#Hypoplastic Nasal Bone
Explore tagged Tumblr posts
Text
UNOSSIFIED NASAL BONE / ABSENT NASAL BONE IN SCAN – WHAT DOES IT MEAN?
Dr. Zohra Ahmad Fetal Medicine Specialist Institute of Human Reproduction
The nasal bone is a paired bone forming the anterior wall of the nasal cavity and is largely responsible for the shape of the nose. The nasal bones are variable in size and form in different individuals. The first trimester scan is an important tool of risk assessment for fetal aneuploidies. The presence or absence of fetal nasal bone is an important secondary marker for risk allocation.
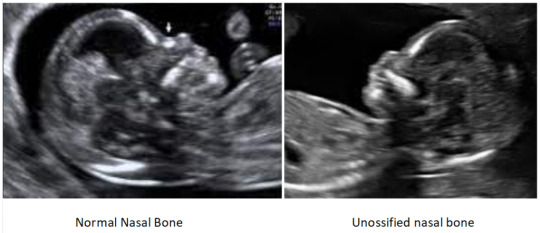
1. What is Unossified / Absent Nasal Bone?
It refers to a finding on ultrasound that nasal bone is not seen or appears small. It is due to delayed ossification of the nasal bone. In the report, it may be mentioned as either unossified, absent, or hypoplastic nasal bone. Unossified and absent nasal bone means that nasal bone is not seen while Hypoplastic nasal bone means nasal bone is seen but very small.
2. Causes of Absent Nasal Bone
It can be due to hypoplasia or delayed ossification of the nasal bone. It can be due to underlying chromosomal abnormality or in 1-2% of normal babies as well.
3. How Common Is Absent Nasal Bone?
It is found in 1-2% of normal babies. Studies have shown that in about 85-90% cases of the isolated absent nasal bone, babies will be normal. However, in about 10-12% of cases, babies may have chromosomal problems including down’s syndrome .
4. When can Unossified Nasal bone be detected?
Unossified nasal bone in the first trimester
It is generally detected in the first trimester that is 11-14 weeks in a special ultrasound scan called First trimester anomaly scan/ First trimester scan or NT/NB scan. It must be done by an expert radiologist or fetal medicine specialist with special training, using a high end ultrasound machine with baby in appropriate position.
Unossified nasal bone in second trimester/20 weeks scan or third trimester
Sometimes if the first trimester scan has not been done, unossified nasal bone can also be first detected later in the second or third trimester. The significance of absent nasal bone in different trimesters is the same.
To read more, Click Here
Consult the best IVF Experts click here —
Best IVF center in Guwahati, Best IVF center in Kolkata
#1st Trimester Scan#Absent Nasal Bone#amniocentesis#Hypoplastic Nasal Bone#Unossified Nasal Bone#First Trimester Scan
0 notes
Text
Facial Reconstruction
Anonymous said to @ask-drferox: Theoretically, is it possible to reconstruct an animal's face, such as a pug, to where they can breathe like normal? I have a pretty good guess that that would be extremely complicated and very expensive, but I thought I'd ask someone that professionally deals with animals in a medical environment
If you mean with selective breeding over multiple generations, that’s certainly possible to do and the way I hope these brachycephalic breeds will go in the future.
If you’re asking whether there are surgical methods to correct the extreme brachycephalic morphology we have bred into dogs, then the answer is ‘sort of but not quite’.
Brachycephalic Airway Syndrome, as we see in breeds like the pug, French bulldog and Boston Terrier, has a number of features all occurring together. There is more information, and some videos, in this post.
The thing is, a brachycephalic dog skull isn’t just a normal dog skull that’s been squished up. Sure, the skull has a shortened length, but the amount of soft tissue hasn’t changed.
Consider these CT images of a brachycephalic dog, and a normal dog skull side by side from the British Veterinary Association.

There is no question that the air ways (black = air) are very different between these dogs. Grey is flesh, and white is bone, and you can see that the tongue is shorter and thicker, as is the soft palate.
If you’re not used to looking at CT scans, here’s a crude color outlined one. I know it looks like it was made in MS Paint, because it was, as I never claimed to be an artist.

I’ll ask you to pay particular attention to the airway - all the black running from mouth and nose down to the trachea on the left.
The lower dog has a airway all the way from its nose, through the nasal turbinates, over the back of the soft palate, past the epiglottis and into the trachea.
The brachycephalic dog does not have any nasal opening to speak of, minimal space in the nasal turbinates, a very thickened soft palate that goes too far back, and even a small trachea. And that’s not to mention the skeletal differences, particularly with the brain case.
In terms of surgical reconstruction, we can:
Open the nostrils (treat stenotic nares)
Shorten the soft palate
Remove any everted laryngeal saccules (bulbs of flesh that get sucked inside out at the larynx)
Remove nasal turbinates (a relatively advanced procedure)
And this makes things better, certainly, but it’s not completely normal, and there still isn’t a technique for correcting the hypoplastic trachea - a wind pipe to the lungs which is two sizes too small.
Oh, and there’s a risk of tissue swelling after surgery, as there always is, but swelling in the airways of a dog with already restricted airways is distinctly not good.
So there are methods, but this is a lot of surgery for an incomplete result, so most of the veterinary proffesion is advocating to just... breed better faces for breathing.
449 notes
·
View notes
Text
Ultrasound Diagnosis of Exomphalos (Omphalocele): Differentiating Exomphalos from Normal Physiologic Gut Herniation and Gastroschisis
Authored by: Jitendra Parmar*

Introduction
An Exomphalos, also known as omphalocele is a congenital midline abdominal wall defect at the base of umbilical cord insertion with herniation of gut and/or liver or occasionally other contents, out of the fetal abdomen. Many chromosomal anomalies are often associated with this embryologic defect [1-6]. A gastroschisis is a ventral abdominal wall defect, almost always to the right of the umbilicus from which it is separated by thin skin bridge, and contains abdominal viscera, most commonly small bowels, stomach and gonads without coverings [7,8]. During first trimester sonogram, normal physiologic herniation of the fetal bowel seen oftenly between 8 and 12 weeks gestational [9]. With careful detail scanning, the sonographer may be able to differentiate normal physiologic gut herniation from early identification of exomphalos and gastroschisis [9,10]. This case report describes a ultrasound diagnosis of exomphalos associated with hypoplastic nasal bone and increased nuchal translucency and discusses how to differentiate exomphalos from normal physiologic gut herniation and gastroschisis.
Case Report
A 27-year-old woman having her first pregnancy, at the 12 weeks + 4 days was referred to the ultrasound (US) department for a routine US without any pertinent past medical history. Transabdominal US, followed by transvaginal US was performed using a 2D (two dimensional) and 3D (three dimensional) ultrasound equipment (Voluson E 10 BT 17; Probe 3.5 MhZ). 2D US showed a single live intrauterine pregnancy with gestational age of 12 weeks + 4 days. A well defined rounded solid homogenous mass lesion, measuring ~ 16 x 15 mm seen at the umbilical cord insertion site (Figure 1). By using Doppler US and 3D US, an exomphalos containing the liver was identified (Figure 2). US also showed increased nuchal translucency, 3.9 mm and a hypoplastic nasal bone (Figure 3). Ductus Venosus shows increased PI (3.06) with reversal of ‘a’ wave (Figure 4). Detailed US for possible associated malformations could not find any other structural defects. The detail ultrasound parameters are described in Table 1. As a result of the sonographic findings the patient terminated the pregnancy, and no follow-up was able to be obtained. It is unknown whether the patient had the products of conception tested for aneuploidy.
BPM: Beats Per Minute
Mm: Millimetre
PI: Pulsatility Index
Discussion
An exomphalos is an encapsulated ventral wall defect containing liver and/or bowel and occurs because of incomplete rotation of bowel returning into the abdominal cavity. The translucent sac, which is composed of the Wharton jelly, amnion and the peritoneum, contains the abdominal viscera with well visualization of the radiation of the umbilical vessels onto the sac wall. In ~ 50% of the cases, the herniated bowel accompanied with the liver, spleen, ovaries or the testes [7,8]. According to contents of herniation, exomphalos may be categorized as those containing both liver and bowel (extracorporeal liver) and those containing only bowel (intracorporeal liver). Intracorporeal exomphalos can only be reliably diagnosed after 12 weeks, because of the difficulty in distinguishing it from physiologic midgut herniation [11,12]; Such exomphalos have a high rate of foetal chomosomal abnormalities [13,14]. Extracorporeal exomphalos can be diagnosed as early as 9 to 10 weeks, and these may also be associated with fetal chromosomal abnormalities and other birth defects [15-17].
Exomphalos frequently have been associated with other conditions, such as congenital heart disease, cleft palate, musculoskeletal abnormalities, intrauterine growth restriction and dental malocclusion. The incidence of associated chromosomal abnormalities is 10-40% that include trisomies 12, 13, 15, 18, and 21.3 [18-22]. A featus found to have an exomphalos has a high mortality rate because of its coexistance with multiple anomalies [9,23]. However, because of improvement in the parenteral nutritional, surgical and the anesthetic management techniques, the survival of the exomphalos cases has increased from 60% during the 1960s to more than 90% at present [7,24].
The diagnosis of exomphalos can be made by ultrasound in 1st trimester; however, two close differentials; gastroschisis and physiologic midgut herniation, need to be excluded by careful and detail imaging. One of the most important and specific finding to differentiate exomphalos from midgut herniation is the visualization of herniated liver associated with gut loop within the herniated sac. Few other significant secondary features that differentiate physiologic midgut herniation from exomphalos: by 12 weeks gestational age, midgut herniation descends back into the abdominal cavity; exomphalos have homogenous appearance, while midgut herniation tends to have heterogenous appearance; exomphalos presents as a large circular shaped lesion measuring more than 7 mm, while midgut herniation presents as a small spherical shaped lesion measuring ~ 4 to 7 mm. Gastroschisis occurs later because the anterior abdominal wall defect before 16th week is very small and anterior abdominal wall muscles and peristaltic waves are visible only in 14th week. Intestinal convolutions pass through a small defect (<1 cm), which is localized to the right of the normal umbilical cord insertion and float freely in the amniotic fluid with no membrane covering the content [25].
In cases with difficulty to identify the exact pathology and to demonstrate the co-existing pathologies, the 3D USG may be helpful. Besides its help as a diagnostic tool, the 3D ultrasound may help the family to understand and realize the situation. The decision process, genetic counselling, perception of the situation and its importance, future planning and the state of the management will be affected by the thorough comprehension and concern of the family. First trimester scanning of the anterior abdominal wall is crucial and at most important. It is important to determine the gestational age of the featus being examined. The sinologist must examine the echogenicity of the herniation at the base of the umbilicus and make a determination as to heterogeneity or homogeneity and whether covering present or absent. Measurements must be taken at the base of the umbilical herniation, if the herniation is too large to be considered a physiologic midgut herniation. As the exomphalos is not usually an isolated finding, a survey must be used to further identified other potential abnormalities.
#open access journals#juniper publisher#global journal of reprodutive medicine#peer review journals#GJORM in juniper publishers
0 notes
Text
Dentists Make a Difference in Pediatric Sleep Apnea Cases
The following article Dentists Make a Difference in Pediatric Sleep Apnea Cases Find more on: EllyMackay.com
Early intervention can mitigate problems such as bedwetting, and dental sleep medicine practitioners are in the perfect place to screen children and treat those referred by physicians with oral appliances.
By Yoona Ha
Diana Batoon, a mother of two from Scottsdale, Ariz, wanted to help her children beat sleep apnea. As a general dentist who’s practiced for over 24 years, Batoon’s experience with following her children’s treatment journey inspired her to learn more about what contributes to upper airway collapse during sleep.
“It was a wakeup call for me when a pulmonologist recommended antidepressants as the treatment option for my 5-year-old son; I didn’t want to start medicating a child at such a young age,” says Batoon, DMD, who practices at Bonita Dental. “Turns out, when we address the breathing habits of the child using behavioral interventions combined with appliance therapy, we could greatly improve the quality of sleep.”
Seeing how her child’s sleep patterns, comorbidities such as eczema, and even performance in school drastically improved, Batoon was inspired to incorporate dental sleep medicine into her practice. Turns out, Batoon is not alone in her experience with children who have sleep apnea.
Aaron Johnson, DDS, a general dentist at Johnson Dental Excellence, also saw his child struggle to breathe during sleep.
“By educating myself further on how dentists can offer treatment for sleep apnea, I realized that sleep apnea is only the end stage of a lifetime of disordered breathing,” says Johnson. “It became my passion to treat these children early so hopefully things like sleep apnea don’t develop later in life.”
Around 90% of children who have sleep-disordered breathing (SDB), which includes obstructive sleep apnea (OSA), are undiagnosed.1 Their symptoms are often mistaken for behavioral issues like attention-deficit hyperactivity disorder.
“It’s not something that can be left alone as something that your child would grow out of,” Batoon says. “As a parent and a dentist, I always let the parents know that early intervention of OSA can change your child’s life for the better.”
Myths Surrounding Symptoms
It’s a common misconception that snoring is the only tell-tale sign of a child who has OSA. On the contrary, a child with OSA can have noisy breathing, restlessness during sleep, and pauses in breathing, which results in fragmented sleep. Other signs and symptoms can develop as a result including bedwetting, sleepwalking, growth impairment, hormonal abnormalities, type 2 diabetes, obesity, and metabolic problems.
Snoring is not always present, particularly if the airway turbulence happens in the lower section of the upper airway behind or below the tongue (versus at the back of the soft palatal area around the uvula).
Joseph Yousefian, DMD, MS, DABO, DABDSM, says dental providers could play a key role in early intervention by understanding how pharyngorofacial disorders can also lead to sleep-disordered breathing in children.
“Clinicians need to evaluate the physiological capability of the upper airway and breathing patterns,” says Yousefian, who practices at Yousefian Orthodontics. “Once a risk is determined, they need to make a referral for proper diagnosis.”
In the past, he adds, dentists have been perceived to be responsible for the health of just the teeth and gums in patients, but a growing number are also screening for potential airways issues.
Stacy Ochoa, DDS, DABDSM, a general dentist at Precision Dental Care, says, “I’d like to see more pediatric patients discussed as part of our conversations about OSA because early intervention is so critical in the development of children.”
Seeing firsthand how her father and her children struggled with OSA and their adherence to CPAP therapy, Ochoa addresses the awareness gap via a membership-only study club for dentists who are interested in pediatric dental sleep medicine. Dubbed “ASAP Pathway,” membership includes on-demand educational videos, a private Facebook group of peers, documents, live webinars, case reviews, and hands-on sessions. “Treating children can be a dynamic process as unlike adults, they’re growing and developing, and it’s exciting to see the growing interest among dentists who want to help their patients grow in the right direction,” Ochoa says.
Echoing Ochoa’s concerns, Yousefian highlights that untreated sleep apnea can become a massive threat to a child’s development. “What we’re really worried about is the neurological impact of the upper airway and sleep-related issues,” he says.
Screening Pediatric Patients
Emily Varsanik, DDS, owner of Davison Dental, says it’s important for dentists to understand that OSA isn’t just an anatomic disease caused by a narrow upper airway. Instead, she looks for multiple factors such as enlarged tonsils, obesity, and genetic conditions that make some groups of children more susceptible than others.
“We not only look at the child’s jaw development and behavior in the office [on whether they display signs of excessive daytime sleepiness] but also ask the parents a series of questions that can clue us in on what’s going on when the child is asleep—this is how screening questionnaires are helpful,” Varsanik says.
A meta-analysis found the Pediatric Sleep Questionnaire (PSQ) to have the best diagnostic accuracy in identifying pediatric sleep-disordered breathing,2 so the PSQ can be a good first step for dental practices to incorporate.
What’s more, screening isn’t limited to questionnaires and clinical predictive tools. For instance, dentists can look for abnormalities in the anatomy and neuromuscular function of the upper airway such as underdeveloped (hypoplastic) maxilla and mandible, which are also commonly found in patients with OSA.
If a child is deemed high risk for sleep-disordered breathing, the next step is to refer to a physician for a definitive diagnosis. And when physicians refer pediatric patients to dentists for treatment, dentists are in a unique position to provide certain therapies.
Therapy Options for Children
Although the standard treatment for OSA has been CPAP therapy, appliance therapy has emerged as a viable treatment option for patients who either have mild OSA or who have trouble tolerating or refuse CPAP. The American Association of Orthodontists (AAO) states, “Possible negative craniofacial consequences of longitudinal usage of PAP on the developing facial structures should be considered.”3
Adenotonsillectomy is frequently leveraged as a surgical treatment option for children whose tonsils obstruct their airway, but some develop a recurrence during adolescence,4 which illustrates how dynamic the condition can be in growing children.
Oral appliances for children have characteristics different from those that treat adult patients. “Unlike adult oral appliances, pediatric appliances take into account that the child is still growing and are designed to move or make [the mandible] develop forward [for patients with mandibular retrusion],” Varsanik says.
The AAO states, “The use of mandibular advancing devices may be prescribed by the physician, and this prescription is not predicated solely on Angle’s classification of occlusion. In this instance, treatment with an oral device is directed primarily toward airway maintenance and less so with dentofacial orthopedic management. Careful monitoring of facial growth and development is important during this time.”3
According to Yousefian, ensuring that the appliance isn’t creating what’s called the “headgear effect” is key. Headgears have been used to correct a misaligned bite by controlling jaw growth. But this control can have a broader impact in the child’s mouth and distort the growth pattern of bones and muscles beyond the upper jaw. As a result, these underdeveloped jaws and dental arches can narrow a child’s facial structure, which in turn overcrowd the tongue and soft tissues in the mouth and can increase the child’s risk of OSA.
“The good news is that the headgear effect is reversible; instead we’re shifting to an approach that expands the upper arches, nasal cavities, and the lower jaw to support the upper airway for the treatment of OSA, known as teledontics,” says Yousefian, who has written a case report on teledontics, appliance therapy that expands the jaw, in an adult patient5 and is editing a book, to publish later this year, on teledontics and telegnathics (jaw surgery) as a treatment for pediatric sleep apnea.
Yousefian says teledontics can be started as a treatment modality for children as young as 3-years-old. Teledontics also can complement telegnathics for sleep apnea. Although the AAO cautions, “Orthognathic surgery usually is not indicated until craniofacial growth is completed….After considering the potential benefits and risks involved (including the need for later surgical revision), orthognathic or telegnathic surgery could be considered.”3
Early Intervention Transforms Lives
The payoff of early intervention with sleep-disordered breathing can be huge. Just ask Johnson and Yousefian, who have also treated their children with appliance therapy.
Successful treatment can look different, depending on how OSA has impacted the patient’s life. Johnson recalls treating an 11-year-old patient who worried about sleeping over at a friend’s house, for fear of wetting the bed. Now that patient can enjoy sleepovers. “That child just turned into a totally different and more confident kid,” Johnson says.
Varsanik shares a story of how grateful her patient’s parents were for helping them understand how OSA was impacting their child’s life. “Being able to give them the clarity on the root cause was extremely powerful,” she says.
In a world with millions of undiagnosed sleep apnea patients, progress in treatment and early interventions can be both promising and daunting, Yousefian says. “I’ve suffered through this issue when my child was suffering in school and dying right in front of my eyes,” Yousefian says. “To help him, I tried to understand the science of sleep medicine and combine it with the science of orthodontics and that’s how I got to where I am today.”
Yoona Ha is a freelance writer and healthcare public relations professional.
REFERENCES
Honaker SM, Meltzer LJ. Sleep in pediatric primary care: A review of the literature. Sleep Med Rev. 2016 Feb;25:31-9.
De Luca Canto G, Singh V, Major MP, et al. Diagnostic capability of questionnaires and clinical examinations to assess sleep-disordered breathing in children: a systematic review and meta-analysis. J Am Dent Assoc. 2014 Feb;145(2):165-78.
American Association of Orthodontists. White paper: Obstructive sleep apnea and orthodontics. 2019 March 15. Available at www1.aaoinfo.org/wp-content/uploads/2019/03/sleep-apnea-white-paper-amended-March-2019.pdf
Guilleminault C, Partinen M, Praud JP, et al. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr. 1989 Jun;114(6):997-9.
Yousefian J, Brown M. Teledontics and telegnathic surgery for treatment of obstructive sleep apnea syndrome utilizing surgically assisted mandibular expansion (SAME). EC Dental Science. 2017;14.1:31-9.
from Sleep Review https://www.sleepreviewmag.com/sleep-disorders/breathing-disorders/obstructive-sleep-apnea/dentists-pediatric-sleep-apnea/
from Elly Mackay - Feed https://www.ellymackay.com/2020/03/15/dentists-make-a-difference-in-pediatric-sleep-apnea-cases/
0 notes