#India based global medical device company
Explore tagged Tumblr posts
Text
Impact of COVID-19 on Medical Device Companies in India
The COVID-19 pandemic has significantly impacted various industries globally, and medical devices companies in India are no exception. The sudden surge in demand for medical equipment, coupled with disruptions in the supply chain, has created both challenges and opportunities for medical device companies in India. This blog explores how the pandemic has affected these companies, highlighting their response and the future outlook.
#Surgical Products#Top MedTech Companies#A global leader in MedTech#surgical technologies and solutions#India based global medical device company#healthcare solutions worldwide#Surgical kits
0 notes
Text
Key Trends Shaping the Medical Plastics Market in 2024 and Beyond
Medical Plastics Industry Overview
The global medical plastics market size is expected to reach USD 87.58 billion by 2030, registering a CAGR of 7.4% from 2024 to 2030, according to a new report by Grand View Research, Inc. The demand for medical device packaging is likely due to the increased need for in-house and advanced medical devices. Plastics including polyethylene, polypropylene, and polycarbonate are increasingly being utilized in the medical device manufacturing The growth of the home healthcare sector on account of the low costs involved as compared to hospital care and intensive care contributes to a rise in the demand for medical devices and multiple hospital associated tests as well.
The growth of the home healthcare sector on account of the low costs involved as compared to hospital care and intensive care has resulted in a rise in the demand for medical devices. The increasing demand for medical devices can also be attributed to the various types of medical tests conducted by hospitals for diagnostic purposes.
Gather more insights about the market drivers, restrains and growth of the Medical Plastics Market
Healthcare spending in the country is likely to accelerate in the near future owing to the growing awareness about healthcare. The rise in demand is driven by the aging population (65 years of age or above), growing prevalence of chronic diseases, infrastructure enhancements and technological advancements in medical plastics market.
The strengthening of the Western European economies is expected to result in an increased provision of healthcare expenditure in the annual budgetary proposals. Moreover, Northern European economies such as Germany, the UK, and Sweden are projected to exhibit an increase in healthcare expenditure, thereby leading to a growth in the production of medical devices. However, the growth is expected to be supported by political unrest in Europe’s transition economies such as Russia and Ukraine.
Browse through Grand View Research's Plastics, Polymers & Resins Industry Research Reports.
The global poly alpha olefin market size was valued at USD 1.48 billion in 2023 and is projected to grow at a CAGR of 2.8% from 2024 to 2030.
The global cross-linked polyethylene market size was valued at USD 8.0 billion in 2023 and is projected to grow at a CAGR of 6.9% from 2024 to 2030.
Medical Plastic Market Segmentation
Grand View Research has segmented the global medical plastic market based on product, application, and region:
Medical Plastics Product Outlook (Volume, Kilotons & Revenue, USD Million, 2018 - 2030)
Polyethylene (PE)
Polypropylene (PP)
Polycarbonate (PC)
Liquid Crystal Polymer (LCP)
Polyphenylsulfone (PPSU)
Polyethersulfone (PES)
Polyethylenimine (PEI)
Polymethyl Methacrylate (PMMA)
Others
Medical Plastics Process Technology Outlook (Volume, Kilotons; Revenue, USD Million, 2018 - 2030)
Extrusion
Injection Molding
Blow Molding
Other
Medical Plastics Application Outlook (Volume, Kilotons & Revenue, USD Million, 2018 - 2030)
Medical Device Packaging
Medical Components
Orthopedic Implant Packaging
Orthopedic Soft Goods
Wound Care
Cleanroom Supplies
BioPharm Devices
Mobility Aids
Sterilization and Infection Prevention
Tooth Implants
Denture Base Material
Other Implants
Others
Medical Plastics Region Outlook (Volume, Kilotons & Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
Germany
UK
France
Italy
Netherland
Asia Pacific
China
India
Japan
Central & South America
Brazil
Argentina
Middle East & Africa
Saudi Arabia
UAE
Key Companies profiled:
Röchling SE & Co. KG
Nolato AB
Saint-Gobain S.A.
SABIC
Orthoplastics Ltd
Eastman Chemical Company
Celanese Corporation
Dow, Inc.
Tekni-Plex, Inc.
Solvay S.A.
HMC Polymers Company Limited
ARAN BIOMEDICAL TEORANTA
Trelleborg Group
Avantor, Inc.
Trinseo
Evonik Industries AG
Key Medical Plastics Company Insights
Some key market players include BASF SE; Celanese Corporation; Evonik Industries AG; SABIC; Dow, Inc.; Solvay S.A.; Trinseo S.A.; and Eastman Chemical Company.
In November 2022, Celanese Corporation announced the acquisition of DuPont's Mobility & Material (M&M) business for USD 11 billion. This strategic move enables Celanese to expand its global reach and enhance its offerings in the environmental sector, particularly in sustainable transportation.
Recent Developments
Some key players operating in market include BASF SE; Celanese Corporation; Evonik Industries AG; SABIC; Dow, Inc.; Solvay S.A.; Trinseo S.A.; Eastman Chemical Company among others.
In February 2023, Cleanse Corporation announced the acquisition of DUPONT's mobility and mobility business for USD 11.00 billion. This strategic move enables Cleanse to expand its global reach and enhance its offerings in the environmental sector, particularly in sustainable transportation.
In June 2023, SABIC acquired Clariant's 50% stake in Scientific Design, a renowned catalysis leader. This acquisition bolstered the non-cyclical, technology-driven business and brought it closer to becoming a leading global specialist.
Order a free sample PDF of the Medical Plastics Market Intelligence Study, published by Grand View Research.
0 notes
Text
LDL Test Market Trends, Growth, and Opportunities 2024

Industrial Snapshot of LDL Test Market
The LDL Test Market Report is a treasured source of insightful data for business strategists. It provides an in-depth assessment of numerous features of industries like market overview, present progress valuations, historical and future Studies, current trends, SWOT valuations, and clients operating in several regions. The study provides valuable information to magnify the understanding, scope, and segments of this report. The report covers a comprehensive analysis of LDL Test Market segmentation, regional and country breakdowns. This research will offer a clear and exact idea about the whole industry to the readers to make beneficial decisions.
According to Straits Research, the global LDL Test market size was valued at USD 8.45 billion in 2023. It is projected to reach from USD 8.96 billion in 2024 to USD 14.45 billion by 2032, growing at a CAGR of 6.15% during the forecast period (2024–2032).
This study pinpoints noteworthy trends influencing the trajectory of the Gesture Recognition market's expansion. Within this recently issued report, crucial dynamics encompassing drivers, limitations, and prospects are underscored. These aspects hold relevance for well-established market entities as well as emerging stakeholders engaged in the realms of production and supply.
Request a Sample Report @ https://straitsresearch.com/report/ldl-test-market/request-sample
Competitive Analysis
The report contains an in-depth analysis of the vendor’s profile, including financial health, business units, key business priorities, SWOT, strategies, and views.
Hoffmann-La Roche Ltd.
Sekisui Diagnostics
Express Biotech International
Sigma-Aldrich Co. LLC
Randox Laboratories Ltd.
Reckon Diagnostics
Quest Diagnostics Incorporated
DiaSys Diagnostics India Private Limited
Eurofins Scientific
Kopibeskyttet Unilabs Labhåndbok
Atlas Medical
Eurolyser Diagnostics GmbH
Laboratory Corporation of America
Diazyme Laboratories Inc.
Thermo Fisher Scientific Inc.
The vendors have been identified based on the portfolio, geographical presence, marketing & distribution channels, revenue generation, and significant R&D investments.
Request Sample Report of Global LDL Test Market @ https://straitsresearch.com/report/ldl-test-market/request-sample
Vendors across different verticals are planning for high investments in this market, and as a result, the market is expected to grow at an impressive rate in the upcoming years. The key players are adopting various organic and inorganic growth strategies such as mergers & acquisitions, collaboration & partnerships, joint ventures, and a few other strategies to be in a strong position in the global market.
Market Segmentation Analysis
The report provides a wide-ranging evaluation of the market, providing in-depth qualitative insights, historical data, and supportable projections along with the assumptions about the LDL Test Market size. The projections featured in the report have been derived using proven research methodologies and assumptions based on the vendor’s portfolio, blogs, white papers, and vendor presentations. Thus, the research report represents every side of the LDL Test Market and is segmented on the basis of regional markets, offerings, applications, and end-users.
By Type
LDL-C
LDL-P
LDL-B
Others
By Component
Devices
Syringes and Needles
Spectrophotometers
Kits and Reagents
Toxicity Assays Kit
Blood Chemistry Assays Kit
Cytotoxicity Assays Kit
Others
By Applications
Atherosclerosis
Obesity
Dyslipidemia
Diabetes
Angina
Stroke Carotid Artery Disease
Peripheral Arterial Disease
Others
By End-User
Hospitals
Clinics
Pharmaceutical and Biotechnological Companies
Diagnostic Centers
Others
Access Detailed Segmentation @ https://straitsresearch.com/report/ldl-test-market/segmentation
Regional Analysis for LDL Test Market
The regional analysis offers a comprehensive view of the LDL Test Market sales and growth across global and country-level markets. It provides volume and market size data by region, with insights into growth trends in countries like the United States, Canada, Germany, France, China, Japan, and more. The analysis also covers major regions such as North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
Benefits
LDL Test Market Industry companies to ensure business continuity with powerful protection by constantly checking the report and representing attractive growth opportunities for the companies. LDL Test Market handles all the needs of the operators by allowing them to improve their services and concentrate on their core business. LDL Test Market Research aims to increase business agility and reduce operational and capital expenditure with improved technology rollouts and capacity planning. The report discusses service types and regions related to this LDL Test Market. Further, the report provides details about the major challenges affecting the market growth.
Buy Now: https://straitsresearch.com/buy-now/ldl-test-market
Other Features of the Report:
Provides a thorough analysis of the key strategies with a focus on the corporate structure, R&D methods, localization strategies, production capabilities, sales, and performance in various companies.
Provides valuable insights into the product portfolio, including product planning, development, and positioning.
Thanks for reading this article; you can also get individual chapter-wise sections or region-wise report versions like North America, Europe, or Asia.
About Us:
StraitsResearch.com is a leading research and intelligence organization, specializing in research, analytics, and advisory services along with providing business insights & research reports.
Contact Us: Email: [email protected] Website: https://straitsresearch.com/
0 notes
Text
IVF Time-lapse Imaging Devices Market Overview: Extensive Evaluation of Market Size, Share, Growth Opportunities
The global IVF time-lapse imaging devices market size is expected to reach USD 1,787.8 million by 2030, based on a new report by Grand View Research, Inc. The market is expected to grow at a CAGR of 23.7% from 2024 to 2030 owing to the rise in infertility cases due to sedentary lifestyles. Infertility is being driven by lifestyle as well as behavioral changes that reflect a rising proportion of working women, advanced maternal and paternal age, reduced marriage rates, and marriage postponement.
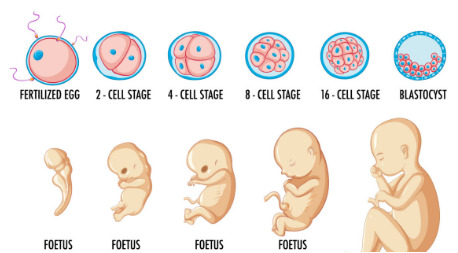
The demand for IVF solutions is on the rise due to the increasing number of people facing issues with naturally conceiving. The rising rate of obesity adds substantially to the incidence of infertility, as obese women are more vulnerable to infertility and uterine disorders. According to the WHO, one out of every six couples experiences issues related to infertility. In India, over 3 million people in India actively sought IVF in 2019. During the COVID-19 pandemic, the IVF market was affected as the number of individuals opting for IVF and IUI procedures reduced significantly. The percentage of people undertaking IVF cycles globally fell by 90%.
IVF Time-lapse Imaging Devices Market Report Highlights
Hospitals & other settings segment dominated the market and accounted for the highest revenue percentage of 77.0% in 2023.
Europe dominated the IVF time-lapse imaging devices market in 2023 with a market share of 36.1% due to increased infertility cases and fertility tourism, driving demand for IVF procedures.
The U.S. accounted for a share of 92.9% in North America in 2023 and attributed to grow as many patients are now practicing the IVF treatment.
Asia Pacific IVF time-lapse imaging devices market is anticipated to witness significant growth in the market with a CAGR of 25.9%.
For More Details or Sample Copy please visit link @: IVF Time-lapse Imaging Devices Market Report
COVID-19 imposed significant changes including paving the way for the sector's digital adoption. To survive and thrive in challenging times, the global IVF market was pushed to prioritize operational efficiency and telehealth implementation. Moreover, since 2019 the fertility market has been one of the top six most-invested treatment areas. As per Crunchbase website data, the global investments in fertility startups have increased by 89 percent from USD 93 million in 2020 to USD 176 million in 2021.
Artificial intelligence can be used to automate processes that are prone to human-derived subjectivity as well as variability. As clinics encounter rising patient demand, which frequently necessitates more and more advanced techniques, there is a clear need to automate as many procedures as possible to ensure the standard treatment quality while optimizing clinic resources. For instance, in April 2021, Life Whisperer announced a notable advancement in using AI to help embryologists rank and choose genetically better and healthier embryos in IVF without invasive procedures. Life Whisperer Genetics, a new AI product, necessitates only camera images of embryos to evaluate their genetic integrity. The AI analyses the physical morphology of the embryo based on the images to ascertain whether the embryo is genetically standard (euploid) and has a complete set of chromosomes.
List of major companies in the IVF Time-lapse Imaging Devices Market
Vitrolife
Esco Medical
Genea BIOMEDX
ASTEC CO., Ltd.
For Customized reports or Special Pricing please visit @: IVF Time-lapse Imaging Devices Market Analysis Report
We have segmented the global IVF time-lapse imaging devices market on the basis of end use and region.
#IVFTechnology#ReproductiveHealth#FertilityCare#MedicalDevices#HealthcareInnovation#EmbryoMonitoring#FertilityClinics#AssistedReproduction#IVFSuccess#EmbryoSelection#ReproductiveTech#FertilitySolutions#MedicalAdvancements#HealthcareTrends#InfertilityCare
0 notes
Text
Pharma Regulatory Consultants: Simplifying Compliance for the Pharmaceutical Industry
The pharmaceutical industry operates under strict regulations to ensure the safety, efficacy, and quality of medicines and healthcare products. Navigating these complex regulatory requirements can be a daunting task for pharmaceutical companies. This is where Pharma Regulatory Consultants come into play, offering specialized expertise to streamline compliance and facilitate market entry.
What Are Pharma Regulatory Consultants?
Pharma regulatory consultants are experts who assist pharmaceutical companies in meeting the legal and regulatory requirements for drug development, approval, manufacturing, and marketing. They provide guidance on domestic and international regulations, helping companies avoid delays and achieve compliance with agencies like the CDSCO in India, FDA in the USA, or EMA in Europe.
Key Services Offered by Pharma Regulatory Consultants
Regulatory Strategy Development
Assessing regulatory requirements based on the target market.
Designing a compliance roadmap for drug approvals.
Regulatory Submissions
Preparing and submitting dossiers, such as Common Technical Documents (CTD) or eCTD, to regulatory bodies.
Managing applications for Investigational New Drugs (IND), New Drug Applications (NDA), or Abbreviated New Drug Applications (ANDA).
Clinical Trial Support
Assisting with clinical trial approvals and ensuring compliance with Good Clinical Practice (GCP) guidelines.
Liaising with ethics committees and regulatory authorities for trial documentation.
Quality Assurance and Audits
Ensuring compliance with Good Manufacturing Practices (GMP) and ISO standards.
Conducting internal audits and preparing for regulatory inspections.
Regulatory Intelligence
Monitoring changes in global pharmaceutical regulations.
Providing insights into new guidelines, trends, or market-specific requirements.
Post-Marketing Compliance
Assisting with pharmacovigilance activities, including adverse event reporting.
Managing lifecycle maintenance, such as renewals, amendments, and labeling updates.
Why Do Companies Need Pharma Regulatory Consultants?
Expertise in Complex Regulations
Regulatory frameworks differ significantly between regions. Consultants possess in-depth knowledge of these complexities, ensuring accurate compliance.
Faster Time-to-Market
With their expertise, consultants minimize errors and delays in submissions, accelerating approval processes.
Cost Efficiency
Avoiding compliance pitfalls and regulatory rejections saves significant resources.
Global Market Access
Consultants help pharmaceutical companies meet international standards, opening doors to global markets.
Industries That Benefit from Pharma Regulatory Consultants
Pharmaceutical Manufacturing: For drug approvals and GMP compliance.
Biotechnology Firms: To navigate biologics and biosimilars regulations.
Medical Device Companies: To meet requirements for combination products.
OTC and Nutraceutical Companies: For compliance with labeling, claims, and advertising standards.
Choosing the Right Pharma Regulatory Consultant
Experience: Look for consultants with proven expertise in your specific domain (e.g., small molecules, biologics, or generics).
Regulatory Knowledge: Ensure familiarity with target market regulations, such as CDSCO, FDA, or EMA requirements.
Client References: Check testimonials or case studies from similar projects.
Proactive Communication: Select a consultant who provides regular updates and clear guidance.
Conclusion
Pharma regulatory consultants are invaluable partners for companies aiming to thrive in a highly regulated industry. From ensuring compliance to streamlining market entry, their expertise helps businesses stay ahead in the competitive pharmaceutical landscape.
Whether you’re a startup developing innovative drugs or an established company expanding globally, a skilled regulatory consultant can make the journey smoother, faster, and more efficient. By partnering with the right consultant, you can focus on delivering quality healthcare solutions while they handle the intricacies of regulatory compliance.
0 notes
Text
One Point One Solutions Limited Reports Robust Growth in Q2 FY25
November 12, 2024, marked a significant milestone for One Point One Solutions Limited, a leading name in next-generation Business Process Management (BPM) services. The company, headquartered in Navi Mumbai, released its financial results for the second quarter ending September 30, 2024, showcasing exceptional performance and notable year-on-year growth across key financial metrics.
The quarterly report highlights One Point One Solutions' continued trajectory of success, with the company recording substantial revenue growth, improved margins, and impressive profit after tax (PAT). The results are a testament to the company's effective strategies and its relentless focus on expanding its footprint across domestic and international markets.
Financial Performance Highlights For Q2 FY25, the company achieved a revenue of ₹62.48 crore, reflecting a remarkable 56.68% growth compared to the ₹39.88 crore revenue recorded in Q2 FY24. Earnings before interest, taxes, depreciation, and amortization (EBITDA) rose to ₹18.57 crore, a 25.22% increase from the ₹14.83 crore recorded in the corresponding quarter of the previous fiscal year.
Profit after tax (PAT) surged by 41.32%, reaching ₹8.38 crore compared to ₹5.93 crore in Q2 FY24. This exceptional performance also saw the company maintaining robust EBITDA and PAT margins at 29.71% and 13.42%, respectively.
Strategic Wins and Global Expansion Beyond the numbers, One Point One Solutions announced several strategic achievements that underscore its growing global presence and operational excellence. Key highlights include:
The signing of a non-binding term sheet to acquire a 100% stake in a BPO firm in Latin America, marking a significant step toward South American market penetration. Securing a strategic client in Tampa, Florida, a leading medical device company, strengthening its U.S. operations. Winning a major contract with a publicly listed Swedish company, with operations in Sweden, India, and the U.S., further expanding its reach in the European market. Its subsidiary, ITCube Solutions, achieving a prominent client win specializing in AI-driven brand threat mitigation tools, showcasing the company’s ability to harness cutting-edge technology. Management Insights Commenting on the results, Mr. Akshay Chhabra, Managing Director of One Point One Solutions, expressed optimism about the company’s growth trajectory. He attributed the strong quarterly performance to successful client acquisitions in Europe and the U.S. and announced plans to operationalize an additional 750 seats in the financial year 2025-26. This expansion is expected to drive a 25% revenue increase.
Mr. Chhabra highlighted the company’s efforts to strengthen its global footprint by incorporating a wholly-owned subsidiary in the U.K. This strategic move positions One Point One Solutions as a significant player in the European market. Additionally, the company is actively exploring acquisitions in Latin America, particularly in Colombia, Mexico, and Costa Rica, to tap into emerging opportunities.
The company is also prioritizing digital transformation by collaborating with AI-focused enterprises. These partnerships aim to enhance operational margins and drive innovative solutions that add value to clients worldwide.
About One Point One Solutions One Point One Solutions Limited is a comprehensive solutions provider in the BPM, IT, and KPO sectors, delivering services across multiple domains including technology, analytics, and process management. With a team of over 5,600 professionals and a global presence spanning the U.S., Europe, and Asia, the company serves diverse industries such as banking, retail, healthcare, and e-commerce.
Recent strategic moves, including the acquisition of ITCube Solutions and the establishment of its U.S.-based subsidiary, have solidified One Point One Solutions’ position as a global leader. By leveraging next-gen technologies such as Generative AI and intelligent automation, the company continues to provide tailored, impactful solutions to its clients.
A Promising Future As One Point One Solutions Limited looks ahead, its focus remains on delivering exceptional client value through organic growth, strategic acquisitions, and cutting-edge technological innovation. The company’s robust financial performance and ambitious global expansion plans underline its commitment to becoming a leader in the BPM and IT services industry.
0 notes
Text
Urinary Catheters Industry Size, Share, Growth, Analysis Forecast to 2030
The global urinary catheters market was valued at USD 5.2 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 5.4% from 2023 to 2030. This growth is driven by an increasing number of patients experiencing Urinary Tract Infections (UTIs), urethral blockages, and tumors in the urinary tract or reproductive organs, along with the rapidly expanding geriatric population. Urinary catheters, which are flexible tubes used to drain urine from the bladder, are commonly made from materials such as plastic, rubber, and silicon. Physicians recommend urinary catheterization for conditions such as Urinary Incontinence (UI), urinary retention, prostate surgery, or other medical issues like spinal cord injury, multiple sclerosis, or dementia, where bladder function may be compromised.
Gather more insights about the market drivers, restrains and growth of the Urinary Catheters Market
Regional Insights:
North America Urinary Catheters Market Trends
North America led the global urinary catheters market in 2022, holding over 34.4% of the total revenue share, with expected continued growth. This dominance is attributed to the increasing prevalence of diseases such as bladder obstruction, UI, Benign Prostate Hyperplasia (BPH), urinary retention, and bladder cancer. In North America, urological conditions are a significant health concern, especially among the elderly. The National Association for Continence reports that individuals aged 40 and above frequently suffer from Overactive Bladder (OAB) and urine urgency. Additionally, the American Society of Nephrology notes that urologic issues rank as the third most common complaint in individuals aged 65 and above, accounting for about 47% of physician visits in the U.S. Furthermore, bladder cancer rates are significant; according to the Canadian Cancer Society, around 12,200 cases were diagnosed in 2020, with 2,600 related deaths. The high incidence of BPH-related surgeries and rising bladder cancer cases are key factors driving market growth in North America.
Asia Pacific Urinary Catheters Market Trends
The Asia Pacific region is projected to be the fastest-growing market for urinary catheters during the forecast period. This growth is fueled by the increasing incidence of spinal cord injuries, BPH, and UTIs, with UTIs being among the most prevalent infectious diseases in the region. UTIs in Asia Pacific lead to high rates of morbidity and mortality, especially in hospitals, and impose a considerable financial burden on communities. Factors like advancing age, constipation, vaginal deliveries, childbirth, obesity, surgery, and chronic respiratory conditions elevate the risk of UTIs, particularly among women, thereby driving demand for urinary catheters in the region. Additionally, increased government spending, investments by leading market players, and awareness initiatives are expected to contribute to market growth. For example, in India, the Urological Society of India developed guidelines to raise awareness about urinary incontinence, while in South Korea, the Korean Incontinence Society promotes awareness regarding pelvic floor and lower urinary tract health issues. Such initiatives are anticipated to drive market expansion in Asia Pacific throughout the forecast period.
Browse through Grand View Research's Category Medical Devices Industry Research Reports.
The global endoscope sterilization market size was estimated at USD 1.20 billion in 2024 and is projected to grow at a CAGR of 9.64% from 2025 to 2030.
The global neuromodulation devices market size was estimated at USD 5,795.81 million in 2024 and is anticipated to grow at a CAGR of 8.51% from 2025 to 2030.
Key Companies & Market Share Insights:
To expand their market share, companies in the urinary catheters industry are continuously introducing new products and expanding their geographical reach. For instance, Japan-based Terumo Corporation acquired Sequent Medical, a U.S.-based medical device manufacturer, through its U.S. subsidiary. This acquisition reflects a broader trend among global players, who are relocating production facilities to different countries to achieve economies of scale.
Furthermore, many manufacturers in the catheter industry form partnerships with major medical device distributors to supply their products either globally or within specific countries. Such strategic alliances are widespread and benefit manufacturers by providing marketing licenses, while also reducing liability in cases of product recall or adverse events. A prominent example is the three-year purchasing agreement signed in March 2019 between Coloplast Corporation and Premier Inc., a U.S.-based healthcare company. Through this agreement, Coloplast’s urology products, including catheters, gained access to Premier’s network alliance, which consists of around 4,000 U.S. hospitals. Premier's integrated supply chain, advisory, and data analytics solutions allow Coloplast to secure supply agreements at lower costs, supporting the company’s market presence and reach in the U.S.
Key Urinary Catheters Companies:
Hollister, Inc.
Medtronic PLC
Boston Scientific Corp.
BD (C.R. Bard, Inc.)
Cook Medical
ConvaTec, Inc.
Teleflex, Inc.
Coloplast
Braun Melsungen AG
Medline Industries, Inc.
J and M Urinary Catheters LLC
Order a free sample PDF of the Urinary Catheters Market Intelligence Study, published by Grand View Research.
0 notes
Text
Urinary Catheters Industry Segmentation, Parameters and Prospects by 2030
The global urinary catheters market was valued at USD 5.2 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 5.4% from 2023 to 2030. This growth is driven by an increasing number of patients experiencing Urinary Tract Infections (UTIs), urethral blockages, and tumors in the urinary tract or reproductive organs, along with the rapidly expanding geriatric population. Urinary catheters, which are flexible tubes used to drain urine from the bladder, are commonly made from materials such as plastic, rubber, and silicon. Physicians recommend urinary catheterization for conditions such as Urinary Incontinence (UI), urinary retention, prostate surgery, or other medical issues like spinal cord injury, multiple sclerosis, or dementia, where bladder function may be compromised.
Gather more insights about the market drivers, restrains and growth of the Urinary Catheters Market
Regional Insights:
North America Urinary Catheters Market Trends
North America led the global urinary catheters market in 2022, holding over 34.4% of the total revenue share, with expected continued growth. This dominance is attributed to the increasing prevalence of diseases such as bladder obstruction, UI, Benign Prostate Hyperplasia (BPH), urinary retention, and bladder cancer. In North America, urological conditions are a significant health concern, especially among the elderly. The National Association for Continence reports that individuals aged 40 and above frequently suffer from Overactive Bladder (OAB) and urine urgency. Additionally, the American Society of Nephrology notes that urologic issues rank as the third most common complaint in individuals aged 65 and above, accounting for about 47% of physician visits in the U.S. Furthermore, bladder cancer rates are significant; according to the Canadian Cancer Society, around 12,200 cases were diagnosed in 2020, with 2,600 related deaths. The high incidence of BPH-related surgeries and rising bladder cancer cases are key factors driving market growth in North America.
Asia Pacific Urinary Catheters Market Trends
The Asia Pacific region is projected to be the fastest-growing market for urinary catheters during the forecast period. This growth is fueled by the increasing incidence of spinal cord injuries, BPH, and UTIs, with UTIs being among the most prevalent infectious diseases in the region. UTIs in Asia Pacific lead to high rates of morbidity and mortality, especially in hospitals, and impose a considerable financial burden on communities. Factors like advancing age, constipation, vaginal deliveries, childbirth, obesity, surgery, and chronic respiratory conditions elevate the risk of UTIs, particularly among women, thereby driving demand for urinary catheters in the region. Additionally, increased government spending, investments by leading market players, and awareness initiatives are expected to contribute to market growth. For example, in India, the Urological Society of India developed guidelines to raise awareness about urinary incontinence, while in South Korea, the Korean Incontinence Society promotes awareness regarding pelvic floor and lower urinary tract health issues. Such initiatives are anticipated to drive market expansion in Asia Pacific throughout the forecast period.
Browse through Grand View Research's Category Medical Devices Industry Research Reports.
The global endoscope sterilization market size was estimated at USD 1.20 billion in 2024 and is projected to grow at a CAGR of 9.64% from 2025 to 2030.
The global neuromodulation devices market size was estimated at USD 5,795.81 million in 2024 and is anticipated to grow at a CAGR of 8.51% from 2025 to 2030.
Key Companies & Market Share Insights:
To expand their market share, companies in the urinary catheters industry are continuously introducing new products and expanding their geographical reach. For instance, Japan-based Terumo Corporation acquired Sequent Medical, a U.S.-based medical device manufacturer, through its U.S. subsidiary. This acquisition reflects a broader trend among global players, who are relocating production facilities to different countries to achieve economies of scale.
Furthermore, many manufacturers in the catheter industry form partnerships with major medical device distributors to supply their products either globally or within specific countries. Such strategic alliances are widespread and benefit manufacturers by providing marketing licenses, while also reducing liability in cases of product recall or adverse events. A prominent example is the three-year purchasing agreement signed in March 2019 between Coloplast Corporation and Premier Inc., a U.S.-based healthcare company. Through this agreement, Coloplast’s urology products, including catheters, gained access to Premier’s network alliance, which consists of around 4,000 U.S. hospitals. Premier's integrated supply chain, advisory, and data analytics solutions allow Coloplast to secure supply agreements at lower costs, supporting the company’s market presence and reach in the U.S.
Key Urinary Catheters Companies:
Hollister, Inc.
Medtronic PLC
Boston Scientific Corp.
BD (C.R. Bard, Inc.)
Cook Medical
ConvaTec, Inc.
Teleflex, Inc.
Coloplast
Braun Melsungen AG
Medline Industries, Inc.
J and M Urinary Catheters LLC
Order a free sample PDF of the Urinary Catheters Market Intelligence Study, published by Grand View Research.
0 notes
Text
Fortrea, a global leader in clinical research and drug development, is hiring Safety Science Specialists in Bangalore, India. This role offers an exciting opportunity for professionals with expertise in pharmacovigilance and clinical trial case processing. If you have over 3 years of experience in adverse event management and safety reporting, Fortrea provides the ideal platform to advance your career in a dynamic and impactful industry. About Fortrea Fortrea is a leading contract research organization (CRO) known for its dedication to scientific rigor and clinical development. With over 19,000 employees operating across 90 countries, Fortrea partners with pharmaceutical, biotechnology, and medical device companies to bring life-changing therapies to patients worldwide. Fortrea focuses on transforming clinical trials through innovative solutions and ensuring patient safety. Job Role: Safety Science Specialist As a Safety Science Specialist at Fortrea, you will be responsible for managing adverse event reports, ensuring timely submission of safety data, and collaborating with various stakeholders to ensure regulatory compliance. This full-time role is based in Bangalore, India, and plays a critical role in maintaining patient safety during clinical trials. Key Responsibilities Manage the receipt and processing of all adverse event reports reported either spontaneously from any source or solicited from a clinical trial. This includes, but is not limited to: Data entry of safety data onto adverse event database(s) and tracking systems Review of adverse events for completeness, accuracy and appropriateness for expedited reporting. Write patient narratives, Code adverse events accurately using MedDRA. Determine listedness against appropriate label (for Marketed products, if applicable) Identifies clinically significant information missing from the reports and ensures its collection; Prepare follow-up correspondence in consultation with the medical staff, as needed. Ensure case receives appropriate medical review. Ensure all cases that require expediting reporting to worldwide regulatory agencies and other required parties are processed swiftly and appropriately within required timelines. Reporting of endpoints to clients, regulatory authorities, ethics committees, investigators, 3rd party vendor, Partner and Fortrea project personnel, if required, within study specified timelines. Submission of expedited Serious Adverse Event (SAE) reports to clients, Regulatory Authorities, Ethics Committees, investigators, 3rd party vendors, Partners and Fortrea project personnel, if required & as agreed with client during study set-up, within study specified timelines. Assist or contribute to Database reconciliation in liaison with Data Management team or clients. Manage processing of Expedited Safety Reports (ESRs), Periodic Safety Reports (PSRs) and submission, includes but not limited to: Maintenance of adverse event tracking systems Set-up and maintenance of project files and central files for documentation Assist with the reporting of ESRs & PSRs to clients, Regulatory Authorities, Ethics Committees, investigators and Fortrea project personnel, as required, within study specified timelines. Perform quality review or peer review of processed reports and support the Line Management with trends and actions needed. Maintains a comprehensive understanding of Standard Operating Procedures (SOPs), Work Instructions (WI), guidance documents and directives associated with safety management, reporting and pharmacovigilance. Assist in the generation and maintenance of the PSS metrics. Support preparation for client meetings and liaise with clients where appropriate. Assist with the set-up of, and the provision of data to Safety Committees/DSMBs as applicable. Prepare and support coordination of safety study files for archiving at completion of projects. Support Root cause analysis and CAPA plan development for the identified quality issues, as needed.
Support and/or participate in audits and inspections including the preparation, as needed. Demonstrate role-specific Core Competencies and company values on a consistent basis ¨ Build and maintain good PSS relationships across functional units. All other duties as needed or assigned. Qualifications Minimum 3+ years of experience in pharmacovigilance, specifically in clinical trial case processing. Knowledge of adverse event reporting, safety database management, and regulatory timelines. Proficiency in MedDRA coding and preparation of patient narratives. Strong attention to detail and ability to manage multiple timelines and reports. Excellent communication skills and the ability to work collaboratively within cross-functional teams. Knowledge of SOPs and Standard Operating Procedures related to pharmacovigilance and safety management. [caption id="attachment_28499" align="aligncenter" width="640"] Safety Science Specialist Job openings in Labcorp Bangalore[/caption] Job Location Location: Bangalore, India (On-site) How to Apply Interested candidates can apply for the Safety Science Specialist position by visiting Fortrea’s career page
0 notes
Text
Medical Equipment Manufacturers In Chennai
Medical Equipment Manufacturers in Chennai: A Comprehensive Overview
Chennai, often regarded as the healthcare hub of South India, is home to a burgeoning medical device and equipment manufacturing industry. With its strong infrastructure, skilled workforce, and supportive government policies, the city has become a focal point for medical device companies. Among the many companies that operate in this sector, Lifesaverinc Group stands out for its contributions to innovative healthcare solutions.
The Medical Equipment Manufacturing Industry in Chennai
Chennai has become a major center for the production of medical equipment, playing a crucial role in the global supply chain. The city's medical manufacturing industry includes a wide range of products, from diagnostic tools and imaging devices to surgical instruments and patient monitoring systems. Over the years, Chennai has attracted both Indian and international companies, thanks to its strategic location, a robust ecosystem for research and development (R&D), and its skilled labor force.
Key Areas of Medical Equipment Manufacturing in Chennai:
Diagnostic Equipment: This includes imaging equipment such as X-rays, MRI machines, and ultrasound devices, which are essential for diagnosing various medical conditions.
Surgical Instruments: Chennai is home to manufacturers that produce high-quality surgical tools used in operations, including forceps, scalpels, and other precision instruments.
Patient Monitoring Devices: These include devices like ECG machines, oxygen concentrators, and blood pressure monitors, which help healthcare providers track and manage patient health.
Therapeutic Devices: This category includes infusion pumps, respiratory devices, and other medical instruments used for patient treatment.
Implants and Prosthetics: Manufacturers in Chennai also produce a range of implants, including joint replacements, dental implants, and orthopedic devices.
Lifesaverinc Group: A Leading Name in Medical Equipment Manufacturing
Among the many companies contributing to the growth of the medical equipment manufacturing industry in Chennai, Lifesaverinc Group has emerged as a key player. The company is known for its high-quality medical devices that cater to a wide range of healthcare needs, from diagnostics to therapy and rehabilitation.
About Lifesaverinc Group
Lifesaverinc Group is a reputed medical equipment manufacturer based in Chennai that specializes in providing a wide array of advanced healthcare solutions. The company’s mission is to improve patient care and safety by offering innovative, reliable, and cost-effective medical equipment. With a focus on research and development, Lifesaverinc Group constantly strives to introduce new technologies that enhance the performance and efficiency of medical devices.
Product Range
Lifesaverinc Group’s product portfolio includes:
Patient Monitoring Systems: These include advanced cardiac monitors, multi-parameter monitors, and pulse oximeters.
Therapeutic Equipment: The company manufactures high-quality infusion pumps, nebulizers, and other therapeutic devices.
Diagnostic Tools: Lifesaverinc is known for its precision diagnostic instruments, such as ECG machines, ultrasound systems, and blood gas analyzers.
Surgical Instruments: They produce a range of surgical instruments used in various medical fields, including orthopedics, cardiology, and general surgery.
Portable Medical Devices: Lifesaverinc Group is also involved in the production of portable medical devices that allow healthcare professionals to monitor and treat patients in diverse settings, such as home care and emergency situations.
Commitment to Quality and Innovation
Lifesaverinc Group is committed to maintaining the highest standards of quality in its products. The company adheres to international regulatory standards and certifications, ensuring that all products meet stringent safety and quality benchmarks. Additionally, Lifesaverinc Group places a strong emphasis on innovation and continuous improvement, often working closely with healthcare professionals to develop products that address the evolving needs of the medical community.
Other Notable Medical Equipment Manufacturers in Chennai
While Lifesaverinc Group is a key player, several other companies also contribute to the city's reputation as a hub for medical device manufacturing. These companies include:
Trivitron Healthcare: A leader in medical technology, Trivitron offers a wide range of products, from imaging devices to laboratory equipment.
Medtronic: A global medical device manufacturer, Medtronic has a significant presence in Chennai, producing advanced healthcare solutions in fields such as cardiac care, diabetes management, and minimally invasive surgery.
Biotronik: A global medical technology company that manufactures cardiovascular devices, including pacemakers, defibrillators, and stents.
Skanray Technologies: Known for its medical imaging equipment and diagnostic devices, Skanray Technologies has become a key player in the Indian medical equipment manufacturing sector.
The Future of Medical Equipment Manufacturing in Chennai
As healthcare needs continue to grow globally, the demand for medical devices and equipment is expected to rise. Chennai’s role as a hub for medical device manufacturing is likely to expand even further in the coming years. The city’s strong industrial base, combined with advancements in technology and R&D, positions it well to cater to both the Indian market and international markets.
Additionally, the government of India has been providing significant support for the medical device industry, including incentives for research and development, subsidies for manufacturing, and streamlined regulatory processes. These measures are expected to further bolster the growth of the medical equipment manufacturing sector in Chennai.
Conclusion
Chennai’s medical equipment manufacturing industry is a dynamic and rapidly growing sector, with companies like Lifesaverinc Group leading the way in providing innovative, high-quality healthcare solutions. With its strong industrial infrastructure, a focus on innovation, and an increasing demand for medical devices globally, Chennai is poised to remain a key player in the global medical device manufacturing industry for years to come.
As more companies emerge and existing ones expand their capabilities, the future looks promising for both the city and the medical industry at large, making it a critical hub for medical device production in India.
0 notes
Text
Lotus Surgicals A Leader among Top Surgical Companies in India
The demand for high-quality surgical products is the highest ever. Lotus Surgicals stands out in this highly competitive landscape with its quality-driven surgical products. Lotus Surgicals is one of the key players in manufacturing high-quality equipment. Lotus Surgicals has grown to be one prominent Surgical product manufacturer in India. Read through this blog to know what makes Lotus Surgicals special amongst fierce competition in the industry.
#India based global medical device company#healthcare solutions worldwide#Surgical kits#broad range of surgical products worldwide#Suture Companies
0 notes
Text
How Clinical Trial Supplies Are Changing the Landscape of Clinical Research
Clinical Trial Supplies Industry Overview
The global clinical trial supplies market size was estimated at USD 3.97 billion in 2030 and is anticipated to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030. Increasing volume of clinical trial studies coupled with the growing complexity in conduction of these trials are some of the major factors driving the market growth.
Globally, an increase in the prevalence of chronic diseases and the rapidly aging population are expected to drive the growth of R&D of biologics, which is expected to further propel the demand for efficient clinical supplies and contribute to the growth of the clinical trial supplies industry. Furthermore, an increase in the demand for orphan drugs and high investment in the R&D of rare diseases are also expected to contribute toward the development of biologic drugs. Thus, owing to these factors, this segment is likely to witness significant growth during the forecast period.
Gather more insights about the market drivers, restrains and growth of the Clinical Trial Supplies Market
For instance, in 2022, Novartis invested around USD 10 billion in research and development. It also secured 23 approvals in the European Union, Japan, China, and the U.S. for new drugs and rare diseases. The company is also conducting 44 ongoing phase III programs in India with 17 clinical programs running in rare diseases such as atypical hemolytic uremic syndrome (aHUS), Immune thrombocytopenic purpura (ITP), spinal muscular atrophy (SMA), and Lupus Nephritis.
Direct-to-Patients (DTP) is an upcoming segment in the distribution of clinical trial supplies, which is expected to be the future model of distribution. DTP is one of the emerging models that involves delivering drugs to patients directly to create patient-centric trials. This would facilitate fewer visits to the site and reduce the burden on participants. The COVID-19 outbreak has led to the increased adoption of such a model, to continue clinical trial studies with minimum disruption. In addition, patient retention and a diverse pool of patients worldwide are some of the notable reasons that can be attributed to the high adoption of this model.
Browse through Grand View Research's Medical Devices Industry Research Reports.
The S. donor egg IVF services market size was estimated at USD 398.5 million in 2024 and is projected to grow at a CAGR of 4.4% from 2025 to 2030
The global electro-medical and electrotherapeutic apparatus market size was estimated at USD 66.2 billion in 2024 and is projected to grow at a CAGR of 6.4% from 2025 to 2030.
Clinical Trial Supplies Market Segmentation
Grand View Research has segmented the global clinical trial supplies market based on clinical phase, product/service, end-use, therapeutic use, and region:
Clinical Trial Supplies Clinical Phase Outlook (Revenue, USD Billion, 2018 - 2030)
Phase I
Phase II
Phase III
Others
Clinical Trial Supplies Product/Service Outlook (Revenue, USD Billion, 2018 - 2030)
Manufacturing
Storage & distribution
Cold chain based
Non-cold chain based
Supply chain management
Clinical Trial Supplies End-Use Outlook (Revenue, USD Billion, 2018 - 2030)
Pharmaceuticals
Biologics
Medical device
Others
Clinical Trial Supplies Therapeutic Use Outlook (Revenue, USD Billion, 2018 - 2030)
Oncology
CNS
Cardiovascular
Infectious disease
Metabolic disorders
Others
Clinical Trial Supplies Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Key Companies profiled:
Almac Group
Biocair
Catalent Inc.
KLIFO
Movianto
PCI Pharma Services
Sharp Services, LLC
Thermo Fischer Scientific Inc.
Marken
PAREXEL International Corporation
Recent Developments
In February 2023,Catalent completed a USD 2.2 million expansion of its clinical supply facility in Singapore. This expansion has enlarged the site's footprint to 31,000 square feet, providing room for installing 35 new freezers dedicated to ultra-low temperature (ULT) storage.
In January 2023,ASLAN Pharmaceuticals and Thermo Fisher Scientific entered into a partnership to manufacture a high concentration formulation of Eblasakimab for upcoming studies. Thermo Fisher Scientific will contribute its expertise in biologic manufacturing and scale-up capacity to oversee a clinical supply of Eblasakimab for the anticipated Phase 3 studies.
In July 2023,Almac Sciences announced the opening of a custom-built GMP warehouse and dispatch hub at Almac Group’s global headquarters in Craigavon, UK. The facility will support all the manufacturing and lab activities of Active Pharmaceutical Ingredients from development to their commercialization.
Order a free sample PDF of the Clinical Trial Supplies Market Intelligence Study, published by Grand View Research.
0 notes
Text
Medical Device Outsourcing Market to Hit $347.63 Billion by 2032
The global Medical Device Outsourcing Market was valued at USD 129.03 Billion in 2024 and it is estimated to garner USD 347.63 Billion by 2032 with a registered CAGR of 11.57% during the forecast period 2024 to 2032.
Are you looking for the Medical Device Outsourcing Market Research Report? You are at the right place. If you desire to find out more data about the report or want customization, Contact us. If you want any unique requirements, please allow us to customize and we will offer you the report as you want.
The global Medical Device Outsourcing Market can be segmented on the basis of product type, Applications, distribution channel, market value, volume, and region [North America, Europe, Asia Pacific, Latin America, Middle East, and Africa]. The Medical Device Outsourcing Industry 2024 report provides a comprehensive overview of critical elements of the industry including drivers, restraints, and management scenarios.
Download Sample PDF: @ https://www.vantagemarketresearch.com/medical-device-outsourcing-market-2383/request-sample
Top Players
Eurofins Scientific (Luxembourg), Integer Holdings Corporation (U.S.), Pace Analytical Services LLC (U.S.), Intertek Group PLC (UK), Plexus Corp. (U.S.), IQVIA Inc. (U.S.), North American Science Associates LLC (U.S.), Charles River Laboratories (U.S.)
Trending 2024: Medical Device Outsourcing Market Report Highlights:
A comprehensive assessment of the parent Industry
Development of key aspects of the business
A study of industry-wide market segments
Evaluation of market value and volume in past, present, and future years
Evaluation of market share
Tactical approaches of market leaders
Innovative strategies that help companies to improve their position in the market
You Can Buy This Report From Here: https://www.vantagemarketresearch.com/buy-now/medical-device-outsourcing-market-2383/0
Analysis Of The Top Companies, Product Types, and Applications In The Market Report:
This report provides sales, revenue growth rate, and verified information about the major players. Also includes a regional analysis and a labor cost analysis, tables, and figures. It also highlights characteristics such as technological growth. The product type segment is expected to continue to maintain its leading position in the future and capture a significant market share based on sales. This report provides analysis, discussion, forecast, and debate on key industry trends, market share estimates, Industry size, and other information. This report also discusses drivers, risks, and opportunities.
Global Medical Device Outsourcing Market report contains detailed data and analysis on the Medical Device Outsourcing Market drivers, restraints, and opportunities. Experts with market and industry knowledge as well as research experience from regional experts validate the report. The Medical Device Outsourcing Market report provides forecast, historical and current revenue for each industry, region, and end-user segment.
Regions Included
-North America [United States, Canada, Mexico]
-South America [Brazil, Argentina, Columbia, Chile, Peru]
-Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
-Middle East & Africa [GCC, North Africa, South Africa]
-Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
Global Medical Device Outsourcing Market report data will help you make more informed decisions. For example, in relation to prices, distribution channels are means of marketing or identifying opportunities to introduce a new product or service. These results will also help you make more informed decisions about your existing operations and activities.
Read Full Research Report with [TOC] @ https://www.vantagemarketresearch.com/industry-report/medical-device-outsourcing-market-2383
You Can Use The Medical Device Outsourcing Market Report To Answer The Following Questions:
What are the growth prospects of the Medical Device Outsourcing Market business?
Who are the key manufacturers in the Medical Device Outsourcing Market space?
What Forecast Period for Global Medical Device Outsourcing Industry Report?
What are the main segments of the global Medical Device Outsourcing Market?
What are the key metrics like opportunities and market drivers?
The Medical Device Outsourcing Market Insights
Product Development/Innovation: Detailed Information On Upcoming Technologies, R&D Activities, And Product Launches In The Market.
Competitive Assessment: In-Depth Assessment Of Market Strategies, Geographic And Business Segments Of Key Market Players.
Market Development: Comprehensive Information On Emerging Markets. This Report Analyzes The Market For Different Segments In Different Regions.
Market Diversification: Comprehensive Information On New Products, Untapped Regions, Latest Developments, And Investments In The Medical Device Outsourcing Market.
Check Out More Reports
Global Photovoltaic Inverter Market : Report Forecast by 2032
Global Industry 4.0 Market: Report Forecast by 2032
Global RF Over Fiber Market: Report Forecast by 2032
Global Trucking Market: Report Forecast by 2032
Global Anti-Vibration Mounts Market: Report Forecast by 2032
#Medical Device Outsourcing Market#Medical Device Outsourcing Market 2024#Global Medical Device Outsourcing Market#Medical Device Outsourcing Market outlook#Medical Device Outsourcing Market Trend#Medical Device Outsourcing Market Size & Share#Medical Device Outsourcing Market Forecast#Medical Device Outsourcing Market Demand#Medical Device Outsourcing Market sales & price
0 notes
Text
Breaking New Ground in Skin Health: Advanced Dermatology Drug Delivery Systems

The global market for advanced dermatology drug delivery devices is set to experience robust growth, with a projected compound annual growth rate (CAGR) of approximately 8% over the forecast period from 2022 to 2028. In 2022, the revenue generated by this market was around USD 5 billion, and it is anticipated to reach nearly USD 8 billion by 2028.
What are Advanced Dermatology Drug Delivery Devices?
Advanced dermatology drug delivery devices are specialized tools designed to administer therapeutic agents directly to or through the skin for treating various dermatological conditions, including acne, psoriasis, eczema, and skin cancers. These devices range from micro-needling systems and transdermal patches to microdermabrasion tools, lasers, and injectables, offering targeted treatment, improved efficacy, and enhanced patient compliance.
Get Sample pages of Report: https://www.infiniumglobalresearch.com/reports/sample-request/42529
Market Dynamics and Growth Drivers
The growth of the advanced dermatology drug delivery devices market is propelled by several key factors:
Rising Prevalence of Dermatological Conditions: Increased cases of skin disorders and conditions such as acne, eczema, and skin cancers are driving the demand for advanced drug delivery systems that offer efficient, localized treatment.
Technological Advancements: Continuous innovation in drug delivery devices, such as needle-free injectors, transdermal patches, and laser-based systems, is enhancing treatment effectiveness and expanding the range of treatable conditions.
Growing Focus on Minimally Invasive and Painless Treatments: Patients are increasingly seeking minimally invasive options with shorter recovery times and minimal pain, contributing to the adoption of advanced drug delivery systems.
Aging Population and Rise in Cosmetic Dermatology: The global aging population and increased interest in skin rejuvenation and anti-aging treatments are driving the market for drug delivery systems that cater to cosmetic dermatology.
Regulatory Approvals and Expanding Applications: Regulatory approvals for advanced dermatology drug delivery devices are creating new opportunities for market players. Devices that deliver drugs for both medical and cosmetic applications are witnessing increased adoption.
Regional Analysis
North America: North America dominates the market due to a high prevalence of dermatological issues, strong healthcare infrastructure, and significant investment in research and development. The U.S., in particular, is a key market with major players and continuous innovations.
Europe: Europe follows North America in terms of market share, driven by the rising demand for advanced dermatological treatments and favorable government regulations supporting innovative healthcare solutions.
Asia-Pacific: The Asia-Pacific region is expected to witness the highest growth rate due to an increasing patient population, rising disposable incomes, and growing awareness of dermatological treatments in countries like China, Japan, and India.
Latin America, Middle East & Africa: These regions are gradually adopting advanced dermatology drug delivery devices due to increasing healthcare investments and a growing focus on improved patient care, particularly in urban areas.
Competitive Landscape
The market for advanced dermatology drug delivery devices is highly competitive, with key players focusing on product development, collaborations, and strategic acquisitions. Notable companies in the market include:
3M: Known for its innovation in transdermal patches and advanced delivery systems.
Johnson & Johnson: Offers a variety of dermatology drug delivery devices targeting both medical and cosmetic applications.
Merz Pharmaceuticals: Provides a range of injectables and topical drug delivery solutions.
Galderma: Specializes in dermatological solutions, including drug delivery devices aimed at treating skin conditions.
Lumenis: Known for its advanced laser systems in dermatology.
These companies are actively working to enhance their devices' effectiveness, increase patient comfort, and broaden their applications.
Report Overview : https://www.infiniumglobalresearch.com/reports/global-advanced-dermatology-drug-delivery-devices-market
Challenges and Opportunities
While the market for advanced dermatology drug delivery devices is expanding, there are challenges, such as the high cost of advanced devices and concerns regarding side effects. Additionally, stringent regulatory requirements for device approval can pose a barrier to market entry.
However, the market also presents opportunities. The integration of artificial intelligence (AI) and smart technologies in dermatology devices is expected to revolutionize the field, enabling personalized treatment options and real-time monitoring. Furthermore, the expansion of teledermatology and home-based treatment devices offers new pathways for growth, particularly in the context of post-pandemic care preferences.
Conclusion
The global advanced dermatology drug delivery devices market is on a positive growth trajectory, expected to increase from USD 5 billion in 2022 to approximately USD 8 billion by 2028, with a CAGR of 8%. Advances in technology, coupled with a rising demand for minimally invasive treatments and the growth of cosmetic dermatology, are likely to drive market expansion. As the market matures, we can expect further innovations aimed at enhancing patient outcomes, comfort, and treatment accessibility.
Discover More of Our Reports
Surgical Navigation Software Market
0 notes
Text
Fiber Optics Industry 2030 Size Outlook, Growth Insight, Share, Trends
The global fiber optics market was valued at USD 8.76 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6.9% from 2023 to 2030. Fiber optics technology, an advanced innovation, has evolved due to extensive research and development by scientists and engineers worldwide. Many connector manufacturers are expanding their product offerings to provide well-protected and precisely aligned fiber optic channels. For example, in December 2022, Microsoft acquired Lumenisity Limited, a UK-based fiber optics supplier, to strengthen its global cloud infrastructure and meet stringent latency and security demands for its Cloud Platform and Services customers.
The fiber optics market is significantly impacted by the combined efforts of businesses to optimize fiber networks, reducing costs associated with operations and maintenance (O&M) and optical distribution network (ODN) construction. Furthermore, government initiatives supporting fiber optic cable deployment have also bolstered market growth. For instance, in July 2022, the Government of India announced the merger of Bharat Sanchar Nigam Ltd (BSNL), a major telecommunications company, with Bharat Broadband Network Ltd (BBNL), a broadband provider, to construct the country’s largest optical fiber cable (OFC) network. This merger gives BSNL control over BBNL’s extensive optical fiber network, which spans 5.67 lakh kilometers across India, aimed at enhancing connectivity.
Gather more insights about the market drivers, restrains and growth of the Fiber Optics Market
The growth of the fiber optics market has also been driven by the extensive use of undersea fiber optic cables. These cables are crucial for enhancing network capacity, spectral efficiency, and rapid data transmission, and they have facilitated technological advancements. For example, in August 2020, the Indian government launched a submarine optical fiber cable project, connecting the Andaman and Nicobar Islands to the mainland. This initiative aims to provide more affordable and improved telecom connectivity to the Union Territory, supporting online education, telemedicine, banking, e-commerce, and tourism for the islands.
Application Segmentation Insights:
The telecom segment was the largest application sector in the fiber optics market in 2022, with a revenue share of 41.7%. Fiber optics technology has promising growth prospects in telecom due to its increasing use in communication and data transmission. It enables high-speed data transfer over short and long distances, making it ideal for the growing demand for cloud-based applications, Video-on-Demand (VoD) services, and audio-video services. Optical fiber technology supports the construction of long-distance telecommunications links with minimal data loss, a crucial benefit for modern telecom infrastructure.
The medical segment is expected to grow significantly due to the rising adoption of fiber optic devices in healthcare. Stringent regulatory requirements enforced by medical associations and government bodies further promote the use of fiber optics in medical applications, facilitating market expansion in this sector over the forecast period.
In the railway industry, fiber optics plays a critical role in track maintenance, enabling effective and low-cost track repairs, which supports growth in this segment. In addition, fiber optics technology in military and aerospace applications is moderately adopted but is expected to witness considerable growth, with its market share likely to increase during the forecast period.
Order a free sample PDF of the Fiber Optics Market Intelligence Study, published by Grand View Research.
#Fiber Optics Market Share#Fiber Optics Market Analysis#Fiber Optics Market Trends#Fiber Optics Market Growth
0 notes
Text
Fiber Optics Market To Observe Strong Development By 2030 - Cost and Profit Status
The global fiber optics market was valued at USD 8.76 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6.9% from 2023 to 2030. Fiber optics technology, an advanced innovation, has evolved due to extensive research and development by scientists and engineers worldwide. Many connector manufacturers are expanding their product offerings to provide well-protected and precisely aligned fiber optic channels. For example, in December 2022, Microsoft acquired Lumenisity Limited, a UK-based fiber optics supplier, to strengthen its global cloud infrastructure and meet stringent latency and security demands for its Cloud Platform and Services customers.
The fiber optics market is significantly impacted by the combined efforts of businesses to optimize fiber networks, reducing costs associated with operations and maintenance (O&M) and optical distribution network (ODN) construction. Furthermore, government initiatives supporting fiber optic cable deployment have also bolstered market growth. For instance, in July 2022, the Government of India announced the merger of Bharat Sanchar Nigam Ltd (BSNL), a major telecommunications company, with Bharat Broadband Network Ltd (BBNL), a broadband provider, to construct the country’s largest optical fiber cable (OFC) network. This merger gives BSNL control over BBNL’s extensive optical fiber network, which spans 5.67 lakh kilometers across India, aimed at enhancing connectivity.
Gather more insights about the market drivers, restrains and growth of the Fiber Optics Market
The growth of the fiber optics market has also been driven by the extensive use of undersea fiber optic cables. These cables are crucial for enhancing network capacity, spectral efficiency, and rapid data transmission, and they have facilitated technological advancements. For example, in August 2020, the Indian government launched a submarine optical fiber cable project, connecting the Andaman and Nicobar Islands to the mainland. This initiative aims to provide more affordable and improved telecom connectivity to the Union Territory, supporting online education, telemedicine, banking, e-commerce, and tourism for the islands.
Application Segmentation Insights:
The telecom segment was the largest application sector in the fiber optics market in 2022, with a revenue share of 41.7%. Fiber optics technology has promising growth prospects in telecom due to its increasing use in communication and data transmission. It enables high-speed data transfer over short and long distances, making it ideal for the growing demand for cloud-based applications, Video-on-Demand (VoD) services, and audio-video services. Optical fiber technology supports the construction of long-distance telecommunications links with minimal data loss, a crucial benefit for modern telecom infrastructure.
The medical segment is expected to grow significantly due to the rising adoption of fiber optic devices in healthcare. Stringent regulatory requirements enforced by medical associations and government bodies further promote the use of fiber optics in medical applications, facilitating market expansion in this sector over the forecast period.
In the railway industry, fiber optics plays a critical role in track maintenance, enabling effective and low-cost track repairs, which supports growth in this segment. In addition, fiber optics technology in military and aerospace applications is moderately adopted but is expected to witness considerable growth, with its market share likely to increase during the forecast period.
Order a free sample PDF of the Fiber Optics Market Intelligence Study, published by Grand View Research.
#Fiber Optics Market Share#Fiber Optics Market Analysis#Fiber Optics Market Trends#Fiber Optics Market Growth
0 notes