#Europe Data for Surgeries Share
Explore tagged Tumblr posts
Text
By: Bernard Lane
Published: Apr 14, 2024
Nine of the 15 gender clinics in a landmark international survey for the Cass review have admitted they do not routinely collect outcome data on their young patients.
This survey, together with a new evaluation of treatment guidelines for gender dysphoria, gives unprecedented insights into the workings of gender clinics around the world offering puberty blockers and cross-sex hormones to minors.
In the 2022-23 survey, six clinics said they “routinely collected some outcome data”: one of these clinics gave no further detail; one noted the number of patients discontinuing treatment; another used measures of quality of life; two were taking part in cohort studies; and the sixth clinic repeated some baseline assessments. Nine clinics acknowledged “not routinely collecting outcome data.”
The report of the survey results1, published by researchers from the University of York earlier this month, identified clinics by country, not name. Of the clinics that took part, Australia and the Netherlands were prominent with five and four clinics respectively.
Poor data collection was central to the controversy over the London-based Tavistock youth gender clinic.
The Cass review had planned to run a data-linkage study—with help from adult gender clinics—to learn the outcomes of the Tavistock’s 9,000-odd former patients.
The missing long-term data would allow clinicians, young patients and parents to make informed decisions about treatment. The review said it was to be the largest study of its kind in the world.
However, six of the seven adult clinics refused to co-operate. One stated reason was that “the study outcomes focus on adverse health events, for which the clinics do not feel primarily responsible.”
Another adult clinic said, “The unintended outcome of the study is likely to be a high-profile national report that will be misinterpreted, misrepresented or actively used to harm patients and disrupt the work of practitioners across the gender dysphoria pathway.”
On April 12, however, The Times newspaper reported that the uncooperative adult clinics had “bowed to pressure to share [the] missing data”.
Mostly medical
In the York University international survey, ordered by the Cass review, all 15 youth gender clinics said they used a multi-disciplinary team, but researchers concluded there was a “paucity” of psychosocial therapy interventions such as psychotherapy or cognitive behaviour therapy. Five clinics did not offer any of these non-medical interventions in-house.
All gender clinics told researchers that “genital reconstructive surgery”—the creation of a pseudo vagina, for example—was “accessible only from age 18.” The youngest age for “masculinising chest surgery” (a double mastectomy) was reported as 16. In fact, there are documented cases in Australia of 15-year-olds approved for transgender mastectomy. Genital surgery is legally available to minors2 in Australia and practised in America.
“Only five clinics reported routine discussion of fertility3 preferences, and only two discussed sexuality4. Finland was the only country to report routinely assessing for history of trauma5,” the final Cass report says in its commentary on the survey.
In separate studies for the Cass review, three independent reviewers evaluated the quality of 21 guidelines for treatment of gender dysphoria in minors.
Included were international guidelines (from the Endocrine Society and the World Professional Association for Transgender Health or WPATH); documents from North America (for example, the 2018 policy statement from the American Academy of Pediatrics); from Europe (the guideline of the UK Royal College of Psychiatrists, for example, and Denmark’s); as well as guidelines from the Asia-Pacific and Africa.
“WPATH has been highly influential in directing international practice, although its guidelines were found by the University of York appraisal process to lack developmental rigour,” the Cass report says.
The York researchers chart patterns of “circular” cross-referencing between guidelines to create a misleading impression of consensus in favour of the medicalised “gender-affirming” treatment approach.
“The guideline appraisal raises serious questions about the reliability of current guidelines. Most guidelines have not followed the international standards for [rigorous and independent] guideline development. Few guidelines are informed by a systematic review of empirical evidence [the gold standard for assessing the evidence supporting a health intervention] and there is a lack of transparency about how recommendations were developed,” the Cass report says.
“Healthcare services and professionals should take into account the variable quality of published guidelines to support the management of children and young people experiencing gender dysphoria. The lack of independence in many national and regional guidelines, and the limited evidence-based underpinning current guidelines, should be considered when utilising these for practice.”
The Cass report says it is “imperative” that gender clinic staff be “cognisant of the limitations in relation to the evidence base and fully understand the knowns and the unknowns.”
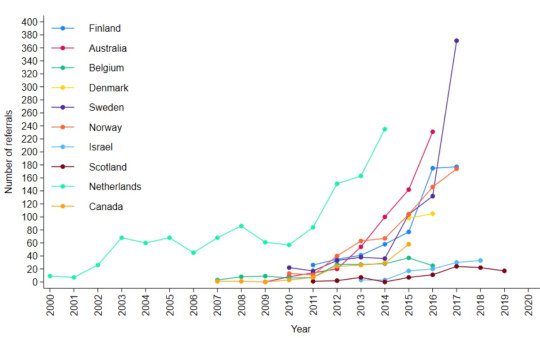
[ Chart: Number of youth gender clinic referrals over time by country. Source: Cass report ]
Bum steer
Staff at the Tavistock clinic misled patients and parents, or failed to correct their misconceptions, according to a new report from the Multi-Professional Review Group (MPRG) given oversight of treatment decisions from 2021.
These shortcomings of clinicians included playing down the extent of the unknowns of hormonal treatment; not explaining that puberty blockers are being used unlicensed and off-label; not challenging the reassuring but false parallel with the licensed use of puberty blockers for precocious (premature) puberty; not discussing the possibility that blockers will pause or slow psychosexual development; and not sharing figures showing the vast majority of children started on puberty blockers will go on to cross-sex hormones supposed to be taken lifelong.
The MPRG was also troubled by clinical documents showing misunderstanding of “the outcome of physical treatments” on the part of patients and parents.
In the York University study of treatment guidelines for gender dysphoria, only two were recommended for use by all three reviewers. These were recent, more cautious policies from Finland and Sweden. Both followed independent systematic reviews showing the evidence base for hormonal and surgical treatment of minors to be very weak and uncertain. Like the Cass review itself, the 2020 Finnish and 2022 Swedish guidelines recognise that puberty blockers are experimental and should not be routine treatment.
Although all the guidelines in the study agreed on the need for a multidisciplinary team to treat gender-distressed minors, the “most striking problem” shown by analysis of these documents was “the lack of any consensus6 on the purpose of the assessment process”, the Cass report says.
“Some guidelines were focused on diagnosis, some on… eligibility for hormones, some on psychosocial assessment, and some on readiness for medical interventions7.
“Only the Swedish and [the 2022] WPATH 8th version guidelines contain detail on the assessment process8. Both recommend that the duration, structure and content of the assessment be varied according to age, complexity and gender development.
“Very few guidelines recommend formal measures/clinical tools to assess gender dysphoria, and a separate analysis demonstrated that the formal measures that exist are poorly validated.”
Nor was there any consensus on “when psychological or hormonal interventions should be offered and on what basis.”
A survey of staff at the Tavistock clinic, undertaken as part of the Cass review, found specialists divided on whether or not “assessment should seek to make a differential diagnosis, ruling out other potential [non-gender9] causes of the child or young person’s distress.”
Arguing for an ambitious research program well beyond a possible clinical trial of puberty blockers, the Cass report says the field of youth gender dysphoria is one of “remarkably weak evidence” where health professionals are “afraid to openly discuss their views” because of vilification and bullying.
“Although some think the clinical approach should be based on a social justice model, the NHS works in an evidence-based way,” the report says.
“The gaps in the evidence base regarding all aspects of gender care for children and young people have been highlighted, from epidemiology through to assessment, diagnosis10 and intervention. It is troubling that so little is known about this cohort and their outcomes.
“Based on a single Dutch study, which suggested that puberty blockers may improve psychological wellbeing for a narrowly defined group of children with gender incongruence [or dysphoria], the practice spread at pace to other countries.
“Some practitioners abandoned normal clinical approaches to holistic assessment, which has meant that this group of [gender-distressed] young people have been exceptionalised compared to other young people with similarly complex presentations.”
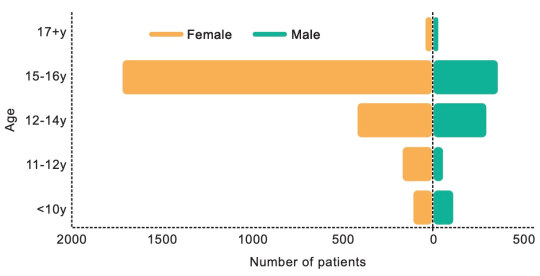
[ Chart: Age and sex on referral to the Tavistock clinic from 2018-2022. Source: Cass report ]
Who to trust?
The Cass report says the missing evidence “makes it difficult to provide adequate information on which a young person and their family can make an informed choice.”
“A trusted source of information is needed on all aspects of medical care, but in particular it is important to defuse/manage expectations that have been built up by claims about the efficacy of puberty blockers.
“The option to provide masculinising or feminising hormones from the age of 16 is available, but the [Cass] review would recommend an extremely cautious clinical approach and a strong clinical rationale for providing hormones before the age of 18. This would keep options open during this important developmental window, allowing time for management of any co-occurring [non-gender] conditions11, building of resilience, and fertility preservation, if required.”
The review stresses that “consent is more than just capacity and competence. It requires clinicians to ensure that the proposed intervention is clinically indicated as they have a duty to offer appropriate treatment. It also requires the patient to be provided with appropriate and sufficient information about the risks, benefits and expected outcomes of the treatment.”
“Assessing whether a hormone pathway is indicated is challenging. A formal diagnosis of gender dysphoria is frequently cited as a prerequisite for accessing hormone treatment. However, it is not reliably predictive of whether that young person will have long-standing gender incongruence in the future, or whether medical intervention will be the best option for them.”
Advocates for the gender-affirming approach assert that detransition and treatment regret are vanishingly rare, whereas suicide risk for those denied medical intervention is claimed to be very high.
The Cass report says: “It has been suggested that hormone treatment reduces the elevated risk of death by suicide in this population, but the evidence found did not support this conclusion.”
“The percentage of people treated with hormones who subsequently detransition remains unknown due to the lack of long-term follow-up studies, although there is suggestion that numbers are increasing.”
The report cites three reasons why the true extent of detransition is unlikely to be clear for some time—patients who decide medicalisation was a mistake may not wish to return to their former clinic to announce this fact; there is a post-treatment honeymoon period and clinicians suggest it may take 5-10 years before a decision to detransition; and the surge in patient numbers only began within the last decade.
Faced with uncertainty and a lack of good evidence, those with responsibility—from health ministers and hospital managers down to gender clinicians—rely on treatment guidelines supposed to advise on clinical practice according to the “best-available” evidence and expert opinion.
In the York University guideline analysis, the 21 documents were rated on six domains, the key two being the rigour of their development and their editorial independence.
“[Rigour] includes systematically searching the evidence, being clear about the link between recommendations and supporting evidence, and ensuring that health benefits, side effects and risks have been considered in formulating the recommendations,” the Cass report says.
Only the Finnish and Swedish guidelines scored above 50 per cent for rigour. Only these two documents, the Cass report says, link “the lack of robust evidence about medical treatments to a recommendation that treatments should be provided under a research framework or within a research clinic. They are also the only guidelines that have been informed by an ethical review conducted as part of the guideline development.”
“Most of the guidelines described insufficient evidence about the risks and benefits of medical treatment in adolescents, particularly in relation to long-term outcomes. Despite this, many then went on to cite this same evidence to recommend medical treatments,” the report says.
“Alternatively, they referred to other guidelines that recommend medical treatments as their basis for making the same recommendations. Early versions of two international guidelines, the Endocrine Society 2009 and WPATH 7th version guidelines, influenced nearly all the other guidelines.
“These two guidelines are also closely interlinked, with WPATH adopting Endocrine Society recommendations, and acting as a co-sponsor and providing input to drafts of the Endocrine Society guideline. The WPATH 8th version cited many of the other national and regional guidelines to support some of its recommendations, despite these guidelines having been considerably influenced by the WPATH 7th version.
“The circularity of this approach may explain why there has been an apparent consensus on key areas of practice despite the evidence being poor.”
Sometimes these gender-affirming guidelines seek to buttress a strong evidence claim with a citation to a study that is weak or involves a different patient group.
The Cass report notes that, “The WPATH 8th version’s narrative on gender-affirming medical treatment for adolescents does not reference its own systematic review [of the evidence], but instead states: ‘Despite the slowly growing body of evidence supporting the effectiveness of early medical intervention, the number of studies is still low, and there are few outcome studies that follow youth into adulthood. Therefore, a systematic review regarding outcomes of treatment in adolescents is not possible’.”
Despite WPATH insisting such an evidence review is not possible, this is precisely what health authorities and experts have undertaken since 2019 in several jurisdictions—Finland, Sweden, the UK National Institute for Health and Care Excellence, Florida, Germany, and University of York research commissioned by the Cass review.
Yet in the 8th and current version of its guideline, WPATH makes the confident statement that, “There is strong evidence demonstrating the benefits in quality of life and well-being of gender-affirming treatments, including endocrine and surgical procedures… Gender-affirming interventions are based on decades of clinical experience and research; therefore, they are not considered experimental, cosmetic, or for the mere convenience of a patient. They are safe and effective at reducing gender incongruence and gender dysphoria”.
But WPATH “overstates the strength of the evidence” for its treatment recommendations, the Cass report says.
--
1 In the survey, there was one clinic each from Belgium, Denmark, Finland, Northern Ireland, Norway and Spain. The response rate was 38 per cent.
2 In Australia there is no good public data on trans surgery for minors.
3 Early puberty blockers followed by cross-sex hormones are expected to sterilise young people and may also impair future sexual function.
4 Some sizeable proportion of gender clinic patients might grow up in healthy bodies and accept their same-sex attraction were it not for trans medicalisation, according to testimony from detransitioners, clinicians’ reports and data.
5 Trauma from a history of sexual abuse, for example, or exposure to domestic violence is thought to be among the many possible underlying causes of what presents as gender dysphoria. The Multi-Professional Review Group (MPRG), given oversight of Tavistock treatment decisions from 2021-23, was troubled by the lack of curiosity by the clinic’s staff about the effect of a child’s “physical or mental illness within the family, abusive or addictive environments, bereavement, cultural or religious background, etc.”
6 Critics of the “gender-affirming” treatment approach say it is not mainstream medicine because the “trans child” in effect self-diagnoses while clinicians avoid differential diagnosis and attribute mental health disorders and other pre-existing issues to a “transphobic” society.
7 “In most cases [at the Tavistock clinic] children and parents were asking to progress on to puberty blockers from the very first appointment”, according to the MPRG.
8 In the MPRG’s opinion, the patient notes from the Tavistock “rarely provide a structured history or physical assessment, however the submissions to the MPRG suggest that the children have a wide range of childhood, familial and congenital conditions.”
9 Once referred to the Tavistock, patients typically were no longer seen by child and adolescent mental health services.
10 According to the MPRG, gender dysphoria in the diagnostic manual DSM-5 “has a low threshold based on overlapping criteria, and is likely to create false positives. Young people who do not go on to have an enduring cross-sex gender identity may have met the criteria in childhood. And early to mid-childhood social transition may be influential in maintaining adherence to the criteria. Sex role and gender expression stereotyping is present within the diagnostic criteria—preferred toys, clothes, etc—not reflecting that many toys, games and activities [today] are less exclusively gendered than in previous decades.”
11 The MPRG said it was “notable that until the child and family’s first appointment at [the Tavistock] they have received little, if any, support from health, social care, or education professionals. Most children and parents have felt isolated and desperate for support and have therefore turned for information to the media and online resources, with many accessing LGBTQ+ and [gender dysphoria] support groups or private providers which appear to be mainly ‘affirmative’ in nature, and children and families have moved forward with social transition. This history/journey is rarely examined closely by [Tavistock clinicians] for signs of difficulty [or] regret.”
==
Critics have described "gender affirming care" - that is, sex-trait modification - as "medical experimentation." This is incorrect. In a medical experiment, you actually collect data and monitor the participants in the experiment. They don't do that. They're cowboys violating all medical ethics - "first, do no harm" - for ideology, money or both.
#Bernard Lane#Cass review#Cass report#Dr. Hilary Cass#Hilary Cass#gender affirming care#gender affirming healthcare#gender affirmation#medical scandal#medical malpractice#sex trait modification#medical corruption#World Professional Association for Transgender Health#WPATH#ethics violations#medical ethics#unethical#gender ideology#gender identity ideology#queer theory#intersectional feminism#religion is a mental illness
13 notes
·
View notes
Text
Heart Defect Closure Devices Market
Heart Defect Closure Devices Market Size, Share, Trends: Abbott Laboratories Leads
Shift Towards Transcatheter Procedures for Heart Defect Closure
Market Overview:
The Heart Defect Closure Devices Market is projected to grow at a CAGR of 9.2% during the forecast period of 2024-2031. The market value is expected to rise from XX USD in 2024 to YY USD by 2031, with North America emerging as the dominant region. Key metrics include increasing prevalence of congenital heart defects, advancements in minimally invasive procedures, and growing geriatric population. The market is experiencing robust growth driven by technological innovations in device design, rising awareness about structural heart diseases, and improving healthcare infrastructure in developing countries.
DOWNLOAD FREE SAMPLE
Market Trends:
The market for heart defect closure devices is rapidly transitioning towards transcatheter procedures, which are less invasive and provide better patient outcomes. This movement is characterized by the development of advanced closure devices that can be delivered via catheters, thus eliminating the need for open-heart surgery. Manufacturers are focusing on developing devices with enhanced deliverability, a smaller profile, and higher closure efficiency. The use of 3D printing technology in device manufacturing enables the creation of patient-specific closure devices, enhancing the success of transcatheter procedures. This trend not only accelerates patient recovery and reduces hospital stays but also expands the range of treatable congenital heart defects, including complex anatomies that were previously untreatable.
Market Segmentation:
Septal occluders dominate the market, accounting for over 45% of total heart defect closure device sales globally. Septal occluders continue to lead the heart defect closure devices market due to their widespread use in treating common congenital heart defects such as atrial septal defects (ASD) and ventricular septal defects (VSD). The popularity of septal occluders can be attributed to their ability to provide a less invasive alternative to open-heart surgery for defect closure, resulting in quicker recovery and fewer complications. Recent market data indicates that demand for septal occluders has increased by 10% year over year in major healthcare markets like the United States and Europe. This growth is driven by advancements in device design, which have led to improved closure rates and reduced procedural complications.
Market Key Players:
Abbott Laboratories
Boston Scientific Corporation
W. L. Gore & Associates
Occlutech
Lifetech Scientific Corporation
Cardia Inc.
Contact Us:
Name: Hari Krishna
Email us: [email protected]
Website: https://aurorawaveintellects.com/
0 notes
Text
Trends in the Intragastric Balloons Market 2021 - 2028
The global intragastric balloons market size is expected to reach USD 100.2 million by 2028, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 11.9% from 2021 to 2028. The rise in obesity and increasing demand for minimally invasive procedures are anticipated to be key market drivers.
Intragastric balloons are one of the most widely adopted endoscopic bariatric therapy devices in clinical settings. This can be attributed to complications associated with surgical weight loss treatments and the low eligibility criteria for surgical options leading to a rise in demand for effective minimally invasive weight-loss treatment options.
The safety concerns associated with the inflatable medical device are anticipated to make the pre-market and post-market scrutiny process of these devices by the regulatory authorities more stringent. For instance, in 2019, Apollo Endosurgery Inc., revised the labeling of its Orbera Intragastric Balloon System to include contradiction clarifications, precautions related to anticholinergic and psychotropic medications, and updated U.S. adverse event tables after it received an FDA safety letter.
Nonetheless, the extensive research activities related development of innovative and novel systems and up-gradation of existing intragastric balloons to overcome the shortcomings of the traditional inflatable medical devices are anticipated to facilitate market growth.
Intragastric Balloons Market Report Highlights
According to The American Society for Metabolic and Bariatric Surgery (ASMBS) data, nearly 5,000 intragastric balloon implantations have been conducted since their U.S. FDA approval
The single segment held the largest revenue share of the market in 2020 while the triple segment is expected to grow at a fast pace
The gas-filled segment is anticipated to witness lucrative growth over the forecast period
The hospital segment held significant revenue share in 2020 while the ambulatory surgical centers segment is expected to grow at a rapid pace
The endoscopy segment dominated the market in 2020 and the pill form segment is expected to witness lucrative growth over the forecast period
North America dominated the market in 2020 due to the presence of a large obese population and high adoption of bariatric procedures. In Asia Pacific, the market is expected to witness remunerative growth over the forecast period
As of January 2019, Apollo Endosurgery has discontinued the sales and distribution of its ReShape Balloon
Intragastric Balloons Market Segmentation
Grand View Research has segmented the intragastric balloons market on the basis of administration, balloon type, filling material, end use, and region:
Intragastric Balloons Administration Outlook (Revenue, USD Million, 2016 - 2028)
Pill Form
Endoscopy
Intragastric Balloons Type Outlook (Revenue, USD Million, 2016 - 2028)
Single
Dual
Triple
Intragastric Balloons Filling Material Outlook (Revenue, USD Million, 2016 - 2028)
Saline Filled
Gas Filled
Intragastric Balloons End-use Outlook (Revenue, USD Million, 2016 - 2028)
Hospitals
Clinics
Ambulatory surgical centers
Intragastric Balloons Regional Outlook (Revenue, USD Million, 2016 - 2028)
North America
US
Canada
Europe
Germany
UK
France
Italy
Spain
The Netherlands
Belgium
Switzerland
Turkey
Poland
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Indonesia
Malaysia
Philippines
Singapore
Latin America
Brazil
Mexico
Argentina
Chile
Colombia
MEA
South Africa
Saudi Arabia
UAE
Israel
Egypt
Order a free sample PDF of the Intragastric Balloons Market Intelligence Study, published by Grand View Research.
0 notes
Text
Challenges and Opportunities in the Multiparameter Patient Monitoring System Market
The global multiparameter patient monitoring system market size is expected to reach USD 18.03 billion by 2030, registering a CAGR of 5.7% according to a new report by Grand View Research, Inc. Increasing health concerns and the resultant requirement to constantly monitor the health parameters of patients, before and after surgery is creating demand for multiparameter patient monitoring systems.
The market growth is also supported by the increasing need for hospital transport, such as intra-hospital or out-of-the-hospital transport. For special examination & therapy, hospitals need intra-hospital transport, which calls for more continuous monitoring of vital signs including blood pressure, blood oxygen saturation by pulse oximetry, heart rate, and electrocardiography.
Healthcare providers are continually looking for innovative medical products to offer superior quality care, which is also expected to drive the market. Mobile solutions and data integration low-acuity monitoring are the significant trends that improve the cost efficiency of multiparameter patient monitoring solutions. Key players are introducing advanced patient monitoring products, such as integrated remote monitoring solutions with added features of greater connectivity through cloud technology and WiFi.
For instance, Radius-7 Pulse Co-Oximeters by Masimo Corp. got U.S. FDA approval in March 2020. It is a wearable patient monitor with flexible functionality of WiFi, Bluetooth, and alarms.
Gather more insights about the market drivers, restrains and growth of the Multiparameter Patient Monitoring System Market
Multiparameter Patient Monitoring System Market Report Highlights
• Portable was the largest device type segment in 2022 accounting for 66.2% due to growing home healthcare and easy mobility across different departments within the hospital
• By acuity level, the high segment has accounted for the highest market share in 2022 due to the increasing prevalence of chronic diseases, such as CVDs, respiratory disorders, and other critical medical conditions, which need continuous monitoring
• By age group, the geriatric segment accounted for the largest segment in 2022 with a share of 53.9% 2021 due to the rapidly growing geriatric population that is susceptible to various chronic disorders, which require continuous patient monitoring
• By end-use, the hospital's segment has accounted for the highest market share in 2022 due to the increasing number of patients being admitted to hospitals due to chronic diseases, injuries, and other emergencies
• North American region contributed the largest share to the market in the year 2022 attributing to the availability of technologically advanced equipment and high-quality healthcare system
Multiparameter Patient Monitoring System Market Segmentation
Grand View Research has segmented the multiparameter patient monitoring system market based on device, acuity level, age group, end-use, and region:
Multiparameter Patient Monitoring System Device Outlook (Revenue, USD Billion, 2018 - 2030)
• Portable
• Fixed
Multiparameter Patient Monitoring System Acuity Level Outlook (Revenue, USD Billion, 2018 - 2030)
• High
• Medium
• Low
Multiparameter Patient Monitoring System Age Group Outlook (Revenue, USD Billion, 2018 - 2030)
• Pediatric
• Adult
• Geriatric
Multiparameter Patient Monitoring System End-Use Outlook (Revenue, USD Billion, 2018 - 2030)
• Hospitals
• Homecare Settings
• Ambulatory Surgical Centers
• Others
Multiparameter Patient Monitoring System Regional Outlook (Revenue in USD Billion 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o U.K.
o Germany
o France
o Italy
o Spain
o Sweden
o Norway
o Denmark
• Asia Pacific
o China
o Japan
o India
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa
o Saudi Arabia
o South Africa
o UAE
o Kuwait
Order a free sample PDF of the Multiparameter Patient Monitoring System Market Intelligence Study, published by Grand View Research.
#Multiparameter Patient Monitoring System Market#Multiparameter Patient Monitoring System Market Size#Multiparameter Patient Monitoring System Market Share#Multiparameter Patient Monitoring System Market Analysis#Multiparameter Patient Monitoring System Market Growth
0 notes
Text
Gastrointestinal Stents Market
Gastrointestinal Stents Market Size, Share, Trends: Boston Scientific Corporation Lead
Rising adoption of self-expanding metal stents (SEMS) drives market growth
Market Overview:
The Gastrointestinal Stents Market is projected to grow at a CAGR of 5.7% from 2024 to 2031. The market value is expected to increase from USD XX billion in 2024 to USD YY billion by 2031. Europe currently dominates the market, with key metrics indicating strong growth in minimally invasive gastrointestinal procedures and an aging population. The market is experiencing significant expansion due to increasing prevalence of gastrointestinal diseases, technological advancements in stent design, and growing preference for minimally invasive procedures.
DOWNLOAD FREE SAMPLE
Market Trends:
The gastrointestinal stents market is witnessing a significant trend towards the adoption of self-expanding metal stents (SEMS). These stents are gaining popularity due to their ability to provide effective and long-lasting relief from various gastrointestinal obstructions. SEMS offer several advantages over traditional plastic stents, including better patency rates, reduced migration risk, and improved patient comfort. The versatility of SEMS in treating both malignant and benign strictures across different segments of the gastrointestinal tract has led to their increased use in clinical practice. This trend is further supported by ongoing innovations in stent materials and designs, which aim to enhance their performance and reduce complications.
Market Segmentation:
Colorectal stents dominate the market, driven by rising incidence of colorectal cancer and bowel obstructions. The colorectal stents segment currently holds the largest market share in the gastrointestinal stents market. This dominance is attributed to the high incidence of colorectal cancer and the increasing use of stents for managing acute malignant colonic obstruction. Colorectal stents offer an effective minimally invasive alternative to emergency surgery, reducing morbidity and improving patient outcomes.
Recent clinical data has shown that the use of colorectal stents as a bridge to surgery in patients with obstructing colorectal cancer can significantly reduce the need for emergency surgeries by up to 80%. The pharmaceutical and medical device industries have been major contributors to the growth of the colorectal stents segment.
Market Key Players:
Boston Scientific Corporation
Cook Medical
Olympus Corporation
ELLA-CS, s.r.o.
Merit Medical Systems, Inc.
Taewoong Medical Co., Ltd.
Contact Us:
Name: Hari Krishna
Email us: [email protected]
Website: https://aurorawaveintellects.com/
0 notes
Text
Laparoscopy Devices Market Analysis Report: Size, Share, and Trends Forecast for the Next Period

The Laparoscopy Devices Market Report for 2024 provides a comprehensive overview of the Laparoscopy Devices Market industry, presenting crucial data and insights into market dynamics, including growth drivers, challenges, and future potential. The report evaluates the Laparoscopy Devices Market Components, focusing on significant opportunities and trends that could shape the industry's trajectory. Key stakeholders such as CEOs, global managers, traders, and analysts will find value in the SWOT analysis, which assesses the competitive strengths, vulnerabilities, opportunities, and threats impacting market players.
According to Straits Research, the global Laparoscopy Devices Market market size was valued at USD 6.96 Billion in 2022. It is projected to reach from USD XX Billion in 2023 to USD 12.80 Billion by 2031, growing at a CAGR of 7% during the forecast period (2023–2031).
Get a Sample PDF/Excel of report starting from USD 995 :https://straitsresearch.com/report/laparoscopy-devices-market/request-sample
Top Key Players of Laparoscopy Devices Market :
B. Braun Melsungen AG
ConMed Corporation
Boston Scientific Corporation
Johnson and Johnson
Medtronic Plc. (Covidien)
Karl Storz SE and CO. KG
Richard Wolf GmbH
Olympus Corporation
Stryker Corporation
and more....
Key Insights from the Laparoscopy Devices Market Report
Market Size Overview: The report provides comprehensive estimates of the Laparoscopy Devices Marketsize, including value and sales volume, for the period.
Market Trends and Dynamics: An analysis of the key drivers, opportunities, challenges, and risks shaping the Laparoscopy Devices Market.
Global Economic and Regional Impact: Evaluation of the effects of global inflation and the Russia-Ukraine conflict on the Laparoscopy Devices Market.
Trade Flow Analysis: Detailed examination of import and export volumes of Laparoscopy Devices Marketacross major regions.
Industry Value Chain: Insight into the Laparoscopy Devices Marketvalue chain, covering raw materials, suppliers, manufacturing processes, distributors, and downstream customers.
Industry News, Policies, and Regulations: Coverage of the latest developments, policies, and regulations impacting the Laparoscopy Devices Market.
Regional Analysis for Laparoscopy Devices Market:
The regional analysis section of the report offers a thorough examination of the global Laparoscopy Devices Market market, detailing the sales growth of various regional and country-level markets. It includes precise volume analysis by country and market size analysis by region for both past and future periods. The report provides an in-depth evaluation of the growth trends and other factors impacting the Laparoscopy Devices Market market in key countries, such as the United States, Canada, Mexico, Germany, France, the United Kingdom, Russia, Italy, China, Japan, Korea, India, Southeast Asia, Australia, Brazil, and Saudi Arabia. Moreover, it explores the progress of significant regional markets, including North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
Laparoscopy Devices Market Segmentations:
By Product Type
Laparoscopes
Energy Devices
Insufflators
Robot-Assisted Surgery Systems
Suction or Irrigation Systems
Closure Devices
Hand Instruments
Access Devices
Accessories
Others
By Applications
General Surgery
Bariatric Surgery
Gynecological Surgery
Urological Surgery
Colorectal Surgery
Others
By End-User
Hospitals
Clinics
Others
Get Detail Market Segmentation :https://straitsresearch.com/report/laparoscopy-devices-market/segmentation
Unit Economics must be known by C-suite professionals:
Cost of Goods Sold (COGS): Includes material, labor, and overhead costs in manufacturing.
R&D Costs: Investment in innovation and compliance with regulations.
Engineering and Design Costs: Resources for design, prototyping, and meeting technical standards.
Production Costs: Specialized manufacturing and quality control expenses.
Supply Chain Costs: Managing procurement and logistics for specialized components.
Testing and Quality Assurance: Costs for ensuring product safety and reliability.
SG&A Costs: Marketing, sales, and administrative expenses.
Revenue per Unit: Income from contracts, services, and licensing.
Gross Margin: Revenue minus COGS, showing unit profitability.
Break-even Analysis: Units or contracts needed to cover total costs.
Customer Acquisition Cost (CAC): Costs to secure new contracts.
Lifetime Value (LTV): Total revenue from a customer over time.
Capital Expenditure (CapEx): Investments in facilities and technology.
Economies of Scale: Cost reductions in larger production runs.
Profit Margin: Final profit after all expenses.
Top Reasons to Choose This Report
Access to Comprehensive Insights: Gain access to extensive analysis, research, and data that are often challenging to gather independently. This report provides valuable information, saving you significant time and effort.
Support for Informed Decisions: Enhance your decision-making process with in-depth insights into market trends, consumer behavior, and key industry factors. This report is essential for strategic planning, including investments, product development, and marketing strategies.
Gain a Competitive Edge: Stay competitive by understanding market dynamics and competitor strategies. The report provides detailed insights into competitor performance and market trends, helping you craft effective business strategies.
Cost-Effective Research Solution: Save on research costs by investing in this report, which offers a detailed and comprehensive analysis of the market. This cost-effective option eliminates the need for extensive independent research.
COVID-19 Aftermath and Geopolitical Influences: Russia-Ukraine Conflict and Middle East Crisis
The report explores the multifaceted impact of COVID-19 on the Laparoscopy Devices Market market, covering both direct and indirect effects across global and local levels. It discusses market size, trends, and growth trajectories in the Laparoscopy Devices Market , classified by type, application, and customer sector. Additionally, it provides a detailed evaluation of market development components before and after the pandemic, supported by a PESTEL analysis to assess key influencers and barriers to market entry. We offer the flexibility to customize the report based on specific regions, applications, or any other statistical details. Our goal is to align our analysis with your specific needs, ensuring a more complete market study. The final report will also examine the impact of the Russia-Ukraine War on the Laparoscopy Devices Market market, assessing how these geopolitical events are influencing current market conditions and future opportunities.
This Report is available for purchase on :https://straitsresearch.com/buy-now/laparoscopy-devices-market
About Us:
Straits Research is a leading research and intelligence organization, specializing in research, analytics, and advisory services along with providing business insights & research reports.
Contact Us: email: [email protected] Address: 825 3rd Avenue, New York, NY, USA, 10022 Tel: +1 646 905 0080 (U.S.) +91 8087085354 (India) +44 203 695 0070 (U.K.)
#Laparoscopy Devices Market#Laparoscopy Devices Market Share#Laparoscopy Devices Market Size#Laparoscopy Devices Market Research#Laparoscopy Devices Industry#What is Laparoscopy Devices?
0 notes
Text
Fusion Biopsy Market worth $0.91 billion in 2029
The report “Fusion Biopsy Market by Route Type (Transrectal, Transperineal), Product (Equipment, Consumables), Application (Prostate Cancer), End User (Hospitals), and Region — Global Forecast to 2029”, is expected to grow from USD 0.65 billion in 2024 to 0.91 billion in 2029 at a CAGR of 7.1% . The increasing prevalence of prostate cancer and the growing geriatric population, the availability of reimbursements & investments by the public and private sectors, and the increasing prevalence of minimally invasive surgery contribute to the market growth. Furthermore, technological advancements in fusion biopsy devices offer growth opportunities for market players in this market.
Browse 191 market data Tables and 45 Figures spread through 196 Pages and in-depth TOC on “Fusion Biopsy Market by Route Type (Transrectal, Transperineal), Product (Equipment, Consumables), Application (Prostate Cancer), End User (Hospitals), and Region — Global Forecast to 2029” View detailed Table of Content here — https://www.marketsandmarkets.com/Market-Reports/fusion-biopsy-market-228439168.html
In 2023, the transrectal segment held the largest share of the fusion biopsy market, by route type segment.
Based on route type, the global fusion biopsy market is segmented into transrectal, transperineal, and other route types. In 2023, transrectal led the fusion biopsy market. Due to its extensive use in the diagnosis of prostate cancer, the transrectal approach accounts for the biggest portion of the fusion biopsy market. Because of its extensive knowledge among urologists, well-established clinical procedures, ease of access to the prostate, and quicker procedure duration, it is the recommended method for fusion biopsy.
“The prostate cancer segment is projected to witness the highest growth rate in the fusion biopsy market, in 2023, by application”
The market for fusion biopsy is divided into segments based on application, such as prostate cancer and others. Due to the rising incidence of prostate cancer worldwide and the growing need for precise diagnostic methods, the prostate cancer segment of the fusion biopsy market is projected to develop at the fastest rate. As prostate cancer remains a leading concern in men’s health, this segment will continue to drive the adoption of fusion biopsy systems worldwide.
Download PDF Brochure- https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=228439168
The Europe market the highest share of fusion biopsy market in 2023.
The global fusion biopsy market is segmented into six distinct regions: North America, Europe, Asia Pacific, Middle East & Africa, Latin America, and GCC Countries. The Europe holds the highest share of 33.7% of global fusion biopsy market in 2023. The high share of Europe market can be attributed to the growing geriatric population, extensive healthcare expenditure and supportive government investments support the fusion biopsy market in this region. High demand for diagnosis of prostate cancer along with supportive regulatory environment in Europe for new technologies has also driven the growth of the market.
Prominent players in the market include Koninklijke Philips N.V. (Netherlands), GE Healthcare (US), KOELIS (France), FUJIFILM Holdings Corporation (Japan), Eigen Health (US), Focal Healthcare (Canada), Esaote SPA (Italy), MTT GmbH (Germany), MedCom (Germany), UC-CARE Medical Systems (US), Biobot Surgical (Singapore), Canon Inc. (Japan), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China).
Recent Developments of Fusion Biopsy Market -FUJIFILM Healthcare announced, effective April 6th, 2023, the release of the new ARIETTA 65 IntuitiveFusion, an MRI-Ultrasound fusion system extending its engineering process to help further enhance efficiency during prostate biopsies. With improved precision, it will enhance prostate biopsy procedures in a seamless way. -September 2023: Koelis DeepHealth has announced a partnership that will see users of the Koelis Trinity 3D Ultrasound Platform have access to DeepHealth’s Prostate AI software for efficient interpretation of the prostate MRI and guidance of prostate fusion biopsies.
0 notes
Text
Industrial Snapshot of Skin Imaging Systems Market

The Skin Imaging Systems Market Report is a treasured source of insightful data for business strategists. It provides an in-depth assessment of numerous features of industries like market overview, present progress valuations, historical and future Studies, current trends, SWOT valuations, and clients operating in several regions. The study provides valuable information to magnify the understanding, scope, and segments of this report. The report covers a comprehensive analysis of Skin Imaging Systems Market segmentation and regional and country breakdowns. This research will offer a clear and exact idea about the whole industry to the readers to make beneficial decisions.
According to Straits Research, the global Skin Imaging Systems market size was valued at USD 163.99 million in 2023. It is projected to reach from USD 176.78 million in 2024 to USD 322.39 million by 2032, growing at a CAGR of 7.8% during the forecast period (2024–2032).
This study pinpoints noteworthy trends influencing the trajectory of the Gesture Recognition market's expansion. Within this recently issued report, crucial dynamics encompassing drivers, limitations, and prospects are underscored. These aspects hold relevance for well-established market entities as well as emerging stakeholders engaged in the realms of production and supply.
Request a Sample Report @ https://straitsresearch.com/report/global-skin-imaging-systems-market/request-sample
Competitive Analysis
The report contains an in-depth analysis of the vendor’s profile, including financial health, business units, key business priorities, SWOT, strategies, and views.
Emage Medical LLC
Courage + Khazaka electronic GmbH
Atys Medical
Longport Inc.
Cortex Technology
Canfield Scientific Inc.
tpm – taberna pro medicum
MetaOptima Technology Inc.
DermSpectra.
The vendors have been identified based on the portfolio, geographical presence, marketing & distribution channels, revenue generation, and significant R&D investments.
Request Sample Report of Global Skin Imaging Systems Market @ https://straitsresearch.com/report/global-skin-imaging-systems-market/request-sample
Vendors across different verticals are planning for high investments in this market, and as a result, the market is expected to grow at an impressive rate in the upcoming years. The key players are adopting various organic and inorganic growth strategies such as mergers & acquisitions, collaboration & partnerships, joint ventures, and a few other strategies to be in a strong position in the global market.
Market Segmentation Analysis
The report provides a wide-ranging evaluation of the market, providing in-depth qualitative insights, historical data, and supportable projections along with the assumptions about the Skin Imaging Systems Market size. The projections featured in the report have been derived using proven research methodologies and assumptions based on the vendor’s portfolio, blogs, white papers, and vendor presentations. Thus, the research report represents every side of the Skin Imaging Systems Market and is segmented on the basis of regional markets, offerings, applications, and end-users.
By Type
Ultrasound
Optical
By Applications
Pigmented Lesions
Psoriasis
Skin Cancer
Plastic and Reconstructive Surgery
Other Applications
By End-User
Hospital
Specialty Clinics
Skin Rejuvenation Centers
Telemedicine Centers
Others
By Sales Channel
Direct
Distribution Channel
Access Detailed Segmentation @ https://straitsresearch.com/report/global-skin-imaging-systems-market/segmentation
Regional Analysis
North America held the largest Skin Imaging Systems Market share in 2018 and is expected to dominate the market during the forecast period. The market will experience a steep rise in the following regions covered- North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
0 notes
Text
The Role of Cardiac Output Monitoring Devices in Modern Healthcare
Cardiac Output Monitoring Devices are essential in healthcare for assessing and monitoring the heart's performance by measuring the amount of blood pumped by the heart per minute. These devices are particularly useful in intensive care units, during surgeries, and in emergency situations, providing real-time data that helps medical professionals make informed decisions about patient care. By monitoring cardiac output, these devices assist in diagnosing, managing, and treating conditions such as heart failure, shock, and other critical illnesses.
The Cardiac Output Monitoring Device Market Size was projected to reach 2.73 billion USD in 2022, according to MRFR analysis. It is anticipated that the market for cardiac output monitoring devices would increase from 2.85 billion USD in 2023 to 4.2 billion USD in 2032. During the projected period (2024-2032), the cardiac output monitoring device market is anticipated to increase at a CAGR of approximately 4.4%.
Size and Market Share of Cardiac Output Monitoring Device
The Cardiac Output Monitoring Device market has experienced steady growth over recent years due to an increasing prevalence of cardiovascular diseases, advances in healthcare technology, and the rising demand for minimally invasive monitoring techniques. The market size for these devices is significant and is projected to continue growing as healthcare providers worldwide emphasize enhanced patient monitoring and outcome optimization. The market share is currently dominated by a few major players, with new competitors entering as demand increases, particularly in regions like North America, Europe, and Asia-Pacific. This growth trajectory is supported by rising healthcare expenditures and the integration of artificial intelligence and machine learning for more precise data analysis in cardiac output monitoring.
Cardiac Output Monitoring Device Analysis
An analysis of the Cardiac Output Monitoring Device market reveals a diverse range of device types, including invasive, minimally invasive, and non-invasive technologies. Invasive devices, such as pulmonary artery catheters, have long been the standard, providing accurate measurements directly from the heart. However, minimally invasive and non-invasive devices, like ultrasound-based Doppler devices, have become increasingly popular due to lower associated risks and improved patient comfort. Key players in the industry are focused on enhancing device accuracy, reducing invasiveness, and ensuring data reliability, which is essential for clinical decision-making. This market analysis also shows that hospitals and diagnostic centers are the primary end-users, with increasing demand from ambulatory care centers and outpatient facilities as cardiac output monitoring technology becomes more portable and user-friendly.
Cardiac Output Monitoring Device Trends
The Cardiac Output Monitoring Device market is influenced by several key trends, including technological advancements, patient preference for non-invasive procedures, and the rise in chronic diseases such as hypertension and diabetes that impact cardiovascular health. Recent developments in digital health technology, such as wearable monitoring devices, are expected to revolutionize the market, offering a convenient option for continuous monitoring without hospital admission. Another significant trend is the integration of artificial intelligence, which allows for more precise data analysis, helping healthcare providers predict outcomes and improve patient management. Moreover, manufacturers are focusing on creating compact, portable devices that can be used in diverse healthcare settings, allowing for more widespread and accessible monitoring solutions.
Reasons to Buy Cardiac Output Monitoring Device Market Reports
Comprehensive Market Insights: Reports offer a detailed understanding of the global Cardiac Output Monitoring Device market, including size, share, trends, and growth projections.
Competitive Analysis: Gain valuable insights into the competitive landscape, identifying key players, recent developments, and potential areas for business expansion.
Technology and Innovation Insights: Stay updated on emerging trends in cardiac output monitoring technology, such as AI integration and portable device innovations.
Investment Opportunities: Understand investment potentials by analyzing market segments, geographical distribution, and demand across various healthcare settings.
Informed Decision Making: Access data-driven insights that help stakeholders make informed decisions regarding product development, marketing strategies, and partnerships.
Recent Developments in Cardiac Output Monitoring Devices
The Cardiac Output Monitoring Device market has witnessed recent advancements aimed at improving accuracy, ease of use, and patient outcomes. Leading companies are incorporating AI algorithms to enhance predictive capabilities, allowing for early detection of potential complications. Non-invasive cardiac output monitoring technologies have also evolved, with innovations such as wearable sensors that provide continuous data with minimal discomfort. Additionally, recent developments in wireless connectivity have enabled remote monitoring, allowing healthcare providers to track patient status from anywhere. With increasing demand, manufacturers are focusing on creating affordable and accessible devices to meet global healthcare needs, especially in low-resource settings. These advancements are set to further propel the growth of the Cardiac Output Monitoring Device market, providing healthcare systems with more effective tools to manage and monitor cardiovascular health.
Related reports:
algae supplements market
automated endoscopy reprocessor market
bacteriological testing market
Top of Form
Bottom of Form
0 notes
Text
Precision Medicine Market - Forecast(2024 - 2030)
Precision Medicine Market Overview
Precision Medicine Market size is $113.76Bn in 2019, growing at a CAGR of 13.2% during the forecast period 2020–2025. Precision medicine is also called personalized medicine or individualized medicine is an approach that protects health and treat diseases taking into account an individual variability in genes, environment and lifestyle for every individual. It includes the use of system biology to determine the reason for an individual patient’s illness at the molecular diagnosis level. It uses advanced technologies in clinical and basic research to develop therapeutics that selectively target panomic analysis and kill cancer cells. It allows doctors and researchers to predict more accurately which treatment and prevention strategies should be adopted for a particular disease or condition.
Sample Request:
Report Coverage

By Indication: Respiratory Disorders, Oncology, Immunology, Central Nervous System (CNS), Infectious Diseases and Others. By Technology: Drug Discovery, Gene Sequencing, Bioinformatics, Big Data Analysis and Others. By Drugs Type: Mepolizumab, Alectinib, Aripiprazole Lauroxil and Others. By End User: Hospitals/Clinics, Pharmaceuticals, Diagnostic Centers and Others By Geography: North America, Europe, Asia-Pacific and Rest of the World
Key Takeaways
Increasing awareness amongst people for early treatment of disease is set to propel the growth of the market.
Increasing prevalence of cancer is the driving factors for the growth of Precision Medicine market.
Increased geriatric population with modernized routine disorders aiding growth towards the market.
Europe region is estimated to record the fastest growth rate during the forecast period 2020–2025.
Inquiry Before Buying:
By Indication — Segment Analysis
In 2019, Oncology segment dominated the Precision Medicine Market in terms of revenue is estimated to grow at a CAGR of 11.2%. Precision medicine helps in the treatment of cancer patients by including surgery, chemotherapy, radiation therapy and immunotherapy depending on the cancerous tumor cell size. Precision medicine gives the information about genetic changes of tumor in individuals which helps in deciding the treatment procedures. Mepolizumab is an effective medicine for breast and lung cancer abetting towards the market’s growth.
Geography — Segment Analysis
In 2019, the North America region dominated Precision Medicine Market in terms of revenue with a market share of 39% owing towards owing to the presence of established payers and an increase in the number of cancer patients in the region. This growth can be attributed towards the increasing research & development initiatives and government support for the improvement of the healthcare sector. U.S holds the biggest market for central nervous system treatment, followed by Canada in North America. The increasing awareness about the health and availability of new treatment methods drives the market in this region is key factors in the growth of the Precision Medicine market. Europe is estimated to record the fastest growth rate during the forecast period 2020–2025.
Drivers — Precision Medicine Market
Increasing In The Prevalence Of Cancer
According to World Health Organization (WHO), in 2018, 9.6 million people worldwide died of cancer. Cancer is said to be one of the leading causes of death globally. The increasing incidence of cancer has increased the need for cancer therapies is rising with the increasing number of cancer cases and deaths caused by genetic cancerous tumors. Government focusing on the drug development for the reduction of cancer cases is the other major factor driving growth. Increasing healthcare expenditure by various countries is also contributing to the market growth.
Schedule a Call :
Challenges — Precision Medicine Market
Cost and Time Associated with Development
High cost is associated with the development and manufacture of genomic precision drugs. The long period of research and development and also the clinical trials take long time. Technologies such as sequencing large amounts of DNA are expensive to carry out (although the cost of sequencing is decreasing quickly) hampering the market’s growth. Strict regulations and patent expiry of various drugs may act key restraining factors for the Precision Medicine Market.
Precision Medicine Industry Outlook
Product Launches was the key strategy of the players in the Precision Medicine Industry. Precision Medicine top 10 companies include Medtronic PLC, Pfizer Inc., Novartis AG, Qiagen NV, Teva Pharmaceuticals, AstraZeneca plc., Takeda Pharmaceutical Company Ltd., Merck& Co. Inc., Teijin Pharma Ltd. and Thermo Fisher Scientific Inc.
Buy Now :
Acquisitions/Product Launches
In January 2020, Merck& Co. Inc acquired ArQule, Inc. This acquisition helped the company in increasing the oncology product production.
In January 2019, Takeda Pharmaceutical Company Ltd acquired Shire plc. This acquisition helped the company in accelerating transformation journey to deliver highly-innovative medicines to patients around the world with expanded scale and geographical footprint.
The precision medicine market is rapidly evolving, driven by advancements in genomics, biotechnology, and data analytics. This innovative approach to healthcare tailors medical treatment to individual characteristics, such as genetic makeup, lifestyle, and environmental factors, rather than a one-size-fits-all model. As the demand for personalized healthcare solutions grows, investments in research and development have surged, leading to the creation of targeted therapies, biomarkers, and companion diagnostics. Major pharmaceutical and biotech companies are increasingly collaborating with academic institutions and research organizations to accelerate the discovery and development of precision medicine therapies, contributing to the market’s growth.
Additionally, the increasing prevalence of chronic diseases, along with rising healthcare costs, is propelling the adoption of precision medicine. Patients are seeking more effective and efficient treatment options, which has resulted in a shift towards value-based care models. Regulatory bodies are also playing a crucial role by providing frameworks for the approval of personalized therapies and diagnostics. As the precision medicine market continues to expand, it presents opportunities for improved patient outcomes, reduced healthcare costs, and enhanced drug efficacy, positioning itself as a key component of the future of healthcare.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Innovations in Ultrasound and Electrosurgical Energy Dissectors Market: Shaping the Future of Minimally Invasive Surgery - UnivDatos
According to a new report published by UnivDatos Markets Insights, the Ultrasound And Electrosurgical Energy Dissectors Market was valued at USD 9 Billion in 2022 & is expected to grow at a CAGR of 8% from 2023-2030. The analysis has been segmented into Type (Ultrasound Devices and Electrosurgical Devices); End User (Hospitals, Ambulatory Surgical Centers, and Others); Region/Country.
The Ultrasound And Electrosurgical Energy Dissectors market report has been aggregated by collecting informative data on various dynamics such as market drivers, restraints, and opportunities. This innovative report makes use of several analyses to get a closer outlook on the opioid market. The Ultrasound And Electrosurgical Energy Dissectors market report offers a detailed analysis of the latest industry developments and trending market factors influencing market growth. Furthermore, this statistical market research repository examines and estimates the Ultrasound And Electrosurgical Energy Dissectors market at the global and regional levels.
Request To Download Sample of This Strategic Report - https://univdatos.com/get-a-free-sample-form-php/?product_id=51051&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Key Market Dynamics
The increasing demand for minimally invasive surgeries is one of the key drivers of the ultrasound and electrosurgical energy dissectors market. Minimally invasive surgeries offer several advantages over traditional open surgeries, such as smaller incisions, reduced blood loss, shorter hospital stays, and quicker recovery times. As a result, there is a growing preference among both patients and healthcare professionals for these less invasive procedures. The increasing adoption of minimally invasive surgeries across a wide range of medical specialties is driving the demand for ultrasound and electrosurgical energy dissectors. For instance, as per the American Society of Plastic Surgeons, about 23.67 Million Minimally-Invasive procedures were performed in America during the year 2022. These procedures are now being used in various fields, including gynecology, urology, general surgery, orthopedics, and cardiovascular surgery. The versatility of these devices and their ability to enhance surgical precision and patient outcomes are contributing to their growing popularity.
Ultrasound And Electrosurgical Energy Dissectors Market Geographical Segmentation Includes:
· North America (U.S., Canada, and the Rest of North America)
· Europe (Germany, UK, Spain, Italy, France, Rest of Europe)
· Asia-Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia-Pacific)
· Rest of the World
Asia-Pacific is expected to witness fast growth in the Ultrasound And Electrosurgical Energy Dissectors market. This is mainly due to the cost-effectiveness of the Ultrasound And Electrosurgical Energy Dissectors and the presence of the countries with emerging economies in the region.
Competitive Landscape
The degree of competition among prominent global companies has been elaborated by analyzing several leading key players operating worldwide. The specialist team of research analysts sheds light on various traits such as global market competition, market share, most recent industry advancements, innovative product launches, partnerships, mergers, or acquisitions by leading companies in the opioid market. The major players have been analyzed by using research methodologies such as Porter’s Five Forces Analysis for getting insight views on global competition.
Ask for Report Customization - https://univdatos.com/get-a-free-sample-form-php/?product_id=51051&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Key questions resolved through this analytical market research report include:
• What are the latest trends, new patterns, and technological advancements in the opioid market?
• Which factors are influencing the Ultrasound And Electrosurgical Energy Dissectors market over the forecast period?
• What are the global challenges, threats, and risks of the opioid market?
• Which factors are propelling and restraining the opioid market?
• What are the demanding global regions of the opioid market?
• What will be the global market size in the upcoming years?
• What are the crucial market acquisition strategies and policies applied by global companies?
• What are the descriptive profiles of key companies along with their SWOT analysis?
We understand the requirement of different businesses, regions, and countries, we offer customized reports as per your requirements of business nature and geography. Please let us know If you have any custom needs.
0 notes
Text
Streamlining Operating Rooms with Advanced Surgical Booms
The global surgical booms market size is estimated to reach USD 618.37 million by 2030, registering a CAGR of 5.21%, according to a new report by Grand View Research, Inc. One of the major factors that drives the market growth includes the increasing adoption of surgical booms throughout the healthcare infrastructure which aims to enhance functionality of operating rooms. Additionally, many hospitals and ambulatory surgery clinics are adopting contemporary equipment management systems. The rising number of surgical procedures performed around the world is creating lucrative growth for the market.
Surgical booms are typically utilized in healthcare facilities that require quick access to medical gases like oxygen, nitrogen, and carbon dioxide as well as electrical power and audio-visual data services. Booms are now vital for organizing and centralizing surgical equipment, keeping cords out of the way to lessen tripping hazards, and providing shelves. These factors would further drive the market growth.
Leading market players are introducing innovative and customizable products which are further boosting the demand for surgical booms. For instance, in Dec 2020, the Skytron LLC launched the freedom boom bringing new and improved qualities like no brakes or buttons to push. The International Society of Aesthetic Plastic Surgery (ISAPS) has revealed the findings of its annual Global Survey on Aesthetic/Cosmetic Procedures, which show that plastic surgeons would perform more than 12.8 million surgical procedures globally in 2021, a 19.3% rise over the previous year.
Surgical Booms Market Segment Highlights
Based on product type the anesthesia booms segment accounted for the largest market share of 52.12% in 2022 as these are frequently used in hospital settings or any hybrid operating room
Based on installation, the roof-mounted segment held the largest market share of 59.87% in 2022 and the growth is attributed to factors such as its convenience and does not require lots of space in the operation room
Based on end-use, the hospitals segment held the largest market share of 29.90% over the forecast period
North America dominated the market at 30.9% in 2022 owing to factors such as favorable reimbursement and government & non-government initiatives to boost research and innovation
Asia Pacific has been estimated to be the fastest growing region due to the large population, increasing healthcare awareness as global economic power shifts away from developed countries towards emerging ones
Browse through Grand View Research's Medical Devices Industry Research Reports.
The global hand-held surgical instruments market size was estimated at USD 5.8 billion in 2024 and is projected to grow at a CAGR of 7.2% from 2025 to 2030.
The global neurosurgical instruments market size was estimated at USD 1.76 billion in 2024 and is projected to grow at a CAGR of 7.00% from 2025 to 2030.
Segments Covered in the Report
This report forecasts revenue growth at global, regional, & country levels as well as provides an analysis on the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global surgical booms market report on the basis of product type, installation, end-use, and region.
Product Type Outlook (Revenue, USD Million, 2018 - 2030)
Equipment Boom
Anesthesia Boom
Other Boom
Installation Outlook (Revenue, USD Million, 2018 - 2030)
Floor Mounted
Roof Mounted
End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Ambulatory Surgical Centers
Dental Clinics
Others
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
Japan
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Order a free sample PDF of the Surgical Booms Market Intelligence Study, published by Grand View Research.
0 notes
Text
Bioresorbable Polymers Market: Advancements and Opportunities
The global bioresorbable polymers market was valued at USD 1.30 billion in 2022 and is projected to experience robust growth, with an expected compound annual growth rate (CAGR) of 13.8% from 2023 to 2030. This growth is primarily driven by several key factors, including a rising global awareness of health and wellness, improvements in healthcare infrastructure, and an increasing number of surgical procedures performed worldwide. According to data from the National Library of Medicine (NLM) in 2020, approximately 310 million major surgeries are conducted globally each year. Out of these, around 20 million surgeries are performed in Europe, while 40 to 50 million surgeries are conducted annually in the U.S. The increasing frequency of surgeries, coupled with a growing demand for advanced medical technologies, is expected to significantly boost the adoption of bioresorbable polymers in healthcare.
Bioresorbable polymers, also known as bioabsorbable polymers, belong to a class of materials that can be gradually broken down and absorbed by the human body over time, making them particularly valuable in medical applications. These polymers are widely used in various fields, with the medical sector being the most prominent. In medical applications, bioresorbable polymers are commonly found in a wide range of devices and implants, including sutures, screws, tissue scaffolds, plates, and stents. The key advantage of using these materials in such applications is that they naturally dissolve in the body, eliminating the need for a second surgery to remove them after the device has fulfilled its purpose.
In addition to their use in medical devices, bioresorbable polymers are also employed in drug delivery systems, such as nanoparticles or microspheres. These drug delivery systems are designed to release medications in a controlled manner over a specific period of time, after which the polymers dissolve safely in the body, removing the need for removal procedures. This controlled release mechanism can enhance the effectiveness of treatments and reduce the risks of side effects, contributing to better patient outcomes.
Gather more insights about the market drivers, restrains and growth of the Bioresorbable Polymers Market
Regional Insights
North America
North America was the dominant region in the global bioresorbable polymers market in 2022, accounting for the largest revenue share of 32.5%. This leading position is primarily due to the increasing demand for bioresorbable polymers in drug delivery and orthopedic applications across the region. The U.S. plays a critical role in driving market growth, serving as a major hub for research and development (R&D) in the healthcare industry. The nation’s strong R&D infrastructure in biotechnology and medical devices further supports the adoption and innovation of bioresorbable polymers. Additionally, the rising demand for orthopedic procedures and drug delivery systems contributes to the sustained growth in polymer consumption in these sectors.
For instance, data from the American Joint Replacement Registry (AJRR) in 2022 reported that more than 2.8 million knee and hip procedures were conducted across 1,250 healthcare institutions in the U.S. This represented a remarkable 14% increase in the total number of procedures compared to the previous year, underscoring the growing demand for medical procedures that benefit from bioresorbable polymers. The U.S. now hosts the world’s largest arthroplasty registry, which highlights the country’s leadership in orthopedic surgeries.
Moreover, the rising healthcare expenditure in the U.S. further fuels the market. According to the American Medical Association, U.S. healthcare spending increased by 2.7% in 2021, reaching USD 4.3 trillion, or USD 12,914 per capita. This substantial investment in healthcare infrastructure and services creates a favorable environment for the growth of bioresorbable polymers, especially in areas like orthopedic implants and drug delivery systems.
Asia Pacific (APAC)
Asia Pacific (APAC) is expected to experience the fastest growth in the bioresorbable polymers market, with a projected CAGR of 15.9% from 2023 to 2030. This rapid growth is driven by several key factors, including the growing geriatric population in countries such as China and Japan, which is increasing the demand for medical procedures. The rise in elderly populations, who often require surgeries, is expected to directly contribute to the need for bioresorbable materials in medical devices and treatments.
In addition to the aging demographic, economic growth in emerging countries within the region, such as India and China, is driving higher consumer disposable income, which is making the APAC market increasingly attractive for bioresorbable polymers. The expansion of healthcare infrastructure across APAC, including the rise in the number of hospitals, favorable reimbursement policies, and supportive government initiatives, further boosts the demand for these materials. Additionally, increased investment in healthcare, along with the growing need for cutting-edge surgery products and advanced medical technologies, positions APAC as a key region for the future growth of bioresorbable polymers.
From 2010 to 2018, countries in the APAC region saw an average annual real health expenditure growth rate of 6.4% per capita in GDP, as reported by the Organization for Economic Cooperation and Development (OECD). Notably, China, Turkmenistan, and Timor Leste experienced rapid growth in health expenditure per capita, which outpaced the growth of GDP per capita. Collaborations between countries like India and the U.S. to promote orthopedic research and education are also expected to boost the demand for bioresorbable polymers. For example, in March 2022, Shalby Advanced Technologies in Gujarat, India, collaborated with Gardner Orthopedics from the U.S. to establish Advanced Orthopedic Centers of Excellence in India and the U.S. These initiatives will likely drive the market for bioresorbable polymers in the APAC region, particularly in orthopedic applications.
Browse through Grand View Research's Plastics, Polymers & Resins Industry Research Reports.
• The global anhydrous hydrogen fluoride market was estimated at USD 3.33 billion in 2024 growing at a CAGR of 6.0% from 2024 to 2030.
• The global polymers & biopolymers in beauty & personal care products market size was estimated at USD 3.29 billion in 2024 and is expected to grow at a CAGR of 4.4% from 2025 to 2030.
Key Companies & Market Share Insights
To strengthen their market positions, companies in the bioresorbable polymers sector are rapidly expanding their production capabilities. With increasing governmental support focused on environmental sustainability and the growing demand for specialized orthopedic and drug delivery applications, industry players are focusing on consumer-specific product positioning strategies to gain deeper penetration in local markets.
For instance, in August 2021, Evonik Industries AG acquired JeNaCell, a German biotech company, to broaden its biomaterials portfolio within the healthcare industry. This acquisition allows Evonik’s Nutrition & Care division to expand its range of natural goods for the medical technology platform, enhancing its position in the bioresorbable polymers market. The expansion is part of Evonik’s broader strategy to transform its portfolio and develop system solutions that meet the growing needs of the healthcare sector.
Some of the major participants in the global bioresorbable polymers market include:
• Corbion NV
• Evonik Industries AG
• Poly-Med Inc.
• Foster Corp.
• Abbott
• KLS Martin Group
• 3D Biotek LLC.
• Sunstar Suisse S.A.
• DSM
• Futerro
Order a free sample PDF of the Bioresorbable Polymers Market Intelligence Study, published by Grand View Research.
#Bioresorbable Polymers Market#Bioresorbable Polymers Market Analysis#Bioresorbable Polymers Market Report#Bioresorbable Polymers Industry
0 notes
Text
Transcutaneous Oxygen Monitor Market Market Size, Share, and Comprehensive Industry Analysis 2024-2032

Transcutaneous Oxygen Monitor Market Insights
Reed Intelligence has recently added a new report to its vast depository titled Global Transcutaneous Oxygen Monitor Market. The report studies vital factors about the Global Transcutaneous Oxygen Monitor Market that are essential to be understood by existing as well as new market players. The report highlights the essential elements such as market share, profitability, production, sales, manufacturing, advertising, technological advancements, key market players, regional segmentation, and many more crucial aspects related to the Transcutaneous Oxygen Monitor Market.
Get Free Sample Report PDF @ https://reedintelligence.com/market-analysis/global-transcutaneous-oxygen-monitor-market/request-sample
Transcutaneous Oxygen Monitor Market Share by Key Players
Radiometer
Perimed AB
Philips
Sentec
Medicap
HumaresCompany seven
Important factors like strategic developments, government regulations, market analysis, end users, target audience, distribution network, branding, product portfolio, market share, threats and barriers, growth drivers, latest trends in the industry are also mentioned.
Transcutaneous Oxygen Monitor Market Segmentation
The report on Global Transcutaneous Oxygen Monitor Market provides detailed segmentation by type, applications, and regions. Each segment provides information about the production and manufacturing during the forecast period of 2024-2032. The application segment highlights the applications and operational processes of the industry. Understanding these segments will help identify the importance of the various factors aiding to the market growth.
The report is segmented as follows:
Segmentation by Type
Wound-healing Monitor
Baby Monitor
Segmentation by Application
Wound Healing
Blood Gas Monitoring
Diabetes
Plastic Surgery
Transcutaneous Oxygen Monitor Market Segmentation by Region
North America
U.S.
Canada
Europe
Germany
UK
France
Asia Pacific
China
India
Japan
Australia
South Korea
Latin America
Brazil
Middle East & Africa
UAE
Kingdom of Saudi Arabia
South Africa
Get Detailed Segmentation @ https://reedintelligence.com/market-analysis/global-transcutaneous-oxygen-monitor-market/segmentation
The market research report on the Global Transcutaneous Oxygen Monitor Market has been carefully curated after studying and observing various factors that determine the growth, such as environmental, economic, social, technological and political status of the regions mentioned. Thorough analysis of the data regarding revenue, production, and manufacturers gives out a clear picture of the global scenario of the Transcutaneous Oxygen Monitor Market. The data will also help key players and new entrants understand the potential of investments in the Global Transcutaneous Oxygen Monitor Market.
Key Highlights
It provides valuable insights into the Global Transcutaneous Oxygen Monitor Market.
Provides information for the years 2024-2032. Important factors related to the market are mentioned.
Technological advancements, government regulations, and recent developments are highlighted.
This report will study advertising and marketing strategies, market trends, and analysis.
Growth analysis and predictions until the year 2032.
Statistical analysis of the key players in the market is highlighted.
Extensively researched market overview.
Buy Transcutaneous Oxygen Monitor Market Research Report @ https://reedintelligence.com/market-analysis/global-transcutaneous-oxygen-monitor-market/buy-now
Contact Us:
Email: [email protected]
#Transcutaneous Oxygen Monitor Market Size#Transcutaneous Oxygen Monitor Market Share#Transcutaneous Oxygen Monitor Market Growth#Transcutaneous Oxygen Monitor Market Trends#Transcutaneous Oxygen Monitor Market Players
0 notes
Text
Precision Medicine Market — Forecast(2024–2030)
Precision Medicine Market Overview

Report Coverage
The report: “Precision Medicine Market — Forecast (2020–2025)”, by IndustryARC covers an in-depth analysis of the following segments of the Precision Medicine Market.
By Indication: Respiratory Disorders, Oncology, Immunology, Central Nervous System (CNS), Infectious Diseases and Others. By Technology: Drug Discovery, Gene Sequencing, Bioinformatics, Big Data Analysis and Others. By Drugs Type: Mepolizumab, Alectinib, Aripiprazole Lauroxil and Others. By End User: Hospitals/Clinics, Pharmaceuticals, Diagnostic Centers and Others By Geography: North America, Europe, Asia-Pacific and Rest of the World
RequestSample
Key Takeaways
Increasing awareness amongst people for early treatment of disease is set to propel the growth of the market.
Increasing prevalence of cancer is the driving factors for the growth of Precision Medicine market.
Increased geriatric population with modernized routine disorders aiding growth towards the market.
Europe region is estimated to record the fastest growth rate during the forecast period 2020–2025.
By Indication — Segment Analysis
In 2019, Oncology segment dominated the Precision Medicine Market in terms of revenue is estimated to grow at a CAGR of 11.2%. Precision medicine helps in the treatment of cancer patients by including surgery, chemotherapy, radiation therapy and immunotherapy depending on the cancerous tumor cell size. Precision medicine gives the information about genetic changes of tumor in individuals which helps in deciding the treatment procedures. Mepolizumab is an effective medicine for breast and lung cancer abetting towards the market’s growth.
Inquiry Before Buying
Geography — Segment Analysis
In 2019, the North America region dominated Precision Medicine Market in terms of revenue with a market share of 39% owing towards owing to the presence of established payers and an increase in the number of cancer patients in the region. This growth can be attributed towards the increasing research & development initiatives and government support for the improvement of the healthcare sector. U.S holds the biggest market for central nervous system treatment, followed by Canada in North America. The increasing awareness about the health and availability of new treatment methods drives the market in this region is key factors in the growth of the Precision Medicine market. Europe is estimated to record the fastest growth rate during the forecast period 2020–2025.
Drivers — Precision Medicine Market
Increasing In The Prevalence Of Cancer
According to World Health Organization (WHO), in 2018, 9.6 million people worldwide died of cancer. Cancer is said to be one of the leading causes of death globally. The increasing incidence of cancer has increased the need for cancer therapies is rising with the increasing number of cancer cases and deaths caused by genetic cancerous tumors. Government focusing on the drug development for the reduction of cancer cases is the other major factor driving growth. Increasing healthcare expenditure by various countries is also contributing to the market growth.
Schedule a Call
Challenges — Precision Medicine Market
Cost and Time Associated with Development
High cost is associated with the development and manufacture of genomic precision drugs. The long period of research and development and also the clinical trials take long time. Technologies such as sequencing large amounts of DNA are expensive to carry out (although the cost of sequencing is decreasing quickly) hampering the market’s growth. Strict regulations and patent expiry of various drugs may act key restraining factors for the Precision Medicine Market.
Buy Now
Precision Medicine Industry Outlook
Product Launches was the key strategy of the players in the Precision Medicine Industry. Precision Medicine top 10 companies include Medtronic PLC, Pfizer Inc., Novartis AG, Qiagen NV, Teva Pharmaceuticals, AstraZeneca plc., Takeda Pharmaceutical Company Ltd., Merck& Co. Inc., Teijin Pharma Ltd. and Thermo Fisher Scientific Inc.
Acquisitions/Product Launches
In January 2020, Merck& Co. Inc acquired ArQule, Inc. This acquisition helped the company in increasing the oncology product production.
In January 2019, Takeda Pharmaceutical Company Ltd acquired Shire plc. This acquisition helped the company in accelerating transformation journey to deliver highly-innovative medicines to patients around the world with expanded scale and geographical footprint.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Global Gelfoam Market 2024 Key Players, Analysis, Share, Trends And Forecast To 2034
The Gelfoam market report offered by Reports Intellect is meant to serve as a helpful means to evaluate the market together with an exhaustive scrutiny and crystal-clear statistics linked to this market. The report consists of the drivers and restraints of the Gelfoam Market accompanied by their impact on the demand over the forecast period. Additionally, the report includes the study of prospects available in the market on a global level.
With tables and figures helping evaluate the Global Gelfoam market, this research offers key statistics on the state of the industry and is a beneficial source of guidance and direction for companies and entities interested in the market. This report comes along with an additional Excel data-sheet suite taking quantitative data from all numeric forecasts offered in the study.
Get Sample PDF Brochure @ https://www.reportsintellect.com/sample-request/2911806
Key players offered in the market: Johnson & Johnson Gelita Pfizer Baxter Ferrosan Medical Devices B Braun Equimedical
Additionally, it takes account of the prominent players of the Gelfoam market with insights including market share, product specifications, key strategies, contact details, and company profiles. Similarly, the report involves the market computed CAGR of the market created on previous records regarding the market and existing market trends accompanied by future developments. It also divulges the future impact of enforcing regulations and policies on the expansion of the Gelfoam Market.
Scope and Segmentation of the Gelfoam Market
The estimates for all segments including type and application/end-user have been provided on a regional basis for the forecast period from 2024 to 2034. We have applied a mix of bottom-up and top-down methods for market estimation, analyzing the crucial regional markets, dynamics, and trends for numerous applications. Moreover, the fastest & slowest growing market segments are pointed out in the study to give out significant insights into each core element of the market.
Gelfoam Market Type Coverage: - Sponge Powder
Gelfoam Market Application Coverage: - Minimally Invasive Surgery General Surgery Others
Regional Analysis:
North America Country (United States, Canada) South America Asia Country (China, Japan, India, Korea) Europe Country (Germany, UK, France, Italy) Other Countries (Middle East, Africa, GCC)
Discount PDF Brochure @ https://www.reportsintellect.com/discount-request/2911806
The comprehensive report provides:
Complete assessment of all opportunities and threats in the global market.
Gelfoam Market recent advancements and major events.
A thorough study of business policies for the growth of the Gelfoam Market leading players.
Concluding study about the growth plot of Gelfoam Market for upcoming years.
Detailed understanding of Gelfoam Market particular drivers, restraints, and major micro markets.
Favorable impression inside vital technological and market latest trends hitting the Gelfoam Market.
Reasons to Purchase Gelfoam Market Research Report
Develop a competitive approach based on the competitive landscape
Build business strategy by identifying the high growth and attractive Gelfoam market classifications
Identify potential business partners, gaining targets and business buyers
Design financial investment policies based on estimated high potential segments
Prepare management and tactical presentations using the Gelfoam market data
Plan for new product promotion and portfolio in advance
Contact Us: [email protected] Phone No: + 1-706-996-2486 US Address: 225 Peachtree Street NE, Suite 400, Atlanta, GA 30303
#Gelfoam Market#Gelfoam Market trends#Gelfoam Market future#Gelfoam Market size#Gelfoam Market growth#Gelfoam Market forecast#Gelfoam Market analysis
0 notes