#Home Testing Kits Market Analyzer Industry
Explore tagged Tumblr posts
Text
#Home Testing Kits Market Size#Home Testing Kits Market Share#Home Testing Kits Market Growth#Home Testing Kits Market Trends#Home Testing Kits Market Forecast Analysis#Home Testing Kits Market Segmentation#Home Testing Kits Market 2024#Home Testing Kits Market CAGR#Home Testing Kits Market Analyzer Industry
0 notes
Text
Blood Bank Equipment Manufacturers In Gurugram
Gurugram, a leading industrial hub in India, is home to a growing number of manufacturers specializing in blood bank equipment. These companies play a crucial role in ensuring the availability of safe blood for medical use by designing, producing, and supplying advanced technologies that streamline the blood donation, storage, and transfusion processes.
Key Equipment Manufactured
Blood bank equipment is integral to maintaining blood safety and quality. The main types of equipment produced include:
Blood Collection Devices: These devices are designed to ensure safe, efficient blood collection. They include blood bags, needles, and collection sets that minimize contamination risks and enhance donor comfort.
Blood Storage Systems: Manufacturers produce refrigerators, freezers, and blood storage cabinets that maintain the optimal temperature for preserving blood products such as whole blood, red blood cells, platelets, and plasma.
Blood Bank Centrifuges: Used for separating components of blood, centrifuges are crucial for separating plasma, platelets, and red blood cells. They ensure efficient processing and storage of blood products.
Blood Bank Analyzers: These advanced devices help in testing blood for compatibility, blood type, and screening for infections. They play a vital role in ensuring the safety of transfusions.
Blood Warmers: Used to safely warm blood before transfusion, these devices are essential for preventing hypothermic complications during critical procedures.
Sterilization Equipment: Ensuring the sterilization of blood collection kits and storage containers is vital for maintaining a sterile environment and preventing infections.
The Importance of Advanced Technology in Blood Banking
The healthcare industry is continuously evolving, and blood banks are no exception. The equipment used in blood banks must comply with rigorous standards for safety, precision, and efficiency. Manufacturers in Gurugram focus on creating cutting-edge technologies that improve the overall performance and safety of blood banks.
Automation and Integration: Automation has revolutionized the blood bank industry by streamlining workflows, reducing human error, and improving efficiency. Manufacturers are increasingly integrating advanced IT solutions, allowing for real-time tracking of blood supplies and enhancing inventory management.
Quality Control: Blood banks rely on equipment that can withstand regular use while maintaining the highest standards of performance. Manufacturers in Gurugram adhere to strict quality control procedures to ensure the longevity and accuracy of their products.
Market Demand and Growth
The demand for blood bank equipment in India, particularly in Gurugram, is growing due to the expansion of healthcare facilities, both public and private. The increase in surgeries, trauma care, and cancer treatments has contributed to a heightened need for blood products, which directly impacts the demand for reliable and efficient equipment.
In addition to the domestic market, manufacturers in Gurugram also export their products internationally, making India an important player in the global blood bank equipment industry.
Conclusion
Blood bank equipment manufacturers in Gurugram are at the forefront of providing essential technologies that support healthcare systems and blood transfusion services. By combining innovation, quality, and adherence to international standards, these manufacturers contribute significantly to the availability of safe and effective blood products, ultimately saving lives.

Blood Bank Equipment Manufacturers In Gurugram
0 notes
Text
Comprehensive Study on the Multiplex Assay Market
The global multiplex assay market size is expected to reach USD 3.87 billion by 2030, registering a CAGR of 14.77% from 2023 to 2030, according to a new report by Grand View Research, Inc. The growing prevalence of infectious diseases is boosting the usage of multiplex assays in clinical trials. For instance, according to the American Cancer Society in 2021, 1.9 million cancer cases were reported along with 6,08,570 deaths in the United States. Multiplex assay aids in the diagnosis of cancer and reduces unnecessary invasive producers. Thus, growing chronic diseases can boost the necessity of these assays and is anticipated to fuel market growth. The increasing adoption of personalized medicine in recent years is another key factor driving the growth.
Personalized medicine is a precise medicine for an individual patient to attain improved treatment options based on the body type and disease risk. These assays ensure to be highly beneficial for the comprehensive diagnosis of personalized medicines. For instance, according to an article published in the MDPI journal in 2020, multiplex immunoassay provides a complete picture of the disease and pathways involved in Rheumatoid Arthritis (RA) and simultaneously analyzes multiple proteins that can yield biomarker signatures of RA subtypes to enable patients to benefit from personalized medicine. During the COVID-19 pandemic, multiplex testing continued to be an essential tool for healthcare professionals in effectively managing the spread of COVID-19. In addition, recently, several private companies have also developed novel versions of multiplex assays.
For instance, in Sept 2020, LabCorp launched an at-home collection version for diagnosis of influenza A/B, COVID-19, and respiratory syncytial virus single-panel tests. Furthermore, the advantages of multiplex assay over singleplex and traditional assay can boost the industry growth in coming years. Several benefits offered by these automated tests include microsampling capability, numerous arrays measured in a single trial, quicker results, high operational efficiency, easy operations, and reduced labor expenses. Thus, the industry has witnessed incremental growth in 2021 and is anticipated to have a similar trend during the forecast period. Moreover, increasing validation of the biomarkers in molecular & protein diagnostics and the rising need for high-throughput and automated systems are expected to create lucrative opportunities during the forecast period.
Multiple biomarker analysis has a wide range of applications in the area of infectious diseases, neurodegenerative diseases, autoimmune diseases, and cancer. Numerous biomarkers are being discovered, and there is a high possibility of the development of novel diagnostics. For instance, Cipla launched RT Direct multiplex PCR kit that delivers quicker results for COVID-19. Such developments can increase the market penetration of multiplex assay during the forecast period. The technological advancements and automation in the multiplex assay, improve the efficiency and speed of delivering accurate results. For instance, in July 2020, Luminex provided xMAP the INTELLIFLEX system to discover novel applications, including the exclusive ability to detect multiple antibodies in a single serology test.
In May 2022, Vela Diagnostics launched a highly automated multiplex PCR-based test for detecting antimicrobial resistance genes and UTI pathogens. Hence novel technological developments can fuel the industry growth in the near future. However, the high cost of equipment can restrict the usage of multiplex assays by researchers and manufacturers in mid and low-income countries. Also, the quality control standards and regulations are more stringent for multiplex assay compared to singlex assay, which can impede the industry growth during the forecast period.
Multiplex Assay Market Report Highlights
By product, the consumables segment held the dominant share in 2022. This is due to the recurring purchase of consumables along with the rise in the number of diagnostic tests
The protein multiplex assay type segment dominated the industry in 2022 due to the increasing focus on proteomics studies for biomarker research and clinical diagnostics
The flow cytometry technology segment held a larger share in 2022. Constant efforts by various companies to launch novel and technological advanced flow cytometer is the key factor driving the segment
The research & development application segment held the largest share in 2022 due to the utilization of these assays in clinical & preclinical stages to evaluate toxicity, immunotherapy success, and drug response biomarkers
The pharmaceutical & biotechnology companies end-user segment led the industry in 2022 due to a rise in pharma & biotech partnerships and collaborations to increase the multiplexing capabilities
North America dominated the global industry in 2022 due to the growing R&D activities as a result of the increasing prevalence of chronic diseases
Asia Pacific is estimated to register the fastest CAGRfrom 2022 to 2030 due to the rising number of hospitals in emerging countries, the developing R&D sector, and the high demand for healthcare infrastructure in the region
Multiplex Assay Market Segmentation
Grand View Research has segmented the global multiplex assay market based on product, type, technology, application, end-user, and region:
Multiplex Assay Product Outlook (Revenue, USD Million, 2018 - 2030)
Consumables
Instruments
Software
Multiplex Assay Type Outlook (Revenue, USD Million, 2018 - 2030)
Protein Multiplex Assays
Planar Protein Assays
Bead-based Protein Assays
Nucleic Acid Multiplex Assays
Planar Protein Assays
Bead-based Protein Assays
Cell-based Multiplex Assays
Multiplex Assay Technology Outlook (Revenue, USD Million, 2018 - 2030)
Flow Cytometry
Fluorescence Detection
Luminescence
Multiplex Real-time PCR
Other Technologies
Multiplex Assay Application Outlook (Revenue, USD Million, 2018 - 2030)
Research & Development
Drug Discovery & Development
Biomarker Discovery & Validation
Clinical Diagnostics
Infectious Diseases
Cancer
Cardiovascular Diseases
Autoimmune Diseases
Nervous System Disorders
Metabolism & Endocrinology Disorders
Other Diseases
Multiplex Assay End-user Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceutical & Biotechnology Companies
Hospitals & Diagnostic laboratories
Research & Academic Institutes
Other End-users
Multiplex Assay Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Asia Pacific
Japan
China
India
South Korea
Australia
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Order a free sample PDF of the Multiplex Assay Market Intelligence Study, published by Grand View Research.
0 notes
Text
In Vitro Diagnostics Market: Revolutionizing Medical Testing
The In Vitro Diagnostics (IVD) Market is at the forefront of medical diagnostics, enabling healthcare providers to detect diseases and conditions through tests performed outside the body. As demand for early detection and accurate diagnostic tools increases, the IVD market is witnessing rapid growth. This article delves into the market segmentation, key growth drivers, and leading companies shaping the industry, providing valuable insights for decision-makers.
Market Overview
According to SkyQuest’s In Vitro Diagnostics Market report, the global IVD market is valued at USD 87.93 Billion in 2023 and is projected to grow at a CAGR of 5.3%. The market’s expansion is driven by the rising prevalence of chronic diseases, the growing importance of early disease detection, and technological advancements in diagnostics.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/in-vitro-diagnostics-market
Market Segmentation
By Product Type:
Reagents & Kits: Essential for a wide variety of diagnostic tests, including immunoassays and molecular diagnostics.
Instruments: Diagnostic tools such as analyzers and automated systems used in laboratories and healthcare settings.
Software & Services: Used for managing data, improving accuracy, and ensuring compliance with regulatory standards.
By Application:
Infectious Diseases: A leading segment, especially with the demand for COVID-19 diagnostics.
Cancer: Rising incidence of cancer has fueled the need for accurate diagnostic tools.
Cardiology: IVD tools for cardiovascular diseases are seeing growing demand due to an increasing global burden.
Diabetes: Blood glucose monitoring systems dominate this segment, with widespread use among diabetic patients.
Autoimmune Diseases: IVD tests play a crucial role in diagnosing autoimmune disorders.
By End-User:
Hospitals & Clinics: Major users of IVD tools, especially for infectious diseases and chronic conditions.
Diagnostic Laboratories: Central hubs for conducting a wide range of tests with high accuracy and reliability.
Research & Academic Institutions: Key in driving innovation and new test development.
Homecare Settings: Growing segment with the rise in at-home diagnostic tools, particularly for chronic conditions.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/in-vitro-diagnostics-market
Key Growth Drivers
Increasing Prevalence of Chronic Diseases: Rising cases of cancer, diabetes, and cardiovascular diseases are driving the demand for IVD tests.
Technological Innovations: Advancements in molecular diagnostics, AI-driven analysis, and automation are enhancing the efficiency and accuracy of diagnostic tools.
Demand for Personalized Medicine: IVD plays a pivotal role in tailoring treatments to individual patients, fueling market growth.
Aging Population: As the global population ages, the demand for diagnostic tests for age-related diseases like Alzheimer’s and cardiovascular conditions is on the rise.
Leading Companies in the Market
SkyQuest’s In Vitro Diagnostics Market report highlights the following key players in the industry:
Roche Diagnostics
Abbott Laboratories
Thermo Fisher Scientific Inc.
Danaher Corporation
Siemens Healthineers
Bio-Rad Laboratories, Inc.
Becton, Dickinson and Company
bioMérieux SA
Qiagen N.V.
Sysmex Corporation
Read More at: - https://www.skyquestt.com/report/in-vitro-diagnostics-market
Challenges and Opportunities
While regulatory hurdles and high costs of advanced diagnostic tools remain challenges, the IVD market offers numerous opportunities. The increasing focus on point-of-care testing and the integration of AI in diagnostics present significant growth avenues for industry players.
Future Outlook
The In Vitro Diagnostics Market is set to experience robust growth as healthcare shifts towards early detection and preventive care. Companies that invest in cutting-edge technologies and innovative diagnostic solutions will be well-positioned to lead in this evolving market.
The In Vitro Diagnostics Market is transforming the healthcare landscape, offering essential tools for early diagnosis and effective treatment planning. Decision-makers who stay ahead of trends and leverage technological advancements will thrive in this rapidly growing industry. For more detailed insights, explore SkyQuest’s In Vitro Diagnostics Market report.
0 notes
Text
Point of Care Diagnostics: Revolutionizing Healthcare with Real-Time Testing
The Advent of Quick and Accurate Medical Testing Point of Care Diagnostics have emerged as a groundbreaking development in the medical field by enabling accurate testing to be done quickly and conveniently. Traditional diagnostic methods usually require samples to be sent to a centralized laboratory for analysis, which can delay vital treatment decisions by several days. However, point-of-care tests provide results within minutes using portable devices, bringing testing closer to the patient. This revolutionary approach is transforming healthcare delivery. Rapid Testing for Better Patient Outcomes By facilitating timely diagnosis, point-of-care testing leads to better patient outcomes. Speedy detection of conditions like infections or chronic diseases allows doctors to prescribe appropriate treatment without delay. For example, point-of-care tests are commonly used in emergency rooms to quickly identify heart attacks, strokes or life-threatening infections. Getting fast diagnostic results is crucial for such medical emergencies as it ensures patients receive the right therapy as soon as possible. The timely administration of antibiotics, anti-clotting medications or other critical treatments improves survival rates and recovery. Patient Comfort and Convenience Besides clinical benefits, Point of Care Diagnostics enhance patient comfort and convenience. People no longer have to wait anxiously for days to learn about their health while potentially worsening conditions go untreated. With devices that analyze samples on-site, patients get actionable results during the same clinical visit when treatment decisions are made. This spares them follow-up trips to the doctor or lab and unnecessary stress. Home testing using self-administered point-of-care kits even allows monitoring health remotely while maintaining independence. Finger-prick blood samples or urine specimens are all that's needed, eliminating difficulties obtaining specimens. More Efficient Use of Resources Speedy diagnostic testing optimizes use of limited healthcare resources. Quick turnaround times avoid unnecessary reliance on expensive treatments initiated just to address uncertainty in diagnoses. Point-of-care devices reduce laboratory workloads too by decentralizing testing. Moreover, decentralized testing is vital for resource-constrained settings like rural areas, refugee camps or developing countries where access to centralized labs is limited. Portable devices overcome infrastructure barriers and enable basic medical services even in remote areas. This promotes healthcare equity globally. A Proliferation of Diagnostic Platforms Rapid technological progress has enabled the development of varied point-of-care testing systems. Examples include paper microfluidic devices, electrochemical sensors, molecular diagnostics platforms and portable ultrasound machines integrated with imaging analysis software. Immunology-based tests detecting proteins or antibodies through lateral flow or microarray methods are commonly used for conditions like infections and cardiac markers. Molecular diagnostic platforms employ techniques like polymerase chain reaction (PCR) for swift nucleic acid amplification and analysis of viruses or genetic markers. Newer technologies like CRISPR gene editing also hold promise as a basis for point-of-care genetic testing. With ongoing research, the types of conditions examinable at the point of care continue expanding in scope and complexity. Get more insights on Point Of Care Diagnostics
Also read related article on Gastroesophageal Reflux Disease Treatment Devices Market
For Deeper Insights, Find the Report in the Language that You want
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)

#Point Of Care Diagnostics#Poc Testing#Rapid Diagnostics#Bedside Testing#Portable Diagnostics#PointOfCare Testing#Poc Devices#NearPatient Testing#Decentralized Diagnostics
0 notes
Text
Buy Hemp Test Kits Online for Accurate Results
The hemp industry is booming, with products ranging from CBD oil to clothing and construction materials. As the market expands, so does the need for reliable and accurate hemp testing. To ensure product quality, safety, and compliance with regulations, many individuals and businesses are turning to online hemp test kits.
Understanding the Importance of Hemp Testing
Hemp, a variety of the Cannabis sativa plant, contains negligible amounts of THC, the psychoactive compound found in marijuana. However, even trace amounts of THC can lead to legal and regulatory issues. This is where hemp testing becomes crucial.
Accurate hemp testing helps to:
Verify THC levels: Ensure that products comply with legal THC limits.
Determine CBD content: Verify the potency and consistency of CBD products.
Identify contaminants: Detect harmful substances like pesticides, heavy metals, and mold.
Protect brand reputation: Maintain consumer trust by guaranteeing product quality.
Facilitate market access: Comply with regulatory requirements for selling hemp products.
How Hemp Test Kits Work
Online hemp test kits offer a convenient and accessible way to analyze hemp samples. These kits typically include the necessary equipment, reagents, and instructions for conducting tests.
The most common tests performed with hemp test kits include:
THC potency testing: Determines the concentration of THC in a sample.
CBD potency testing: Measures the amount of CBD present in a product.
Terpene profiling: Identifies the aromatic compounds that contribute to hemp's flavor and aroma.
Contaminant screening: Checks for the presence of harmful substances like pesticides and heavy metals.
While at-home test kits can provide preliminary results, it's essential to note that they may not offer the same level of accuracy and precision as laboratory-based testing. For official compliance purposes or when precise results are required, it's recommended to send samples to a certified laboratory.
Choosing the Right Hemp Test Kit
With numerous hemp test kits available online, selecting the right one can be overwhelming. Consider the following factors when making a decision:
Test parameters: Determine which components you need to test (THC, CBD, terpenes, contaminants).
Kit accuracy: Research the kit's precision and reliability.
Ease of use: Choose a kit that matches your technical expertise.
Cost: Compare prices and consider the overall value of the kit.
Customer reviews: Read feedback from other users to assess the kit's performance.
It's also crucial to purchase test kits from reputable suppliers to ensure the quality and accuracy of the results.
Interpreting Test Results
Once you have conducted a test, it's essential to understand the results. Test kits typically provide clear instructions on how to interpret the data. However, it's advisable to consult with a hemp industry expert or a laboratory for further analysis and guidance.
If you identify any issues with your test results, such as high THC levels or the presence of contaminants, it's crucial to take appropriate action to protect your product and your business. This may involve remedying the problem, discarding the affected product, or seeking professional advice.
The Role of Laboratories in Hemp Testing
While at-home test kits can be useful for initial screening, laboratories offer the highest level of accuracy and reliability for hemp testing. Accredited laboratories employ advanced analytical techniques and experienced personnel to provide comprehensive test results.
If you need official test results for regulatory compliance or legal purposes, it's essential to send your samples to a certified laboratory. These laboratories can also provide detailed reports and documentation to support your claims.
Conclusion
Investing in hemp test kits is a proactive step towards ensuring the quality and safety of your hemp products. By understanding the importance of testing, choosing the right kit, and interpreting results accurately, you can build trust with your customers and protect your business.
Remember, while at-home test kits can be a valuable tool, they should not replace laboratory testing for critical quality control and compliance purposes.
0 notes
Text
Lateral Flow Assay Market Overview, Key Players & Growth Forecast 2023 to 2031
Astute Analytica created the Global Lateral Flow Assay Market Research Report based on a thorough understanding of the client’s needs.
This research offers details on the regional and worldwide market conditions as they are right now. This offers insightful information on the world market. This industry analysis report provides specifics on market forces, market constraints, and their impact on market demand going forward. The market research provides a broad overview of this sector.
The Global Lateral Flow Assay Market, analyzed by Astute Analytica, was valued at US$ 9,535.1 Mn in 2022 and is estimated to reach US$ 14,819.4 Mn by 2031. The market is recording a CAGR of 5.3% over the forecast period 2023–2031.
Download the Comprehensive PDF Strategic Report for Exclusive Details: https://www.astuteanalytica.com/request-sample/lateral-flow-assays-market
The report includes data on businesses, app categories, nations, and geographies. These reports include data on trade, investments, sales, and turnover. The impact of COVID-19 on the upstream, midstream, and downstream businesses is examined via market research. This research uses statistics to analyze a number of different aspects of the industry.
The report on the worldwide Lateral Flow Assay Market looks at prospective expansion in various applications and geographical areas. The impact of industry demography and industry growth are examined in this study. The study looks at preferred channels, domain drivers, and market dynamics in emerging markets. There are also restraints in it. Prices, revenues, revenue growth, and production costs were all analyzed.
The elements in this study have the potential to significantly impact market expansion throughout the projected period. Access to the necessary data is made simple by the availability of a market dashboard with all the specifics. There are various components to the Lateral Flow Assay Market Analysis and Future Outlook. This report contains facts and information that can help businesses decide more wisely and increase their return on investment (ROI). CAGR figures are used in this study to quantify changes in or increases in product demand throughout a predicted period.
Leading Companies
Abbott Laboratories
bioMerieux S.A
Bio-Rad Laboratories Inc.
Danaher Corporation
F. Hoffmann-La Roche Ltd.
Hologic Inc.
Merck KGaA
PerkinElmer Inc.
Qiagen N.V.
Quidel Corporation
Siemens Healthineers
Thermo Fisher Scientific, Inc.,
Other Prominent Players
Explore the Full Comprehensive Report Here: https://www.astuteanalytica.com/industry-report/lateral-flow-assays-market
Segmentation Outline
The following are the different segments of the Global Lateral flow assay market:
By Product & Services:
Readers
Bench-top Readers
Hand-held Readers
LFA Kits
Test Strips
Dipsticks
Cassettes
Lancets
By Indications:
Infectious Diseases
Mosquito-borne Diseases
Streptococcus Infections
Sexually Transmitted Diseases
Hepatitis
Tuberculosis
Asthma
Pneumonia
Sepsis
Gastrointestinal Infections
Others
Pregnancy Test
Drug of Abuse Testing
By Technique:
Sandwich Assays
Competitive Assays
Multiplex Detection Assays
By End User:
Hospitals & Clinics
Diagnostics Laboratories
Home Care Settings
Pharmaceuticals & Biotechnology Companies
Other
By Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Supermarkets/ Hypermarkets
e-Commerce
By Region:
North America
The U.S.
Canada
Mexico
Europe
Western Europe
The UK
Germany
France
Italy
Spain
Rest of Western Europe
Eastern Europe
Poland
Russia
Rest of Eastern Europe
Asia Pacific
China
India
Japan
Australia & New Zealand
ASEAN
Rest of Asia Pacific
Middle East & Africa (MEA)
UAE
Saudi Arabia
South Africa
Rest of MEA
South America
Argentina
Brazil
Rest of South America
Important Developments in the Lateral Flow Assay Market:
The report examines manufacturers’ profiles, news, main companies, and revenue.
To demonstrate the intense competition that the top manufacturers in the world deal with.
Presenting the market by kind and application, along with each type’s revenue, sales, and growth rates.
An examination of the major nations in North America according to manufacturer, application, and kind. Southeast Asia and Europe. Latin America. Sales, turnover, and market shares for manufacturers.
The report examines the manufacturing expenses, raw resources, and production process.
Grab Your Sample PDF Report for Deeper Understanding: https://www.astuteanalytica.com/request-sample/lateral-flow-assays-market
About Astute Analytica: Astute Analytica is a renowned global analytics and advisory company, having swiftly established a strong reputation through the tangible and impressive results delivered to our valued clients. Our commitment lies in providing unparalleled, in-depth, and remarkably accurate estimates and projections to demanding clients across diverse industries, such as technology, healthcare, chemicals, semiconductors, FMCG, and more, drawing from a wide spectrum of satisfied and returning customers from around the world.
Our clients benefit greatly from our comprehensive analysis of the complex business environment, meticulously examining existing and emerging possibilities in different segments, technological advancements, growth estimations, and strategic choices. In essence, we offer a complete package, empowering our clients to make well-informed decisions, seize highly profitable opportunities, and overcome formidable challenges.
Our strength lies in our highly qualified, competent, and experienced team of professionals, comprising proficient business analysts, economists, consultants, and technology experts. As a customer-centric organization, you, our esteemed patron, are our top priority. When you choose to engage with us, rest assured that you will receive the best cost-effective and value-added package tailored to your needs.
To connect with us, feel free to use the following contact details:
Phone number: +18884296757
Email: [email protected]
Website: https://www.astuteanalytica.com/
LinkedIn | Twitter | YouTube | Facebook
#LateralFlowAssaysMarket#LateralFlowAssaysMarketSize#LateralFlowAssaysMarketShare#LateralFlowAssaysMarketTrends#LateralFlowAssaysMarketDemand#LateralFlowAssaysMarketForecast#LateralFlowAssaysMarketAnalysis#LateralFlowAssaysMarketGrowth
0 notes
Text
Africa IVD Market to be Worth $1.78 Billion by 2029
According to a new market research report titled, ‘Africa In Vitro Diagnostics Market by Product & Services, Technology (Immunoassay, Point of Care, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), and Customer Type - Forecast to 2029,’ published by Meticulous Research®, the Africa in vitro diagnostics market is expected to reach $1.78 billion by 2029, at a CAGR of 4.8% from 2022 to 2029.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In vitro diagnostics (IVD) are tests performed on samples of blood or tissue taken from the human body to detect a wide range of diseases. These tests help monitor overall health to prevent and treat various diseases. IVD involves the use of various reagents, assays, and kits based on technologies such as immunoassay, whole blood glucose monitoring, molecular diagnostics, point of care, clinical chemistry, hematology, coagulation & hemostasis, critical care, and urinalysis.
High Prevalence of Infectious Diseases Driving the Growth of the Africa IVD Market
Diagnostic tests play a crucial role in the medical care of patients with infections. The demand for IVD kits used to diagnose infectious diseases is high in Africa due to the increasing awareness regarding emerging and re-emerging infectious diseases and the adoption of advanced molecular testing and lab-on-chip technology. The burden of diseases such as HIV, tuberculosis, malaria, viral hepatitis, and neglected tropical diseases (NTDs) is large in Africa. For example, according to WHO, in 2020, 25.6 million people were living with HIV in the African Region. The annual number of new HIV infections also increased in the Middle East and North Africa (source: UNAIDS data 2021).
In addition, there is a frequent outbreak of endemic diseases in Africa. For instance, Lassa fever is endemic in Nigeria and continues to be reported throughout the country. According to Nigeria Centre for Disease Control and Prevention (NCDC). In January 2022, 981 suspected and 211 confirmed cases of Lassa fever were reported in Nigeria. There were 40 deaths reported among confirmed cases, indicating a case fatality rate of 19%. Thus, the high burden of infectious diseases increases the demand for IVD tests in Africa.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5415
Africa IVD Market: Future Outlook
The Africa IVD market is segmented based on Product Category (Reagents, Assays, and Kits, Instruments, and Software & Services), Technology (Immunoassay, Whole Blood Glucose Monitoring, Point-of-Care Diagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, Coagulation & Hemostasis, Critical Care, Urinalysis, and Other IVD Technologies), Application (Infectious Diseases, Oncology, Diabetes, Endocrinology, Cardiology, and Other Applications), Diagnostic Approach (Laboratory Testing, Point-of-Care Testing, and OTC/Self Testing), and Customer Type (Hospital Labs, Private Labs, Home Care/Self Testing, Government, and Other Customer Types), and Geography (South Africa, Egypt, Nigeria, Kenya, Algeria, Tanzania, Morocco, Tunisia, and the rest of Africa). The study also evaluates industry competitors and analyzes their market shares.
Based on product category, the reagents, assays, and kits segment is slated to register the fastest growth rate during the forecast period. In Africa, infectious diseases cause chronic illnesses, premature deaths, and loss of productivity, affecting the region's economic growth. Thus, the emerging threats of infectious diseases drive the demand for IVD in Africa. The frequent use of reagents for diagnosing diseases and the easy availability & accessibility of these tests are the major factors driving the growth of this segment.
Based on technology, the point of care segment is slated to register the fastest growth rate during the forecast period. The rising geriatric population, the growing prevalence of chronic diseases, and the rising incidence of infectious diseases are driving the growth of this segment. The increasing burden of chronic diseases and pathogen outbreaks has significantly increased the demand for PoC diagnostics in Africa. The increased need for early and accurate diagnosis, precise confirmation of clinical findings, and immediate & informed decision-making on treating diseases contributes to the growth of the PoC segment.
Based on application, the oncology segment is slated to register the fastest growth rate during the forecast period. Cancer involves a complex set of genetic alternations and subsequent changes in gene expression, leading to the uncontrolled proliferation of cells. In cancer diagnostics, early diagnosis and knowing the exact nature of the tumor are of prime importance. Early cancer detection and access to effective anti-cancer treatment can result in higher rates of survival and a better quality of life. According to GLOBOCAN 2020, 1.1 million new cases and 711,429 deaths were recorded in Africa. The growing demand for personalized medicines in cancer therapy, the increasing prevalence of cancer, and the rising demand for rapid cancer diagnosis tests drive the growth of the IVD market for oncology applications.
Based on diagnostic approach, the OTC/self-testing segment is slated to register the fastest growth rate during the forecast period. In Africa, over-the-counter (OTC)/self-testing is promoted for diseases, such as HIV and COVID-19, due to the high prevalence of infectious diseases. Self-testing reduces the burden on healthcare workers and the system. The OTC/self-testing kits are diagnostic tests that can be purchased from any pharmacy store and used by the patient. These tests can give rapid results and can be taken from anywhere.
Quick Buy – Africa IVD Market Research Report: https://www.meticulousresearch.com/Checkout/47708335
Based on customer type, the home care/self-testing segment is slated to register the fastest growth rate during the forecast period. The growth of this segment is attributed to the increasing need to monitor and manage chronic diseases, the growing awareness about home healthcare testing products, and the reduced waiting time and healthcare costs offered by self-testing kits.
This research report analyzes the market across major African countries and provides a comprehensive analysis of South Africa, Algeria, Nigeria, Kenya, Morocco, Tunisia, Tanzania, Egypt, and the rest of Africa.
Key companies operating in the Africa IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Scope of the Report
Africa IVD Market, by Product Category
Reagents, Assays, and Kits
Instruments
Software & Services
Africa IVD Market, by Technology
Immunoassay
Whole Blood Glucose Monitoring
Point-of-Care Diagnostics
Clinical Chemistry
Molecular Diagnostics
Hematology
Coagulation & Hemostasis
Critical Care
Urinalysis
Other IVD Technologies
Note: Other technologies include microscopy, hybridization, and loop-mediated amplification.
Africa IVD Market, by Application
Infectious Diseases
Oncology
Diabetes
Endocrinology
Cardiology
Other Applications
Note: Other applications include nephrology, toxicology, gastroenterology, neonatal, genetic, and neurological disorders.
Africa IVD Market, by Diagnostic Approach
Laboratory Testing
Point-of-Care Testing
OTC/Self-testing
Africa IVD Market, by Customer Type
Hospital Labs
Private Labs
Home Care/Self Testing
Government
Other Customer Types
Note: Other customer types include long-term care facilities, academic & research institutes, ambulatory care centers, and transfusion laboratories.
Africa IVD Market, by Country
South Africa
Egypt
Nigeria
Kenya
Algeria
Tanzania
Morocco
Tunisia
Rest of Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In Vitro Diagnostic (IVD) Reagents Market - https://www.meticulousresearch.com/product/in-vitro-diagnostic-reagents-market-5110
0 notes
Text
Water Tester: Ensuring the Quality of Your Water
Water is an essential resource for all living beings. It is not only vital for our survival but also plays a crucial role in maintaining our overall health and well-being. However, with increasing pollution and contamination, the quality of water has become a growing concern. This is where a water tester comes into play. In this blog, we will explore the significance of water testing and the various tools available, such as water quality testers, water testing kits, and water TDS meters.
Water testing is the process of analyzing the chemical, physical, and biological properties of water to determine its quality. It helps in identifying potential contaminants, such as bacteria, chemicals, heavy metals, and other harmful substances that may pose a threat to human health. By conducting regular water tests, you can ensure that the water you consume is safe and free from any pollutants.
One of the most commonly used tools for water testing is the water quality tester. This device measures various parameters, including pH levels, temperature, conductivity, and total dissolved solids (TDS). It provides accurate readings, allowing you to assess the overall quality of your water. A water quality tester is easy to use and provides quick results, making it an ideal tool for both professionals and homeowners.
Another popular option for water testing is a water testing kit. These kits usually come with a set of test strips or reagents that can detect specific contaminants. They are designed to be user-friendly, enabling anyone to perform basic water tests at home. With a water testing kit, you can check for common pollutants like chlorine, lead, nitrates, and bacteria. These kits are cost-effective and provide a convenient solution for regular water testing.
For a more detailed analysis of water quality, a water TDS meter is an excellent choice. TDS stands for Total Dissolved Solids, which refers to the concentration of inorganic salts, minerals, and metals present in water. A TDS meter measures the electrical conductivity of water and converts it into a TDS reading. This reading indicates the overall purity of the water. A high TDS value may indicate the presence of contaminants, while a low TDS value suggests cleaner water. TDS meters are widely used in various industries, including agriculture, aquaculture, and hydroponics.
When it comes to purchasing water testing equipment, it's essential to consider factors such as accuracy, durability, and price. The market offers a wide range of options, from basic testers to advanced meters. The prices of water testing devices vary depending on their features and capabilities. However, it is crucial to invest in a reliable and accurate device, as compromising on quality may lead to inaccurate results and potential health risks.
In conclusion, water testing is crucial to ensure the quality and safety of the water we consume. With the help of water testers, such as water quality testers, water testing kits, and water TDS meters, we can identify potential contaminants and take necessary measures to address them. Regular water testing is essential for both residential and commercial purposes, as it helps in maintaining a healthy and sustainable water supply. So, don't wait any longer – invest in a water tester today and take control of your water quality.
0 notes
Text

KDM Global is a well-known producer, supplier, and exporter of lab testing equipment, air pollution instruments, dust monitoring equipment, and pollution monitoring equipment at the best prices in Delhi NCR, India.
The business has been able to establish a solid foundation in this cutthroat sector because to the unwavering determination and passion of our mentor Mr.Mayank. We have been motivated to work hard by his consistent inspiration and upbeat attitude.
In manufacturing companies across a wide range of industries, testing has always been a crucial process, with the final product's quality entirely reliant on the type of testing equipment employed. The output drawn from the total production is improved by the equipment's increased accuracy and precision.As a result, KDM Global is acting responsibly for industries who need air pollution monitoring systems, impact testing equipment, bomb calorimeters, humidity chambers, etc. to increase the availability of high-quality testing equipment. Being a producer and wholesaler, we have integrated ourselves into the industry and market that needs the most inventive testing equipment. Our product line for our clients includes customised machines, lab testing equipment, environment testing equipment, and electronic testing equipment.
Equipment for measuring and testing the environment
Our business has had tremendous success by producing and offering a wide range of top-notch environment testing equipments. We offer benzene, mixed, gaseous, fine particulate, high volume air, convenient sampler, and stack monitoring kit in this selection of air pollution monitoring equipment.These are made for a variety of uses, including balances, evaporators, biofuel analyzers, temperature chambers, particle counts, stability chambers, and more. Additionally, temperature and humidity levels can both be tested using environment testing equipment. Our products are renowned for their top performance and extreme durability. available for purchase with a variety of features, patterns, sizes, and forms.
Our Goals and Mission
We entered the market with the intention of developing into a trustworthy source with the mission of raising the standards of testing equipment being offered on the Indian market. To that aim, we are working diligently on R&D and bringing the most affordable items.
• Keep products readily available;
• Speak with as many people as possible
For more details contact us:
Website: https://kdmglobal.business.site
Email : [email protected]
Website : https://sites.google.com/view/kdmglobal/home
Contact :8218470498
Follow Us On :
Facebook : https://www.facebook.com/testingequipmentkdm
Twitter : https://twitter.com/global_kdm
LinkedIn : https://www.linkedin.com/company/91037160/admin/
YouTube : https://www.youtube.com/channel/UCXSi4eywyPih3FPziTIT5Jg
Pinterest : https://in.pinterest.com/kdmglobaltesting/
Instagram : https://www.instagram.com/kdmglobalsales/
Tumblr : https://www.tumblr.com/blog/kdmglobal
Flickr : https://www.flickr.com/people/197865063@N04/
Reddit : https://www.reddit.com/user/kdmglobaltesting
Quora : https://www.quora.com/profile/KDM-Global-1
0 notes
Text
Rapid Diagnostic Kits Market for Indian Ocean Region Countries Worth $1.75 Billion by 2029
Rapid Diagnostic Kits Market for Indian Ocean Region Countries Worth $1.75 Billion by 2029
According to this latest publication from Meticulous Research®, the rapid diagnostics kits market for Indian Ocean region countries is expected to register a CAGR of 1.6% from 2022 to 2029 to reach $1.75 billion by 2029. The growth of this market is driven by the growing demand for PoC diagnostics, the high prevalence of chronic diseases coupled with the increasing geriatric population, the high prevalence of infectious diseases, the increasing need for rapid decision-making, and the outbreak of the COVID-19 pandemic.
Download Research PDF @ https://www.meticulousresearch.com/download-sample-report/cp_id=5418
The Rapid Diagnostics Kits Market for Indian Ocean Region Countries is segmented by Product (Professional Kits and OTC Kits), Platform (Immunoassays, Molecular Detection Tests, and Other Rapid Diagnostic Platforms), Application (Blood Glucose Testing, Cardiac Metabolism Testing, Infectious Diseases Testing, Pregnancy & Fertility Testing, Hematology Testing, Tumor/Cancer Markers Testing, Urinalysis, Cholesterol Testing, and Other Tests), End User (Hospitals & Clinics, Diagnostic Laboratories, Home Care/Self-testing, and Other End Users), and Geography. The study also evaluates industry competitors and analyzes their key growth strategies in the market.
Based on product, in 2022, the professional kits segment is expected to account for the larger share of the rapid diagnostic kits market in Indian Ocean region countries. The large market share of this segment is attributed to the higher preference for PoC testing, the high prevalence of chronic diseases, and technological advancements in rapid diagnostics, supporting the high adoption of professional kits.
Based on platform, in 2022, the immunoassays segment is expected to account for the largest share of the rapid diagnostic kits market in Indian Ocean region countries. The large market share of this segment is mainly attributed to the high prevalence of chronic and infectious diseases, technological innovations, coupled with the advantages offered by the rapid immunoassay tests, such as cost-effectiveness and at-home testing.
Based on application, in 2022, the infectious diseases testing segment is expected to account for the largest share of the rapid diagnostic kits market for Indian Ocean region countries. Infectious diseases are transmissible or communicable illnesses that can spread quickly and become epidemics. It is crucial to quickly diagnose initial infections and prevent further spread through in-vitro diagnosis. The high prevalence of infectious diseases in the countries of the Indian Ocean region is a key factor in the large share of the segment. For instance, around 2.4 million people were infected with HIV in India in 2021.
𝐐𝐮𝐢𝐜𝐤 Buy 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐑𝐞𝐩𝐨𝐫𝐭: https://www.meticulousresearch.com/Checkout/75587733
Based on end user, in 2022, the hospitals & clinics segment is expected to account for the largest share of the rapid diagnostic kits market for Indian Ocean region countries. Factors attributing to this segment's largest share include a high preference for hospitals & clinics for primary care across the countries in the Indian Ocean region.
This research report provides a comprehensive analysis of the rapid diagnostic kits market in France, India, Australia, Singapore, Indonesia, Madagascar, Mauritius, Réunion, Comoros, Mayotte, and Seychelles. India is expected to grow with the fastest growth over the forecast period. Factors contributing to the growth include the rising hospitalization due to various diseases, the increasing geriatric population, and increasing healthcare access & expenditure.
Key Players:
Key companies operating in the rapid diagnostic kits market for Indian Ocean region countries are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), Bio-Rad Laboratories, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), ACON Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), Thermo Fisher Scientific Inc. (U.S.), bioMérieux S.A. (France), Alfa Scientific Designs, Inc. (U.S.), BTNX Inc. (Canada), Meridian Bioscience, Inc. (U.S.), and Trinity Biotech plc (Ireland).
To gain more insights into the market with a detailed table of content and figures, click here:https://www.meticulousresearch.com/product/rapid-diagnostics-kits-market-for-indian-ocean-region-countries-5418
0 notes
Text
Led Engin Products
Subscribe to the Fulham publication, Illuminations, for product news, occasion updates, rising tendencies in the lighting business, and extra. A Light Engine drives the LEDs by regulating energy, managing thermals, and offering safety. Our Light Engines easily integrate into new and present light fixtures.
MRO company Interline Brands can also be owned by The Home Depot, with 70 distribution facilities across the United States. The Home Depot, Inc., commonly generally known as Home Depot, is the most important residence improvement retailer in the United States, supplying instruments, construction products, and services.
The firm is headquartered in incorporated Cobb County, Georgia, with an Atlanta mailing address. Mark Larson, who joined Digi-Key in 1976 as its general manager, became president in 1985. He has led the company from its initial concentrate on the hobbyist market to the expanded promote it serves at present.
The company color is a bright orange , on signs, tools and employee aprons. It was announced in August 2014 that Craig Menear will take over for Frank Blake as CEO while Blake will remain the chairman of the board.
According to an August 2016 report by Bloomberg Businessweek, aggressive price-chopping decisions that began in 2000 when Lee Scott took over as CEO of the company led to a big enhance in crime in shops throughout the United States.
While these selections succeeded in growing earnings 23% within the decade that adopted, in addition they led to an increase in each theft and violent crime. In April 2019 Walmart Inc.
announced plans to increase the usage of robots in shops in order to enhance and monitor stock, clear flooring and unload trucks, a part of the corporate's effort to decrease its labor prices. David Merriman, Joseph Persky, Julie Davis and Ron Baiman did a examine in Economic Development Quarterly outlining the impacts of Walmart in Chicago.
Fulham is dedicated to intelligent, sustainable lighting options that give their users the ability to manage their mild. Quality is excellent they usually add an excellent stage of safety with the brightness of the L.E.D.'s.
Bought with the Plug-n-Play Lighting Installation Kit (P/N bought individually) so they operate as turn indicators as well as operating lights. Only draw back is the added expense of buying the loom, however the excellent news is I can join a further 3 Goldstrike lighting products to the identical loom as it has 4 retailers.
As of September 16, 2012, all seven of the box stores in China had been shut down. The Home Depot has no quick plans to additional broaden its specialty stores in China. The firm is taking a "wait-and-see" angle towards the Chinese market, however does not want to completely pull out because re-entry into the market would be very costly.
Walmart Discount Stores, additionally branded as simply "Walmart", are low cost department stores with sizes varying from 30,000 to 221,000 sq. feet , with the average retailer covering 106,000 square toes . Some newer and reworked low cost stores have an expanded grocery department, just like Target's PFresh department.
S-LCD was owned by Samsung (50% plus one share) and Sony (50% minus one share) and operates its factories and facilities in Tanjung, South Korea.
As of 26 December 2011, it was announced that Samsung had acquired the stake of Sony on this joint venture. In 1980s, Samsung Electronics began to speculate heavily in analysis and growth, investments that had been pivotal in pushing the corporate to the forefront of the global electronics trade.
In 1982, it constructed a tv assembly plant in Portugal; in 1984, a plant in New York; in 1985, a plant in Tokyo; in 1987, a facility in England; and another facility in Austin, Texas, in 1996. As of 2012, Samsung has invested greater than US$thirteen,000,000,000 in the Austin facility, which operates underneath the name Samsung Austin Semiconductor.
Walmart started changing workers who count currency by hand with machines that count 8 payments per second and three,000 cash a minute.
The processing machines, positioned in the back of stores, permit cashiers to course of the money for digital depositing. In April 2011, Walmart acquired Kosmix to develop software for analyzing real-time data streams.
As the largest retailer in the U.S., Walmart collects and analyzes a considerable amount of client data. The huge data sets are mined for use in predictive analytics, which permit the company to optimize operations by predicting customer's habits.
Following the deadly police shooting of Walter Wallace Jr. in October 2020, Walmart quickly eliminated gun and ammunition displays in thousands of shops throughout the U.S. from sales floors, grounding their reason in issues of civil unrest. Walmart struggled to export its brand elsewhere as it rigidly tried to breed its model overseas.
In 2012, The Home Depot conceded that it misread the nation's appetite for do-it-yourself products. Additionally, The Home Depot promotes compact fluorescent mild bulbs in its stores.
As part of this effort, the company created the largest recycling program within the United States for the bulbs.In March 2013, Home Depot places in Canada stopped accepting compact fluorescent light bulbs for recycling.
Home Depot stores average 105,000 ft2 in dimension and are organized warehouse-fashion, stocking a wide variety of provides. Home Depot's two largest stores are positioned in Vauxhall, New Jersey, which encompasses 217,000 ft2 of area, and in Anaheim Hills, California, the place it encompasses 204,000 ft2. led engine
The mixed firm is focused on optical solutions and serves the complete worth chain in sensing, visualization and illumination, from emitters to sensors and software. In 1998 Osram acquired the lamp enterprise of ECE Industries India Ltd at a value of $9.55 million.
After a bidding warfare with Bain Capital, Osram was taken over by the Austrian company ams AG in July 2020 and a majority of shares was acquired. That’s why Google engineers spend every single day testing it, conducting tons of of thousands of experiments yearly, resulting in thousands of enhancements.
1 note
·
View note
Text
In Vitro Diagnostics Market: Revolutionizing Healthcare with Advanced Diagnostics
The In Vitro Diagnostics (IVD) market plays a pivotal role in the healthcare industry, offering essential tools for disease diagnosis, treatment monitoring, and overall patient care. With advancements in diagnostic technology and the rising demand for personalized medicine, the IVD market is experiencing rapid growth. This article provides a detailed overview of the market trends, segmentation, key drivers, and leading companies in the IVD industry, offering valuable insights for decision-makers.
Market Overview
According to SkyQuest's In Vitro Diagnostics Market report, the global market is currently valued at USD 87.93 Billion in 2023, with a projected CAGR of 5.3% over the forecast period. The market is driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and the growing demand for early and precise disease detection.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/in-vitro-diagnostics-market
Market Segmentation
By Product Type:
Reagents and Kits: Essential components used in diagnostic procedures across a range of diseases.
Instruments: Include advanced diagnostic tools like analyzers, molecular diagnostic machines, and point-of-care devices.
Software & Services: Diagnostic software for accurate test results and integrated solutions for laboratories.
By Technology:
Immunoassays: Widely used for infectious diseases and cancer diagnosis.
Molecular Diagnostics: Key in genetic testing and precision medicine applications.
Clinical Chemistry: Essential for routine testing and biomarker identification.
Microbiology: Used to identify pathogens and guide antibiotic therapies.
Hematology: Focuses on blood-related diagnostics such as complete blood count (CBC).
Others: Encompasses emerging technologies like proteomics and metabolomics.
By Application:
Infectious Diseases: Dominating the market due to the global rise in bacterial, viral, and fungal infections.
Oncology: Growing demand for early cancer diagnostics and targeted treatments.
Cardiology: Vital in diagnosing cardiovascular diseases and risk factors.
Diabetes: Includes blood glucose monitoring and HbA1c testing for diabetes management.
Other Applications: Includes diagnostics for autoimmune diseases, nephrology, and neurology.
By End-User:
Hospitals and Clinics: Major centers for diagnostic testing and patient care.
Diagnostic Laboratories: Specializing in high-volume testing across various disease areas.
Homecare Settings: Growing segment due to increasing demand for at-home diagnostic kits.
Academic and Research Institutes: Driving innovation in diagnostic tools and techniques.
Key Growth Drivers
Rising Prevalence of Chronic Diseases: Increasing cases of cancer, diabetes, and cardiovascular diseases fuel the demand for diagnostic tools.
Advancements in Technology: Development of rapid, accurate, and minimally invasive diagnostic methods is boosting the market.
Growing Demand for Personalized Medicine: The focus on tailored treatments and early diagnosis is driving the adoption of molecular diagnostics.
Expansion of Point-of-Care Testing (POCT): The shift towards decentralized testing is increasing the demand for portable and easy-to-use diagnostic devices.
Read More at: - https://www.skyquestt.com/report/in-vitro-diagnostics-market
Leading Companies in the Market
SkyQuest’s In Vitro Diagnostics Market report identifies key players that are shaping the market, including:
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
Danaher Corporation
Thermo Fisher Scientific
bioMérieux SA
Becton, Dickinson and Company
QIAGEN
Sysmex Corporation
Agilent Technologies
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/in-vitro-diagnostics-market
Challenges and Opportunities
The IVD market faces challenges such as stringent regulatory frameworks and high costs of diagnostic devices, especially in emerging markets. However, opportunities lie in the growing adoption of telemedicine, increased government funding for healthcare infrastructure, and technological advancements that enable faster and more precise diagnostic results.
Future Outlook
The In Vitro Diagnostics Market is expected to experience robust growth as healthcare providers increasingly rely on advanced diagnostics for effective patient care. Companies that invest in innovation and cater to the rising demand for point-of-care and home-based testing will have a competitive edge in this dynamic market.
As diagnostics play a critical role in healthcare, the In Vitro Diagnostics Market is poised for significant growth. Decision-makers should stay informed about emerging trends and technological advancements to leverage the full potential of this market. For more detailed insights and strategies, consult SkyQuest’s comprehensive In Vitro Diagnostics Market report.
0 notes
Link
Anticoagulation therapy market size is projected to grow at a compound annual growth rate of 6% over the forecast period of 2021 to 2028.
0 notes
Text
Business Outlook of NORTH AMERICA Women’s Health Diagnostics Market
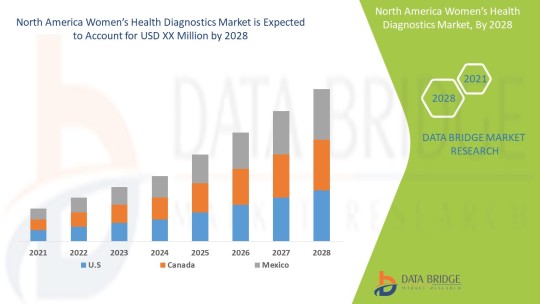
The women’s health diagnostics market is expected to witness market growth at a rate of 5.90% in the forecast period of 2021 to 2028. Data Bridge Market Research report on women’s health diagnostics market provides analysis and insights regarding the various factors expected to be prevalent throughout the forecast period while providing their impacts on the market’s growth.
The universal women’s health diagnostics market survey report offers important statistics on the market status of producers and offers useful advice and direction for businesses and individuals interested in the industry. Research and analysis about the key developments in the market, major competitors and detailed competitor analysis covered in this industry report helps businesses imagine the bigger picture of the market place and products which ultimately assists in defining superior business strategies. The report aids in assessing production processes, major issues, and solutions to mitigate the development risk. Women’s health diagnostics market report brings about the list of top competitors and presents the insights on strategic industry analysis of the key factors influencing the market. An influential women’s health diagnostics market report makes available notable data, present market trends, future events, market environment, technological innovation, forthcoming technologies and the technical advancement in the relevant industry. This report makes it easy to know about the market strategies that are being adopted by the competitors and leading organizations. This industry analysis report also provides the understanding of most affecting driving and restraining forces in the market and its impact on the global market. More importantly, to comprehend the future outlook and prospects for the market, women’s health diagnostics market business report is very useful.
North America Women’s Health Diagnostics Market Scope and Market Size
The women’s health diagnostics market is segmented on the basis of application and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries, and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
On the basis of application, the women’s health diagnostics market is segmented into osteoporosis testing, OVC testing, cervical cancer testing, breast cancer testing, pregnancy and fertility testing, prenatal genetic screening and carrier testing, infectious disease testing, STD testing and ultrasound tests. Osteoporosis testing is further segmented into bone densitometry and in vitro blood tests. OVC testing is further segmented into OVC tumor marker tests, OVC diagnostic imaging tests and other OVC tests. Cervical cancer testing is further segmented into pap smears and HPV testing. Breast cancer testing is further segmented into mammography, breast cancer tumor marker tests, biopsies and other breast cancer tests. Pregnancy and fertility testing is further segmented into lab-based testing, pregnancy testing and ovulation prediction kits and fertility monitors. Prenatal genetic screening and carrier testing is further segmented into CF testing, Down’s syndrome and Edwards’ syndrome testing, TORCH testing and other prenatal genetic disease tests. Infectious disease testing is further segmented into MRSA testing, UTI testing, hepatitis testing, tuberculosis testing and other infectious disease tests. STD testing is further segmented into CT/NG testing, HIV testing and other STD tests. Ultrasound tests are further segmented into breast imaging and OB/GYN imaging.
On the basis of end user, the women’s health diagnostics market is segmented into hospitals, diagnostic and imaging centers, clinics and home care setting.
Get the free sample copy of complete report here:
Market Analysis and Insights: North America Women’s Health Diagnostics Market
The women’s health diagnostics market is expected to witness market growth at a rate of 5.90% in the forecast period of 2021 to 2028. Data Bridge Market Research report on women’s health diagnostics market provides analysis and insights regarding the various factors expected to be prevalent throughout the forecast period while providing their impacts on the market’s growth. The rise in healthcare sector globally is escalating the growth of women’s health diagnostics market.
Women’s health is defined as the diagnosis and treatment of conditions and diseases that may affect a woman’s health physically as well as emotionally. Women tend to have issues such as sexually transmitted infections (STIs), breast cancer, birth control, gynecology disorders, and ovarian cancer, among other female cancers. These diagnostics are utilized to analyze and detect these health issues in women such as cervical cancer, bone density screening and breast cancer screening.
North America Women’s Health Diagnostics Market Country Level Analysis
The women’s health diagnostics market is analyzed and market size insights and trends are provided by country, application and end user as referenced above.
The countries covered in the North America women’s health diagnostics market report are U.S., Canada and Mexico in North America.
North America Women’s Health Diagnostics Market Share Analysis
The women’s health diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to women’s health diagnostics market.
Leading Players in women’s health diagnostics market
The major players covered in the women’s health diagnostics market report are Siemens, Hologic, Inc., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Abbott, BD, F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., Koninklijke Philips N.V., NeuroLogica Corp., Shimadzu Corporation, bioMérieux SA, Carestream Health, MEDGYN PRODUCTS, INC., URIT MEDICAL ELECTRONIC CO., LTD, COOK, Cardinal Health, PerkinElmer Inc., GENERAL ELECTRIC, Quest Diagnostics Incorporated, Danaher, Sysmex Corporation, Hitachi, Ltd., Canon Inc., FUJIFILM Holdings Corporation and others. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
MAJOR TOC OF THE REPORT
Chapter One: Introduction
Chapter Two: Market Segmentation
Chapter Three: Market Overview
Chapter Four: Executive Summary
Chapter Five: Premium Insights
Chapter Six: women's health diagnostics market
Get full Access of report
Get TOC Details
Browse Related Reports@
Global Radiology Services Market – Industry Trends and Forecast to 2029
Europe Prefilled Syringes Market – Industry Trends and Forecast to 2028
North America Prefilled Syringes Market – Industry Trends and Forecast to 2028
Global Prefilled Syringes Market – Industry Trends and Forecast to 2029
North America Optical Fiber Components Market – Industry Trends and Forecast to 2028
Global Smart Hospital Market – Industry Trends and Forecast to 2029
Global Artificial Intelligence (AI) in Drug Discovery Market – Industry Trends and Forecast to 2029
Global Aldose Reductase Inhibitor Market - Industry Trends and Forecast to 2027
Global Cardiovascular Information Systems Market – Industry Trends and Forecast to 2029
Global Cyclooxygenase 2 Inhibitor Market – Industry Trends and Forecast to 2029
Global Photophobia Drug Market – Industry Trends and Forecast to 2028
Global Automotive Intelligent Park Assist Systems Market – Industry Trends and Forecast to 2028
Middle East and Self-Organizing Network (SON) Market – Industry Trends and Forecast to 2027
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Women’s Health Diagnostics Market#Women’s Health Diagnostics Market scope#Women’s Health Diagnostics Market share#Women’s Health Diagnostics Market trend#Women’s Health Diagnostics Market analysis#Women’s Health Diagnostics Market forecast
0 notes
Text
Africa IVD Market to be Worth $1.78 Billion by 2029 – Exclusive Report by Meticulous Research®
According to a new market research report titled, ‘Africa In Vitro Diagnostics Market by Product & Services, Technology (Immunoassay, Point of Care, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), and Customer Type – Forecast to 2029,’ published by Meticulous Research®, the Africa in vitro diagnostics market is expected to reach $1.78 billion by 2029, at a CAGR of 4.8% from 2022 to 2029.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In vitro diagnostics (IVD) are tests performed on samples of blood or tissue taken from the human body to detect a wide range of diseases. These tests help monitor overall health to prevent and treat various diseases. IVD involves the use of various reagents, assays, and kits based on technologies such as immunoassay, whole blood glucose monitoring, molecular diagnostics, point of care, clinical chemistry, hematology, coagulation & hemostasis, critical care, and urinalysis.
High Prevalence of Infectious Diseases Driving the Growth of the Africa IVD Market
Diagnostic tests play a crucial role in the medical care of patients with infections. The demand for IVD kits used to diagnose infectious diseases is high in Africa due to the increasing awareness regarding emerging and re-emerging infectious diseases and the adoption of advanced molecular testing and lab-on-chip technology. The burden of diseases such as HIV, tuberculosis, malaria, viral hepatitis, and neglected tropical diseases (NTDs) is large in Africa. For example, according to WHO, in 2020, 25.6 million people were living with HIV in the African Region. The annual number of new HIV infections also increased in the Middle East and North Africa (source: UNAIDS data 2021).
In addition, there is a frequent outbreak of endemic diseases in Africa. For instance, Lassa fever is endemic in Nigeria and continues to be reported throughout the country. According to Nigeria Centre for Disease Control and Prevention (NCDC). In January 2022, 981 suspected and 211 confirmed cases of Lassa fever were reported in Nigeria. There were 40 deaths reported among confirmed cases, indicating a case fatality rate of 19%. Thus, the high burden of infectious diseases increases the demand for IVD tests in Africa.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5415
Africa IVD Market: Future Outlook
The Africa IVD market is segmented based on Product Category (Reagents, Assays, and Kits, Instruments, and Software & Services), Technology (Immunoassay, Whole Blood Glucose Monitoring, Point-of-Care Diagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, Coagulation & Hemostasis, Critical Care, Urinalysis, and Other IVD Technologies), Application (Infectious Diseases, Oncology, Diabetes, Endocrinology, Cardiology, and Other Applications), Diagnostic Approach (Laboratory Testing, Point-of-Care Testing, and OTC/Self Testing), and Customer Type (Hospital Labs, Private Labs, Home Care/Self Testing, Government, and Other Customer Types), and Geography (South Africa, Egypt, Nigeria, Kenya, Algeria, Tanzania, Morocco, Tunisia, and the rest of Africa). The study also evaluates industry competitors and analyzes their market shares.
Based on product category, the reagents, assays, and kits segment is slated to register the fastest growth rate during the forecast period. In Africa, infectious diseases cause chronic illnesses, premature deaths, and loss of productivity, affecting the region’s economic growth. Thus, the emerging threats of infectious diseases drive the demand for IVD in Africa. The frequent use of reagents for diagnosing diseases and the easy availability & accessibility of these tests are the major factors driving the growth of this segment.
Based on technology, the point of care segment is slated to register the fastest growth rate during the forecast period. The rising geriatric population, the growing prevalence of chronic diseases, and the rising incidence of infectious diseases are driving the growth of this segment. The increasing burden of chronic diseases and pathogen outbreaks has significantly increased the demand for PoC diagnostics in Africa. The increased need for early and accurate diagnosis, precise confirmation of clinical findings, and immediate & informed decision-making on treating diseases contributes to the growth of the PoC segment.
Based on application, the oncology segment is slated to register the fastest growth rate during the forecast period. Cancer involves a complex set of genetic alternations and subsequent changes in gene expression, leading to the uncontrolled proliferation of cells. In cancer diagnostics, early diagnosis and knowing the exact nature of the tumor are of prime importance. Early cancer detection and access to effective anti-cancer treatment can result in higher rates of survival and a better quality of life. According to GLOBOCAN 2020, 1.1 million new cases and 711,429 deaths were recorded in Africa. The growing demand for personalized medicines in cancer therapy, the increasing prevalence of cancer, and the rising demand for rapid cancer diagnosis tests drive the growth of the IVD market for oncology applications.
Based on diagnostic approach, the OTC/self-testing segment is slated to register the fastest growth rate during the forecast period. In Africa, over-the-counter (OTC)/self-testing is promoted for diseases, such as HIV and COVID-19, due to the high prevalence of infectious diseases. Self-testing reduces the burden on healthcare workers and the system. The OTC/self-testing kits are diagnostic tests that can be purchased from any pharmacy store and used by the patient. These tests can give rapid results and can be taken from anywhere.
Quick Buy – Africa IVD Market Research Report: https://www.meticulousresearch.com/Checkout/47708335
Based on customer type, the home care/self-testing segment is slated to register the fastest growth rate during the forecast period. The growth of this segment is attributed to the increasing need to monitor and manage chronic diseases, the growing awareness about home healthcare testing products, and the reduced waiting time and healthcare costs offered by self-testing kits.
This research report analyzes the market across major African countries and provides a comprehensive analysis of South Africa, Algeria, Nigeria, Kenya, Morocco, Tunisia, Tanzania, Egypt, and the rest of Africa.
Key companies operating in the Africa IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Scope of the Report
Africa IVD Market, by Product Category
Reagents, Assays, and Kits
Instruments
Software & Services
Africa IVD Market, by Technology
Immunoassay
Whole Blood Glucose Monitoring
Point-of-Care Diagnostics
Clinical Chemistry
Molecular Diagnostics
Hematology
Coagulation & Hemostasis
Critical Care
Urinalysis
Other IVD Technologies
Note: Other technologies include microscopy, hybridization, and loop-mediated amplification.
Africa IVD Market, by Application
Infectious Diseases
Oncology
Diabetes
Endocrinology
Cardiology
Other Applications
Note: Other applications include nephrology, toxicology, gastroenterology, neonatal, genetic, and neurological disorders.
Africa IVD Market, by Diagnostic Approach
Laboratory Testing
Point-of-Care Testing
OTC/Self-testing
Africa IVD Market, by Customer Type
Hospital Labs
Private Labs
Home Care/Self Testing
Government
Other Customer Types
Note: Other customer types include long-term care facilities, academic & research institutes, ambulatory care centers, and transfusion laboratories.
Africa IVD Market, by Country
South Africa
Egypt
Nigeria
Kenya
Algeria
Tanzania
Morocco
Tunisia
Rest of Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
0 notes