#FDA 510k submission
Text
1 note
·
View note
Text
FDA 510k Clearance for Medical Device and IVDs
FDA 510k - An Overview
FDA 510k is a file containing sufficient information about a device to demonstrate that the medical device is at least as safe and effective as legally marketed devices that are not subjected to PMA. Organizations planning to launch Class I, II, and III devices in the United States intended for human use must submit a 510(k) if pre-market approval is not required.
Most class 1 devices are exempt from FDA 510k requirements. During the review of the file, if the FDA finds the device to be substantially equivalent, it will grant the FDA 510k clearance for medical devices with a ‘(k)’ number.
We offer technical and scientific assistance in identifying a suitable predicate device, regulation number, and device code, along with file drafting, e-copy conversion, and submission through the US Agent service. Few manufacturers opt for Q submission before final submission.
Role of I3CGlobal Consultants
As a prominent FDA 510k Consultants on a global scale, we have gained the trust of more than 350 plus manufacturers and specification developers. Our team consists of highly skilled subject-matter experts, assuring timely file preparation for 510(k) clearance irrespective of the manufacturer’s size or the regulatory knowledge of their internal team.
With an impressive 85% success rate, a track record of over 350+ clearances worldwide, and a history of serving more than 150+ manufacturers and specification developers since 1999, we proudly stand out from others in terms of quality and service cost.
Our dedication goes beyond mere verbal or email communication; we painstakingly assemble the complete files on behalf of our clients and take full responsibility until the 510k approval is obtained.
F
DA 510k Predicate Device
The term Predicate Device refers to a FDA 510k or PMA-cleared and legally marketed device in the USA with the same (a) intended use, (b) indications for use, and (c) similar technological characteristics as the device in question. It is crucial to note that the predicate device must be a single unit, if possible, and currently available in the USA market without any product recalls in the past years.
Substantial Equivalence Definition
It is a must to demonstrate substantial equivalence to a legally US-marketed device with similar intended use. Proving substantial equivalence to the reference device affirms that the subject device is as safe and effective as the predicate device. The key criteria for determining Substantial Equivalence include establishing similarity are the following, but are not limited to
Intended use,
Design,
Safety,
Adherence to standards,
Labelling,
Biological Compatibility,
Performance characteristics,
Materials used in the construction/ chemical composition,
Manufacturing Methods,
FDA 510k Predicate Device
The term Predicate Device refers to a FDA 510k or PMA-cleared and legally marketed device in the USA with the same (a) intended use, (b) indications for use, and (c) similar technological characteristics as the device in question. It is crucial to note that the predicate device must be a single unit, if possible, and currently available in the USA market without any product recalls in the past years.
Substantial Equivalence Definition
It is a must to demonstrate substantial equivalence to a legally US-marketed device with similar intended use. Proving substantial equivalence to the reference device affirms that the subject device is as safe and effective as the predicate device. The key criteria for determining Substantial Equivalence include establishing similarity are the following, but are not limited to
Intended use,
Design,
Safety,
Adherence to standards,
Labelling,
Biological Compatibility,
Performance characteristics,
Materials used in the construction/ chemical composition,
Manufacturing Methods,
Get more Information : https://www.i3cglobal.com/fda-510k/
0 notes
Text
FDA 510k — An Overview
FDA 510k is a file containing sufficient information about a device to demonstrate that the medical device is at least as safe and effective as legally marketed devices that are not subjected to PMA. Organizations planning to launch Class I, II, and III devices in the United States intended for human use must submit a 510(k) if pre-market approval is not required.
Most class 1 devices are exempt from 510k requirements. During the review of the file, if the FDA finds the device to be substantially equivalent, it will grant the FDA 510k clearance for medical devices with a ‘(k)’ number
We offer technical and scientific assistance in identifying a suitable predicate device, regulation number, and device code, along with file drafting, e-copy conversion, and submission through the US Agent service. Few manufacturers opt for Q submission before final submission.
Different Types of 510k
Traditional 510k is an original submission that normally has to be provided for the medical device, that requires 510k clearance according to 21 CFR 807. It can also be used to submit if there is any change in the previously cleared device. It generally takes 90 days for the 510k submission.
Abbreviated 510k submission is appropriate when FDA guidance exists for specific medical devices for the demonstration of compliance and any voluntary FDA-recognized consensus standards are available. It covers traditional, as well as a brief report on the usage of FDA guidance documents and FDA-approved standards.
Special 510k submission is for an already cleared device with some changes that don’t require the full review but only the summary document. These changes include indications of use, design, and labelling, but they should not affect the safety or performance of the new, modified device, and well-established methods.
1 note
·
View note
Text
Medical Device Regulatory Publishing - FDA 510k Submission | MakroCare
MakroCare provides comprehensive Medical Device Regulatory Submission Consultant & FDA 510k publishing services. Our team makes sure that your product complies.
#medical devices#regulatorychallenges#regulatorycompliance#healthcareinnovation#industrystandards#complianceassurance#medicaldevicequality#continuousimprovement
0 notes
Text
510(k) Submission & FDA 510k Submission - Whether manufacturing a medical device or using a CMO, medical device consultants can prove valuable to 510k submission and FDA inspection readiness. More info: https://pharmdevgroup.com/medical-device-consultant-review-quality-management-system-help-with-510k-submission/
0 notes
Text
Choosing the Right USFDA Consulting Firm for medical devices
The medical device industry is rapidly growing, and with it, the importance of regulatory compliance. The United States Food and Drug Administration (USFDA) is responsible for the regulation and approval of medical devices in the United States. USFDA Consulting Firms and USFDA Regulatory Consultants can assist medical device companies in navigating the complex regulatory landscape to ensure compliance with USFDA regulations.

USFDA regulatory consultants are experts in the field of medical device regulations. They provide a range of services to medical device companies, including regulatory compliance, product registration, and product approval. Consulting firms can help companies navigate the complex and ever-changing FDA regulatory landscape, ensuring that their products meet the necessary standards.
This article will explore the factors to consider when choosing the right USFDA Consulting Firm for medical devices. We will discuss the importance of experience, expertise, and reputation in selecting a consulting firm. Additionally, we will highlight the key services provided by USFDA Consulting Firms and how they can benefit medical device companies.
Experience is a critical factor to consider when choosing a USFDA consulting firm. Look for a firm that has a proven track record of successfully guiding medical device companies through the regulatory process. A firm with a long history of working with medical device companies is likely to have a deep understanding of the regulatory requirements and challenges associated with the industry.
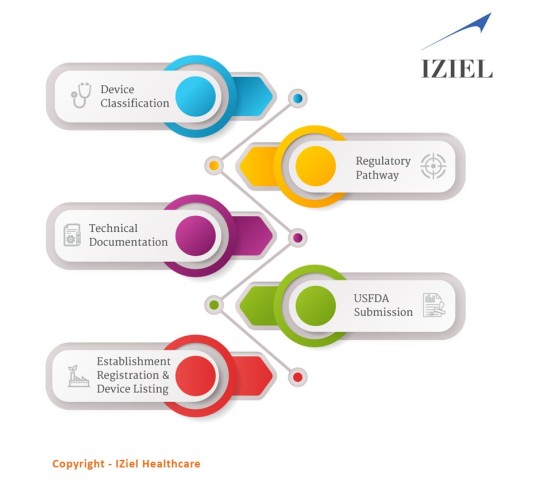
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
Medical Device FDA 510 k consulting company | Operon Strategist
What Does a FDA 510( k) Clearance mean?
Medical devices for human use within the United States must be submitted to the FDA to determine if the product is safe and effective. To do this, in utmost cases a company must compare their product to one formerly cleared by the FDA and give substantiation that their product is “ substantially equivalent ” to the preliminarily cleared( legally marketed) device. To be mainly original, the product must meet criteria for the same intended use, have the same technology or( slightly) different technology but produces similar end results, and be safe and effective.1 Once “pre-market clearance ” is took from the FDA the device can be distributed commercially immediately.
There are important differences between “ cleared ” and “ approved ”. When a medical device is cleared, this means it has experienced a 510( k) submission, which FDA has reviewed and handed clearance. For Class III medical devices to be legally marketed they must suffer a rigorous review and approval process orpre-market approval( PMA).
In the United States, this frequently means submitting a 510( k). A 510( k) is a structured package of information about your device and its performance and safety that you submit to the Food and Drug Administration( FDA) for “ clearance ” before you can deal your device in the U.S. In order to receive clearance from the FDA, your 510( k) will need to demonstrate that your medical device is substantially equivalent to another legally marketed device (called a predicate device).
What Exactly is Substantial Equivalence?
Now that we know what a 510( k) is, let’s talk about the substantial equivalence standard. You ’ll recall from the intro that your 510( k) must show that the new( or modified) device is substantially equivalent to at least one other legally marketed device, called a predicate device. Substantial equivalence looks at the intended use and the technological characteristics of the two devices.
Operon Strategist is a leading medical device consultant providing FDA 510k Clearance process consulting to the customers to register SBU( Small Business Unit), if applicable. Take out the testing demand of the product, creation of the dossier, resolving the queries and after completion of all the conditioning, the customer receives the US FDA 510 k premarket approval. We also help with the establishment enrollment and device listings to make suitable the supply of medical devices in the US.
We helps during the process of submitting operations for class I, II, III medical devices at any stages of the product development. Our team of good experts will successfully prepare and submit FDA medical device regulatory documents for theU.S and international customers. These operations include
510( k) Premarket Notifications
Premarket Approval Applications( PMAs)
De Novo Request( Application)
513( g) Requests for Classification
Investigational Device Exemption Applications( IDEs
Investigational New Drug Applications( INDs)
510( k) Premarket Notifications
Operon Strategist provides answers to related questions
Which groups( Class I, II, and III) do we require an FDA 510( k)?
How many stages consist of the 510( k) application process?
A class I, II, and III device intended for human use, for which a Premarket Approval FDA operation( PMA) isn't needed, must submit a 510( k) to FDA unless the device is pure from 510( k) conditions of the Federal Food, Drug, and Cosmetic Act( the FD&C Act) and doesn't exceed the limitations of exemptions in.9 of the device classification regulation chapters(e.g., 21 CFR862.9, 21 CFR864.9). Technically, under the 510( k) process, the FDA doesn't “ authorize ” medical devices and IVDs; the FDA issues a “ clearance ” or “ Approval ” for deal in the United States. typically, the FDA shall be subject to a provision of 510( k), should manufacturers intend to deal the Class II Medical Devices and some needed Class I and III bias or IVDs on the US market. Apre-market 510( k) approval is also required for already approved medical devices( Predicate) if the manufacturer( s) modifies the technology or changed the intent of device operation in a way that significantly affects patient safety or device performance.
The 510( k) is generally the most effective route to market clearance in the U.S. because you show your device is safe and effective based on this substantial equivalence standard, rather of demanding to present more expansive clinical trial data.
There are three types of 510( k) Traditional, shortened, and Special. This eBook will begin with a general overview of the 510( k) process, including its purpose and benefits. Next, we will explore the Traditional 510( k) and the sections and factors needed in depth. Finally, we will look at the Special and shortened 510( k).
When is a 510( k) needed?
A 510( k) is needed for medium risk devices that have a predicate on the market which can be used to demonstrate the safety and effectiveness of the new device. Meanwhile, a PMA is needed for high- risk or new devices which bear a advanced position of scrutiny to be verified safe and effective.
Complaints
Still, the end user can file a complaint with the FDA or the manufacturer, If a FDA 510( k) cleared medical device isn't performing as intended.
The user or client can also file a complaint with the FDA if it knows that the manufacturer or his representative or distributor is making false marketing claims or incorrectly promoting the performance of their device. This is a straightforward process to insure that medical devices on the market are being held to strict standards to make sure it's being used safely and effectively
Things to Consider
Before choosing a product with a FDA 510( k) clearance, it's important to understand what the product is claiming to do. Similar products may not perform exactly the same due to differences in technology or manufacturing. It's also important to make sure to read the indications for use to know how to use the product properly. Eventually, test data that has been transferred to the FDA should be available to the end user, frequently in a confirmation guide or test report.
Operon Strategist is a medical device regulatory consulting company which provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers.
1 note
·
View note
Photo

ISO 9001 implementation
#ISO13485 and ISO 14791implementation#ISO 9001 implementation#Portable Oxygen Concentrators#EU MDR medical device#FDA 510k submission
1 note
·
View note
Text

FDA 510 k Clearance & Premarket Approval
Operon Strategist is a medical device regulatory consulting company which provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers.
Contact details –
Phone no - 93702 83428
Mail id – [email protected]
#fda 510 k clearance#510 k clearance#510k certification#FDA 510k submission#510 k approval#fda 510k#fda 510k consultant#fda design control#premarket approval#510k consultant#FDA 510k Guideline
0 notes
Link
0 notes
Text
Comprehensive 510k Submission

Introduction
When it comes to getting medical devices approved for the market in the United States, understanding the 510k submission process is crucial. This pathway ensures that new devices are safe and effective by comparing them to previously approved products. But what exactly is a 510k submission, and why is it so important?
Understanding 510k Submission
The 510k submission, named after Section 510(k) of the Food, Drug, and Cosmetic Act, is a premarket submission made to the FDA. This submission demonstrates that the device to be marketed is at least as safe and effective, substantially equivalent, to a legally marketed device that is not subject to premarket approval (PMA).
Why 510k Submission is Essential
Regulatory Compliance
Regulatory compliance is a non-negotiable aspect of the medical device industry. Without FDA clearance through a 510k submission, a medical device cannot legally be marketed in the U.S. This compliance ensures devices meet strict safety and effectiveness standards.
Market Access
A successful 510k submission opens the doors to the lucrative U.S. medical device market. This not only provides significant revenue opportunities but also enhances the credibility and reputation of the device manufacturer.
Key Components of a 510k Submission
Device Description
A detailed description of the device, including its design, materials, and intended use, forms the foundation of the submission.
Predicate Device Comparison
This involves comparing the new device to a predicate device to establish substantial equivalence. The comparison covers design, function, and intended use.
Performance Testing
Performance testing data, including bench tests, animal tests, and clinical trials, demonstrate the device’s safety and effectiveness.
Labeling and Instructions for Use
Clear labeling and comprehensive instructions for use ensure the end-user understands how to safely and effectively operate the device.
Role of FDA in 510k Submission
FDA Review Process
The FDA’s review process involves evaluating the submitted data to determine if the new device is substantially equivalent to the predicate device.
Interaction with FDA
Maintaining open communication with the FDA can help address any questions or concerns that arise during the review process.
Conclusion
The 510k submission process is a critical component of bringing new medical devices to market in the U.S. Understanding this process, preparing thoroughly, and engaging effectively with the FDA can significantly enhance the chances of a successful submission.
0 notes
Link
Freyr provides regulatory support for medical device manufacturers in 510k submission (510 k premarket notification) to USFDA, which include predicate device identification, 510k application compilation, gap analysis, publishing, creation & validation of e-copy, device listing for compliant market entry.
0 notes
Text
Modernization of 510(k) – A major milestone to safer and better healthcare
The FDA regulates over 190,000 distinct medical devices, with approximately 3,000 low and moderate-risk devices receiving FDA's 510(k) clearance on average annually, accounting for 80% of total approvals. The 510(k) pathway, established in 1976, undergoes constant refinement by the FDA to ensure practicality and transparency.
In November 2018, as part of its Medical Device Safety Action Plan, the FDA announced changes to the 510(k) clearance pathway.
Commissioner Scott Gottlieb emphasized the need to promote innovation and safety by encouraging reliance on modern predicate devices or objective performance criteria.
Notably, 20% of 510(k)s were approved based on predicates older than ten years, prompting the FDA to modernize the process by making certain devices ineligible as predicates and requiring updated data submissions.
The FDA is also developing an alternate 510(k) pathway based on contemporary standards rather than outdated predicates, aiming to foster innovation and facilitate access to advanced technology.
Key implications for medical device manufacturers include the elimination of old predicates, the requirement for objective testing evidence aligned with modern technology, and the FDA's plans to finalize guidance emphasizing safety and performance criteria.
Read Full Article: Modernization of 510(k) – A major milestone to safer and better healthcare
As regulations evolve, companies can rely on regulatory partners like Elexes to stay updated and navigate changes effectively. For more information, interested parties can contact Elexes at [email protected].
#fda#510k#medical device#regulatory#quality#modernization#510k medical device#medical devices#medicaldevicecompany 510k submission
0 notes
Link
#Medical device classification#510k submission#Medical device recalls#Combination products#IDE submission#Pre-market requirements#Post-market requirements#De Novo submission#Pre-market approvals (PMA)#Medical device registration & listing#Importing/ exporting medical devices#FDA communications
0 notes
Text
Do medical devices need to be approved by the FDA?
Medical Device Manufacturers require USFDA Approvals to sell their products in USA. USFDA differentiates product approvals in Class I, II &III depending upon the risk associated. The submissions include self-certification, 510(k) and PMA depending upon the class of the product.
The FDA medical devices have been classified into 3 classes.
Class I: They are low-risk devices.
Class II: They are medium-moderate risk devices.
Class III: They are of high risk, generally life supporting and life-sustaining.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
Regulatory Data and Info management for Med Device success
With EU MDR changing the plain field of med devices to a whole a new level, other countries are following similar path of increasing demands in product safety, tractability, performance areas
Companies not organised on information management with a system will end up duplicating lot of activities leading to loss of productivity and increase risk of non-compliance
There are 3 categories of Challenges grouped by:
https://www.ddismart.com/blog/regulatory-data-and-info-management-for-med-device-success

Process Challenges:
Training must be required to simplify the complexity of multiple system & user interface. As a result, users work outside of the system using local file shares or email to collaborate.
To check the work status and to create report most of the companies depends on separate reporting tool or manual spreadsheets.
System Challenges:
Medical device regulation is much versatile. Upgrading a system with updated integrations is challenging and expensive as well.
Systems that lie behind corporate firewalls are difficult to outsource to business partners or service providers.
Compliance Challenges:
Users develop manual tracking spreadsheets when planning & tracking capabilities aren’t part of a content management system.
Many times users share information and documents via email that cause inconsistent use of document template and it is much difficult to Re-import.
Importance of a RIM system
Medical device registrations differ from country to country, with the difference in FDA’s 510K (Class I and II devices) and Marketer Approval (Class III devices) processes from the EU’s CE Marking process. The current regulatory environment in which the medical devices and diagnostics companies are competing is complex.
There is a higher demand for regulatory and compliance information required to support submissions 510 (k), PMA, De Novo and HDE.A cloud based RIM can effectively help management of product registration, commitments and regulatory submissions to medical device and diagnostics companies. This unified, single-source system provides a global user base with real-time information necessary to ensure the quality of the product and the registration.
Following capabilities should be present in an effective regulatory information management system
Identifies device-specific global regulatory requirements
Assembles product information into country-specific TF template
Controls the dossier configurations for internal review and external review
Manages changes and revisions to TFs and product information
Generates compliant submission documents in various HA/NB formats
Tracks & retains submission status& manage commitments
Decreases effort and calendar time to replicate regulatory submission between products and regulatory agencies
Provides clear oversight of original and life cycle submissions
RIM in future:
With the versatile and novel reporting requirements set out by regulatory authorities, the plea to need Regulatory Information Management System (RIMS) is emerging brassier.The medical device market is progressively adopting regulations for UDI, eIFU, electronic submissions, others. Making these things happen without a proper RIM system is very challenging.
At the same time, Medical device companies themselves are now realizing the strategically important role that various forms of product data could play in the future; enhancing new productivity, efficiency and create competitive differentiation.
#regulatory information management system software#Regulatory Information Management Software#regulatory information management system rims
1 note
·
View note