#Heart Defect Closure Devices Market Growth
Explore tagged Tumblr posts
Text
Understanding Metastatic HER2-Positive Breast Cancer
What is Metastatic HER2-Positive Breast Cancer?
HER2-positive breast cancer is a subtype identified by an overexpression of the HER2 protein, which accelerates cancer cell growth. When the cancer extends beyond the breast and nearby lymph nodes to distant organs, it is classified as metastatic HER2-positive breast cancer. Due to its aggressive nature, this condition typically requires specialized HER2-targeted therapies.
Symptoms of HER2-Positive Breast Cancer
The symptoms of HER2-positive breast cancer depend on the organs affected. Common signs include:
Persistent cough
Bone pain
Shortness of breath
Severe headaches
Jaundice
Unexplained weight loss Other indicators may include skin changes, swollen lymph nodes, and chronic fatigue. Early detection and symptom management are crucial for improving patient outcomes.
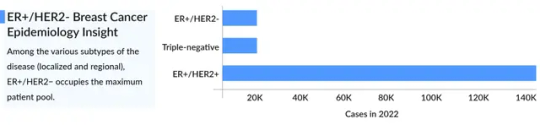
Prevalence of HER2-Positive Breast Cancer Cases
HER2-positive breast cancer accounts for approximately 15-20% of all breast cancer cases. A significant portion of these cases progress to stage 4 HER2-positive breast cancer, contributing to the overall HER2-positive metastatic breast cancer landscape. While advancements in treatment have led to better survival rates, this form of cancer remains a global challenge.
HER2-Targeted Therapies for Metastatic HER2-Positive Breast Cancer
Several leading pharmaceutical companies, such as Roche, Merck, and Immunomedics, have developed innovative HER2-targeted therapies. Notable treatments include:
Trastuzumab (Herceptin)
Pertuzumab (Perjeta)
Trastuzumab deruxtecan (Enhertu) These therapies have significantly improved survival rates and quality of life. Researchers are also exploring immunotherapies and combination treatments for enhanced effectiveness.
The HER2 Breast Cancer Pipeline: Future Prospects
The HER2 breast cancer pipeline continues to expand with promising new therapies undergoing clinical trials. Research efforts focus on:
Novel HER2 inhibitors
Antibody-drug conjugates
Advanced immunotherapies Companies like Roche and Merck are at the forefront of HER2-positive metastatic breast cancer research, aiming to develop more effective treatment options.
Conclusion
Advancements in the HER2-positive breast cancer market are revolutionizing treatment approaches, offering hope for improved survival rates. As HER2-positive metastatic breast cancer research progresses, early detection and targeted therapy remain crucial in managing the disease effectively. With ongoing clinical trials and new therapeutic innovations, the future holds promise for better treatment options and improved patient outcomes.
Another Reports Offered By Delveinsight
Liquid Biopsy for Cancer Diagnostics Market | Plasmodium Vivax Malaria Market | Polycystic Ovarian Syndrome Market | Short Bowel Syndrome Drugs Market | Somatotropin Deficiency Market | Temporomandibular Disorders Market | Testicular Neoplasm Market |Venous Ulcer Market | Adeno-Associated Viruses (AAV) Gene Therapy Market | Blastomycosis Market | Carcinoid Syndrome Market | Congenital Heart Defect Market | CXCR Inhibitors Market | Hip Replacement Devices Market | Myeloproliferative Neoplasms Market | Nocturia Market | Percutaneous Arterial Closure Device Market | Peripheral SpA Market | Psoriasis Vulgaris Market | Radial Artery Compression Device Market | Schistosomiasis Market | Type 1 Diabetes Market | Vital Sign Monitors Devices Market | Atherosclerosis Market | Avascular Necrosis Market | Gene Therapy in CNS Disorder Market | Pediatric Neuroblastoma Market | Spinal Trauma Devices Market | Surgical Lasers Market | Thyroid Cancer Market | Ventral Hernia Market
Contact Information
Kanishk
0 notes
Text
0 notes
Text
Heart Defect Closure Devices Market
Heart Defect Closure Devices Market Size, Share, Trends: Abbott Laboratories Leads
Shift Towards Transcatheter Procedures for Heart Defect Closure
Market Overview:
The Heart Defect Closure Devices Market is projected to grow at a CAGR of 9.2% during the forecast period of 2024-2031. The market value is expected to rise from XX USD in 2024 to YY USD by 2031, with North America emerging as the dominant region. Key metrics include increasing prevalence of congenital heart defects, advancements in minimally invasive procedures, and growing geriatric population. The market is experiencing robust growth driven by technological innovations in device design, rising awareness about structural heart diseases, and improving healthcare infrastructure in developing countries.
DOWNLOAD FREE SAMPLE
Market Trends:
The market for heart defect closure devices is rapidly transitioning towards transcatheter procedures, which are less invasive and provide better patient outcomes. This movement is characterized by the development of advanced closure devices that can be delivered via catheters, thus eliminating the need for open-heart surgery. Manufacturers are focusing on developing devices with enhanced deliverability, a smaller profile, and higher closure efficiency. The use of 3D printing technology in device manufacturing enables the creation of patient-specific closure devices, enhancing the success of transcatheter procedures. This trend not only accelerates patient recovery and reduces hospital stays but also expands the range of treatable congenital heart defects, including complex anatomies that were previously untreatable.
Market Segmentation:
Septal occluders dominate the market, accounting for over 45% of total heart defect closure device sales globally. Septal occluders continue to lead the heart defect closure devices market due to their widespread use in treating common congenital heart defects such as atrial septal defects (ASD) and ventricular septal defects (VSD). The popularity of septal occluders can be attributed to their ability to provide a less invasive alternative to open-heart surgery for defect closure, resulting in quicker recovery and fewer complications. Recent market data indicates that demand for septal occluders has increased by 10% year over year in major healthcare markets like the United States and Europe. This growth is driven by advancements in device design, which have led to improved closure rates and reduced procedural complications.
Market Key Players:
Abbott Laboratories
Boston Scientific Corporation
W. L. Gore & Associates
Occlutech
Lifetech Scientific Corporation
Cardia Inc.
Contact Us:
Name: Hari Krishna
Email us: [email protected]
Website: https://aurorawaveintellects.com/
0 notes
Text
Transseptal Access Systems Market Key Players, Industry Overview, Applications and Analysis 2032
The Transseptal Access Systems Market is a specialized sector within the medical device industry that focuses on devices and tools used to access the left atrium of the heart through the interatrial septum, a thin wall that separates the heart's upper chambers. Transseptal access is a critical procedure in various cardiovascular interventions, including catheter-based ablation for arrhythmias, structural heart procedures like atrial septal defect (ASD) and patent foramen ovale (PFO) closure, as well as left atrial appendage occlusion to reduce the risk of stroke in atrial fibrillation patients. The market for these systems has witnessed substantial growth due to the increasing prevalence of cardiovascular diseases and the expanding array of minimally invasive cardiac procedures.
One of the key drivers of growth in the Transseptal Access Systems Market is the rising incidence of atrial fibrillation (AF), a common cardiac arrhythmia. As AF is associated with an elevated risk of stroke, patients often require interventions like left atrial appendage occlusion, which necessitates transseptal access. Furthermore, advancements in catheter-based therapies for structural heart conditions, such as ASD and PFO closures, have expanded the applications of transseptal access systems. The market has responded with innovations in device design and improved safety profiles, making these procedures more accessible and less invasive for patients.
The Transseptal Access Systems Market also benefits from ongoing technological advancements. Novel catheter designs, real-time imaging technologies, and navigation systems have significantly improved the precision and safety of transseptal procedures. These innovations not only enhance the overall patient experience but also reduce the procedural risk for physicians. Additionally, the market is witnessing a shift towards the development of integrated systems that offer comprehensive solutions for transseptal access, further simplifying and streamlining these complex procedures.
For More Info@ https://www.prnewswire.com/news-releases/transseptal-access-systems-market-is-projected-to-grow-at-a-cagr-of-104-during-2017-to-2025---persistence-market-research-665416633.html
Despite the positive growth prospects, the market faces challenges related to safety and the learning curve for physicians adopting these procedures. Ensuring the proper training and proficiency of healthcare professionals using transseptal access systems is crucial to minimizing complications and optimizing patient outcomes. Regulatory and reimbursement hurdles also need to be addressed to ensure widespread adoption of these devices. In conclusion, the Transseptal Access Systems Market is expected to continue its expansion, driven by the increasing prevalence of cardiovascular diseases, technological innovations, and the growing acceptance of minimally invasive cardiac procedures.
0 notes
Text
0 notes
Text
0 notes
Text
Global Heart Defect Closure Devices Market 2019-2023

AF is the most commonly encountered arrhythmia in clinical practice. It is associated with increased morbidity, especially stroke and heart failure, and mortality. AF is becoming a major public health burden across the world, and its prevalence is expected to increase due to the rapidly growing geriatric population, especially in developing countries. Other risk factors of AF such as high blood pressure (BP), obesity, diabetes, heart failure, ischemic heart disease, chronic kidney disease (CKD), and the heavy consumption of alcohol are also on the rise. Hence, the rising prevalence of AF will increase the demand for LAA closure therapy. Which will drive the growth of the global heart defect closure devices market. Our analysts have predicted that the heart defect closure devices market will register CAGR of over 29% by 2023.
Request Sample Report @ https://www.acquiremarketresearch.com/sample-request/718
Market Overview
Technological advances in heart defect closure
There had been several significant technological advances in all forms of cardiovascular care. Especially in the treatment of structural heart disease, in the last decade. The advances in catheterization techniques, medical imaging, and devices have increased the adoption of minimally invasive procedures. Therefore, interventional cardiology procedures have now become the standard of care to treat ASD and PFO.
Shortage of skilled surgeons to perform heart defect closure procedures
There is a global shortage of cardiologists, including general cardiologists, electrophysiologists: interventional cardiologists, both coronary and peripheral, and pediatric cardiologists. This shortage is attributed to the aging cardiology community, limited growth in fellowship positions, increasing burden of cardiovascular diseases (CVDs): and evolving Medical Devices & Consumables reforms.
For the detailed list of factors that will drive and challenge the growth of the heart defect closure devices market during the 2019-2023, view our report.
Competitive Landscape
The market appears to be moderately concentrated and with the presence of few vendors. This market research report will help clients identify new growth opportunities and design unique growth strategies by providing a comprehensive analysis of the markets competitive landscape and offering information on the products offered by companies.
More Info and TOC @ https://www.acquiremarketresearch.com/industry-reports/global-heart-defect-closure-devices-market-2019-2023/718/
Table of Contents:
PART 01: EXECUTIVE SUMMARY
PART 02: SCOPE OF THE REPORT
- 2.1 Preface
- 2.2 Preface
- 2.3 Currency conversion rates for US$
PART 03: MARKET LANDSCAPE
- Market ecosystem
- Market characteristics
- Market segmentation analysis
PART 04: MARKET SIZING
- Market definition
- Market sizing 2018
- Market size and forecast 2018-2023
PART 05: FIVE FORCES ANALYSIS
- Bargaining power of buyers
- Bargaining power of suppliers
- Threat of new entrants
- Threat of substitutes
- Threat of rivalry
- Market condition
PART 06: MARKET SEGMENTATION BY PRODUCT
- Market segmentation by product
- Comparison by product
- LAA closure devices - Market size and forecast 2018-2023
- PFO closure devices - Market size and forecast 2018-2023
- ASD closure devices - Market size and forecast 2018-2023
- Other heart defect closure devices - Market size and forecast 2018-2023
- Market opportunity by product
Request for Discount @ https://www.acquiremarketresearch.com/discount-request/718
About Acquire Market Research: Acquire Market Research is a shrine of world-class research reports from around the world and we offer you only the best in the Industry when it comes to research. At Acquire, every data need will be catered to and met with a powerful world of choices. "We understand the integral role data plays in the growth of Business empires."
Contact Us: 555 Madison Avenue, 5th Floor, Manhattan, New York, 10022 USA Phone No.: +1 (800) 663-5579 Email ID:[email protected]
#Heart Defect Closure Devices Market#Heart Defect Closure Devices Market Trends#Heart Defect Closure Devices Market Research#Heart Defect Closure Devices Market Growth#Heart Defect Closure Devices Market Share
0 notes
Text
Global Congenital Heart Defect Closure Devices Market Size, Share, Trends and Forecast by 2027
The recent report on “Global Congenital Heart Defect Closure Devices Market Report 2021 by Key Players, Types, Applications, Countries, Market Size, Forecast to 2027” offered by Axel Reports, comprises a comprehensive investigation into the geographical landscape, industry size along with the revenue estimation of the business. Additionally, the report also highlights the challenges impeding market growth and expansion strategies employed by leading companies in the “Congenital Heart Defect Closure Devices Market”.
An exhaustive competition analysis that covers insightful data on industry leaders is intended to help potential market entrants and existing players in competition with the right direction to arrive at their decisions. Market structure analysis discusses in detail Congenital Heart Defect Closure Devices companies with their profiles, revenue shares in the market, comprehensive portfolio of their offerings, networking and distribution strategies, regional market footprints, and much more.
Download Sample PDF+ All Related Graphs & Charts (Including COVID19 Impact Analysis) @: https://axelreports.com/request-sample/67099
Global Market Segmentation by Top Key-Players: St.Jude Medical Edwards Lifesciences Gore Medical Siemens Healthcare Boston Scientific GE Healthcare Abbott
Market segments by Types of, the report covers- Atrial Septal Defect Ventricular Septal Defect Patent Foramen Ovale Patent Foramen Ovale Other Market segments by Applications of, the report covers- Hospitals Clinics Other
(Note: The sample of this report is updated with COVID-19 impact analysis before delivery)
Key Questions Covered in the Report :
What is the total market value of the Global Congenital Heart Defect Closure Devices Market report?
What would be the forecast period in the market report?
What is the market value of the Global Congenital Heart Defect Closure Devices Market in 2021?
What is the Key Industry Leader’s opinion for the Global Congenital Heart Defect Closure Devices?
Which is the base year calculated in the Global Congenital Heart Defect Closure Devices Market Report?
What are the key trends in the Global Congenital Heart Defect Closure Devices Market Report?
What are the market values/growth % of emerging countries?
Which market holds the maximum market share of the Global Congenital Heart Defect Closure Devices Market?
Some Point from Table of Content:
Market Overview: It includes six chapters, research scope, major manufacturers covered, market segments by type, Congenital Heart Defect Closure Devices market segments by application, study objectives, and years considered.
Market Landscape: Here, the competition in the Worldwide Congenital Heart Defect Closure Devices Market is analyzed, by price, revenue, sales, and market share by company, market rate, competitive situations Landscape, and latest trends, merger, expansion, acquisition, and market shares of top companies.
Profiles of Manufacturers: Here, leading players of the global Congenital Heart Defect Closure Devices market are studied based on sales area, key products, gross margin, revenue, price, and production.
Market Status and Outlook by Region: In this section, the report discusses about gross margin, sales, revenue, production, market share, CAGR, and market size by region. Here, the global Congenital Heart Defect Closure Devices Market is deeply analysed on the basis of regions and countries such as North America, Europe, China, India, Japan, and the MEA.
Application or End User: This section of the research study shows how different end-user/application segments contribute to the global Congenital Heart Defect Closure Devices Market.
Market Forecast: Production Side: In this part of the report, the authors have focused on production and production value forecast, key producers forecast, and production and production value forecast by type.
Research Findings and Conclusion: This is one of the last sections of the report where the findings of the analysts and the conclusion of the research study are provided.
Do You Have Any Query Or Specific Requirement? Ask to Our Industry Expert @ https://axelreports.com/enquiry-before-buying/67099
Note: This content doesn’t contain all the Information of the Report please fill the form (via link) and get all interesting information just one click in PDF with the latest update with chart and Table of Content. Any special requirements about this report, please let us know and we can provide custom report.
ABOUT US:
Axel Reports has the most comprehensive collection of market research products and services available on the web. We deliver reports from virtually all major publications and refresh our list regularly to provide you with immediate online access to the world’s most extensive and up-to-date archive of professional insights into global markets, companies, goods, and patterns.
Contact: Axel Reports Akansha G (Knowledge Partner) Office No- B 201 Pune, Maharashtra 411060 Phone: US +18488639402 Web: https://axelreports.com/
#congenital heart defect closure devices#heart defect closure devices#congenital heart defect closure devices market
1 note
·
View note
Text
Heart Defect Closure Devices Market 2018-2022 Growth, Trends and Demands Research Report
Heart Defect Closure Devices Market 2018-2022 Growth, Trends and Demands Research Report
Heart Defect Closure Devices Report by Material, Application, and Geography – Global Forecast to 2022 is a professional and in-depth research report on the world’s major regional market conditions, focusing on the main regions (North America, Europe and Asia-Pacific) and the main countries (United States, Germany, united Kingdom, Japan, South Korea and China).
Download Sample Copy: https://www.al…
View On WordPress
#Heart Defect Closure Devices market growth#Heart Defect Closure Devices market size#Heart Defect Closure Devices#Heart Defect Closure Devices Industry#Heart Defect Closure Devices Market#Heart Defect Closure Devices market 2018#Heart Defect Closure Devices market 2022#Heart Defect Closure Devices market analysis#Heart Defect Closure Devices market demands#Heart Defect Closure Devices market forecast#Heart Defect Closure Devices market study#Heart Defect Closure Devices market trends#Heart Defect Closure Devices Research Report#Heart Defect Closure Devices sector
0 notes
Text
Asia-Pacific Heart Defect Closure Devices Market- Worldwide Analysis of Company Profile’s Forecast to 2018-2023
Asia-Pacific Heart Defect Closure Devices Market- Worldwide Analysis of Company Profile’s Forecast to 2018-2023
MarketResearchNest.com adds “Asia-Pacific Heart Defect Closure Devices Market Analysis 2012-2017 and Forecast 2018-2023” new report to its research database.The records spread across 122 with more than one tables and figures in it. Description:- Heart defect closure devices are permanent implants designed to close defects between chambers of the heart or a patent ductus arteriosus. These are…
View On WordPress
#Heart Defect Closure Devices#Heart Defect Closure Devices Industry#Heart Defect Closure Devices Industry Analysis#Heart Defect Closure Devices Market#Heart Defect Closure Devices Market Analysis#Heart Defect Closure Devices Market Growth#Heart Defect Closure Devices Market Share
0 notes
Text
Global Structural Heart Devices Market to Grow at a CAGR of 11.1% during Forecast Period
Global Structural Heart Devices Market is flourishing owing to the rising prevalence of various structural heart diseases, a surge in expanded indication approval for various transcatheter structural heart products, and an increase in the geriatric population burdened with various degenerative valvular heart diseases…
A recent study conducted by the strategic consulting and market research firm, BlueWeave Consulting, revealed that the Global Structural Heart Devices Market was worth USD 11.63 billion in the year 2021. The market is projected to grow at a CAGR of 11.1%, earning revenues of around USD 24.16 billion by the end of 2028. The Global Structural Heart Devices Market is booming because of the rising structural heart disease cases. Moreover, these diseases are mostly associated with heart valves or tissues. These diseases are known to be congenital, meaning they can be present from birth, though some structural heart diseases develop later in life. Additionally, the increase in demand for highly efficient procedures among people, the growing preference for minimally invasive structural heart therapeutic products among the value-oriented patient population, and the increase in R&D spending for developing innovative and technologically advanced products all contribute to the structural heart devices market's growth. Furthermore, Global Structural Heart Devices Market is one of the most emerging markets that grow continuously owing to the fast integration of new technologies such as the Internet of Things (IoT), Artificial Intelligence (AI), cloud computing, and others. However, Low cardiac surgery affordability and accessibility in developing countries are expected to limit market growth in the coming years. However, a shortage of skilled personnel may have an impact on overall procedural volumes, slowing the rate of market growth during the forecast period (2022-2028).
The Number of Patients Suffering from Structural Heart Disease is Increasing.
Because of the increased number of patients suffering from structural heart diseases, there has been an increase in demand for structural heart devices. Because these disorders are generally congenital, they are very common in newborns. According to The Nemours Foundation, approximately one in every 100 newborns has congenital heart defects, which can range from mild to severe. According to Micro Interventional Devices, Inc., structural heart defects affect approximately 60 million people in the United States, or 20-25 percent of the adult population. According to the Center for Structural Heart Disease at Henry Ford Hospital (CSHD), approximately 250,000 patients are diagnosed with mitral valve diseases each year at Henry Ford Hospital. Furthermore, 1.75 million people in Europe have aortic stenosis. These statistics demonstrate the need for structural heart treatment devices, providing impetus to develop new techniques for heart defect repair and replacement. TAVR procedures were approved in Europe in 2007, and in the United States in 2012. Furthermore, each year, approximately 80,000 patients undergo mitral valve repair or valve replacement surgery. All these factors boost the growth of the Global Structural Heart Devices Market during the forecast period (2022-2028).

Request for Sample Report @ https://www.blueweaveconsulting.com/report/structural-heart-devices-market/report-sample
Demand for minimally invasive procedures is increasing
Because structural cardiac procedures and devices are less intrusive than other procedures, such as open-heart operations, they are highly desired. Professionals in cardiology routinely perform minimally invasive procedures to replace the aortic, mitral, and artificial heart valves to treat disorders with the structural heart. Additionally, innovative advancements in the structural heart treatment and device markets, such as left arterial appendage closure devices, aortic valve replacement valves, and the introduction of biological and tissue valves, are anticipated to offer potential prospects in the projected future.
Challenge: The Structural Heart Device Approval Process is Rigorous.
Most countries have stringent approval processes for structural heart devices because these are highly specialized devices that are implanted in the body. According to the FDA, structural heart devices are class III devices, whereas these devices are CE mark classified as class IIa (heart valve occluders and testers) and class III (for prosthetic and biological heart valves). Furthermore, the procedure is time-consuming and expensive. According to the Advanced Medical Technology Association, FDA-linked approval stages account for approximately 77 percent and 80 percent of the total cost of commercializing a class II and class III medical device in the market, respectively.
Segmental Coverage
The Global Structural Heart Devices Market is divided into Replacement procedures are further subdivided into TAVR (transcatheter aortic valve replacement) procedures and SAVR (surgical aortic valve replacement) procedures, while repair procedures are further subdivided into Closure Procedures, Annuloplasty, Valvuloplasty, TMVR Procedures, and Others. During the forecast period, the replacement procedures segment will have the largest market share (2022-2028). The segment's growth can be attributed to the long-term durability of these procedures, as well as the widespread preference for transcatheter replacement. During the forecast period, all of these factors contribute to the growth of the Global Structural Heart Devices Market (2022-2028).
Please Visit Press Release of the Global Structural Heart Devices Market: https://www.blueweaveconsulting.com/press-release/global-structural-heart-devices-market-to-grow-at-a-cagr-of-11-1-during-forecast-period
Impact of COVID-19 on Global Structural Heart Devices Market
Around the world, the COVID-19 pandemic has affected several different businesses. Governments all across the world enacted stringent lockdown regulations and social segregation standards to slow the pandemic's quick spread. Manufacturing plants all across the world were closed during the pandemic's early phases. The commercialization of the structural heart devices market may also be significantly delayed as a result of the economic crisis that followed the pandemic. Supply chain interruptions presented several difficulties for market participants. However, things will get better over the projection period (2022-2028) as more supplies become available in the second half of 2022.
Competitive Landscape
The leading market players in the Global Structural Heart DevicesMarket are Abbott Laboratories, Affluent Medical SA, Artivion Inc, AtriCure Inc, CORONEO Inc, Braile Biomedica, Boston Scientific Corporation, Getinge AB, Jc Medical Inc, Johnson and Johnson Inc, and other prominent players
The Global Structural Heart DevicesMarket is highly fragmented with the presence of several manufacturing companies in the country. The market leaders retain their supremacy by spending on research and development, incorporating cutting-edge technology into their goods, and releasing upgraded items for customers. Various tactics, including strategic alliances, agreements, mergers, and partnerships, are used.
Don’t miss the business opportunity in the Global Structural Heart Devices Market. Consult our analysts to gain crucial insights and facilitate your business growth.
The in-depth analysis of the report provides information about growth potential, upcoming trends, and statistics of the Global Structural Heart DevicesMarket. It also highlights the factors driving forecasts of total market size. The report promises to provide recent technology trends in the Global Structural Heart DevicesMarket and industry insights to help decision-makers make sound strategic decisions. Furthermore, the report also analyzes the growth drivers, challenges, and competitive dynamics of the market.
Please Find Below Some Related Report:
Surgical Robotics Market- Global Industry Size, Share, Trend Analysis and Forecast Report, 2018-2028
Saudi Arabia Digital Pathology Market- Size, Share, Trend Analysis and Forecast Report, 2018-2028
Europe Dental Implants Market- Size, Share, Trend Analysis and Forecast Report, 2018-2028
Pharmaceutical Excipients Market- Global Industry Size, Share, Growth, Opportunity and Forecast, 2018-2028
Veterinary Infectious Disease Diagnostics Market- Global Industry Size, Share, Growth, Opportunity and Forecast, 2018-2028
About Us
BlueWeave Consulting provides comprehensive Market Intelligence (MI) Solutions to businesses regarding various products and services online and offline. We offer all-inclusive market research reports by analyzing both qualitative and quantitative data to boost the performance of your business solutions. BWC has built its reputation from the scratch by delivering quality inputs and nourishing long-lasting relationships with its clients. We are one of the promising digital MI solutions companies providing agile assistance to make your business endeavors successful.
Contact Us:
BlueWeave Consulting & Research Pvt. Ltd
+1 866 658 6826 | +1 425 320 4776 | +44 1865 60 0662
https://www.linkedin.com/company/blueweaveconsulting/
0 notes
Text
Structural Heart Devices Market Size, Revenue Growth Factors & Trends, Key Player Strategy Analysis Till 2027

Structural heart diseases are problems associated with the valves or tissues of the heart. These diseases may be congenital or might be acquired later in life. Structural heart diseases include cardiomyopathy, myocarditis, aortic valve stenosis, heart valve disease, atrial septal defect, mitral valve regurgitation, and others. As per the latest report of Market Research Future (MRFR), the structural heart devices market is poised to expand at CAGR of 10.4% over the forecast period.
The structural heart devices market is primarily driven by a radical rise in the incidence rate of non-coronary heart disorders, increased demand for minimally invasive surgical procedures, and improving reimbursement schemes. In the past few years, tremendous advances have been achieved with respect to the understanding of these diseases, and accordingly, therapeutic modalities have been developed. A number of clinical trials and technology developments are underway, which is likely to provide the structural heart devices market opportunities for growth in the coming years.
Segmentation
The structural heart devices market has been segmented based on type, indication, procedure, and end-user.
By type, the structural heart devices market has been segmented into heart valve devices, occluders and delivery systems, annuloplasty rings, accessories, and other devices. The heart valve devices segment has been further segmented into transcatheter heart valves and surgical heart valves. The surgical heart valves sub-segment has been further segmented into tissue heart valves and mechanical heart valves.
By indication, the structural heart devices market has been segmented into valvular heart disease, cardiomyopathy, congenital heart defects, and others. The valvular heart disease segment has been further segmented into regurgitation and stenosis.
By procedure, the structural heart devices market has been segmented into Replacement Procedures and repair procedures. The Replacement Procedures segment has been further segmented into TAVR procedures and SAVR procedures. The repair procedures segment has been further segmented into closure procedures, annuloplasty, valvuloplasty, and TMVR procedures.
By end-user, the structural heart devices market has been segmented into hospitals, ambulatory surgery centers, and others.
Regional Analysis
Region-wise, the structural heart devices market has been segmented into North America, Europe, the Middle East & Africa (MEA), and Asia Pacific (APAC).
North America is the dominant market for structural heart devices. High prevalence of cardiovascular disorders, fast uptake of the latest technologies, and high healthcare expenditure are the factors that are driving the North America market. Additionally, favorable reimbursement policies for cardiac surgeries also support the growth of the market.
Europe accounts for the second most significant share of the global structural heart devices market. The growth of the Europe market can be attributed to the high incidence rate of cardiovascular disorders in the region, which requires surgeries and advanced treatment procedures for remediation. Other driving factors include high healthcare expenditure, the prevalence of obesity and diabetes, rise in geriatric population, and the presence of robust healthcare infrastructure.
The APAC structural heart devices market is anticipated to expand at the fastest CAGR over the forecast period. Expanding base of population suffering from diabetes, obesity, and other cardiac disorders are influencing the growth of the market.
The MEA structural heart devices market is likely to capture the smallest share of the market over the forecast period. The market growth is subdued due to low healthcare expenditure and low healthcare penetration in the underdeveloped regions of Africa.
Competitive Landscape
Boston Scientific Corporation (US), Comed BV, Edwards Lifesciences Corporation (US), Cook Group Incorporated (US), Medtronic plc (Ireland), W. L. Gore & Associates, Inc. (US), Biomerics, Endologix Inc. (US)., ST. Jude Medical, LivaNova plc (UK), CardioKinetix, JenaValve Technology, Inc., and Abbott (US) are the key players in the structural heart devices market.
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients.
Contact us:
Market Research Future (part of Wantstats Research and Media Private Limited),
99 Hudson Street, 5Th Floor,
New York, New York 10013
United States of America
+1 628 258 0071
Email: [email protected]
0 notes
Link
0 notes
Text
Structural Heart Devices Market Outlook, Trends, Forecast of Top Countries 2020-2027
Structural Heart Devices Market Scope
Market Research Future (MFRF) studied the global structural heart devices market 2021 for the analysis period till 2023. As per MRFR analysis, the structural heart devices market is expected to expand at 10.4% CAGR through the forecast tenure (from 2018 to 2023). By 2023, the structural heart devices market value is expected to touch number.
Structural Heart Devices Market Drivers and Restrains
The surge in cases of congenital cardiac defects and the increase in need for effective diagnosis for the early detection of such disease can favor the expansion of structural heart device market in the near future. The rise in cases of atrial septal defect, paravalvular leak, ventricular septal defect, arterial or venous fistula, congenital heart disease, and patent foramen oval is creating the demand for minimally invasive techniques, which, in turn, can drive the need for structural heart devices. Thus, can prompt the market upsurge in the analysis tenure. The availability of advanced products, such as; disruptive technology assisted structural heart valves for treating patients can promote the market growth in the years to come. On the contrary, the high expense of structural device is expected to limit the adoption of such device that can restrain the market upsurge.
Segment Analysis of Structural Heart Devices Market
The segment study of the global structural heart devices market is based on type, procedure, indication, and end-user.
The type-based segments of the structural heart device market are occludes and delivery systems, heart valve devices, annuloplasty rings, and accessories among other devices. The segment of heart valve devices consists surgical heart valves and transcatheter heart valves. The segment of surgical heart valves further studies mechanical heart valves and tissue heart valves. The increase in utility of transcatheter heart valves is expected to prompt the market growth.
The indication-based segments of the structural heart device market are valvular heart disease, congenital heart defects, and cardiomyopathy among others. The segment of valvular heart disease studies stenosis and regurgitation. The cardiomyopathy segment can rise at decent CAGR over the review period.
Get Free Sample of This Report at: https://www.marketresearchfuture.com/sample_request/6385
The procedure-based segments of the structural heart device market are repair procedures and Replacement Procedures. The segment of replacement procedures is SAVR procedures and TAVR procedures. The segment of the repair procedures is assessed for closure procedures, valvuloplasty, annuloplasty, and TMVR procedures. The increase in adoption of TMVR procedures is likely to garner high revenue in the years to come.
The end-users-based segments of the structural heart device market are ambulatory surgery centres, and hospitals among others. The segment of ambulatory surgery centres is likely to thrive in the evaluation period.
Regional Analysis of Structural Heart Devices Market
In the Americas, the market of structural heart devices is expected to earn considerable revenue over the analysis tenure for several factors. Increase in rate of surgeries due to pathological conditions, such as; diabetes, and high blood pressure among others can contribute to the expansion of the regional market. Other factors, such as; rise in healthcare awareness, the adoption of disruptive technology, rise in medical tourism, and increase in disposable income can promote North America structural heart device market.
In Europe, the market of structural heart devices market is expected to garner high revenue by 2023. Out of other EU regions, Germany is expected to contribute significantly to the regional market are earn decent revenue by 2023. The upsurge of EU structural heart device market is likely to thrive owing to increase in expansion of elderly population and rise in number of people suffering from obesity and diabetes in this region.
In Asia Pacific region, the market of structural heart devices is likely to earn considerable revenue in the review period owing to the surge in cardiac problem patient population. The structural heart device market in all regions of APAC is anticipated to garner huge revenue in the near future. In the Middle East Asia and Africa, the structural heart device market is likely to expand at sluggish pace due to low per capita income and stringent government policies.
Browse Full Report with TOC at: https://www.marketresearchfuture.com/reports/structural-heart-devices-market-6385
Structural Heart Devices Market Key Players
Edwards Lifesciences Corporation (US), LivaNova plc (UK), Biomerics, ST. Jude Medical, Medtronic plc (Ireland), Abbott (US), Comed BV, Boston Scientific Corporation (US), JenaValve Technology, Inc., CardioKinetix, Endologix Inc. (US)., Cook Group Incorporated (US), and W. L. Gore & Associates, Inc. (US) are some reputed companies in the global structural heart devices market that are listed by MRFR in the report.
#Structural Heart Devices Market#Structural Heart Devices Market Analysis#Structural Heart Devices Market Growth#Structural Heart Devices Market Size
0 notes
Text
Global Structural Heart Devices Market Size, Share, Industry Growth Analysis by Types, Applications and Key Players
Structural Heart Devices Market Analysis
The global structural heart devices market is gaining enough weightage and is eyeing to grow at a phenomenal 10.4% CAGR over the predicted years (2018-2023) owing to uncompromising increase in aortic stenosis, mitral regurgitation and other heart related ailments. Structural heart diseases simply put refer to the cardiac defects that happens by birth which means it is congenital by nature and involves the abnormalities that takes place in the heart vessels and valves due to wear or tear resulting from some diseases. This is a non-coronary heart abnormality that does not impact the heart’s blood vessels. The common structural heart condition includes congenital heart disease, venous/arterial fistulae, paravalvular leak, ventricular septal defect, patent foramen oval and atrial septal defect.
Get customized Sample with complete Toc, Inclusive of COVID-19 Industry Analysis @ https://www.marketresearchfuture.com/sample_request/6385
There are many factors that is driving the growth of the structural heart devices market. Some of these factors as per the Market Research Future (MRFR) report include upsurge in structural heart diseases, favorable reimbursement scenario in structural heart devices and heart procedures, regulatory approvals for advanced and new structural heart devices, rising awareness regarding structural heart diseases, demand for minimally invasive techniques, increasing number of surgeries owing to factors such as high blood pressure and diabetes, rise in disposal income, medical tourism, technological advancement, increasing healthcare awareness, rise in elderly population, increasing patient population and changing lifestyle. On the other hand, factors such as inaccessibility of cardiac surgeries, low affordability and lack of skilled personnel may slow the structural heart devices market pace over the predicted years.
Structural Heart Devices Market Key Players
Key players profiled in the structural heart devices market growth include W. L. Gore & Associates, Inc. (US), Endologix Inc. (US)., CardioKinetix, Cook Group Incorporated (US), JenaValve Technology, Inc., Comed BV, ST. Jude Medical, LivaNova plc (UK), Boston Scientific Corporation (US), Abbott (US), Medtronic plc (Ireland), and Edwards Lifesciences Corporation (US).
Dec 2018- Keystone Heart is all set to enroll patients in the United States for testing the efficacy of the TriGuard 3 product. It is waiting for an approval from the FDA by the end of 2019 and is also expecting the CE Mark approval.
Structural Heart Devices Market Segmentation
Market Research Future report offers an all-inclusive segmental analysis of the structural heart devices market on the basis of type, indication, procedure and end-user.
Based on type, it is segmented into accessories, annuloplasty rings, delivery systems, occluders, heart valve devices and others. The heart valve devices are further segmented into surgical heart valves and transcatheter heart valves. The surgical heart valves are again segmented into mechanical heart valves and tissue heart valves. Of these, the heart valves devices is anticipated to grow at the highest CAGR over the predicted years.
Based on indication, the structural heart devices market is segmented into congenital heart defects, cardiomyopathy, valvular heart disease and others. The valvular heart disease is further segmented into stenosis and regurgitation. Of these, valve regurgitation is expected to grow at a favorable CAGR.
Based on procedure, it is segmented into repair procedures and replacement procedures. The replacement procedures are segmented into SAVR procedures and TAVR procedures. The repair procedures are further segmented into TMVR procedures, valvuloplasty, annuloplasty and closure procedures. Of these, the replacement procedures will have the maximum share in the market.
Based on end-users, the structural heart devices market is segmented into ambulatory surgery centers, hospitals and others. Of these, hospitals will have the largest share.
Structural Heart Devices Market Regional Analysis
Based on region, the structural heart devices market covers growth opportunities and latest trends across North America, Europe, Asia Pacific and the Middle East and Africa. Of these, North America will remain at the forefront owing to increasing number of surgeries owing to factors such as high blood pressure, diabetes and others, advancement in technology, increasing healthcare awareness and rise in disposable income. The structural heart devices market in Europe will have the second largest share with the soaring demand for structural heart devices in Germany.
The market in the European region is anticipated to expand owing to obesity, prevalence of diabetes and rise in elderly population. In Asia Pacific, the structural heart devices market is expected to grow at the fastest pace due to increasing patient population, growing elderly population and changing lifestyle. On the other hand, the structural heart devices market in the Middle East and Africa will have minimum share owing to low per capita income and stringent government policies.
Get Premium Research Report, Inclusive of COVID-19 Impact Analysis, Find more information @ https://www.marketresearchfuture.com/reports/structural-heart-devices-market-6385
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by Components, Application, Logistics and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions.
In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.
Contact:
Akash Anand
Market Research Future
+1 646 845 9312
Email: [email protected]
NOTE: Our team of researchers are studying Covid-19 and its impact on various industry verticals and wherever required we will be considering covid-19 footprints for a better analysis of markets and industries. Cordially get in touch for more details.
0 notes
Text
Congenital Heart Defect Closure Devices Market 2021 - Global Research, Recent Trends and Growth Forecast To 2026
Congenital Heart Defect Closure Devices Market 2021 – Global Research, Recent Trends and Growth Forecast To 2026
The research report “Global Congenital Heart Defect Closure Devices Market – Industry Analysis 2021-2026” covers all the major trends and drivers playing a key part in the development of the Life Sciences industry. The analysis gives an extensive investigation of market growth in terms of value (US$ Mn) and volume (units) throughout the above forecast period. The report emphasizes market dynamics…

View On WordPress
0 notes