#Heparinoids
Explore tagged Tumblr posts
Text
About 1.8 million people worldwide are bitten by snakes each year. Of those, up to 138,000 die and another 400,000 end up with permanent scarring and disability. Many cobras have tissue-damaging venoms that can't be treated with current antivenoms. We have discovered that cheap, readily available blood-thinning medications can be repurposed as antidotes for these venoms. Using CRISPR gene-editing technology we learned more about how these venoms attack our cells, and found out that a common class of drugs called heparinoids can protect tissue from the venom. Our research is published today in Science Translational Medicine.
Continue Reading.
133 notes
·
View notes
Text
Vera shrugged gamely and took a cursory glance over those tightly coiled braids and the rather obvious contusions. Whether it was a lack of tact or an attempted slight, there was no telling. Either way, Vera was primarily concerned about her patient’s pain level with swelling like that. She turned and opened the leftmost cabinet to fish out a little bottle of heparinoid. “This will reduce bruising and soothe soreness, if you’d like,” she said, placing the bottle beside the exam table.
Then, she turned to wash her hands thoroughly in the sink. “I’ll be running you through the usual tests today. Bloodwork. Medical history. You know the drill.” She dried her hands and snapped on a pair of blue nitrile gloves. “There is a little something extra,” Vera added, full attention on Atalanta. “I have to get to know the entire team fast. Before they throw us into the field.”
She sat down on the small swivel chair, mindful of her hands. “During this session, I’d like us to ask each other questions. Nothing terrible,” Vera followed quickly. “Little things are fine. Stupid questions are fine. We’ll go back and forth. If you don’t like a question, you can pass or ask another question of your own or omit details you don’t think are important. Same with me. The goal is not to make either of us uncomfortable. Just don’t lie. Be honest and we’ll get on fine.”
She stood and removed her stethoscope. “I’ll start. Is it alright if I begin the physical exam?”

Nadia was never going to be stoked for a doctor's visit, and one with a Foundation doctor fell even lower on her list. But, it was just the routine drudgery: usual exam and questions, boxes checked, and then Nadia could get back to... What was on the schedule today? Walking Club bullshit and the defense seminar. Endless fun.
She was early to the appointment and spent a few minutes undoing her braids and then resecuring them even more tightly than the first pass. Too late she realized, with the braids, the tank top and oversized flannel she was wearing exposed a bit too much of her neck. Fuck it, not like she was going to go back to her bunk and change just because--
Before her mind could ramble any further, Nadia reached out and knocked on the door. She didn't respond to Dr Nair's pleasantries beyond a shallow nod. Hauling herself onto the exam table, Nadia brought her feet up and crossed her legs like a kindergartener at circle time.
Like before, during introductions, Nadia felt a riot of anxious keening swell in her mind. All she could see was that moment before everything went to shit that night, Dr Nair and Dalton greeting Guin and all of them smiling. Dalton standing tall and alive and--
"Let's just. Get this over with, okay?"

23 notes
·
View notes
Text
Yantai Dongcheng Pharmaceutical Group Co Ltd Company Profile - Overview - GlobalData
Yantai Dongcheng Pharmaceutical Group Co Ltd (Yantai Dongcheng Pharma), formerly Yantai Dongcheng Biochemicals Co Ltd, is a developer, manufacturer, and marketer of biochemical raw materials, chemical synthetic drugs, and Chinese medicines. The company offers its products under categories including heparin series, chondroitin sulfate series, collagen, hyaluronic acid, glucosamine series, heparinoid and cytochrome C.
0 notes
Text
Chitosan Derivatives Market, Size, Share, Growth, Trends 2021-2028
The global chitosan derivatives market size was valued at US$ YY billion in 2020 and is estimated to reach US$ YY billion by 2028, growing at a CAGR of YY % during the forecast period (2021-2028).
Chitosan Derivatives, because of their capacity to penetrate GI barriers, induce mucoadhesion, and aid in transitory openings of tight junctions. Chitosan is a linear polysaccharide made up of N-acetyl-D-glucosamine and -linked D-glucosamine that are randomly dispersed.Its derivatives are extremely important in oral administration.
Download Free Sample: https://www.datamintelligence.com/download-sample/chitosan-derivatives-market
Market Dynamics
The application of chitosan in biomedicine and the use of chitosan for oral insulin delivery is estimated to drive the market.
The application of chitosan in biomedicine is estimated to drive the market
Chitosan is made via the deacetylation of chitin, a naturally occurring protein. It has active functional groups that are susceptible to chemical reactions allowing for chitosan derivatives. Modification of chitosan has been a major focus of chitosan research, with results demonstrating improved solubility, pH-sensitive targeting and a greater number of delivery systems, among other things.
Antibacterial materials are a novel class of functional materials that have the capability of killing or suppressing germs. Antibacterial materials, such as antibacterial plastics, antibacterial fibres and fabrics, antibacterial ceramics, and antibacterial methicillin, are a type of new functional material that can inhibit or kill. Chitosan and chitosan derivatives have long been utilised as antibacterial agents that are non-toxic or low-toxic. Chitosan is one of them. For example, quaternized chitosan has dramatically improved antibacterial activity compared to chitosan and can be employed in anti-inflammatory medications or as a filler fibre in wound dressing materials.
Chitosan and quaternized chitosan have yet to be proven to have antibacterial properties. There are just three possibilities. (1) Because chitosan and chitosan derivatives are positively charged, and bacteria are negatively charged, they attract and interact with one another due to electrostatic adsorption; (2) after adsorbing bacteria, chitosan and chitosan derivatives enter the inside of bacterial cells and bind to DNA, interfering with bacterial DNA transcription, resulting in bacterial death. The other application of chitosan is in bone tissue engineering. In bone tissue engineering, Carboxymethyl chitosan is a regularly used material. CMCS can also be used to construct nanofiber scaffolds in addition to the applications shown in CMCS. For the development of periosteal mimics in BTE, TMC and heparinoid are routinely employed materials.
The use of chitosan for oral insulin delivery is estimated to drive the chitosan derivatives market in the forecast period
Diabetes mellitus is a chronic endocrine disorder that affects over 400 million people worldwide. Patients with poorly regulated blood glucose levels are at risk of developing life-threatening consequences including cardiovascular disease, neuropathy, retinopathy, and even death. Subcutaneous, parenteral insulin is still the most prevalent form of insulin therapy today. Patients find oral insulin treatment to be beneficial and convenient. Oral insulin administration, unlike injection, replicates the physiological process of endogenous insulin secretion. Due to the hostile physiological environment through the gastrointestinal track, oral insulin has a low absorption (less than 2%). (GIT). Using insulin encapsulation into nanoparticles as an advanced technique, several attempts have been undertaken over the last few decades to create an effective oral insulin formulation with high bioavailability. Nanoparticles as a delivery mechanism for oral insulin administration have been created using a variety of natural polymers.
Because of its appealing qualities, such as biodegradability, biocompatibility, bioactivity, nontoxicity, and polycationic nature, chitosan, a natural polymer, has been extensively investigated. Several researches have been undertaken to assess the capabilities of chitosan and chitosan derivatives-based nanoparticles for oral insulin administration. Nanocarriers can increase insulin absorption via the GI tract, transport insulin to the bloodstream, and lower blood glucose levels. Although significant flaws in the technology, chitosan and chitosan derivative-based nanoparticles are highly attractive candidates for oral insulin delivery. The U.S FDA has approved that chitosan is safe in the use of foods and drugs.
The challenges faced by chitosan derivatives are estimated to hamper the chitosan derivatives market
Because chitosan is insoluble in water and most organic solvents, its applicability scope and fields of use are severely limited. Only when chitosan is in the polycationic form and at pH values below the pKa does it have antibacterial properties. The electrostatic interactions between polycationic chitosan molecules and negatively charged cell envelopes are responsible for chitosan's antibacterial properties. If the positive charge of chitosan is neutralised, food components such as NaCl, proteins, and carbohydrates have a negative impact on its activity. In order to manufacture safe and effective chitosan products, a lot of research has been done recently. However, the poor stability of chitosan-based systems limits its practical application; hence, establishing an adequate shelf-life for chitosan formulations has become a major difficulty. Controlling environmental parameters, altering production settings (e.g., temperature), introducing a suitable stabilising component, producing chitosan blends with another polymer, or modifying the chitosan structure using chemical or ionic agents can all help to improve stability.
For instance, even though chitosan is a unique and adaptable chemical with a wide range of applications in the pharmaceutical and biomedical disciplines, there are few pharmaceutical products based on it (only hemostatic dressings, wound-healing preparations, and nutraceutical goods are available). This could be due to chitosan's high hygroscopicity and the fact that chitosan material isolated from different sources varies greatly in molecular weight and molecular weight distribution, degree of deacetylation, and purity level. Furthermore, chitosan's high vulnerability to external influences and processing conditions (such as heating or freezing) can cause structural stress and polymer degradation.
COVID-19 Impact Analysis
COVID-19 has affected the healthcare industry. To stop its development, government-imposed lockdown. Chitin and chitosan biopolymers, as well as their derivatives have been widely studied for their antibacterial characteristics. They have the ability to stop a wide spectrum of bacteria and fungus from growing. The antibacterial activities of chitin or chitosan as direct antimicrobial agents have been investigated against a variety of diseases. They exert antibacterial actions by interacting with the negative surfaces of bacterial membranes due to the presence of NH3+ groups. Some investigations have attempted to determine whether chitosan nanoparticle technology can prevent coronavirus infection. However, more research is needed to create a completely validated method based on the application of these nanoparticles and the specified polymers against COVID-19. Chitosan and its derivatives have a lot of potential for therapeutic use against COVID-19 soon. Hence, the chitosan derivatives market is estimated to see a positive impact in the forecast period.
Segment Analysis
By Derivative Type:
· Carboxymethyl Chitosan
· Chitosan HCl
· Chitosan Quaternary Ammonium Salt
· Hydroxypropyl Chitosan
By End-use:
· Pharmaceutical and Bio-pharmaceutical
· Biotechnology
· Cosmetics
· Other
Geographical Analysis
North America region is estimated to dominate the global chitosan derivatives market
More pharmaceutical and biopharmaceutical research and development in this region is estimated to dominate the chitosan derivatives market in this region.
The United States Food and Drug Administration (FDA) has approved that chitosan is safe in the use of foods and drugs. The American Society of Testing Materials (ASTM F04 division IV) is working to develop standards for tissue-created medical goods. The F2103 guidance explains how to evaluate chitosan salts for usage in medical applications. Furthermore, chitosan hydrochloride (a chitosan derivative) has been added to the European Pharmacopeia.
The biopharmaceutical industry in the United States stands out as leading research and development (R&D) and advanced manufacturing industry at a time when economic competitiveness at the national and state levels is recognised to be strongly rooted in the ability to advance innovation-based industries. Over the last 30 years, the United States has cemented its position as the world's leading biopharmaceutical innovator. Today, that global leadership is based on a strong foundation of U.S. enterprises that perform and support sophisticated R&D as well as maintain a broad and large-scale supply chain for biopharmaceutical research, production, and distribution. The United States is the world's largest biopharmaceutical market, accounting for almost a third of global sales, and the world's leader in biopharmaceutical research and development. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), American companies do more than half of all pharmaceutical R&D ($75 billion) and own the intellectual property rights to the majority of new drugs.
Competitive Landscape
The major key players in the global chitosan derivatives market are FMC Corp, Kitozyme, Kunpoong Bio, BIO21, Heppe Medical Chitosan, Yaizu Suisankagaku, Golden-Shell, Lushen Bioengineering, AK BIOTECH, Zhejiang New Fuda Ocean Biotech, Weifang Sea Source Biological Products, Qingdao Honghai Biotech, Haidebei Marine Bioengineering, Jiangsu, Aoxin Biotechnology and Jinhu Crust Product.
FMC Corp
Overview: FMC Corporation is a global agricultural sciences firm dedicated to assisting growers in the production of food, feed, fibre and fuel for an ever-increasing global population while adapting to changing environmental conditions. Growers, crop advisers, turf and pest management specialists can more affordably address their most serious difficulties with FMC's revolutionary crop protection solutions, including biologicals, crop nutrition, digital, and precision agriculture.
capacity Product Portfolio: The company comprises of Crop Protection, Biologicals, Precision Agriculture, Plant Health, Agricultural Sciences, and Agriculture Chemicals
Key Development: In 2021, FMC Corporation formed a strategic partnership with UPL Ltd., a global provider of sustainable agriculture products and solutions, to extend Rynaxypyr active access to producers around the world and increase manufacturing.
View Full Report: https://www.datamintelligence.com/research-report/chitosan-derivatives-market
Enquiry Before Buying: https://www.datamintelligence.com/enquiry/chitosan-derivatives-market
About Us
DataM Intelligence was incorporated in the early weeks of 2017 as a Market Research and Consulting firm with just two people on board. Within a span of less than a year, we have secured more than 100 unique customers from established organizations all over the world.
For more information:
Sai Kiran
Sales Manager at DataM Intelligence
Email: [email protected]
Tel: +1 877 441 4866
Website: www.datamintelligence.com
0 notes
Text
The Importance of Drinking Water to Treat Swollen Legs and Feet
Swelling of the ft and ankles is medically called edema. It can have an effect on any a part of the frame aleven though it's miles maximum normally visible withinside the legs, ft, ankles, hands and hands. Edema can be because of sure scientific situations (liver disease, kidney disease, coronary heart failure or pregnancy) or because of sure medicinal drugs. Individuals affected by venous insufficiency might also revel in edema.

“Usually, those who be afflicted by heaviness and leg swelling make the error of now no longer ingesting sufficient water – says Dr. Marco Setti, Head of Vascular Surgery at Humanitas Gavazzeni. Instead, it's miles important to introduce fluids into the frame through consuming plenty of greens and end result in addition to ingesting herbal water. It is secure to anticipate from scientific help that phlebotonic medicinal drugs can assist enhance blood circulation. The unique lotions that incorporate phlebotonics and heparinoids lessen the “heat” this is manifested withinside the calves and might assist make a distinction even supposing it's miles most effective a minimum distinction.”
How does edema occur?
Edema happens whilst water leaks from the small blood vessels withinside the frame. The kidneys begin to react through maintaining greater sodium and water. The fluid then builds up in surrounding tissues, main to swelling. In such instances, it's miles important to motel to decrease sodium ingredients and beverages.
Mild instances of edema may also end result from the following:
Salty ingredients
Pregnancy
Premenstrual symptoms and symptoms and symptoms
Prolonged sitting
Certain medicinal drugs which could motive edema include:
Steroids
Estrogens
High blood stress medicinal drugs
Diabetes medicinal drugs
More excessive situations which could motive edema include:
Kidney damage
Kidney disease
Liver cirrhosis
Heart failure
Helpful guidelines to alleviate ache withinside the legs
“A few useful guidelines on relieving ache whilst you be afflicted by swollen ft and legs is to exercise, preserve energetic and drink masses of water. At night, you ought to additionally preserve your legs raised through resting them on a smooth pillow – provides Dr. Setti. Unfortunately, for women, hormonal troubles associated with pregnancy, using delivery manipulate capsules or different comparable troubles lead them to greater at risk of venous insufficiency than men. Edema impacts as many as 7 out of 10 women.”

Treatment commonly relies upon on looking after the underlying motive through:
Limiting sodium intake (stopping the frame from maintaining extra water)
Eating healthful ingredients
Drinking masses of fluids (1.5-2 liters of water a day)
Avoiding a sedentary lifestyle
Moving the ankles greater often
“When you're taking lengthy trips through plane, vehicle or train, your limbs are pressured into small areas or crouched positions. In such instances, it's miles advocated to put on rest stockings for the duration of the period of the experience with a purpose to enhance venous circulation. For people with episodes of thrombus phlebitis, it's miles cautioned to seek advice from a specialist. Sun publicity is authorized in such instances however with moderation. In instances of phlebitis inflammation, solar publicity is genuinely prohibited. Finally, it's miles cautioned to stroll alongside the water or swim even as you're at sea. Such sports produce a herbal and healthful hydro rub down at the frame
More.. best mineral water, natural mineral water 500ml, mineral water bottle price ,natural mineral water, pure mineral water
0 notes
Photo

とてもよくわかる>RT http://pic.twitter.com/sbTAPlRhnw
— |Ω| |憑き/橙色の((((00ヤ (@heparinoid) January 4, 2017
0 notes
Text
Damage to sebaceous gland and the efficacy of moisturizer after whole breast radiotherapy: a randomized controlled trial
Abstract
Background
We conducted a randomized trial to evaluate the efficacy of heparinoid moisturization for radiation dermatitis. We report the time-course of sebum content after whole breast radiotherapy (WBRT) and the efficacy of heparinoid moisturizer.
Methods
Patients receiving adjuvant breast RT were randomly assigned into three groups; prophylaxis, post-WBRT and control groups. Patients used moisturizer on the irradiated breast from the beginning of RT in the prophylaxis group, 2 weeks post-RT in the post-WBRT group, and no moisturizer in the control group. Sebum content of the irradiated and non-irradiated breast was measured to assess sebaceous gland damage. Sebum composition was also analyzed.
Results
A total of 76 patients were analyzed; 30 in the post-WBRT group, 32 in the control group, 14 in the prophylaxis group. The sebum content in the irradiated breast significantly decreased after WBRT in the post-WBRT and control groups. The decrease was sustained in the control group. In the non-irradiated breast, sebum content also decreased after WBRT in the post-WBRT and control groups. After moisturizer application, sebum content by sebumeter returned to pre-RT level in the post-WBRT group, while the decrease was sustained in the control group. Sebum content measured by evaporative light scattering detector and sebumeter was similar in the control group, but the dissociation was observed after moisturizer application in the post-WBRT group. The proportion of wax esters decreased in the irradiated breast after WBRT.
Conclusions
Radiotherapy significantly reduced sebum content in both irradiated and non-irradiated breast, indicating that RT caused quantifiably persistent sebaceous gland damage in irradiated sites and the surrounding tissue. Combined with the results from our previous study, heparinoid moisturizer treatment effectively prevents water loss by retaining oil contents on the skin surface.
Trial registration
UMIN, UMIN000005532. Registered 1 April 2011.
http://bit.ly/2Ghb612
0 notes
Text
Assessment of an Extended Interval Fondaparinux Dosing Regimen for Venous Thromboembolism Prophylaxis in Critically Ill Patients with Severe Renal Dysfunction Using Antifactor Xa Levels
Abstract
Objective
Pharmacologic options for venous thromboembolism (VTE) prophylaxis are often limited in critically ill patients due to thrombocytopenia and multisystem organ dysfunction. Fondaparinux offers potential advantages in the critically ill; however, it is currently contraindicated in severe renal dysfunction (SRD). We evaluated antifactor Xa levels in critically ill patients with SRD who were receiving an extended interval dosing regimen of fondaparinux for VTE prophylaxis.
Methods
A prospective, single arm, interventional study was conducted at two academic hospitals of the Detroit Medical Center. Eligible patients were in the intensive care unit, had an estimated creatinine clearance less than30 ml/min, and had either acute kidney injury or end-stage renal disease; several patients were on renal replacement therapy. Fondaparinux was administered at an extended interval dosing regimen of 2.5 mg subcutaneously every 48 hours. Fondaparinux peak and trough antifactor Xa levels were obtained. Lower extremity venous duplex studies were performed at baseline and study completion to assess for deep vein thrombosis (DVT), and patients were monitored for bleeding complications.
Results
Thirty two patients were enrolled. Patients received a median of 4 doses (interquartile range 2-5) of fondaparinux. Fondaparinux peak (n=98) and trough (n=86) antifactor Xa levels were 0.36 ± 0.18 mg/L and 0.17 ± 0.11 mg/L (mean ± SD), respectively, and were similar to levels reported in patients with normal renal function receiving conventional once-daily dosing. No lower extremity DVTs or suspected VTE events occurred. Two patients (6%) had significant bleeding events.
Conclusions
In critically ill patients with SRD, an extended interval fondaparinux dosing regimen of 2.5 mg every 48 hours for VTE prophylaxis achieved peak and trough antifactor Xa levels similar to those reported in noncritically ill patients with normal renal function receiving once-daily fondaparinux. This regimen offers an alternative for patients with SRD when heparinoids must be avoided.
This article is protected by copyright. All rights reserved.
from # All Medicine by Alexandros G. Sfakianakis via alkiviadis.1961 on Inoreader http://ift.tt/2xn4gP5 from OtoRhinoLaryngology - Alexandros G. Sfakianakis via Alexandros G.Sfakianakis on Inoreader http://ift.tt/2xneizG
0 notes
Text
Hyaluronik Asit Bazlı Dermal Dolgu Uygulamaları - Hyaluronidaz Enzimi ile İstenmeyen Etkinin Ortadan Kaldırılması
Estetik amaçlı dolgu uygulamaları, yüzdeki ince kırışıklıkların, derin çizgilerin, göz altındaki çökmelerin giderilmesi ve/ veya yüzün yeniden yapılandırılması amaçlarıyla yapılmaktadır; daha dolgun dudak ve daha belirgin elmacık kemikleri, ellerin, boyun ve dekolte bölgelerinin daha pürüzsüz ve genç bir görünüme sahip olması ve yüzün daha dolgun, daha parlak ve daha canlı bir görünüme sahip olması için en çok tercih edilen dolgu türü hyaluronik asit bazlı dermal dolgulardır.
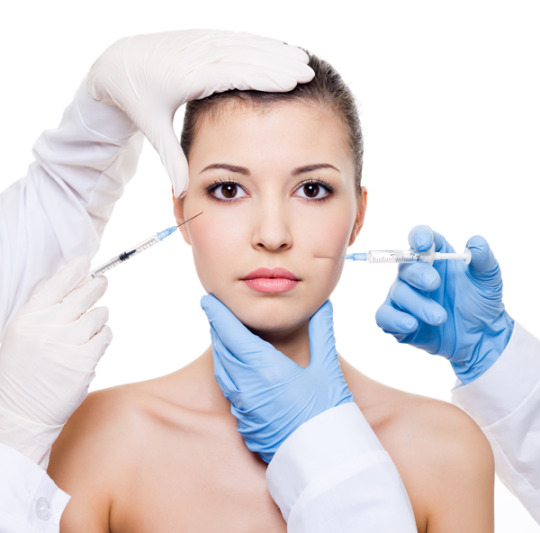
Alın kırışıklıkları, glabellar kırışıklıklar, periorbital kırışıklıklar ve peribukkal kırışıklıkların giderilmesinde genellikle 18-20 mg/ml yoğunlukta hyaluronik asit bazlı dermal dolgunun kullanımı yeterlidir. Glabellar kırışıklıklar ve peribukkal kırışıklıklar daha derin ise 20mg/ml yoğunluktan daha fazla bir yoğunluğa sahip (22 – 24 mg/ml) bir dolgu daha derine uygulanabilir.
Nazolabial katlantıların, hoşnutsuzluk çizgilerinin giderilmesinde, dudak hacminin arttırılmasında 22 -24 mg/ml yoğunluğa sahip dolguların kullanımı idealdir. Yanak hacminin arttırılması, elmacık kemiklerinin belirginleştirilmesi için ise yoğunluğu en az 24 mg/ml olan ve çapraz bağ yapısı daha yoğun olan ürünler tercih edilmelidir.
Dolgu uygulamalarında en riskli alan ise oldukça hassas olan göz çevresidir. Güvenli bölge sınırını aşmadan, oldukça yüzeysel bir şekilde yoğunluğu 18 mg/ml i aşmayan, çapraz bağ yapısı oldukça zayıf olan ya da hiç çapraz bağ içermeyen düz bağlı saf hyaluronik asidin mezodolgu uygulamaları tercih edilmelidir.
Dolgu seçimine ne kadar dikkat edilirse edilsin (özellikle bakteriyel fermantasyon ile üretilmiş, BDDE çapraz bağlayıcı ajanı içeren ve viskozitesi rahat uygulama sağlayan kaliteli bir dolgu tercih edilse bile) maalesef bir takım kontraendikasyonlar – yan etkiler gelişebilmektedir.
Hastaların dolgu enjeksiyonundan önceki 2-3 içerisinde aspirin, non-steroid antienflamatuvar kullanımından, C vitamini takviyesinden, yoğun çay-kahve tüketiminden kaçınması gerektiği konusunda bilgilendirilmeleri son derece önemlidir.
Enjeksiyon sonrası, masaj yapılması, Arnica veya türevi antienflatuvar ve heparinoid içeren bir krem sürülmesi son derece önemlidir. Ayrıca optimal ve uzun süreli bir etkinin sağlanabilmesi için, enjeksiyon sonrası hastanın 1-2 gün süresince; güneş, yetersiz uyku, sigara ve alkol tüketimi, özellikle yüz bölgesini kapsayan aşırı egzersizden kaçınması konusunda bilgilendirilmesi gerekmektedir.
Hamile veya emziren kadınlar, akne veya herpes gibi enflamatuvar ve/veya enfesiyöz tipte cilt sorunları olanlarda hyaluronik asit bazlı dolguların yaratabileceği etkilere dair yeterince klinik çalışma bulunmadığı için, kullanımı pek önerilmemektedir.
Yine hastanın patolojik geçmiş öyküsü de son derece önemlidir; hipertrofik yara geliştirme eğilimi, otoimmün hastalıklar, immünoterapi uygulaması, hyaluronik aside karşı aşırı duyarlılık hususlarında hastanın tıbbi geçmişi ayrıntılı bir şekilde sorgulanmalıdır.
Ayrıca, dolgu uygulamalarının hemen sonrasında; lazer, kimyasal peeling veya dermabrazyon işlemleri aynı seansta yapılmamalı; bu tarz uygulamalar için dolgu enjeksiyonundan sonra en az 1 hafta geçmesi beklenmelidir.
Hyaluronik asit bazlı dolgularda alerjik reaksiyon oluşması riski daha çok hyaluronik asit kaynağı ve kullanılan çapraz bağlama ajanı ile doğrudan alakalıdır. Dolgu seçimine ne kadar dikkat edilirse edilsin (özellikle bakteriyel fermantasyon ile üretilmiş, BDDE çapraz bağlayıcı ajanı içeren ve viskozitesi rahat uygulama sağlayan kaliteli bir dolgu tercih edilse bile) maalesef bir takım yan etkiler gelişebilmektedir. Enjeksiyondan sonra, kaşınma veya ağrıyla ilişkilendirilebilecek enflamatuvar reaksiyonlar (kızarıklık, ödem, eritem..) nadiren de olsa görülebilir. Ancak bu tarz yan etkiler en geç 1 hafta içerisinde son bulur.
Dolgu uygulamalarında ürüne bağlı olarak, uygulama yapılan hastaya bağlı olarak yada uygulamaya bağlı olarak nadiren de olsa sertlikler veya nodüller gözlemlenebilir. Enjeksiyon sonrası yapılan masajlar, enflamatuvar granülomalar gibi komplikasyonların görülmesini büyük ölçüde engeller.
Hasta konforunu arttırmak adına, lidokain, tetrakain gibi anestezik madde içeren dolgular da kullanıma sunulmuştur. Ancak bu tarz dolgularda şöyle bir dezavantaj bulunmaktadır; eritem ve morarma riski daha yüksektir, özellikle uygulama esnasında aynı bölgeye tekrar enjeksiyon yapıldığı takdirde ciddi morluklar oluşur ve kanama gözlemlenir. Ayrıca bazı hastalarda saf hyaluronik asit bazlı dolgunun alerjik etki göstemediği ancak, anestezik madde içeren hyaluronik asit bazlı dolguların alerjik etki gösterdiği gözlemlenmiştir. O nedenle, lokal olarak anestezik bir krem ile hastanın acı hissinin baskılanması daha uygun bir yöntemdir. Hyaluronik asit dolguların üretimlerinde, zaten ilgi tamponlar kullanılarak pH 7 olarak ayarlanmaktadır; enjeksiyonunda acı ve yanma hissi oldukça azdır; ancak dudak ve göz çevresinde hassasiyet biraz daha fazladır. O nedenle, anestezik bir krem ile lokal anestezi sağlanması tavsiye edilmektedir.
Bazı dolgu uygulamalarında, enjeksiyon bölgesinde nadiren de olsa renklenmeler görülebilir; geri dönüşümlüdür, bir hafta içerisinde düzelme beklenir.
Dolgu uygulamaları esnasında dolgunun etkisi hemen gözlemlenir, ancak ilk etapta iğnenin yaratmış olduğu travma neticesinde şişlikler ve ödem oluşacağından asıl etki 3-7 gün içerisinde gözlemlenir. O nedenle kontrol ve/veya korreksiyon 1 hafta sonra yapılır. Hipokorreksiyon durumunda aynı dolgu (çapraz bağ ajanı açısından önemlidir) ya da düz bağlı saf hyaluronik asit ile nokta enjeksiyonu yapılabilir. Aşırı şişkinliğin gözlemlendiği hiperkorreksiyon durumunda ise termal tedavi, özellikle radyofrekans uygulaması önerilir ve implantın merkezine hyaluronidaz enzim enjeksiyonu yapılabilir. Genellikle, piyasada bulunan hyaluronidaz enzimleri 100 U/ml ya da 150 U/ml formundadır. İlk işlemde 0,1-0,2 ml hyaluronidaz enzimi, dolgunun yapıldığı derinlikle aynı derinliğe nokta enjeksiyonu şeklinde uygulanmalı ve mutlaka masaj yapılmalıdır. Tek seferde 0,5 ml den fazla hyaluronidaz enzimleri verilmemeli ve asıl sonucu görmek adına en az 3 gün beklenmelidir. Eğer gerek görülür ise 2. enzim enjeksiyonu 3 gün sonra yine 0,1-0,2 ml olarak uygulanmalıdır. Dolgunun gözlemlenen fazla etkisinin veya yarattığı nodüllerin giderilmesinde; hem radyofrekans hem de enzim enjeksiyonu yapılacak ise; dokuda yaratılacak ısı etkisiyle çapraz bağ yapısı zayıflatılarak, su tutucu, hacim arttırıcı etki termal etki ile azaltılacaksa bu uygulama daha önce yapılmalı, en az 15-20 dakika sonrasında enzim enjeksiyonu yapılmalıdır; çünkü ısı enzimin yapısını bozar ve aktivitesini zayıflatır. Enzim enjeksiyonundan hemen sonra ise termal etki yaratacak hiçbir işlem uygulanmamalıdır.
#dermal dolgu#asit bazlı dermal dolgu#hyaluronik asit bazlı dermal dolgu#hyaluronidaz enzimleri#hyaluronidaz enzimi
0 notes
Text
New Post has been published on Pharmapedia
New Post has been published on https://pharmapedia.pw/2017/06/11/%d8%b2%d8%a7%d8%af%d9%8a%d9%85%d8%a7-%d8%ac%d9%8a%d9%84-zadema-gel/
زاديما جيل Zadema Gel | لعلاج الكدمات والتورمات والالتواءات

زاديما جيل Zadema Gel
زاديما جيل Zadema Gel | لعلاج الكدمات والتورمات والالتواءات
المادة الفعالة في زاديما جيل Zadema Gel :
يحتوي زاديما جيل Zadema Gel علي ثلاثة مواد هي Aescine + Diethylamine salicylate + heparinoid
دواعي الاستخدام :
يستخدم زاديما جيل Zadema Gel في علاج الكدمات ، الالتواءات ، التجمعات ��لدموية ، الام الرقبة والعمود الفقري ، التهاب الاوتار ، التهاب الاوردة والدوالي ، اللومباجو ، عرق النسا
موانع الاستخدام :
يمنع استخدام زاديما جيل Zadema Gel في حالة اذا كانت لديك حساسية لاحدي المكونات او جميعها
فولتارين جيل
0 notes
Text
Anti-cancer effect of dung beetle glycosaminoglycans on melanoma
Abstract
Background
Dung beetle glycosaminoglycan is known to possess anti-aging activities. However, its anti-cancer mechanisms are not fully elucidated yet. The objective of this study was to evaluate the anti-cancer effect of insect-derived polymer dung beetle glycosaminoglycan (GAG) after intraperitoneally injecting it to melanoma mice induced by B16F10 cells.
Methods
To determine molecular mechanism involved in the anti-cancer effect of dung beetle GAG, its origin N-glycan under 3KD Dalton was assayed for melanoma cell cytotoxicity. Quantitative comparisons of adhesive molecule on extracellular matrix and activities of tissue inhibitor of metalloprotease 2 (TIMP-2) were also investigated. In vivo anti-cancer effect of dung beetle GAG on solid tumor size, survival time and gene-expression profiles was also assayed using B10F10 melanoma mice model. Mice with induced melanoma were then treated with Catharsius molossus (dung beetle) GAG (CaG) at 5 mg/kg for 8 weeks to investigate its anti-cancer effects compared to bumblebee (Bombus ignitus) queen glycosaminoglycan (IQG) and Huechys sanguinea glycosaminoglycan (HEG).
Results
These N-glycans derived from these GAG were composed of many linear heparinoid polysaccharides, polymers with hexose and N-acetylhexose. Adminstration with these GAGs increased survival time and decreased melanoma sizes in mice, in accordance with their inhibitory effects on cell growth ratio of melanoma B16F10. In addition, treatment with N-glycans derived from theses glycosaminoglycan increased activities of TIMP-2 in HMVEC cells pretreated with TNF-alpha and in melanoma cells, suggesting that they had anti-inflammatory and anticancer activities. In DNA microarray results, compared to control, CaG treated mouse group showed upregulation of 192 genes including collagen,typeI,alpha1 (Col1a1), consistent with the highly increased in vitro extracellular matrix (ECM) adhesion on collagen 1 and up-regulation of heparanase (Hpse). After treatment with CaG, a total of 152 genes were down-regulated, including nuclear RNA export factor (Nxf3) and hyaluronan proteoglycan link protein1 (Hapln1).
Conclusions
Glycosaminoglycan, CaG can strengthen ECM by increasing activity of TIMP-2 and adhesion activity on collagen known to inhibit changes of ECM, leading to tumor cell invasion and progression.
http://bit.ly/2R8fO7k
0 notes