#HomogeneousMixtures
Explore tagged Tumblr posts
Text
youtube
#ChemistrySolutions#ScienceInAction#MoleculesAtWork#SolventAndSolute#SolutionScience#ChemicalMixtures#LearnChemistry#HomogeneousMixtures#ChemistryExplained#SolutionsInChemistry#MolarityMatters#ScienceMadeSimple#ChemicalReactions#ExploringSolutions#SolvingChemistry#Youtube
0 notes
Text
What is a Heterogeneous and Homogeneous Mixtures?
While learning Chemistry, students will come across mixtures that contain, the combination of one or more elements or substances. And thus, for understanding these mixtures, and find out their various parameters such as Melting and Boiling Point. Students must have a good understanding of Heterogeneous and Homogeneous mixtures, and their differences, while also listing out their examples.
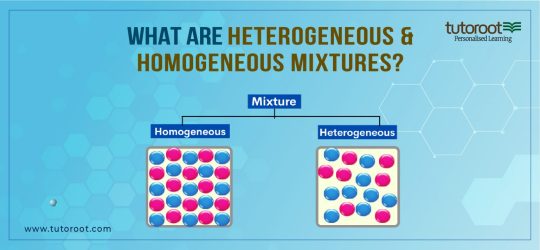
What is a Heterogeneous Mixture?
A heterogeneous Mixture is defined as the type of mixture where there is no uniformity, as different types of components are mixed in it. However, unlike other mixtures, the components in the Heterogeneous and Homogeneous mixture can be observed or detected quickly, thus making it easier to separate the components because of no uniformity. Some of the popular examples of Heterogeneous Mixtures are Suspensions and Colloids, as well as Soil.
What is a Homogeneous Mixture?
Another type of mixture, where the components are uniformly distributed, is referred to as the Homogeneous Mixture. Moreover, the mixture cannot be generally observed or detected as the components cannot be identified or separated because of the uniformity. Thus, in reality, the components in the mixture, have different compositions. Alloys and Solutions are some of the common examples of the Homogeneous Mixture.
If you are facing trouble studying any other topics in Chemistry subject, then you can join the Online Interactive Classes offered by the Tutoroot platform. Through these courses, the students can access various benefits such as Exclusive Doubt Sessions, Customizable Time Tables, and Access to Best Educational Material, Top Staff Guidance, and many more.
0 notes
Text
What are the Differences between Heterogeneous and Homogeneous Mixtures?
There are multiple differences between Homogeneous and Heterogeneous mixtures, which we are going to explain briefly here in this section. Differences such as,
Heterogeneous Mixture
Definition: A mixture is made of different types of components and is not uniform.
Visibility: These mixtures can be easily viewed through the naked eye.
Particle Size: The size of the particles in this mixture is very large.
Physical Properties: All the components in the Heterogeneous mixture have different physical properties.
Examples: Stone pieces, salt and water mixture, grains, pulses, etc.
Homogeneous Mixture
Definition: While this type of mixture is observed when the different components have a uniform composition.
Visibility: Whereas the Homogeneous mixture cannot be observed or detected through the naked eye.
Particle Size: However, inhomogeneous mixture, the particle size is limited to the molecular or atomic level.
Physical Properties: And all the components in a Homogeneous mixture have similar physical properties.
Examples: Air, salt, and sugar solution.
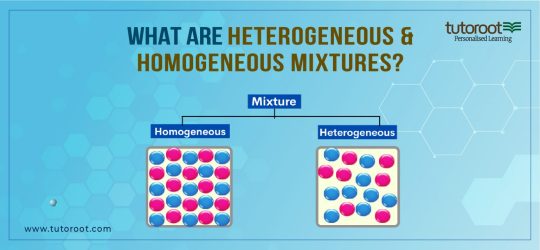
Students must have a good understanding of Heterogeneous and Homogeneous mixtures, and their differences, while also listing out their examples. And here in the article below, you can all abohese mixtures in much more detail. If you are facing trouble studying any other topics in Chemistry subject, then you can join the Online Interactive Classes offered by the Tutoroot platform. Through these courses, the students can access various benefits such as Exclusive Doubt Sessions, Customizable Time Tables, and Access to Best Educational Material, Top Staff Guidance, and many more.
0 notes