#Global Glucose monitoring kits Market Demand
Explore tagged Tumblr posts
Text
Point-of-Care Testing Market Size, Share, Trends, Growth and Competitor Analysis
Point-of-Care Testing (POCT) Market - Size, Share, Demand, Industry Trends and Opportunities
Global Point-of-Care Testing (POCT) Market, By Product (Glucose Monitoring Products, Cardiometabolic Testing Products, Infectious Disease Testing Products, Pregnancy and Fertility Testing Products, Tumor/Cancer Marker Testing Products, Urinalysis Testing Products, Cholesterol Testing Products, Hematology Testing Products, Drugs-of-Abuse Testing Products, Fecal Occult Testing Products, Rapid Coagulation Testing, Other POC Products), Application (Blood Transfusion, Cardiac Monitoring, Coagulation, Blood Glucose, Haematology, Non- Invasive SPO2 Monitoring, Non- Invasive PCO2 Monitoring, Whole Blood Analysis, Vital Sign Monitoring, Others), Platform (Lateral Flow Assays/Immunochromatography Tests, Dipsticks, Microfluidics, Molecular Diagnostics, Immunoassays), Prescription Mode (Prescription-Based Testing, OTC Testing), Testing Type (Immunoassays, Cell-Based Assays, Nucleic Acid Amplification Testing, Clinical Chemistry Assays, Hematology), End-User (Hospitals, Clinics, Laboratories, Home Care, Ambulatory Surgery Centers, Others), Distribution Channel (Direct Tender, Retail Pharmacies) – Industry Trends.
Access Full 350 Pages PDF Report @
**Segments**
- **Product Type**: The Point-of-Care Testing (POCT) market can be segmented based on product types such as Glucose Monitoring, Infectious Disease Testing, Cardiometabolic Testing, Pregnancy and Fertility Testing, Coagulation Testing, Hematology Testing, Urinalysis Testing, Cholesterol Testing, Drug Abuse Testing, Cancer Marker Testing, and Others.
- **End-User**: The market can also be segmented by end-users, including Hospitals, Home Care Settings, Clinics, Diagnostic Laboratories, and Assisted Living Healthcare Facilities.
- **Mode of Delivery**: The Point-of-Care Testing market can be categorized based on the mode of delivery into OTC Testing Kits and Prescriptions Tests. This segment looks at how the tests are accessed and utilized by patients and healthcare providers.
**Market Players**
- **Roche Diagnostics**: Roche is a key player in the POCT market, offering a wide range of testing solutions across various disease areas. They are known for their innovative technologies and strong presence in the healthcare industry.
- **Abbott Laboratories**: Abbott is another major player in the POCT market, with a focus on delivering accurate and reliable testing solutions. Their portfolio includes a diverse range of products catering to different medical needs.
- **Siemens Healthineers**: Siemens is a prominent company in the healthcare sector, providing advanced POCT solutions that enable faster and more efficient testing processes. They are recognized for their cutting-edge technologies and commitment to improving patient care.
- **Danaher Corporation**: Danaher is a multinational conglomerate with a strong presence in the POCT market through its diverse portfolio of diagnostic products. They are known for their high-quality testing solutions and continuous innovation in the field.
- **F. Hoffmann-La Roche AG**: Roche AG is a global leader in healthcare, with a significant market share in the POCT segment. Their comprehensive range of testing products and services cater to the evolving needs of healthcare providers andRoche Diagnostics is a prominent player in the Point-of-Care Testing (POCT) market, known for its extensive range of testing solutions across various disease areas. The company's commitment to innovation has enabled them to develop cutting-edge technologies that set them apart from competitors. Roche's strong presence in the healthcare industry positions them as a key player in the global POCT market, with a focus on delivering accurate and reliable testing solutions to meet the evolving needs of healthcare providers and patients. Their comprehensive portfolio of products, including Glucose Monitoring, Infectious Disease Testing, Cardiometabolic Testing, and Cancer Marker Testing, caters to a wide range of medical requirements.
Abbott Laboratories is another major player in the POCT market, recognized for its focus on providing high-quality and reliable testing solutions. The company's diverse range of products, spanning different medical needs such as Pregnancy and Fertility Testing, Hematology Testing, and Urinalysis Testing, showcases their commitment to addressing various healthcare challenges. Abbott's reputation for accuracy and efficiency in diagnostic testing positions them as a key competitor in the global POCT market, with a strong presence in hospitals, clinics, and diagnostic laboratories worldwide.
Siemens Healthineers stands out in the POCT market for its advanced solutions that enable faster and more efficient testing processes. The company's innovative technologies and commitment to improving patient care have solidified their position as a leading player in the industry. Siemens' focus on developing cutting-edge POCT solutions, including Coagulation Testing, Cholesterol Testing, and Drug Abuse Testing, reflects their dedication to meeting the diverse needs of healthcare providers and patients. With a strong presence in home care settings and assisted living healthcare facilities, Siemens Healthineers continues to drive advancements in POCT technology.
Danaher Corporation's multinational conglomerate status and diverse portfolio of diagnostic products have established them as a significant player in the POCT market. The company's reputation for high-quality testing solutions and continuous innovation sets them apart from competitors. Dan**Segments:**
- **Global Point-of-Care Testing (POCT) Market, By Product**: Glucose Monitoring Products, Cardiometabolic Testing Products, Infectious Disease Testing Products, Pregnancy and Fertility Testing Products, Tumor/Cancer Marker Testing Products, Urinalysis Testing Products, Cholesterol Testing Products, Hematology Testing Products, Drugs-of-Abuse Testing Products, Fecal Occult Testing Products, Rapid Coagulation Testing, Other POC Products - **Application**: Blood Transfusion, Cardiac Monitoring, Coagulation, Blood Glucose, Haematology, Non-Invasive SPO2 Monitoring, Non-Invasive PCO2 Monitoring, Whole Blood Analysis, Vital Sign Monitoring, Others - **Platform**: Lateral Flow Assays/Immunochromatography Tests, Dipsticks, Microfluidics, Molecular Diagnostics, Immunoassays - **Prescription Mode**: Prescription-Based Testing, OTC Testing - **Testing Type**: Immunoassays, Cell-Based Assays, Nucleic Acid Amplification Testing, Clinical Chemistry Assays, Hematology - **End-User**: Hospitals, Clinics, Laboratories, Home Care, Ambulatory Surgery Centers, Others - **Distribution Channel**: Direct Tender, Retail Pharmacies
In the Point-of-Care Testing (POCT) market, the segments play a crucial role in understanding the diverse landscape of testing solutions available to healthcare providers and patients. Product type segmentation provides insight into
Global Point-of-Care Testing (POCT) Market survey report analyses the general market conditions such as product price, profit, capacity, production, supply, demand, and market growth rate which supports businesses on deciding upon several strategies. Furthermore, big sample sizes have been utilized for the data collection in this business report which suits the necessities of small, medium as well as large size of businesses. The report explains the moves of top market players and brands that range from developments, products launches, acquisitions, mergers, joint ventures, trending innovation and business policies.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Point-of-Care Testing (POCT) Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Point-of-Care Testing (POCT) Market.
The following are the regions covered in this report.
North America [U.S., Canada, Mexico]
Europe [Germany, UK, France, Italy, Rest of Europe]
Asia-Pacific [China, India, Japan, South Korea, Southeast Asia, Australia, Rest of Asia Pacific]
South America [Brazil, Argentina, Rest of Latin America]
The Middle East & Africa [GCC, North Africa, South Africa, Rest of the Middle East and Africa]
Browse Trending Reports:
Field Hockey Equipment Market Software-Defined Networking (SDN) Orchestration Market Radio Frequency Identification RFID Tags for Livestock Management Market Colposcopy Market Marine Fuel Injection Market Enhanced Fire Protection Systems Market Moisture Wicking Socks Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]
0 notes
Text
Disposable Point-of-Care Testing (POCT) Devices Market Insights: Growth, Share, Value, Size, and Analysis
"Disposable Point-of-Care Testing (POCT) Devices Market Size, Share, and Trends Analysis Report—Industry Overview and Forecast to 2030
The Rapid Diagnostic Test Market is witnessing strong growth across multiple sectors, including [industry name], where demand is rising due to innovation and market expansion. Market research data indicates that businesses in the Single-Use POCT Market are adapting to regulatory changes, sustainability initiatives, and evolving consumer behaviors. Companies in the Portable Lab Testing Market are leveraging big data and analytics to understand trends, optimize supply chains, and improve service offerings. As competition increases, firms operating in the On-the-Spot Medical Testing Market are investing in strategic market research to gain insights into emerging opportunities, industry challenges, and future business models shaping the Mobile Health Testing Market.
The Disposable Point-of-Care Testing (POCT) Devices Market is poised for significant growth, with a market outlook highlighting substantial growth potential driven by emerging opportunities in key sectors. This report provides strategic insights, demand dynamics, and revenue projections, offering a comprehensive view of the future landscape, technology disruptions, and adoption trends shaping the industry’s ecosystem evaluation. According to Data Bridge Market Research Data Bridge Market Research analyses that the Global Disposable Point-of-Care Testing (POCT) Devices Market which was USD 32960 Billion in 2022 is expected to reach USD 54140.25 Million by 2030 and is expected to undergo a CAGR of 6.40% during the forecast period of 2022 to 2030
In today's dynamic business landscape, understanding the nuances of specific sectors is paramount. The Quick-Response Diagnostic Kits Market presents a compelling case study for any organization seeking to navigate its complexities. We've observed a surge in interest surrounding the Handheld Diagnostic Solutions Market, driven by evolving consumer behaviors and technological advancements. This market, characterized by its unique challenges and opportunities, demands a keen, analytical eye. Our deep dive into the At-Site Testing Devices Market reveals patterns and trends that are crucial for strategic decision-making. We aim to provide clarity on the evolving terrain of the Urgent Care POCT Market, helping businesses understand the current realities of the market. The intricacies of the Fast-Acting Diagnostic Devices Market are becoming more apparent.
Our comprehensive Disposable Point-of-Care Testing (POCT) Devices Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-disposable-poct-devices-market
**Segments**
- **Product Type**: The disposable POCT devices market can be segmented based on product type, which includes blood glucose monitoring kits, infectious disease testing kits, pregnancy and fertility testing kits, cardiometabolic monitoring kits, urinalysis testing kits, and other types of kits. These different product types cater to varying medical needs and offer convenience, accuracy, and ease of use for point-of-care testing.
- **End User**: Another important segmentation factor is the end user of disposable POCT devices. This can include hospitals, clinics, diagnostic laboratories, home care settings, and other healthcare facilities. The adoption of disposable POCT devices varies across these end user segments based on factors such as volume of testing, ease of use, cost-effectiveness, and scalability of the testing solution.
- **Distribution Channel**: The market for disposable POCT devices can also be segmented based on distribution channels. These channels include direct sales to end users, online sales platforms, retail pharmacies, and other distribution channels. The choice of distribution channel can impact the accessibility of these devices to healthcare providers and end users, influencing market penetration and sales growth.
**Market Players**
- **Abbott Laboratories**: Abbott is a key player in the disposable POCT devices market, offering a wide range of testing kits for various medical conditions. The company's focus on innovation, quality, and accessibility has helped it maintain a strong position in the market.
- **Roche Diagnostics**: Roche Diagnostics is another prominent player in the disposable POCT devices market, known for its high-quality testing solutions and reliable performance. The company's continuous investment in research and development ensures a competitive product portfolio.
- **Siemens Healthineers**: Siemens Healthineers is a leading provider of disposable POCT devices, offering innovative testing kits for diverse medical applications. The company's strong global presence and emphasis on technological advancements contribute to its market leadership.
- **BD (Becton, Dickinson and Company)**: BD is a well-established player inBD (Becton, Dickinson and Company) is a well-established player in the disposable POCT devices market, with a strong reputation for its high-quality medical products and solutions. The company's extensive experience and expertise in developing innovative testing kits for a wide range of medical conditions have positioned it as a key player in the market. BD's commitment to research and development ensures the continuous enhancement of its product portfolio to address the evolving needs of healthcare providers and patients.
One of the key strengths of BD in the disposable POCT devices market is its comprehensive product offerings that cater to various medical applications. The company provides a diverse range of testing kits, including blood glucose monitoring kits, infectious disease testing kits, pregnancy and fertility testing kits, cardiometabolic monitoring kits, and other types of kits. This wide product range allows BD to target different segments of the market and meet the specific requirements of healthcare facilities and end users.
BD's strong distribution network is another factor that contributes to its success in the disposable POCT devices market. The company utilizes multiple distribution channels, including direct sales, online platforms, retail pharmacies, and other channels, to ensure the accessibility of its products to healthcare providers and end users. By leveraging these channels effectively, BD can enhance market penetration and reach a broader customer base, driving sales growth and market share expansion.
In addition to its product offerings and distribution capabilities, BD's focus on quality and innovation sets it apart in the disposable POCT devices market. The company prioritizes product quality and reliability, ensuring that its testing kits deliver accurate and consistent results for point-of-care testing. Furthermore, BD invests heavily in research and development to drive innovation and stay ahead of market trends, allowing it to introduce new and advanced solutions that address emerging needs in healthcare.
As the demand for disposable POCT devices continues to grow, BD is well-positioned to capitalize on market opportunities and sustain its competitive edge. The company's strong brand reputation, extensive product portfolio, robust distribution network, commitment to quality and innovation**Market Analysis**
The disposable POCT devices market is experiencing rapid growth driven by factors such as the increasing prevalence of chronic diseases, the rising demand for rapid diagnostic solutions, and the emphasis on decentralized healthcare delivery. The segmentation of the market based on product type, end user, and distribution channels allows for a more targeted approach to cater to the diverse needs of healthcare providers and patients. Blood glucose monitoring kits, infectious disease testing kits, and cardiometabolic monitoring kits are among the key product types driving market growth as they offer convenience, accuracy, and ease of use for point-of-care testing.
Key market players in the disposable POCT devices industry include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and BD (Becton, Dickinson and Company). These companies have established strong positions in the market through their focus on innovation, quality, and accessibility of testing solutions. For example, BD's comprehensive product offerings and robust distribution network have positioned it as a leader in the market, allowing it to target different market segments effectively and meet the specific requirements of healthcare facilities and end users.
Other notable market players in the disposable POCT devices market include Danaher, F. Hoffmann-La Roche Ltd., Trinity Biotech Ireland, Cardinal Health, Thermo Fisher Scientific, and Meridian Bioscience, among others. The presence of these key players in the market signifies intense competition and ongoing advancements in testing technology. As the demand for disposable POCT devices continues to rise, market players are
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Disposable Point-of-Care Testing (POCT) Devices Market : https://www.databridgemarketresearch.com/reports/global-disposable-poct-devices-market/companies
Key Questions Answered by the Global Disposable Point-of-Care Testing (POCT) Devices Market Report:
What are the key consumer preferences and buying behaviors in the Disposable Point-of-Care Testing (POCT) Devices Market?
How does the Disposable Point-of-Care Testing (POCT) Devices Market compare to other related markets in terms of growth and investment potential?
What is the role of research and development (R&D) in shaping the future of the Disposable Point-of-Care Testing (POCT) Devices Market?
How do geopolitical factors and trade policies affect the Disposable Point-of-Care Testing (POCT) Devices Market?
What are the top trends shaping the competitive landscape of the Disposable Point-of-Care Testing (POCT) Devices Market?
How are companies in the Disposable Point-of-Care Testing (POCT) Devices Market addressing environmental and sustainability concerns?
What are the short-term and long-term growth opportunities in the Disposable Point-of-Care Testing (POCT) Devices Market?
How will shifts in global supply chains impact the Disposable Point-of-Care Testing (POCT) Devices Market?
What are the expected market dynamics over the next five to ten years?
What are the key sustainability trends influencing the Disposable Point-of-Care Testing (POCT) Devices Market?
Which companies are investing the most in R&D, and how does it influence the market?
What are the key challenges for companies in scaling operations within the Disposable Point-of-Care Testing (POCT) Devices Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/north-america-aws-managed-services-markethttps://www.databridgemarketresearch.com/reports/global-eco-fiber-markethttps://www.databridgemarketresearch.com/reports/global-geospatial-imagery-analytics-markethttps://www.databridgemarketresearch.com/reports/global-green-plant-based-proteins-markethttps://www.databridgemarketresearch.com/reports/global-skin-biopsy-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 982
✉ Email: [email protected]
Tag
Disposable Point-of-Care Testing (POCT) Devices Market Size, Disposable Point-of-Care Testing (POCT) Devices Market Share, Disposable Point-of-Care Testing (POCT) Devices Market Trend, Disposable Point-of-Care Testing (POCT) Devices Market Analysis, Disposable Point-of-Care Testing (POCT) Devices Market Report, Disposable Point-of-Care Testing (POCT) Devices Market Growth, Latest Developments in Disposable Point-of-Care Testing (POCT) Devices Market, Disposable Point-of-Care Testing (POCT) Devices Market Industry Analysis, Disposable Point-of-Care Testing (POCT) Devices Market Key Players, Disposable Point-of-Care Testing (POCT) Devices Market Demand Analysis"
#Rapid Diagnostic Test Market#Single-Use POCT Market#Portable Lab Testing Market#On-the-Spot Medical Testing Market#Mobile Health Testing Market#Quick-Response Diagnostic Kits Market#Handheld Diagnostic Solutions Market#At-Site Testing Devices Market#Urgent Care POCT Market#Fast-Acting Diagnostic Devices Market
0 notes
Text
Remote Patient Monitoring Software Development: Transforming the Future of Healthcare

The healthcare sector is undergoing a remarkable transformation, with technology-driven care taking center stage. At the forefront of this change is Remote Patient Monitoring Software Development . This innovative approach enables doctors to keep an eye on patients’ vital signs, manage chronic conditions, and track treatment progress from afar, all while enhancing the quality and accessibility of care. By harnessing the power of modern technology, healthcare providers can reach beyond the confines of traditional clinical environments, ensuring timely interventions and ultimately improving patient outcomes.
Industry Growth Stats: The Rise of Remote Patient Monitoring Software Development
The RPM market has experienced unprecedented growth over the past few years. In 2023, the market was valued at approximately $25 billion and is expected to exceed $100 billion by 2030, growing at a compound annual growth rate (CAGR) of 18%. This surge highlights the increasing demand for remote healthcare solutions and the role of RPM in meeting global health challenges.
Key Drivers Behind the Growth
Several factors contribute to the rapid adoption of RPM solutions:
Chronic Diseases: The rise in chronic illnesses like diabetes and hypertension requires continuous monitoring.
Aging Population: Older adults need consistent care, often from home.
Technological Advancements: Innovations in IoT, AI, and wearable tech fuel RPM capabilities. Pandemic Influence: COVID-19 accelerated the shift to remote care models for safety and efficiency.
What is Remote Patient Monitoring Software Development in Healthcare?
Remote Patient Monitoring Software Development involves creating platforms that collect and transmit patient health data in real time. These systems are integrated with wearable devices, mobile apps, and sensors that measure vital signs. The data is securely sent to healthcare professionals, allowing them to track patient health trends and provide timely medical advice or interventions. A trusted Remote Patient Monitoring Software Development company will expertly guide you through these considerations, ensuring you receive a cost-effective, high-quality solution tailored perfectly to your unique healthcare needs.
Types of RPM Solutions
Wearable Devices Integration: Smartwatches and fitness bands tracking heart rate, sleep, and activity.
Chronic Disease Management Tools: Specialized tools for conditions like diabetes and COPD.
Mobile Health Applications: Apps for medication reminders, telehealth, and real-time communication.
Home Health Monitoring Kits: Devices for at-home blood pressure, glucose, and oxygen level checks.
Key Benefits of Remote Patient Monitoring Software DevelopmentEnhanced Patient Care and Engagement
Patients are empowered to take control of their health. Real-time feedback and reminders encourage adherence to treatment plans, improving outcomes.
Real-Time Data and Predictive Analytics
Healthcare providers can leverage real-time data to make informed decisions quickly. Predictive analytics help in anticipating complications before they arise.
Cost-Effectiveness for Healthcare Providers
RPM reduces the need for frequent in-person visits, lowering operational costs and freeing up resources for critical care cases.
The Future of Remote Patient Monitoring Software Development
Integration with AI and IoT
The future of RPM lies in its ability to integrate seamlessly with Artificial Intelligence and Internet of Things devices. AI enhances decision-making, while IoT ensures real-time connectivity.
Regulatory and Compliance Trends
New regulations are shaping RPM’s development, focusing on data privacy, security, and interoperability standards like HL7 and FHIR.
What is the Cost of Remote Patient Monitoring Software Development?
The cost of developing Remote Patient Monitoring Software Development may cost between $25,000 — $50,000 based on features.
It varies based on several other factors:
Features and Functionalities: Basic apps with standard monitoring features may cost between $25,000 — $50,000. More complex solutions integrating AI, analytics, and custom dashboards can range from $70,000 to $150,000+.
Device Integrations: RPM software often needs to integrate with various wearable and medical devices, which can affect development time and cost.
Data Security and Compliance: Ensuring HIPAA, GDPR, and other regulatory compliance adds to the development cost but is essential for safeguarding patient data.
Customization and Scalability: Tailored solutions designed for specific healthcare providers or patient populations may increase costs but offer long-term value.
A reliable Remote Patient Monitoring Software Development Company will help you navigate these factors and deliver cost-effective, high-quality solutions aligned with your needs.
How a Remote Patient Monitoring Software Development Company Can Help?
Custom Solutions Tailored to Your Business
Partnering with a professional Remote Patient Monitoring Software Development company ensures that your solution is specifically designed to meet your organization’s needs. From user-friendly interfaces to specialized features, these companies deliver custom-built platforms that align with your workflow and patient demographics.
Support and Maintenance Services
Post-development, continuous support is vital. RPM companies offer regular updates, bug fixes, security patches, and feature enhancements to ensure your software remains effective, compliant, and scalable.
ConclusionRemote Patient Monitoring Software Development is transforming the way we deliver healthcare, making it more efficient, accessible, and centered around patients. By integrating RPM, healthcare providers can boost outcomes, lower costs, and provide care that reaches beyond the clinic. Collaborating with a knowledgeable Remote Patient Monitoring Software Development company ensures you have the right resources and guidance to excel in this rapidly changing digital health environment. The future of healthcare is remote, real-time, and data-focused — are you prepared to embrace it?
FAQWhat is the primary function of RPM software?
RPM software monitors patient health remotely, providing real-time data to healthcare providers for better decision-making.
How secure is Remote Patient Monitoring Software?
Highly secure. Top RPM systems comply with HIPAA, GDPR, and other international data protection standards to ensure patient data confidentiality.
What industries use RPM outside healthcare?
Beyond healthcare, RPM technology is used in fitness, eldercare, insurance, and occupational health sectors.
How long does it take to develop Remote Patient Monitoring software?
Development timelines vary but typically range between 4 to 9 months, depending on complexity.
Can RPM software integrate with existing systems?
Yes, most RPM platforms are designed to integrate with Electronic Health Records (EHRs), hospital management systems, and other healthcare IT solutions.
Is RPM software scalable?
Absolutely. Modern RPM solutions can scale to meet growing patient bases and incorporate new features as needed.
Also read — Develop a Medicine Delivery App like 1mg — Cost, Features, and Guide
0 notes
Text
Beyond the Basics: Revolutionizing the Blood Testing Landscape
The global blood testing market size is estimated to reach USD 160.50 billion by 2030, registering a CAGR of 8.83% from 2025 to 2030, according to a new report by Grand View Research, Inc. The growth of the market is attributable to various factors including the growing demand for the identification of infectious agents and increased healthcare spending by government and regulatory bodies. Furthermore, the rising prevalence of infectious diseases, such as diabetes, COVID-19, and cardiovascular diseases, is anticipated to drive market growth. For instance, according to the CDC in 2022, approximately 37.3 million people were diagnosed with diabetes in the United States, accounting for 11.3 % of the population.
This increasing prevalence of diabetes creates a significant demand for blood tests, thus contributing to the growth of the market. Furthermore, technological advancement in the products used for blood testing fuels the growth of the market. The availability of portable glucose meters that enable patients to perform routine checkups conveniently and anywhere has contributed to an expansion of the market. In addition, the monitoring of diabetes is crucial prior to any surgical intervention for patients with diabetes, which further drives the demand for these products. The introduction of new products is anticipated to fuel market growth over the projection period.
For instance, in May 2022, Labcorp introduced an at-home collection kit with Labcorp OnDemand, which allows individuals to measure their hemoglobin A1c (HbA1c) levels from a small blood sample to monitor their sugar levels. Key organizations, such as the American Red Cross, the New York Blood Center, America's Blood Centers, Armed Services Blood Program, AABB, and Blood Centers of America, play a crucial role in providing information on plasma collection and delivery. The rise in blood donations is expected to further drive the demand for testing products used in pre-transplant and post-transplant procedures, thereby boosting market growth.
Blood Testing Market Report Highlights
The glucose testing segment held the largest revenue share in 2024. The segment growth is supported by the increasing prevalence of both type I and type II diabetes, along with the growing number of cases related to hereditary diabetes
Continuous advancements in technology have led to the development of innovative blood testing methods and devices. These advancements, such as point-of-care testing, wearable devices, and improved laboratory testing techniques, drive market growth.
North America held the largest revenue share in 2024 and is expected to maintain its position throughout the forecast period; however, Asia Pacific is expected to grow at a significant rate from 2025 to 2030
Key players operating are focusing on product launches and geographical expansion to maintain their presence. For instance, in March 2023, Apple made notable progress in the development of non-invasive blood glucose monitoring technology, enabling individuals with diabetes and other conditions to measure their blood glucose levels without the need for skin pricks or traditional blood testing methods
In June 2022, Apollo Cancer Centers, in collaboration with Datar Cancer Genetics, introduced a blood test capable of detecting early-stage breast cancers in asymptomatic individuals.
Blood Testing Market Segmentation
Grand View Research has segmented the global blood testing market based on test type, and region:
Blood Testing Test Type Outlook (Revenue, USD Million, 2018 - 2030)
Glucose Testing
A1C Testing
Direct LDL Testing
Lipid Panel Testing
Prostate-specific Antigen Testing
COVID-19 Testing
BUN Testing
Vitamin-D Testing
Thyroid-stimulating Hormone (TSH)
Serum Nicotine/Cotinine
High sensitivity CRP Testing
Testosterone Testing
ALT Testing
Cortisol Testing
Creatinine Testing
AST Testing
Others Blood Tests
Blood Testing Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
UK
Germany
France
Italy
Spain
Sweden
Norway
Denmark
Russia
Asia Pacific
Japan
China
India
Australia
Thailand
South Korea
Singapore
Latin America
Brazil
Argentina
MEA
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players
Abbott
Hoffmann-La Roche Ltd
Bio-Rad Laboratories, Inc.
bioMerieux
Quest Diagnostics Incorporated
Biomerica
BD
Siemens Healthineers AG
Danaher Corporation
Trinity Biotech Plc
Order a free sample PDF of the Blood Testing Market Intelligence Study, published by Grand View Research.
0 notes
Text
In Vitro Diagnostics Market: Revolutionizing Medical Testing
The In Vitro Diagnostics (IVD) Market is at the forefront of medical diagnostics, enabling healthcare providers to detect diseases and conditions through tests performed outside the body. As demand for early detection and accurate diagnostic tools increases, the IVD market is witnessing rapid growth. This article delves into the market segmentation, key growth drivers, and leading companies shaping the industry, providing valuable insights for decision-makers.
Market Overview
According to SkyQuest’s In Vitro Diagnostics Market report, the global IVD market is valued at USD 87.93 Billion in 2023 and is projected to grow at a CAGR of 5.3%. The market’s expansion is driven by the rising prevalence of chronic diseases, the growing importance of early disease detection, and technological advancements in diagnostics.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/in-vitro-diagnostics-market
Market Segmentation
By Product Type:
Reagents & Kits: Essential for a wide variety of diagnostic tests, including immunoassays and molecular diagnostics.
Instruments: Diagnostic tools such as analyzers and automated systems used in laboratories and healthcare settings.
Software & Services: Used for managing data, improving accuracy, and ensuring compliance with regulatory standards.
By Application:
Infectious Diseases: A leading segment, especially with the demand for COVID-19 diagnostics.
Cancer: Rising incidence of cancer has fueled the need for accurate diagnostic tools.
Cardiology: IVD tools for cardiovascular diseases are seeing growing demand due to an increasing global burden.
Diabetes: Blood glucose monitoring systems dominate this segment, with widespread use among diabetic patients.
Autoimmune Diseases: IVD tests play a crucial role in diagnosing autoimmune disorders.
By End-User:
Hospitals & Clinics: Major users of IVD tools, especially for infectious diseases and chronic conditions.
Diagnostic Laboratories: Central hubs for conducting a wide range of tests with high accuracy and reliability.
Research & Academic Institutions: Key in driving innovation and new test development.
Homecare Settings: Growing segment with the rise in at-home diagnostic tools, particularly for chronic conditions.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/in-vitro-diagnostics-market
Key Growth Drivers
Increasing Prevalence of Chronic Diseases: Rising cases of cancer, diabetes, and cardiovascular diseases are driving the demand for IVD tests.
Technological Innovations: Advancements in molecular diagnostics, AI-driven analysis, and automation are enhancing the efficiency and accuracy of diagnostic tools.
Demand for Personalized Medicine: IVD plays a pivotal role in tailoring treatments to individual patients, fueling market growth.
Aging Population: As the global population ages, the demand for diagnostic tests for age-related diseases like Alzheimer’s and cardiovascular conditions is on the rise.
Leading Companies in the Market
SkyQuest’s In Vitro Diagnostics Market report highlights the following key players in the industry:
Roche Diagnostics
Abbott Laboratories
Thermo Fisher Scientific Inc.
Danaher Corporation
Siemens Healthineers
Bio-Rad Laboratories, Inc.
Becton, Dickinson and Company
bioMérieux SA
Qiagen N.V.
Sysmex Corporation
Read More at: - https://www.skyquestt.com/report/in-vitro-diagnostics-market
Challenges and Opportunities
While regulatory hurdles and high costs of advanced diagnostic tools remain challenges, the IVD market offers numerous opportunities. The increasing focus on point-of-care testing and the integration of AI in diagnostics present significant growth avenues for industry players.
Future Outlook
The In Vitro Diagnostics Market is set to experience robust growth as healthcare shifts towards early detection and preventive care. Companies that invest in cutting-edge technologies and innovative diagnostic solutions will be well-positioned to lead in this evolving market.
The In Vitro Diagnostics Market is transforming the healthcare landscape, offering essential tools for early diagnosis and effective treatment planning. Decision-makers who stay ahead of trends and leverage technological advancements will thrive in this rapidly growing industry. For more detailed insights, explore SkyQuest’s In Vitro Diagnostics Market report.
0 notes
Text
The global demand for Point-Of-Care Diagnostics / Testing (POCT) was valued at USD 45618.5 Million in 2023 and is expected to reach USD 118599.8 Million in 2032, growing at a CAGR of 11.20% between 2024 and 2032.The Point-of-Care (POC) diagnostics/testing market has emerged as a transformative force in healthcare, promising to deliver rapid and accurate diagnostic results at the patient's bedside or near the site of care. This market is experiencing significant growth due to technological advancements, rising prevalence of chronic diseases, and the increasing need for rapid diagnostics in remote and resource-limited settings.
Browse the full report at https://www.credenceresearch.com/report/point-of-care-diagnostics-or-testing-market
Introduction to Point-of-Care Diagnostics
Point-of-care diagnostics refers to medical diagnostic testing performed outside the traditional laboratory setting, typically at or near the site of patient care. These tests enable healthcare providers to make immediate clinical decisions, thus improving patient outcomes and streamlining the care process. POC diagnostics encompass a broad range of tests, including blood glucose monitoring, pregnancy tests, infectious disease detection, and cholesterol testing, among others.
Market Dynamics
Technological Advancements: The rapid advancements in technology have been a key driver of the POC diagnostics market. Innovations such as microfluidics, lab-on-a-chip technology, and portable diagnostic devices have made it possible to conduct complex tests with minimal sample volumes and faster turnaround times. These technologies not only enhance the accuracy and reliability of POC tests but also make them more accessible and user-friendly.
Rising Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases such as diabetes, cardiovascular diseases, and infectious diseases has fueled the demand for POC diagnostics. These conditions require continuous monitoring and timely interventions, which POC tests can provide. For instance, the global rise in diabetes cases has led to a surge in demand for blood glucose monitoring devices.
Need for Rapid Diagnostics in Remote Areas: In many parts of the world, particularly in low- and middle-income countries, access to centralized laboratory facilities is limited. POC diagnostics bridge this gap by providing essential diagnostic services in remote and resource-limited settings. This capability is crucial for managing infectious diseases and other health conditions in areas with limited healthcare infrastructure.
Key Market Segments
The POC diagnostics market is segmented based on product type, technology, end-user, and region.
Product Type: The market includes a variety of POC testing products such as glucose monitoring kits, infectious disease testing kits, pregnancy and fertility testing kits, cholesterol testing kits, hematology testing kits, and others. Among these, glucose monitoring kits hold a significant share due to the high prevalence of diabetes.
Technology: Based on technology, the market is segmented into lateral flow assays, dipsticks, microfluidics, molecular diagnostics, and immunoassays. Lateral flow assays are widely used due to their simplicity, low cost, and rapid results.
End-User: The end-users of POC diagnostics include hospitals and clinics, home care settings, diagnostic centers, and research laboratories. Hospitals and clinics are the major end-users due to the high volume of patients and the need for rapid diagnostic results.
Region: Geographically, the market is divided into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. North America holds the largest market share due to advanced healthcare infrastructure, high adoption of new technologies, and the presence of major market players. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing awareness about POC diagnostics, and improving healthcare facilities.
Challenges and Future Prospects
Despite the promising growth, the POC diagnostics market faces several challenges. These include regulatory hurdles, the high cost of advanced diagnostic devices, and issues related to the accuracy and reliability of some POC tests. Moreover, there is a need for greater integration of POC diagnostic data with electronic health records to ensure seamless patient care.
Looking ahead, the POC diagnostics market is poised for substantial growth, driven by continuous technological advancements, increasing healthcare needs, and the ongoing efforts to improve access to quality healthcare services worldwide. The integration of artificial intelligence and machine learning in POC diagnostics is expected to further enhance diagnostic accuracy and personalized patient care.
Key Players
Abbott (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
B.D. (U.S.)
Siemens Healthcare Private Limited (Germany)
Bio-Rad Laboratories, Inc. (U.S.)
Danaher (U.S.)
Sekisui Diagnostics (U.S.)
Trinity Biotech (Ireland)
bioMérieux (France)
EKF Diagnostics (Germany)
AccuBioTech Co., Ltd. (China)
Instrumentation Laboratory (U.S.)
Beckman Coulter, Inc. (U.S.)
PTS Diagnostics (U.S.)
Nova Biomedical (U.S.)
Chembio Diagnostics, Inc. (U.S.)
Quidel Corporation (U.S.)
Sienco, Inc (U.S.)
Segmentation
By Technology:
Lateral Flow Assays (LFAs)
Dipsticks
Microfluidics/ Lab-on-a-Chip (LOC)
Biosensors
Molecular Diagnostics
Immunodiagnostics
Hematology Analyzers
Coagulation Monitoring
Others
By End-User:
Hospitals and Clinics
Physician Offices
Ambulatory Care Settings
Home Care
Diagnostic Laboratories
Research Institutes
By Application:
Infectious Disease Testing
HIV
Influenza
Hepatitis
Sexually Transmitted Infections (STIs)
Others
Cardiology
Diabetes Monitoring
Coagulation Monitoring
Pregnancy and Fertility Testing
Cancer Markers
Urinalysis
Cholesterol Testing
Others
By Product Type:
Blood Glucose Monitoring Kits
Cardiac Marker Testing Kits
Infectious Disease Testing Kits
Pregnancy and Fertility Testing Kits
Hematology Testing Kits
Coagulation Testing Kits
Cholesterol Testing Kits
Urinalysis Testing Kits
Drug-of-Abuse Testing Kits
Others
By Platform:
Desktop/ Benchtop Analyzers
Handheld/ Portable Analyzers
By Distribution Channel:
Hospitals and Clinics
Pharmacies
Online Retailers
Diagnostic Centers
By Infectious Disease Testing Type:
HIV POCT
Influenza POCT
Hepatitis POCT
Sexually Transmitted Infections (STIs) POCT
Others
By Cardiac Marker Testing Type:
Troponin Testing
BNP Testing
Others
By Pregnancy and Fertility Testing Type:
Pregnancy Testing Kits
Ovulation Prediction Kits
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/point-of-care-diagnostics-or-testing-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Global Disposable Medical Sensors Market Insights : Revolutionizing Healthcare
The global disposable medical sensors market was valued at USD 9.52 billion in 2022 and is projected to grow at a CAGR of 18.45% from 2023 to 2030. This growth can be attributed to technological advancements, increasing demand for health data tracking, and the need for low-cost medical devices. The rising prevalence of chronic diseases worldwide is expected to drive the demand for disposable medical sensors. According to a report by Front. Public Health in 2020, chronic non-communicable diseases (NCDs) accounted for approximately 80% of mortality among Chinese adults aged 60, with Ischemic heart disease, stroke, Chronic Obstructive Pulmonary Disease (COPD), and Type 2 diabetes being the most common conditions. The growing adoption of disposable medical sensors in healthcare settings is likely to contribute to the market's expansion in the coming years.
Click here for full report:
https://www.pharmanucleus.com/reports/disposable-medical-sensors
Market Insights:
In the global disposable medical sensors market, the diagnostic segment led the market in 2022, accounting for over 39.38% of the global revenue. Disposable medical sensors are widely used in diagnostic devices such as surgical tools, endoscopy equipment, spirometry devices, and medical imaging devices, enhancing their capabilities and enabling early disease detection. Advanced sensors, such as carbon nanotube-based biosensors, are being used to detect microorganisms like S. aureus and E. coli, providing faster and more accurate results at a lower cost. The integration of wireless communication and biosensors is creating growth opportunities in the diagnostic field.
The increasing prevalence of chronic diseases and the demand for diagnostics are driving the adoption of disposable medical sensors. Diagnostic devices are crucial for monitoring symptoms and signs of chronic diseases like diabetes. Companies are developing innovative solutions to improve diagnostic capabilities, such as portable genetic material amplification systems for point-of-care molecular diagnostic detection. The patient monitoring segment is expected to have the highest compound annual growth rate (CAGR) during the forecast period. Medical sensors are utilized in patient monitoring devices like pulse oximeters and blood pressure monitors. Advancements in technology and product launches, such as next-generation monitoring biosensors by Philips, contribute to improved quality of care.
The demand for disposable medical sensors is projected to increase due to their various advantages, leading to a rise in R&D investments and collaborations between companies. For example, Rockley Photonics Holdings Limited and Medtronic are collaborating to implement Medtronic's solutions with Rockley's Bioptx biomarker sensing technology in healthcare settings. This collaboration aims to provide real-time, non-invasive monitoring of individuals' well-being and health, enabling proactive healthcare and personalized care based on actionable data. Such initiatives are expected to drive the growth of the patient monitoring segment in the near future.
Click here for full report:
https://www.pharmanucleus.com/reports/disposable-medical-sensors
Product Insights:
In 2022, the biosensors segment held the largest market share, accounting for over 49.70% of the market. Biosensors are sensors designed to detect analytes by collecting biological components and utilizing a physiochemical detector. The signals from the analyte are detected, measured, and displayed on the device through associated electronics. Various types of biosensors are used, including electronic, amperometric, blood glucose, potentiometric, conduct metric, thermometric, optical, fiber optic lactate, immune, and piezoelectric biosensors. Biosensors have applications in patient monitoring and diagnostics, and the increasing demand for rapid and accurate diagnostic kits is driving the growth of biosensors. Innovations in biosensor technology are expected to lead to the development of advanced biosensors for faster diagnosis in the future.
The image sensors segment is projected to have the highest compound annual growth rate (CAGR) during the forecast period. Image sensors convert light waves into signals to create images and find extensive use in diagnostic devices such as endoscopy equipment and electronic imaging devices. There are two types of image sensors: charge-coupled devices (CCD) and complementary metal oxide semiconductors (CMOS). CMOS image sensors are more commonly used due to their lower power consumption and faster diagnostic speed. They are primarily utilized in x-ray imaging, minimally invasive surgery, endoscopy, and ocular surgery. Technological advancements and the demand for higher resolution are expected to drive market growth in the image sensors segment.
Click here for Free sample request:
https://www.pharmanucleus.com/request-sample/1180
Market Segmentation:
The strip sensors segment held the largest market share in 2022, accounting for over 38.76% of the market. Strip sensors are primarily used in blood glucose monitoring, disease testing, and magnetic nanoparticles. Their dominance in the market is attributed to increasing demand and their widespread usage in diagnostic applications. Strip sensors offer the advantage of faster results, which contributes to their popularity. Furthermore, the market is driven by the growing demand for self-diagnosis and home-based medical devices.
The ingestible sensors segment is projected to have the highest compound annual growth rate (CAGR) during the forecast period. Ingestible sensors are small chips enclosed in capsules that are swallowed, allowing for detection of any abnormalities in the body and transmission of the data to an external device. These sensors find applications in endoscopy, controlled drug delivery, and patient monitoring. The rising prevalence of chronic diseases and the need for invasive diagnostic testing are driving the growth of ingestible sensors. Additionally, the accuracy of results obtained through ingestible sensors further fuels their demand in the market.
Due to the various advantages of ingestible sensors, the demand for these devices is anticipated to increase during the forecast period. Increasing R&D investments and collaborations between companies are also expected to propel market growth. In July 2021, according to Medtronic plc, the FDA cleared the use of two LINQ II insertable cardiac monitors with the AI AccuRhythm algorithms. When the AI AccuRhythm algorithms are made accessible on the CareLink Network later this year, all LINQ II implants in the U.S will be able to use them.
Regional Analysis:
North America dominated the global disposable medical sensors market in 2022, holding the largest revenue share of over 41.18%. This can be attributed to several factors, including the region's well-established healthcare infrastructure, high healthcare spending, the presence of monopolistic market players, and the rapid adoption of cutting-edge technologies. The market in North America is highly developed and is expected to be driven by the increasing uptake of patient monitoring and homecare devices for routine, ongoing, and long-term patient monitoring, which helps reduce the frequency of hospital visits.
Favorable reimbursement policies are expected to further fuel market expansion in North America. Additionally, the rising prevalence of lifestyle-related health issues, accidents, and sports injuries contribute to the growth potential in the region. The increasing use of mobile surgery centers and the demand for effective emergency care are also anticipated to boost the market during the forecast period. Moreover, the COVID-19 pandemic has highlighted the importance of disposable medical sensors in enabling remote patient monitoring and care at home, leading to increased adoption of these devices.
The Asia-Pacific region is projected to experience the fastest growth in the global disposable medical sensors market during the forecast period. The region witnesses a significant demand for disposable medical sensor equipment due to the increasing prevalence of cardiac disorders, particularly in countries like China and India, which also have high diabetes rates. The rising burden of diabetes in the region is driving the demand for home-based disposable medical sensors, creating new market opportunities. Additionally, the presence of major companies in the disposable medical sensors market further contributes to the region's market share and growth potential.
#Disposable Medical Sensors Market#Healthcare Technology#Sensor Innovations#Global Market Trends#Medical Device Industry#Sensor Solutions#Market Insights
0 notes
Text
Telehealth and Beyond: How Smart Home Medical Devices Are Transforming Healthcare
Introduction
The smart home medical device market is experiencing exponential growth, redefining the way healthcare is delivered and monitored. These innovative devices, equipped with advanced technologies, have the potential to revolutionize the healthcare industry by providing convenient, cost-effective, and real-time solutions for monitoring and managing medical conditions. As our world becomes increasingly connected, the smart home medical device market is poised for substantial expansion, promising improved patient outcomes, reduced healthcare costs, and enhanced quality of life.
Market Overview
The smart home medical device market encompasses a wide range of devices designed to monitor, diagnose, and manage various health conditions. These devices leverage cutting-edge technologies, such as the Internet of Things (IoT), artificial intelligence (AI), and remote connectivity, to provide accurate, real-time data to patients, caregivers, and healthcare providers. From wearable fitness trackers to in-home diagnostic tools, the market offers a diverse array of products and solutions to cater to different healthcare needs.
Key Factors Driving Market Growth
1. Aging Population: The global population is aging, leading to an increased demand for healthcare services. Smart home medical devices offer a solution to address the needs of the elderly by enabling remote monitoring, medication reminders, and fall detection.
2. Chronic Disease Management: The prevalence of chronic diseases like diabetes, hypertension, and heart disease is on the rise. Smart devices enable individuals to manage these conditions more effectively by tracking vital signs, blood glucose levels, and medication adherence.
3. Telehealth: The growth of telehealth services has accelerated the adoption of smart home medical devices. These devices seamlessly integrate with telehealth platforms, allowing patients to consult with healthcare professionals remotely.
4. Health and Fitness Trends: Consumers are increasingly focused on health and fitness, creating a market for wearables that monitor physical activity, sleep patterns, and more. These devices motivate individuals to take a proactive approach to their well-being.
5. COVID-19 Pandemic: The pandemic highlighted the importance of remote monitoring and diagnosis. Smart thermometers, pulse oximeters, and home-based test kits became essential tools for monitoring symptoms and managing the spread of the virus.
Market Segmentation
The smart home medical device market can be segmented into various categories:
1. Wearable Devices: These include smartwatches, fitness bands, and other wearables that track physical activity, heart rate, sleep, and other health metrics.
2. Monitoring and Diagnostic Devices: Devices like blood pressure monitors, glucometers, and thermometers provide real-time data for tracking vital signs and health conditions.
3. Medication Management: Smart pill dispensers and medication reminder devices help users adhere to their medication schedules.
4. Telehealth and Remote Monitoring Tools: These include video consultation platforms and devices for remote health monitoring, such as connected scales and ECG monitors.
5. Home Health and Wellness Products: Smart scales, air quality monitors, and devices that promote general health and well-being fall under this category.
Challenges and Concerns
While the smart home medical device market holds immense potential, it also faces some challenges:
1. Data Security: Storing sensitive health data in the cloud raises concerns about data security and privacy. Ensuring robust encryption and compliance with data protection regulations is crucial.
2. User Adoption: Some users, especially the elderly, may find it challenging to adopt and adapt to new technologies. User-friendly designs and comprehensive support are vital to overcome this hurdle.
3. Regulatory Compliance: Navigating the complex landscape of healthcare regulations and ensuring FDA approval or other relevant certifications can be a significant challenge for device manufacturers.
4. Interoperability: Ensuring that smart medical devices can seamlessly integrate with various healthcare systems and platforms is essential for their success.
Future Outlook
The smart home medical device market is set for continued expansion. As technology advances and becomes more accessible, these devices will become integral to preventive healthcare and chronic disease management. The integration of AI for predictive analytics and the growth of 5G networks for faster, more reliable data transfer will further enhance the capabilities of smart medical devices.
Conclusion
The smart home medical device market is on a trajectory of rapid growth, with innovation and technology driving its expansion. These devices are changing the way healthcare is delivered, making it more patient-centric, accessible, and convenient. As we continue to navigate an aging population, the rise of chronic diseases, and the ever-evolving landscape of healthcare, smart home medical devices are positioned to play a pivotal role in the future of healthcare.
0 notes
Text
Home Diagnostics Market Size, Share | Global Report 2030
The usage of diagnostic tests that may be completed in the convenience of one’s own home, without the necessity of a healthcare provider or laboratory setting, is referred to as the “home diagnostics market.” Due to a number of variables, such as increased consumer desire for convenience, rising healthcare expenses, and technological improvements, this industry has experienced considerable expansion in recent years.
Make The Smart Decision. Download A Free Sample Of Our Report @ https://cognizancemarketresearch.com/request/home-diagnostics-market/
The Global Home Diagnostics Market was valued at US$ 5,927.00 million in 2022 and is anticipated to reach US$ 8,285.16 million by the end of 2030 expected to register a CAGR of 4.3% from 2023 to 2030 (forecast period).
Pregnancy tests, blood glucose monitors, cholesterol testing, infectious illness diagnostics, and a host of other goods are available on the market. Product kind, test type, and end-user are the three primary categories into which these items can be divided.
The section by product type consists of software, consumables, and diagnostic tools. Tests for infectious diseases, cholesterol, diabetes, pregnancy, and other conditions are included in the test type segment. The end-user category consists of various entities such as homes, hospitals, diagnostic labs, and others. Assuring the accuracy and dependability of home-based testing is one of the main issues the industry for home diagnostics faces. To provide reliable findings, manufacturers must make sure their goods adhere to tight regulatory standards and give customers clear instructions.
The development of wearable technology and other technological developments, such as the creation of digital health platforms, are propelling the home diagnostics market’s expansion. These innovations provide patients more control over their healthcare by enabling remote monitoring and management of health issues.
The home diagnostics market is anticipated to expand significantly over the next few years, with North America and Asia Pacific projected to have the strongest growth rates. The market is expected to rise as a result of factors like rising knowledge of preventative healthcare, an increase in the prevalence of chronic diseases, and an ageing population.
https://cognizancemarketresearch.com/wp-content/uploads/2023/09/Home-Diagnostics-Market.png
Factors responsible for the boost in the Home Diagnostics Market:
Several causes, such as the following, have contributed to the tremendous growth of the home diagnostics market in recent years:
Convenience: Chronic diseases are becoming more common, which has boosted the demand for home diagnostic testing kits. Chronic disorders include diabetes, cancer, and cardiovascular diseases. Patients can use these kits to keep tabs on their health and make educated decisions.
Convenience and cost-effectiveness: Home diagnostic testing kits provide patients with privacy and convenience by enabling them to self-test without having to travel to a healthcare centre. These kits are typically less expensive than standard laboratory testing as well.
Technological developments: The creation of more precise and dependable home diagnostics testing kits is the result of the development of cutting-edge technologies like biosensors and mobile health applications. Additionally, these developments have made it possible for platforms for digital health to be better integrated.
COVID-19 pandemic: As a way to identify and track the spread of the virus, the COVID-19 pandemic has increased demand for home-based testing kits. Home diagnostics testing kits have been developed and adopted more quickly as a result of this.
Ageing Population: As the world’s population ages, there will be an increasing demand for home diagnostic testing kits since older people are more likely to have chronic diseases that need ongoing monitoring. By enabling this population to better manage their diseases, the adoption of home diagnostic testing kits can enhance their quality of life.
Make The Smart Decision. Download A Free Sample Of Our Report @ https://cognizancemarketresearch.com/request/home-diagnostics-market/
Emerging Markets for Home Diagnostics Market:
The Asia-Pacific region is one of the home diagnostics market’s emerging markets. The region is anticipated to increase significantly over the next few years as a result of the rising prevalence of chronic diseases, rising healthcare costs, and growing public awareness of the advantages of early disease identification.
In addition, the market is anticipated to rise as home diagnostics is becoming more popular in nations like China and India, where there is a shortage of healthcare workers and infrastructure. The market is also benefited by the growing number of alliances and partnerships between major players and regional businesses, which is assisting in the expansion of the region’s supply of cost-effective and dependable home diagnostic products.
India is a newly developing market for home diagnostics. The population of the nation is increasing quickly, and chronic diseases like diabetes and cardiovascular disease are very common. Additionally, the country is putting more of an emphasis on remote patient monitoring and preventive healthcare. The government has started a number of programmes to encourage the use of digital health technologies, such as home diagnostics, and has also loosened restrictions to make it simpler to obtain medical equipment.
Additionally, the region’s adoption of home diagnostics is anticipated to be further boosted by the growing usage of telemedicine and digital health solutions, which allow patients to remotely monitor their health and communicate their diagnostic findings with medical professionals. Overall, the Asia-Pacific region offers the home diagnostics market a sizable potential opportunity, and major players are increasingly concentrating on growing their presence in this region.
0 notes
Text
Global Self-Testing Market Is Estimated To Witness High Growth Owing To Increasing Awareness and Technological Advancements
The global self-testing market is estimated to be valued at US$ 20,117.5 million in 2022 and is expected to exhibit a CAGR of 8.7% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights. Market Overview: The self-testing market encompasses a wide range of products that allow individuals to perform diagnostic tests conveniently at home or in other non-clinical settings. These products include blood glucose monitors, pregnancy test kits, cholesterol monitoring devices, HIV test kits, and many others. Self-testing products provide individuals with the ability to monitor their health conditions regularly and take necessary actions to manage their well-being effectively. Market Dynamics: The global self-testing market is driven by two primary factors. Firstly, increasing awareness among individuals about the importance of timely diagnosis and self-monitoring of health conditions is contributing to the growth of the market. Self-testing empowers individuals to take control of their health and make informed decisions about their lifestyle and treatment options. Secondly, technological advancements in self-testing devices have led to the development of more accurate and user-friendly products. For example, the introduction of smartphone-compatible devices and wearable technologies has made self-testing easier and more accessible. These devices allow individuals to track their health data over time and share it with healthcare professionals for better management of their conditions. Segment Analysis: The Self-Testing Market Growth can be segmented based on product type, end-user, and region. Among the product types, blood glucose monitors dominate the market due to the high prevalence of diabetes worldwide. The ease of use and instant results provided by these devices make them highly popular among individuals with diabetes. In terms of end-users, the home diagnostics segment is expected to dominate the market. The ability to perform tests conveniently at home without the need for a visit to a healthcare facility is driving the demand for self-testing products among individuals. PEST Analysis: Political: Government initiatives promoting self-care and self-management of health conditions are expected to drive the growth of the self-testing market. For example, various healthcare programs and policies encourage individuals to take responsibility for their health and actively participate in managing their conditions. Economic: The increasing healthcare costs and the need for cost-effective healthcare solutions are driving the demand for self-testing products. These products offer a more affordable alternative to regular visits to healthcare facilities for diagnostic tests. Social: The growing awareness about the importance of preventive healthcare and the availability of self-testing products have contributed to a change in social behavior. Individuals are now more proactive in monitoring their health and taking necessary actions to prevent or manage diseases. Technological: Technological advancements have revolutionized the self-testing market. The integration of wireless and smartphone technologies in self-testing devices has made them more user-friendly and accessible. Moreover, the development of portable and point-of-care testing devices has further enhanced the convenience of self-testing. Key Takeaways: - The global self-testing market is expected to witness high growth, exhibiting a CAGR of 8.7% over the forecast period. The increasing awareness among individuals about the importance of timely diagnosis and self-monitoring of health conditions is driving the growth of the market. - North America is currently the dominating region in the global self-testing market, with the highest adoption rate of self-testing products. The region's well-established healthcare infrastructure and the increasing prevalence of chronic diseases contribute to its leadership position.
0 notes
Text
Rapid Diagnostics Market Size, Share & Forecast: 2028
Rapid Diagnostics Market by Product (Glucose, Infectious Disease (Hepatitis C, Influenza), Coagulation), Platform (Microfluidics, Immunoassay), Mode of Purchase (Prescription, OTC), Enduser (Hospital, e-comm, Home Care), and Region (North America, Europe, Asia-Pacific, Middle East and Africa and South America)
Market Overview:
The Rapid Diagnostics market size is projected to reach a CAGR of 11.6% from 2022 to 2028.
Rapid Diagnostics are the medical techniques which are used for the diagnosis and the detection of the disease but are way to perform and are quick. These techniques play major role in the situation wherein the preliminary or the emergency situation similar to the ongoing pandemic. These test kits tend to provide the results in either 20 minutes or the same day.
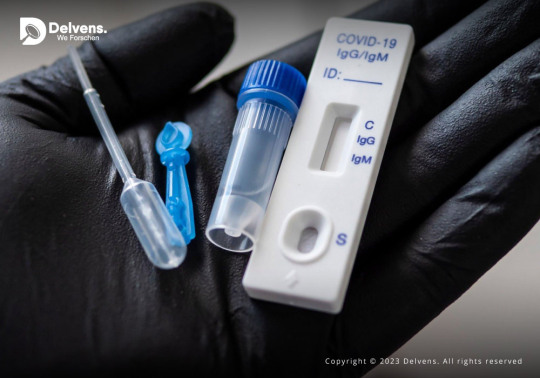
The technological advancements in the sector along with the surge in the incidents of the infectious disease are some of the factors that have supported long-term expansion for Rapid Diagnostics Market.
COVID-19 had a positive effect on the market, as because the global demand has increased to various folds and also the shortage of lab-based testing facilities the demand for rapid diagnostics was increased.
Request Research Sample Pages: https://www.delvens.com/get-free-sample/rapid-diagnostics-market-trends-forecast-till-2028
Regional Analysis
North America is the most rapidly growing market and offers a huge opportunity for the industry, whose growth is driven by the incidents of lifestyle disorder becoming popular in the region and also the presence of the key players in the region.
Competitive Landscape
Key Players
Roche Diagnostics
Siemens Healthineers
Danaher Corporation
Becton Dickinson and Company
Abbott Laboratories
Quidel Diagnostics
Chembio Diagnostics
EKF Diagnostics
Trinity Biotech
Fluxergy
Recent Developments
In June 2019, FDA gave the approval to Roche diagnostics for VENTANA PD-L1 (SP142) Assay.
Make an Inquiry Before the Purchase: https://www.delvens.com/Inquire-before-buying/rapid-diagnostics-market-trends-forecast-till-2028
Reasons to Acquire
Increase your understanding of the market for identifying the best and suitable strategies and decisions on the basis of sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends and factors
Gain authentic and granular data access for Rapid Diagnostics Market so as to understand the trends and the factors involved behind changing market situations
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns
Report Scope
Rapid Diagnostics Market is segmented into product, platform, mode of purchase, end users and region.
On the basis of Product
Glucose Monitoring Products
Cardiometabolic Monitoring Products
Infectious Disease Testing Products
Coagulation Monitoring Products
Pregnancy & Fertility Testing Products
Fecal Occult Testing Products
Hematology Testing Products
Tumor/Cancer Marker Testing Products
Urinalysis Testing Products
Drugs-of-abuse Testing Products
Cholesterol Testing Products
Other POC Products
On the basis of Purchase
OTC Testing Products
Prescription-based Testing Products
On the basis of End-Users
Outpatient & Ambulatory Care Facilities
Hospitals & Critical Care Centers
Home Care Settings 5
Other End Users
On the basis of Region
Asia Pacific
North America
Europe
South America
Middle East & Africa
Purchase the Research Report: https://www.delvens.com/checkout/rapid-diagnostics-market-trends-forecast-till-2028
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Browse For More Related Reports:
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
+44-20-8638-5055
0 notes
Photo

Landscape Of The Global Point Care Diagnostics Market Outlook: Ken Research Buy Now The point-of-care diagnostic test is a medical diagnostic test accompanied outside the laboratory at or near the place where the patient is accepting the care and also defined as bedside testing or near-patient testing.
#Abbott Laboratories Point of Care Diagnostics Market#Cardiometabolic monitoring kits Market#Coagulation monitoring kits Market Value#Drugs of abuse testing kits Sale Market#France Point of Care Diagnostics Market#Global Glucose monitoring kits Market Demand#Global POC Coagulation Testing Market Dynamics#Global Point of Care Diagnostics Market Forecast#Global Point of Care Diagnostics Market Revenue#Global Point of Care Diagnostics Market Scope#Global Urinalysis testing kits Market Revenue#India Point of Care Diagnostics Market#Infectious disease testing kits Market#Japan Cholesterol testing kits Market#Johnson & Johnson Point of Care Diagnostics Market#MEA Point of Care Diagnostics Market Growth#Nova Biomedical Point of Care Diagnostics Market#Online Point of Care Diagnostics Market Sale#Online sale of Point of Care Diagnostics Market#Point of Care Diagnostics Industry Research Report#Point of Care Diagnostics Market Analysis#Point of Care Diagnostics Market Demand#Point of Care Diagnostics Market Distributors#Point of Care Diagnostics Market Drivers#Point of Care Diagnostics Market Future#Point of Care Diagnostics Market in China#Point of Care Diagnostics Market Key Players#Point of Care Diagnostics Market Manufacturers#Point of Care Diagnostics Market Online Players#Point of Care Diagnostics Market opportunities
0 notes
Text
In Vitro Diagnostics Market: Revolutionizing Healthcare with Advanced Diagnostics
The In Vitro Diagnostics (IVD) market plays a pivotal role in the healthcare industry, offering essential tools for disease diagnosis, treatment monitoring, and overall patient care. With advancements in diagnostic technology and the rising demand for personalized medicine, the IVD market is experiencing rapid growth. This article provides a detailed overview of the market trends, segmentation, key drivers, and leading companies in the IVD industry, offering valuable insights for decision-makers.
Market Overview
According to SkyQuest's In Vitro Diagnostics Market report, the global market is currently valued at USD 87.93 Billion in 2023, with a projected CAGR of 5.3% over the forecast period. The market is driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and the growing demand for early and precise disease detection.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/in-vitro-diagnostics-market
Market Segmentation
By Product Type:
Reagents and Kits: Essential components used in diagnostic procedures across a range of diseases.
Instruments: Include advanced diagnostic tools like analyzers, molecular diagnostic machines, and point-of-care devices.
Software & Services: Diagnostic software for accurate test results and integrated solutions for laboratories.
By Technology:
Immunoassays: Widely used for infectious diseases and cancer diagnosis.
Molecular Diagnostics: Key in genetic testing and precision medicine applications.
Clinical Chemistry: Essential for routine testing and biomarker identification.
Microbiology: Used to identify pathogens and guide antibiotic therapies.
Hematology: Focuses on blood-related diagnostics such as complete blood count (CBC).
Others: Encompasses emerging technologies like proteomics and metabolomics.
By Application:
Infectious Diseases: Dominating the market due to the global rise in bacterial, viral, and fungal infections.
Oncology: Growing demand for early cancer diagnostics and targeted treatments.
Cardiology: Vital in diagnosing cardiovascular diseases and risk factors.
Diabetes: Includes blood glucose monitoring and HbA1c testing for diabetes management.
Other Applications: Includes diagnostics for autoimmune diseases, nephrology, and neurology.
By End-User:
Hospitals and Clinics: Major centers for diagnostic testing and patient care.
Diagnostic Laboratories: Specializing in high-volume testing across various disease areas.
Homecare Settings: Growing segment due to increasing demand for at-home diagnostic kits.
Academic and Research Institutes: Driving innovation in diagnostic tools and techniques.
Key Growth Drivers
Rising Prevalence of Chronic Diseases: Increasing cases of cancer, diabetes, and cardiovascular diseases fuel the demand for diagnostic tools.
Advancements in Technology: Development of rapid, accurate, and minimally invasive diagnostic methods is boosting the market.
Growing Demand for Personalized Medicine: The focus on tailored treatments and early diagnosis is driving the adoption of molecular diagnostics.
Expansion of Point-of-Care Testing (POCT): The shift towards decentralized testing is increasing the demand for portable and easy-to-use diagnostic devices.
Read More at: - https://www.skyquestt.com/report/in-vitro-diagnostics-market
Leading Companies in the Market
SkyQuest’s In Vitro Diagnostics Market report identifies key players that are shaping the market, including:
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
Danaher Corporation
Thermo Fisher Scientific
bioMérieux SA
Becton, Dickinson and Company
QIAGEN
Sysmex Corporation
Agilent Technologies
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/in-vitro-diagnostics-market
Challenges and Opportunities
The IVD market faces challenges such as stringent regulatory frameworks and high costs of diagnostic devices, especially in emerging markets. However, opportunities lie in the growing adoption of telemedicine, increased government funding for healthcare infrastructure, and technological advancements that enable faster and more precise diagnostic results.
Future Outlook
The In Vitro Diagnostics Market is expected to experience robust growth as healthcare providers increasingly rely on advanced diagnostics for effective patient care. Companies that invest in innovation and cater to the rising demand for point-of-care and home-based testing will have a competitive edge in this dynamic market.
As diagnostics play a critical role in healthcare, the In Vitro Diagnostics Market is poised for significant growth. Decision-makers should stay informed about emerging trends and technological advancements to leverage the full potential of this market. For more detailed insights and strategies, consult SkyQuest’s comprehensive In Vitro Diagnostics Market report.
0 notes
Text
At-Home Health Testing Market Unidentified Segments – The Biggest Opportunity Of 2023
At-Home Health Testing Market Comprehensive Study is an expert and top to bottom investigation on the momentum condition of the Global At-Home Health Testing industry with an attention on the Global market. The report gives key insights available status of the Global At-Home Health Testing producers and is an important wellspring of direction and course for organizations and people keen on the business. By and large, the report gives an inside and out understanding of 2021-2027 worldwide At-Home Health Testing Market covering extremely significant parameters. Some key Players in This Report Include Roche Holding AG (Switzerland)
Siemens Healthineers (Germany)
Thermo Fisher Scientific (United States)
BD (United States)
Abbott Laboratories (United States)
Synlab Group (Germany)
Quidel Corporation
Everly Well, Inc. (United States)
Chembio Diagnostics (United States)
Cardinal Health (United States)
Biosynex (France)
At-home medical tests are kits that can be purchased online or at a local pharmacy or supermarket. The kits allow you to test for, screen for, or monitor certain diseases and conditions in the comfort of your own home. At-home testing is a growing part of health care that, like telemedicine, has gained popularity during the COVID-19 pandemic. Direct-to-consumer (DTC) at-home tests cover a wide range of test types. Pregnancy tests, glucose tests, faecal occult blood tests, infectious disease tests, and many other common at-home tests. Market Trends: Emergence of Covid-19 At-Home Test Kits
Rising Prevalence of Customizable Home Test Kits
Market Drivers: Increase in Adoption of Self-Help and Do-It-Yourself (DIY) Test Kits
Growing Demand of Self-Diagnosis for Diabetes
Market Challenges: Home Test Kits are Limited to taking Sample Blood and Saliva
Lack of Trained Professional to Perform Proper Tests
Market Opportunities: Increasing Demand for Point-of-Care Diagnostics
Rising Concerns of Infectious Diseases
The Global At-Home Health Testing Market segments and Market Data Break Down by Usage (Disposable, Reusable), Distribution Channel (Online {Brand Website, E-Commerce Website}, Offline {Retail Pharmacies, Drug Stores, Supermarket/Hypermarket}), Sample Type (Blood, Urine, Saliva, Others), Form Type (Cassette, Strip, Midstream, Test Panel, Cup, Others), Test Type (Glucose Monitoring Devices, Pregnancy Test, HIV Test Kits, Cholesterol Detection Kits, Covid-19 Test Kits, Others)
Presented By
AMA Research & Media LLP
0 notes
Text
The global glycated albumin assay market was valued at USD 325.4 million in 2023 and is poised to grow at a CAGR of 9.2% from 2023 to 2032.The Glycated Albumin Assay market is an emerging segment within the diagnostic industry, crucial for monitoring and managing diabetes. Glycated albumin is a glycoprotein formed when glucose binds to serum albumin, providing a shorter-term measure of blood glucose levels compared to hemoglobin A1c. This assay is particularly valuable for patients with conditions affecting red blood cells, offering a reliable alternative for diabetes management. The growing prevalence of diabetes globally, alongside advancements in diagnostic technologies, has significantly boosted the market for glycated albumin assays.
Browse the full report at https://www.credenceresearch.com/report/glycated-albumin-assay-market
Market Dynamics
1. Rising Prevalence of Diabetes: The increasing global incidence of diabetes is a primary driver for the glycated albumin assay market. According to the International Diabetes Federation, the number of adults living with diabetes is expected to rise to 643 million by 2030. This surge necessitates reliable diagnostic tools for effective disease management, thereby propelling the demand for glycated albumin assays.
2. Technological Advancements: Continuous advancements in diagnostic technologies have enhanced the accuracy and efficiency of glycated albumin assays. Innovations such as automated analyzers, improved assay kits, and point-of-care testing devices are making the testing process quicker and more accessible, further fueling market growth.
3. Advantages Over Traditional Methods: Glycated albumin assays offer several benefits over traditional glucose monitoring methods. Unlike hemoglobin A1c, which reflects average glucose levels over 2-3 months, glycated albumin provides insights into the previous 2-3 weeks, allowing for more immediate adjustments in treatment plans. This feature is particularly beneficial for patients with fluctuating glucose levels or those undergoing changes in therapy.
Challenges and Opportunities
Despite its promising growth, the glycated albumin assay market faces challenges such as the high cost of advanced diagnostic equipment and limited awareness in developing regions. Additionally, the lack of standardization in glycated albumin measurement can hinder market expansion.
However, opportunities abound with the increasing adoption of point-of-care testing and the integration of artificial intelligence and machine learning in diagnostics. These advancements are expected to streamline the testing process, reduce costs, and improve accuracy, thereby driving market growth.
Future Prospects
The future of the glycated albumin assay market looks promising, with continuous innovations and expanding applications in diabetes management and beyond. Increasing investment in healthcare infrastructure, particularly in emerging economies, and growing awareness about the importance of early and accurate diabetes diagnosis will further propel market growth. As the healthcare industry moves towards personalized medicine, glycated albumin assays are set to play a crucial role in providing tailored treatment plans for diabetes patients, ensuring better outcomes and improved quality of life.
Key Players
Asahi Kasei Pharma
Diazyme Laboratories
LifeSpan BioSciences
Biomatik Corporation
Crystal Chem, Inc.
Simes Sikma Biochem BV
Epinex Diagnostics, Inc.
Randox Laboratories
Abnova Corporation
Diagnostic Systems GmbH
Ethos Biosciences, Inc.
Zivak Technologies, USA
Abbexa Ltd.
Elabscience Biotechnology Inc.
Bioatlas Manufacturing
Exocell Inc.
MicroCoat Biotechnologie GmbH
Cusabio Technology, LLC
Biocore Co., Ltd.
BioVision Inc
Market Segmentation
The glycated albumin assay market can be segmented based on product type, application, end-user, and region.
1. By Product Type: The market includes assay kits and analyzers. Assay kits are widely used in clinical laboratories and research settings, while analyzers are becoming increasingly popular due to their automation capabilities and higher throughput.
2. By Application: The primary application of glycated albumin assays is in diabetes management. However, they are also used in research settings for studying glycation processes and their impact on various diseases.
3. By End-User: Major end-users include hospitals, diagnostic laboratories, academic and research institutes, and point-of-care testing facilities. Diagnostic laboratories hold the largest market share due to the high volume of tests conducted.
4. By Region: The market is geographically segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America currently dominates the market due to the high prevalence of diabetes and well-established healthcare infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate, driven by increasing healthcare awareness and rising diabetes cases.
Browse the full report at https://www.credenceresearch.com/report/glycated-albumin-assay-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Diabetes Care Devices Market Worth $31.9 Billion by 2028 – Exclusive Report by Meticulous Research®
According to a new market research report, titled “Diabetes Care Devices Market by Type [Diabetes Monitoring (CGM, Test Strips, Self-Monitoring Blood Glucose Meters, Lancets, Haemoglobin A1C Testing Kits)], [Insulin Delivery (Syringes, Pens, Pumps, Jet Injectors)] – Global Forecast to 2028” published by Meticulous Research®, the diabetes care devices market is expected to grow at a CAGR of 6.7% from 2021 to reach $31.9 billion by 2028.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=3438
As the prevalence of diabetes is increasing globally, self-management of diabetic treatments has become necessary to avoid long-term complications and fatalities. Diabetes care devices play an important role in managing diabetes as they enable patients to record and track their blood glucose levels all day long and administer the insulin doses according to the insulin levels.
The key factors driving the overall diabetes care devices market are the rising obesity cases, unhealthy and sedentary lifestyle, increasing prevalence of diabetes, and development of new insulin delivery systems. However, some limitations of diabetes care devices are their high costs and accessibility in low-middle income countries.
Impact of COVID-19 on Diabetes Care Devices Market
The COVID-19 pandemic has severely impacted the diabetes care devices market. COVID-19 patients had higher blood glucose levels due to the use of steroids for the COVID-19 treatment. In 2020, the increase in the number of individuals suffering from diabetes due to COVID-19 expanded the income of the central participants in the diabetes care division. Many players in the market presented new progressed diabetes care devices and association arrangements for diabetes care devices.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=3438
Diabetes Care Devices Market Overview
The diabetes care devices market is segmented based on type: (diabetes monitoring devices and insulin delivery devices) and geography (North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa). The study also evaluates industry competitors and analyzes their market share at a global level.
In 2021, the diabetes monitoring devices segment is estimated to account for the largest share of the overall diabetes care devices market. This is mainly attributed to the increased adoption of test strips and self-monitoring blood glucose meters and increasing demand for continuous glucose monitoring with rising awareness towards the disease in developing countries. Diabetes monitoring devices can be utilized to monitor blood glucose values and help the patient adjust their dietary intake, physical activity, and insulin doses to improve glycemic control on a regular basis.
Diabetes monitoring devices are further segmented into continuous blood glucose monitoring devices, test strips, lancets, self-monitoring blood glucose meters, and hemoglobin A1C testing kits. The test strips segment is expected to dominate the diabetes monitoring devices market in 2021, primarily due to the frequent usage of test strips for both continuous and self-glucose monitoring and is widely used by patients in developing countries.
Moreover, insulin delivery devices also are further segmented into insulin syringes, insulin pens, insulin pumps, and insulin jet injectors. In 2021, the insulin pens segment is expected to dominate the insulin delivery devices market. This can be primarily attributed to its advantages over traditional syringe insulin delivery methods, including improved patient satisfaction and adherence, user-friendly, greater ease of use, superior accuracy for delivering small insulin doses, greater social acceptability, and less reported injection pain. However, insulin pumps are the fastest-growing segment due to the advantages such as predictable insulin delivery, precise delivery of insulin, and reduction of wide fluctuation in blood glucose. It also mimics the normal pancreas’s release of insulin.
Quick Buy – Diabetes Care Devices Market Research Report: https://www.meticulousresearch.com/Checkout/33395503
Geographically, in 2021, North America is estimated to account for the largest share of the global diabetes care devices market, followed by Europe and Asia-Pacific. Factors such as the increasing prevalence of diabetes with the rising need to self-monitor and treat the disease, increasing prevalence of major risk factors of diabetes such as obesity and sedentary lifestyle, increasing geriatric population, and growing government initiatives promoting diabetes care are driving the growth of the diabetes care devices market in North America. However, Asia-Pacific region is expected to grow at the highest CAGR during the forecast period. Growth in this market is expected to be driven by the increasing prevalence of diabetes with the rising need to self-monitor the treatments, increasing prevalence of obesity and sedentary lifestyle, growing geriatric population, and government initiatives promoting diabetes care.
The report also includes an extensive assessment of the key strategic developments of leading market participants in the industry over the past four years. In recent years, the diabetes care devices market has witnessed several new product launches, approvals, agreements, collaborations, partnerships, expansions, and acquisitions. For instance, in May 2021, Medtronic PLC (Ireland) received CE Mark approval for the expanded functionality of its InPen and Guardian 4 sensors. Moreover, in February 2020, Abbott Laboratories (U.S.) partnered with Insulet Corporation (U.S.) to combine its leading continuous glucose monitoring (CGM) technology with Insulet’s Omnipod Horizon Automated Insulin Delivery System.
Some of the key players operating in the global diabetes care devices market are Novo Nordisk A/S (Denmark), Ypsomed AG (Switzerland), Abbott Laboratories (U.S.), Ascensia Diabetes Care Holdings AG (Switzerland), F. Hoffmann-La Roche Ltd (Switzerland), LifeScan IP Holdings, LLC (U.S.), Medtronic PLC (Ireland), Becton, Dickinson and Company (U.S.), Terumo Corporation (Japan), Sanofi AS (France), and DexCom, Inc. (U.S.), among others.
The diabetes care devices market is dominated by Abbott Laboratories (U.S.), Medtronic PLC (Ireland), DexCom, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Ascensia Diabetes Care (Switzerland), LifeScan IP Holding, LLC (U.S.), Becton, Dickinson and Company (U.S.), Insulet Corporation (U.S.), Novo Nordisk A/S (Denmark), and Tandem Diabetes Care, Inc. (U.S.). Together, these companies held around 80% share of the global diabetes care devices market in 2020.
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/diabetes-care-devices-market-3438
Scope of the Report:
Diabetes Care Devices Market, by Type
Diabetes Monitoring Devices
Insulin Delivery Devices
Diabetes Care Devices Market, by Geography
North America
U.S.
Canada
Europe
Germany
U.K.
France
Italy
Spain
Rest of Europe (RoE)
Asia-Pacific (APAC)
China
Japan
India
Rest of APAC (RoAPAC)
Latin America
Middle East & Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=3438
1 note
·
View note