#Multi-parameter Monitor
Explore tagged Tumblr posts
Text
Multi-para Bedside Monitor Display=800-times-600-pixels-screen-resolution; Parameter=; SpO2=; Pulse rate=; NIBP=;Shop Online at Medzer.com

#Patient Monitor#Multi-parameter Monitor#Vital sign Monitor#remote patient monitoring#patient monitoring system#patient monitor machine#medzer
0 notes
Text
Multi-Parameter Equipment
Our multi parameter vital signs monitor is a cutting-edge device designed to provide comprehensive health monitoring. With its advanced technology, this equipment is capable of detecting various cardiovascular parameters, ensuring a thorough assessment of an individual's health status.
Types of neonatal multi parameter monitor in Jamr
Our multi-parameter equipment integrates essential vital sign monitoring functions, such as blood pressure measurement, heart rate monitoring, oxygen saturation levels, ECG testing, AFib detection, and atherosclerosis detection. This comprehensive multi-parameter vital signs monitor allows healthcare providers to efficiently monitor multiple aspects of a patient’s health simultaneously, saving valuable time and resources. With accurate and real-time data, healthcare professionals can make informed decisions and deliver comprehensive care to their patients.
Important Parameters of Cardiovascular Health
Identifying potential issues of cardiovascular health is crucial for maintaining a healthy heart. There are several important parameters that healthcare professionals use to assess the risk of cardiovascular disease. These include blood pressure, ECG, SpO2, AFib, and arteriosclerosis.
Blood pressure is a measure of the force of blood against the walls of arteries and is a critical indicator of heart health. SpO2 is a measure of the oxygen saturation level in the blood and is used to assess respiratory function. ECG is a test that records the electrical activity of the heart and can detect abnormalities in heart rhythm. AFib, or atrial fibrillation, is a type of irregular heartbeat that can lead to blood clots and stroke. Arteriosclerosis is a condition in which the arteries become narrow and hardened, which can increase the risk of heart attack and stroke.
By monitoring these parameters, healthcare professionals can identify potential issues with cardiovascular health and develop treatment plans to prevent or manage cardiovascular disease. It is important for individuals to schedule regular check-ups with their healthcare provider to ensure that their heart is healthy and functioning properly.

0 notes
Text
Enhancing Patient Care with Multi-Parameter Monitoring
Our patient monitors go beyond basic tracking, offering a wide array of parameters to ensure thorough patient care. Learn about the significance of 5 para and 7 para patient monitors, and explore our product range designed to cater to various medical scenarios and requirements.

0 notes
Text
Buy Veterinary Multi Parameter Monitor
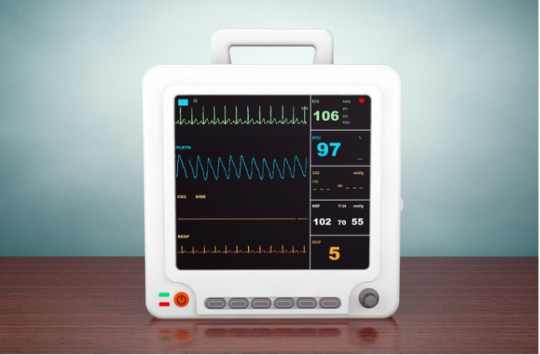
In a world where pets are cherished members of the family, providing them with the best healthcare is paramount. The Veterinary Multi-Parameter Monitor is a sophisticated piece of equipment that enables veterinarians to comprehensively assess the health status of animals.
The Veterinary Multi-Parameter Monitor is a cutting-edge medical device that amalgamates various monitoring tools into one compact unit.
It continuously measures and records a range of vital parameters, enabling veterinarians to closely observe the physiological state of animals.
Call - +31404020858
To know about products, visit our website
0 notes
Text
Multiparameter Patient Monitors
Lepu Portable Patient Monitors are designed to be compact and lightweight, making them easy to transport and use in various healthcare settings. They typically feature a built-in rechargeable battery, allowing for continuous monitoring even in remote or emergency situations.
Lepu Multiparameter Patient Monitors Types
Lepu Medical Grade AIView VX Tablet Patient Monitor Portable Multiparameter Monitor Vital Signs Monitor with Touch Screen for Hospital Clinic Ward and Home Use
Lepu AIView VX has a minimalist and unique design, advanced technology, and innovative patented design to streamline caregivers' workflow and improve work efficiency.
Lepu Medical AiView V12 Multiparameter Patient Monitor Portable All-in-one Vital Signs Monitor with AI Analysis Diagnosis Touch Screen for Hospital ICU Clinical Ambulance and Home Use
Lepu's latest generation of AI intelligent multi-parameter monitors, available in both 13.3-inch (V12) and 11.6-inch (V10) versions, feature a hardware design optimized for ergonomics, as well as a software design optimized for flat interactive experiences. These monitors are able to meet the unique needs of various hospital departments, greatly transforming the working modes of medical staff and delivering an unparalleled user experience.
Lepu Medical Grade K15/K12/K10 All-in-one Patient Monitor Portable Multiparameter Monitor with Touch Screen for Hospital ICU Clinical Home
15"/12"/10.4" high-resolution display touch screen. Comprehensive calculations for clinical application.

1 note
·
View note
Text
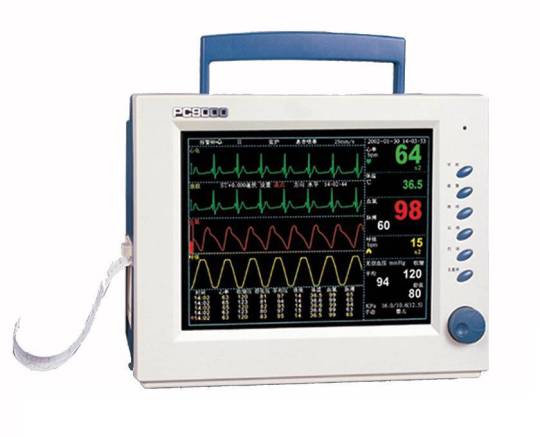
Top Features of the 1PC-Multi-Parameter Patient Monitor.
0 notes
Text

#water quality monitor#water Analyzer#water quality analyzer#water Analyzers#turbidity analyzer#water analysis equipment#tss meter#TSS meter#water hardness meter#water hardness test meter#multi-parameter water quality meter#cod analyzer#COD analyzer#ph meter#PH meter#water distiller equipment#water particle tester#auto titration meter#auto titration equipment#dew point meter#Puc monitor#puc device#puc monitor#vehicle emission#emission control
0 notes
Text
Research Log #P5-00436
FACILITY: [REDACTED] DATE: [REDACTED] CASE: #E2756895 ATTENDING: [REDACTED] UNIT: WARD 92 OBJECTIVE: Behavioral Compliance Induction
TIME: [09:45:00]
SUBJECT #1138-B7 was brought to the operating theater, prepped and draped in the usual fashion. Intravenous access was established using a 20-gauge catheter inserted into the left antecubital vein. Electrodes were placed on the scalp for continuous EEG monitoring. Additional sensors were attached to record heart rate, respiratory rate, and galvanic skin response (GSR).
Subject presents as a 25 year old male, physically healthy, baseline vitals recorded WNL. Subject exhibited signs of anxiety and resistance, which were managed by the use of sedatives (2 mg Midazolam IV).
[09:53:11]: Subject questioned to establish baseline cognitive and physiological parameters. Orientation, recall, and basic comprehension intact.
[10:00:00]: Infusion of proprietary psychotropic agent PCA-35 initiated at a rate of 5 mL/min.
[10:03:48]: Subject displays signs of restlessness. Cortical activation indicated by increased uptake on EEG. Subject gives responses to verbal stimuli and reports a sensation of lightheadedness.
[10:04:25]: Subject complains of stinging sensation and bittersweet taste. Noted slight tremor in extremities and increased heart rate. GSR indicates heightened anxiety.
[10:05:13]: Subject questioned to establish cognitive and physiological parameters. Noted delayed responses. Subject struggles to follow simple instructions, becomes distracted, provides incoherent explanations of surroundings, misinterprets questions.
[10:09:32]: Subject begins to exhibit signs of altered perception, including auditory hallucinations and delirium. EEG shows increased theta wave activity. Physical agitation observed; restraints effective in maintaining Subject's position. Subject too agitated for cognitive and physiological testing.
[10:14:45]: Administration of compound #GS-P5R initiated at 12 L/min via inhalation mask to reduce anxiety and stabilize neural response. Infusion of PCA-35 increased to 7.5 mL/min.
[10:19:48]: Subject's responses to verbal and physical stimuli decrease significantly. Continued monitoring shows stable vitals but increased physical rigidity. Administered 1 mg Lorazepam IV to reduce muscle tension.
[10:24:22]: Subject’s speech becomes slurred and incoherent. Noted disorientation to stimuli, increased muscle laxity. Decrease in heart rate and blood pressure.
[10:33:14]: Subject enters a semi-catatonic state. Eyes remain open but unresponsive to visual stimuli. Pupils equal but dilated. EEG shows dominant delta wave activity.
[10:42:28]: Subject displays signs of decreased neural responsiveness. Decreased pupillary reaction, continued slow rolling movement of the eyes, jerky movement of the whole body (hypnic jerks). Persistent drooling noted.
[10:45:04]: Infusion of PCA-13 reduced to 1 mL/min. Administration of compound #GS-P5R reduced to 2 L/min via nasal cannula.
[10:50:34]: Subject engaged with repetitive commands in accordance to Behavioral Compliance Protocols. Verbal cues, electronic conditioning, and multi-sensory stimuli reinforcement prove ineffective. Subject remains largely non-reactive.
[10:57:55]: Subject’s eyes remain unfocused with significant drooping. Attempts to direct gaze result in brief eye opening, followed by rapid drooping. Subject mumbles incoherently.
[10:58:06]: Speculum applied to maintain eyelid retraction for continuous observation and responsiveness testing. Subject demonstrates minimal resistance; remains in stuporous state. Droplets of propriety psychotic #3A administered to each eye. Immediate increase in pupil dilation and noticeable twitching observed.
[11:00:17]: Visual stimulus presented. Subject's eyes remain fixed and extremely dilated. Noted tremors in hands, erratic breathing patterns, increase in heart rate. Subject occasionally mumbles with extreme delay in response latency to verbal and physical testing.
[11:05:23]: Subject engaged with repetitive commands in accordance to Behavioral Compliance Protocols. Verbal cues, electronic conditioning, and multi-sensory stimuli reinforcement prove insignificant. Subject displays significant cognitive impairment, involuntary reflexes, significant drooling, and uncoordinated movements.
[11:10:19]: Increased auditory and visual stimuli introduced to enhance command comprehension of Behavioral Compliance Protocols. Subject displays signs of severe neural suppression. EEG findings variable and nonspecific, low voltage and slow irregular activity nonreactive to sensory stimuli.
[11:15:52]: Subject engaged with high-intensity visual stimuli (rapid flashing) and continuous auditory commands. Subject shows brief eye fixation on visual stimulus, with occasional facial twitching. Overall response is characterized by slow, inconsistent movements and frequent confusion. Subject’s attempts to respond are sporadic, sluggish, and incoherent.
[11:20:14]: Administered low-frequency auditory tones and ambient lighting. Subject displays intermittent eye tracking and reflexive vocalizations. Eyes lubricated to prevent irritation; speculum remains in place. Despite the high level of impairment, occasional partial compliance with commands noted.
[11:30:31]: Subject provided with 500 mL saline IV to maintain hydration. Subject engaged with repetitive commands in accordance to Compliance Protocols. Verbal cues, electronic conditioning, and multi-sensory stimuli reinforcement prove moderately effective as demonstrated by increased uptake seen on EEG. Noted severe motor function impairment, persistent drooling, disorientation.
[11:37:48]: Visual and auditory stimuli calibrated to induce deep trance state in preparation for Hypnotic Compliance Protocols. Subject's head and neck stabilized to ensure alignment with visual stimuli. Monitored vital signs remain stable but indicate persistent sedation effects. Subject remains largely unresponsive, exhibiting only involuntary reflexes and intense eye fixation on visual stimulus.
[12:00:00]: End of Behavioral Compliance Induction log. Subject's transition to hypnotic phase officially logged and observed.
TRANSFER OF CARE: [REDACTED]
#whump#whump community#medical whump#hospital whump#sedation whump#tw experimentation#drug whump#hypnosis#lab rat
68 notes
·
View notes
Text

The DP2003 Multi-Parameter Patient Monitor features a 12.1” high-resolution LED display with LCD backlight, a modern 120mm slim design, and comprehensive monitoring of ECG, NIBP, SpO2, TEMP, PR/HR, and RESP. https://www.narang.com/icu-equipments/patient-monitor/DP2003.php
3 notes
·
View notes
Text
Unveiling the Technical Wizardry of Geek Robocook Electric Pressure Cooker: Your Gateway to Effortless Cooking
In the world of kitchen appliances, Geek Robocook Electric Pressure Cooker stands out as a technological marvel—a culinary wizard that simplifies meal preparation while delivering delicious results. In this comprehensive guide, we'll dive deep into the technical aspects of Geek Robocook, exploring its innovative features and functionalities that make it the ultimate choice for anyone seeking effortless cooking without sacrificing taste or quality.
Smart Cooking Technology: Simplifying Culinary Complexity
Geek Robocook Electric Pressure Cooker isn't just another kitchen gadget—it's a smart cooking companion designed to simplify even the most complex culinary tasks. At its core lies innovative technology that revolutionizes the cooking experience, ensuring that every meal is a masterpiece of flavor and convenience.

1. Multi-Functional Versatility: Geek Robocook isn't limited to just pressure cooking. Its versatility shines through a plethora of cooking modes, including slow cooking, sautéing, steaming, and more. This multi-functionality allows users to explore a wide range of recipes and cooking techniques with ease, all from a single appliance. Whether you're craving a hearty stew, a fluffy batch of rice, or a succulent roast, Geek Robocook has you covered.
2. Precision Cooking Algorithms: Behind the scenes, Geek Robocook employs sophisticated algorithms to ensure precise cooking times and temperatures for optimal results. These algorithms take into account factors such as ingredient type, quantity, and desired doneness to deliver consistent and delicious meals every time. Whether you're cooking for one or entertaining a crowd, you can trust that Geek Robocook will deliver perfect results with pinpoint accuracy.
3. Adaptive Cooking Technology: Geek Robocook's adaptive cooking technology sets it apart from traditional pressure cookers. By constantly monitoring internal temperature and pressure levels, Geek Robocook adjusts cooking parameters in real-time to achieve the desired outcome. This means no more undercooked or overcooked meals—just perfectly cooked dishes that are sure to impress even the most discerning palate.
Intuitive User Experience: Navigating the Culinary Landscape
While Geek Robocook boasts advanced technology under the hood, its user experience remains refreshingly intuitive. Whether you're a seasoned chef or a kitchen novice, you'll find Geek Robocook's interface easy to navigate and understand, empowering you to unleash your culinary creativity with confidence.

1. Clear and Concise Controls: Geek Robocook's control panel features clearly labeled buttons and intuitive icons, making it easy to select cooking modes, adjust settings, and monitor progress at a glance. With just a few taps, you can customize cooking parameters to suit your preferences and dietary needs, ensuring that every meal is tailored to perfection.
2. Guided Cooking Programs: For those moments when inspiration is lacking, Geek Robocook offers a variety of guided cooking programs to spark your creativity. These pre-programmed recipes provide step-by-step instructions and cooking tips for a wide range of dishes, taking the guesswork out of meal preparation and empowering you to explore new flavors and cuisines with ease.
3. Interactive Feedback: Geek Robocook goes beyond mere functionality to provide interactive feedback and guidance throughout the cooking process. Visual indicators, audible alerts, and on-screen prompts keep you informed and engaged every step of the way, ensuring a seamless and enjoyable cooking experience from start to finish.
Safety and Reliability: Cooking with Confidence
In addition to its advanced features and intuitive interface, Geek Robocook prioritizes safety and reliability to provide users with peace of mind in the kitchen. With a robust set of safety features and built-in safeguards, Geek Robocook ensures that cooking is not only convenient and enjoyable but also safe and worry-free.

1. Pressure Release Mechanism: Geek Robocook's pressure release mechanism allows for safe and controlled depressurization after cooking, preventing accidents and ensuring user safety at all times. Whether you're releasing pressure manually or allowing it to dissipate naturally, you can trust that Geek Robocook has your back when it comes to safety.
2. Lid Lock Detection: Geek Robocook's lid lock detection feature prevents the cooker from being opened while under pressure, minimizing the risk of accidental spills or burns. This ensures that you can safely handle the cooker during and after cooking without fear of injury, allowing for a stress-free cooking experience for users of all skill levels.
3. Overheat Protection: With built-in overheat protection, Geek Robocook automatically shuts off or reduces heat output in the event of overheating, preventing damage to the appliance and ensuring safe operation. This added layer of protection gives users peace of mind, allowing them to cook with confidence knowing that Geek Robocook has their safety in mind.
Real User Testimonials: From Kitchen Challenges to Culinary Triumphs
But don't just take our word for it—let's hear from some satisfied users who have experienced the technical brilliance of Geek Robocook firsthand:
"I was initially skeptical about investing in a pressure cooker, but Geek Robocook has exceeded all my expectations. Its smart cooking technology and intuitive interface have made meal preparation a breeze, even on the busiest of days. Whether I'm cooking a quick weeknight dinner or experimenting with new recipes, Geek Robocook consistently delivers delicious results with minimal effort." - Jennifer L.
"As someone who loves to cook but doesn't always have the time or energy to devote to it, Geek Robocook has been a game-changer. Its precision cooking algorithms and adaptive technology ensure that every meal turns out perfectly, regardless of my level of expertise. Plus, its safety features give me peace of mind, allowing me to cook with confidence knowing that I'm in good hands." - Michael T.
Conclusion: Elevate Your Culinary Experience with Geek Robocook
In conclusion, Geek Robocook Electric Pressure Cooker is not just a kitchen appliance—it's a culinary companion that empowers users to unleash their creativity and enjoy delicious meals with ease. With its smart cooking technology, intuitive user experience, and emphasis on safety and reliability, Geek Robocook sets the standard for modern cooking appliances, making it the ultimate choice for anyone seeking convenience, versatility, and exceptional results in the kitchen. Ready to take your cooking to the next level? Visit the Geek Robocook website today and discover why Geek Robocook is the best option for your culinary journey. Join the ranks of satisfied customers who have embraced the technical brilliance of Geek Robocook and transformed their cooking experience for the better.
2 notes
·
View notes
Text
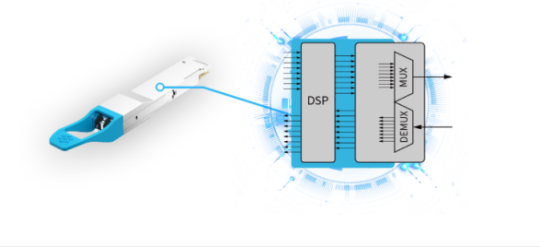
Closer Look at 400G QSFP-DD Optical Transceiver Module
Recent years have seen the evolution towards the 5G, Internet of Things (IoT), and cloud computing, increasing pressure on data centers to ramp up both network capacity to 400G and driving providers to search for new solutions to achieve their 400G Data Center Interconnects (DCIs). 400G QSFP-DD optical transceiver module is becoming one of the most popular cutting-edge 400G DCI solutions. This article will provide a basic understanding of QSFP-DD optical transceiver modules.

What is the QSFP-DD Optical Transceiver Module?
Quad small form-factor pluggable-double density (QSFP-DD) is designed with eight lanes that operate at up to 25 Gbps via NRZ modulation or 50 Gbps via PAM4 modulation. It supports data rates of 200 Gbps or 400 Gbps and doubles the density. QSFP-DD is backward compatible with current 40G and 100G QSFPs. It is compliant with IEEE802.3bs and QSFP-DD MSA standards. QSFP-DD is available in single-mode (SM) and multi-mode (MM) fiber optic cables and includes digital diagnostics monitoring (DDM) to monitor parameters such as power, temperature, and voltage.

Types of 400G QSFP-DD Optical Transceiver Module
400G SR8 QSFP-DD
400G SR8 QSFP-DD optical transceiver module is suitable for short-distance interconnection or multi-channel data communication. The transmission rate is up to 425Gbps, and the central wavelength is 850nm. The transmission distance is up to 70m or 100m through multi-mode OM3 and OM4 fiber.
400G DR4 QSFP-DD
400G DR4 QSFP-DD optical transceiver module achieves the transmission over single-mode fiber (SMF) with an MPO-12 connector. It supports a max transmission distance of 500m on SMF.
400G FR4 QSFP-DD
400G FR4 QSFP-DD optical transceiver module supports link lengths of up to 2km SMF with a duplex LC connector. It uses wavelength division multiplexing(CWDM ) technology, eight channels of 53Gbps PAM4 signals on the electrical side, and four channels of 106Gbps PAM4 signals on the optical side, which is twice the rate of the electrical side.
400G LR4 QSFP-DD
400G LR4 QSFP-DD optical transceiver module is designed with a built-in Gearbox chip that multiplexes the two channels' electrical input data into a single-channel outputs signal and then modulates it to the optical receiver end. The digital signal processor (DSP) basis gearbox converts eight channels of 25GBaud PAM4 signals into four channels of 50GBaud (PAM4) over an SMF cable with duplex LC connectors. It supports a transmission distance of up to 10km.
400G LR8 QSFP-DD
The 400GBASE-LR8 optical transceiver module supports link lengths of up to 10km over a standard pair of G.652 SMF with duplex LC connectors.
400G ER8 QSFP-DD
The 400G ER8 QSFP-DD optical transceiver module supports link lengths of up to 40km over a standard pair of G.652 SMF with duplex LC connectors.
400G ER4 QSFP-DD
400G ER4 QSFP-DD optical transceiver module supports link lengths of up to 40km over a standard pair of G.652 SMF with duplex LC connectors. It has only four wavelengths for 4 LAN WDM channels 1295.56/1300.05/1304.58/1309.14nm.
Advantages of 400G QSFP-DD Optical Transceiver Module
Backward compatibility: Allowing the QSFP-DD to support existing QSFP modules (QSFP+, QSFP28, QSFP56, etc.). It provides flexibility for end-users and system designers.
Adopting the 2x1 stacked integrated cage/connector to support the one-high cage connector and two-high stack cage connector system.
SMT connector and 1xN cage design: Enable thermal support of at least 12W per module. The higher thermal reduces the requirement for heat dissipation capabilities of transceivers, thus reducing some unnecessary costs.
ASIC design: Supporting multiple interface rates and fully backward compatible with QSFP+ and QSFP28 modules, thus reducing port and equipment deployment costs.
Applications
400G QSFP-DD optical transceiver module is used in 400G Ethernet, data centers, telecommunications networks, cloud networks, Infiniband interconnects, high-performance computing networks, 5G, 4K video, IoT, etc.
Conclusion
400G QSFP-DD optical transceiver module offers high speed, performance, scalability, and low power in 400G Ethernet. Sun Telecom specializes in providing one-stop total fiber optic solutions for all fiber optic application industries worldwide. Contact us if any needs.
2 notes
·
View notes
Text
Buy Veterinary Multi Parameter Monitor

In a world where pets are cherished members of the family, providing them with the best healthcare is paramount. The Veterinary Multi-Parameter Monitor is a sophisticated piece of equipment that enables veterinarians to comprehensively assess the health status of animals.
The Veterinary Multi-Parameter Monitor is a cutting-edge medical device that amalgamates various monitoring tools into one compact unit.
It continuously measures and records a range of vital parameters, enabling veterinarians to closely observe the physiological state of animals.
Call - +31404020858
To know about products, visit our website
0 notes
Text
Next-Gen Cable Machinery: Advancements Driving the Industry Forward

The cable manufacturing industry is evolving rapidly, driven by technological advancements, automation, and the increasing demand for high-quality, durable cables. From telecommunications to power distribution, industries worldwide rely on efficient and innovative cable production solutions.
With the rise of Industry 4.0, smart manufacturing, and sustainability, next-generation cable machinery is transforming production processes, enhancing efficiency, and improving product quality. In this article, we explore the latest advancements in cable machinery and how they are shaping the future of the industry.
Cable Machinery Manufacturers
1. Automation and Smart Manufacturing
Automation has revolutionized cable production, minimizing human intervention while improving speed and precision. Next-gen cable machinery integrates Artificial Intelligence (AI), Internet of Things (IoT), and Machine Learning (ML) to streamline operations and optimize performance.
Key Innovations in Automation:
✅ AI-Driven Quality Control: AI-powered sensors and cameras detect defects in real-time, reducing waste and ensuring consistent quality. ✅ IoT-Enabled Monitoring: Smart machines can collect data, monitor performance, and send alerts for maintenance, preventing downtime. ✅ Robotics in Cable Assembly: Automated robotic arms enhance precision in cutting, stripping, and assembling cables.
By leveraging automation, manufacturers can achieve higher efficiency, lower production costs, and improved product consistency.
2. High-Speed Extrusion Technology
Extrusion is a critical process in cable manufacturing, involving the coating of conductors with insulation and protective layers. Next-generation high-speed extrusion machines are revolutionizing the process with:
🔹 Energy-efficient heating systems that reduce power consumption. 🔹 Advanced cooling mechanisms for better insulation adhesion. 🔹 Multi-layer extrusion technology for producing complex cable structures in a single pass.
These advancements enhance productivity, reduce material waste, and improve cable durability, making them essential for modern production lines.
Wire Machinery Manufacturers
3. Precision Wire Drawing and Stranding Techniques
Wire drawing and stranding are fundamental processes in cable manufacturing, determining the strength and conductivity of the final product. The latest innovations include:
🔹 Microfine wire drawing: Enables ultra-thin yet highly conductive wires for specialized applications like 5G and aerospace. 🔹 Torsion-free stranding technology: Ensures uniform strand distribution, reducing internal stress and enhancing cable flexibility. 🔹 Automatic tension control systems: Optimize wire tension, preventing breakage and improving consistency.
These developments contribute to higher-quality cables with superior electrical performance and durability.
4. Sustainable and Eco-Friendly Manufacturing
Sustainability is a major focus in cable production, with manufacturers adopting green technologies to reduce environmental impact. Innovations in this space include:
✅ Recyclable and biodegradable insulation materials that replace traditional PVC and polyethylene. ✅ Energy-efficient machinery that consumes less power while maintaining high output. ✅ Closed-loop water cooling systems that minimize water wastage in extrusion and cooling processes.
By embracing eco-friendly practices, cable manufacturers not only reduce carbon footprints but also comply with global sustainability standards.
Cable Machinery Manufacturers
5. Digital Twin Technology in Cable Manufacturing
A Digital Twin is a virtual replica of a physical manufacturing process, enabling real-time monitoring and simulation of production scenarios. This innovation is transforming the cable machinery industry by:
🔹 Predicting machine failures through AI-driven diagnostics. 🔹 Optimizing production parameters before implementing changes in real-time operations. 🔹 Enhancing training and safety by simulating complex processes before execution.
By integrating Digital Twin technology, manufacturers can improve operational efficiency, reduce downtime, and enhance overall productivity.
6. Advanced Testing and Quality Assurance Systems
Next-gen cable machinery comes equipped with state-of-the-art testing and inspection systems to meet strict industry standards. Some of the latest innovations include:
✅ High-speed spark testers that instantly detect insulation defects. ✅ Real-time X-ray and laser measurement systems for precise diameter and thickness control. ✅ Automated tensile and bending tests to ensure flexibility and durability.
These advancements guarantee high-quality, defect-free cables that meet global safety and performance regulations.
7. The Rise of Smart Factory Integration
Modern cable manufacturing facilities are evolving into smart factories, where interconnected machines and data-driven processes enhance production efficiency. Key aspects of smart factory integration include:
🔹 Cloud-based data analytics for real-time decision-making. 🔹 Automated material handling systems for seamless production flow. 🔹 Cybersecurity enhancements to protect manufacturing data from digital threats.
With smart factories, cable manufacturers can increase productivity, reduce operational costs, and improve product traceability.
Wire Machinery Manufacturers
Conclusion
The future of cable manufacturing is being shaped by automation, digitalization, sustainability, and smart technologies. With next-generation cable machinery, manufacturers can produce stronger, more efficient, and environmentally friendly cables while maintaining cost-effectiveness and high quality.
As the demand for high-performance cables continues to grow, embracing these advancements will be essential for staying competitive in the industry. Now is the time to invest in next-gen cable machinery to drive innovation and efficiency forward.
🔹 Is your cable manufacturing process ready for the future? Explore the latest advancements today!
#Wire Machine Manufacturers#Wire Machinery Manufacturers#Cable Machinery Manufacturers#Cable Making Machine Manufacturers#Wire Making Machine Manufacturers
0 notes
Text
Cardiac Monitoring Devices Market: Trends, Growth Drivers, and Future Outlook
The global cardiac monitoring devices market is experiencing significant growth, driven by the increasing prevalence of cardiovascular diseases (CVDs), technological advancements, and the rising adoption of remote patient monitoring systems. According to a report by SNS Insider, the market size was valued at USD 29.15 billion in 2023 and is projected to reach USD 48.58 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 5.84% during the forecast period 2024-2032.
Market Segmentation:
The cardiac monitoring devices market is segmented based on type, product type, application, and region.
By Type:
Cardiovascular Devices
Multi-Parameter ECG Monitors
Patient Monitoring Devices
Ambulatory Cardiac Monitoring
Cardiac Monitors
By Product Type:
Resting ECG Devices
Stress ECG Devices
Holter Monitors
Event Monitors
Implantable Loop Recorders
Cardiac Output Monitoring Devices
By Application:
Coronary Heart Disease
Arrhythmia
Heart Failure
Others
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1021
Regional Analysis:
North America holds a significant share of the cardiac monitoring devices market, attributed to advanced healthcare infrastructure, high prevalence of CVDs, and early adoption of innovative technologies. Europe follows, with substantial growth driven by increasing healthcare expenditure and awareness programs. The Asia-Pacific region is anticipated to witness the fastest growth during the forecast period, owing to a rising geriatric population, improving healthcare facilities, and growing awareness about early disease detection.
Key Players
1. Medtronic
Reveal LINQ
HCT (Heart Failure Monitoring)
CareLink
2. Abbott
Freestyle Libre
Confirm Rx
Tavi
3. Boston Scientific Corporation
LUX-Dx
HeartLogic
ACUITY
4. iRhythm Technologies, Inc.
Zio XT
5. GE Healthcare
CardioSoft
MAC 400
CASE System
6. Biotronik, Inc.
BioMonitor 2
Edora 8/6/4
Visions 2
7. SCHILLER Healthcare India Pvt. Ltd
CARDIOVIT AT-102 G2
CARDIOVIT FT-1
8. Koninklijke Philips N.V.
Philips HeartStart
IntelliVue Patient Monitoring
BioTel Heart
9. MicroPort Scientific Corporation
Apollo
Renata
10. Honeywell Life Care Solutions
LifeStream
AMT Cardiac Monitoring System
Care Assure
Key Highlights:
Technological advancements, such as wearable electrocardiogram (ECG) monitors and artificial intelligence (AI)-powered diagnostic tools, are enhancing real-time and remote cardiac monitoring.
The adoption of remote patient monitoring (RPM) systems is increasing, allowing healthcare providers to monitor patients' cardiac health outside clinical settings, thereby reducing hospital readmissions.
The global aging population is contributing to market growth, as older adults are more susceptible to cardiovascular diseases.
Future Outlook:
The cardiac monitoring devices market is poised for sustained growth, driven by continuous technological innovations and a growing emphasis on preventive healthcare. The integration of AI and machine learning in diagnostic tools is expected to further enhance the accuracy and efficiency of cardiac monitoring. Additionally, the expansion of telemedicine and RPM systems will facilitate better patient management and early detection of cardiac conditions, ultimately improving patient outcomes.
Conclusion:
The global cardiac monitoring devices market is on a robust growth trajectory, with significant advancements in technology and increasing awareness about cardiovascular health. Stakeholders, including healthcare providers, manufacturers, and investors, are well-positioned to benefit from the evolving landscape of cardiac care.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Bone Densitometer Market Size
Tissue Diagnostics Market Share
Bone Densitometer Market Size
#Cardiac Monitoring Devices Market#Cardiac Monitoring Devices Market Share#Cardiac Monitoring Devices Market Size#Cardiac Monitoring Devices Market Trends
0 notes
Text

Abstract The demand for efficient and durable chargers has surged with the widespread use of electronic devices. Conducting a charger speed test is critical in evaluating a charger’s performance, reliability, and safety. The LISUN SY65240T Charger Aging Test Rack (Constant Temperature) offers a comprehensive solution for testing chargers under controlled conditions. This paper explores the significance of charger speed testing, highlights the features of the SY65240T system, and demonstrates its application in ensuring chargers meet industry standards. Introduction In today’s technology-driven world, chargers are essential for powering devices from smartphones to industrial equipment. Evaluating a charger’s performance requires understanding its charging speed, durability, and response to environmental factors. The charger speed test involves analyzing how fast and efficiently a charger delivers power to a device while maintaining safety and longevity. LISUN’s SY65240T Charger Aging Test Rack is designed to simulate real-world conditions during these tests. By operating in a constant-temperature environment, this equipment provides accurate and repeatable results, ensuring compliance with global quality standards. Importance of Charger Speed Testing A charger speed test goes beyond merely measuring how quickly a device recharges; it also evaluates: • Charging Efficiency: The conversion of electrical energy into usable charge without significant losses. • Heat Management: Excess heat can damage devices and reduce their lifespan. Testing helps identify thermal limits. • Durability Under Load: Continuous high-speed charging can wear out components. Aging tests reveal potential failure points. • Safety Compliance: Fast chargers must meet safety regulations to prevent overheating, short circuits, and electrical hazards. Overview of the LISUN SY65240T Charger Aging Test Rack The LISUN SY65240T Charger Aging Test Rack is a state-of-the-art system designed for conducting comprehensive charger speed tests and aging evaluations. Key Features: • Constant Temperature Control: Maintains stable conditions to test chargers in high-precision environments. • Simultaneous Multi-Unit Testing: Accommodates multiple chargers, enabling batch testing for manufacturers. • Adjustable Voltage and Current Ranges: Supports diverse charger types, including fast chargers for smartphones, laptops, and electric vehicles. • Real-Time Monitoring: Captures critical data such as voltage fluctuations, current stability, and temperature variations. • Automatic Shutoff for Safety: Protects against overheating or electrical failures during extended tests. SY65240T_Constant Temperature Charger Aging Test Rack Technical Specifications: Parameter Details Input Voltage Range 220V ±10% Output Capacity Customizable (10–240 units) Temperature Range Room temperature to 50°C Data Logging Integrated software system Methodology for Charger Speed Test Using the SY65240T Preparation: • Select chargers for testing, ensuring they span a range of models and capacities. • Configure the SY65240T system to match the test parameters, including voltage, current, and temperature. Testing Process: • Step 1: Measure baseline charging speed for each charger in standard conditions. • Step 2: Introduce variable loads to simulate real-world usage. • Step 3: Monitor heat generation and charging efficiency over time. • Step 4: Conduct aging tests by operating chargers continuously for 72–120 hours. Data Collection and Analysis: • Use real-time monitoring to gather data on voltage stability, current output, and temperature. • Compare performance metrics against manufacturer specifications. Reporting: • Generate detailed reports, including graphs and tables, summarizing the findings of the charger speed test. Results and Discussion A typical charger speed test using the SY65240T revealed the following insights: • Charging Speed Consistency: Most chargers maintained their advertised speed during initial testing but showed variability under higher loads. • Temperature Stability: Fast chargers exhibited significant heating, but the SY65240T effectively identified thermal limits. • Durability Under Stress: Aging tests highlighted potential points of failure, such as component degradation and output instability. Sample Data: Charger Model Initial Speed (W) Speed After 72 Hours (W) Temperature Increase (°C) Model A 25 24 12 Model B 45 43 18 Model C 65 60 25 The table illustrates that while most chargers maintained their speed, some experienced minor efficiency drops due to thermal stress. Applications and Benefits The SY65240T Charger Aging Test Rack supports manufacturers, quality control teams, and R&D departments by offering: • Improved Product Design: Insights from the charger speed test can help refine heat dissipation and circuit design. • Enhanced Customer Satisfaction: Ensures chargers meet performance claims, reducing customer complaints and returns. • Regulatory Compliance: Assists manufacturers in meeting international safety and performance standards. Conclusion The charger speed test is indispensable for ensuring chargers meet the growing demands for efficiency, durability, and safety. The LISUN SY65240T Charger Aging Test Rack provides a reliable platform for conducting these tests with precision and consistency. By leveraging advanced features like constant temperature control and real-time monitoring, it equips manufacturers to deliver superior products that excel in the competitive electronics market. Keywords: charger speed test, charger performance, LISUN SY65240T Charger Aging Test Rack, constant temperature testing Read the full article
0 notes
Text

DP2003 Multi-Parameter Patient Monitor features a 12.1” high-resolution LED display with LCD backlight, a modern 120mm slim design, and capabilities for ECG, NIBP, SpO2, TEMP, PR/HR, and RESP, with optional functions such as EtCO2, 2IBP, and a recorder, offering 168 hours of graphic and tabular trends for all parameters ... https://www.narang.com/icu-equipments/patient-monitor/DP2003.php
3 notes
·
View notes