#Led Light Therapy Malvern
Explore tagged Tumblr posts
Text
Led Light Therapy Malvern | Malvern Skin Studio
Led Light Therapy Malvern | LED light therapy can help treat a variety of dermatological conditions and may speed up wound healing. Learn more about at-home and medical treatments here.
1 note
·
View note
Text
Novel Poly(Vinyl caprolactum-co-Sodiumacrylate) Microspheres for Controlled Release of 5-Fluorouracil
INTRODUCTION Controlled delivery of drugs by means of biodegradable polymers began in the 1970s and continued to expand rapidly with numerous novel products1,2. The controlled release technology has lead to the development of newer methods of drug administration as well as the design and application of different types of CR formulations for effective targeting of certain drugs to the site of action. In particular, biodegradable polymeric systems have led to the development of CR dosage formulations to achieve the desired therapeutic results to obtain maximum dose regimen with minimum side effects3. The release of drug from a polymer matrix occurs due to the transport of drug to the surrounding medium system by the molecular diffusion mechanism. The CR systems offer many advantages over the conventional dosage forms, including improved efficacy, reduced toxicity as well as improved patient compliance and convenience4-6. Among the various types of polymers employed, hydrophilic biopolymers are quite suitable for oral applications7 due to their several inherent advantages over the synthetic polymers. Drug targeting to a specific tissue or organ has been the subject of creative and innovative research in medicinal and pharmaceutical chemistry since the beginning of the twentieth century. In many diseases (e.g. cancer, AIDS, rheumatoid arthritis, etc.) a considerable therapeutic advantage could be gained if drugs were delivered more selectively and in a controlled manner to their target sites. More particularly, it is conventionally accepted that efficient, compliant and reliable therapy requires that the drug reside as long as its therapeutic action is needed at a specific site, where it acts (by systemic absorption, binding, inhibition, etc.) as intact molecules. This concept has led to the development of a variety of physically based controlled release dosage forms such as drug dispersible matrices, coated tablets or particles, microcapsules. The development of an appropriate delivery system will first require a proper consideration of three related factors; the properties of the drug; the disease and the destination in the body. Over the past few years, stimuli-responsive (sensitive) polymers have become the object of intensive study due to their ability to change drastically their physical state under minute changes in external environment such as temperature, pH, ionic strength, light illumination, etc. Recently, chromatographic8,9 drug delivery10,11 membrane technology12,13 and kinetic inhibition14 applications were reported. Poly(N-isopropylacrylamide)15-17 (PNIPA) and poly(N-vinyl caprolactam)18,19 (PVCL) were intensively investigated due to their thermo-sensitive properties since these are water soluble at low temperature. However, they exhibit lower critical solution temperature (LCST) in water and undergo a coil-to-globule transition and aggregation at higher temperatures. For PNIPA the coil-to-globule transition occurs at around 32°C. PVCL is a homolog of poly(N-vinylpyrrolidone) (PVP), which is a biocompatible polymer widely used in medicine and pharmaceutics20. PVCL combines the useful and important properties of PVP and PNIPAm. It is a biocompatible polymer with a phase transition in the region of physiological temperature (30-37 °C). Such properties make it a prospective material in designing CR systems. Further, the incorporation of ionic hydrophilic moieties into the PolyVCL hydrogel networks would enhance the LCST and the gels become sensitive towards PH, whereas hydrophobic moieties decrease the LCST. Liu et al.21 found that salts of acrylic acid monomers are strong electrolytes, which are completely ionized in water, and their copolymeric units increased the swelling characteristics to a greater extent. 5-Fluorouracil is an acidic, water-soluble22,23, hydrophilic, is an antineoplastic drug used extensively in clinical chemotherapy for the treatment of solid tumors. It has been widely used in drug administration due to its large number of secondary effects that accompany its conventional administration. We present here the development of 5-fluorouracil-loaded poly(vinyl caprolactam-co-Sodium acrylate) microspheres for investigating its slow release characteristics. The plasma lifetime of 5-Fu is 1-1.2 hand it needs to extend for its effective therapy. The microspheres prepared were characterized by particle size analyzer, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). The in vitro release studies have been performed in 7.4 pH buffer solution at 25 0C and 370C to extend to the release rates of the drug. MATERIALS AND METHOD Materials Vinyl caprolactam (VC) was purchased from Aldrich Chemicals, Milwaukee, WI USA. Sodium acrylate (SA), N, N¢-methylene bisacrylamide (NNMBA), sodium lauryl sulfate, potassium persulfate, and calcium chloride were all purchased from s.d. fine chemicals, Mumbai, India. 5-Fluorouracil was purchased from MP Biochemicals, Eschwege, Germany. Synthesis of poly(vinyl caprolactam-co-sodium acrylate) microspheres Sodium lauryl sulfate (1g) was dissolved in 80 ml of water taken in a three-necked round bottom flask equipped with a mechanical stirrer, a condenser, and a gas inlet to maintain the inert nitrogen atmosphere. The flask was immersed in an oil bath with a thermostatic control to maintain the desired temperature accurate to ± 1oC. The solution was stirred at 800 rpm speed until it became clear and 100 mg of potassium persulfate was added. The required amount of SA, VC, crosslinking agent, NNMBA and 5-Fluorouracil were dissolved separately in 20 ml of water. This mixture was added to the reaction mixture drop-wise using a dropping funnel and the reaction was continued for 8 h at 700C to obtain the maximum yield. The reaction mixture was taken out after 8 h and added to 1% calcium chloride solution drop-wise to break the emulsion24. Particles were then isolated by centrifuging the product at the rotor speed of 12,000 rpm, washed with water and dried under vacuum at 400C for 24 h. Conversion of Copolymer The yield of copolymeric microspheres was determined gravimetrically. After copolymerization, the latex solution was added to 1 % calcium chloride solution and centrifuged to isolate the particles from the mixture. The copolymeric microspheres were washed several times successively with water and methanol solvents to remove the remaining monomer and initiator and then dried in a vacuum oven at 500C until attainment of constant weight. The % conversion of monomers was calculated as: % Conversion = (W/M) ×100 Where W is the weight of the dry copolymer obtained from the latex sample and M is the weight of the monomers taken. The yield of copolymeric microspheres varied between 80 and 85 % for various formulations prepared in this study. pH and Temperature Sensitive Nature of Copolymer Microspheres

percentages of swelling ratio (% SR) pH and temperature sensitivity of copolymer microspheres were studied through swelling experiments. First, the microspheres were immersed in a buffer solution with various pH values (pH buffer solutions were prepared using NaH2PO4, Na2HPO4, NaCl and NaOH solution and pH values were measured using ELICO pH meter, India) at 30oC for 12 h. The swollen MGs were taken out for every 30 min and removed surface adhered buffer solution using tissue paper. The MGs were further immersed in various buffer solutions to reach equilibrium swelling. Swelling experiments were carried out in water by mass measurements at various temperatures to study temperature responsive behavior of microspheres. The percentages of swelling ratio (% SR) were calculated using the following equations. Where, Ws is the weight of swollen gel at time t, and Wd is the dry weight of the hydrogel. Mass measurements were made on a digital ADAMS microbalance (Model AF 210L, U.K) with a sensitivity of 0.01 mg. Each value was averaged over three parallel measurements. Statistical analysis was performed using one-way ANOVA way in ORIGIN 8.0. All quantitative data are presented as means + standard deviation. Differential Scanning Calorimetry (DSC) Studies Differential scanning calorimetric (DSC) curves were recorded on a Rheometric scientific differential scanning calorimeter (Model-DSC SP, UK). The instrument was calibrated using indium as the standard. Samples were heated in sealed aluminum pans between 300 and 400oC at the heating rate of 10oC/min under inert nitrogen purge gas at the rate of 20 ml/min. Scanning Electron Microscopic (SEM) Studies Morphology of the microspheres was confirmed by scanning electron microscopy (SEM). Micrographs of the dry microspheres in powder form, dispersed in acetone, were all recorded using Leica 400, Cambridge, UK instrument. Particle Size Analysis Size distribution of the microspheres was determined using the particle size analyzer (Mastersizer 2000, Malvern Instruments, UK) equipped with the dry accessory system. Estimation of Drug Loading and Encapsulation Efficiency Loading efficiency of 5-FU in the microspheres was determined spectrophotometrically. About 10 mg of the drug-loaded core-shell microspheres were placed in 10 ml of buffer solution and stirred vigorously for 48 h to extract the drug from the microspheres. The solution was filtered and assayed by UV spectrophotometer (model Anthelme, Secomam, Dumont, France) at the fixed lmax value of 270 nm. The results of % drug loading and encapsulation efficiency were calculated, respectively using Equations. (1) and (2). These data are compiled in Tables 1 and 2, respectively. Table 1: Results of % encapsulation efficiency and mean diameter of poly(VC-co-SA) microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil Sample code % Vinyl Caprolactum (VC) % SA % NNMBA % 5-FU
% Encapsulation efficiency ± SD
Mean particle diameter (mm) ± SD VCSA-1 20 80 1 5 70 ± 1 29 ± 6 VCSA-2 20 80 1 10 74 ± 2 31 ± 8 VCSA-3 20 80 1 15 78 ± 2 34 ± 6 VCSA-4 20 80 2 10 75 ± 9 28 ± 4 VCSA-5 20 80 3 10 71 ± 8 16 ± 2 VCSA-6 10 90 1 10 68 ± 6 30 ± 4 VCSA-7 30 70 1 10 71 ± 5 24 ± 1 VCSA-8 00 100 1 10 72 ± 1 22 ± 8 Table 2: Release kinetics parameters of microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil at 370C Formulation codes K x 102 n Correlation coefficient ‘r’ VCSA-1 0.008 0.74 0.972 VCSA-2 0.023 0.57 0.999 VCSA-3 0.026 0.55 0.999 VCSA-4 0.021 0.57 0.996 VCSA-5 0.011 0.66 0.971 VCSA-6 0.014 0.64 0.979 VCSA-7 0.011 0.71 0.978 VCSA-8 0.027 0.59 0.990
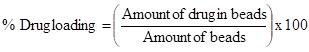
% Drug Loading
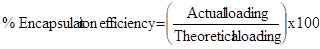
$ Encapsulation Efficiency
In-vitro Release Study
Dissolution was carried out using Tablet dissolution tester (Lab India, Mumbai, India) equipped with eight baskets. Dissolution rates were measured at 370C under 100 rpm speed. Drug release from the microspheres was studied in 7.4 pH phosphate buffer solution. Aliquot samples were withdrawn at regular time intervals and analyzed by UV spectrophotometer as explained before. RESULTS AND DISCUSSION pH and Temperature Responsive Behavior of Microspheres Figure 1 (a) shows the swelling ratio of microspheres at various pH solutions. As we can clearly see that the swelling ratio of microspheres slowly increases when pH increases up to 5.0 after that it increases rapidly up to pH 8. Because at low pH i.e.,
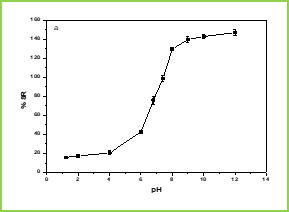
Figure 1.(a)

Figure 1.(b) Figure 1. Swelling studies of MGs (a) various pH conditions, and (b) different temperatures The effect of temperature on the equilibrium swelling ratios for microspheres is shown in Figure 1(b) The swelling ratio of microspheres is higher at low temperature ( LCST). This is because below LCST VCL contains a hydrophilic group (-CONH-) and hydrophobic isopropyl group present in the linear polymer chain. So, the hydrophilic group in the polymer structure will form an intermolecular hydrogen bond with surrounding water at low temperature (below the gel transition temperature); above LCST the hydrogen bonds are broken and the water molecules are expelled from the polymer. These two results make the water molecule inside the gel change from a bound state to a free State and release from the gel. This phenomenon makes the swelling ratios of the microspheres decrease rapidly at the gel transition temperature. Differential scanning calorimetry (DSC) DSC tracings of pure 5-fluorouracil, drug-loaded microspheres, and plain microspheres are displayed in Figure 2. The pure 5-FU exhibits a sharp peak at 285oC (curve c) is due to polymorphism and melting. However, this peak has not appeared in the case of drug-loaded microspheres (curve b) which confirms that the drug is molecularly dispersed in the polymeric microspheres.

Figure 2: DSC thermograms of (a) plain Poly(VC-co-SA) microspheres (c) 5-FU loaded Poly(VC-co-SA) microspheres and (c) 5-FU

Figure 3: Scanning electron micrographs of Poly(VC-co-SA) microspheres Scanning Electron Microscopic (SEM) Studies Figure 3. shows the morphology of microspheres. The formed copolymer particles are spherical with the diameters of around 10 mm. Laser Particle Size Analyzer
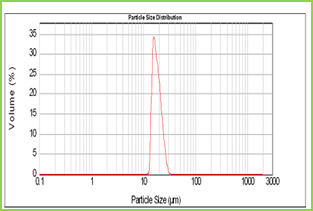
Figure 4: Particle size distribution curve of Poly(VC-co-SA) microspheres Results of the mean particle size with standard errors are presented in Table 1, while the size distribution curve for a typical formulation containing SA-5 is displayed in Figure 4. It is found that size distribution is broad and volume means diameter of the particle is around 16 mm. The particle size of different formulations containing different amounts of drug, crosslinking agent and different ratios of VC-co-SA are given in Table 1. The particle size of formulations containing different amounts of crosslinking agent (NNMBA) i.e., 1, 2 and 3 % are 34, 28 and 16, respectively. The particle size decreased with increasing amount of crosslinking due to the formation of a rigid structure due to a reduction in chain length of the polymer formed. Encapsulation Efficiency Results of encapsulation efficiencies are given in Table 1. The % encapsulation efficiency varied depending upon the initial loading of the drug. In general, for formulations VCSA-1, VCSA-2 and VCSA-3, the % encapsulation efficiency increased systematically with increasing drug content of the matrices. At higher amount of crosslinking agent i.e., 2 % or 3 % of NNMBA in the matrix, the % encapsulation efficiency decreased. The highest % encapsulation efficiency of 79 was observed for VCSA-3 containing 15 % of 5-FU with a higher amount of SA in the copolymer matrix and its size was also highest i.e., 34 mm. Drug Release Kinetics
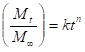
cumulative release data While studying the drug release from the polymer matrices, it has been the usual practice to analyze the release data using the empirical relationship proposed by Ritger and Peppas25. In the present study, we have analyzed the cumulative release data using26. Here, the ratio, Mt/M∞ represents the fractional drug release at the time, t; k is a constant characteristic of the drug-polymer system and n is an empirical parameter characterizing the release mechanism. Using the least-squares procedure, we have estimated the values of n and k for all the nine formulations at a 95% confidence limit; these data are given in Table 2 at 370C. If the values of n = 0.5, then drug diffuses and releases out of the microsphere matrix following the Fickian diffusion. If n > 0.5, anomalous or non-Fickian transport occurs. For n = 1, non-Fickian or more commonly called Case II release kinetics is operative. The values of n ranging between 0.5 and 1 indicate the anomalous type transport27. The values of k and n have shown a dependence on the extent of crosslinking, % drug loading and SA content of the matrix. Values of n for microspheres prepared by varying the amount of SA 90, 80 and 70 % in the microspheres of by keeping 5-FU (10 %) and 1 % NNMBA, ranged from 0.70 to 0.56 leading to a shift of transport from Fickian to anomalous type. The 5-FU-loaded particles have the n values ranging from 0.55 to 0.73, indicating the shift from erosion type release to a swelling-controlled non-Fickian type of mechanism. This could be possibly due to a reduction in the regions of low microviscosity and closure of microcavities in the swollen state. Similar findings have been observed elsewhere, wherein the effect of different polymer ratios on dissolution kinetics was observed. On the other hand, the values of k are quite smaller for drug-loaded microspheres, suggesting their lesser interactions compared to microspheres containing varying amount of SA.
Effect of Sodium Acrylate Content
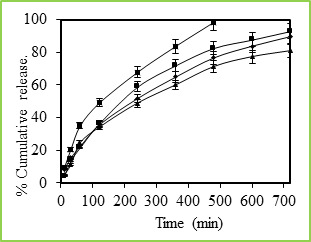
Figure 5: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of acrylamide at 37 0C, Symbols: (■)100 %, (■)30 %, (•) 20 % and (▲) 10 % Figure 5 shows the in vitro release data of 5-fluorouracil from poly(VC-co-SA) particles performed with particles taking the different ratio of SA. These data show that higher amount of SA containing particles have more encapsulation efficiencies and also release studies have shown that higher amounts SA containing particles have shown prolonged release characteristics than the microspheres containing lower amounts of SA. Generally, the drug release pattern depends upon factors like particle size, crystallinity, surface character, molecular weight, polymer composition, swelling ratio, degradation rate, drug binding affinity, the rate of hydration of polymeric materials, etc.27. In the release behavior of poly(VC-co-SA) system, one can consider the binding affinity of drug and polymer swelling property of SA. A rapid release of more than 98% of the drug was observed within 12 h. from the microspheres containing a lower amount of SA, indicating on the interaction between the two polymers. Effect of Temperature
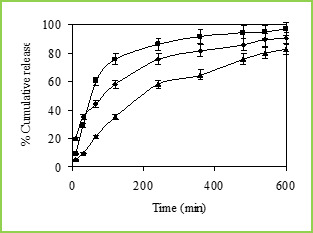
Figure 6: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of Vinyl Caprolactum at 25 0C, Symbols: (■)10 %, (•) 20 % and (▲) 30 %.
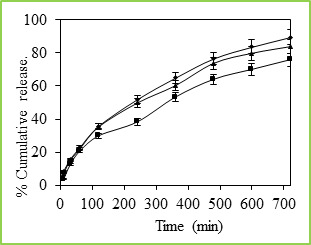
Figure 7: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of crosslinking agent at 37 0C, Symbols: (■) 3%, (▲) 2% and (•) 1 % The cumulative release data vs time curves for varying amounts of vinyl caprolactam are displayed in Figure 6 at 250C. Drug release profiles exhibited drastic changes by variations in temperature from 370 to 250C as shown in Figures 4 and 5, respectively. It may be noticed that drug was released slowly at 370C i.e., above the LCST of 320C, but the release was much faster at 250C i.e., below the LCST than at 370C. This is due to the fact that at a higher temperature, the surface of microspheres would shrink, causing the drug to migrate toward the surface of the microspheres as seen by the initial burst effect during the dissolution experiments (Figure 6 and 7). However, dense surfaces of the microspheres will prohibit the release of more amount of drug. At lower temperatures, the already shrunken surface layer starts to re-swell, which would allow the drug to be released after a certain period of time, depending upon the minimum time required for re-swelling of the surface. Thus, the time required for drug release was accelerated as a result of cooling below the LCST, which further slowed down upon reheating. Microspheres were thus found to be sensitive to changes in temperature. At 250C (in the swollen state), the release rate and the total amount of drug release were considerably higher than those found at 370C (in a collapsed state). Drug molecules entrapped inside the polymer network will diffuse out of the microspheres, since they quickly get hydrated in the swollen state. In contrast, at 370C, the network structure is collapsed and exhibits a lesser tendency to uptake water or buffer solution, leading to a decrease in drug diffusion rate. Effect of Crosslinking Agent The % cumulative release vs time curves for varying amounts of NNMBA are displayed in Figure 7. The % cumulative release is quite fast and large at the lower amount of NNMBA, whereas release is quite slower at a higher amount of NNMBA. The cumulative release is somewhat smaller when a lower amount of NNMBA was used probably because, at higher concentration of NNMBA, polymeric chains would become rigid due to the contraction of microvoids, thus decreasing the % cumulative release of 5-FU through the polymeric matrices. As expected, the release becomes slower at a higher amount of NNMBA but becomes faster at a lower amount of NNMBA. Effect of Drug Concentration

Figure 8 % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of 5-FU at 37 0C, Symbols: (■) 15 %, (•) 10 % and (▲) 5 % Figure 8 displays the release profiles of poly(VC-co-SA) microspheres that are loaded with different amounts of 5-FU. Notice that initially, during the first hour, the release is quite fast in all the formulations, but later it slowed down. The similar findings were observed in earlier literature of 5-fluorouracil loaded microspheres of a different kind . Release data suggest that those formulations containing the highest amount of drug (i.e., 15 wt. %) displayed higher release rates than those containing smaller amounts of 5-FU (i.e., 10 and 5 wt. %). A prolonged and slow release was observed for the formulation containing a lower amount of 5-FU (i.e., 5 wt. %) at 370C; this is due to the large free volume spaces available in the matrix through which, a lesser number of 5-FU molecules would transport. Notice that for all the 5-FU-loaded formulations, the almost complete release of 5-FU was achieved after 720 min. CONCLUSION Poly(vinyl caprolactam-Sodium acrylate) copolymeric microspheres crosslinked with N, N¢-methylene bisacrylamide were prepared by free radical emulsion polymerization. The microspheres have been characterized by differential scanning calorimetry (DSC) and x-ray diffractometry (x-RD) to understand the drug dispersion in microspheres. Microspheres with different copolymer compositions were prepared in yields of 80-85 %. DSC indicated a uniform distribution of 5-fluorouracil particles in microspheres, whereas SEM suggested a spherical structure of the microspheres with the slight rough surface. The in vitro drug release indicated that particle size and release kinetics depend upon copolymer composition, amount of crosslinking agent and amount of 5-fluorouracil present in the microspheres. REFERENCES Dunn, R. L., & Ottenbrite, R. M. (Eds.). (1991). Polymeric drugs and drug delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1991-0469 El-Nokaly, M. A., Piatt, D. M., & Charpentier, B. A. (1993). Polymeric delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1993-0520 Garcı́a, O., Blanco, M. D., Martı́n, J. A., & Teijón, J. M. (2000). 5-Fluorouracil trapping in poly (2-hydroxyethyl methacrylate-co-acrylamide) hydrogels: in vitro drug delivery studies. European polymer journal, 36(1), 111-122. https://doi.org/10.1016/S0014-3057(99)00037-3 Uhrich, K. E., Cannizzaro, S. M., Langer, R. S., & Shakesheff, K. M. (1999). Polymeric systems for controlled drug release. Chemical reviews, 99(11), 3181-3198. https://doi.org/10.1021/cr940351u , PMid:11749514 Işiklan, N. (2006). Controlled release of insecticide carbaryl from sodium alginate, sodium alginate/gelatin, and sodium alginate/sodium carboxymethyl cellulose blend beads crosslinked with glutaraldehyde. Journal of applied polymer science, 99(4), 1310-1319. https://doi.org/10.1002/app.22012 Vaithiyalingam, S., Nutan, M., Reddy, I., & Khan, M. (2002). Preparation and characterization of a customized cellulose acetate butyrate dispersion for controlled drug delivery. Journal of pharmaceutical sciences, 91(6), 1512-1522. https://doi.org/10.1002/jps.10155 , PMid:12115850 Xing, L., Dawei, C., Liping, X., & Rongqing, Z. (2003). Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. Journal of Controlled Release, 93(3), 293-300. https://doi.org/10.1016/j.jconrel.2003.08.019 , PMid:14644579 Hosoya, K., Kubo, T., Takahashi, K., Ikegami, T., & Tanaka, N. (2002). Novel surface modification of polymer-based separation media controlling separation selectivity, retentivity and generation of electroosmotic flow. Journal of Chromatography A, 979(1-2), 3-10. https://doi.org/10.1016/S0021-9673(02)01255-4 Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai, N., Kikuchi, A., Sakurai, Y., & Okano, T. (1996). Temperature-responsive chromatography using poly (N-isopropylacrylamide)-modified silica. Analytical Chemistry, 68(1), 100-105. https://doi.org/10.1021/ac950359j , PMid:21619225 Vihola, H., Laukkanen, A., Hirvonen, J., & Tenhu, H. (2002). Binding and release of drugs into and from thermosensitive poly (N-vinyl caprolactam) nanoparticles. European journal of pharmaceutical sciences, 16(1-2), 69-74. https://doi.org/10.1016/S0928-0987(02)00076-3 Torres-Lugo, M., & Peppas, N. A. (1999). Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules, 32(20), 6646-6651. https://doi.org/10.1021/ma990541c Kirsh, Y. E., Vorobiev, A. V., Yanul, N. A., Fedotov, Y. A., & Timashev, S. F. (2001). Facilitated acetylene transfer through membranes composed of sulfonate-containing aromatic polyamides and poly-N-vinylamides involving nanocluster silver. Separation and purification technology, 22, 559-565. https://doi.org/10.1016/S1383-5866(00)00138-6 Hester, J. F., Olugebefola, S. C., & Mayes, A. M. (2002). Preparation of pH-responsive polymer membranes by self-organization. Journal of Membrane Science, 208(1-2), 375-388. https://doi.org/10.1016/S0376-7388(02)00317-4 Lederhos, J. P., Long, J. P., Sum, A., Christiansen, R. L., & Sloan Jr, E. D. (1996). Effective kinetic inhibitors for natural gas hydrates. Chemical Engineering Science, 51(8), 1221-1229. https://doi.org/10.1016/0009-2509(95)00370-3 Coughlan, D. C., Quilty, F. P., & Corrigan, O. I. (2004). Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly (N-isopropylacrylamide) hydrogels. Journal of Controlled Release, 98(1), 97-114. https://doi.org/10.1016/j.jconrel.2004.04.014 , PMid:15245893 Schild, H. G. (1992). Poly (N-isopropylacrylamide): experiment, theory and application. Progress in polymer science, 17(2), 163-249. https://doi.org/10.1016/0079-6700(92)90023-R Kirsh, Y. E., Yanul, N. A., & Kalninsh, K. K. (1999). Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. European polymer journal, 35(2), 305-316. https://doi.org/10.1016/S0014-3057(98)00114-1 Meeussen, F., Nies, E., Berghmans, H., Verbrugghe, S., Goethals, E., & Du Prez, F. (2000). Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer, 41(24), 8597-8602. https://doi.org/10.1016/S0032-3861(00)00255-X Lozinsky, V. I., Simenel, I. A., Kurskaya, E. A., Kulakova, V. K., Galaev, I. Y., Mattiasson, B., ... & Khokhlov, A. R. (2000). Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer, 41(17), 6507-6518. https://doi.org/10.1016/S0032-3861(99)00844-7 S. Barabas In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H. F., Bicales, N. M., Overberger, C. C., Menges, G., Eds.; John Wiley & Sons: New York, 1985; Vol. 17, pp 225-226. Liu, J.L. Velada, M.B.Huglin, Polymer 40 (1999) 4299. https://doi.org/10.1016/S0032-3861(98)00458-3, https://doi.org/10.1016/S0032-3861(99)00081-6, https://doi.org/10.1016/S0032-3861(98)00387-5, https://doi.org/10.1016/S0032-3861(98)00533-3, https://doi.org/10.1016/S0032-3861(99)00101-9, https://doi.org/10.1016/S0032-3861(98)00758-7, https://doi.org/10.1016/S0032-3861(98)00660-0, https://doi.org/10.1016/S0032-3861(98)00858-1 Yuksel, D. E. A. (1999). Preparation of spray-dried microspheres of indomethacin and examination of the effects of coating on dissolution rates. Journal of microencapsulation, 16(3), 315-324. https://doi.org/10.1080/026520499289040, PMid:10340217 Sairam, M., Babu, V. R., Naidu, B. V. K., & Aminabhavi, T. M. (2006). Encapsulation efficiency and controlled release characteristics of crosslinked polyacrylamide particles. International journal of pharmaceutics, 320(1-2), 131-136. https://doi.org/10.1016/j.ijpharm.2006.05.001, PMid:16766148 Babu, V. R., Sairam, M., Hosamani, K. M., & Aminabhavi, T. M. (2006). Development of 5-fluorouracil loaded poly (acrylamide-co-methylmethacrylate) novel core-shell microspheres: In vitro release studies. International journal of pharmaceutics, 325(1-2), 55-62. https://doi.org/10.1016/j.ijpharm.2006.06.020, PMid:16884868 Ritger, P. L., & Peppas, N. A. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of controlled release, 5(1), 37-42. https://doi.org/10.1016/0168-3659(87)90035-6 Harogoppad, S. B., & Aminabhavi, T. M. (1991). Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8-C16). Macromolecules, 24(9), 2598-2605. https://doi.org/10.1021/ma00009a070 Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (1996). Biomaterials science: an introduction to materials in medicine. Elsevier New York, 347-356. Read the full article
0 notes
Text
Novel Poly(Vinyl caprolactum-co-Sodiumacrylate) Microspheres for Controlled Release of 5-Fluorouracil
INTRODUCTION Controlled delivery of drugs by means of biodegradable polymers began in the 1970s and continued to expand rapidly with numerous novel products1,2. The controlled release technology has lead to the development of newer methods of drug administration as well as the design and application of different types of CR formulations for effective targeting of certain drugs to the site of action. In particular, biodegradable polymeric systems have led to the development of CR dosage formulations to achieve the desired therapeutic results to obtain maximum dose regimen with minimum side effects3. The release of drug from a polymer matrix occurs due to the transport of drug to the surrounding medium system by the molecular diffusion mechanism. The CR systems offer many advantages over the conventional dosage forms, including improved efficacy, reduced toxicity as well as improved patient compliance and convenience4-6. Among the various types of polymers employed, hydrophilic biopolymers are quite suitable for oral applications7 due to their several inherent advantages over the synthetic polymers. Drug targeting to a specific tissue or organ has been the subject of creative and innovative research in medicinal and pharmaceutical chemistry since the beginning of the twentieth century. In many diseases (e.g. cancer, AIDS, rheumatoid arthritis, etc.) a considerable therapeutic advantage could be gained if drugs were delivered more selectively and in a controlled manner to their target sites. More particularly, it is conventionally accepted that efficient, compliant and reliable therapy requires that the drug reside as long as its therapeutic action is needed at a specific site, where it acts (by systemic absorption, binding, inhibition, etc.) as intact molecules. This concept has led to the development of a variety of physically based controlled release dosage forms such as drug dispersible matrices, coated tablets or particles, microcapsules. The development of an appropriate delivery system will first require a proper consideration of three related factors; the properties of the drug; the disease and the destination in the body. Over the past few years, stimuli-responsive (sensitive) polymers have become the object of intensive study due to their ability to change drastically their physical state under minute changes in external environment such as temperature, pH, ionic strength, light illumination, etc. Recently, chromatographic8,9 drug delivery10,11 membrane technology12,13 and kinetic inhibition14 applications were reported. Poly(N-isopropylacrylamide)15-17 (PNIPA) and poly(N-vinyl caprolactam)18,19 (PVCL) were intensively investigated due to their thermo-sensitive properties since these are water soluble at low temperature. However, they exhibit lower critical solution temperature (LCST) in water and undergo a coil-to-globule transition and aggregation at higher temperatures. For PNIPA the coil-to-globule transition occurs at around 32°C. PVCL is a homolog of poly(N-vinylpyrrolidone) (PVP), which is a biocompatible polymer widely used in medicine and pharmaceutics20. PVCL combines the useful and important properties of PVP and PNIPAm. It is a biocompatible polymer with a phase transition in the region of physiological temperature (30-37 °C). Such properties make it a prospective material in designing CR systems. Further, the incorporation of ionic hydrophilic moieties into the PolyVCL hydrogel networks would enhance the LCST and the gels become sensitive towards PH, whereas hydrophobic moieties decrease the LCST. Liu et al.21 found that salts of acrylic acid monomers are strong electrolytes, which are completely ionized in water, and their copolymeric units increased the swelling characteristics to a greater extent. 5-Fluorouracil is an acidic, water-soluble22,23, hydrophilic, is an antineoplastic drug used extensively in clinical chemotherapy for the treatment of solid tumors. It has been widely used in drug administration due to its large number of secondary effects that accompany its conventional administration. We present here the development of 5-fluorouracil-loaded poly(vinyl caprolactam-co-Sodium acrylate) microspheres for investigating its slow release characteristics. The plasma lifetime of 5-Fu is 1-1.2 hand it needs to extend for its effective therapy. The microspheres prepared were characterized by particle size analyzer, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). The in vitro release studies have been performed in 7.4 pH buffer solution at 25 0C and 370C to extend to the release rates of the drug. MATERIALS AND METHOD Materials Vinyl caprolactam (VC) was purchased from Aldrich Chemicals, Milwaukee, WI USA. Sodium acrylate (SA), N, N¢-methylene bisacrylamide (NNMBA), sodium lauryl sulfate, potassium persulfate, and calcium chloride were all purchased from s.d. fine chemicals, Mumbai, India. 5-Fluorouracil was purchased from MP Biochemicals, Eschwege, Germany. Synthesis of poly(vinyl caprolactam-co-sodium acrylate) microspheres Sodium lauryl sulfate (1g) was dissolved in 80 ml of water taken in a three-necked round bottom flask equipped with a mechanical stirrer, a condenser, and a gas inlet to maintain the inert nitrogen atmosphere. The flask was immersed in an oil bath with a thermostatic control to maintain the desired temperature accurate to ± 1oC. The solution was stirred at 800 rpm speed until it became clear and 100 mg of potassium persulfate was added. The required amount of SA, VC, crosslinking agent, NNMBA and 5-Fluorouracil were dissolved separately in 20 ml of water. This mixture was added to the reaction mixture drop-wise using a dropping funnel and the reaction was continued for 8 h at 700C to obtain the maximum yield. The reaction mixture was taken out after 8 h and added to 1% calcium chloride solution drop-wise to break the emulsion24. Particles were then isolated by centrifuging the product at the rotor speed of 12,000 rpm, washed with water and dried under vacuum at 400C for 24 h. Conversion of Copolymer The yield of copolymeric microspheres was determined gravimetrically. After copolymerization, the latex solution was added to 1 % calcium chloride solution and centrifuged to isolate the particles from the mixture. The copolymeric microspheres were washed several times successively with water and methanol solvents to remove the remaining monomer and initiator and then dried in a vacuum oven at 500C until attainment of constant weight. The % conversion of monomers was calculated as: % Conversion = (W/M) ×100 Where W is the weight of the dry copolymer obtained from the latex sample and M is the weight of the monomers taken. The yield of copolymeric microspheres varied between 80 and 85 % for various formulations prepared in this study. pH and Temperature Sensitive Nature of Copolymer Microspheres

percentages of swelling ratio (% SR) pH and temperature sensitivity of copolymer microspheres were studied through swelling experiments. First, the microspheres were immersed in a buffer solution with various pH values (pH buffer solutions were prepared using NaH2PO4, Na2HPO4, NaCl and NaOH solution and pH values were measured using ELICO pH meter, India) at 30oC for 12 h. The swollen MGs were taken out for every 30 min and removed surface adhered buffer solution using tissue paper. The MGs were further immersed in various buffer solutions to reach equilibrium swelling. Swelling experiments were carried out in water by mass measurements at various temperatures to study temperature responsive behavior of microspheres. The percentages of swelling ratio (% SR) were calculated using the following equations. Where, Ws is the weight of swollen gel at time t, and Wd is the dry weight of the hydrogel. Mass measurements were made on a digital ADAMS microbalance (Model AF 210L, U.K) with a sensitivity of 0.01 mg. Each value was averaged over three parallel measurements. Statistical analysis was performed using one-way ANOVA way in ORIGIN 8.0. All quantitative data are presented as means + standard deviation. Differential Scanning Calorimetry (DSC) Studies Differential scanning calorimetric (DSC) curves were recorded on a Rheometric scientific differential scanning calorimeter (Model-DSC SP, UK). The instrument was calibrated using indium as the standard. Samples were heated in sealed aluminum pans between 300 and 400oC at the heating rate of 10oC/min under inert nitrogen purge gas at the rate of 20 ml/min. Scanning Electron Microscopic (SEM) Studies Morphology of the microspheres was confirmed by scanning electron microscopy (SEM). Micrographs of the dry microspheres in powder form, dispersed in acetone, were all recorded using Leica 400, Cambridge, UK instrument. Particle Size Analysis Size distribution of the microspheres was determined using the particle size analyzer (Mastersizer 2000, Malvern Instruments, UK) equipped with the dry accessory system. Estimation of Drug Loading and Encapsulation Efficiency Loading efficiency of 5-FU in the microspheres was determined spectrophotometrically. About 10 mg of the drug-loaded core-shell microspheres were placed in 10 ml of buffer solution and stirred vigorously for 48 h to extract the drug from the microspheres. The solution was filtered and assayed by UV spectrophotometer (model Anthelme, Secomam, Dumont, France) at the fixed lmax value of 270 nm. The results of % drug loading and encapsulation efficiency were calculated, respectively using Equations. (1) and (2). These data are compiled in Tables 1 and 2, respectively. Table 1: Results of % encapsulation efficiency and mean diameter of poly(VC-co-SA) microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil Sample code % Vinyl Caprolactum (VC) % SA % NNMBA % 5-FU
% Encapsulation efficiency ± SD
Mean particle diameter (mm) ± SD VCSA-1 20 80 1 5 70 ± 1 29 ± 6 VCSA-2 20 80 1 10 74 ± 2 31 ± 8 VCSA-3 20 80 1 15 78 ± 2 34 ± 6 VCSA-4 20 80 2 10 75 ± 9 28 ± 4 VCSA-5 20 80 3 10 71 ± 8 16 ± 2 VCSA-6 10 90 1 10 68 ± 6 30 ± 4 VCSA-7 30 70 1 10 71 ± 5 24 ± 1 VCSA-8 00 100 1 10 72 ± 1 22 ± 8 Table 2: Release kinetics parameters of microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil at 370C Formulation codes K x 102 n Correlation coefficient ‘r’ VCSA-1 0.008 0.74 0.972 VCSA-2 0.023 0.57 0.999 VCSA-3 0.026 0.55 0.999 VCSA-4 0.021 0.57 0.996 VCSA-5 0.011 0.66 0.971 VCSA-6 0.014 0.64 0.979 VCSA-7 0.011 0.71 0.978 VCSA-8 0.027 0.59 0.990

% Drug Loading

$ Encapsulation Efficiency
In-vitro Release Study
Dissolution was carried out using Tablet dissolution tester (Lab India, Mumbai, India) equipped with eight baskets. Dissolution rates were measured at 370C under 100 rpm speed. Drug release from the microspheres was studied in 7.4 pH phosphate buffer solution. Aliquot samples were withdrawn at regular time intervals and analyzed by UV spectrophotometer as explained before. RESULTS AND DISCUSSION pH and Temperature Responsive Behavior of Microspheres Figure 1 (a) shows the swelling ratio of microspheres at various pH solutions. As we can clearly see that the swelling ratio of microspheres slowly increases when pH increases up to 5.0 after that it increases rapidly up to pH 8. Because at low pH i.e.,

Figure 1.(a)

Figure 1.(b) Figure 1. Swelling studies of MGs (a) various pH conditions, and (b) different temperatures The effect of temperature on the equilibrium swelling ratios for microspheres is shown in Figure 1(b) The swelling ratio of microspheres is higher at low temperature ( LCST). This is because below LCST VCL contains a hydrophilic group (-CONH-) and hydrophobic isopropyl group present in the linear polymer chain. So, the hydrophilic group in the polymer structure will form an intermolecular hydrogen bond with surrounding water at low temperature (below the gel transition temperature); above LCST the hydrogen bonds are broken and the water molecules are expelled from the polymer. These two results make the water molecule inside the gel change from a bound state to a free State and release from the gel. This phenomenon makes the swelling ratios of the microspheres decrease rapidly at the gel transition temperature. Differential scanning calorimetry (DSC) DSC tracings of pure 5-fluorouracil, drug-loaded microspheres, and plain microspheres are displayed in Figure 2. The pure 5-FU exhibits a sharp peak at 285oC (curve c) is due to polymorphism and melting. However, this peak has not appeared in the case of drug-loaded microspheres (curve b) which confirms that the drug is molecularly dispersed in the polymeric microspheres.

Figure 2: DSC thermograms of (a) plain Poly(VC-co-SA) microspheres (c) 5-FU loaded Poly(VC-co-SA) microspheres and (c) 5-FU

Figure 3: Scanning electron micrographs of Poly(VC-co-SA) microspheres Scanning Electron Microscopic (SEM) Studies Figure 3. shows the morphology of microspheres. The formed copolymer particles are spherical with the diameters of around 10 mm. Laser Particle Size Analyzer

Figure 4: Particle size distribution curve of Poly(VC-co-SA) microspheres Results of the mean particle size with standard errors are presented in Table 1, while the size distribution curve for a typical formulation containing SA-5 is displayed in Figure 4. It is found that size distribution is broad and volume means diameter of the particle is around 16 mm. The particle size of different formulations containing different amounts of drug, crosslinking agent and different ratios of VC-co-SA are given in Table 1. The particle size of formulations containing different amounts of crosslinking agent (NNMBA) i.e., 1, 2 and 3 % are 34, 28 and 16, respectively. The particle size decreased with increasing amount of crosslinking due to the formation of a rigid structure due to a reduction in chain length of the polymer formed. Encapsulation Efficiency Results of encapsulation efficiencies are given in Table 1. The % encapsulation efficiency varied depending upon the initial loading of the drug. In general, for formulations VCSA-1, VCSA-2 and VCSA-3, the % encapsulation efficiency increased systematically with increasing drug content of the matrices. At higher amount of crosslinking agent i.e., 2 % or 3 % of NNMBA in the matrix, the % encapsulation efficiency decreased. The highest % encapsulation efficiency of 79 was observed for VCSA-3 containing 15 % of 5-FU with a higher amount of SA in the copolymer matrix and its size was also highest i.e., 34 mm. Drug Release Kinetics

cumulative release data While studying the drug release from the polymer matrices, it has been the usual practice to analyze the release data using the empirical relationship proposed by Ritger and Peppas25. In the present study, we have analyzed the cumulative release data using26. Here, the ratio, Mt/M∞ represents the fractional drug release at the time, t; k is a constant characteristic of the drug-polymer system and n is an empirical parameter characterizing the release mechanism. Using the least-squares procedure, we have estimated the values of n and k for all the nine formulations at a 95% confidence limit; these data are given in Table 2 at 370C. If the values of n = 0.5, then drug diffuses and releases out of the microsphere matrix following the Fickian diffusion. If n > 0.5, anomalous or non-Fickian transport occurs. For n = 1, non-Fickian or more commonly called Case II release kinetics is operative. The values of n ranging between 0.5 and 1 indicate the anomalous type transport27. The values of k and n have shown a dependence on the extent of crosslinking, % drug loading and SA content of the matrix. Values of n for microspheres prepared by varying the amount of SA 90, 80 and 70 % in the microspheres of by keeping 5-FU (10 %) and 1 % NNMBA, ranged from 0.70 to 0.56 leading to a shift of transport from Fickian to anomalous type. The 5-FU-loaded particles have the n values ranging from 0.55 to 0.73, indicating the shift from erosion type release to a swelling-controlled non-Fickian type of mechanism. This could be possibly due to a reduction in the regions of low microviscosity and closure of microcavities in the swollen state. Similar findings have been observed elsewhere, wherein the effect of different polymer ratios on dissolution kinetics was observed. On the other hand, the values of k are quite smaller for drug-loaded microspheres, suggesting their lesser interactions compared to microspheres containing varying amount of SA.
Effect of Sodium Acrylate Content

Figure 5: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of acrylamide at 37 0C, Symbols: (■)100 %, (■)30 %, (•) 20 % and (▲) 10 % Figure 5 shows the in vitro release data of 5-fluorouracil from poly(VC-co-SA) particles performed with particles taking the different ratio of SA. These data show that higher amount of SA containing particles have more encapsulation efficiencies and also release studies have shown that higher amounts SA containing particles have shown prolonged release characteristics than the microspheres containing lower amounts of SA. Generally, the drug release pattern depends upon factors like particle size, crystallinity, surface character, molecular weight, polymer composition, swelling ratio, degradation rate, drug binding affinity, the rate of hydration of polymeric materials, etc.27. In the release behavior of poly(VC-co-SA) system, one can consider the binding affinity of drug and polymer swelling property of SA. A rapid release of more than 98% of the drug was observed within 12 h. from the microspheres containing a lower amount of SA, indicating on the interaction between the two polymers. Effect of Temperature

Figure 6: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of Vinyl Caprolactum at 25 0C, Symbols: (■)10 %, (•) 20 % and (▲) 30 %.

Figure 7: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of crosslinking agent at 37 0C, Symbols: (■) 3%, (▲) 2% and (•) 1 % The cumulative release data vs time curves for varying amounts of vinyl caprolactam are displayed in Figure 6 at 250C. Drug release profiles exhibited drastic changes by variations in temperature from 370 to 250C as shown in Figures 4 and 5, respectively. It may be noticed that drug was released slowly at 370C i.e., above the LCST of 320C, but the release was much faster at 250C i.e., below the LCST than at 370C. This is due to the fact that at a higher temperature, the surface of microspheres would shrink, causing the drug to migrate toward the surface of the microspheres as seen by the initial burst effect during the dissolution experiments (Figure 6 and 7). However, dense surfaces of the microspheres will prohibit the release of more amount of drug. At lower temperatures, the already shrunken surface layer starts to re-swell, which would allow the drug to be released after a certain period of time, depending upon the minimum time required for re-swelling of the surface. Thus, the time required for drug release was accelerated as a result of cooling below the LCST, which further slowed down upon reheating. Microspheres were thus found to be sensitive to changes in temperature. At 250C (in the swollen state), the release rate and the total amount of drug release were considerably higher than those found at 370C (in a collapsed state). Drug molecules entrapped inside the polymer network will diffuse out of the microspheres, since they quickly get hydrated in the swollen state. In contrast, at 370C, the network structure is collapsed and exhibits a lesser tendency to uptake water or buffer solution, leading to a decrease in drug diffusion rate. Effect of Crosslinking Agent The % cumulative release vs time curves for varying amounts of NNMBA are displayed in Figure 7. The % cumulative release is quite fast and large at the lower amount of NNMBA, whereas release is quite slower at a higher amount of NNMBA. The cumulative release is somewhat smaller when a lower amount of NNMBA was used probably because, at higher concentration of NNMBA, polymeric chains would become rigid due to the contraction of microvoids, thus decreasing the % cumulative release of 5-FU through the polymeric matrices. As expected, the release becomes slower at a higher amount of NNMBA but becomes faster at a lower amount of NNMBA. Effect of Drug Concentration

Figure 8 % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of 5-FU at 37 0C, Symbols: (■) 15 %, (•) 10 % and (▲) 5 % Figure 8 displays the release profiles of poly(VC-co-SA) microspheres that are loaded with different amounts of 5-FU. Notice that initially, during the first hour, the release is quite fast in all the formulations, but later it slowed down. The similar findings were observed in earlier literature of 5-fluorouracil loaded microspheres of a different kind . Release data suggest that those formulations containing the highest amount of drug (i.e., 15 wt. %) displayed higher release rates than those containing smaller amounts of 5-FU (i.e., 10 and 5 wt. %). A prolonged and slow release was observed for the formulation containing a lower amount of 5-FU (i.e., 5 wt. %) at 370C; this is due to the large free volume spaces available in the matrix through which, a lesser number of 5-FU molecules would transport. Notice that for all the 5-FU-loaded formulations, the almost complete release of 5-FU was achieved after 720 min. CONCLUSION Poly(vinyl caprolactam-Sodium acrylate) copolymeric microspheres crosslinked with N, N¢-methylene bisacrylamide were prepared by free radical emulsion polymerization. The microspheres have been characterized by differential scanning calorimetry (DSC) and x-ray diffractometry (x-RD) to understand the drug dispersion in microspheres. Microspheres with different copolymer compositions were prepared in yields of 80-85 %. DSC indicated a uniform distribution of 5-fluorouracil particles in microspheres, whereas SEM suggested a spherical structure of the microspheres with the slight rough surface. The in vitro drug release indicated that particle size and release kinetics depend upon copolymer composition, amount of crosslinking agent and amount of 5-fluorouracil present in the microspheres. REFERENCES Dunn, R. L., & Ottenbrite, R. M. (Eds.). (1991). Polymeric drugs and drug delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1991-0469 El-Nokaly, M. A., Piatt, D. M., & Charpentier, B. A. (1993). Polymeric delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1993-0520 Garcı́a, O., Blanco, M. D., Martı́n, J. A., & Teijón, J. M. (2000). 5-Fluorouracil trapping in poly (2-hydroxyethyl methacrylate-co-acrylamide) hydrogels: in vitro drug delivery studies. European polymer journal, 36(1), 111-122. https://doi.org/10.1016/S0014-3057(99)00037-3 Uhrich, K. E., Cannizzaro, S. M., Langer, R. S., & Shakesheff, K. M. (1999). Polymeric systems for controlled drug release. Chemical reviews, 99(11), 3181-3198. https://doi.org/10.1021/cr940351u , PMid:11749514 Işiklan, N. (2006). Controlled release of insecticide carbaryl from sodium alginate, sodium alginate/gelatin, and sodium alginate/sodium carboxymethyl cellulose blend beads crosslinked with glutaraldehyde. Journal of applied polymer science, 99(4), 1310-1319. https://doi.org/10.1002/app.22012 Vaithiyalingam, S., Nutan, M., Reddy, I., & Khan, M. (2002). Preparation and characterization of a customized cellulose acetate butyrate dispersion for controlled drug delivery. Journal of pharmaceutical sciences, 91(6), 1512-1522. https://doi.org/10.1002/jps.10155 , PMid:12115850 Xing, L., Dawei, C., Liping, X., & Rongqing, Z. (2003). Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. Journal of Controlled Release, 93(3), 293-300. https://doi.org/10.1016/j.jconrel.2003.08.019 , PMid:14644579 Hosoya, K., Kubo, T., Takahashi, K., Ikegami, T., & Tanaka, N. (2002). Novel surface modification of polymer-based separation media controlling separation selectivity, retentivity and generation of electroosmotic flow. Journal of Chromatography A, 979(1-2), 3-10. https://doi.org/10.1016/S0021-9673(02)01255-4 Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai, N., Kikuchi, A., Sakurai, Y., & Okano, T. (1996). Temperature-responsive chromatography using poly (N-isopropylacrylamide)-modified silica. Analytical Chemistry, 68(1), 100-105. https://doi.org/10.1021/ac950359j , PMid:21619225 Vihola, H., Laukkanen, A., Hirvonen, J., & Tenhu, H. (2002). Binding and release of drugs into and from thermosensitive poly (N-vinyl caprolactam) nanoparticles. European journal of pharmaceutical sciences, 16(1-2), 69-74. https://doi.org/10.1016/S0928-0987(02)00076-3 Torres-Lugo, M., & Peppas, N. A. (1999). Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules, 32(20), 6646-6651. https://doi.org/10.1021/ma990541c Kirsh, Y. E., Vorobiev, A. V., Yanul, N. A., Fedotov, Y. A., & Timashev, S. F. (2001). Facilitated acetylene transfer through membranes composed of sulfonate-containing aromatic polyamides and poly-N-vinylamides involving nanocluster silver. Separation and purification technology, 22, 559-565. https://doi.org/10.1016/S1383-5866(00)00138-6 Hester, J. F., Olugebefola, S. C., & Mayes, A. M. (2002). Preparation of pH-responsive polymer membranes by self-organization. Journal of Membrane Science, 208(1-2), 375-388. https://doi.org/10.1016/S0376-7388(02)00317-4 Lederhos, J. P., Long, J. P., Sum, A., Christiansen, R. L., & Sloan Jr, E. D. (1996). Effective kinetic inhibitors for natural gas hydrates. Chemical Engineering Science, 51(8), 1221-1229. https://doi.org/10.1016/0009-2509(95)00370-3 Coughlan, D. C., Quilty, F. P., & Corrigan, O. I. (2004). Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly (N-isopropylacrylamide) hydrogels. Journal of Controlled Release, 98(1), 97-114. https://doi.org/10.1016/j.jconrel.2004.04.014 , PMid:15245893 Schild, H. G. (1992). Poly (N-isopropylacrylamide): experiment, theory and application. Progress in polymer science, 17(2), 163-249. https://doi.org/10.1016/0079-6700(92)90023-R Kirsh, Y. E., Yanul, N. A., & Kalninsh, K. K. (1999). Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. European polymer journal, 35(2), 305-316. https://doi.org/10.1016/S0014-3057(98)00114-1 Meeussen, F., Nies, E., Berghmans, H., Verbrugghe, S., Goethals, E., & Du Prez, F. (2000). Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer, 41(24), 8597-8602. https://doi.org/10.1016/S0032-3861(00)00255-X Lozinsky, V. I., Simenel, I. A., Kurskaya, E. A., Kulakova, V. K., Galaev, I. Y., Mattiasson, B., ... & Khokhlov, A. R. (2000). Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer, 41(17), 6507-6518. https://doi.org/10.1016/S0032-3861(99)00844-7 S. Barabas In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H. F., Bicales, N. M., Overberger, C. C., Menges, G., Eds.; John Wiley & Sons: New York, 1985; Vol. 17, pp 225-226. Liu, J.L. Velada, M.B.Huglin, Polymer 40 (1999) 4299. https://doi.org/10.1016/S0032-3861(98)00458-3, https://doi.org/10.1016/S0032-3861(99)00081-6, https://doi.org/10.1016/S0032-3861(98)00387-5, https://doi.org/10.1016/S0032-3861(98)00533-3, https://doi.org/10.1016/S0032-3861(99)00101-9, https://doi.org/10.1016/S0032-3861(98)00758-7, https://doi.org/10.1016/S0032-3861(98)00660-0, https://doi.org/10.1016/S0032-3861(98)00858-1 Yuksel, D. E. A. (1999). Preparation of spray-dried microspheres of indomethacin and examination of the effects of coating on dissolution rates. Journal of microencapsulation, 16(3), 315-324. https://doi.org/10.1080/026520499289040, PMid:10340217 Sairam, M., Babu, V. R., Naidu, B. V. K., & Aminabhavi, T. M. (2006). Encapsulation efficiency and controlled release characteristics of crosslinked polyacrylamide particles. International journal of pharmaceutics, 320(1-2), 131-136. https://doi.org/10.1016/j.ijpharm.2006.05.001, PMid:16766148 Babu, V. R., Sairam, M., Hosamani, K. M., & Aminabhavi, T. M. (2006). Development of 5-fluorouracil loaded poly (acrylamide-co-methylmethacrylate) novel core-shell microspheres: In vitro release studies. International journal of pharmaceutics, 325(1-2), 55-62. https://doi.org/10.1016/j.ijpharm.2006.06.020, PMid:16884868 Ritger, P. L., & Peppas, N. A. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of controlled release, 5(1), 37-42. https://doi.org/10.1016/0168-3659(87)90035-6 Harogoppad, S. B., & Aminabhavi, T. M. (1991). Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8-C16). Macromolecules, 24(9), 2598-2605. https://doi.org/10.1021/ma00009a070 Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (1996). Biomaterials science: an introduction to materials in medicine. Elsevier New York, 347-356. Read the full article
0 notes
Text
Novel Poly(Vinyl caprolactum-co-Sodiumacrylate) Microspheres for Controlled Release of 5-Fluorouracil
INTRODUCTION Controlled delivery of drugs by means of biodegradable polymers began in the 1970s and continued to expand rapidly with numerous novel products1,2. The controlled release technology has lead to the development of newer methods of drug administration as well as the design and application of different types of CR formulations for effective targeting of certain drugs to the site of action. In particular, biodegradable polymeric systems have led to the development of CR dosage formulations to achieve the desired therapeutic results to obtain maximum dose regimen with minimum side effects3. The release of drug from a polymer matrix occurs due to the transport of drug to the surrounding medium system by the molecular diffusion mechanism. The CR systems offer many advantages over the conventional dosage forms, including improved efficacy, reduced toxicity as well as improved patient compliance and convenience4-6. Among the various types of polymers employed, hydrophilic biopolymers are quite suitable for oral applications7 due to their several inherent advantages over the synthetic polymers. Drug targeting to a specific tissue or organ has been the subject of creative and innovative research in medicinal and pharmaceutical chemistry since the beginning of the twentieth century. In many diseases (e.g. cancer, AIDS, rheumatoid arthritis, etc.) a considerable therapeutic advantage could be gained if drugs were delivered more selectively and in a controlled manner to their target sites. More particularly, it is conventionally accepted that efficient, compliant and reliable therapy requires that the drug reside as long as its therapeutic action is needed at a specific site, where it acts (by systemic absorption, binding, inhibition, etc.) as intact molecules. This concept has led to the development of a variety of physically based controlled release dosage forms such as drug dispersible matrices, coated tablets or particles, microcapsules. The development of an appropriate delivery system will first require a proper consideration of three related factors; the properties of the drug; the disease and the destination in the body. Over the past few years, stimuli-responsive (sensitive) polymers have become the object of intensive study due to their ability to change drastically their physical state under minute changes in external environment such as temperature, pH, ionic strength, light illumination, etc. Recently, chromatographic8,9 drug delivery10,11 membrane technology12,13 and kinetic inhibition14 applications were reported. Poly(N-isopropylacrylamide)15-17 (PNIPA) and poly(N-vinyl caprolactam)18,19 (PVCL) were intensively investigated due to their thermo-sensitive properties since these are water soluble at low temperature. However, they exhibit lower critical solution temperature (LCST) in water and undergo a coil-to-globule transition and aggregation at higher temperatures. For PNIPA the coil-to-globule transition occurs at around 32°C. PVCL is a homolog of poly(N-vinylpyrrolidone) (PVP), which is a biocompatible polymer widely used in medicine and pharmaceutics20. PVCL combines the useful and important properties of PVP and PNIPAm. It is a biocompatible polymer with a phase transition in the region of physiological temperature (30-37 °C). Such properties make it a prospective material in designing CR systems. Further, the incorporation of ionic hydrophilic moieties into the PolyVCL hydrogel networks would enhance the LCST and the gels become sensitive towards PH, whereas hydrophobic moieties decrease the LCST. Liu et al.21 found that salts of acrylic acid monomers are strong electrolytes, which are completely ionized in water, and their copolymeric units increased the swelling characteristics to a greater extent. 5-Fluorouracil is an acidic, water-soluble22,23, hydrophilic, is an antineoplastic drug used extensively in clinical chemotherapy for the treatment of solid tumors. It has been widely used in drug administration due to its large number of secondary effects that accompany its conventional administration. We present here the development of 5-fluorouracil-loaded poly(vinyl caprolactam-co-Sodium acrylate) microspheres for investigating its slow release characteristics. The plasma lifetime of 5-Fu is 1-1.2 hand it needs to extend for its effective therapy. The microspheres prepared were characterized by particle size analyzer, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). The in vitro release studies have been performed in 7.4 pH buffer solution at 25 0C and 370C to extend to the release rates of the drug. MATERIALS AND METHOD Materials Vinyl caprolactam (VC) was purchased from Aldrich Chemicals, Milwaukee, WI USA. Sodium acrylate (SA), N, N¢-methylene bisacrylamide (NNMBA), sodium lauryl sulfate, potassium persulfate, and calcium chloride were all purchased from s.d. fine chemicals, Mumbai, India. 5-Fluorouracil was purchased from MP Biochemicals, Eschwege, Germany. Synthesis of poly(vinyl caprolactam-co-sodium acrylate) microspheres Sodium lauryl sulfate (1g) was dissolved in 80 ml of water taken in a three-necked round bottom flask equipped with a mechanical stirrer, a condenser, and a gas inlet to maintain the inert nitrogen atmosphere. The flask was immersed in an oil bath with a thermostatic control to maintain the desired temperature accurate to ± 1oC. The solution was stirred at 800 rpm speed until it became clear and 100 mg of potassium persulfate was added. The required amount of SA, VC, crosslinking agent, NNMBA and 5-Fluorouracil were dissolved separately in 20 ml of water. This mixture was added to the reaction mixture drop-wise using a dropping funnel and the reaction was continued for 8 h at 700C to obtain the maximum yield. The reaction mixture was taken out after 8 h and added to 1% calcium chloride solution drop-wise to break the emulsion24. Particles were then isolated by centrifuging the product at the rotor speed of 12,000 rpm, washed with water and dried under vacuum at 400C for 24 h. Conversion of Copolymer The yield of copolymeric microspheres was determined gravimetrically. After copolymerization, the latex solution was added to 1 % calcium chloride solution and centrifuged to isolate the particles from the mixture. The copolymeric microspheres were washed several times successively with water and methanol solvents to remove the remaining monomer and initiator and then dried in a vacuum oven at 500C until attainment of constant weight. The % conversion of monomers was calculated as: % Conversion = (W/M) ×100 Where W is the weight of the dry copolymer obtained from the latex sample and M is the weight of the monomers taken. The yield of copolymeric microspheres varied between 80 and 85 % for various formulations prepared in this study. pH and Temperature Sensitive Nature of Copolymer Microspheres

percentages of swelling ratio (% SR) pH and temperature sensitivity of copolymer microspheres were studied through swelling experiments. First, the microspheres were immersed in a buffer solution with various pH values (pH buffer solutions were prepared using NaH2PO4, Na2HPO4, NaCl and NaOH solution and pH values were measured using ELICO pH meter, India) at 30oC for 12 h. The swollen MGs were taken out for every 30 min and removed surface adhered buffer solution using tissue paper. The MGs were further immersed in various buffer solutions to reach equilibrium swelling. Swelling experiments were carried out in water by mass measurements at various temperatures to study temperature responsive behavior of microspheres. The percentages of swelling ratio (% SR) were calculated using the following equations. Where, Ws is the weight of swollen gel at time t, and Wd is the dry weight of the hydrogel. Mass measurements were made on a digital ADAMS microbalance (Model AF 210L, U.K) with a sensitivity of 0.01 mg. Each value was averaged over three parallel measurements. Statistical analysis was performed using one-way ANOVA way in ORIGIN 8.0. All quantitative data are presented as means + standard deviation. Differential Scanning Calorimetry (DSC) Studies Differential scanning calorimetric (DSC) curves were recorded on a Rheometric scientific differential scanning calorimeter (Model-DSC SP, UK). The instrument was calibrated using indium as the standard. Samples were heated in sealed aluminum pans between 300 and 400oC at the heating rate of 10oC/min under inert nitrogen purge gas at the rate of 20 ml/min. Scanning Electron Microscopic (SEM) Studies Morphology of the microspheres was confirmed by scanning electron microscopy (SEM). Micrographs of the dry microspheres in powder form, dispersed in acetone, were all recorded using Leica 400, Cambridge, UK instrument. Particle Size Analysis Size distribution of the microspheres was determined using the particle size analyzer (Mastersizer 2000, Malvern Instruments, UK) equipped with the dry accessory system. Estimation of Drug Loading and Encapsulation Efficiency Loading efficiency of 5-FU in the microspheres was determined spectrophotometrically. About 10 mg of the drug-loaded core-shell microspheres were placed in 10 ml of buffer solution and stirred vigorously for 48 h to extract the drug from the microspheres. The solution was filtered and assayed by UV spectrophotometer (model Anthelme, Secomam, Dumont, France) at the fixed lmax value of 270 nm. The results of % drug loading and encapsulation efficiency were calculated, respectively using Equations. (1) and (2). These data are compiled in Tables 1 and 2, respectively. Table 1: Results of % encapsulation efficiency and mean diameter of poly(VC-co-SA) microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil Sample code % Vinyl Caprolactum (VC) % SA % NNMBA % 5-FU
% Encapsulation efficiency ± SD
Mean particle diameter (mm) ± SD VCSA-1 20 80 1 5 70 ± 1 29 ± 6 VCSA-2 20 80 1 10 74 ± 2 31 ± 8 VCSA-3 20 80 1 15 78 ± 2 34 ± 6 VCSA-4 20 80 2 10 75 ± 9 28 ± 4 VCSA-5 20 80 3 10 71 ± 8 16 ± 2 VCSA-6 10 90 1 10 68 ± 6 30 ± 4 VCSA-7 30 70 1 10 71 ± 5 24 ± 1 VCSA-8 00 100 1 10 72 ± 1 22 ± 8 Table 2: Release kinetics parameters of microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil at 370C Formulation codes K x 102 n Correlation coefficient ‘r’ VCSA-1 0.008 0.74 0.972 VCSA-2 0.023 0.57 0.999 VCSA-3 0.026 0.55 0.999 VCSA-4 0.021 0.57 0.996 VCSA-5 0.011 0.66 0.971 VCSA-6 0.014 0.64 0.979 VCSA-7 0.011 0.71 0.978 VCSA-8 0.027 0.59 0.990

% Drug Loading

$ Encapsulation Efficiency
In-vitro Release Study
Dissolution was carried out using Tablet dissolution tester (Lab India, Mumbai, India) equipped with eight baskets. Dissolution rates were measured at 370C under 100 rpm speed. Drug release from the microspheres was studied in 7.4 pH phosphate buffer solution. Aliquot samples were withdrawn at regular time intervals and analyzed by UV spectrophotometer as explained before. RESULTS AND DISCUSSION pH and Temperature Responsive Behavior of Microspheres Figure 1 (a) shows the swelling ratio of microspheres at various pH solutions. As we can clearly see that the swelling ratio of microspheres slowly increases when pH increases up to 5.0 after that it increases rapidly up to pH 8. Because at low pH i.e.,

Figure 1.(a)

Figure 1.(b) Figure 1. Swelling studies of MGs (a) various pH conditions, and (b) different temperatures The effect of temperature on the equilibrium swelling ratios for microspheres is shown in Figure 1(b) The swelling ratio of microspheres is higher at low temperature ( LCST). This is because below LCST VCL contains a hydrophilic group (-CONH-) and hydrophobic isopropyl group present in the linear polymer chain. So, the hydrophilic group in the polymer structure will form an intermolecular hydrogen bond with surrounding water at low temperature (below the gel transition temperature); above LCST the hydrogen bonds are broken and the water molecules are expelled from the polymer. These two results make the water molecule inside the gel change from a bound state to a free State and release from the gel. This phenomenon makes the swelling ratios of the microspheres decrease rapidly at the gel transition temperature. Differential scanning calorimetry (DSC) DSC tracings of pure 5-fluorouracil, drug-loaded microspheres, and plain microspheres are displayed in Figure 2. The pure 5-FU exhibits a sharp peak at 285oC (curve c) is due to polymorphism and melting. However, this peak has not appeared in the case of drug-loaded microspheres (curve b) which confirms that the drug is molecularly dispersed in the polymeric microspheres.

Figure 2: DSC thermograms of (a) plain Poly(VC-co-SA) microspheres (c) 5-FU loaded Poly(VC-co-SA) microspheres and (c) 5-FU

Figure 3: Scanning electron micrographs of Poly(VC-co-SA) microspheres Scanning Electron Microscopic (SEM) Studies Figure 3. shows the morphology of microspheres. The formed copolymer particles are spherical with the diameters of around 10 mm. Laser Particle Size Analyzer

Figure 4: Particle size distribution curve of Poly(VC-co-SA) microspheres Results of the mean particle size with standard errors are presented in Table 1, while the size distribution curve for a typical formulation containing SA-5 is displayed in Figure 4. It is found that size distribution is broad and volume means diameter of the particle is around 16 mm. The particle size of different formulations containing different amounts of drug, crosslinking agent and different ratios of VC-co-SA are given in Table 1. The particle size of formulations containing different amounts of crosslinking agent (NNMBA) i.e., 1, 2 and 3 % are 34, 28 and 16, respectively. The particle size decreased with increasing amount of crosslinking due to the formation of a rigid structure due to a reduction in chain length of the polymer formed. Encapsulation Efficiency Results of encapsulation efficiencies are given in Table 1. The % encapsulation efficiency varied depending upon the initial loading of the drug. In general, for formulations VCSA-1, VCSA-2 and VCSA-3, the % encapsulation efficiency increased systematically with increasing drug content of the matrices. At higher amount of crosslinking agent i.e., 2 % or 3 % of NNMBA in the matrix, the % encapsulation efficiency decreased. The highest % encapsulation efficiency of 79 was observed for VCSA-3 containing 15 % of 5-FU with a higher amount of SA in the copolymer matrix and its size was also highest i.e., 34 mm. Drug Release Kinetics

cumulative release data While studying the drug release from the polymer matrices, it has been the usual practice to analyze the release data using the empirical relationship proposed by Ritger and Peppas25. In the present study, we have analyzed the cumulative release data using26. Here, the ratio, Mt/M∞ represents the fractional drug release at the time, t; k is a constant characteristic of the drug-polymer system and n is an empirical parameter characterizing the release mechanism. Using the least-squares procedure, we have estimated the values of n and k for all the nine formulations at a 95% confidence limit; these data are given in Table 2 at 370C. If the values of n = 0.5, then drug diffuses and releases out of the microsphere matrix following the Fickian diffusion. If n > 0.5, anomalous or non-Fickian transport occurs. For n = 1, non-Fickian or more commonly called Case II release kinetics is operative. The values of n ranging between 0.5 and 1 indicate the anomalous type transport27. The values of k and n have shown a dependence on the extent of crosslinking, % drug loading and SA content of the matrix. Values of n for microspheres prepared by varying the amount of SA 90, 80 and 70 % in the microspheres of by keeping 5-FU (10 %) and 1 % NNMBA, ranged from 0.70 to 0.56 leading to a shift of transport from Fickian to anomalous type. The 5-FU-loaded particles have the n values ranging from 0.55 to 0.73, indicating the shift from erosion type release to a swelling-controlled non-Fickian type of mechanism. This could be possibly due to a reduction in the regions of low microviscosity and closure of microcavities in the swollen state. Similar findings have been observed elsewhere, wherein the effect of different polymer ratios on dissolution kinetics was observed. On the other hand, the values of k are quite smaller for drug-loaded microspheres, suggesting their lesser interactions compared to microspheres containing varying amount of SA.
Effect of Sodium Acrylate Content

Figure 5: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of acrylamide at 37 0C, Symbols: (■)100 %, (■)30 %, (•) 20 % and (▲) 10 % Figure 5 shows the in vitro release data of 5-fluorouracil from poly(VC-co-SA) particles performed with particles taking the different ratio of SA. These data show that higher amount of SA containing particles have more encapsulation efficiencies and also release studies have shown that higher amounts SA containing particles have shown prolonged release characteristics than the microspheres containing lower amounts of SA. Generally, the drug release pattern depends upon factors like particle size, crystallinity, surface character, molecular weight, polymer composition, swelling ratio, degradation rate, drug binding affinity, the rate of hydration of polymeric materials, etc.27. In the release behavior of poly(VC-co-SA) system, one can consider the binding affinity of drug and polymer swelling property of SA. A rapid release of more than 98% of the drug was observed within 12 h. from the microspheres containing a lower amount of SA, indicating on the interaction between the two polymers. Effect of Temperature

Figure 6: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of Vinyl Caprolactum at 25 0C, Symbols: (■)10 %, (•) 20 % and (▲) 30 %.

Figure 7: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of crosslinking agent at 37 0C, Symbols: (■) 3%, (▲) 2% and (•) 1 % The cumulative release data vs time curves for varying amounts of vinyl caprolactam are displayed in Figure 6 at 250C. Drug release profiles exhibited drastic changes by variations in temperature from 370 to 250C as shown in Figures 4 and 5, respectively. It may be noticed that drug was released slowly at 370C i.e., above the LCST of 320C, but the release was much faster at 250C i.e., below the LCST than at 370C. This is due to the fact that at a higher temperature, the surface of microspheres would shrink, causing the drug to migrate toward the surface of the microspheres as seen by the initial burst effect during the dissolution experiments (Figure 6 and 7). However, dense surfaces of the microspheres will prohibit the release of more amount of drug. At lower temperatures, the already shrunken surface layer starts to re-swell, which would allow the drug to be released after a certain period of time, depending upon the minimum time required for re-swelling of the surface. Thus, the time required for drug release was accelerated as a result of cooling below the LCST, which further slowed down upon reheating. Microspheres were thus found to be sensitive to changes in temperature. At 250C (in the swollen state), the release rate and the total amount of drug release were considerably higher than those found at 370C (in a collapsed state). Drug molecules entrapped inside the polymer network will diffuse out of the microspheres, since they quickly get hydrated in the swollen state. In contrast, at 370C, the network structure is collapsed and exhibits a lesser tendency to uptake water or buffer solution, leading to a decrease in drug diffusion rate. Effect of Crosslinking Agent The % cumulative release vs time curves for varying amounts of NNMBA are displayed in Figure 7. The % cumulative release is quite fast and large at the lower amount of NNMBA, whereas release is quite slower at a higher amount of NNMBA. The cumulative release is somewhat smaller when a lower amount of NNMBA was used probably because, at higher concentration of NNMBA, polymeric chains would become rigid due to the contraction of microvoids, thus decreasing the % cumulative release of 5-FU through the polymeric matrices. As expected, the release becomes slower at a higher amount of NNMBA but becomes faster at a lower amount of NNMBA. Effect of Drug Concentration

Figure 8 % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of 5-FU at 37 0C, Symbols: (■) 15 %, (•) 10 % and (▲) 5 % Figure 8 displays the release profiles of poly(VC-co-SA) microspheres that are loaded with different amounts of 5-FU. Notice that initially, during the first hour, the release is quite fast in all the formulations, but later it slowed down. The similar findings were observed in earlier literature of 5-fluorouracil loaded microspheres of a different kind . Release data suggest that those formulations containing the highest amount of drug (i.e., 15 wt. %) displayed higher release rates than those containing smaller amounts of 5-FU (i.e., 10 and 5 wt. %). A prolonged and slow release was observed for the formulation containing a lower amount of 5-FU (i.e., 5 wt. %) at 370C; this is due to the large free volume spaces available in the matrix through which, a lesser number of 5-FU molecules would transport. Notice that for all the 5-FU-loaded formulations, the almost complete release of 5-FU was achieved after 720 min. CONCLUSION Poly(vinyl caprolactam-Sodium acrylate) copolymeric microspheres crosslinked with N, N¢-methylene bisacrylamide were prepared by free radical emulsion polymerization. The microspheres have been characterized by differential scanning calorimetry (DSC) and x-ray diffractometry (x-RD) to understand the drug dispersion in microspheres. Microspheres with different copolymer compositions were prepared in yields of 80-85 %. DSC indicated a uniform distribution of 5-fluorouracil particles in microspheres, whereas SEM suggested a spherical structure of the microspheres with the slight rough surface. The in vitro drug release indicated that particle size and release kinetics depend upon copolymer composition, amount of crosslinking agent and amount of 5-fluorouracil present in the microspheres. REFERENCES Dunn, R. L., & Ottenbrite, R. M. (Eds.). (1991). Polymeric drugs and drug delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1991-0469 El-Nokaly, M. A., Piatt, D. M., & Charpentier, B. A. (1993). Polymeric delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1993-0520 Garcı́a, O., Blanco, M. D., Martı́n, J. A., & Teijón, J. M. (2000). 5-Fluorouracil trapping in poly (2-hydroxyethyl methacrylate-co-acrylamide) hydrogels: in vitro drug delivery studies. European polymer journal, 36(1), 111-122. https://doi.org/10.1016/S0014-3057(99)00037-3 Uhrich, K. E., Cannizzaro, S. M., Langer, R. S., & Shakesheff, K. M. (1999). Polymeric systems for controlled drug release. Chemical reviews, 99(11), 3181-3198. https://doi.org/10.1021/cr940351u , PMid:11749514 Işiklan, N. (2006). Controlled release of insecticide carbaryl from sodium alginate, sodium alginate/gelatin, and sodium alginate/sodium carboxymethyl cellulose blend beads crosslinked with glutaraldehyde. Journal of applied polymer science, 99(4), 1310-1319. https://doi.org/10.1002/app.22012 Vaithiyalingam, S., Nutan, M., Reddy, I., & Khan, M. (2002). Preparation and characterization of a customized cellulose acetate butyrate dispersion for controlled drug delivery. Journal of pharmaceutical sciences, 91(6), 1512-1522. https://doi.org/10.1002/jps.10155 , PMid:12115850 Xing, L., Dawei, C., Liping, X., & Rongqing, Z. (2003). Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. Journal of Controlled Release, 93(3), 293-300. https://doi.org/10.1016/j.jconrel.2003.08.019 , PMid:14644579 Hosoya, K., Kubo, T., Takahashi, K., Ikegami, T., & Tanaka, N. (2002). Novel surface modification of polymer-based separation media controlling separation selectivity, retentivity and generation of electroosmotic flow. Journal of Chromatography A, 979(1-2), 3-10. https://doi.org/10.1016/S0021-9673(02)01255-4 Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai, N., Kikuchi, A., Sakurai, Y., & Okano, T. (1996). Temperature-responsive chromatography using poly (N-isopropylacrylamide)-modified silica. Analytical Chemistry, 68(1), 100-105. https://doi.org/10.1021/ac950359j , PMid:21619225 Vihola, H., Laukkanen, A., Hirvonen, J., & Tenhu, H. (2002). Binding and release of drugs into and from thermosensitive poly (N-vinyl caprolactam) nanoparticles. European journal of pharmaceutical sciences, 16(1-2), 69-74. https://doi.org/10.1016/S0928-0987(02)00076-3 Torres-Lugo, M., & Peppas, N. A. (1999). Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules, 32(20), 6646-6651. https://doi.org/10.1021/ma990541c Kirsh, Y. E., Vorobiev, A. V., Yanul, N. A., Fedotov, Y. A., & Timashev, S. F. (2001). Facilitated acetylene transfer through membranes composed of sulfonate-containing aromatic polyamides and poly-N-vinylamides involving nanocluster silver. Separation and purification technology, 22, 559-565. https://doi.org/10.1016/S1383-5866(00)00138-6 Hester, J. F., Olugebefola, S. C., & Mayes, A. M. (2002). Preparation of pH-responsive polymer membranes by self-organization. Journal of Membrane Science, 208(1-2), 375-388. https://doi.org/10.1016/S0376-7388(02)00317-4 Lederhos, J. P., Long, J. P., Sum, A., Christiansen, R. L., & Sloan Jr, E. D. (1996). Effective kinetic inhibitors for natural gas hydrates. Chemical Engineering Science, 51(8), 1221-1229. https://doi.org/10.1016/0009-2509(95)00370-3 Coughlan, D. C., Quilty, F. P., & Corrigan, O. I. (2004). Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly (N-isopropylacrylamide) hydrogels. Journal of Controlled Release, 98(1), 97-114. https://doi.org/10.1016/j.jconrel.2004.04.014 , PMid:15245893 Schild, H. G. (1992). Poly (N-isopropylacrylamide): experiment, theory and application. Progress in polymer science, 17(2), 163-249. https://doi.org/10.1016/0079-6700(92)90023-R Kirsh, Y. E., Yanul, N. A., & Kalninsh, K. K. (1999). Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. European polymer journal, 35(2), 305-316. https://doi.org/10.1016/S0014-3057(98)00114-1 Meeussen, F., Nies, E., Berghmans, H., Verbrugghe, S., Goethals, E., & Du Prez, F. (2000). Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer, 41(24), 8597-8602. https://doi.org/10.1016/S0032-3861(00)00255-X Lozinsky, V. I., Simenel, I. A., Kurskaya, E. A., Kulakova, V. K., Galaev, I. Y., Mattiasson, B., ... & Khokhlov, A. R. (2000). Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer, 41(17), 6507-6518. https://doi.org/10.1016/S0032-3861(99)00844-7 S. Barabas In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H. F., Bicales, N. M., Overberger, C. C., Menges, G., Eds.; John Wiley & Sons: New York, 1985; Vol. 17, pp 225-226. Liu, J.L. Velada, M.B.Huglin, Polymer 40 (1999) 4299. https://doi.org/10.1016/S0032-3861(98)00458-3, https://doi.org/10.1016/S0032-3861(99)00081-6, https://doi.org/10.1016/S0032-3861(98)00387-5, https://doi.org/10.1016/S0032-3861(98)00533-3, https://doi.org/10.1016/S0032-3861(99)00101-9, https://doi.org/10.1016/S0032-3861(98)00758-7, https://doi.org/10.1016/S0032-3861(98)00660-0, https://doi.org/10.1016/S0032-3861(98)00858-1 Yuksel, D. E. A. (1999). Preparation of spray-dried microspheres of indomethacin and examination of the effects of coating on dissolution rates. Journal of microencapsulation, 16(3), 315-324. https://doi.org/10.1080/026520499289040, PMid:10340217 Sairam, M., Babu, V. R., Naidu, B. V. K., & Aminabhavi, T. M. (2006). Encapsulation efficiency and controlled release characteristics of crosslinked polyacrylamide particles. International journal of pharmaceutics, 320(1-2), 131-136. https://doi.org/10.1016/j.ijpharm.2006.05.001, PMid:16766148 Babu, V. R., Sairam, M., Hosamani, K. M., & Aminabhavi, T. M. (2006). Development of 5-fluorouracil loaded poly (acrylamide-co-methylmethacrylate) novel core-shell microspheres: In vitro release studies. International journal of pharmaceutics, 325(1-2), 55-62. https://doi.org/10.1016/j.ijpharm.2006.06.020, PMid:16884868 Ritger, P. L., & Peppas, N. A. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of controlled release, 5(1), 37-42. https://doi.org/10.1016/0168-3659(87)90035-6 Harogoppad, S. B., & Aminabhavi, T. M. (1991). Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8-C16). Macromolecules, 24(9), 2598-2605. https://doi.org/10.1021/ma00009a070 Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (1996). Biomaterials science: an introduction to materials in medicine. Elsevier New York, 347-356. Read the full article
0 notes
Text
Novel Poly(Vinyl caprolactum-co-Sodiumacrylate) Microspheres for Controlled Release of 5-Fluorouracil
INTRODUCTION Controlled delivery of drugs by means of biodegradable polymers began in the 1970s and continued to expand rapidly with numerous novel products1,2. The controlled release technology has lead to the development of newer methods of drug administration as well as the design and application of different types of CR formulations for effective targeting of certain drugs to the site of action. In particular, biodegradable polymeric systems have led to the development of CR dosage formulations to achieve the desired therapeutic results to obtain maximum dose regimen with minimum side effects3. The release of drug from a polymer matrix occurs due to the transport of drug to the surrounding medium system by the molecular diffusion mechanism. The CR systems offer many advantages over the conventional dosage forms, including improved efficacy, reduced toxicity as well as improved patient compliance and convenience4-6. Among the various types of polymers employed, hydrophilic biopolymers are quite suitable for oral applications7 due to their several inherent advantages over the synthetic polymers. Drug targeting to a specific tissue or organ has been the subject of creative and innovative research in medicinal and pharmaceutical chemistry since the beginning of the twentieth century. In many diseases (e.g. cancer, AIDS, rheumatoid arthritis, etc.) a considerable therapeutic advantage could be gained if drugs were delivered more selectively and in a controlled manner to their target sites. More particularly, it is conventionally accepted that efficient, compliant and reliable therapy requires that the drug reside as long as its therapeutic action is needed at a specific site, where it acts (by systemic absorption, binding, inhibition, etc.) as intact molecules. This concept has led to the development of a variety of physically based controlled release dosage forms such as drug dispersible matrices, coated tablets or particles, microcapsules. The development of an appropriate delivery system will first require a proper consideration of three related factors; the properties of the drug; the disease and the destination in the body. Over the past few years, stimuli-responsive (sensitive) polymers have become the object of intensive study due to their ability to change drastically their physical state under minute changes in external environment such as temperature, pH, ionic strength, light illumination, etc. Recently, chromatographic8,9 drug delivery10,11 membrane technology12,13 and kinetic inhibition14 applications were reported. Poly(N-isopropylacrylamide)15-17 (PNIPA) and poly(N-vinyl caprolactam)18,19 (PVCL) were intensively investigated due to their thermo-sensitive properties since these are water soluble at low temperature. However, they exhibit lower critical solution temperature (LCST) in water and undergo a coil-to-globule transition and aggregation at higher temperatures. For PNIPA the coil-to-globule transition occurs at around 32°C. PVCL is a homolog of poly(N-vinylpyrrolidone) (PVP), which is a biocompatible polymer widely used in medicine and pharmaceutics20. PVCL combines the useful and important properties of PVP and PNIPAm. It is a biocompatible polymer with a phase transition in the region of physiological temperature (30-37 °C). Such properties make it a prospective material in designing CR systems. Further, the incorporation of ionic hydrophilic moieties into the PolyVCL hydrogel networks would enhance the LCST and the gels become sensitive towards PH, whereas hydrophobic moieties decrease the LCST. Liu et al.21 found that salts of acrylic acid monomers are strong electrolytes, which are completely ionized in water, and their copolymeric units increased the swelling characteristics to a greater extent. 5-Fluorouracil is an acidic, water-soluble22,23, hydrophilic, is an antineoplastic drug used extensively in clinical chemotherapy for the treatment of solid tumors. It has been widely used in drug administration due to its large number of secondary effects that accompany its conventional administration. We present here the development of 5-fluorouracil-loaded poly(vinyl caprolactam-co-Sodium acrylate) microspheres for investigating its slow release characteristics. The plasma lifetime of 5-Fu is 1-1.2 hand it needs to extend for its effective therapy. The microspheres prepared were characterized by particle size analyzer, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). The in vitro release studies have been performed in 7.4 pH buffer solution at 25 0C and 370C to extend to the release rates of the drug. MATERIALS AND METHOD Materials Vinyl caprolactam (VC) was purchased from Aldrich Chemicals, Milwaukee, WI USA. Sodium acrylate (SA), N, N¢-methylene bisacrylamide (NNMBA), sodium lauryl sulfate, potassium persulfate, and calcium chloride were all purchased from s.d. fine chemicals, Mumbai, India. 5-Fluorouracil was purchased from MP Biochemicals, Eschwege, Germany. Synthesis of poly(vinyl caprolactam-co-sodium acrylate) microspheres Sodium lauryl sulfate (1g) was dissolved in 80 ml of water taken in a three-necked round bottom flask equipped with a mechanical stirrer, a condenser, and a gas inlet to maintain the inert nitrogen atmosphere. The flask was immersed in an oil bath with a thermostatic control to maintain the desired temperature accurate to ± 1oC. The solution was stirred at 800 rpm speed until it became clear and 100 mg of potassium persulfate was added. The required amount of SA, VC, crosslinking agent, NNMBA and 5-Fluorouracil were dissolved separately in 20 ml of water. This mixture was added to the reaction mixture drop-wise using a dropping funnel and the reaction was continued for 8 h at 700C to obtain the maximum yield. The reaction mixture was taken out after 8 h and added to 1% calcium chloride solution drop-wise to break the emulsion24. Particles were then isolated by centrifuging the product at the rotor speed of 12,000 rpm, washed with water and dried under vacuum at 400C for 24 h. Conversion of Copolymer The yield of copolymeric microspheres was determined gravimetrically. After copolymerization, the latex solution was added to 1 % calcium chloride solution and centrifuged to isolate the particles from the mixture. The copolymeric microspheres were washed several times successively with water and methanol solvents to remove the remaining monomer and initiator and then dried in a vacuum oven at 500C until attainment of constant weight. The % conversion of monomers was calculated as: % Conversion = (W/M) ×100 Where W is the weight of the dry copolymer obtained from the latex sample and M is the weight of the monomers taken. The yield of copolymeric microspheres varied between 80 and 85 % for various formulations prepared in this study. pH and Temperature Sensitive Nature of Copolymer Microspheres

percentages of swelling ratio (% SR) pH and temperature sensitivity of copolymer microspheres were studied through swelling experiments. First, the microspheres were immersed in a buffer solution with various pH values (pH buffer solutions were prepared using NaH2PO4, Na2HPO4, NaCl and NaOH solution and pH values were measured using ELICO pH meter, India) at 30oC for 12 h. The swollen MGs were taken out for every 30 min and removed surface adhered buffer solution using tissue paper. The MGs were further immersed in various buffer solutions to reach equilibrium swelling. Swelling experiments were carried out in water by mass measurements at various temperatures to study temperature responsive behavior of microspheres. The percentages of swelling ratio (% SR) were calculated using the following equations. Where, Ws is the weight of swollen gel at time t, and Wd is the dry weight of the hydrogel. Mass measurements were made on a digital ADAMS microbalance (Model AF 210L, U.K) with a sensitivity of 0.01 mg. Each value was averaged over three parallel measurements. Statistical analysis was performed using one-way ANOVA way in ORIGIN 8.0. All quantitative data are presented as means + standard deviation. Differential Scanning Calorimetry (DSC) Studies Differential scanning calorimetric (DSC) curves were recorded on a Rheometric scientific differential scanning calorimeter (Model-DSC SP, UK). The instrument was calibrated using indium as the standard. Samples were heated in sealed aluminum pans between 300 and 400oC at the heating rate of 10oC/min under inert nitrogen purge gas at the rate of 20 ml/min. Scanning Electron Microscopic (SEM) Studies Morphology of the microspheres was confirmed by scanning electron microscopy (SEM). Micrographs of the dry microspheres in powder form, dispersed in acetone, were all recorded using Leica 400, Cambridge, UK instrument. Particle Size Analysis Size distribution of the microspheres was determined using the particle size analyzer (Mastersizer 2000, Malvern Instruments, UK) equipped with the dry accessory system. Estimation of Drug Loading and Encapsulation Efficiency Loading efficiency of 5-FU in the microspheres was determined spectrophotometrically. About 10 mg of the drug-loaded core-shell microspheres were placed in 10 ml of buffer solution and stirred vigorously for 48 h to extract the drug from the microspheres. The solution was filtered and assayed by UV spectrophotometer (model Anthelme, Secomam, Dumont, France) at the fixed lmax value of 270 nm. The results of % drug loading and encapsulation efficiency were calculated, respectively using Equations. (1) and (2). These data are compiled in Tables 1 and 2, respectively. Table 1: Results of % encapsulation efficiency and mean diameter of poly(VC-co-SA) microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil Sample code % Vinyl Caprolactum (VC) % SA % NNMBA % 5-FU
% Encapsulation efficiency ± SD
Mean particle diameter (mm) ± SD VCSA-1 20 80 1 5 70 ± 1 29 ± 6 VCSA-2 20 80 1 10 74 ± 2 31 ± 8 VCSA-3 20 80 1 15 78 ± 2 34 ± 6 VCSA-4 20 80 2 10 75 ± 9 28 ± 4 VCSA-5 20 80 3 10 71 ± 8 16 ± 2 VCSA-6 10 90 1 10 68 ± 6 30 ± 4 VCSA-7 30 70 1 10 71 ± 5 24 ± 1 VCSA-8 00 100 1 10 72 ± 1 22 ± 8 Table 2: Release kinetics parameters of microspheres with different amounts of crosslinking agent, monomer concentration and 5-fluorouracil at 370C Formulation codes K x 102 n Correlation coefficient ‘r’ VCSA-1 0.008 0.74 0.972 VCSA-2 0.023 0.57 0.999 VCSA-3 0.026 0.55 0.999 VCSA-4 0.021 0.57 0.996 VCSA-5 0.011 0.66 0.971 VCSA-6 0.014 0.64 0.979 VCSA-7 0.011 0.71 0.978 VCSA-8 0.027 0.59 0.990

% Drug Loading

$ Encapsulation Efficiency
In-vitro Release Study
Dissolution was carried out using Tablet dissolution tester (Lab India, Mumbai, India) equipped with eight baskets. Dissolution rates were measured at 370C under 100 rpm speed. Drug release from the microspheres was studied in 7.4 pH phosphate buffer solution. Aliquot samples were withdrawn at regular time intervals and analyzed by UV spectrophotometer as explained before. RESULTS AND DISCUSSION pH and Temperature Responsive Behavior of Microspheres Figure 1 (a) shows the swelling ratio of microspheres at various pH solutions. As we can clearly see that the swelling ratio of microspheres slowly increases when pH increases up to 5.0 after that it increases rapidly up to pH 8. Because at low pH i.e.,

Figure 1.(a)

Figure 1.(b) Figure 1. Swelling studies of MGs (a) various pH conditions, and (b) different temperatures The effect of temperature on the equilibrium swelling ratios for microspheres is shown in Figure 1(b) The swelling ratio of microspheres is higher at low temperature ( LCST). This is because below LCST VCL contains a hydrophilic group (-CONH-) and hydrophobic isopropyl group present in the linear polymer chain. So, the hydrophilic group in the polymer structure will form an intermolecular hydrogen bond with surrounding water at low temperature (below the gel transition temperature); above LCST the hydrogen bonds are broken and the water molecules are expelled from the polymer. These two results make the water molecule inside the gel change from a bound state to a free State and release from the gel. This phenomenon makes the swelling ratios of the microspheres decrease rapidly at the gel transition temperature. Differential scanning calorimetry (DSC) DSC tracings of pure 5-fluorouracil, drug-loaded microspheres, and plain microspheres are displayed in Figure 2. The pure 5-FU exhibits a sharp peak at 285oC (curve c) is due to polymorphism and melting. However, this peak has not appeared in the case of drug-loaded microspheres (curve b) which confirms that the drug is molecularly dispersed in the polymeric microspheres.

Figure 2: DSC thermograms of (a) plain Poly(VC-co-SA) microspheres (c) 5-FU loaded Poly(VC-co-SA) microspheres and (c) 5-FU

Figure 3: Scanning electron micrographs of Poly(VC-co-SA) microspheres Scanning Electron Microscopic (SEM) Studies Figure 3. shows the morphology of microspheres. The formed copolymer particles are spherical with the diameters of around 10 mm. Laser Particle Size Analyzer

Figure 4: Particle size distribution curve of Poly(VC-co-SA) microspheres Results of the mean particle size with standard errors are presented in Table 1, while the size distribution curve for a typical formulation containing SA-5 is displayed in Figure 4. It is found that size distribution is broad and volume means diameter of the particle is around 16 mm. The particle size of different formulations containing different amounts of drug, crosslinking agent and different ratios of VC-co-SA are given in Table 1. The particle size of formulations containing different amounts of crosslinking agent (NNMBA) i.e., 1, 2 and 3 % are 34, 28 and 16, respectively. The particle size decreased with increasing amount of crosslinking due to the formation of a rigid structure due to a reduction in chain length of the polymer formed. Encapsulation Efficiency Results of encapsulation efficiencies are given in Table 1. The % encapsulation efficiency varied depending upon the initial loading of the drug. In general, for formulations VCSA-1, VCSA-2 and VCSA-3, the % encapsulation efficiency increased systematically with increasing drug content of the matrices. At higher amount of crosslinking agent i.e., 2 % or 3 % of NNMBA in the matrix, the % encapsulation efficiency decreased. The highest % encapsulation efficiency of 79 was observed for VCSA-3 containing 15 % of 5-FU with a higher amount of SA in the copolymer matrix and its size was also highest i.e., 34 mm. Drug Release Kinetics

cumulative release data While studying the drug release from the polymer matrices, it has been the usual practice to analyze the release data using the empirical relationship proposed by Ritger and Peppas25. In the present study, we have analyzed the cumulative release data using26. Here, the ratio, Mt/M∞ represents the fractional drug release at the time, t; k is a constant characteristic of the drug-polymer system and n is an empirical parameter characterizing the release mechanism. Using the least-squares procedure, we have estimated the values of n and k for all the nine formulations at a 95% confidence limit; these data are given in Table 2 at 370C. If the values of n = 0.5, then drug diffuses and releases out of the microsphere matrix following the Fickian diffusion. If n > 0.5, anomalous or non-Fickian transport occurs. For n = 1, non-Fickian or more commonly called Case II release kinetics is operative. The values of n ranging between 0.5 and 1 indicate the anomalous type transport27. The values of k and n have shown a dependence on the extent of crosslinking, % drug loading and SA content of the matrix. Values of n for microspheres prepared by varying the amount of SA 90, 80 and 70 % in the microspheres of by keeping 5-FU (10 %) and 1 % NNMBA, ranged from 0.70 to 0.56 leading to a shift of transport from Fickian to anomalous type. The 5-FU-loaded particles have the n values ranging from 0.55 to 0.73, indicating the shift from erosion type release to a swelling-controlled non-Fickian type of mechanism. This could be possibly due to a reduction in the regions of low microviscosity and closure of microcavities in the swollen state. Similar findings have been observed elsewhere, wherein the effect of different polymer ratios on dissolution kinetics was observed. On the other hand, the values of k are quite smaller for drug-loaded microspheres, suggesting their lesser interactions compared to microspheres containing varying amount of SA.
Effect of Sodium Acrylate Content

Figure 5: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of acrylamide at 37 0C, Symbols: (■)100 %, (■)30 %, (•) 20 % and (▲) 10 % Figure 5 shows the in vitro release data of 5-fluorouracil from poly(VC-co-SA) particles performed with particles taking the different ratio of SA. These data show that higher amount of SA containing particles have more encapsulation efficiencies and also release studies have shown that higher amounts SA containing particles have shown prolonged release characteristics than the microspheres containing lower amounts of SA. Generally, the drug release pattern depends upon factors like particle size, crystallinity, surface character, molecular weight, polymer composition, swelling ratio, degradation rate, drug binding affinity, the rate of hydration of polymeric materials, etc.27. In the release behavior of poly(VC-co-SA) system, one can consider the binding affinity of drug and polymer swelling property of SA. A rapid release of more than 98% of the drug was observed within 12 h. from the microspheres containing a lower amount of SA, indicating on the interaction between the two polymers. Effect of Temperature

Figure 6: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of Vinyl Caprolactum at 25 0C, Symbols: (■)10 %, (•) 20 % and (▲) 30 %.

Figure 7: % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of crosslinking agent at 37 0C, Symbols: (■) 3%, (▲) 2% and (•) 1 % The cumulative release data vs time curves for varying amounts of vinyl caprolactam are displayed in Figure 6 at 250C. Drug release profiles exhibited drastic changes by variations in temperature from 370 to 250C as shown in Figures 4 and 5, respectively. It may be noticed that drug was released slowly at 370C i.e., above the LCST of 320C, but the release was much faster at 250C i.e., below the LCST than at 370C. This is due to the fact that at a higher temperature, the surface of microspheres would shrink, causing the drug to migrate toward the surface of the microspheres as seen by the initial burst effect during the dissolution experiments (Figure 6 and 7). However, dense surfaces of the microspheres will prohibit the release of more amount of drug. At lower temperatures, the already shrunken surface layer starts to re-swell, which would allow the drug to be released after a certain period of time, depending upon the minimum time required for re-swelling of the surface. Thus, the time required for drug release was accelerated as a result of cooling below the LCST, which further slowed down upon reheating. Microspheres were thus found to be sensitive to changes in temperature. At 250C (in the swollen state), the release rate and the total amount of drug release were considerably higher than those found at 370C (in a collapsed state). Drug molecules entrapped inside the polymer network will diffuse out of the microspheres, since they quickly get hydrated in the swollen state. In contrast, at 370C, the network structure is collapsed and exhibits a lesser tendency to uptake water or buffer solution, leading to a decrease in drug diffusion rate. Effect of Crosslinking Agent The % cumulative release vs time curves for varying amounts of NNMBA are displayed in Figure 7. The % cumulative release is quite fast and large at the lower amount of NNMBA, whereas release is quite slower at a higher amount of NNMBA. The cumulative release is somewhat smaller when a lower amount of NNMBA was used probably because, at higher concentration of NNMBA, polymeric chains would become rigid due to the contraction of microvoids, thus decreasing the % cumulative release of 5-FU through the polymeric matrices. As expected, the release becomes slower at a higher amount of NNMBA but becomes faster at a lower amount of NNMBA. Effect of Drug Concentration

Figure 8 % Cumulative release of 5-fluorouracil through Poly(VC-co-SA) microspheres containing different amount of 5-FU at 37 0C, Symbols: (■) 15 %, (•) 10 % and (▲) 5 % Figure 8 displays the release profiles of poly(VC-co-SA) microspheres that are loaded with different amounts of 5-FU. Notice that initially, during the first hour, the release is quite fast in all the formulations, but later it slowed down. The similar findings were observed in earlier literature of 5-fluorouracil loaded microspheres of a different kind . Release data suggest that those formulations containing the highest amount of drug (i.e., 15 wt. %) displayed higher release rates than those containing smaller amounts of 5-FU (i.e., 10 and 5 wt. %). A prolonged and slow release was observed for the formulation containing a lower amount of 5-FU (i.e., 5 wt. %) at 370C; this is due to the large free volume spaces available in the matrix through which, a lesser number of 5-FU molecules would transport. Notice that for all the 5-FU-loaded formulations, the almost complete release of 5-FU was achieved after 720 min. CONCLUSION Poly(vinyl caprolactam-Sodium acrylate) copolymeric microspheres crosslinked with N, N¢-methylene bisacrylamide were prepared by free radical emulsion polymerization. The microspheres have been characterized by differential scanning calorimetry (DSC) and x-ray diffractometry (x-RD) to understand the drug dispersion in microspheres. Microspheres with different copolymer compositions were prepared in yields of 80-85 %. DSC indicated a uniform distribution of 5-fluorouracil particles in microspheres, whereas SEM suggested a spherical structure of the microspheres with the slight rough surface. The in vitro drug release indicated that particle size and release kinetics depend upon copolymer composition, amount of crosslinking agent and amount of 5-fluorouracil present in the microspheres. REFERENCES Dunn, R. L., & Ottenbrite, R. M. (Eds.). (1991). Polymeric drugs and drug delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1991-0469 El-Nokaly, M. A., Piatt, D. M., & Charpentier, B. A. (1993). Polymeric delivery systems. American Chemical Society. https://doi.org/10.1021/bk-1993-0520 Garcı́a, O., Blanco, M. D., Martı́n, J. A., & Teijón, J. M. (2000). 5-Fluorouracil trapping in poly (2-hydroxyethyl methacrylate-co-acrylamide) hydrogels: in vitro drug delivery studies. European polymer journal, 36(1), 111-122. https://doi.org/10.1016/S0014-3057(99)00037-3 Uhrich, K. E., Cannizzaro, S. M., Langer, R. S., & Shakesheff, K. M. (1999). Polymeric systems for controlled drug release. Chemical reviews, 99(11), 3181-3198. https://doi.org/10.1021/cr940351u , PMid:11749514 Işiklan, N. (2006). Controlled release of insecticide carbaryl from sodium alginate, sodium alginate/gelatin, and sodium alginate/sodium carboxymethyl cellulose blend beads crosslinked with glutaraldehyde. Journal of applied polymer science, 99(4), 1310-1319. https://doi.org/10.1002/app.22012 Vaithiyalingam, S., Nutan, M., Reddy, I., & Khan, M. (2002). Preparation and characterization of a customized cellulose acetate butyrate dispersion for controlled drug delivery. Journal of pharmaceutical sciences, 91(6), 1512-1522. https://doi.org/10.1002/jps.10155 , PMid:12115850 Xing, L., Dawei, C., Liping, X., & Rongqing, Z. (2003). Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. Journal of Controlled Release, 93(3), 293-300. https://doi.org/10.1016/j.jconrel.2003.08.019 , PMid:14644579 Hosoya, K., Kubo, T., Takahashi, K., Ikegami, T., & Tanaka, N. (2002). Novel surface modification of polymer-based separation media controlling separation selectivity, retentivity and generation of electroosmotic flow. Journal of Chromatography A, 979(1-2), 3-10. https://doi.org/10.1016/S0021-9673(02)01255-4 Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai, N., Kikuchi, A., Sakurai, Y., & Okano, T. (1996). Temperature-responsive chromatography using poly (N-isopropylacrylamide)-modified silica. Analytical Chemistry, 68(1), 100-105. https://doi.org/10.1021/ac950359j , PMid:21619225 Vihola, H., Laukkanen, A., Hirvonen, J., & Tenhu, H. (2002). Binding and release of drugs into and from thermosensitive poly (N-vinyl caprolactam) nanoparticles. European journal of pharmaceutical sciences, 16(1-2), 69-74. https://doi.org/10.1016/S0928-0987(02)00076-3 Torres-Lugo, M., & Peppas, N. A. (1999). Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules, 32(20), 6646-6651. https://doi.org/10.1021/ma990541c Kirsh, Y. E., Vorobiev, A. V., Yanul, N. A., Fedotov, Y. A., & Timashev, S. F. (2001). Facilitated acetylene transfer through membranes composed of sulfonate-containing aromatic polyamides and poly-N-vinylamides involving nanocluster silver. Separation and purification technology, 22, 559-565. https://doi.org/10.1016/S1383-5866(00)00138-6 Hester, J. F., Olugebefola, S. C., & Mayes, A. M. (2002). Preparation of pH-responsive polymer membranes by self-organization. Journal of Membrane Science, 208(1-2), 375-388. https://doi.org/10.1016/S0376-7388(02)00317-4 Lederhos, J. P., Long, J. P., Sum, A., Christiansen, R. L., & Sloan Jr, E. D. (1996). Effective kinetic inhibitors for natural gas hydrates. Chemical Engineering Science, 51(8), 1221-1229. https://doi.org/10.1016/0009-2509(95)00370-3 Coughlan, D. C., Quilty, F. P., & Corrigan, O. I. (2004). Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly (N-isopropylacrylamide) hydrogels. Journal of Controlled Release, 98(1), 97-114. https://doi.org/10.1016/j.jconrel.2004.04.014 , PMid:15245893 Schild, H. G. (1992). Poly (N-isopropylacrylamide): experiment, theory and application. Progress in polymer science, 17(2), 163-249. https://doi.org/10.1016/0079-6700(92)90023-R Kirsh, Y. E., Yanul, N. A., & Kalninsh, K. K. (1999). Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. European polymer journal, 35(2), 305-316. https://doi.org/10.1016/S0014-3057(98)00114-1 Meeussen, F., Nies, E., Berghmans, H., Verbrugghe, S., Goethals, E., & Du Prez, F. (2000). Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer, 41(24), 8597-8602. https://doi.org/10.1016/S0032-3861(00)00255-X Lozinsky, V. I., Simenel, I. A., Kurskaya, E. A., Kulakova, V. K., Galaev, I. Y., Mattiasson, B., ... & Khokhlov, A. R. (2000). Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer, 41(17), 6507-6518. https://doi.org/10.1016/S0032-3861(99)00844-7 S. Barabas In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H. F., Bicales, N. M., Overberger, C. C., Menges, G., Eds.; John Wiley & Sons: New York, 1985; Vol. 17, pp 225-226. Liu, J.L. Velada, M.B.Huglin, Polymer 40 (1999) 4299. https://doi.org/10.1016/S0032-3861(98)00458-3, https://doi.org/10.1016/S0032-3861(99)00081-6, https://doi.org/10.1016/S0032-3861(98)00387-5, https://doi.org/10.1016/S0032-3861(98)00533-3, https://doi.org/10.1016/S0032-3861(99)00101-9, https://doi.org/10.1016/S0032-3861(98)00758-7, https://doi.org/10.1016/S0032-3861(98)00660-0, https://doi.org/10.1016/S0032-3861(98)00858-1 Yuksel, D. E. A. (1999). Preparation of spray-dried microspheres of indomethacin and examination of the effects of coating on dissolution rates. Journal of microencapsulation, 16(3), 315-324. https://doi.org/10.1080/026520499289040, PMid:10340217 Sairam, M., Babu, V. R., Naidu, B. V. K., & Aminabhavi, T. M. (2006). Encapsulation efficiency and controlled release characteristics of crosslinked polyacrylamide particles. International journal of pharmaceutics, 320(1-2), 131-136. https://doi.org/10.1016/j.ijpharm.2006.05.001, PMid:16766148 Babu, V. R., Sairam, M., Hosamani, K. M., & Aminabhavi, T. M. (2006). Development of 5-fluorouracil loaded poly (acrylamide-co-methylmethacrylate) novel core-shell microspheres: In vitro release studies. International journal of pharmaceutics, 325(1-2), 55-62. https://doi.org/10.1016/j.ijpharm.2006.06.020, PMid:16884868 Ritger, P. L., & Peppas, N. A. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of controlled release, 5(1), 37-42. https://doi.org/10.1016/0168-3659(87)90035-6 Harogoppad, S. B., & Aminabhavi, T. M. (1991). Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8-C16). Macromolecules, 24(9), 2598-2605. https://doi.org/10.1021/ma00009a070 Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (1996). Biomaterials science: an introduction to materials in medicine. Elsevier New York, 347-356. Read the full article
0 notes
Text
What are the Advantages of Skin Needling Melbourne?
Needling is extremely popular in healthy skin and in light of current circumstances. The cycle includes rolling a wand or pen fastened with smaller than expected needles to make minuscule injuries. These injuries are minuscule and quite shallow; however, your body reacts with caution, sending in mending factors that lift skin strength, brilliance, and wellbeing.
Micro needling makes your skin gleam after only one treatment and keeps on boosting the presence of your composition for quite a long time thereafter. The advantages of skin needling Melbourne are many. Here are the most eminent.
Reduces the appearance of lines and wrinkles-
Nobody needs to look older than they are. Yet, untimely maturing that presents as scarcely discernible differences and wrinkles do precisely that. The small wounds from a needling therapy support collagen and elastin creation to battle lines and wrinkles.
Collagen and elastin are compounds in your skin that add construction and strength, loaning an energetic quality. The led light therapy Malvern likewise invigorate your body to deliver new skin cells, making scarce differences, crow's feet, and forehead wrinkles less obvious.
Scar treatment-
Because needling stimulates collagen and elastin production, it’s also super effective in addressing acne and other scars on your skin. The only type of scar not possible to treat is keloid or raised, scars.
Sun damage-
Needling is powerful in diminishing the presence of sun harm, especially hyperpigmentation and age spots. If you have a smeared, tarnish appearance, which is presumably an aftereffect of an excessive number of long periods of sunning, micro-needling and it is capacity to invigorate new collagen and skin cells can revitalize your watch and surprisingly out your tone.
Anti-aging-
Maturing doesn't simply appear as wrinkles and staining. As you get more seasoned, your skin loses its flexibility, so it seems careless. Maturing skin may likewise look dull and come up short on a glossy shine. Microneedling can change this.
The development of collagen that happens because of the small injuries can improve skin construction to battle hanging. Also, only 24 hours following a meeting, you experience a brilliant, new glow.
Shrinks pores-
Despite the way that skin needling Melbourne includes poking holes in your skin, it doesn't build the size of your pores. Indeed, it assists your pores with seeming more modest. When the collagen around your pores is invigorated, the territory around each pore plumps, making the actual pore nearly vanish.
Improves the Effectiveness of topical products-
Post-microneedling is the ideal chance to apply hostile to aging medicines, creams, and other skin medicines that lift skin wellbeing and appearance. The miniature openings get creams, serums, and gels all the more promptly and convey them more profound into your dermis than when applied consistently.
Rosacea reduction-
The ruddiness and skin thickening of rosacea can be tended to with skin needling. If you experience the ill effects of this skin condition, you experience a rushed breakdown of collagen. Led light therapy Malvern animates the development of collagen to balance and supplant this breakdown, so your skin looks less kindled and bothered.
1 note
·
View note
Text
How is Led light Therapy effective for skins?
LED is another light source that has entered the application field in the previous ten years. It has been utilized in the clinical field for around six years, showing significant helpful impacts in a brief timeframe. The Led light Therapy Malvern is now utilized in the clinical field, incorporate red, blue, and purple. The adequacy of LED in sanitization and cleansing, injury recuperating, wound Therapy, aggravation end, edema decrease, and photodynamic cancer therapy has been ultimately confirmed in the clinical field.
LED Light Therapy is otherwise called PDT (Photo Dynamic Therapy). It depends on LED'S (Light Emitting Diodes) that transmit electromagnetic waves at various wavelengths to enter the skin and give energy to the cells. It is this energy that advances cell digestion.
LED Light Therapy treatment attempts to develop skin conditions further. Deductively talking, singular LED lights infiltrate the subcutaneous tissue to empower the mitochondria in cells; thus, they produce more ATP (Adenosine triphosphate), which causes the cell to duplicate quicker. This general activity prompts a miniature flow of blood, which brings about new collagen and elastin development.
If the frequency transmitted is in the noticeable range somewhere in the range of 400nm and 800nm, then, at that point, the shading will be a clear blue, red, yellow, or green light. On the off chance that the frequency discharged isn't in the noticeable range, the light won't be apparent, like Infra-Red light. This is because skin reacts distinctively, relying upon the frequencies utilized. This implies that treatment of different skin conditions can happen contingent upon the determination of shade of light.
Each tone infiltrates skin at an alternate profundity, with Infra-Red (IR) being the most profound – making it ideal for wound mending and irritation. Skin break-out is best treated with Blue Light as it focuses on the top layer to obliterate microbes. At the same time, barely recognizable differences and wrinkles fit Red Light which enters further. The Green Light's frequency sits consummately among Red and Blue and attempts to develop lymph flow further. By stirring these tones up, we can likewise deliver Yellow Light for skin cell resistance, mending and conditioning, and Pink Light to firm skin and decrease scarce differences and wrinkles.
LED Therapy is non-obtrusive, non-ablative, protected, and regular. It doesn't contain any UVA, UVB, or bright light. The treatment fills in as an independent treatment or notwithstanding other signature or clinical medicines. LED Therapy after a facial treatment broadens the advantages of the facial, advancing by and significant skin wellbeing and revival.
Customary LED medicines give continuous improvements to the skin, smoothing, generally speaking, complexion as the collagen and elastin strands are framed. In addition, by animating the skin cell metabolic capacity, the skin's composition seems better and more uniform with fewer lines, wrinkles, and quicker mending of skin conditions.
LED treatment is sans torment, substance-free, ok for all skin types, and an unwinding, charming treatment to have. The Led light Therapy Malvern East requires 20 minutes by and large, albeit this can change. We suggest a hand or foot knead during the LED treatment for a superior experience.
0 notes
Text
How is Led light Therapy effective for skins?
LED is another light source that has entered the application field in the previous ten years. It has been utilized in the clinical field for around six years, showing significant helpful impacts in a brief timeframe. The Led light Therapy Malvern is now utilized in the clinical field, incorporate red, blue, and purple. The adequacy of LED in sanitization and cleansing, injury recuperating, wound Therapy, aggravation end, edema decrease, and photodynamic cancer therapy has been ultimately confirmed in the clinical field.
0 notes
Text
Role Of Led Light Therapy Malvern For Your Skin Treatment
Led light therapy, Malvern utilizes controlled light waves to treat various sicknesses and illnesses. Every frequency of light enters a particular profundity of skin tissue, and, subsequently, various frequencies of lights can be utilized to treat various conditions. The LED lights generally utilized in this field contain complex semiconductors, which convert electrical flows into muddled tight range light, considering a more serious shading or lightwave recurrence. The LED lights we use at Freedom Float have numerous shading settings, so you can handle the settings relying upon what you need to accomplish. You can likewise request that a colleague assist you with choosing your light therapy settings.
The Benefits of LED Therapy
As the light frequency enhances, so does the profundity of the entrance. This light is consumed by receptors in the skin, like effective skin health management, and each shade of light invigorates an alternate reaction in the skin. Light, which is photon energy, can regulate or change your science. Light receptors in particles respond to fluctuating frequencies, which is why various shades of light have diverse skin benefits.
As per Dermatologist Howard Sobel, Led light therapy Malvern is appropriate for all skin types and tones. These medicines are easy, don't cause any copies or skin harm, and are non-obtrusive with practically no vacation and distress. Indeed, skin regularly looks emphatically sparkling when the LED veil is eliminated.
The recuperating properties of LED make it ideal for use after in-office methods like strips, lasers, and miniature needling. It works by transmitting infrared lights in various frequencies, which have diverse skin benefits. The skin utilizes light as a wellspring of energy to fuel the maintenance and revival of harmed cells.
As indicated by Beauty Therapist Malvern East, a partner teacher of dermatology, light therapy is an immensely under-used region in medication. Driven light therapy is altering home healthy skin therapy. He clarifies that through an interaction called photograph biomodulation, light modifies natural material. As indicated by Jagdeo, red light infiltrates the skin further than apparent light and animates the mitochondria, which has a calming and restoring impact. Collagen is underlying the dermis; the skin is quieted, and wrinkles in the end lessen. Blue light doesn't enter the skin as profoundly yet destroys skin inflammation causing microscopic organisms on a superficial level. The science on the green light isn't as strong; however, in principle, it targets melanocytes, debilitating abundance melanin creation.
Light Therapy Masks
Numerous corrective brands have dispatched compact hand-held variants of in-office LED medicines for safe use at home. The gadgets guarantee to target everything from hanging and wrinkling skin to skin break out and irritation. These FDA-endorsed gadgets utilize red, blue, yellow, and green to treat an assortment of issues. At the point when home-utilize LED light veils were acquainted with the market, they turned into a hit on Instagram, getting supports from superstars like Jessica Alba and Kourtney Kardashian.
Red: most at-home LED veils offer a red light setting. At the lighter finish of the range, red light relieves aggravation and redness, while more profound shades enter the skin to incite cell fix and dissemination, bringing about a plumper, more dynamic appearance. Red light speaks with skin fibroblasts to build collagen creation, making skin firmer over the long haul. Red light likewise diminishes irritation, which can help quiet rosacea, skin break out, and expands dissemination, which can carry supplements to the skin and support hair development.
Driven light therapy is an easy, non-obtrusive skin therapy whereby an individual's face (or at times different body pieces) is presented to a variety of Red, Blue and Near-Infrared LED lights. Pamela clarifies: With the Beauty Therapist Malvern East, drove light therapy utilizes photon energy to recover cells, lessen irritation, mend wounds, dissipate melanocyte bunches, diminish skin inflammation, and increment skin restoration. Blue, Red, and Near-Infrared work in a mix with one another.
0 notes
Text
Role of Led light therapy Malvern for your skin treatment
As per Dermatologist Howard Sobel, Led light therapy Malvern is appropriate for all skin types and tones. These medicines are easy, don't cause any copies or skin harm, and are non-obtrusive with practically no vacation and distress. Indeed, skin regularly looks emphatically sparkling when the LED veil is eliminated.
0 notes
Text
Benefits of getting Led light therapy Malvern
Skin specialists share each easily overlooked detail you need to answer these best-in-class skin health management treatments. This incorporates at-home laser hair expulsion microcurrent treatments and progressively, Led light therapy Malvern.
0 notes
Text
How Led light therapy Malvern East Improve your Skin?
LED light therapy Malvern East is a famous non-invasive skin treatment for skin inflammation, sun harm, wounds, and other skin issues. Individuals can decide to have LED light treatment at a dermatologist's office or to utilize a gadget at home.
The treatment utilizes differing frequencies of light to trigger the skin's characteristic recuperating cycles to fix the skin. A few medicines are important to get results.
0 notes
Text
How Led light therapy Malvern East Improve your Skin?
LED light therapy Malvern East is a famous non-invasive skin treatment for skin inflammation, sun harm, wounds, and other skin issues. Individuals can decide to have LED light treatment at a dermatologist's office or to utilize a gadget at home.
The treatment utilizes differing frequencies of light to trigger the skin's characteristic recuperating cycles to fix the skin. A few medicines are important to get results.
In this article, we investigate how LED light treatment functions and who may be a decent competitor. We additionally cover the conceivable results.
Benefits of LED Light Therapy
Smooths fine lines and wrinkles
Reduces inflammation
Improves acne scars
Prevents breakouts by killing the acne-causing bacteria
Promotes circulation
Stimulates collagen production
Reduces inflammation
Brightens skin
What is Light Therapy?
Light travels at a scope of frequencies cooperating with the actual world contrastingly and each lightwave recurrence show a unique tone. On the off chance that you've at any point heard the expression, "eat a rainbow" you may realize that distinctive shaded food varieties have explicit supplements and advantages to human and creature wellbeing. A comparable rule applies to light treatment (here and there known as phototherapy or chromotherapy). Hire the acne treatment Malvern to rid of acne.
The light treatment utilizes controlled light waves to treat various sicknesses and infections. Every frequency of light enters a particular profundity of skin tissue and, subsequently, various frequencies of lights can be utilized to treat various conditions. The LED lights that are generally utilized in this field are contained complex semiconductors, which convert electrical flows into mixed up limited range light, considering a more extreme tone, or lightwave recurrence.
How to use it?
LED light therapy Malvern East can happen at an expert's office or home utilizing a home gadget. During an expert treatment, the dermatologist may request that the individual rests under LED lights, or they may utilize a LED wand on the skin.
Every meeting keeps going roughly 20 minutes, and up to 10 meetings might be important. After this, an individual may have to return intermittently for support meetings.
At-home LED gadgets might be more helpful because no arrangements are important. In any case, they might be less powerful than proficient medicines.
When utilizing an at-home gadget, it is imperative to adhere to the maker's directions. These gadgets ordinarily come as a cover that an individual applies to the face for a few minutes or a wand that they use on the skin. Driven light treatment is reasonable for use on any body part, including the face, hands, neck, and chest.
LED light treatment has all the earmarks of being a protected treatment for a few skin conditions, including skin break out, skin maturing, skin wounds, and different issues.
Examination demonstrates that this treatment offers promising outcomes, even though individuals ought not to anticipate a 100% improvement. Likewise, the outcomes are not generally lasting, so follow-up medicines might be fundamental.
Acne treatment Malvern is commonly more powerful than at-home medicines.
0 notes
Text
Know Everything About Skin Needling Melbourne | Malvernskinstudio
Skin Needling, otherwise called Dermal Rolling, has been all the rage in the most recent couple of years and keeps on acquiring fame. Healthy skin and magnificence lovers the same depend on it. In any case, how does Skin Needling Melbourne respond, and what would it be able to treat?
What can Skin Needling treat?
Skin Needling is the one-stop hostile to maturing skin treatment! It animates the creation of your collagen to make smoother, better skin. Skin Needling benefits incorporate lessening the presence of:
• Acne scarring
• Fine lines
• Pigmentation
• Enlarged pores
• Scarring
• Stretch marks
The outcomes are further developed skin surface, all the more even complexion and an overall sound shine.
How frequently would it be advisable for me to seek Skin Needling medicines?
This relies upon your singular treatment plan and your treatment objectives. Overall, we suggest 3-6 medicines with every treatment divided 4 a month and a half separated. Note, every individual will fluctuate somewhat, and our skin advisor will inform you of the suggested number regarding medicines at your free meeting.
How does skin needling work?
As each fine needle penetrates the skin, it makes a channel or miniature injury. The microchannels made precipitously close following 10 minutes with the goal that epidermal boundary honesty stays flawless.
The needles harm shallow dermal collagen strands and little veins setting off a controlled course of irritation, including the arrival of platelet-inferred development factors changing development factor-alpha and beta (TGF β-3), connective tissue enacting protein, connective tissue development factor, and fibroblast development factor. These lead to the creation of new collagen, elastin, and vessels.
Neovascularisation and neocollagenesis lead to thickened skin and decreased scars, with the further developed skin surface, immovability, and hydration.
Advantages of skin needling
Skin needling's advantages are recorded beneath.
• It is utilized to limit scars from skin break out, medical procedures, warm consumption, chickenpox, or injury.
• Skin needling is utilized for facial revival, less discernible differences and kinks, and further developing complexion, surface, and pigmentation.
• There is a decreased danger of hyperpigmentation and scarring with skin needling contrasted with more intrusive strategies, and accordingly, it is more secure on the ethnic or brown complexion.
• It is reasonable for slender and touchy skin.
• The treatment can be acted in an office setting and needn't bother with any broad extraordinary preparing or costly instruments.
• The method is very much endured and all around acknowledged by patients.
• It is practical and should be possible on spaces of skin that are not reasonable for stripping or laser reemerging, for example, close to eyes.
• As went against ablative laser restoring, the epidermis stays flawless and isn't harmed. Thus, the activity can be securely rehashed if necessary.
• Treatment doesn't bring about a boundary line between treated and untreated skin, as ordinarily happens with other reemerging systems. This considers explicit spaces of scarring to be treated without the need to 'mix' or 'quill' at the treatment edges.
• The patient can continue normal exercises within a couple of days, contingent upon the profundity of infiltration of the needles. Led Light Therapy Malvern Treatment alternatives like laser reemerging or dermabrasion are regularly connected with impressive horribleness and vacation from day by day exercises of the patient in the post-treatment time frame.
2 notes
·
View notes
Text
What are the Facial Benefits by Beauty Therapist Malvern East?
Facial massages are treatments you can do with a beauty therapist Malvern East or all alone. The procedure includes invigorating pressing factors focuses on the face, neck, and shoulders.
You can utilize moisturizers, oils, or purifying ointments with facial back rubs, just as a face roller or a level gua sha device.
Facial massage advances sound skin while loosening up your facial muscles. It has an unwinding and restoring impact, assisting you with looking and feel much improved.
Regardless of whether you need to utilize facial back rub only for unwinding or to treat a particular condition, there are a lot of methods to attempt. Keep perusing to investigate a portion of the advantages of facial back rub, how you can do it all alone, and when it's ideal to visit an expert.
Read on to learn what some of the research and anecdotal evidence has to say about the benefits of facial massage.
Anti-aging and wrinkles-
One of the primary advantages of facial massage is its capacity to improve the general appearance of skin.
A little 2017 study by led light therapy Malvern inspected the viability of facial back rub that incorporated an animating back rub gadget. Members utilized an enemy of maturing gadget and cream all over and neck for about two months. The impacts of the cream were improved when utilized with the facial back rub. Upgrades were found in wrinkles, skin hanging, and texture.
Sinus pressure-
However long it is anything but an irresistible case or during an intense phase of sinusitis, you can utilize massage to calm sinus pressing factors, distress, and clog.
Sinus massage may likewise help advance the seepage of bodily fluid, ease migraines, and lift dissemination. More top to bottom logical examinations is expected to affirm and develop the impacts of sinus pressure rub.
Acne-
Stimulating the skin through massage may help advance blood dissemination and decrease the presence of skin inflammation. The explicit examination that gives proof of facial back rub in improving skin break out is restricted.
A few groups depend on doing an olive oil back rub to treat skin break out. Results shift, so if it's something you're keen on attempting, test it out on a little region prior to rubbing your whole face. Book an appointment with the best beauty therapist Malvern East.
Glowing skin-
Facial massage might be only the ticket on the off chance that you need brilliant, gleaming skin. An examination from 2002 tracked down that 59% of ladies who had a facial back rub detailed a sensation of newness and revival.
Around 54% announced flexible skin, while 50% experienced skin fixing. A lot of recounted proof recommends that invigorating your facial muscles can help fix skin, ease tight muscles, and lift circulation. Facial massage might be only the ticket on the off chance that you need brilliant, sparkling skin. Exploration from 2002 tracked down that 59% of ladies who had a facial back rub detailed a sensation of newness and revival.
Around 54% detailed graceful skin, while 50% experienced skin fixing. A lot of narrative proof recommends that animating your facial muscles can help fix skin, mitigate tight muscles, and lift course.
0 notes
