, such as the development of more sensitive and accurate diagnostic tests, have also contributed to the growth of this market. Additionally, government initiatives to promote early disease detection and prevention have further boosted the demand for IVD raw materials. Market Key Trends: One key trend in the IVD raw materials market is the increasing adoption of molecular diagnostics. Molecular diagnostics is a branch of in vitro diagnostics that involves the detection and measurement of genetic markers or biomarkers associated with diseases. This technology allows for faster and more accurate diagnosis, leading to better patient outcomes. For example, polymerase chain reaction (PCR) is widely used for the detection of infectious diseases, genetic disorders, and cancer. The growing applications of molecular diagnostics are driving the demand for specialized raw materials such as nucleic acid extraction kits, enzymes, and reagents. PEST Analysis: Political: The IVD raw materials market is influenced by government regulations and policies related to healthcare and the medical device industry. Changes in regulations can impact the market dynamics and the entry of new players in the market. Economic: The economic factors that affect the IVD raw materials market include healthcare expenditure, reimbursement policies, and income levels of individuals. Increasing healthcare spending and favorable reimbursement policies are expected to drive market growth. Social: The increasing prevalence of chronic diseases and the aging population are key social factors driving the demand for in vitro diagnostic products and, in turn, the IVD raw materials market. Technological: Technological advancements in molecular diagnostics, such as the development of next-generation sequencing (NGS) and digital PCR technologies, are driving innovation in the IVD raw materials market. Key Takeaways: Paragraph 1: The global IVD Raw Materials Market Analysis is expected to witness high growth, exhibiting a CAGR of 5.8% over the forecast period, due to increasing demand for in vitro diagnostic products. The rising incidence of chronic diseases and the aging population are driving the demand for accurate and reliable diagnostic tests. Paragraph 2: In terms of regional analysis, North America is expected to dominate the IVD raw materials market due to the presence of key market players, well-established healthcare infrastructure, and high healthcare expenditure. The Asia Pacific region is projected to be the fastest-growing market due to the increasing healthcare spending, growing awareness about early disease detection, and improving healthcare infrastructure. Paragraph 3: Key players operating in the global IVD raw materials market include Aalto Bio Reagents, Fapon Biotech, Fujirebio, Merck KgaA, F. Hoffmann-La Roche, and Thermo Fisher Scientific, among others. These companies are focused on product development, mergers and acquisitions, and collaborations to expand their market presence.
#IVD Raw Materials#IVD Raw Materials Market#IVD Raw Materials Market Share#IVD Raw Materials Market Size#IVD Raw Materials Market Demand#Pharmaceutical
0 notes
Link
HAIFA, Israel, July 1, 2019 /PRNewswire/ -- MeMed Ltd., the developer of a cutting-edge immune system-based diagnostic that distinguishes between bacterial and viral infections at the point of care, today announced its recognition as a 2019 World Economic Forum Technology Pioneer, one of 56 companies selected worldwide.
The Technology Pioneers are a global community of early- to growth-stage companies poised to transform a wide range of industries and have a significant impact on business and society. The World Economic Forum selected MeMed for its ground-breaking approach to helping doctors avoid prescribing ineffective medicines and treatments, thus combating the rise of drug-resistant pathogens.
"We're excited to welcome MeMed to this year's innovative class of technology pioneers," says Fulvia Montresor, Head of Technology Pioneers at the World Economic Forum. "MeMed and its fellow pioneers are leaders in using novel technologies to transform their industries. We see great potential for these next generation companies to shape solutions to global challenges and improve society for years to come."
MeMed's mission is to translate the immune system's complex signals into simple diagnostic insights that can be used to transform the way infectious diseases and inflammatory disorders are diagnosed and treated, profoundly benefiting patients at both the individual and societal level. The Israel-based company's breakthrough immune-based protein signature MeMed BV™ quickly and reliably determines whether an infection is caused by bacteria that will respond to an antibiotic, or by a virus which antibiotics cannot treat. MeMed BV™ has been validated by an unprecedented level of high-quality data from double-blinded clinical studies conducted worldwide.
"It's an honor to be recognized as a 2019 Technology Pioneer by the World Economic Forum and join a community of mission-driven companies working to improve lives on a global scale," said Dr. Eran Eden, Co-Founder and CEO of MeMed. "Our goal is to develop solutions to the world's greatest healthcare challenges and make a meaningful difference in patients' lives, beginning with antimicrobial resistance."
Dr. Kfir Oved, MeMed's Co-Founder and CTO, added: "We're delighted by this recognition from the World Economic Forum. It reaffirms our commitment to enabling broad access to quick, effective diagnostic tests and a cultural paradigm shift away from overprescribing antibiotics. These are critical steps to preventing the emergence and spread of drug-resistant bacteria and protecting public health."
Drug-resistant diseases cause at least 700,000 deaths worldwide every year, "a figure that could increase to 10 million deaths globally per year by 2050," according to a United Nations report released in April 2019. To accelerate efforts to combat this threat, MeMed also developed MeMed Key™, an easy to use platform that measures proteins with high precision at the point of care and runs MeMed BV™ in 15 minutes. The platform will also support the expansion of MeMed's pipeline of innovative diagnostic tests, which integrate machine learning capabilities with immune-based measurements, to address a range of clinical challenges.
MeMed joins a diverse cohort of 2019 Technology Pioneers selected by an international committee of more than 59 academics, entrepreneurs, venture capitalists and corporate executives brought together by the World Economic Forum. Past honorees include Airbnb, Editas Medicine, Foundation Medicine, Google, Kickstarter, Mozilla, Proteus Digital Health, Spotify and Twitter.
Eden will participate in the World Economic Forum Annual Meeting of the New Championsin Dalian, China, July 1-3, 2019. His presentation on the public health concerns that stem from misusing and overprescribing antibiotics will take place at 11:45 am (local time) on Wednesday, July 3, 2019.
About The World Economic Forum: The World Economic Forum, committed to improving the state of the world, is the International Organization for Public-Private Cooperation. The Forum engages the foremost political, business and other leaders of society to shape global, regional and industry agendas. (www.weforum.org).
About MeMed: MeMed is the developer of a cutting-edge immune system-based diagnostic that distinguishes between bacterial and viral infections at the point of care. Our mission is to translate the immune system's complex signals into simple diagnostic insights that can be used to transform the way infectious diseases and inflammatory disorders are diagnosed and treated, profoundly benefiting patients at both the individual and population levels. We developed and validated MeMed BV™, our pioneering immune-based protein signature, over the course of decade-long collaborations with leading academic and commercial partners, providing physicians with an indispensable tool in the fight against resistant strains of bacteria – one of the biggest healthcare challenges of our time. An ELISA format of MeMed BV™, called ImmunoXpert™, is cleared for use in the European Union (CE-IVD), Switzerland and Israel and is currently in pilot distribution in these markets. MeMed also developed MeMed Key™, a groundbreaking platform that makes it possible to precisely measure multiple proteins and signatures, both existing and new, within minutes at the point of care. Today, we are expanding our network of partnerships with internationally renowned academic, commercial and government stakeholders to advance, validate and facilitate the global availability of our platform and tests. For additional information on MeMed, please visit http://www.me-med.com.
MeMed Contacts:
Media: Adee Mor, VP Marketing, MeMed [email protected]
Investor: Kfir Emmer, VP Finance, MeMed +972-4-8500302, [email protected]
4 notes
·
View notes
Text
Detailed Report on Biomarker Market | BIS Research

Biomarkers are measurable indicators found in biological materials such as blood, urine, tissues, or other bodily fluids, whose presence, absence, or quantity can signify normal biological processes, pathogenic processes, or pharmacological responses to therapeutic interventions.
The global Biomarker Market was valued at $24.80 billion in 2023 and is expected to reach $53.20 billion by 2033, growing at a CAGR of 7.93% between 2023 and 2033.
Biomarker Market Overview
The biomarkers market is a dynamic and rapidly evolving sector within the healthcare industry that encompasses a wide range of activities related to the discovery, development, and commercialization of biomarkers. Biomarkers, which are measurable indicators found in biological materials, play a crucial role in medical research, clinical diagnostics, and drug development.
Key Components of Biomarker Market
Biomarker Discovery and Development
Diagnostics Tests and Tools
Clinical Applications
Drug Development and Personalized Medicines
Regulatory and Compliance Framework
Applications of Biomarker Market
Disease Diagnosis
Prognosis and Risk Management
Therapeutic Monitoring
Personalized Medicine
Drug Development
Companion Diagnostics
Veterinary Vaccines
Download the report and get to know the interesting facts Click Here !
Market Drivers
Advancement in Technology
Increasing Prevalence of Chronic Diseases
Emphasis on Personalized Medicines
Aging Population
Rise in Healthcare Expenditure
Grab a look at our sample page click here!
Market Segmentation
1 By Type
Biomarker Type
2 By Application
Diagnostics
Drug Development and Discovery
Personalized Medicines
3 By Disease Identification
Next Generation Sequencing
Polymerase Chain Reaction
Immunoassays
Bioinformatics
4 By Region
North America
Europe
Asia Pacific
Latin America
Click here to visit our Precision medicine page !
Uses of Biomarker Market
Disease Diagnosis
Prognosis and Risk Management
Therapeutic Monitoring
Personalized Medicine
Key Players in Biomarker Market
Abbott Laboratories
Agilent Technologies, Inc.
ALCEN
Recent Developments in the Global Biomarker Market
•In August 2023, Quest Diagnostics launched the AD-Detect test for Alzheimer’s disease in the U.S., offering consumers the first opportunity to acquire and evaluate a blood-based biomarker test for assessing the potential risks of developing AD
•In September 2023, Becton, Dickinson and Company partnered with Navigate BioPharma Services, Inc. to develop and commercialize flow cytometry-based companion diagnostics and clinical decision tools. The collaboration combined Navigate BioPharma's expertise in biomarker assay design for clinical trials with BD's extensive portfolio of flow cytometry instruments, reagents, software, and in vitro diagnostics (IVD) development services.
Key Question Answers
QWhat are the major market drivers, challenges, and opportunities in the global Biomarker Market Biomarkers market?
Q What are the business development strategies, such as business expansion, acquisitions, and funding, which are implemented by the major players to sustain in the competitive market?
Q Which is the dominant product and service type developed by the leading and emerging players for Biomarker Market Biomarkers?
QHow is each segment of the market expected to grow during the forecast period from 2023 to 2033?
Conclusion
The Biomarkers Market is a rapidly evolving and essential component of modern healthcare and biomedical research. Its growth is driven by several factors, including the increasing prevalence of chronic diseases, advancements in technology, and the rising emphasis on personalized medicine.
0 notes
Text
Brexit draft agreement for medical devices: What does it mean?
The UK government has released a draft agreement regarding the withdrawal of the UK from the European Association along with a joint statement and diagram of a political declaration on the future relationship between the UK and the EU.
The draft withdrawal document comprises of 585 pages and contains numerous segments detailing what will happen to certain ventures should this document be officially agreed as the Brexit deal.
Brexit draft agreement for medical devices The part covering Medical Devices Industry Top M&A deals Q3 2022 records five regulations and orders that will be in place for the medical gadget industry in case of this deal.
For more insights on the sub-sectors of M&A deals in the medical devices sector in Q3 2022, download a free report sample
Be that as it may, what do each of the regulations and mandates actually mean? Coming up next is a summary of each list item in the medical devices category and an indication of what the Brexit draft agreement means for medical devices. The document recommends that all of the accompanying will in any case be in place after Brexit has happened:
Board Mandate 93/42/EEC of 14 June 1993 concerning medical devices
This is an order which is planned to harmonize the laws relating to medical devices inside the EU. Having this order in place after Brexit will mean that if a manufacturer could jump at the chance to legally place a medical gadget on the UK market then it must in any case meet the requirements of the MD Mandate.
Order 98/79/EC of the European Parliament and of the Committee of 27 October 1998 on in vitro diagnostic medical devices
In vitro diagnostic medical devices (IVDs, for example, pregnancy tests and HIV tests, are dependent upon this order assuming manufacturers want their devices to be available in the EU. The mandate controls things like what classifies an IVD, what accessories can be utilized with them and essential requirements of the devices. The draft agreement states that this will in any case apply to new IVDs after Brexit.
Board Mandate 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices
This order applies to active implantable devices. Its most memorable goal is to harmonize the regulatory environment across the European Economic Area while enabling the free movement of products inside the EU. The order sets the essential safety requirements in terms of capability, sterility, material compatibility, marking, client guidelines, plan documentation, CE marking, requirements for type approval, creation quality management, clinical investigation and manufacturer registration.
Regulation (EU) 2017/745 of the European Parliament and of the Chamber of 5 April 2017 on medical devices, amending Order 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Gathering Mandates 90/385/EEC and 93/42/EEC6
This mandate establishes a regulatory framework for medical devices that is expected to safeguard general health and safety while supporting the competitiveness of the market. It will come completely into force in 2020 and places limitations and announcing requirements on the substances utilized in the plan and manufacturing cycle of medical devices. It aims to lessen the potential dangers presented by around 2,000 substances.
Regulation (EU) 2017/746 of the European Parliament and of the Committee of 5 April 2017 on in vitro diagnostic medical devices and repealing Mandate 98/79/EC and Commission Choice 2010/227/EU1
This regulation is similar to the one above and regards the new general health and safety decides on medical devices that will come into force in 2020.
0 notes
Text
Meticulous Research® Forecasts Middle East IVD Market to Reach $2.15 Billion by 2031
The report highlights market dynamics, growth drivers, key challenges, and opportunities across the region.
Meticulous Research®, a globally recognized leader in market intelligence, has released a new report titled, "Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2031." According to the findings, the Middle East in vitro diagnostics (IVD) market is projected to reach $2.15 billion by 2031, growing at a compound annual growth rate (CAGR) of 4.1% from 2024 to 2031.
Key Market Drivers
The growth of the Middle East IVD market is driven by several factors, including the rising prevalence of chronic and infectious diseases, increased demand for point-of-care (PoC) and rapid diagnostic solutions, expanding healthcare expenditures, and an aging population. Moreover, growing healthcare awareness, especially in countries such as the UAE and Saudi Arabia, is also contributing to market expansion. However, the high costs associated with IVD tests and inconsistencies in results from rapid diagnostic tests present challenges to sustained growth.
Download Sample Report Here: https://www.meticulousresearch.com/download-sample-report/cp_id=5764
Emerging Opportunities
Increasing awareness around early disease detection and personalized medicine, particularly in the UAE and Saudi Arabia, is creating opportunities for market players. These regions are witnessing an uptick in the adoption of advanced diagnostic technologies, which is expected to fuel demand in the coming years. Nonetheless, issues such as false positives in immunoassays and PoC tests remain significant hurdles that stakeholders need to address.
Competitive Landscape
The Middle East IVD market is dominated by global healthcare giants such as Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), and Siemens Healthineers AG (Germany). Other key players include QIAGEN N.V. (Netherlands), Thermo Fisher Scientific Inc. (U.S.), and Bio-Rad Laboratories, Inc. (U.S.), among others.
Request Sample Report Here: https://www.meticulousresearch.com/request-sample-report/cp_id=5764
Market Segmentation Insights
The report provides a detailed breakdown of the Middle East IVD market based on various parameters:
Offerings: The kits and reagents segment is expected to register the highest growth rate due to their extensive use in diagnosing chronic and infectious diseases. This is attributed to the increasing volume of tests for diseases such as HIV and influenza, alongside growing consumer awareness of self-testing options.
Technologies: Molecular diagnostics, including PCR and next-generation sequencing (NGS), is expected to capture the largest market share in 2024, driven by the rising prevalence of communicable and non-communicable diseases and the need for high-volume, accurate testing.
Applications: Infectious disease testing is set to dominate the market, with respiratory infections, hepatitis, HIV, and sexually transmitted diseases (STDs) being the primary drivers. The region's aging population and increasing healthcare demand are also contributing to the rise in testing.
Diagnostic Approach: Point-of-care testing is expected to exhibit the highest growth, propelled by the increasing burden of chronic diseases and the launch of innovative PoC diagnostics by market leaders.
End Users: Hospitals and clinics will continue to be the largest segment, performing a wide range of diagnostic tests for various medical conditions, particularly in urban areas where access to healthcare services is more robust.
Access Full Report Details Here : https://www.meticulousresearch.com/request-sample-report/cp_id=5764
Country Insights
Among the Middle Eastern countries, Saudi Arabia is projected to dominate the IVD market in 2024, largely due to its rapidly aging population, the high incidence of chronic and infectious diseases, and the growing adoption of self-testing. The Kingdom’s healthcare sector is also experiencing a boost thanks to its Vision 2030 strategy, which aims to elevate healthcare standards and increase investments in medical technologies. Healthcare expenditure in Saudi Arabia is expected to grow annually by 3.0% from 2022 to 2025, further supporting market growth.
Conclusion
The Middle East IVD market presents lucrative opportunities for growth, driven by evolving healthcare needs, technological advancements, and rising awareness around early disease detection. However, market players must navigate challenges related to pricing and diagnostic accuracy to fully capitalize on the region’s potential.
Quick Buy: https://www.meticulousresearch.com/Checkout/69732912
About Meticulous Research®
Meticulous Research® is a globally recognized market research and consulting firm known for delivering in-depth insights across a variety of industries. We provide high-quality market intelligence and strategic guidance to clients across the globe.
Contact Information Email: [email protected] Sales Inquiry: +1-646-781-8004 Connect with us on LinkedIn: Meticulous Research® LinkedIn
0 notes
Text
In Vitro Diagnostics Quality Control Market Research Report and Industry Analysis By 2026
The global IVD quality control market size is expected to reach USD 1.24 billion by 2026, according to a new report by Grand View Research, Inc. The market is projected to witness a CAGR of 4.1% over the forecast period. Continually evolving technology-oriented changes in the diagnostics field and growing need to ensure patient safety necessitate the implementation of quality assurance programs in various medical disciplines including radiology and Point-of-Care (PoC) devices. Patients rely on self-testing IVD devices for long-term disease management and hence it is important for such devices to be checked, in terms of result reproducibility and validity, to guarantee patient safety.

Rising number of certified clinical laboratories offering dependable IVD-based diagnostic services directly correlates with increased patient confidence, thus driving the market. In addition to quality assessments, amendments to the regulatory framework are made intermittently to enhance the existing standards with the main objective of safeguarding qualitative superiority of the diagnostic services. In May 2016, the European Union passed an agreement to update the pre-existing regulations pertaining to IVD devices, wherein the updates were in concern with raising the patient safety levels, particularly for disabled persons. The presence of third-party agencies for independent assessment of the IVD devices is expected to elevate the current safety standards, which will drive the In Vitro Diagnostics (IVD) Quality Control market further.
Further key findings from the study suggest:
• In 2018, the clinical chemistry was the second-largest application segment of the global IVD market, in terms of market share
• Demand for preventive medicine and rapid transformation of clinical laboratories into highly automated and efficient businesses are some of the factors for the segment’s growth
• Molecular diagnostics is projected to be the fastest-growing segment due to increasing technical complexity of molecular diagnostic testing and need for quality evaluation to ensure standards
• These tests are of prime importance as the outcomes enable healthcare practitioners make critical treatment decisions
• Hospitals was the largest segment in 2018 due to the presence of advanced technology-based devices like Next Generation Sequencing (NGS) and microarrays, and rising applications of the optimized quality-control procedures
• North America was the dominant regional market in 2018 due to the presence of over 150,000 registered diagnostics labs and is likely to maintain the dominance throughout the forecast years
• Siemens Healthcare GmbH; Roche Diagnostics; Alere, Inc.; Abbott Laboratories, Inc.; Qiagen N.V.; Bio-Rad Laboratories, Inc.; Quidel Corp.; Becton, Dickinson and Company; bioMerieux, Inc.; Sysmex Corp.; Sero AS; and Thermo Fisher Scientific, Inc. are some of the key companies in the global market
The global In Vitro Diagnostics (IVD) and IVD quality control market combines to account for USD 112.79 billion in revenue in 2021, which is expected to reach USD 114.73 billion by 2030, growing at a cumulative rate of 0.2% over the forecast period.
In Vitro Diagnostics Quality Control Market Segmentation:
Grand View Research has segmented the global IVD quality control market on the basis of application, type, end use, and region:
IVD Quality Control Application Outlook (Revenue, USD Million, 2014 – 2026)
• Immunochemistry
• Hematology
• Clinical Chemistry
• Molecular Diagnostics
• Coagulation
• Microbiology
• Others
IVD Quality Control Type Outlook (Revenue, USD Million, 2014 – 2026)
• Quality Control
Quality Controls, by Type
Plasma-based Control
Serum-based Control
Whole Blood-based Control
Others
Quality Controls, by Application
Immunochemistry
Hematology
Clinical Chemistry
Molecular Diagnostics
Coagulation
Microbiology
Others
• Quality Assurance Services
Immunochemistry
Hematology
Clinical Chemistry
Molecular Diagnostics
Coagulation
Microbiology
Others
• Data Management
Clinical Chemistry
Immunochemistry
Hematology
Molecular Diagnostics
Coagulation
Microbiology
Others
IVD Quality Control End Use Outlook (Revenue, USD Million, 2014 – 2026)
• Hospitals
• Laboratories
• Home-care
• Others
IVD Quality Control Regional Outlook (Revenue, USD Million, 2014 – 2026)
• North America
U.S.
Canada
• Europe
U.K.
Germany
France
Spain
Italy
Russia
• Asia Pacific
Japan
China
India
South Korea
Singapore
Australia
• Latin America
Brazil
Argentina
• Middle East & Africa
South Africa
UAE
Saudi Arabia
Order a free sample of this Intelligence study @ https://www.grandviewresearch.com/sector-report/in-vitro-diagnostics-ivd-quality-control-industry-data-book/request/rs1
#In Vitro Diagnostics Quality Control Market#In Vitro Diagnostics Quality Control Market Research#In Vitro Diagnostics Quality Control Market Report#In Vitro Diagnostics Quality Control Market analysis#In Vitro Diagnostics Quality Control Industry
0 notes
Text
Next-generation Sequencing (NGS) Market Valued at $6.3 Billion in 2021 and is anticipated to expand at a CAGR of 16% by 2026

Next-generation sequencing is a technique is used to determine the sequence of DNA or RNA in order to examine genetic variations linked to diseases or other biological phenomena. The term "NGS" refers to a set of technologies that utilize massively parallel sequencing to produce millions of short read sequences more quickly and with a significantly higher throughput.
The Global Next-generation Sequencing (NGS) Market valued at $6.3 billion (2021) is set to witness a significant growth rate of 16% in the next 5 years. Some of the key factors driving the global next-generation sequencing (NGS) market include the quick advancements in sequencing platforms, increasing applications of NGS, rising demand for NGS services, development of advanced bioinformatics tools, high investments in R&D, and growing incidence of cancer and infectious diseases.
Technological developments in sequencing platforms to fuels the Next-generation Sequencing (NGS) Market Demand
Continuous developments in sequencing technology have enabled to develop efficient, portable, and easy-to-use NGS sequencers to deliver rapid and precise results while also reducing turnaround times. Such advancements tend to provide competitive edge to manufacturers and therefore, major players are constantly focusing on investing in research activities for the creation of new products to strengthen their positions in this high growth market.
Here is a list of a few technological developments:
In March 2022, Element Biosciences Inc. launched its benchtop sequencer - Element AVITI System, that offers combination of performance, cost, and flexibility in an unprecedented way. This system consists of a benchtop NGS instrument and related consumables
In December 2021, Singular Genomics Systems, Inc. commercially launched its G4 platform, a powerful benchtop sequencer featuring a new high-performance chemistry with advanced engineering to give precision, flexibility, speed, and power for a wide range of applications, including cancer and immunology research
In December 2021, Roche launched its AVENIO Edge System, a next-generation platform to assist sequencing laboratories and researchers to reduce human error, further providing fast, precise, and reliable results
Explore Premium Report on Next-generation Sequencing (NGS) Market @ https://meditechinsights.com/next-generation-sequencing-ngs-market/
Partnership and collaboration to increase the acceptance and research outcomes for diagnostic applications
The next-generation sequencing market has seen a number of partnerships and collaborations by market players to improve the adoption of this technology for clinical applications and co-develop advanced diagnostic solutions.
The following is a list of some of the strategic initiatives that boost the adoption of NGS for diagnostic applications:
In January 2022, Illumina entered into multi-year partnership with Agendia N.V., a precision oncology provider for breast cancer, to co-develop in vitro diagnostic (IVD) tests for oncology testing using NGS technology
In July 2021, Qiagen partnered with Sysmex for the development and marketing of cancer companion diagnostic (CDx) solutions, leveraging Sysmex’s Plasma-Safe-SeqS technology for NGS
In April 2021, Thermo Fisher Scientific collaborated with Mayo Clinic, a non-profit organisation to accelerate access to innovative diagnostic solutions for haematology, allergy, oncology, and autoimmunity diagnostics, leveraging NGS technology
In January 2021, Illumina announced precision oncology partnerships with Merck, Bristol Myers Squibb, Myriad Genetics, and Kura Oncology for advancements in comprehensive genomic profiling using TruSight Oncology product portfolio
In August 2020, Oxford Nanopore Technologies entered into an agreement with the UK’s Department of Health and Social Care, to roll out its LamPORE SARS-CoV-2 tests across NHS testing laboratories
Competitive Landscape Analysis of Next-generation Sequencing (NGS) Market
The global next-generation sequencing (NGS) market is marked by the presence of key players such as Illumina (US), Thermo Fisher Scientific (US), Pacific Biosciences (US), Oxford Nanopore Technologies (UK), Hoffmann-La Roche AG (Switzerland), QIAGEN (Germany), and others.
For More Detailed Insights, Contact Us @ https://meditechinsights.com/contact-us/
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
0 notes
Text
Global Next-generation Sequencing Market valued at $6.3 billion (2021), is set to witness a lucrative growth of 16%
Next-generation sequencing is a technique for determining the sequence of DNA or RNA in order to investigate genetic variations linked to diseases or other biological phenomena. NGS refers to a set of technologies that use massively parallel sequencing to generate millions of short read sequences in a shorter time and with considerably higher throughput.
Recent developments in sequencing platforms, increasing applications of NGS, rising demand for NGS services, development of advanced bioinformatics tools and growing incidence of cancer and infectious diseases are the key factors driving the growth of the global next-generation sequencing market.
Technological advancements in Sequencing Platforms
Continuous advances in sequencing technology have enabled to develop efficient, portable, and easy-to-use NGS sequencers to deliver rapid and precise results while also reducing turnaround times. Such advancements tend to provide competitive edge to manufacturers and therefore, major players are continuously focusing on investing in research activities for new product development to strengthen their positions in this high growth market. Some of the technological advancements are listed below:
In March 2022, Element Biosciences Inc. launched its benchtop sequencer - Element AVITI System, that offers combination of performance, cost, and flexibility in an unprecedented way. This system consists of a benchtop NGS instrument and related consumables.
In December 2021, Singular Genomics Systems, Inc. commercially launched its G4 platform, a powerful benchtop sequencer featuring a new high-performance chemistry with advanced engineering to give precision, flexibility, speed, and power for a wide range of applications, including cancer and immunology research.
Partnership and collaboration to boost adoption and research outcomes for diagnostic applications
The next-generation sequencing market has witnessed a number of partnerships and collaborations by key market players to enhance the adoption of this technology for clinical applications and co-develop advanced diagnostic solutions. The strategic initiatives that boost the adoption of NGS for diagnostic applications are listed below:
In January 2022, Illumina entered into multi-year partnership with Agendia N.V., a precision oncology provider for breast cancer, to co-develop in vitro diagnostic (IVD) tests for oncology testing using NGS technology.
In July 2021, Qiagen partnered with Sysmex for the development and marketing of cancer companion diagnostic (CDx) solutions, leveraging Sysmex’s Plasma-Safe-SeqS technology for NGS.
In April 2021, Thermo Fisher Scientific collaborated with Mayo Clinic, a non-profit organisation to accelerate access to innovative diagnostic solutions for haematology, allergy, oncology, and autoimmunity diagnostics, leveraging NGS technology.
Competitive Landscape Analysis: Next-Generation Sequencing Market
The global next-generation sequencing market is marked by the presence of leading market players such as Illumina (US), Thermo Fisher Scientific (US), Pacific Biosciences (US), Oxford Nanopore Technologies (UK), Hoffmann-La Roche AG (Switzerland), QIAGEN (Germany), and others.
Get Customized Report on Next-Generation Sequencing Market @ https://meditechinsights.com/next-generation-sequencing-ngs-market/
#next generation sequencing#next generation#sequencing#Next-Generation Sequencing Market#healthcare#Next-Generation Sequencing Market Size#Next-Generation Sequencing Market Growth#Next-Generation Sequencing#bioinformatics
0 notes
Text
In Vitro Diagnostics Market Share, Industry Growth, Trend, Key Companies by 2028
The Global in vitro diagnostics industry Market report published by Reports and Data is a concise summary on the in vitro diagnostics industry industry and offers deep insights into the industry’s core structure and mechanism. The report digs into the key segments and sub-segments of the industry and offers a thorough study of the industry’s leading regional markets, competitive scenario, product and application segments, technology landscape, sales & distribution networks, and key industry statistics. Market insights included in the report have been compiled through extensive research, detailed market surveys, and expert interviews.
Key market dynamics illustrated in the report include market share, market size, revenue growth drivers, restraints, opportunities, threats, challenges, emerging market trends, product innovations, and industry revenue growth rate. Other imperative factors highlighted in the report are volatility in demand and supply graphs, production & consumption patterns, paradigm shifts in consumer preferences, import/export analysis, and a multitude of macro-economic and micro-economic growth indicators. Going ahead, the report elaborates on the highly competitive environment of the in vitro diagnostics industry industry and discusses the strategic initiatives undertaken by each market player, including partnerships & collaborations, mergers & acquisitions, joint ventures, government & corporate deals, and new product launches.
Get a sample of the report @ https://www.reportsanddata.com/sample-enquiry-form/4520
Some Key Factors Contributing to the Global Pharma & Healthcare Market Growth
Unprecedented revenue growth of the global pharma & healthcare industry is attributed to factors such as rising prevalence of chronic and acute diseases worldwide, increasing geriatric population, rising awareness of health & wellness among consumers, and growing demand for more advanced healthcare services. Increasing demand for advanced drugs and therapeutics, growing availability of next-generation diagnostics and treatment options – especially in developing countries like India and China – rise in R&D activities and clinical trials in the pharmaceutical and biotechnology sectors, increasing public and private investments in healthcare research projects, and rising consumer expenditure on healthcare are among the other significant factors contributing to the industry revenue growth.
Top Players in the Global in vitro diagnostics industry Market:
Company 1
Company 2
Company 3
Company 4
Company 5
Company 6
Company 7
Company 8
Company 9
Company 10
Company 11
Company 12
Others
The coronavirus pandemic has had a drastic impact on the global healthcare industry, with rising cases of COVID-19 worldwide, substantially growing hospital admission and readmission rates, and rising demand for telehealth and telemedicine services for remote patient monitoring. Furthermore, rising focus on development of rapid COVID-19 diagnostics such as the RT-PCR test kits, increased government funding for vaccine development, stringent regulatory norms and protocols for COVID-19 safety, and increasing sales of COVID-19 safety equipment, such as N-95 masks, face shields, PPE kits, and hand sanitizers, have driven the global pharma & healthcare industry revenue growth over the recent past.
To know more about the report @ https://www.reportsanddata.com/report-detail/in-vitro-diagnostics-ivd-market
in vitro diagnostics industry Market Segmentation:
Product Type Outlook (Revenue, USD Billion; 2018-2028)
Reagents
Instruments
Software and Services
Others
Technology Type Outlook (Revenue, USD Billion; 2018-2028)
Immunoassay/ Immunochemistry
Enzyme-linked Immunosorbent Assay (ELISA)
Radioimmunoassay
Rapid Tests
Western Blotting
Enzyme-linked Immunospot Assays
Biochemistry/Clinical Chemistry
Molecular Diagnostics
Polymerase Chain Reaction (PCR)
Isothermal Nucleic Acid Amplification Technology
Microarray
Hybridization
DNA Sequencing & Next-generation Sequencing
Microbiology
Hematology
Coagulation/Hemostasis
Urinalysis
Others
Application Outlook (Revenue, USD Billion; 2018-2028)
Infectious Diseases
Oncology
Endocrinology
Diabetes
Cardiology
Nephrology
Others
End-use Outlook (Revenue, USD Billion; 2018-2028)
Laboratories
Large/Reference Laboratories
Medium-sized Laboratories
Small Laboratories
Hospitals
Academics
Point-Of-Care Testing
Patient Self-Testing
Others
Global in vitro diagnostics industry Market Report: Regional Segmentation
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
Request customization of the report @ https://www.reportsanddata.com/request-customization-form/4520
Frequently Asked Questions Answered in the Report:
What is the estimated revenue growth rate of the global in vitro diagnostics industry market over the forecast period?
What are the major factors driving the global market revenue growth?
Which are the leading manufacturers and suppliers in the global in vitro diagnostics industry market?
Which regional market is expected to lead in terms of revenue share in the global in vitro diagnostics industry market over the forecast years?
What are the key outcomes of SWOT analysis and Porter’s Five Forces analysis of the market?
Thank you for reading our report. Please connect with us to know more about the report and its customization feature. Our team will ensure the report is well suited to meet your requirements.
About Reports and Data
Reports and Data is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyze consumer behavior shifts across demographics, across industries, and help clients to make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Products, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Reports and Data has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
0 notes
Text
Molecular Diagnostics Market Analysis, Opportunity and Forecast 2020 to 2030
Global Molecular Diagnostics Market: Snapshot
Rising demand for molecular diagnostics solutions and technologies from industries such as in vitro diagnostics, personalized medicine, research and treatment of a variety of diseases has allowed a vast rise in growth opportunities for the global molecular diagnostics market in the past few years. Molecular diagnostics has led to advances in medical research and treatment of a number of diseases and continues to one of the leading enablers of innovative, accurate, highly sensitive, quick, and economic diagnosis of several diseases. These benefits of molecular diagnostics continue to drive the market.
Download PDF Brochure: https://www.tmrresearch.com/sample/sample?flag=B&rep_id=1886
Geographically, North America leads the global market of molecular diagnostics and is expected to dominate with a large share in the revenue pie of the global market throughout the report’s forecast period. In Asia Pacific, which has emerged as one of the most promising markets for molecular diagnostics owing to the rising patient pool of chronic diseases and an improving healthcare infrastructure in emerging economies, India, Japan, and China are expected to present considerable growth opportunities.
Remarkable expansion in diagnostic tests for infectious and genetic diseases is an excellent growth opportunity for the global molecular diagnostics market. In terms of technology, the segment of biochips/microarrays has reported the fastest growth pace over the report’s review period and is expected to expand favorably through the report’s forecast period. The report offers key insights about molecular diagnostics along with a detailed analysis of the factors affecting the growth of this market. Qualitative and quantitative insights on this market have also been presented through this research report.
Global Molecular Diagnostics Market: Overview
Molecular diagnostics refers to a collection of techniques used to analyze biological markers in the individual’s genetic code. The technique is used in diagnosing and monitoring diseases, detecting risks, and deciding suitable therapies for individual patients. It finds applications in blood screening, oncology, microbiology, and genetic tests. The technologies commonly employed in this technique are polymerase chain reaction (PCR), hybridization, isothermal nucleic acid amplification technology (INAAT), and DNA sequencing and next-generation sequencing. Some of the products and services used in the technique are instruments, reagents and kits, and software and services.
Global Molecular Diagnostics Market: Key Trends
The transformative and dynamic nature of molecular diagnostics has facilitated it in evolving as an attractive segment in the global in vitro diagnostics (IVD) industry. It has paved way for advancements in medical research and treatment of numerous diseases. The global demand for molecular diagnostics methodologies is largely supplemented by high sensitivity, accuracy, easy workflow, cost-effective testing, and fast turnaround time offered by these testing modalities.
In addition, these merits have created a gateway for personalized medicines, thus providing momentum to the global market. Moreover, the increasing prevalence of various types of cancer and infectious diseases is augmenting the market. Furthermore, reforms in the U.S. reimbursement scenario are working in favor of the overall market. However, delays in approvals of new molecular diagnostic tests due to the complex regulatory framework are limiting the market from realizing its full potential.
Global Molecular Diagnostics Market: Market Potential
Acquisitions and partnerships have emerged as a common trend in the global molecular diagnostics market over the past few years and are anticipated to continue in the coming years as well. For instance, in September 2016, Danaher acquired molecular diagnostics firm Cepheid for approximately US$4 bn. Another case in point is Qiagen N.V., which in July 2015 collaborated with multiplex clinical molecular diagnostics and multiplex molecular technologies developer Seegene Inc. Such deals are likely to make way for the development of novel diagnostics, which will not only help players in broadening their product offerings but also in making the future of the overall market bright.
Global Molecular Diagnostics Market: Geographical Segmentation
In terms of geography, the report presents an analysis of the market in North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America. North America will represent a large share in the market throughout the forecast period. The growth of the region can be attributed to the presence of well-established and advanced laboratory accreditation infrastructure, government initiatives promoting PoC facilities and awareness regarding the benefits of early diagnosis, and the increasing expenditure on healthcare.
Asia Pacific is expected to register a significant CAGR during the same period, with emerging countries such as India and China being the sights of high growth. The improving healthcare infrastructure, high unmet needs, and growing geriatric populations are attracting global players to invest in the region. The increasing external funding for clinical studies is likely to drive the growth of the region.
Global Molecular Diagnostics Market: Competitive Landscape
A raft of players in the global molecular diagnostics market is entering into strategic partnerships with biotech firms to provide novel diagnostics solutions in order to enhance their visibility. Companies are investing hefty funds in research and development activities to introduce cost-effective and innovative products that will help them in expanding their product portfolio. Several participants are focusing towards business expansion through mergers and acquisitions, which is likely to intensify the competition in the market in the near future.
Some of the prominent companies operating in the market are Abbott Molecular, F. Hoffmann-La Roche Ltd, Johnson & Johnson Services Inc., Bio-Rad Laboratories, Inc., Alere Inc., Dako, Siemens Healthcare, Novartis AG, Becton, Dickinson and Company, Bayer AG, Danaher Corporation, Hologic, Inc. (Gen Probe), and Qiagen.
Key questions answered in this report
What are the diverse growth parameters influencing the market?
Which regions will contribute largely to the growth of the market
What are the recent innovations and technological advancements in the market?
What are the emerging trends across the market?
How has COVID-19 affected the market?
What will be the post-pandemic scenario of the market?
What are the major threats that will dent the growth prospects of the market?
For Full TOC:
https://www.tmrresearch.com/sample/sample?flag=B&rep_id=1886
About Us:
TMR Research is a premier provider of customized market research and consulting services to business entities keen on succeeding in today’s supercharged economic climate. Armed with an experienced, dedicated, and dynamic team of analysts, we are redefining the way our clients’ conduct business by providing them with authoritative and trusted research studies in tune with the latest methodologies and market trends.
Contact Us:
Rohit Bhisey
Head Internet Marketing
Tel: +1-415-520-1050
Website: https://www.tmrresearch.com/
0 notes
Text
In Vitro Diagnostics Quality Control Market Size Estimated to Reach $1.2 Billion by 2025
In Vitro Diagnostics (IVD) Quality Control Market size is estimated to reach $1.2 Billion by 2025 and is poised to grow at a CAGR of 4.1% during the forecast period 2020-2025. In Vitro Diagnostics (IVD) Quality Control are samples/materials used to validate the reliability of IVD testing system to ensure accuracy of test results and evaluate the impact of factors such as environmental conditions and operator’s performance on test results. Continually evolving technology-oriented changes in the diagnostics field and growing need to ensure patient safety necessitate the implementation of quality assurance programs in various medical disciplines including radiology and Point-of-Care (PoC) devices. Rising number of certified clinical laboratories offering dependable IVD-based diagnostic services directly correlates with increased patient confidence, thus driving the market. In addition to quality assessments, amendments to the regulatory framework are made intermittently to enhance the existing standards with the main objective of safeguarding qualitative superiority of the diagnostic services.
By Technology- Segment Analysis
The global In Vitro Diagnostics (IVD) Quality Control market based on the technology has Immunochemistry, Clinical Chemistry, Molecular Diagnostics, Microbiology, Hematology, Coagulation & Hemostasis, and Other Technologies. The immunochemistry registers for the highest market share in 2019 and is set to continue for the forecast period (2020-2025) owing to its importance in identifying the molecular mechanism of various antigens, antibodies, and their interactions that affect the immune system. The molecular diagnostics segment is poised to witness the highest CAGR of 4.8% over the forecast period 2020-2025 owing to a rise in the adoption of advanced technologies such as Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS).
Request for Sample of the Report @ https://www.industryarc.com/pdfdownload.php?id=16822
Report Price: $ 4500 (Single User License)
By End Use – Segment Analysis
The global In Vitro Diagnostics (IVD) Quality Control market based on end-use has Hospitals, Clinical Laboratories, Academic and Research Institutes, other end users. Hospitals segment registers for the highest market share in 2019 and is set to continue for the forecast period (2020-2025). The segment is set to emerge owing to the rising number of admissions and better reliability of healthcare professionals on clinical diagnosis for deciding treatment alternatives. Hospitals have a separate department for diagnostic services. The tests and instruments in this department need to be monitored regularly, which is poised to augment the market growth.
By Geography - Segment Analysis
Based on geographical analysis, the global In Vitro Diagnostics quality control market is segmented into North America, Asia-Pacific, Europe, and Rest of world. North America has accounted for largest market share of 48% in terms of revenue in 2019, and estimated to maintain its dominance over the forecast period 2020-2025, owing to the presence of the U.S. FDA and huge number of accredited diagnostic laboratories including strong QC regulation systems. The Asia pacific is estimated to be the fastest-growing region over the forecast period 2020-2025 as the region has a high potential for this market owing to increasing number of product manufacturing companies.
Drivers – In Vitro Diagnostics (IVD) Quality Control Market
Rise in clinical laboratories
The demand for In Vitro Diagnostics (IVD) quality control market is driving forward owing to the rising number of accredited clinical laboratories. Besides, increasing demand for third-party quality controls, and rising prevalence of chronic and infectious diseases are acting as drivers for supporting the growth of the In Vitro Diagnostics (IVD) quality control market.
Talk to one of our sales representative about the full report by providing your details in the link below:
https://www.industryarc.com/support.php?id=16822
Challenges – In Vitro Diagnostics (IVD) Quality Control Market
High cost of IVD quality control services
Factors such as high cost of the In Vitro Diagnostics (IVD) quality control services and unfavourable reimbursement policies are hampering the market growth. Owing to the additional costs involved in the quality control process, and budget constraints in hospitals and laboratories, it has become a challenge for the growth of the market in the forecast period (2020-2025).
In Vitro Diagnostics (IVD) Quality Control Market Industry outlook
Product launches, mergers and acquisitions, joint ventures, and geographical expansions are key strategies adopted by players in the In Vitro Diagnostics (IVD) Quality Control Market. Key companies of this market Abbott Laboratories, Bio-Rad Laboratories Inc, F. Hoffmann-La Roche AG, Quidel Corporation, bioMerieux Inc, Qnostics Ltd, Microbiologics Inc, SeraCare Life Sciences Inc, Thermo Fisher Scientific, ZeptoMetrix Corporation.
Acquisitions/Product Launches
In October 2019, Thermo Fisher Scientific launched Acrometrix BCR-ABL Panel, a new external molecular quality control panel for the analytical validation of BCR-ABL test methods.
In February 2018, Qnostics (UK) and Randox Laboratories Ltd. (UK) entered into a partnership wherein Randox Laboratories to sell and distribute Qnostics’ molecular range of products, which strengthened Qnostics’ market penetration.
Key Takeaways
Geographically, the North America accounted for the largest share of the market owing to extraordinary development and increased number of diagnostic centres.
High cost of In Vitro Diagnostics (IVD) control in hospitals and laboratories poses a significant challenge for the In Vitro Diagnostics quality control market.
The third-party controls segment accounted for the largest market share in 2019 and is anticipated to grow with fastest CAGR. The large share of this segment is mainly attributed to the increasing demand for third-party quality controls owing to their unbiased test results.
Rise in demand for quality assessment support and immediate diagnosis systems are estimated to contribute to the expansion of the worldwide market during the forecast period 2020-2025.
Related Reports :
A. In Vitro Diagnostic (IVD) Tests Market
https://www.industryarc.com/Report/19282/in-vitro-diagnostic-tests-market.html
B. In Vitro Fertilization (IVF) Market
https://www.industryarc.com/Report/15071/in-vitro-fertilization-ivf-market.html
About IndustryARC: IndustryARC primarily focuses on Cutting Edge Technologies and Newer Applications market research. Our Custom Research Services are designed to provide insights on the constant flux in the global supply-demand gap of markets. Our strong team of analysts enables us to meet the client research needs at a rapid speed, with a variety of options for your business. Any other custom requirements can be discussed with our team, drop an e-mail to [email protected] to discuss more about our consulting services.
#in vitro diagnostics (ivd) quality controls market#in vitro diagnostics (ivd) quality controls market price
0 notes