#Multiple Myeloma Market Trends
Explore tagged Tumblr posts
Text
BMS vs. Janssen: Who Will Prevail in the Race for Multiple Myeloma Treatment Dominance?

The landscape of multiple myeloma treatment has experienced unprecedented growth and innovation in recent years, thanks to advancements in therapeutic options and a deeper understanding of the disease. Among the key players in this evolving market, Bristol Myers Squibb (BMS) and Janssen Pharmaceuticals have emerged as leaders, each vying for a dominant position. This article explores their competitive strategies, product portfolios, and future prospects as they navigate the complexities of the multiple myeloma treatment market throughout this decade.
The Current Landscape of Multiple Myeloma Treatments
Multiple myeloma, characterized by the abnormal growth of plasma cells in the bone marrow, has traditionally been challenging to treat. However, the development of new therapies has significantly improved patient outcomes. The current treatment landscape includes several categories of drugs, including:
Proteasome Inhibitors: These agents, such as bortezomib and carfilzomib, disrupt the degradation of proteins in cancer cells, effectively inducing cell death.
Immunomodulatory Drugs (IMiDs): Drugs like lenalidomide and pomalidomide have shown efficacy in stimulating the immune system to fight cancer cells while simultaneously inhibiting their growth.
Monoclonal Antibodies: Agents such as daratumumab and elotuzumab are designed to specifically target myeloma cells, enhancing the immune response against them.
CAR-T Cell Therapies: Innovative treatments that engineer a patient's T cells to specifically attack cancer cells have gained traction in managing relapsed cases.
Given the increasing prevalence of multiple myeloma, driven by an aging population, the competition among pharmaceutical companies to develop effective therapies has intensified, with BMS and Janssen at the forefront.
Bristol Myers Squibb: Driving Innovation in Oncology
Bristol Myers Squibb has established itself as a formidable force in the oncology space, particularly in multiple myeloma treatment. The company's flagship drug, Revlimid (lenalidomide), remains a standard of care and has significantly improved survival rates for patients. Looking ahead, BMS is dedicated to expanding its treatment offerings through:
Advanced CAR-T Cell Therapies: BMS is pioneering CAR-T therapies, notably Abecma (idecabtagene vicleucel), which targets BCMA (B-cell maturation antigen) and has shown promise in treating patients with relapsed or refractory multiple myeloma.
Innovative Monoclonal Antibodies: The company is actively investigating new monoclonal antibodies to improve treatment outcomes and target various mechanisms of resistance.
Combination Treatment Strategies: BMS is focused on developing combination therapies to enhance efficacy and counteract treatment resistance, positioning itself well for future success.
BMS’s commitment to research and innovation is evident in its collaborations and partnerships that aim to broaden treatment access and improve patient outcomes.
Janssen Pharmaceuticals: A Comprehensive Approach to Treatment
Janssen, a subsidiary of Johnson & Johnson, is a powerful contender in the multiple myeloma treatment arena. The company’s diverse portfolio of therapies has redefined treatment options for patients. Key products include:
Darzalex (daratumumab): This pioneering monoclonal antibody has become a cornerstone therapy for multiple myeloma, providing significant survival benefits and becoming standard practice for many patients.
Ninlaro (ixazomib): An oral proteasome inhibitor that simplifies treatment regimens, enhancing patient adherence and overall satisfaction.
Carvykti (ciltacabtagene autoleucel): A newly approved CAR-T therapy that targets BCMA and offers new hope for patients with limited treatment options.
Janssen's strategic focus on clinical research and real-world evidence ensures that its therapies meet the evolving needs of patients and healthcare providers. The company is committed to exploring combination therapies to optimize treatment outcomes.
Comparative Analysis: Strengths, Weaknesses, and Market Strategies
Pipeline Robustness:
BMS boasts a strong pipeline, particularly in CAR-T cell therapies, positioning it favorably for treating relapsed cases.
Janssen's diverse approach, incorporating monoclonal antibodies and oral therapies, allows it to cater to a broader patient demographic.
Market Penetration and Access:
BMS has made significant strides in securing market access for its therapies, but faces competition from Janssen’s established distribution channels.
Janssen's vast resources enable it to reach a wide patient population, bolstering its competitive advantage.
Clinical Research and Development:
Both companies are heavily invested in clinical trials to expand indications and improve treatment efficacy. Janssen's focus on combination therapies may yield quicker results in enhancing patient outcomes.
Future Outlook: Who Will Lead the Market?
As the decade unfolds, both BMS and Janssen are poised to significantly impact the multiple myeloma treatment landscape. The competition between these two companies will be shaped by several key factors, including:
Advancements in Treatment Paradigms: The introduction of innovative therapies and combination regimens will influence clinician preferences and treatment guidelines.
Patient-Centric Approaches: Companies that effectively communicate the benefits of their therapies and ensure patient access will likely gain a competitive edge.
Regulatory Approvals: Timely approvals for new therapies and indications can drastically alter market dynamics, providing significant competitive advantages.
Conclusion
The rivalry between BMS and Janssen in the multiple myeloma treatment market is intense, with both companies poised to lead through their innovative therapies and extensive pipelines. While BMS focuses on advancing its CAR-T cell offerings, Janssen’s diverse product portfolio positions it well for continued growth. Ultimately, the next decade will be defined by advancements in treatment options, patient accessibility, and the adaptability of these companies to meet the evolving needs of patients and healthcare providers. The competition for dominance in the multiple myeloma treatment market is just beginning, and the outcome will significantly influence the future of cancer care.
#multiple myeloma#multiple myeloma Market#multiple myeloma Forecast#multiple myeloma Companies#multiple myeloma Drugs#multiple myeloma Therapies#multiple myeloma Epidemiology#multiple myeloma Pipeline#multiple myeloma Market Size#multiple myeloma Market Trends
0 notes
Link
0 notes
Text
SLAMF7 Inhibitors Market Size, Target Population, Competitive Landscape, and Forecast to 2034
The SLAMF7 inhibitors market represents a growing segment in immuno-oncology, particularly for hematological malignancies. As therapies targeting SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7) continue to evolve, their market potential is increasingly recognized. This article explores the current and future outlook of the SLAMF7 inhibitors market, focusing on its size, target population, competitive landscape, and market trends through 2034.
SLAMF7 Inhibitors Market Size and Growth Dynamics
The SLAMF7 inhibitors market is expected to expand significantly by 2034, driven by the increasing prevalence of cancers such as multiple myeloma and rising adoption of targeted therapies. The global demand for novel, effective treatments is steering investments in research and development, which is also supported by government initiatives and partnerships between academia and the pharmaceutical industry.
Key SLAMF7 Inhibitors market growth drivers include:
- Rising Incidence of Multiple Myeloma: Multiple myeloma remains one of the primary indications for SLAMF7-targeting therapies. As incidence rates climb globally, so does the demand for targeted treatment options.
- Adoption of Immunotherapies: Immunotherapy is becoming a cornerstone in cancer treatment, with SLAMF7 inhibitors offering a promising approach by enhancing immune response against malignant cells.
- Expanding Application Areas: Beyond multiple myeloma, research is exploring the potential of SLAMF7 inhibitors in other cancers and autoimmune conditions, which could further boost market growth.
Regions such as North America and Europe dominate the market due to advanced healthcare infrastructure and early adoption of innovative therapies. However, the Asia-Pacific region is anticipated to see the fastest growth, spurred by improving healthcare systems and increasing awareness of targeted cancer treatments.
Request for a sample report @ https://www.delveinsight.com/sample-request/slamf7-inhibitors-market-forecast
SLAMF7 Inhibitors Target Population
SLAMF7 inhibitors primarily target patients with:
- Multiple Myeloma: SLAMF7 is highly expressed in myeloma cells, making it an effective target for therapies.
- Other Hematological Malignancies: Research is ongoing into their use for treating lymphomas and leukemias.
- Potential Non-Cancer Indications: Studies suggest potential in autoimmune diseases, further broadening the addressable patient population.
With an aging global population and improved diagnostic capabilities, the target pool for SLAMF7 inhibitors is likely to grow, presenting significant opportunities for market expansion.
SLAMF7 Inhibitors Competitive Landscape
The SLAMF7 inhibitors market is competitive, with several pharmaceutical giants and biotech firms actively engaged in the development and commercialization of these therapies. The competitive dynamics are defined by innovations in combination therapies, improved drug delivery mechanisms, and expansion into broader therapeutic areas.
Download sample report @ https://www.delveinsight.com/sample-request/slamf7-inhibitors-market-forecast
Key SLAMF7 Inhibitors Companies and Products
- Bristol Myers Squibb (Empliciti - Elotuzumab): As a pioneering SLAMF7-targeting therapy approved for multiple myeloma, Empliciti remains a cornerstone product. Its clinical success underscores the therapeutic value of SLAMF7 inhibitors.
- Emerging Biotechs: Smaller companies are also contributing to innovation in this space, with a focus on enhancing drug efficacy and patient outcomes through next-generation SLAMF7 inhibitors.
SLAMF7 Inhibitors Research and Development Trends
- Combination Therapies: SLAMF7 inhibitors are increasingly being used in combination with other immunomodulators or checkpoint inhibitors to improve treatment efficacy.
- Pipeline Developments: A robust pipeline of SLAMF7-targeting drugs reflects ongoing efforts to expand indications and overcome resistance mechanisms in cancer cells.
SLAMF7 Inhibitors Technological Advancements and Innovations
The SLAMF7 inhibitors market benefits from advancements in biotechnology and precision medicine:
- Enhanced Antibody Engineering: The development of bispecific antibodies targeting SLAMF7 and other immune receptors is a significant area of focus.
- Biomarker Identification: Precision medicine approaches are leveraging biomarkers to identify patients most likely to benefit from SLAMF7 therapies.
- Improvements in Drug Delivery: Innovations in delivery systems aim to reduce dosing frequency and improve patient compliance.
These advancements not only enhance therapeutic outcomes but also improve the accessibility and affordability of these treatments.
SLAMF7 Inhibitors Market Challenges
Despite its promise, the SLAMF7 inhibitors market faces several challenges:
1. High Development Costs: R&D for immuno-oncology therapies is resource-intensive, which impacts pricing and market penetration.
2. Therapeutic Resistance: The development of resistance to SLAMF7-targeting therapies requires continuous innovation to maintain efficacy.
3. Limited Awareness in Emerging Markets: While awareness is growing, it remains a barrier in regions with underdeveloped healthcare infrastructure.
Efforts to address these issues include collaboration between industry stakeholders, patient advocacy, and initiatives to improve access in low- and middle-income countries.
SLAMF7 Inhibitors Market Forecast to 2034
The SLAMF7 inhibitors market is projected to grow at a robust compound annual growth rate (CAGR) through 2034. Key growth drivers include:
- Expanding Indications: The use of SLAMF7 inhibitors in non-oncological conditions could significantly expand the market.
- Strategic Collaborations: Partnerships between pharmaceutical companies and research institutions are expected to accelerate innovation and market entry.
- Regulatory Approvals: Anticipated approvals of pipeline drugs will add to the therapeutic arsenal and drive market growth.
While North America and Europe will continue to lead in terms of market share, Asia-Pacific is poised to emerge as a significant player due to its rapidly evolving healthcare landscape.
The SLAMF7 inhibitors market is on a trajectory of rapid growth, fueled by its proven efficacy in managing hematological malignancies and its expanding role in immunotherapy. Innovations in drug development, coupled with efforts to address challenges such as cost and access, will be critical in unlocking the market's full potential.
For a deeper dive into the SLAMF7 inhibitors market, including detailed insights into its competitive landscape and future trends, visit the [DelveInsight SLAMF7 Inhibitors Market Forecast Report](https://www.delveinsight.com/report-store/slamf7-inhibitors-market-forecast).
0 notes
Text
Multiple Myeloma Market Segmentation Analysis, Prominent Regions, and Forecast to 2032
Multiple Myeloma is a type of blood cancer that affects plasma cells, a form of white blood cell responsible for producing antibodies. These malignant plasma cells accumulate in the bone marrow, hindering the production of healthy blood cells and damaging bones. This condition is often accompanied by symptoms like bone pain, anemia, fatigue, and kidney problems. Though the exact cause of multiple myeloma remains unclear, advancements in treatment options have significantly improved survival rates. With the development of novel therapies such as targeted drugs, immunotherapy, and stem cell transplants, patients are experiencing better outcomes and an enhanced quality of life.
The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.
Future Scope
The future of multiple myeloma treatment lies in personalized medicine and the use of cutting-edge therapies that target specific molecular and genetic factors of the disease. With ongoing research in immunotherapy, particularly CAR-T cell therapy and bispecific antibodies, the goal is to enhance the body’s immune response to the cancer cells, offering more effective and less toxic treatment options. Precision medicine, which tailors treatment to an individual’s genetic makeup, will continue to play a pivotal role in improving outcomes for multiple myeloma patients. Additionally, combination therapies that integrate multiple drug classes are expected to further advance the standard of care, reducing the risk of relapse and improving long-term remission rates.
Trends
One of the most significant trends in multiple myeloma treatment is the shift towards immunotherapy. This approach, which includes drugs like monoclonal antibodies and CAR-T cell therapy, enhances the immune system’s ability to target and destroy myeloma cells. Another growing trend is the use of minimal residual disease (MRD) testing, which measures the number of cancer cells remaining after treatment. MRD testing allows for more accurate monitoring of disease progression and helps tailor therapy decisions to achieve deeper remission. Additionally, advances in drug development, including the introduction of oral therapies, are making treatment more convenient for patients while maintaining efficacy.
Applications
The primary application of multiple myeloma treatments is to slow disease progression, alleviate symptoms, and improve overall survival rates. Treatments include chemotherapy, stem cell transplantation, immunotherapy, and targeted therapies like proteasome inhibitors and immunomodulatory drugs. These therapies work to reduce the number of cancer cells, manage bone damage, and prevent complications like infections. Supportive care, such as bone-strengthening treatments and pain management, plays a critical role in improving patients' quality of life. Early detection through regular monitoring and genetic testing is also key in optimizing treatment outcomes.
Key Points
Multiple Myeloma is a blood cancer that affects plasma cells and leads to symptoms like bone pain, anemia, and kidney issues.
Future treatments focus on personalized medicine, immunotherapy, and precision medicine tailored to individual genetic factors.
Trends include the rise of immunotherapy, minimal residual disease testing, and the development of more convenient oral therapies.
Treatments aim to slow disease progression, alleviate symptoms, and improve patient survival rates.
Early detection and supportive care are crucial in managing multiple myeloma effectively.
Conclusion
Multiple myeloma treatment has seen remarkable progress in recent years, with new therapies offering hope for improved survival and quality of life. As research continues, the focus on personalized and targeted treatments will drive the next wave of innovation, ensuring better outcomes for patients. With advancements in immunotherapy and precision medicine, the future looks promising for those diagnosed with this challenging condition.
0 notes
Text
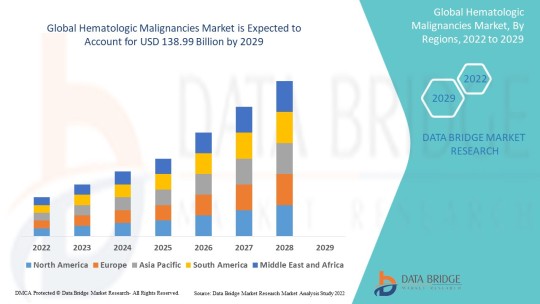
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- **Type**: The hematologic malignancies market can be segmented based on the type of malignancy, including leukemia, lymphoma, and multiple myeloma. Leukemia is a cancer of the blood cells, while lymphoma affects the lymphatic system. Multiple myeloma, on the other hand, is a cancer that forms in a type of white blood cell called a plasma cell.
- **Treatment**: Segmentation based on treatment modalities includes chemotherapy, immunotherapy, targeted therapy, stem cell transplant, and others. Chemotherapy is a common treatment for hematologic malignancies that involves the use of drugs to kill cancer cells. Immunotherapy utilizes the body's immune system to fight cancer cells, while targeted therapy focuses on specific molecules involved in cancer growth.
- **End-User**: The market can also be segmented by end-user, such as hospitals, specialty clinics, research institutes, and others. Hospitals are the primary point of care for hematologic malignancies patients, where they receive diagnosis, treatment, and follow-up care. Specialty clinics may offer specialized treatments or clinical trials for these conditions.
**Market Players**
- **Roche**: A leading player in the hematologic malignancies market, Roche offers a range of innovative therapies and diagnostic tools for leukemia, lymphoma, and multiple myeloma. The company's commitment to research and development has resulted in groundbreaking treatments that improve patient outcomes.
- **Johnson & Johnson**: With a focus on cutting-edge oncology therapies, Johnson & Johnson has made significant advancements in the treatment of hematologic malignancies. The company's portfolio includes novel drugs that target specific cancer pathways, providing new options for patients.
- **Novartis**: Known for its expertise in precision medicine, Novartis has developed several targeted therapies for hematologic malignancies. By identifying genetic mutations driving cancer growth, Novartis delivers personalized treatments that are more effective and less toxic for patients.
- **AbbVie**:AbbVie is a key player in the hematologic malignancies market, known for its strong focus on developing innovative therapies for various types of blood cancers. The company's robust pipeline includes potential treatments for leukemia, lymphoma, and multiple myeloma, leveraging cutting-edge technologies and research to address unmet medical needs in this space. AbbVie's commitment to oncology research and development has led to the introduction of novel treatment options that aim to improve patient outcomes and quality of life.
In the competitive landscape of the hematologic malignancies market, AbbVie distinguishes itself through a combination of strategic partnerships, investments in research, and a patient-centric approach to drug development. The company's collaborative efforts with academic institutions, research organizations, and other industry partners have resulted in the acceleration of novel therapeutic solutions for blood cancers. By prioritizing patient needs and engaging in meaningful dialogue with healthcare providers, AbbVie continues to shape the future of hematologic oncology with a focus on personalized medicine and targeted therapies.
AbbVie's portfolio of hematologic malignancy treatments encompasses a diverse range of modalities, including small molecule inhibitors, monoclonal antibodies, and immunotherapies. These innovative therapies target specific pathways and molecular mechanisms involved in the development and progression of blood cancers, offering new hope for patients who may not have responded to conventional treatments. By leveraging its expertise in precision medicine and biomarker-driven approaches, AbbVie continues to advance the field of hematologic oncology with a strong emphasis on tailored treatment regimens that consider individual patient characteristics and disease profiles.
In addition to its focus on drug development, AbbVie also plays a crucial role in raising awareness about hematologic malignancies and promoting early detection and diagnosis. Through educational initiatives, patient advocacy programs, and community engagement efforts, the company strives to empower patients, caregivers, and healthcare professionals with the knowledge and resources needed to effectively manage blood cancers. By fostering a culture of collaboration and knowledge-sharing, AbbVie contributes to the overall**Global Hematologic Malignancies Market Analysis**
- **Type**: The global hematologic malignancies market, segmented by type, includes leukemia, lymphoma, and multiple myeloma. With advancements in precision medicine and targeted therapies, the market is witnessing a shift towards personalized treatment regimens tailored to the specific type of malignancy, driving growth in the segment.
- **Therapy Type**: The market segmented by therapy type comprises chemotherapy, immunotherapy, and targeted therapy, among others. The rising prevalence of hematologic malignancies and the increasing adoption of novel treatment approaches are driving the demand for innovative therapies, leading to significant market growth in this segment.
- **Diagnosis**: Diagnostic modalities such as blood tests, biopsies, imaging tests, and others play a crucial role in the early detection and management of hematologic malignancies. The emphasis on early diagnosis and personalized medicine is driving the market for diagnostic tools, contributing to the overall growth of the hematologic malignancies market.
- **Route of Administration**: Different routes of administration, including oral, parenteral, and others, offer varied options for delivering hematologic malignancy treatments. The convenience and efficacy of different administration routes influence patient compliance and treatment outcomes, shaping the market dynamics in this segment.
- **Dosage Form**: The market segmented by dosage form includes tablets, capsules, injections, and others. The availability of diverse dosage forms caters to patient preferences and treatment needs, promoting adherence and enhancing the overall therapeutic outcomes in
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
0 notes
Text
0 notes
Text
Carfilzomib Market Overview and Regional Outlook Study 2024 – 2034
Carfilzomib Market Defination:
TheCarfilzomib Market refers to the economic and clinical landscape surrounding the pharmaceutical drug carfilzomib. Carfilzomib is a proteasome inhibitor used primarily in the treatment of multiple myeloma, a type of cancer affecting plasma cells in bone marrow. It functions by selectively inhibiting the proteasome, a complex protein-degrading machinery essential for cell function and survival. This inhibition leads to the accumulation of proteins within cancer cells, triggering cell death through apoptosis.
Print This Guide for Future Reference:https://wemarketresearch.com/reports/request-free-sample-pdf/carfilzomib-market/1493
Exploring the Carfilzomib Market: Advancements in Multiple Myeloma Treatment
In the realm of oncology, particularly in the treatment landscape of multiple myeloma, carfilzomib has emerged as a cornerstone therapy, offering new hope and improved outcomes for patients. This blog delves into the dynamic carfilzomib market, examining its impact, current trends, challenges, and future prospects.
Understanding Carfilzomib
Carfilzomib is a proteasome inhibitor approved for the treatment of relapsed or refractory multiple myeloma. It works by selectively and irreversibly binding to the 20S proteasome, disrupting protein degradation in cancer cells and inducing apoptosis. Approved by the FDA in 2012, carfilzomib has since been integrated into treatment protocols, often in combination with other agents like lenalidomide and dexamethasone.
Market Dynamics
Current Landscape: The Carfilzomib Market is driven by its efficacy in treating relapsed or refractory multiple myeloma, particularly in patients who have received prior therapies. Its mechanism of action and clinical benefits have positioned it as a valuable option in the treatment algorithm for multiple myeloma.
Treatment Advancements: Clinical studies have demonstrated that carfilzomib-based regimens prolong progression-free survival and overall survival compared to traditional therapies. Its approval marked a significant advancement in the management of multiple myeloma, offering a targeted approach to combating the disease.
Competitive Environment: Within the proteasome inhibitor class, carfilzomib competes with bortezomib and ixazomib, each offering unique profiles in terms of efficacy, safety, and administration convenience. Ongoing research aims to optimize carfilzomib’s use through novel combinations and sequencing strategies to maximize patient benefit.
Clinical Applications
Approved Indications: Carfilzomib is primarily indicated for use in combination with other agents for the treatment of relapsed or refractory multiple myeloma. Clinical trials are also exploring its potential in newly diagnosed patients and maintenance therapy settings, broadening its scope of application.
Future Directions: Research efforts are focused on expanding carfilzomib’s indications and understanding its synergies with emerging therapies such as immunomodulators, monoclonal antibodies, and cellular therapies like CAR-T cells. These endeavors aim to further improve treatment outcomes and offer personalized therapeutic approaches.
Carfilzomib Market Challenges and Opportunities
Challenges: Economic considerations remain a significant challenge in the adoption of carfilzomib, given its high cost as a biologic therapy. Managing treatment-related adverse events, such as cardiovascular complications and hematologic toxicities, also requires vigilant monitoring and proactive management strategies.
Opportunities: Advances in biomarker identification and personalized medicine offer opportunities to tailor carfilzomib-based therapies to individual patient profiles. Moreover, ongoing research into combination therapies and novel formulations aims to enhance efficacy while minimizing adverse effects, thereby improving patient adherence and outcomes.
Patient Impact and Healthcare Considerations
Patient Experience: For patients diagnosed with relapsed or refractory multiple myeloma, carfilzomib represents a crucial treatment option that can potentially extend survival and improve quality of life. Education and support programs play a vital role in helping patients manage treatment-related challenges and adhere to therapy.
Healthcare System Implications: Integrating carfilzomib into clinical practice requires healthcare providers to navigate complex treatment algorithms and ensure appropriate patient monitoring. Collaboration among multidisciplinary teams, including oncologists, hematologists, and supportive care specialists, is essential for optimizing patient care and outcomes.
Regulatory and Market Access
Regulatory Landscape: Regulatory approvals and reimbursement policies influence the accessibility of cCarfilzomib Market in different regions. Streamlining regulatory processes and demonstrating cost-effectiveness through real-world evidence are crucial for enhancing market access and patient affordability.
Market Expansion: As clinical data continues to evolve and new indications are explored, the carfilzomib market is poised for growth. Market expansion strategies should prioritize evidence-based medicine and stakeholder collaboration to drive adoption and improve patient access.
Conclusion
In conclusion, the carfilzomib market represents a significant advancement in the treatment of multiple myeloma, reflecting the transformative impact of targeted therapies in oncology. Its approval and integration into treatment protocols underscore a shift towards personalized medicine and multidisciplinary care approaches that optimize patient outcomes.
While challenges such as economic considerations and treatment-related adverse events persist, ongoing research and collaborative efforts among stakeholders are paving the way for continued innovation and improvement inCarfilzomib-Based Therapies. By addressing these challenges proactively, healthcare providers and pharmaceutical companies can ensure that carfilzomib realizes its full potential in improving the lives of patients battling multiple myeloma.
Stay informed and engaged with the latest developments in the carfilzomib market to contribute to advancements in oncology and patient-centered care.
0 notes
Text
Bortezomib Market Estimated to Witness High Growth Owing to Rising Adoption of Proteasome Inhibitors

The bortezomib market is primarily driven by high incidence and prevalence of multiple myeloma across the globe. Bortezomib, which is commonly sold under the brand name Velcade among others, is a proteasome inhibitor primarily used for the treatment of multiple myeloma and mantle cell lymphoma.
The global proteasome inhibitor drug market size is valued at approximately US$ 24.54 million in 2024 and is expected to register a CAGR of 4.7% over the forecast period of 2024-2031. The introduction of novel proteasome inhibitors and their increasing adoption in the treatment of cancer are the major factors anticipated to propel market growth. Key Takeaways
Key players operating in the bortezomib market are Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, and Chengdu Aslee Biopharmaceuticals.
The introduction of generic versions of Bortezomib Market Demand has led to increased adoption and lowered treatment costs. Moreover, ongoing clinical trials evaluating the efficacy of bortezomib in other cancer indications are expected to expand the eligible patient pool. Technological advancements in proteasome inhibitor development focused on overcoming resistance, reducing toxicity, and novel delivery systems are further anticipated to support market growth. Market Drivers
The primary factors driving the growth of the global bortezomib market include rising prevalence of multiple myeloma globally, increasing adoption of proteasome inhibitors in treatment regimens, availability of generic versions, and ongoing clinical research evaluating the efficacy of bortezomib in other cancer indications. Additionally, improving healthcare infrastructure and expenditures in emerging economies will further support the market growth during the forecast period. Current challenges in Bortezomib Market
The Bortezomib Market Size And Trends faces several challenges primarily due to the presence of alternative therapeutic options for treating multiple myeloma (MM). Some of the major challenges include increasing generic competition from drugs like ixazomib and daratumumab which are leading to lower sales of bortezomib drugs. Further, the patents of bortezomib drugs have expired in several regions making them available in generic forms at lower costs. This increasing availability of low-cost generics is a major challenge faced by innovator bortezomib drug companies. Additionally, the adverse side effects associated with bortezomib drugs like neuropathy and thrombocytopenia require close patient monitoring during treatment posing operational challenges. Stringent regulations for drug approval is another regulatory challenge for new market entrants. SWOT Analysis
Strength: Well-established drug with proven efficacy and safety profile in treating MM. It was the first proteasome inhibitor approved and remains a standard of care. Weakness: Patent expiry has led to availability of low-cost generics reducing sales of innovator brands. Further, it causes serious side effects like neuropathy requiring cautious use. Opportunity: Emerging economies with growing cancer burden and healthcare spending present an opportunity. Combination therapies with other anti-MM drugs can boost its use further. Threats: Increasing competition from newer oral proteasome inhibitors and monoclonal antibody based therapies poses pricing and market share threats. Stringent regulations for approval delays market entry of new players.
Geographical regions with high market concentration
In terms of value, North America accounts for the largest share of over 40% of the global bortezomib market led by the US. This is due to established healthcare infrastructure and higher adoption of innovative therapies. Europe is the second major regional market with a value share of over 30% supported by favourable reimbursement policies. The Asia Pacific region is projected to be the fastest growing market during the forecast period due to rising healthcare expenditure, growing cancer incidence and increasing demand for cancer treatments from middle-income countries like China and India. Fastest growing geographical region
The Asia Pacific region is poised to exhibit the highest growth rate during the forecast period in the global bortezomib market. This is attributed to rising disposable incomes, growing awareness about cancer treatments, expansion of healthcare facilities and increasing private sector investment in pharmaceutical research in emerging economies like China and India. Large patient pools undergoing cancer treatment in Asia present lucrative opportunities for bortezomib drug makers looking to tap high future growth potential in this region. Get More Insights On, Bortezomib Market For More Insights Discover the Report In language that Resonates with you French, German, Italian, Russian, Chinese, Korean About Author: Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Bortezomib Market Demand#Bortezomib Market Size#Bortezomib Market Trends#Bortezomib Market Forcast#Bortezomib#Bortezomib Market
0 notes
Text
Velcade Market Size, Share, Industry Trends, and Forecast 2032
Introduction
Velcade, also known by its generic name bortezomib, is a proteasome inhibitor used primarily in the treatment of multiple myeloma and mantle cell lymphoma. Since its approval, Velcade has significantly impacted the oncology market, offering a promising therapeutic option for patients with these malignancies. The global Velcade market is characterized by its dynamic nature, driven by advancements in oncology research, increasing incidence of hematologic cancers, and a growing focus on personalized medicine.
Market Size and Growth Dynamics
Velcade Market Size was estimated at 1.76 (USD Billion) in 2023. The Velcade Market Industry is expected to grow from 1.83(USD Billion) in 2024 to 2.5 (USD Billion) by 2032. The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). This growth is fueled by the rising prevalence of multiple myeloma and other related cancers, which are increasingly being diagnosed due to advances in diagnostic technologies. Furthermore, the aging population, which is more susceptible to cancer, is also contributing to the expanding market.
North America holds the largest market share, attributed to the region's advanced healthcare infrastructure, high adoption rates of new therapies, and a strong focus on research and development. Europe follows closely, driven by similar factors. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, spurred by increasing healthcare expenditures, improving access to cancer treatments, and rising awareness about hematologic cancers.
Market Share Analysis
Velcade, originally developed by Millennium Pharmaceuticals and marketed by Takeda Oncology, has maintained a dominant position in the proteasome inhibitor segment. Its efficacy, safety profile, and first-mover advantage have contributed to its strong market share. However, with the expiration of key patents, the market has seen the entry of generic versions, which has led to increased competition and a shift in market dynamics.
The introduction of generics has made the treatment more accessible, particularly in developing regions, but it has also put pressure on the market share of branded Velcade. Despite this, the brand continues to hold a significant share due to its established presence and the trust it has garnered among healthcare professionals.
Industry Trends
Several key trends are shaping the Velcade market:
Rise of Combination Therapies: The use of Velcade in combination with other drugs, such as lenalidomide and dexamethasone, is becoming increasingly common. These combination therapies have shown improved efficacy and are becoming a standard of care in multiple myeloma treatment protocols.
Focus on Personalized Medicine: With the growing emphasis on personalized medicine, there is a trend towards tailoring treatments based on individual patient profiles, which includes genetic makeup and disease characteristics. This approach is expected to drive demand for Velcade as part of personalized treatment regimens.
Increased Research and Development: The oncology sector continues to see significant investment in research and development, leading to the discovery of new therapeutic targets and treatment options. While this fosters innovation, it also intensifies competition as new drugs enter the market.
Patent Expirations and Generic Competition: The expiration of Velcade’s patents has opened the market to generic competition, leading to reduced prices and increased accessibility. This trend is expected to continue, particularly in cost-sensitive markets.
Expanding Indications: Research is ongoing to explore the potential of Velcade in treating other cancers and conditions beyond multiple myeloma and mantle cell lymphoma. If successful, these efforts could lead to new indications, further driving market growth.
Forecast Through 2032
The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). Key drivers of this growth include the increasing global cancer burden, advancements in cancer therapy, and the expansion of healthcare infrastructure in emerging markets.
However, challenges such as the rising cost of cancer treatment, the availability of alternative therapies, and regulatory hurdles may pose risks to market expansion. Additionally, the shift towards biosimilars and generics is likely to impact the market dynamics, particularly in terms of pricing and market share distribution.
The forecast period is also expected to witness a greater emphasis on real-world evidence and outcome-based reimbursement models, which could influence the adoption of Velcade and its competitors.
Conclusion
The Velcade market is poised for significant growth in the coming years, driven by a combination of factors including rising cancer incidence, advancements in treatment protocols, and the expanding reach of healthcare services. While challenges such as generic competition and pricing pressures exist, the overall outlook remains positive, with opportunities for growth in emerging markets and through the development of new therapeutic indications. As the oncology landscape continues to evolve, Velcade is expected to remain a key player, contributing to improved outcomes for patients worldwide.
0 notes
Text
Globalization and Market Expansion in Multiple Myeloma

The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.The Multiple Myeloma market is characterized by a dynamic interplay of research, treatment advancements, and patient-centric care initiatives. As one of the most prevalent hematologic cancers, it continuously draws attention from pharmaceutical innovators and healthcare providers alike. Recent years have witnessed a surge in targeted therapies, immunotherapies, and personalized medicine approaches tailored to combatting its complexities. This evolving landscape not only fosters competition among biopharmaceutical companies but also emphasizes the importance of early detection and multidisciplinary treatment strategies. With ongoing clinical trials promising novel therapeutic avenues, the Multiple Myeloma market remains poised for further breakthroughs in extending patient survival and enhancing quality of life.
The Multiple Myeloma Market research study for the term also includes a variety of business opportunities and growth potential. A business plan detailing market risks and constraints as well as the effects of various regulatory regimes is given to executives by the market research. This is carried out to assist companies in reaching their main goals and making better judgments.
Get Sample Copy Of This Report @ https://www.snsinsider.com/sample-request/3490
Market Segmentation
By Type
Chemotherapy
Monoclonal Antibody
Protease Inhibitors
Others
By Disease Type
Smoldering Multiple Myeloma
Active Multiple Myeloma
By End User
Clinics
Hospitals
Others
Regional Outlook
The geographical categories that make up the Multiple Myeloma Market each have their own revenue, market share, sales, and growth rates. Among the important geographical areas covered in the market analysis are Europe, Asia-Pacific, South America, North America, and the Middle East and Africa. Latin America is expected to have a small market share in value, while North America is forecast to maintain its global leadership position and have a significant market share in both volume and value.
COVID-19 Impact Analysis
In the first half of 2020, the COVID-19 virus started to spread over the world, infecting millions of people and forcing major nations to implement work stoppage and foot restrictions. Nearly every area of the economy has suffered, with the exception of medical goods and equipment for life support, including the Multiple Myeloma Market .
Competitive Landscape
The competitive analysis section of the global Multiple Myeloma Market offers details and insights on the participants. Among the details provided are information on competition, a market overview by business status, and revenue projections by region. These businesses use a variety of strategies, such as product launches, partnerships, alliances, technology advancements, and contracts, to boost market income.
Conclusion
The market research is supported by first-hand experience, qualitative and quantitative analysis by industry analysts, and comments from key market players and actors in the value chain. The study investigates parent industry trends, micro and macroeconomic data, governing factors, and market attractiveness on a segment-by-segment basis. The study also illustrates how different market factors might have a qualitative impact on market segmentation based on geography and Multiple Myeloma Market.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Flash Chromatography Market Size
Cystic Fibrosis Market Size
Cancer Biopsy Market Size
Glaucoma Therapeutics Market Size
Genomics Services Market Size
0 notes
Text
Revolutionizing Multiple Myeloma Treatment: The Rise of Bispecific Antibodies

Multiple myeloma (MM), a complex and incurable blood cancer, has long posed challenges for both patients and healthcare providers. Historically, treatment options have included chemotherapy, immunomodulatory drugs, proteasome inhibitors, and stem cell transplants. However, the recent introduction of bispecific antibodies marks a significant breakthrough in the treatment landscape, offering new hope for those battling this formidable disease.
Understanding Multiple Myeloma
Multiple myeloma develops from abnormal plasma cells in the bone marrow, resulting in the production of irregular proteins that can lead to various complications, including bone damage, kidney dysfunction, and immune system impairment. The prognosis for multiple myeloma patients has traditionally been grim, with a five-year survival rate of around 50%. Nonetheless, advancements in treatment strategies have improved outcomes and bispecific antibodies are leading this charge.
What Are Bispecific Antibodies?
Bispecific antibodies are engineered proteins designed to bind to two different antigens simultaneously. This unique capability allows them to redirect immune cells, such as T-cells, to target and destroy cancer cells more effectively. Unlike traditional monoclonal antibodies, which focus on a single antigen, bispecific antibodies can engage multiple pathways in the immune response, enhancing their therapeutic potential.
The Promise of Bispecific Antibodies in MM
Recent clinical trials have showcased the efficacy of bispecific antibodies in treating multiple myeloma. Among the most promising candidates, bispecific T-cell engagers (BiTE) have demonstrated significant anti-tumor activity in heavily pre-treated patients. By connecting T-cells to myeloma cells, BiTE antibodies can initiate a powerful immune response, resulting in reduced tumor burden and improved patient outcomes.
A landmark study featuring a bispecific antibody targeting BCMA (B-cell maturation antigen) revealed impressive results, with a high overall response rate and many patients achieving complete or partial remission. This breakthrough has catalyzed further research and development in this promising area, with several bispecific antibodies currently in clinical trials.
Advantages Of Traditional Therapies
The advent of bispecific antibodies brings several advantages compared to traditional treatments for multiple myeloma:
Targeted Action: Bispecific antibodies can precisely target cancer cells while sparing healthy cells, potentially reducing side effects and improving tolerability.
Enhanced Efficacy: By engaging multiple pathways in the immune system, these antibodies may enhance overall treatment effectiveness, leading to better outcomes.
Combination Potential: Bispecific antibodies can be combined with existing therapies, such as checkpoint inhibitors or other immunotherapies, to create synergistic effects that further boost treatment responses.
Accessibility: Designed to work with the patient’s immune system, bispecific antibodies may offer treatment options for those unresponsive to conventional therapies.
Challenges and Future Directions
Despite their promise, several challenges remain with bispecific antibodies. The complexity of the immune response and potential adverse effects, such as cytokine release syndrome, necessitate careful patient management and monitoring during treatment. Additionally, determining the optimal treatment regimen and its sequencing with existing therapies remains an area of active research.
Looking forward, the future of multiple myeloma treatment featuring bispecific antibodies is bright. Ongoing clinical trials are crucial for establishing the long-term efficacy and safety profiles of these therapies. As researchers explore innovative treatment strategies, bispecific antibodies could play a central role in transforming the management of multiple myeloma, offering renewed hope to patients and their families.
Conclusion
The rise of bispecific antibodies signifies a revolution in the treatment of multiple myeloma, presenting exciting possibilities for improved patient outcomes. As research progresses and these therapies become standard practice, we may soon witness a significant transformation in the management of this challenging disease, ultimately enhancing the quality of life and increasing survival rates for those affected by multiple myeloma.
#Multiple Myeloma#Multiple Myeloma Market#Multiple Myeloma Forecast#Multiple Myeloma Companies#Multiple Myeloma Drugs#Multiple Myeloma Therapies#Multiple Myeloma Epidemiology#Multiple Myeloma Pipeline#Multiple Myeloma Market Size#Multiple Myeloma Market Trends
0 notes
Text
Minimal Residual Disease Testing Market Future Trends to Look Out | Bis Research
Minimal Residual Disease Testing is a sophisticated diagnostic technique used primarily in oncology to detect and quantify residual cancer cells that may remain in the body during or after treatment.
The Global Minimal Residual Disease Testing Market is a rapidly growing segment in the healthcare industry, driven by the increasing demand for accurate and sensitive methods to monitor and manage cancer patients.
The Minimal Residual Disease Testing market was valued at $1.67 billion in 2023 and is expected to reach $6.67 billion by 2033, growing at a CAGR of 14.81% between 2023 and 2033.
MRD Testing Overview
Minimal Residual DiseaseTesting refers to the detection and quantification of residual cancer cells that remain in a patient after treatment, which are below the detection threshold of conventional diagnostic methods. MRD testing is particularly significant in hematologic malignancies such as leukemia, lymphoma, and multiple myeloma, where even a small number of remaining cancer cells can lead to relapse.
Have a look at our sample page here !
Market Segmentation
By Technology
By Target Detection
By End Users
By Region
China dominated the Asia-Pacific Minimal Residual Disease Testing Market in 2022 with a share of 36.08%. Although the market is expected to remain in a strong growth phase due to the massively growing number of cancer cases and the rising health-related awareness among people in Asia-Pacific, a significant barrier to the increasing adoption is an uneven economic balance among countries within the region.
Importance of Minimal Residual Disease Testing Market
Assessing Treatment Response
Predicting Relapse
Tailoring Therapy
Key Factors
The Minimal Residual Disease Testing Market has experienced significant growth in recent years, driven by several key factors like
advancements in technology
rising cancer burden,
clinical evidence supporting MRD monitoring
Key Players In the Minimal Residual Disease Testing Market includes
QIAGEN N.V.
Thermo Fisher Scientific Inc.
Sysmex Corporation
Mission Bio
OPKO Health
Bio-Rad Laboratories, Inc
ICON plc
Hoffmann-La Roche
and many others
Techniques used in Minimal Residual Disease Testing
Flow Cytometry - This technique uses fluorescent antibodies to identify cancer-specific markers on the surface of cells.
Polymerase Chain Reaction- PCR amplifies cancer-specific genetic sequences, allowing for the detection of one cancer cell among a million normal cells.
Next Generation Sequencing - NGS provides detailed genetic information by sequencing DNA or RNA at high depth, offering unparalleled sensitivity and the ability to identify clonal diversity and mutations.
Digital Droplet PCR- A more recent advancement, ddPCR partitions the sample into thousands of droplets and performs PCR on each droplet individually, providing high sensitivity and precise quantification.
Applications for Minimal Residual Disease Testing Market
Treatment Response Monitoring
Relapse Prediction
Treatment Decision-making
Prognostic Assessment
Clinical Trials and drug development
Minimal Residual Disease Testing Market Dynamics
Market Drivers
Advent of MRD and its Awareness among Consumers
Increasing Incidence of Cancer Cases Demanding MRD
Rise in administration of solid tumors
Expanding Medicare Coverage for MRD
Recent Developments in the Minimal Residual Disease Testing Market
•Quest Diagnostics acquired Haystack Oncology, expanding its oncology portfolio with the inclusion of advanced liquid biopsy technology. This addition aimed to enhance personalized cancer care by offering highly sensitive diagnostic capabilities. Integrated DNA Technologies launched the Archer FUSIONPlex Core Solid Tumor Panel, a pioneering cancer research testing solution that has been enhanced and fine-tuned to include a broader range of single nucleotide variant (SNV) and indel coverage.
Visit our precision medicine page here!
Key Questions Answered
Q What is MRD ?
Minimal residual disease (MRD) testing is a supplementary approach to detect extremely low levels of blood cancer cells and solid tumors following the treatment of conditions such as acute and chronic leukemia, lymphoma, or multiple myeloma. MRD specifically pertains to the minute population of cancer cells that persist in the body despite achieving complete remission (CR) through chemotherapy or stem cell transplantation.
Q What kinds of New Strategies are adopted by the existing market players to strengthen their positions in the Industry ?
The global MRD market is currently witnessing several developments, primarily aimed at introducing new products and services. Major manufacturers of MRD products, along with the service providers, are actively undertaking significant business strategies to translate success in research and development into the commercial clinical setting.
Conclusion
In conclusion, Minimal Residual testing is a powerful tool in modern oncology, offering the potential to significantly improve patient outcomes through more precise and personalized treatment strategies.
0 notes
Text
Oncology Drugs Market Growth, Trends, Size, Share, Demand And Top Growing Companies 2031

In a landscape where the battle against cancer rages on, advancements in healthcare systems, public health measures, and novel pharmaceutical therapies have ushered in a new era of hope. According to the National Cancer Institute, the United States saw an estimated 1,806,590 new cancer cases and approximately 606,520 deaths due to the disease in 2020. However, over the past five decades, cancer survival rates have soared from 50% in 1970 to an impressive 70%, thanks to a trifecta of progress.
For more information: https://www.fairfieldmarketresearch.com/report/oncology-drugs-market
Unprecedented Growth Trajectory: The global oncology therapy sales are forecasted to surpass US$ 300 billion by 2026, with oncology contributing 21.7% to total pharmaceutical sales. Fueling this growth are the top 10 pharmaceutical companies, which have declared oncology as their key focus area, driving multibillion-dollar M&A deals and strategic collaborations. Pfizer's acquisition of Array BioPharma for US$11 billion in 2019 and AbbVie's strategic partnership with Genmab for a bispecific antibody development deal worth US$3 billion are testament to this focus.
Diverse Indications Drive Demand: While oncology represents over 20 different indications, a significant portion of revenue stems from just five of them: breast cancer, multiple myeloma, non-small-cell lung carcinoma (NSCLC), prostate cancer, and non-Hodgkin's lymphoma (NHL), which collectively accounted for approximately 65% of the market in 2020. Moreover, with breast, lung, and colorectal cancers expected to collectively account for ~50% of all new cancer diagnoses by 2026, the demand for innovative therapies continues to surge.
Disruptive Trends Reshape Landscape: Innovation in oncology is accelerating, with disruptive technologies such as cell therapy, RNA therapy, viral vectors, and stem cell therapy gaining traction. Recent approvals of CAR-T cell therapies like Kymriah and Yescarta for acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) respectively signal a new frontier in cancer treatment. Precision medicine is also driving progress, with over 160 oncology biomarkers approved by 2019, paving the way for more targeted and effective therapies.
Impact of COVID-19: Despite remarkable progress, oncology has been among the worst-hit therapeutic areas amid the COVID-19 pandemic. Decreased demand for physician-administered products, disruptions in cancer screenings, and a decline in new clinical trials have posed significant challenges. However, the industry remains resilient, adapting to the evolving landscape and ensuring continued innovation.
Immuno-Oncology Leads the Way: Immuno-oncology sales are expected to soar to ~US$ 95 billion by 2026, with agents and protein kinase inhibitors comprising ~65% of sales. With over 550 active cell- and gene-therapy agents under clinical development, the future of cancer treatment looks promising. Investments in combination studies and the exploration of new mechanisms underscore the industry's commitment to advancing immuno-oncology therapies.Roche and Keytruda: Leading the Charge: In a highly concentrated market where the top 10 companies capture over 75% of the market value, F. Hoffmann-La Roche AG (Roche) and Merck & Co. stand out as leaders. While Roche maintains its global leadership position, Merck's Keytruda is poised to become the world's top-selling oncology
0 notes
Text
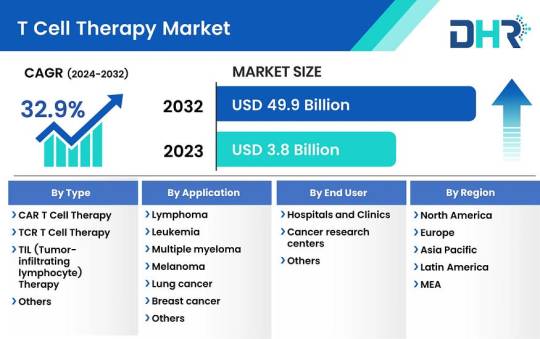
T Cell Therapy Market Size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032
The T cell therapy market size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032 at a CAGR of 32.9%.
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/t-cell-therapy-market-3114
Top Companies are:
· Novartis AG
· Gilead Sciences, Inc.
· Bristol-Myers Squibb Company
· Adaptimmune Therapeutics plc
· Amgen Inc.
· Atara Biotherapeutics, Inc.
· Autolus Therapeutics plc
· bluebird bio, Inc.
· Cellectis S.A.
· Iovance Biotherapeutics, Inc.
· Kite Pharma (a Gilead Company)
· Tmunity Therapeutics, Inc.
Market Segmentations:
By Type-
CAR T cell therapy
TCR T cell therapy
TIL (Tumor-infiltrating lymphocyte) therapy
Others
By Application-
Lymphoma
Leukemia
Multiple myeloma
Melanoma
Lung cancer
Breast cancer
Colorectal cancer
Autoimmune disorders
Infectious diseases
Others
By End User-
Hospitals and Clinics
Cancer research centers
Others
Regional Analysis:
The dominance of the T Cell Therapy market in North America is underpinned by the presence of established biopharmaceutical firms, a robust clinical trial infrastructure, and a favorable regulatory landscape. Among North American countries, the United States stands out as the primary contributor, buoyed by the FDA’s approval of several CAR T cell therapies such as Kymriah, Yescarta, and Abecma for treating hematological malignancies. The U.S. National Library of Medicine’s database reveals an extensive presence of over 1,000 active clinical trials dedicated to evaluating T cell therapies, underscoring the region’s steadfast commitment to research and development in this field.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- Leukemia: Leukemia, a type of hematologic malignancy, is characterized by the rapid production of abnormal white blood cells in the bone marrow, leading to complications in the immune system's function. The leukemia segment is significant in the hematologic malignancies market, with a high prevalence globally. Factors such as genetic predisposition, exposure to radiation, and certain chemotherapy drugs contribute to the development of leukemia.
- Lymphoma: Lymphoma is another key segment in the hematologic malignancies market, affecting the lymphatic system and lymphoid tissues. There are two main types of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is characterized by the presence of Reed-Sternberg cells, while non-Hodgkin lymphoma comprises a diverse group of lymphomas with varying characteristics and prognosis. The lymphoma segment is witnessing advancements in treatment options, including immunotherapy and targeted therapies.
- Myeloma: Multiple myeloma is a type of hematologic malignancy that affects plasma cells in the bone marrow. This segment of the market is characterized by the abnormal production of monoclonal proteins, leading to bone damage, renal complications, and other symptoms. The myeloma segment has seen significant progress in treatment modalities, including proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies. The market for myeloma therapies continues to expand, with a focus on improving patient outcomes and quality of life.
**Market Players**
- Roche: Roche is a prominent player in the hematologic malignancies market, offering a range of innovative therapies for leukemia, lymphoma, and myeloma. The company's portfolio includes targeted therapies, immunotherapies, and personalized medicine options for patients with hematologic malignancies. Roche invests heavily in research and development to introduce novel treatments and improve existing standards of care for these conditions.
- Novartis: Novartis isIn the competitive landscape of the hematologic malignancies market, Roche and Novartis stand out as key market players with a significant presence and impact on the industry. Roche, a Swiss multinational healthcare company, has established itself as a leader in providing innovative therapies for leukemia, lymphoma, and myeloma. With a diverse portfolio that includes targeted therapies, immunotherapies, and personalized medicine options, Roche continues to drive advancements in treatment options for patients with hematologic malignancies. The company's strong focus on research and development enables it to introduce novel treatments that address unmet medical needs and improve patient outcomes in this complex and challenging disease area.
Novartis, another major player in the hematologic malignancies market, has made substantial contributions to advancing the field of oncology with its portfolio of innovative therapies. The company's commitment to developing cutting-edge treatments for leukemia, lymphoma, and myeloma has made it a key player in the market. Novartis's emphasis on precision medicine and personalized treatment approaches has led to the development of targeted therapies that aim to improve the efficacy and safety profiles of treatments for hematologic malignancies. By investing in research and collaborations with key stakeholders in the healthcare ecosystem, Novartis continues to drive progress in addressing the evolving needs of patients with these complex diseases.
Both Roche and Novartis play a vital role in shaping the hematologic malignancies market through their focus on innovation, research, and patient-centric approaches to therapy development. These companies leverage their expertise in oncology and biotechnology to bring forward novel treatment options that have the potential to transform the standard of care for patients with leukemia, lymphoma, and myeloma. In addition to developing new therapies, Roche and Novartis also engage in strategic partnerships, regulatory initiatives, and patient advocacy efforts to drive awareness, access, and affordability of hematologic malignancy treatments on a global scale.
As the landscape of hematologic malignancies continues to evolve with advancements in technology**Segments:**
- Leukemia: Leukemia, a type of hematologic malignancy, is a significant segment in the market due to its high prevalence globally and the complexities associated with the rapid production of abnormal white blood cells. Factors such as genetic predisposition and exposure to certain chemicals play a role in the development of leukemia. The market for leukemia treatments is driven by continuous research and development efforts to improve patient outcomes and quality of life.
- Lymphoma: Lymphoma, affecting the lymphatic system, comprises Hodgkin lymphoma and non-Hodgkin lymphoma. Advancements in treatment options, including immunotherapy and targeted therapies, have significantly impacted the lymphoma market. The focus on personalized medicine and precision therapies is shaping the future of lymphoma treatment, with a strong emphasis on improving therapeutic efficacy and reducing adverse effects for patients.
- Myeloma: Multiple myeloma, characterized by the abnormal production of monoclonal proteins, poses challenges such as bone damage and renal complications. The myeloma segment has witnessed remarkable progress in treatment modalities, with the introduction of novel therapies such as proteasome inhibitors and monoclonal antibodies. The market for myeloma therapies is expanding, driven by the need to address unmet medical needs and enhance patient care.
**Global Hematologic Malignancies Market:** - By Type: Leukemia, Lymphoma, Myeloma - Therapy Type: Chemotherapy, Immunotherapy, Targeted Therapy
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"
0 notes