#Global Rare Disease Therapeutics Market
Text
Global Antisense and RNAi therapeutics Market Share, Industry Trends, Regional Analysis, Growth Factors and Competitive Analysis by Players
The global antisense and RNA interference therapeutics market is poised for remarkable growth, with projections indicating the market will expand from USD 4.15 billion in 2023 to USD 18.48 billion by 2032. This growth reflects a robust compound annual growth rate (CAGR) of 18.05% over the forecast period from 2024 to 2032, driven by advances in genetic therapies and the rising prevalence of chronic diseases.
Antisense and RNAi therapeutics represent cutting-edge biotechnological approaches that target and regulate gene expression at the molecular level, offering innovative treatments for a range of genetic disorders, cancers, and neurodegenerative diseases. By silencing or modulating the expression of disease-causing genes, these therapies have the potential to address conditions previously considered untreatable.
Key Drivers of Market Growth
Rising Prevalence of Genetic and Chronic Diseases: The increasing incidence of genetic disorders, neurodegenerative diseases, and cancer has led to a surge in demand for novel therapeutic approaches. Antisense and RNAi therapies offer targeted treatment options by selectively silencing disease-related genes. Conditions such as Huntington's disease, Duchenne muscular dystrophy, and various cancers have become key targets for these innovative therapies, contributing to the expansion of the market.
Advancements in Drug Development and Delivery Technologies: Recent technological advancements in RNA delivery systems, such as lipid nanoparticles and conjugated oligonucleotides, have significantly improved the stability, efficacy, and safety of antisense and RNAi therapeutics. These innovations have led to a more streamlined drug development process and increased the number of promising therapies reaching clinical trials. As more RNA-based treatments are approved and commercialized, the market is expected to experience accelerated growth.
Growing Investment in Biotechnology and Genomic Medicine: Substantial investments from both public and private sectors in biotechnology and genomic medicine are playing a crucial role in the market’s expansion. Governments, research institutions, and pharmaceutical companies are increasingly focusing on gene therapies and precision medicine to address complex diseases. These investments are fueling research and development in antisense and RNAi therapeutics, paving the way for more clinical applications and breakthroughs.
Favorable Regulatory Landscape: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have been supportive of antisense and RNAi therapeutics, expediting approvals for novel drugs targeting rare diseases. This favorable regulatory environment is encouraging pharmaceutical companies to invest in these technologies, contributing to the accelerated commercialization of therapies.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4493
Challenges and Opportunities
Despite the significant growth potential, challenges persist in the antisense and RNAi therapeutics market. Issues related to off-target effects, delivery efficiency, and the high cost of treatment development continue to pose hurdles. However, ongoing research aimed at improving RNA delivery systems and reducing side effects is expected to mitigate these challenges, unlocking further market potential.
Additionally, the shift toward personalized medicine, where therapies are tailored to the genetic profile of individual patients, presents a significant opportunity for the antisense and RNAi market. Personalized approaches allow for more targeted and effective treatments, particularly in oncology and rare genetic disorders, positioning the market for sustained growth.
Regional Insights
North America leads the global antisense and RNAi therapeutics market, owing to its strong biotechnology sector, cutting-edge research facilities, and favorable regulatory environment. The region’s dominance is bolstered by the presence of key market players, ongoing clinical trials, and increasing healthcare expenditure.
Europe is also a significant contributor to market growth, driven by rising government support for biotechnology research and a growing focus on gene therapy. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, spurred by expanding healthcare infrastructure, increasing investments in biotechnology, and a rising focus on precision medicine in countries like China, Japan, and South Korea.
Future Outlook
The future of the antisense and RNAi therapeutics market looks promising, with continued advancements in genetic medicine, personalized therapies, and RNA delivery technologies. With several RNA-based treatments already approved and more in the pipeline, the market is set to experience robust growth in the coming years. The projected CAGR of 18.05% between 2024 and 2032 signals strong investor confidence and significant opportunities for innovation.
In conclusion, the antisense and RNAi therapeutics market is on the cusp of a major expansion, driven by advances in gene-silencing technologies, increasing demand for targeted therapies, and strong industry support. From a valuation of USD 4.15 billion in 2023, the market is expected to reach USD 18.48 billion by 2032, revolutionizing the landscape of therapeutic development for genetic and chronic diseases.
Other Trending Reports
Smart Fertility Tracker Market Size
Venous Thromboembolism Treatment Market Size
Automated Liquid Handling Technologies Market Size
Digestive Health Supplements Market Size
0 notes
Text
Blood Group Typing Market Analysis: New Opportunities in the Healthcare Sector

The Blood Group Typing Market is expected to grow at a rapid pace over the coming years. In 2023, the market was valued at USD 1.9 billion, and by 2030, it's projected to surpass USD 3.3 billion, growing at a compound annual growth rate (CAGR) of 8.2%. This surge in market value highlights the increasing demand for blood typing in various healthcare sectors.
What is Blood Group Typing?
Blood group typing is the process of determining an individual's blood group, typically classified into A, B, AB, or O types. This crucial medical procedure ensures compatibility for blood transfusions, organ transplants, and managing pregnancies where blood group incompatibility may lead to complications. It also plays an important role in research, diagnostics, and therapeutics.
Why is the Blood Group Typing Market Growing?
Several factors contribute to the rapid growth of the blood group typing market:
Rising Demand for Blood Transfusions: The growing number of surgeries and trauma cases has driven the need for safe and compatible blood transfusions.
Advancements in Technology: With the advent of advanced testing technologies, blood typing has become quicker and more accurate.
Increasing Prevalence of Chronic Diseases: As chronic diseases like cancer and cardiovascular conditions become more common, the need for blood transfusions, organ transplants, and diagnostic tests rises.
Government Initiatives: Government health agencies across the globe are launching campaigns to improve healthcare infrastructure, which includes better access to blood banks and safe transfusion practices.
Access Full Report @ https://intentmarketresearch.com/latest-reports/blood-group-typing-market-3123.html
Key Segments of the Blood Group Typing Market
1. Based on Products
Consumables: Reagents, anti-sera, and red blood cells that are essential for blood typing procedures.
Instruments: Machines used in hospitals and laboratories for blood typing tests.
Services: Outsourcing services for hospitals and laboratories to perform blood group testing.
2. Based on Test Type
Antibody Screening: Detects antibodies in the blood that may cause immune reactions.
HLA Typing: Human leukocyte antigen typing is often used in organ transplantation to ensure compatibility.
ABO Blood Group Typing: The most common test, determining whether a person has blood type A, B, AB, or O.
Crossmatching: Ensures compatibility between donor and recipient blood before a transfusion.
3. Based on Technique
PCR-Based Testing: Polymerase chain reaction (PCR) testing is becoming popular due to its accuracy in detecting rare blood types.
Microarray Testing: A cutting-edge technique that offers detailed insights into blood group antigens.
Gel Agglutination: A traditional method where blood cells clump together to reveal their type.
4. Based on End-User
Hospitals and Clinics: The largest users of blood typing services, especially in emergency care, surgeries, and transfusions.
Blood Banks: Critical for maintaining safe blood supplies for hospitals and clinics.
Diagnostic Laboratories: Play a key role in providing accurate blood typing for various medical needs.
Research Institutes: Blood typing is essential in biomedical research and the development of new medical treatments.
Geographical Insights into the Blood Group Typing Market
North America
North America dominates the global blood group typing market, thanks to its advanced healthcare infrastructure and high demand for blood transfusions. The U.S. leads the region with significant investments in healthcare research and technological advancements.
Europe
Europe holds the second-largest market share, driven by increased demand for organ transplants and blood donations. Countries like Germany, the UK, and France are key players in the region.
Asia-Pacific
Asia-Pacific is the fastest-growing market, with a rapidly improving healthcare infrastructure, especially in countries like China, India, and Japan. Increased government initiatives and rising awareness of safe blood transfusion practices are fueling growth in this region.
Latin America and the Middle East
These regions are experiencing moderate growth, with improving healthcare systems and increased focus on safe transfusion practices. Brazil, Saudi Arabia, and the UAE are emerging as key markets.
Download Sample Report @ https://intentmarketresearch.com/request-sample/blood-group-typing-market-3123.html
Technological Advancements in Blood Group Typing
Technological innovations have dramatically improved the accuracy and speed of blood group typing. Some of the most noteworthy advancements include:
Automation: Automated blood typing machines reduce human error and streamline the testing process.
DNA-Based Testing: Genetic testing methods like PCR and next-generation sequencing (NGS) have made it easier to identify rare blood types.
Artificial Intelligence: AI-driven platforms can now analyze blood typing data more efficiently, predicting rare blood types and crossmatching results with greater precision.
Challenges in the Blood Group Typing Market
Despite its rapid growth, the blood group typing market faces several challenges:
High Cost of Advanced Technologies: While innovative testing methods improve accuracy, they also raise costs, limiting access in some regions.
Limited Awareness in Low-Income Countries: In developing countries, there is still a lack of awareness regarding the importance of safe blood transfusions.
Regulatory Hurdles: Compliance with government regulations varies by region, sometimes slowing the approval process for new technologies.
Key Players in the Blood Group Typing Market
Several companies are leading the charge in the global blood group typing market:
Bio-Rad Laboratories: Specializing in diagnostics and testing solutions.
Grifols: A global leader in plasma-derived medicines and transfusion diagnostics.
Ortho Clinical Diagnostics: A top player in immunohematology and blood typing products.
Immucor: Focuses on improving transfusion and transplantation diagnostics.
Future Outlook of the Blood Group Typing Market
The future of the blood group typing market looks promising, with continued growth expected through 2030. Some key trends include:
Increased Investment in R&D: Research into advanced blood typing methods will likely continue, focusing on automation, accuracy, and cost reduction.
Emerging Markets: Countries with developing healthcare systems are investing in better blood typing technologies, creating new growth opportunities.
Personalized Medicine: The rise of personalized treatments will likely drive demand for more precise blood typing methods to ensure compatibility in therapies like immunotherapy and gene editing.
Conclusion
The global blood group typing market is on a path of exponential growth, driven by technological advancements, rising demand for blood transfusions, and government initiatives. With the market projected to surpass USD 3.3 billion by 2030, the future looks bright for companies and healthcare providers in this field. As the industry continues to innovate, the accuracy and accessibility of blood group typing will only improve, saving more lives and ensuring safer medical procedures.
FAQs
What is the CAGR of the blood group typing market from 2024 to 2030?
The market is expected to grow at a CAGR of 8.2% during this period.
What is the importance of blood group typing?
Blood group typing ensures compatibility for blood transfusions, organ transplants, and other medical procedures, reducing the risk of complications.
Which regions are leading the blood group typing market?
North America holds the largest market share, followed by Europe and the rapidly growing Asia-Pacific region.
What are the major technologies used in blood group typing?
Key technologies include PCR-based testing, microarray testing, and gel agglutination methods.
What are the major challenges facing the blood group typing market?
Challenges include the high cost of advanced technologies, limited awareness in developing regions, and regulatory hurdles.
About Us
Intent Market Research (IMR) is dedicated to delivering distinctive market insights, focusing on the sustainable and inclusive growth of our clients. We provide in-depth market research reports and consulting services, empowering businesses to make informed, data-driven decisions.
Our market intelligence reports are grounded in factual and relevant insights across various industries, including chemicals & materials, healthcare, food & beverage, automotive & transportation, energy & power, packaging, industrial equipment, building & construction, aerospace & defense, and semiconductor & electronics, among others.
We adopt a highly collaborative approach, partnering closely with clients to drive transformative changes that benefit all stakeholders. With a strong commitment to innovation, we aim to help businesses expand, build sustainable advantages, and create meaningful, positive impacts.
Contact Us
US: +1 463-583-2713
0 notes
Text
Menkes Disease Treatment Market Will Grow At Highest Pace Owing To Rising Orphan Drug Designations

Menkes disease is an X-linked neurodegenerative disorder caused by mutations in the ATP7A gene. Symptoms include sparse, brittle hair; impaired vascular tissue growth; low serum copper levels; and delayed development. Treatment for Menkes disease primarily involves replacement of copper through intravenous administration of copper histidine. Currently, there are only a few FDA-approved treatment options for Menkes disease. Patients primarily rely on off-label use of existing copper histidine products.
The Menkes Disease Market size is estimated to be valued at US$ 165.1 million in 2024 and is expected to exhibit a CAGR of 6.6% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the Menkes disease treatment market are Fortress Biotech, Inc., Teva Pharmaceutical Industries Ltd., Amerigen Pharmaceuticals Limited, Mylan N.V., and Bausch Health Companies Inc. Fortress Biotech launched MNK-6105, a novel formulation of copper histidine for the treatment of Menkes disease in 2020.
The growing diagnosis of Menkes disease, especially in developed regions, is expected to drive the demand for treatment drugs. According to CDC, an estimated 1 in 34,000 to 39,000 newborn males are affected by Menkes disease each year in the United States.
Technological advancements in drug design and manufacturing, such as modified drug delivery systems and novel formulations, are generating lucrative opportunities in the market. Companies are focusing on developing orphan drugs with improved bioavailability and fewer side effects.
Market Trends
Increasing collaborations between biotech companies and research institutes is one of the key trends witnessed in the market. For instance, in 2019 Fortress Biotech partnered with the University of Connecticut to develop treatments for rare genetic disorders like Menkes disease.
Emergence of novel drug formulations is another major trend. MNK-6105 developed by Fortress Biotech is a nano-particle formulation of copper histidine, resulting in enhanced absorption and lowering the dosing frequency.
Market Opportunities
Orphan drug designations by regulatory bodies provide significant market opportunities. Drugs in development for rare diseases like Menkes enjoy benefits like tax credits, waiver of application fees, and market exclusivity for a period of 7 years in the US.
Geographic expansion in developing Asian countries and Latin America presents untapped growth prospects. Availability of low-cost manufacturing infrastructure and emergence of local biotech companies also supports market expansion.
Impact Of COVID-19 On Menkes Disease Market Growth
The outbreak of COVID-19 pandemic has adversely impacted the growth of Menkes disease market globally. Several factors such as restrictions on non-essential medical services, disruptions in supply chain, difficulty in conducting clinical trials, etc contributed to slowed market growth during the crisis. However, with gradual lifting of lockdowns and restoration of healthcare facilities post-recovery from first and second waves, the market is expected to regain lost momentum. Teleconsultations helped to sustain continuity of care for Menkes disease patients during lockdowns. Development of vaccines and treatments have improved disease management, though challenges persist in some geographical regions with limited resources. In the post-pandemic scenario, partnerships involving stakeholders would be pivotal to ensure uninterrupted access to diagnostics and therapeutics. Long-term strategies focusing on decentralized community-based services may help boost early diagnosis and effective treatment interventions.
Menkes Disease Market In North America
North America holds the major share of the Menkes Disease Market in terms of value. This can be mainly attributed to factors such as availability of advanced healthcare infrastructure, growing healthcare expenditure, presence of major market players, and high diagnosis and treatment rates in the region. The United States commands the lion's share within the North America regional market. Favorable reimbursement policies have ensured high adoption of screening and treatment options for Menkes disease. Furthermore, continuous research activities have led to development of new treatment approaches, driving the regional market growth. Other countries in North America such as Canada are also lucrative markets expected to register significant growth over the forecast period.
Fastest Growing Region - Asia Pacific
The Asia Pacific region is poised to witness the fastest growth in the Menkes disease market during the forecast period. This growth can be credited to rising medical tourism, increasing patient population due to growing birth rates, and improving healthcare expenditures in emerging economies of the region. In addition, heightened awareness about early diagnosis and availability of generic medications are supporting the Asia Pacific market expansion. Countries such as China and India with their large population bases and significant improvements in healthcare access present immense growth opportunities. Initiatives by governments towards strengthening newborn screening programs would boost early diagnosis and treatment uptake. Overall, the Asia Pacific Menkes disease market is anticipated to attract higher investments from global players over the coming years.
Get more insights on this topic: https://www.trendingwebwire.com/menkes-disease-market-estimated-to-witness-high-growth-owing-to-advancements-in-gene-therapy/
Author Bio
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups. (LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
What Are The Key Data Covered In This Menkes Disease Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Menkes Disease Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Menkes Disease Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Menkes Disease Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Menkes Disease Market vendors
FAQ’s
Q.1 What are the main factors influencing the Menkes Disease Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Menkes Disease Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Menkes Disease Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Menkes Disease Market Trend#Menkes Disease Market Size#Menkes Disease Market Information#Menkes Disease Market Analysis#Menkes Disease Market Demand
0 notes
Text
Global Rare Disease Treatment Market Outlook, Trends And Future Opportunities (2023-2030)
Global Rare Disease Treatment Market is growing at a CAGR of 11.6% over the next 5 years. Roche Ltd., Pfizer, Inc., PTC Therapeutics, AstraZeneca, Novartis AG are the major companies operating in Global Rare Disease Treatment Market
Global Rare Disease Treatment Market Outlook
0 notes
Text
Exploring the Gene Therapy Market: Breakthroughs and Opportunities
The global gene therapy market is expected to reach USD 18.20 billion by 2030, registering a CAGR of 18.88% from 2024 to 2030, according to a new report by Grand View Research, Inc. The development of the market is owing to an increase in the number of gene therapy-based discoveries, increasing investment in this sector, and rising approval of gene therapy products. According to the WHO, 10 to 20 new cell and gene therapies are expected to be approved each year by 2025.

Continuous developments in recombinant DNA technology are anticipated to enhance the efficiency of gene therapy in the coming years. Hence, ongoing progresses in recombinant DNA technology are anticipated to expand the number of ongoing clinical trials for gene therapy. Primarily, these advancements are taking place in the context of various gene-editing tools and expression systems to augment the R&D for products. The advent of CRISPR/Cas9 nuclease, ZFN, and TALEN allows easy & precise genome editing. As a result, in recent times, the gene-editing space has witnessed a substantial number of research activities, which, in turn, is expected to influence the growth of the gene therapy market.
The growth of the gene therapy market is expected to be majorly benefitted from the increasing prevalence of cancer. The ongoing increase in cancer patients and related death per year emphasizes the essential for the development of robust treatment solutions. In 2020, there were around 18.1 million new cases of cancer worldwide. 9.3 million of these cases involved men, while 8.8 million involved women. Continuing developments in tumor genetic studies have delivered substantial information about cancer-related molecular signatures, which in turn, is expected to support ongoing clinical trials for cancer therapeutics.
With rising demand for robust disease treatment therapies, companies have focused their efforts to accelerate R&D for effective genetic therapies that target the cause of disease at a genomic level. . Furthermore, the U.S. FDA provides constant support for innovations in this sector via a number of policies with regard to product manufacturing. In January 2020, the agency released six final guidelines on the manufacturing and clinical development of safe and efficient products.
Furthermore, facility expansion for cell and gene therapies is one of the major factors driving the gene therapy market growth. Several in-house facilities and CDMOs for gene therapy manufacturing have begun investing to enhance their production capacity, which, in turn, is anticipated to create lucrative opportunities for market players. For instance, in April 2022, the FDA approved commercial licensure approval to Novartis for its Durham, N.C. site. This approval permits the 170,000 square-foot facility to make, test, and issue commercial Zolgensma, as well as manufacture therapy products for current & upcoming clinical trials.
For More Details or Sample Copy please visit link @: Gene Therapy Market Report
Gene Therapy Market Report Highlights
The AAV segment shows a significant revenue contribution of 22% in 2023. Several biopharma companies are offering their viral vector platform for the development of AAV-based gene therapy product.
By indication, the spinal muscular atrophy (SMA) segment dominated the market in 2023 with a share of 46.8%. Although SMA is a rare disorder, it is one of the most common fatal inherited diseases of infancy.
The Beta-Thalassemia Major/SCD segment is anticipated to register the fastest CAGR of 38.3% over the forecast period. Gene therapy for SCD and β-thalassemia is based on transplantation of gene-modified hematopoietic stem cells.
North America dominated the market in 2023 with the largest revenue share of 65.2% in 2023. This region is expected to become the largest routine manufacturer of gene therapy in terms of the number of approvals and revenue generated during the forecast period.
Europe is estimated to be the fastest-growing regional segment from 2024 to 2030. This is attributed to its large population with unmet medical needs and increasing demand for novel technologies in the treatment of rare but increasingly prevalent diseases.
Gain deeper insights on the market and receive your free copy with TOC now @: Gene Therapy Market Report
We have segmented the global gene therapy market report based on indication, vector type, route of administration, and region.
#GeneTherapy#PersonalizedMedicine#BiotechInnovation#GeneticEngineering#HealthcareRevolution#MedicalBreakthroughs#TherapeuticAdvances#Biopharmaceuticals#RareDiseaseTreatment#GeneticResearch
0 notes
Text
The global market for Pompe disease therapeutics is projected to expand at a compound annual growth rate (CAGR) of 5.00%, from an estimated USD 1584.2 million in 2023 to USD 2457.61 million in 2032.Pompe disease, a rare inherited lysosomal storage disorder, has garnered significant attention within the medical community due to its severe implications and the complexity of its treatment. The disease, characterized by the buildup of glycogen in the body's cells, leads to progressive muscle weakness and respiratory difficulties, often proving fatal without appropriate intervention. The global market for Pompe disease therapeutics has been evolving rapidly, driven by advances in biotechnology, increasing awareness, and growing investments in rare disease research. This article delves into the current trends, challenges, and future prospects of the Pompe disease therapeutics market.
Browse the full report at https://www.credenceresearch.com/report/global-pompe-disease-therapeutics-market
Market Overview
The global Pompe disease therapeutics market is primarily dominated by enzyme replacement therapy (ERT), which has been the cornerstone of treatment since its introduction. Myozyme (alglucosidase alfa), developed by Sanofi Genzyme, remains the leading ERT for Pompe disease, significantly improving patient outcomes, especially in infantile-onset Pompe disease. However, the high cost of ERT, coupled with the lifelong need for treatment, presents substantial economic challenges for patients and healthcare systems.
In addition to ERT, the market is witnessing the emergence of gene therapy as a promising treatment option. Gene therapy aims to correct the underlying genetic defect responsible for Pompe disease by introducing a functional copy of the GAA gene. Several companies are currently in various stages of clinical trials, with AT845 (Astellas Gene Therapies) and ACTUS-101 (Actus Therapeutics) being notable examples. These advancements underscore a shift towards more innovative and potentially curative treatment modalities.
Key Market Drivers
1. Technological Advancements: The rapid progress in genetic engineering and biotechnology has been instrumental in the development of novel therapeutics for Pompe disease. Gene therapy, in particular, is expected to revolutionize the treatment landscape by offering a one-time, potentially curative option.
2. Increasing Awareness and Diagnosis: Growing awareness among healthcare professionals and patients has led to earlier diagnosis and treatment of Pompe disease. Newborn screening programs, particularly in developed countries, have been pivotal in identifying Pompe disease early, allowing for timely intervention.
3. Rising Investment in Rare Disease Research**: The pharmaceutical industry's increasing focus on rare diseases has spurred significant investment in the development of therapies for Pompe disease. This is reflected in the growing number of clinical trials and the entry of new market players, including biotech startups and established pharmaceutical companies.
Challenges Facing the Market
1. High Treatment Costs: The high cost of existing treatments, particularly ERT, remains a significant barrier to access, especially in low- and middle-income countries. The affordability of new therapies, including gene therapy, will be a crucial factor in determining their market penetration.
2. Regulatory Hurdles: The regulatory approval process for novel therapies, particularly gene therapies, is complex and stringent. Ensuring the safety and efficacy of these treatments requires extensive clinical trials, which can delay market entry and increase development costs.
3. Limited Patient Population: As a rare disease, Pompe disease has a limited patient population, which can restrict the commercial viability of new therapeutics. This makes it challenging for companies to recoup the high costs associated with drug development.
Regional Market Insights
The United States and Europe currently dominate the Pompe disease therapeutics market, driven by robust healthcare infrastructure, high awareness, and supportive regulatory frameworks. These regions have been at the forefront of newborn screening programs, which have played a critical role in early diagnosis and treatment initiation. Asia-Pacific, however, is emerging as a key market due to increasing healthcare expenditure, rising awareness, and improving access to advanced therapies.
Future Prospects
The future of the Pompe disease therapeutics market looks promising, with ongoing research and development expected to yield more effective and accessible treatments. Gene therapy holds the potential to transform the treatment paradigm, offering hope for a cure rather than lifelong management. Additionally, advancements in personalized medicine may lead to more tailored and effective treatment approaches.
Key Players
Amicus Therapeutics, Inc. (U.S.)
Audentes Therapeutics (U.S.)
Novartis AG (Switzerland)
Fresenius Kabi AG (Germany)
Akorn Incorporated (U.S.)
Teva Pharmaceutical Industries Ltd (Israel)
Mylan N.V (U.S.)
Johnsons & Johnsons Services Inc (U.S.)
F. Hoffman-La Roche Ltd. (Switzerland)
Danaher (U.S.)
B.D. (U.S.)
Chembio Diagnostics (U.S.)
EKF Diagnostics (U.K.)
Trinity Biotech plc (Ireland)
Instrumentation Laboratory (U.S.)
Nova Biomedical (U.S.)
PTS Diagnostics (U.S.)
Sekisui Diagnostics (U.S.)
Thermo Fisher Scientific (U.S.)
bioMérieux S.A. (France)
Others
Segmentation
By Therapy Type
Enzyme Replacement Therapy (ERT)
Recombinant Enzymes
Long-Acting ERT
Novel ERT Formulations
Gene Therapy
Viral Vector-Based Gene Therapy
Non-Viral Gene Therapy
Small Molecule Therapies
Chaperone Therapies
Substrate Reduction Therapies (SRT)
Supportive Therapies
Symptomatic Treatment
Nutritional Support
By Route of Administration
Intravenous (IV) Administration
Oral Administration
By End-User
Hospitals and Clinics
Specialty Clinics
Home Care Settings
By Region
North America
U.S.
Canada
Mexico
Europe
Germany
France
UK.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
The Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/global-pompe-disease-therapeutics-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Base Editing Market Size To Reach USD 681.24 Million By 2030

Base Editing Market Growth & Trends
The global base editing market size is anticipated to reach USD 681.24 million by 2030 and is anticipated expected to expand at a CAGR of 14.63% during the forecast period, according to a new report by Grand View Research, Inc. Increased government funding, a growing focus on genomic studies, and the rising prevalence of rare diseases contribute to market growth. Moreover, the increased application of genomic research in therapeutics to cure chronic and rare diseases, immunodeficiency, and cardiovascular disorders is expected to drive market growth further.
The increased application of genomic research in therapeutics has significantly advanced the field of medicine, leading to more personalized and effective treatments for various diseases. CRISPR-based genome editing technology has been applied to precise genome editing techniques such as base editing. Base editing allows for the targeted conversion of one base pair to another without generating double-strand breaks. Furthermore, Using CRISPR-based genome editing technology, creating animal models of human diseases has become more accessible, faster, and more flexible. The application of base editing in monkeys has shown promising results in genetic research and potential therapeutic interventions. Recent research has shown that base editing technology can be used in monkeys to study the causes and treatment of STXBP1 encephalopathy. The successful outcomes demonstrate that this technology is effective in creating effectively creates primate models of human genetic disorders.
The COVID-19 pandemic has significantly impacted the base editing market, leading to an increased emphasis on genomic studies and gene editing technologies. The global healthcare crisis has highlighted the significance of advanced medical solutions, resulting in a surge of interest and investment in technologies such as base editing, which have the potential to revolutionize healthcare treatments. Moreover, the pandemic has increased the emphasis on precision medicine and targeted therapies. Base editing technologies enable precise modifications at the DNA or RNA level, allowing for tailored treatments for specific genetic diseases. This aligns with the growing trend towards personalized medicine for effective and targeted intervention.
However, similar to like other genome editing techniques, safety is one of the primary ethical concerns with base editing technology is safety. The potential for off-target effects and unintended consequences due to inaccuracies in the editing process raises significant safety issues. In the case of base editing, ensuring the accuracy and specificity of edits is crucial to prevent unintended mutations that could have harmful effects on harm an organism or future generations.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/base-editing-market-report
Base Editing Market Report Highlights
Products segment dominated the product & services segment in 2023 owing to the launch of innovative products and platforms in cell and gene therapeutics.
The Pplatform segment dominated the product segment with the largest revenue share of 44.82%, as base editing platforms enable precise single-base changes in genomic DNA
In 2023, drug discovery & development dominated the application segment and held the largest market share of 52.08%, and is expected to grow at the highest CAGR over the forecast period.
In 2023, the DNA base editing dominated the type segment, and is expected to grow at the highest CAGR of 15.39% from 2024 to 2030.
Pharmaceutical & biotechnology companies segment dominated the end-use market with the largest share in 2023, driven by new product launches and research activities in the field of genomics.
North America region dominated the base editing market with a share of 40.26%, owing to factors such as the increasing funding for research & development, government support for quality healthcare, etc..
Market players operating in the base editing market include Danaher; ElevateBio; Merck KGaA; Revvity; Maravai LifeSciences; GenScript; Beam Therapeutics; Intellia Therapeutics, Inc.; Cellectis; Creative Biogene; Bio Palette Co., Ltd
Base Editing Market Segmentation
For the purpose of this report, Grand View Research has segmented the base editing market on the basis of based on product & services, application, type, end-use, and region:
Base Editing Products & Services Outlook (Revenue, USD Million, 2018 - 2030)
Product
Platform
Kits & Reagents
Plasmids
Base Editing Libraries
Services
gRNA Design
Cell Line Engineering
Base Editing Application Outlook (Revenue, USD Million, 2018 - 2030)
Drug Discovery & Development
Agriculture
Veterinary
Base Editing Type Outlook (Revenue, USD Million, 2018 - 2030)
DNA Base Editing
RNA Base Editing
Base Editing End-u-use Outlook (Revenue, USD Million, 2018 - 2030)
Academic & Research Institutes
Contract Research Organizations
Pharmaceutical & Biotechnology Companies
Base Editing Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Mexico
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Argentina
MEA
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players in the Base Editing Market
ElevateBio
Merck KGaA
Revvity
Maravai LifeSciences
GenScript
Beam Therapeutics
Intellia Therapeutics, Inc.
Cellectis
ElevateBio
Creative Biogene
Bio Palette Co., Ltd
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/base-editing-market-report
#Base Editing Market#Base Editing Market Share#Base Editing Market Size#Base Editing Market Trends#Base Editing Market Forecast
0 notes
Text
Epidermolysis Bullosa Market will grow at highest pace owing to increasing pipeline drugs developments
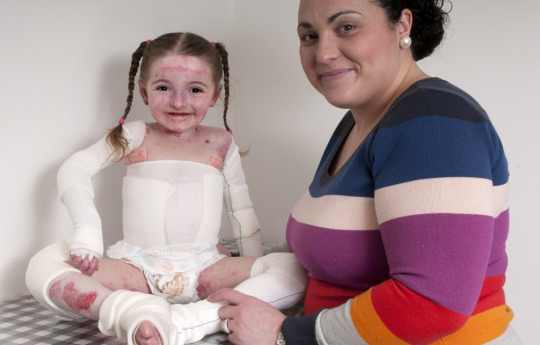
The global epidermolysis bullosa market is estimated to exhibit a CAGR of 11% over the forecast period 2023 to 2030. Epidermolysis bullosa (EB) is a group of rare genetic disorders that causes easy blistering of the skin and mucous membranes from mechanical friction or trauma. The major symptoms include blistering and separating of the top layer of the skin. There is currently no cure for EB, but palliative care options aim to prevent and treat complications.
Key Takeaways
Key players operating in the epidermolysis bullosa market are Amryt Pharma, Abeona Therapeutics, Castle Creek Pharmaceuticals, RegeneRx, Krystal Biotech, RHEACELL GmbH, Holostem Terapie Avanzate, StemRim/Shionogi, and Phoenix Tissue Repair. Amryt Pharma and Abeona Therapeutics are conducting late stage clinical trials for their respective pipeline drugs. The global EB patient population is growing steadily due to increasing disease awareness and diagnosis rate. Several companies are investing in R&D to develop drugs targeting specific EB types and improving treatment outcomes. Advancements in cell and gene-based therapies provide hope for a potential cure for EB in the long run.
Market Trends
Growing Pipeline of Drugs: There are several drugs under development targeting different types and symptoms of EB. Key companies like Amryt Pharma, Castle Creek Pharmaceuticals, and RegeneRx have drugs in clinical trials which can potentially provide symptom relief and improve wound healing. This will drive significant market growth over the forecast period.
Increasing Role of Cell and Gene Therapies: Researchers are exploring cell and gene-based therapies as potential cure for EB. RHEACELL GmbH and Hologem Terapie Avanzate are developing engineered skin equivalents using gene-corrected cells. StemRim/Shionogi and Phoenix Tissue Repair are researching cell therapies derived from stem cells. Advancements in these novel therapies will open new opportunities.
Market Opportunities
Focus on Rare EB Types: While drugs are being developed for generalized DEB, research on therapies for rarer EB types like JEB and Kindler syndrome still lag behind. Developing treatment options for these underserved populations will enable access to effective care.
Combination Therapy Approaches: Combining a topical drug or cream with cell/gene therapy may improve outcomes by delivering benefits via two mechanisms of action. Companies can collaborate to develop optimized treatment regimens maximizing efficacy.
Impact of COVID-19 on Epidermolysis Bullosa Market Growth
The COVID-19 pandemic has significantly impacted the growth of the epidermolysis bullosa market. Treatment delays and the shift of healthcare resources towards COVID-19 treatment reduced access to care for epidermolysis bullosa patients during this period. Hospitalizations for epidermolysis bullosa fell drastically as people avoided hospitals due to infection risk. This posed challenges for wound management and other disease interventions. Additionally, supply chain disruptions affected the availability of certain drugs and therapies.
#Epidermolysis Bullosa Market Share#Epidermolysis Bullosa Market Trend#Epidermolysis Bullosa Market Growth
0 notes
Text
Personalized Medicine Market Size, Share & Review 2024-2030
Personalized Medicine Industry Overview
The global personalized medicine market was valued at USD 529.28 billion in 2023 and is projected to grow at a CAGR of 8.20% from 2024 to 2030. The personalized medicine market is driven majorly by the growing demand for novel drug discovery to combat the rising incidence of cancers and other diseases across the globe. Moreover, numerous collaborations among researchers and market players are also anticipated to have a positive impact on the personalized medicine market growth.
For instance, in February 2023, Roche extended its partnership with Janssen Biotech Inc., intensifying efforts in the development of companion diagnostics for targeted therapies. This expanded collaboration encompasses various precision technologies, such as immunohistochemistry, digital pathology, next-generation sequencing, polymerase chain reaction, and immunoassays, fostering advancements in research and innovation
Gather more insights about the market drivers, restrains and growth of the Personalized Medicine Market
One of the most important factors expected to have a significant impact on the market is how much and to what extent the growth of Next-Generation Sequencing (NGS) will affect the adoption of personalized medicine(PM) in the coming seven years. The exponentially decreasing cost of sequencing whole genomes and technological advancements in NGS in a way with Moore’s law for semiconductors in the field of life sciences. For instance, as per the Medical Device Network article published in 2023, sequencing costs have significantly decreased over time as a result of increased competition and advancements in technology.
The increasing prevalence of rare diseases is also anticipated to boost the demand for growth of the market. The increasing level of understanding and correlation of characteristics of the human genome paved the way for efforts in devising various precision medicine and therapeutic exercises. For instance, in September 2022, a research study carried out at the University of California at Irvine, proposed a novel technique for the management of inherited retinal diseases (IRDs) by using precision genome editing that is very specific to an individual’s requirements.
Companion diagnostics are tests or assays that are specifically designed to identify biomarkers for patient stratification, ensuring that the right patients receive the right therapies at the right time. Many companies are embracing this approach to tailor treatments based on individual patient characteristics, optimizing therapeutic outcomes while minimizing potential adverse effects. For instance, in November 2023, Foundation Medicine announced a partnership with Pierre Fabre Laboratories aimed at advancing the development of FoundationOneCDx and FoundationOneLiquidCDx, which are high-quality genomic tests. The goal is to establish these tests as companion diagnostics for novel targeted therapies designed to treat individuals diagnosed with Non-Small Cell Lung Cancer (NSCLC).
Personalized medicine is poised to reshape the healthcare landscape in the coming years, fueled by four prominent trends. This evolution is driven by decision support techniques utilizing the potential of the human genome, the integration of big data analytics and machine learning in healthcare practices, reimbursement strategies promoting preventative care within health systems, and the introduction of advanced tools facilitating increased data accessibility and interoperability. For instance, in June 2023, Dartmouth inaugurated its Center for Precision Health and Artificial Intelligence (CPHAI) propelled by an initial USD 2 million funding. CPHAI is dedicated to advancing interdisciplinary research exploring the application of artificial intelligence and biomedical data in enhancing personalized medicine and health outcomes. Emphasizing the importance of maintaining ethical standards in health AI, the center aims to leverage AI's transformative potential in addressing real-world clinical challenges, improving patient outcomes, and ensuring equitable healthcare access.
Browse through Grand View Research's Biotechnology Industry Research Reports.
The global plasmid purification market size was estimated at USD 1.72 billion in 2023 and is projected to grow at a CAGR of 11.60% from 2024 to 2030.
The global enzymatic DNA synthesis market size was estimated at USD 232.4 million in 2023 and is projected to grow at a CAGR of 26.4% from 2024 to 2030.
Key Personalized Medicine Company Insights
Some key players operating in the market include Abbott; GE Healthcare., Inc.; Illumina, Inc., and Danaher Corporation. Established players focus majorly on innovation & technology advancements to develop cutting-edge diagnostic solutions and partner with emerging players to leverage their technology. Mature players also have a strong global presence with a diverse portfolio of genetic testing products and a well-established brand reputation which gives them a competitive edge.
Emerging players however focus on launching products in limited countries and then expanding regionally. Some operating strategies also include strategic partnerships, acquisitions, or collaborations to enhance their capabilities and market presence. Additionally, these players may be more flexible and agile than established players in terms of responding and changing to market needs and demand, allowing them to quickly adapt and develop new technologies.
Key Personalized Medicine Companies:
The following are the leading companies in the personalized medicine market. These companies collectively hold the largest market share and dictate industry trends.
GE Healthcare
Illumina, Inc.
ASURAGEN, INC.
Abbott
Dako A/S
Exact Sciences Corporation
Danaher Corporation (Cepheid, Inc.)
Decode Genetics, Inc.
QIAGEN
Exagen Inc.
Precision Biologics
Celera Diagnostics LLC.
Biogen
Genelex
International Business Machines Corporation (IBM)
Genentech, Inc.
23andMe, Inc.
Recent Developments
In September 2023, A Memorandum of Understanding (MOU) was signed by Agilent Technologies & Advanced Cell Therapy and Research Institute, Singapore (ACTRIS) to advance in gene and cell therapy over the next 3 years.
In July 2023, As a part of Illumina's oncology product portfolio, Pillar Biosciences and Illumina formed a strategic partnership to commercialize Pillar's suite of oncology assays worldwide. Completing the agreement will lead to an unparalleled offering of additional Next-Generation Sequencing (NGS) solutions, improving patient access to personalized cancer treatment solutions.
In June 2023, GE Healthcare and DePuy Synthes signed a distribution agreement to expand the reach of OEC 3D Imaging System and product offerings of DePuy Synthes to more surgeons & patients in the U.S.
In June 2023, Exact Sciences Corp. collaborated independently with two distinguished healthcare institutions at the forefront of cancer research. The agreements seek to increase access to genomic information in order to enhance patient care.
Order a free sample PDF of the Personalized Medicine Market Study, published by Grand View Research.
0 notes
Text
The Gene Synthesis Market in the Next Decade: Trends and Insights
The global gene synthesis market, valued at USD 2.28 billion in 2023, is projected to expand significantly, reaching USD 9.64 billion by 2032. This rapid growth reflects a compound annual growth rate (CAGR) of 17.41% over the forecast period from 2024 to 2032, driven by advancements in biotechnology and a growing need for customized genes for various applications.
Gene synthesis, a process by which artificial genes are designed and assembled in laboratories, is revolutionizing industries from pharmaceuticals to agriculture. The growing demand for innovative therapies, improved agricultural products, and industrial biotechnology solutions is propelling the market for synthetic genes.
Key Market Drivers
Advancements in Drug Discovery and Development The pharmaceutical industry is one of the largest consumers of gene synthesis services. With the growing focus on precision medicine and the development of gene-based therapies, the need for custom gene sequences has risen dramatically. Gene synthesis is playing a critical role in the discovery of new drugs, the development of biologics, and the creation of innovative therapies for various genetic disorders, cancers, and infectious diseases.
Expanding Applications in Agriculture In agriculture, gene synthesis is being used to create genetically modified organisms (GMOs) that can enhance crop yields, improve resistance to pests and diseases, and increase tolerance to environmental stress. As the global population grows and demand for sustainable agriculture intensifies, gene synthesis offers a pathway to develop more resilient and productive crops, reducing reliance on chemical pesticides and fertilizers.
Growth in Synthetic Biology Synthetic biology, an interdisciplinary field that combines biology and engineering, is transforming industries by allowing the creation of new biological parts, systems, and devices. Gene synthesis is a foundational tool in synthetic biology, enabling scientists to design and assemble genes with specific functions. From developing biofuels to creating sustainable chemicals, synthetic biology applications are driving demand for gene synthesis services.
Rising Demand for Personalized Medicine The rise of personalized medicine, which tailors treatments to individual patients based on their genetic makeup, is contributing to the growth of the gene synthesis market. Custom-designed genes are being used to develop patient-specific therapies, particularly in oncology and rare genetic disorders. As gene editing technologies such as CRISPR become more widespread, gene synthesis will play a key role in developing personalized treatments.
Decreasing Costs and Increased Efficiency Technological advancements have significantly reduced the cost of gene synthesis, making it more accessible to researchers and companies. The introduction of high-throughput synthesis platforms has improved the speed and efficiency of producing synthetic genes, further fueling market growth. This reduction in cost is enabling smaller biotechnology firms and academic institutions to take advantage of gene synthesis technologies.
Get Free Sample Report: https://www.snsinsider.com/sample-request/4521
Market Segmentation
The global gene synthesis market is segmented based on product type, application, end user, and region.
By Product Type:
Gene Fragments
Gene fragments, which are smaller, partial gene sequences, are commonly used in applications such as DNA cloning and PCR (polymerase chain reaction). These short, customized sequences allow researchers to test and modify genes efficiently, contributing to their widespread use in laboratories.
Complete Genes
Complete synthetic genes are in high demand for various research and therapeutic applications. From creating new proteins to developing cell therapies, full-length synthetic genes enable researchers to conduct a wide range of experiments and tests.
Custom Gene Synthesis Services
Custom gene synthesis services allow customers to design and order gene sequences that meet their specific requirements. These services are essential for pharmaceutical companies, academic researchers, and biotechnology firms that need precise genetic sequences for their projects.
By Application:
Drug Discovery and Development
The pharmaceutical sector remains the largest application area for gene synthesis, using synthetic genes to identify drug targets, develop biologics, and create therapeutic interventions for genetic disorders and cancer.
Agriculture and Biotechnology
In agriculture, gene synthesis is being employed to create genetically modified plants with improved traits, while in industrial biotechnology, synthetic genes are used to develop enzymes and bio-based chemicals.
Diagnostics and Therapeutics
Gene synthesis is also used in the development of diagnostics tools and therapeutics, including gene therapies, which target specific genetic mutations.
By End User:
Pharmaceutical and Biotechnology Companies
Pharmaceutical and biotechnology companies are the primary end-users of gene synthesis services, utilizing synthetic genes in drug development, biologics manufacturing, and personalized medicine research.
Academic and Research Institutes
Academic and research institutes are leveraging gene synthesis for various research purposes, including genetic engineering, synthetic biology, and gene function studies.
Contract Research Organizations (CROs)
CROs play an essential role in providing outsourced research services, including gene synthesis, to pharmaceutical and biotechnology companies. As drug discovery becomes more complex, many companies turn to CROs for efficient and cost-effective gene synthesis solutions.
Regional Analysis
North America
North America leads the gene synthesis market, driven by the region’s strong biotechnology and pharmaceutical sectors. The presence of key market players, robust research and development activities, and increasing government funding for genomic research contribute to the region's dominance. The U.S. is expected to maintain its leadership position throughout the forecast period.
Europe
Europe is the second-largest market for gene synthesis, with significant contributions from countries like Germany, the U.K., and France. The region’s focus on personalized medicine and synthetic biology is driving the demand for gene synthesis services. European initiatives aimed at improving healthcare infrastructure and promoting innovation in biotechnology are further fueling market growth.
Asia-Pacific
The Asia-Pacific region is projected to experience the fastest growth in the gene synthesis market, driven by increasing investments in biotechnology research, a growing pharmaceutical industry, and government initiatives to promote synthetic biology. Countries like China, Japan, and India are emerging as key players in the market, offering substantial opportunities for growth.
Key Market Players
The global gene synthesis market features several prominent players, including:
Thermo Fisher Scientific Inc.
As a global leader in the life sciences industry, Thermo Fisher provides gene synthesis services and products to researchers worldwide, supporting drug development, diagnostics, and synthetic biology applications.
GenScript Biotech Corporation
GenScript is a leading provider of gene synthesis services, offering high-quality synthetic genes for research and industrial applications.
Integrated DNA Technologies (IDT)
IDT is known for its advanced gene synthesis solutions, serving customers in pharmaceuticals, biotechnology, and academia.
Twist Bioscience Corporation
Twist Bioscience specializes in high-throughput gene synthesis, enabling the rapid production of synthetic genes for drug discovery, diagnostics, and industrial biotechnology.
Future Outlook
The gene synthesis market is poised for remarkable growth over the next decade, fueled by advancements in synthetic biology, personalized medicine, and biotechnology. As technological innovations continue to lower costs and improve efficiency, gene synthesis will become increasingly accessible to a wider range of industries, driving innovation and new product development.
0 notes
Text
Plasma Fractionation Market: Fueling Growth in Protein Therapeutics

The plasma fractionation market is poised for substantial growth, expected to expand from USD 31.7 billion in 2023 to USD 50.1 billion by 2030, with a projected compound annual growth rate (CAGR) of 7.8%. Plasma fractionation plays a critical role in the healthcare sector by enabling the extraction of various plasma-derived proteins for therapeutic use. This article explores the dynamics of this rapidly growing market, the driving forces behind its expansion, and the future outlook.
Market Size and Growth Projections
As of 2023, the plasma fractionation market stands at USD 31.7 billion, reflecting the increasing demand for plasma-derived products used in treating immune deficiencies, rare diseases, and trauma-related conditions. Projections estimate that by 2030, the market will grow to USD 50.1 billion, driven by advances in fractionation technology, a growing global population, and rising healthcare needs.
What is Plasma Fractionation?
Plasma fractionation is the process of separating plasma into its key components, such as immunoglobulins, albumin, and coagulation factors, which can then be used for various therapeutic applications. The process involves several stages of purification, extraction, and concentration, ensuring that high-quality plasma-derived products are available for medical use.
Access Full Report @ https://intentmarketresearch.com/latest-reports/plasma-fractionation-market-3065.html
Major Drivers of Growth in the Plasma Fractionation Market
Several factors are contributing to the rapid expansion of the plasma fractionation market:
Increasing Prevalence of Immune and Blood-Related Diseases
The growing incidence of conditions such as primary immune deficiencies (PID), hemophilia, and other blood disorders has increased the demand for plasma-derived therapies.
Rising Demand for Plasma-Derived Products
Plasma-derived products like albumin and immunoglobulins are widely used in treating immune system disorders and trauma patients, driving up demand.
Advancements in Technology
Improvements in fractionation technology have enabled more efficient processing and the development of novel therapies.
Challenges Facing the Plasma Fractionation Market
While the market is growing rapidly, several challenges remain:
Supply Chain Limitations
The collection of human plasma is a time-consuming process, and there are limitations on how much can be safely collected, affecting supply.
Regulatory Challenges
Navigating the complex regulatory landscape can delay product approvals and market access, especially in regions with stringent guidelines.
Key Segments in the Plasma Fractionation Market
By Product Type
Immunoglobulins: Used in treating immune deficiencies and autoimmune diseases.
Albumin: Widely used in trauma care and surgery.
Coagulation Factors: Essential for treating hemophilia and other clotting disorders.
By Application
Therapeutics: Plasma-derived products are critical in the treatment of immune deficiencies and blood disorders.
Diagnostics: Plasma components are also used in diagnostic tests.
By End-User
Hospitals and Clinics: Major consumers of plasma-derived therapies.
Research Institutes: Involved in plasma fractionation research and development.
Regional Analysis of the Plasma Fractionation Market
North America
North America is the largest market for plasma fractionation, thanks to the high prevalence of immune deficiencies and strong healthcare infrastructure.
Europe
Europe follows closely, driven by government initiatives to promote plasma donation and technological advancements.
Asia-Pacific
Asia-Pacific is experiencing rapid growth due to increasing healthcare expenditure and greater awareness of plasma-derived therapies.
Rest of the World
Other regions are showing steady growth, though access to plasma therapies remains limited in some developing countries.
Download Sample Report @ https://intentmarketresearch.com/request-sample/plasma-fractionation-market-3065.html
Plasma Fractionation Technology and Process
The process of plasma fractionation involves the separation of blood plasma into its various components through techniques like centrifugation and chromatography. These processes are becoming more efficient due to innovations in the field, which help meet the rising demand for plasma-derived products.
Plasma-Derived Products and Their Applications
Immunoglobulins: Widely used for treating immune deficiencies and autoimmune disorders.
Albumin:Used in critical care settings to restore blood volume in trauma patients.
Coagulation Factors:Essential for patients with hemophilia and other blood-clotting disorders.
Emerging Products:New plasma-derived products are continuously being developed to address unmet medical needs.
Therapeutic Uses of Plasma-Derived Products
Treatment of Immune Deficiencies: Plasma-derived immunoglobulins are life-saving for patients with weak immune systems.
Use in Trauma and Critical Care: Albumin is a critical component in treating patients who have lost large amounts of blood.
Applications in Rare Diseases: Many rare diseases, such as hemophilia, rely on plasma-derived products for effective treatment.
Regulatory Landscape in the Plasma Fractionation Market
FDA and EMA Guidelines: Regulatory bodies like the FDA in the U.S. and the EMA in Europe have stringent guidelines for the production and distribution of plasma-derived products, ensuring safety and efficacy.
Global Regulatory Trends: Emerging markets are adopting similar regulatory frameworks to ensure the safe introduction of plasma therapies.
Competitive Landscape
The plasma fractionation market is highly competitive, with major players like Grifols, CSL Behring, and Takeda dominating. These companies are investing heavily in R&D and engaging in mergers and acquisitions to strengthen their market position.
The Future of Plasma Fractionation
Emerging Trends and Innovations: The future of plasma fractionation looks promising, with developments in personalized medicine, gene therapy, and advancements in fractionation technology set to revolutionize the market.
Personalized Medicine and Plasma Fractionation: With the rise of personalized medicine, plasma therapies are becoming more tailored to individual patient needs, improving treatment outcomes.
Opportunities for Investors in the Plasma Fractionation Market
Growth Sectors for Investment: Investors can find lucrative opportunities in the growing demand for immunoglobulins and coagulation factors, as well as emerging plasma-based treatments.
Strategic Partnerships: Collaborations between pharmaceutical companies and research institutes are driving innovation and expanding the market potential.
Conclusion and Market Outlook
The plasma fractionation market is on an upward trajectory, driven by advances in technology, increasing demand for plasma-derived products, and a growing number of patients requiring these therapies. As the market continues to expand, it offers significant opportunities for healthcare providers, pharmaceutical companies, and investors.
Contact Us
US: +1 463-583-2713
0 notes
Text
Gene Switch Market To Grow Significantly Due To Advancements In CRISPR Technology

The Gene Switch Market is estimated to gain significant traction over the forecast period owing to advancements in CRISPR technology allowing precise genome editing.
The Gene Switch Market involves products and tools used to control the expression of genes in cells and organisms. Genome editing involves precisely altering DNA sequences and modifying gene function. Technologies such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9 system are commonly used gene switches that help control genes in cell therapies, drug discovery, bioprocessing, and plant and animal breeding. They provide temporal and quantitative control over gene expression, allowing modulation of gene expression levels. Gene switches find widespread application in controlling cell signaling pathways, investigating gene function, metabolic engineering, synthetic biology, and tissue engineering.
The Gene Switch Market is estimated to be valued at US$ 0.78 Bn in 2024 and is expected to exhibit a CAGR of 11.% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the Gene Switch Market are CRISPR Therapeutics, Editas Medicine, Sangamo Therapeutics, Intellia Therapeutics, Sana Biotechnology. CRISPR Therapeutics and Editas Medicine are leaders in developing CRISPR/Cas9-based therapies. CRISPR Therapeutics’ CTX001 is undergoing clinical trials for treating sickle cell disease and beta-thalassemia. Editas Medicine is developing in vivo CRISPR medicines for treating leucine-rich repeat kinase 2 (LRRK2) gene-associated Parkinson's disease.
The Gene Switch Market is witnessing increasing demand driven by genome editing applications in drug discovery, biotechnology research, synthetic biology, and agriculture. Gene switches allow understanding disease mechanisms and developing therapies by modulating gene function. Growing R&D targeting rare diseases and viruses is supporting Gene Switch Market growth.
Technological advancements such as enhanced precision and efficiency of genome editing driven by modified CRISPR systems, base editors, and prime editors are expanding the repertoire of programmable gene switches. These innovations are improving applications of genome editing in developing therapies and bioproduction cell lines.
Market Trends
Increasing focus on cell and gene therapies: Advancements in gene switches are enabling development of multiple cell and gene therapies targeting cancers, genetic disorders, and infectious diseases. This is a key trend driving increased adoption of programmable gene switches.
Growing synthetic biology applications: Design and construction of novel genetic circuits using programmable gene switches enables applications such as development of sensing and detection technologies. This growing field is creating opportunities for Gene Switch Market players.
Market Opportunities
Precision medicine: Advancements in gene switches can facilitate development of precision drugs for addressing disease heterogeneity at the molecular level. Tailored treatments are an emerging opportunity.
Agricultural biotechnology: Development of stress-resistant and high-yield crops using gene switches can address global food security challenges. Gene editing in livestock and fish offers market prospects.
Impact Of COVID-19 On Gene Switch Market Growth
The COVID-19 pandemic has significantly impacted the growth of the Gene Switch Market. Some of the major effects of the virus outbreak on the market include:
- Disruption in Research & Development Activities: Nationwide lockdowns and social distancing norms enforced by governments across the globe led to closure of research laboratories and delay in ongoing clinical trials for gene therapies utilizing gene switches. This hampered innovations and new product development efforts of companies in the short term.
- Change in Funding Priorities: Several private and government funding bodies in different countries shifted their research funding priorities towards developing diagnostics, vaccines and therapeutics for COVID-19. This led to temporary reduced financing availability for non-COVID gene therapy projects utilizing gene switches.
- Supply Chain Disruptions: Movement restrictions and shuttering of manufacturing units globally disrupted Gene Switch Market supply chains in the initial months of the pandemic. It affected raw material procurement as well as finished product distribution for several players.
- Economic Uncertainty Impacting Investments: The widespread economic recession caused by the public health crisis negatively impacted investment sentiments particularly in the biotechnology sector. It deterred capital commitments towards R&D and commercialization activities in the Gene Switch Market in 2020.
While short term challenges emerged, the pandemic has highlighted the need for development of advanced gene therapies utilizing gene switches for treatment of genetic disorders. With resumption of business activities and relaxation of lockdowns, the market is expected to get back on the growth trajectory over the coming years bolstered by accelerated research in cell and gene therapies for various diseases.
Geographical Regions Concentrated In Gene Switch Market
North America accounts for the largest share of the Gene Switch Market in terms of value. Availability of advanced research infrastructure and high healthcare expenditure are major factors contributing to concentration of market in the region. The United States stands out as the dominant country-level market within North America attributed to presence of several global players as well as supportive regulatory environment for clinical research.
Fastest Growing Region In Gene Switch Market
Asia Pacific region is projected to witness the fastest growth in the Gene Switch Market during the forecast period led by China, Japan and South Korea. Increasing government funding for medical research, rising healthcare investments, developing regional capabilities in genetic engineering and growing incidence of genetic disorders are some of the key aspects fueling robust regional expansion. A rapidly developing biotechnology industry and growing pool of clinical research organizations are facilitating technology adoption, driving the Asia Pacific market for gene switches at a higher growth rate compared to mature Western markets.
Get more insights on this topic: https://www.pressreleasebulletin.com/gene-switch-market-is-estimated-to-witness-high-growth-owing-to-genome-editing-technologies/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
What Are The Key Data Covered In This Gene Switch Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Gene Switch Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Gene Switch Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Gene Switch Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Gene Switch Market vendors
FAQ’s
Q.1 What are the main factors influencing the Gene Switch Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Gene Switch Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Gene Switch Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Gene Switch Market Trend#Gene Switch Market Size#Gene Switch Market Information#Gene Switch Market Analysis#Gene Switch Market Demand
0 notes
Text
Meticulous Research® Releases In-Depth Analysis on Global Clinical Trials Market, Forecasting Growth to $102.20 Billion by 2031
Meticulous Research®, a renowned global market research company, has published a comprehensive report titled, "Clinical Trials Market Size, Share, Forecast, & Trends Analysis by Service (Consulting, Patient Recruitment, Data Management, Regulatory, Site Support), Therapeutic Area (Oncology, Cardiology, Diabetes, Dermatology), Phase, End User - Global Forecast to 2031." This report provides a detailed examination of the global clinical trials market, which is projected to reach $102.20 billion by 2031, expanding at a CAGR of 7.2% from 2024 to 2031.
Key Market Drivers and Challenges
The clinical trials market is experiencing robust growth due to several pivotal factors:
Focus on Rare Diseases: An increasing emphasis on developing treatments for rare diseases is driving market growth.
Adoption of Personalized Medicine: The growing adoption of personalized medicine is fueling demand for specialized clinical trials.
R&D Expenditure: Rising investments in research and development are accelerating the number of clinical trials conducted globally.
Improved Recruitment Processes: Enhanced recruitment and retention strategies are leading to more efficient clinical trials.
Download Sample Report Here: https://www.meticulousresearch.com/download-sample-report/cp_id=5934
However, the market faces challenges such as the time-intensive nature of clinical trials, stringent regulations, and evolving legal frameworks, which add complexity to the process.
Emerging Opportunities and Trends
The clinical trials market is poised for significant growth opportunities, particularly in the following areas:
Patient-Centric Trials: The integration of the Internet of Medical Things (IoMT) is enabling more patient-centered approaches in clinical trials.
Cloud-Based Pharmacovigilance: Advancements in cloud technology are revolutionizing pharmacovigilance practices, ensuring better safety monitoring.
AI Integration: The use of artificial intelligence in clinical trials, from patient recruitment to data analytics, is transforming the industry.
Notable market trends include the incorporation of digital health technologies, wearable devices, automation, and decentralized clinical trials. Additionally, AI-driven modeling for patient enrichment and recruitment is gaining traction, alongside AI-enabled analytics for clinical trial management.
Leading Industry Players
The global clinical trials market is dominated by key players, including:
Laboratory Corporation of America Holdings (U.S.)
Medpace, Inc. (U.S.)
Charles River Laboratories International, Inc. (U.S.)
IQVIA Inc. (U.S.)
Parexel International Corporation (U.S.)
Syneos Health (U.S.)
ICON plc (Ireland)
WuXi AppTec Co., Ltd. (China)
Thermo Fisher Scientific Inc. (U.S.)
Fortrea Inc. (U.S.)
Celerion Inc. (U.S.)
Novotech Health Holdings (Australia)
SGS Société Générale de Surveillance SA (Switzerland)
CTI Clinical Trial and Consulting, Inc. (U.S.)
Linical USA, Inc. (U.S.)
Quick Buy: https://www.meticulousresearch.com/Checkout/42238521
Market Segmentation and Regional Insights
The clinical trials market is segmented into phases, service types, therapeutic areas, end users, and geography. Key segments include:
Phase III Trials: Expected to dominate in 2024 with a market share exceeding 68.4%, driven by the large number of participants, extended trial durations, and significant financial investments.
Oncology Therapeutics: Forecasted to lead the therapeutic areas with over 33.2% market share in 2024, propelled by the rising incidence of cancer and increased research efforts.
Geographically, North America is set to maintain the largest market share, accounting for over 41.6% in 2024. This dominance is attributed to substantial R&D investments, the early adoption of new technologies in clinical trials, and the presence of major market players. For instance, Syneos Health (U.S.) and uMotif Limited (U.K.) have recently collaborated to enhance clinical trials through digital innovations, including Electronic Patient-reported Outcomes (ePRO) and Electronic Clinical Outcome Assessments (eCOA).
Request Sample Report Here: https://www.meticulousresearch.com/request-sample-report/cp_id=5934
Key Questions Addressed in the Report:
What are the high-growth market segments in terms of phase, service type, therapeutic area, end user, and region/country?
What was the historical market size for clinical trials globally?
What are the market forecasts and estimates for 2024–2031?
What are the major drivers, restraints, challenges, opportunities, and trends in the global clinical trials market?
Who are the key competitors, and what strategies do they employ?
What are the recent developments and geographical trends in the market?
Contact Meticulous Research®
For further inquiries or detailed insights:
Email: [email protected]
Phone: +1-646-781-8004
0 notes
Text
Veterinary Clinical Trials Market Future Outlook: Analyzing Size, Share, and Growth Patterns
The global veterinary clinical trials market size is expected to reach USD 8.99 billion by 2030, exhibiting a CAGR of 9.0% from 2024 to 2030, according to a new report by Grand View Research, Inc. The key factors driving industry growth include increased R&D expenditure by key players, rising prevalence of chronic diseases in animals, advancements in veterinary medicines, and rise in strategic alliances. For instance, in April 2024, Boehringer Ingelheim, a biopharmaceutical company focused on both human and animal health invested USD 593.5 Mn in animal health R&D to accelerate veterinary clinical trials as well as to develop innovative veterinary medicine.
Veterinary Clinical Trials Market Report Highlights
Ongoing advancements in veterinary medicine drive the demand for veterinary clinical trials to improve animal health and welfare. Additionally, growing R&D investments by key players to innovate veterinary medicine are expected to boost market demand.
By animal type,companion animal segment dominated the market in 2023 and is projected to witness the fastest growth rate of 9.5% in the coming years. Furthermore, among companion animals, canines segment is holding highest market share in 2023.
In terms of indication, oncology segment dominated the market with a share of 27.51% in 2023, owing to the rising prevalence of cancers in animals and growing R&D investment for veterinary oncology. Whereas internal medicine segment is expected to grow at the fastest rate of over 10.2% in the coming years.
Based on the sponsor, pharmaceutical and biopharmaceutical companies segment dominated the market in 2023, and the academics and research centers segment is expected to grow at the fastest rate of over 9.8% in the coming years.
Medicines accounted for the largest market share by intervention type in 2023. However, medical device segment is estimated to witness the highest growth rate of around 10.0% in the near future.
In 2023, North America held the highest share of 33.70% of the market by region.The Asia Pacific region is expected to grow at the fastest rate of over 10.1% in the coming years.
For More Details or Sample Copy please visit link @: Veterinary Clinical Trials Market Report
Additionally, an increasing number of clinical trials for rare diseases in pets is expected to drive veterinary clinical trial market. Veterinary clinics and research institutions are undertaking specialized clinical trial initiatives focused specifically on rare canine cancers. These trials aim to investigate novel treatment approaches, including targeted therapies, immunotherapies, and precision medicine techniques designed for individual dogs' genetic profiles. For instance, in March 2024, Vivesto AB initiated its Paccal Vet clinical trial for dogs with splenic hemangiosarcoma, marking a significant step toward addressing this challenging cancer type in canines. The trial aims to assess the safety and efficacy of Paccal Vet, formulated with XR-17 technology, with promising results potentially leading to further pivotal studies.
There is a growing focus on preventive care in veterinary medicine. Clinical trials aimed at developing preventive treatments, such as vaccines and early diagnostic tools, are becoming more common. Furthermore, veterinary clinics and research institutions are launching specialized clinical trial initiatives focused specifically on rare canine cancers. These trials aim to investigate novel treatment approaches, including targeted therapies, immunotherapies, and precision medicine techniques tailored to individual dogs' genetic profiles.
List of Key Players in the Veterinary Clinical Trials Market
Charles River Laboratory
IDEXX Laboratories, Inc.
Boehringer Ingelheim International GmbH.
Argenta
Bioagile Therapeutics Pvt. Ltd.
Veterinary Research Management
Merck & Co., Inc.
Labcorp Drug Development
OCR - Oncovet Clinical Research
Vivesto AB
Vetbiolix
#VeterinaryClinicalTrialsMarket#VeterinaryCRO#VeterinaryClinicalStudies#VeterinaryClinicalInvestigation#ClinicalTrials#VeterinaryCenter#VeterinaryMedicine#AnimalHealth#Biomarkers#VeterinaryCare#VeterinaryClinics
0 notes
Text
The global market for Gaucher disease therapeutics is projected to expand at a compound annual growth rate (CAGR) of 2.50%, from an estimated USD 1358.2 million in 2023 to USD 1696.20 million in 2032.Gaucher disease, a rare genetic disorder, is caused by the deficiency of the enzyme glucocerebrosidase, leading to the accumulation of harmful substances in certain organs, primarily the spleen, liver, and bone marrow. The disease manifests in various forms, ranging from mild to severe, and can significantly impact the quality of life. The market for Gaucher disease therapeutics has seen substantial growth due to advancements in treatment options, increased awareness, and the rising prevalence of the condition.
Browse the full report at https://www.credenceresearch.com/report/global-gaucher-disease-therapeutics-market
Market Overview
The global Gaucher disease therapeutics market has witnessed significant growth over the past decade. The market is driven by several factors, including the rising prevalence of Gaucher disease, increased R&D activities, and the availability of advanced therapies. According to recent estimates, Gaucher disease affects approximately 1 in 40,000 to 1 in 60,000 individuals globally. However, in certain populations, such as the Ashkenazi Jewish community, the prevalence is significantly higher, with about 1 in 850 individuals affected.
The market is segmented based on treatment type, including enzyme replacement therapy (ERT), substrate reduction therapy (SRT), and others. ERT is currently the most widely used treatment option, with drugs like Cerezyme (imiglucerase) and Vpriv (velaglucerase alfa) dominating the market. SRT, which includes drugs like Cerdelga (eliglustat), is also gaining traction as a viable alternative, especially for patients who may not respond well to ERT.
Key Market Drivers
1. Rising Prevalence and Diagnosis Rates: With the advent of more sophisticated diagnostic tools and increased awareness among healthcare professionals and patients, Gaucher disease is being diagnosed more frequently. This has led to an increase in the number of patients seeking treatment, thereby driving market growth.
2. Advancements in Treatment Options: The introduction of novel therapies, such as gene therapy and personalized medicine, has opened new avenues for the treatment of Gaucher disease. These advancements are expected to provide more effective and targeted treatment options, thus boosting market growth.
3. Increased R&D Activities: Pharmaceutical companies and research institutions are investing heavily in R&D to develop new and improved therapies for Gaucher disease. This has led to the introduction of several new drugs in the market and the expansion of the existing therapeutic pipeline.
4. Government Support and Initiatives: Governments and non-profit organizations are playing a crucial role in raising awareness about Gaucher disease and providing support for research and treatment. Various initiatives aimed at improving access to treatment and reducing the cost burden on patients have also contributed to market growth.
Challenges and Restraints
While the Gaucher disease therapeutics market is poised for growth, it is not without challenges. One of the primary challenges is the high cost of treatment. Enzyme replacement therapy, for instance, can cost upwards of $200,000 per year, making it unaffordable for many patients, particularly in low- and middle-income countries. Additionally, the rarity of the disease poses challenges in terms of conducting large-scale clinical trials and obtaining regulatory approvals.
Another significant challenge is the limited availability of treatment options in certain regions. While North America and Europe are well-established markets for Gaucher disease therapeutics, access to treatment in regions like Asia-Pacific, Latin America, and Africa remains limited. This disparity in access is a major barrier to market growth in these regions.
Regional Insights
North America currently holds the largest share of the Gaucher disease therapeutics market, followed by Europe. This dominance can be attributed to the presence of well-established healthcare infrastructure, high awareness levels, and the availability of advanced treatment options. The Asia-Pacific region, however, is expected to witness the fastest growth during the forecast period. This growth is driven by increasing healthcare spending, rising awareness, and the growing focus of pharmaceutical companies on expanding their presence in emerging markets.
Future Outlook
The future of the Gaucher disease therapeutics market looks promising, with several new therapies expected to receive regulatory approval in the coming years. Gene therapy, in particular, holds immense potential for revolutionizing the treatment landscape. Additionally, the increasing focus on personalized medicine and the development of therapies tailored to individual patient needs is expected to further drive market growth.
Key Players
Novartis AG (Switzerland)
Johnson & Johnson Private Limited (U.S.)
Teva Pharmaceutical Industries Ltd. (Ireland)
Merck and Co., Inc. (U.S.)
Allergan (Ireland)
Pfizer Inc. (U.S.)
GlaxoSmithKline plc (U.K.)
Sanofi (France)
Merck KGaA (Germany)
Abbott (U.S.)
Boehringer Ingelheim International GmbH. (Germany)
Takeda Pharmaceutical Company Limited (Japan)
Amicus Therapeutics, Inc. (U.S.)
Moderna, Inc. (U.S.)
Greenovation Biotech GmbH (Germany)
Biomarin (U.S.)
JCR Pharmaceuticals Co., Ltd (Japan)
ISU ABXIS (South Korea)
Idorsia Pharmaceuticals Ltd (Switzerland)
AVROBIO, Inc. (U.S.)
Resverlogix Corp. (Canada)
Enzyvant (U.S.)
CHIESI Farmaceutici SpA (Italy)
Others
Segmentation
By Therapy Type
Enzyme Replacement Therapy (ERT)
Recombinant Enzymes
Long-Acting ERT
Novel ERT Formulations
Substrate Reduction Therapy (SRT)
Pharmacological Chaperones
Substrate Inhibitors
Gene Therapy
Viral Vector-Based Gene Therapy
Non-Viral Gene Therapy
Supportive Therapies
Symptomatic Treatment
Nutritional Support
By Route of Administration
Intravenous (IV) Administration
Oral Administration
By End-User
Hospitals and Clinics
Specialty Clinics
Home Care Settings
By Region
North America
U.S
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
The Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/global-gaucher-disease-therapeutics-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes
Text
Biopharmaceuticals Market Size, Share, Segments, Top Companies, Future Prospects, Application and Forecast by 2031
The Biopharmaceuticals Market has been a subject of considerable interest and investment in recent years, as advancements in biotechnology continue to redefine the landscape of healthcare and pharmaceuticals. With the potential to revolutionize treatment approaches and address unmet medical needs, biopharmaceuticals have emerged as a critical component of modern medicine. The Global Biopharmaceuticals market, analyzed and explored by Metastat Insight, reflects the growing significance of these innovative therapies in shaping the future of healthcare.
Get Free Sample Report @ https://www.metastatinsight.com/request-sample/2731
Top Companies
Abbvie Inc, Amgen Inc., AstraZeneca PLC, Biogen Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, F Hoffmann-La Roche AG, GlaxoSmithKline PLC, Merck & Co., Inc., Novartis AG, Novo Nordisk A/S.
Biopharmaceuticals, also known as biologics, encompass a diverse range of therapeutic products derived from biological sources such as living organisms, cells, tissues, or genetic material. Unlike traditional small molecule drugs, biopharmaceuticals offer unique advantages, including greater specificity, enhanced efficacy, and reduced side effects. This has led to their widespread adoption across various disease areas, including oncology, autoimmune disorders, infectious diseases, and rare genetic conditions.
The growth of the Global Biopharmaceuticals market can be attributed to several factors. Firstly, the increasing prevalence of chronic and complex diseases worldwide has created a pressing need for more effective and targeted treatment options. Biopharmaceuticals, with their ability to precisely target disease pathways and modulate the immune system, offer promising solutions for patients with challenging medical conditions.
Browse Complete Report @ https://www.metastatinsight.com/report/biopharmaceuticals-market
Moreover, advancements in biotechnology and genetic engineering have fueled the development of innovative biopharmaceuticals with enhanced therapeutic properties. From monoclonal antibodies and recombinant proteins to gene and cell therapies, the repertoire of biopharmaceutical products continues to expand, driving growth and innovation in the market.
0 notes