#Eukaryotic translation initiation factor 4E
Explore tagged Tumblr posts
Text
Inhibition of EIF4E Downregulates VEGFA and CCND1 Expression to Suppress Ovarian Cancer Tumor Progression by Jing Wang in Journal of Clinical Case Reports Medical Images and Health Sciences
Abstract
This study investigates the role of EIF4E in ovarian cancer and its influence on the expression of VEGFA and CCND1. Differential expression analysis of VEGFA, CCND1, and EIF4E was conducted using SKOV3 cells in ovarian cancer patients and controls. Correlations between EIF4E and VEGFA/CCND1 were assessed, and three-dimensional cell culture experiments were performed. Comparisons of EIF4E, VEGFA, and CCND1 mRNA and protein expression between the EIF4E inhibitor 4EGI-1-treated group and controls were carried out through RT-PCR and Western blot. Our findings demonstrate elevated expression of EIF4E, VEGFA, and CCND1 in ovarian cancer patients, with positive correlations. The inhibition of EIF4E by 4EGI-1 led to decreased SKOV3 cell clustering and reduced mRNA and protein levels of VEGFA and CCND1. These results suggest that EIF4E plays a crucial role in ovarian cancer and its inhibition may modulate VEGFA and CCND1 expression, underscoring EIF4E as a potential therapeutic target for ovarian cancer treatment.
Keywords: Ovarian cancer; Eukaryotic translation initiation factor 4E; Vascular endothelial growth factor A; Cyclin D1
Introduction
Ovarian cancer ranks high among gynecological malignancies in terms of mortality, necessitating innovative therapeutic strategies [1]. Vascular endothelial growth factor (VEGF) plays a pivotal role in angiogenesis, influencing endothelial cell proliferation, migration, vascular permeability, and apoptosis regulation [2, 3]. While anti-VEGF therapies are prominent in malignancy treatment [4], the significance of cyclin D1 (CCND1) amplification in cancers, including ovarian, cannot be overlooked, as it disrupts the cell cycle, fostering tumorigenesis [5, 6]. Eukaryotic translation initiation factor 4E (EIF4E), central to translation initiation, correlates with poor prognoses in various cancers due to its dysregulated expression and activation, particularly in driving translation of growth-promoting genes like VEGF [7, 8]. Remarkably, elevated EIF4E protein levels have been observed in ovarian cancer tissue, suggesting a potential role in enhancing CCND1 translation, thereby facilitating cell cycle progression and proliferation [9]. Hence, a novel conjecture emerges: by modulating EIF4E expression, a dual impact on VEGF and CCND1 expression might be achieved. This approach introduces an innovative perspective to impede the onset and progression of ovarian cancer, distinct from existing literature, and potentially offering a unique therapeutic avenue.
Materials and Methods
Cell Culture
Human ovarian serous carcinoma cell line SKOV3 (obtained from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) was cultured in DMEM medium containing 10% fetal bovine serum. Cells were maintained at 37°C with 5% CO2 in a cell culture incubator and subcultured every 2-3 days.
Three-Dimensional Spheroid Culture
SKOV3 cells were prepared as single-cell suspensions and adjusted to a concentration of 5×10^5 cells/mL. A volume of 0.5 mL of single-cell suspension was added to Corning Ultra-Low Attachment 24-well microplates and cultured at 37°C with 5% CO2 for 24 hours. Subsequently, 0.5 mL of culture medium or 0.5 mL of EIF4E inhibitor 4EGI-1 (Selleck, 40 μM) was added. After 48 hours, images were captured randomly from five different fields—upper, lower, left, right, and center—using an inverted phase-contrast microscope. The experiment was repeated three times.
GEPIA Online Analysis
The GEPIA online analysis tool (http://gepia.cancer-pku.cn/index.html) was utilized to assess the expression of VEGFA, CCND1, and EIF4E in ovarian cancer tumor samples from TCGA and normal samples from GTEx. Additionally, Pearson correlation coefficient analysis was employed to determine the correlation between VEGF and CCND1 with EIF4E.
RT-PCR
RT-PCR was employed to assess the mRNA expression levels of EIF4E, VEGF, and CCND1 in treatment and control group samples. Total RNA was extracted using the RNA extraction kit from Vazyme, followed by reverse transcription to obtain cDNA using their reverse transcription kit. Amplification was carried out using SYBR qPCR Master Mix as per the recommended conditions from Vazyme. GAPDH was used as an internal reference, and the primer sequences for PCR are shown in Table 1.
Amplification was carried out under the following conditions: an initial denaturation step at 95°C for 60 seconds, followed by cycling conditions of denaturation at 95°C for 10 seconds, annealing at 60°C for 30 seconds, repeated for a total of 40 cycles. Melting curves were determined under the corresponding conditions. Each sample was subjected to triplicate experiments. The reference gene GAPDH was used for normalization. The relative expression levels of the target genes were calculated using the 2-ΔΔCt method.
Western Blot
Western Blot technique was employed to assess the protein expression levels of EIF4E, VEGF, and CCND1 in the treatment and control groups. Initially, cell samples collected using RIPA lysis buffer were lysed, and the total protein concentration was determined using the BCA assay kit (Shanghai Biyuntian Biotechnology, Product No.: P0012S). Based on the detected concentration, 20 μg of total protein was loaded per well. Electrophoresis was carried out using 5% stacking gel and 10% separating gel. Subsequently, the following primary antibodies were used for immune reactions: rabbit anti-human polyclonal antibody against phospho-EIF4E (Beijing Boao Sen Biotechnology, Product No.: bs-2446R, dilution 1:1000), mouse anti-human monoclonal antibody against EIF4E (Wuhan Sanying Biotechnology, Product No.: 66655-1-Ig, dilution 1:5000), mouse anti-human monoclonal antibody against VEGFA (Wuhan Sanying Biotechnology, Product No.: 66828-1-Ig, dilution 1:1000), mouse anti-human monoclonal antibody against CCND1 (Wuhan Sanying Biotechnology, Product No.: 60186-1-Ig, dilution 1:5000), and mouse anti-human monoclonal antibody against GAPDH (Shanghai Biyuntian Biotechnology, Product No.: AF0006, dilution 1:1000). Subsequently, secondary antibodies conjugated with horseradish peroxidase (Shanghai Biyuntian Biotechnology, Product No.: A0216, dilution 1:1000) were used for immune reactions. Finally, super-sensitive ECL chemiluminescence reagent (Shanghai Biyuntian Biotechnology, Product No.: P0018S) was employed for visualization, and the ChemiDocTM Imaging System (Bio-Rad Laboratories, USA) was used for image analysis.
Statistical Analysis
GraphPad software was used for statistical analysis. Data were presented as (x ± s) and analyzed using the t-test for quantitative data. Pearson correlation analysis was performed for assessing correlations. A significance level of P < 0.05 was considered statistically significant.
Results
3D Cell Culture of SKOV3 Cells and Inhibitory Effect of 4EGI-1 on Aggregation
In this experiment, SKOV3 cells were subjected to 3D cell culture, and the impact of the EIF4E inhibitor 4EGI-1 on ovarian cancer cell aggregation was investigated. As depicted in Figure 1, compared to the control group (Figure 1A), the diameter of the SKOV3 cell spheres significantly decreased in the treatment group (Figure 1B) when exposed to 4EGI-1 under identical culture conditions. This observation indicates that inhibiting EIF4E expression effectively suppresses tumor aggregation.
Expression and Correlation Analysis of VEGFA, CCND1, and EIF4E in Ovarian Cancer Samples
To investigate the expression of VEGFA, CCND1, and EIF4E in ovarian cancer, we utilized the GEPIA online analysis tool and employed the Pearson correlation analysis method to compare expression differences between tumor and normal groups. As depicted in Figures 2A-C, the results indicate significantly elevated expression levels of VEGFA, CCND1, and EIF4E in the tumor group compared to the normal control group. Notably, the expression differences of VEGFA and CCND1 were statistically significant (p < 0.05). Furthermore, the correlation analysis revealed a positive correlation between VEGFA and CCND1 with EIF4E (Figures 2D-E), and this correlation exhibited significant statistical differences (p < 0.001). These findings suggest a potential pivotal role of VEGFA, CCND1, and EIF4E in the initiation and progression of ovarian cancer, indicating the presence of intricate interrelationships among them.
EIF4E, VEGFA, and CCND1 mRNA Expression in SKOV3 Cells
To investigate the function of EIF4E in SKOV3 cells, we conducted RT-PCR experiments comparing EIF4E inhibition group with the control group. As illustrated in Figure 3, treatment with 4EGI-1 significantly reduced EIF4E expression (0.58±0.09 vs. control, p < 0.01). Concurrently, mRNA expression of VEGFA (0.76±0.15 vs. control, p < 0.05) and CCND1 (0.81±0.11 vs. control, p < 0.05) also displayed a substantial decrease. These findings underscore the significant impact of EIF4E inhibition on the expression of VEGFA and CCND1, indicating statistically significant differences.
Protein Expression Profiles in SKOV3 Cells with EIF4E Inhibition and Control Group
Protein expression of EIF4E, VEGFA, and CCND1 was assessed using Western Blot in the 4EGI-1 treatment group and the control group. As presented in Figure 4, the expression of p-EIF4E was significantly lower in the 4EGI-1 treatment group compared to the control group (0.33±0.14 vs. control, p < 0.001). Simultaneously, the expression of VEGFA (0.53±0.18 vs. control, p < 0.01) and CCND1 (0.44±0.16 vs. control, p < 0.001) in the 4EGI-1 treatment group exhibited a marked reduction compared to the control group.
Discussion
EIF4E is a post-transcriptional modification factor that plays a pivotal role in protein synthesis. Recent studies have underscored its critical involvement in various cancers [10]. In the context of ovarian cancer research, elevated EIF4E expression has been observed in late-stage ovarian cancer tissues, with low EIF4E expression correlating to higher survival rates [9]. Suppression of EIF4E expression or function has been shown to inhibit ovarian cancer cell proliferation, invasion, and promote apoptosis. Various compounds and drugs that inhibit EIF4E have been identified, rendering them potential candidates for ovarian cancer treatment [11]. Based on the progressing understanding of EIF4E's role in ovarian cancer, inhibiting EIF4E has emerged as a novel therapeutic avenue for the disease. 4EGI-1, a cap-dependent translation small molecule inhibitor, has been suggested to disrupt the formation of the eIF4E complex [12]. In this study, our analysis of public databases revealed elevated EIF4E expression in ovarian cancer patients compared to normal controls. Furthermore, through treatment with 4EGI-1 in the SKOV3 ovarian cancer cell line, we observed a capacity for 4EGI-1 to inhibit SKOV3 cell spheroid formation. Concurrently, results from PCR and Western Blot analyses demonstrated effective EIF4E inhibition by 4EGI-1. Collectively, 4EGI-1 effectively suppresses EIF4E expression and may exert its effects on ovarian cancer therapy by modulating EIF4E.
Vascular Endothelial Growth Factor (VEGF) is a protein that stimulates angiogenesis and increases vascular permeability, playing a crucial role in tumor growth and metastasis [13]. In ovarian cancer, excessive release of VEGF by tumor cells leads to increased angiogenesis, forming a new vascular network to provide nutrients and oxygen to tumor cells. The formation of new blood vessels enables tumor growth, proliferation, and facilitates tumor cell dissemination into the bloodstream, contributing to distant metastasis [14]. As a significant member of the VEGF family, VEGFA has been extensively studied, and it has been reported that VEGFA expression is notably higher in ovarian cancer tumors [15], consistent with our public database analysis. Furthermore, elevated EIF4E levels have been associated with increased malignant tumor VEGF mRNA translation [16]. Through the use of the EIF4E inhibitor 4EGI-1 in ovarian cancer cell lines, we observed a downregulation in both mRNA and protein expression levels of VEGFA. This suggests that EIF4E inhibition might affect ovarian cancer cell angiogenesis capability through downregulation of VEGF expression.
Cyclin D1 (CCND1) is a cell cycle regulatory protein that participates in controlling cell entry into the S phase and the cell division process. In ovarian cancer, overexpression of CCND1 is associated with increased tumor proliferation activity and poor prognosis [17]. Elevated CCND1 levels promote cell cycle progression, leading to uncontrolled cell proliferation [18]. Additionally, CCND1 can activate cell cycle-related signaling pathways, promoting cancer cell growth and invasion capabilities [19]. Studies have shown that CCND1 gene expression is significantly higher in ovarian cancer tissues compared to normal ovarian tissues [20], potentially promoting proliferation and cell cycle progression through enhanced cyclin D1 translation [9]. Our public database analysis results confirm these observations. Furthermore, treatment with the EIF4E inhibitor 4EGI-1 in ovarian cancer cell lines resulted in varying degrees of downregulation in CCND1 mRNA and protein levels. This indicates that EIF4E inhibition might affect ovarian cancer cell proliferation and cell cycle progression through regulation of CCND1 expression.
In conclusion, overexpression of EIF4E appears to be closely associated with the clinical and pathological characteristics of ovarian cancer patients. In various tumors, EIF4E is significantly correlated with VEGF and cyclin D1, suggesting its role in the regulation of protein translation related to angiogenesis and growth [9, 21]. The correlation analysis results in our study further confirmed the positive correlation among EIF4E, VEGFA, and CCND1 in ovarian cancer. Simultaneous inhibition of EIF4E also led to downregulation of VEGFA and CCND1 expression, validating their interconnectedness. Thus, targeted therapy against EIF4E may prove to be an effective strategy for treating ovarian cancer. However, further research and clinical trials are necessary to assess the safety and efficacy of targeted EIF4E therapy, offering more effective treatment options for ovarian cancer patients.
Acknowledgments:
Funding: This study was supported by the Joint Project of Southwest Medical University and the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (Grant No. 2020XYLH-043).
Conflict of Interest: The authors declare no conflicts of interest.
#Ovarian cancer#Eukaryotic translation initiation factor 4E#Vascular endothelial growth factor A#Cyclin D1#Review Article in Journal of Clinical Case Reports Medical Images and Health Sciences .#jcrmhs
2 notes
·
View notes
Text
4EBP2 Antibody
4EBP2 Antibody Catalog number: B2020487 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 25 ug Molecular Weight or Concentration: N/A Supplied as: Liquid Applications: molecular tool for various biochemical applications Storage: 2-8°C Keywords: Eukaryotic translation initiation factor 4E-binding protein 2, eIF4E-binding protein 2 Grade: Biotechnology grade. All products are…
0 notes
Text
Modeling the Structure and DAP5 Binding Site of the FGF-9 5 UTR #RNA Utilized in Cap-Independent Translation [Article]
Cap-independent, or eukaryotic initiation factor (eIF) 4E-independent, translation initiation in eukaryotes requires scaffolding protein eIF4G or its homolog, death-associated protein 5 (DAP5). eIF4G associates with the 40S ribosomal subunit, recruiting the ribosome to the RNA transcript. A subset of RNA transcripts, such as fibroblast growth factor 9 (FGF-9), contain 5’ untranslated regions (5’ UTRs) that directly bind DAP5 or eIF4GI. Internal-ribosome-entry-site (IRES)-like cap-independent translation initiation does not require an unpaired 5’ end for eIF binding, as these eIFs recruit the 40S ribosome at or near the start codon. For viral mRNA, eIF recruitment usually utilizes RNA structure, such as a pseudoknot or stem loops, and the RNA helicase eIF4A is required for DAP5- or 4G-mediated translation, suggesting these 5’ UTRs are structured. However, for cellular IRES-like translation, no consensus RNA structures or sequences have yet been identified for eIF binding. FGF-9 is a member of a subset of mRNAs that are cap-independently upregulated in breast and colorectal #cancer cells, likely using an IRES-like mechanism. However, the DAP5 binding site within the FGF-9 5’ UTR is unknown. Moreover, DAP5 binds to other, dissimilar 5’ UTRs, some of which require proximity to an unpaired, accessible 5’ end to stimulate cap-independent translation. Using SHAPE-seq, we modeled the 186-nt FGF-9 5’ UTR RNA’s complex secondary structure in vitro. Further, DAP5 footprinting, toeprinting, and UV-crosslinking experiments identify DAP5-RNA interactions. Modeling of FGF-9 5’ UTR tertiary structure aligns DAP5-interacting nucleotides on one face of the predicted structure. We propose that RNA structure involving tertiary folding, rather than a conserved sequence or secondary structure, acts as a DAP5 binding site. DAP5 appears to contact nucleotides near the start codon. Our findings offer a new perspective in the hunt for cap-independent translational enhancers. Structural, rather than sequence-specific, eIF binding sites may act as attractive chemotherapeutic targets or as dosage tools for mRNA-based therapies. http://rnajournal.cshlp.org/cgi/content/short/rna.080013.124v1?rss=1&utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
PROTEIN METABOLISM IN CANCER CACHEXIA
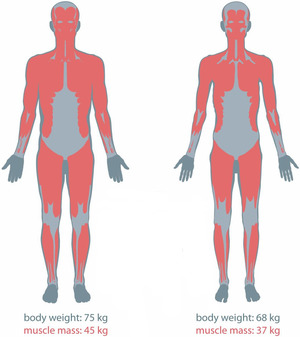
There are metabolic abnormalities seen in individuals with cancer cachexia that arise from malnutrition and malignancy. Protein turnover is evident and high in cancer cachectic patients. Muscle protein synthesis is suppressed during their initial stages of weight loss and is further reduced as their condition progresses. Moreover, protein degradation activation and disrupted oxidative metabolism in skeletal muscles also continue to develop. Thus, protein and amino acid metabolism is clearly affected. These results to the progressive loss of muscle mass, which is the most prominent feature of cancer cachexia (Dodesini et al, 2009). Muscle depletion also reduces chemotherapy effectiveness, increases susceptibility to treatment toxicity, decreases physical function, and impairs psychosocial ability.
The loss of skeletal muscle in cancer patients are due to anorexia and early satiety, reduced muscle protein synthesis, and increased muscle protein breakdown. Inflammation has also negatively contributed to these mechanisms. Inflammatory cytokines such as IL-6 and TNFα contribute to the effects of inflammation on muscle protein metabolism through several pathways.
Impaired muscle protein synthesis
Cancer cachexia disrupts protein translation at several regulatory steps such as initiation, elongation, and termination. The phosphorylation of eIF2��, which impairs translation initiation and overall protein synthesis, is elevated (Hardee, Montalvo, & Carson, 2017). Anabolic and catabolic hormones and energy balance influence signal transduction pathways that regulate protein synthesis via mTOR-related signal transduction pathways. This pathway is the most predominant signaling regulator of translation initiation and are activated by insulin and IGF-1 (Blackwell, 2017). It is also major mediator of anabolic responses in skeletal muscle. Specifically, mTOR activation by insulin or amino acids activates the downstream targets eukaryotic initiation factor binding protein 4E and ribosomal S6 kinase 1, which promote translation initiation, and eukaryotic elongation factor, which stimulates elongation. This pathway is inhibited in cancer cachexia, which corresponds to disrupted S6K1 and 4EBP-1 regulation. A decrease in mTOR activation is also due to AMPK and systemic inflammation. AMPK inhibits mTOR by preventing the interaction with both p70S6K and 4E-BP1 (Durham, Dillon, & Sheffield-Moore, 2009).
Glutamine is the most abundant amino acid in the body. However, glutamine depletion is observed in Cancer Cachexia since glutamine is a regulator of muscle protein synthesis and this is inhibited in the condition. Furthermore, glutamine is a principal fuel for most rapidly proliferating cancers. Tumor cells are major glutamine consumers and they compete with the host for circulating glutamine. Thus, there are changes in glutamine metabolism resulting in glutamine depletion developing with progressive tumor growth (Bode et al, 1996).
Stimulated muscle protein breakdown
There are three main degradation pathways in the skeletal muscle to account for protein degradation. These are ubiquitin-mediated proteasome degradation (UPR), autophagy, and calcium-activated protease calpains.
The over activation of proteolysis is the primary pathway of protein degradation through muscle wasting in Cancer Cachexia (Blackwell, 2017). Muscle protein degradation in cachexia is mainly mediated by the ubiquitin proteasome system which is induced through the activation of E3 ligases, MAFbx, and MurF-1. The FOXO signaling pathway also contributes through the induction of the transcription of these ubiquitin ligases. Thus, the inhibition of FOXO transcription activity prevents muscle fiber atrophy during cachexia (Camargo, 2015). Many of these effects occur through activation of NF-κB by upstream factors such as TNF-α and PIF (Durham, Dillon, & Sheffield-Moore, 2009). During Cachexia, skeletal muscle specifically upregulates muscle specific UPR system, in particular by promoting ubiquitin-ligase MurF1 and Atrogin-1 expression. UPR upregulation is shown by Atrogin-1 messenger RNA and increased protein ubiquitylation. The expression of this ubiquitin ligase is mainly regulated by the transcription factor FoxO3a (FOXO) (Porporato, 2016).
Aside from UPR, the role of autophagy in mediating skeletal muscle wasting is also evident (Porporato, 2016). Autophagy is increased and upregulated in Cancer Cachexia. It is a process of lysosomal degradation where aggregated protein and damaged organelles are engulfed by an autophagosome prior to ultimate joining with lysosome and degradation. These is also contributed by an increase in Reactive Oxygen Species (ROS) production in skeletal muscle (Blackwell, 2017).
Inflammation
Inflammation initiates central anorexic pathways that limit dietary consumption of nutrients. As amino acids are required for muscle protein synthesis, the reduced availability of amino acids in individuals with Cancer Cachexia mainly contributes to their inflammation-induced loss of skeletal muscle mass. Moreover, the reduced supply of amino acids and anorexic response also reduces the exposure to insulin which stimulates muscle protein synthesis. (Durham, Dillon, & Sheffield-Moore, 2009). In relation to insulin resistance, insulin action is not fully able to control catabolic processes in the muscle in Cachectic individuals (Dodesini et al, 2009).
Although liver contributes to cachexia by increasing energy expenditure through gluconeogenesis and reducing VLDL circulation, it worsens inflammation by secreting acute phase proteins and reducing albumin secretion, a process mostly driven by IL-6 and TNFα. This contributes to muscular protein breakdown and adipocytes lipolysis in affected individuals (Porporato, 2016).
Inflammatory-mediated signaling muscle protein synthesis by several mechanisms. TNFα is the most characterized cytokine in cachexia, which promotes anorexia and skeletal muscle wasting mainly through the NF-kB pathway. It activates the transcription factor NF-κB, which inhibits the synthesis of the muscle-specific transcription factor MyoD. TNF-α also influences the anabolic mTOR signaling pathway. It synergizes with interferon gamma and IL-1 in promoting muscle wasting (Porporato, 2016). Inflammatory proteins such as IL-6 also reduce the activation of mTOR directly and indirectly through phosphorylation of AMPK. The infusion of IL-6 increases whole body protein turnover and reduces circulating amino acid concentrations (Durham, Dillon, & Sheffield-Moore, 2009).
References:
Photo retrieved from: https://images.app.goo.gl/kwtG3Tzjmw2Mw6RAA
Blackwell, Thomas Allen, "RNA Sequencing in the Development of Cancer-Cachexia" (2017). Theses and Dissertations. 2434. http://scholarworks.uark.edu/etd/2434
Bode, B. P., Fischer, C., Abcouwer, S., Wasa, M., & Souba, W. W. (1996). Glutamine and Cancer Cachexia. Protein and Amino Acid Metabolism in Cancer Cachexia Medical Intelligence Unit, 139–170. doi: 10.1007/978-3-662-22346-8_11
Dodesini, A. R., Benedini, S., Terruzzi, I., Sereni, L. P., & Luzi, L. (2009). Protein, glucose and lipid metabolism in the cancer cachexia: A preliminary report. Acta Oncologica, 46(1), 118–120. doi: 10.1080/02841860600791491
Durham, W. J., Dillon, E. L., & Sheffield-Moore, M. (2009). Inflammatory burden and amino acid metabolism in cancer cachexia. Current opinion in clinical nutrition and metabolic care, 12(1), 72–77. https://doi.org/10.1097/MCO.0b013e32831cef61
Hardee, J. P., Montalvo, R. N., & Carson, J. A. (2017). Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal Muscle Oxidative Metabolism. Oxidative Medicine and Cellular Longevity, 2017, 1–14. doi: 10.1155/2017/8018197
Porporato, P. E. (2016). Understanding cachexia as a cancer metabolism syndrome. Oncogenesis, 5(2). doi: 10.1038/oncsis.2016.3
0 notes
Text
MTOR COORDINATES PROTEIN SYNTHESIS, MITOCHONDRIAL ACTIVITY AND PROLIFERATION.
mTORC1 stimulates protein synthesis and other anabolic processes to fuel cellular growth and proliferation, and is sensitive to acute rapamycin treatment.
In most cell types,mTORC2 is insensitive to acute rapamycin treatment, regulates cytoskeletal organization, phosphorylates AGC kinases such as SGK1 and AKT, and has been implicated in the degradation of newly synthesized polypeptides.
mTORC1activation leads to phosphorylation of a number of substrates including eukaryotic translation initiation factor 4E (eIF4E)-binding proteins (4E-BPs), ribosomal pro-tein S6 kinases (S6Ks), LARP1, Atg13,ULK1/2, which results in the upregulation of anabolic processes such as protein and lipid synthesis and inhibition of autophagy.

0 notes
Text
Kaempferol promotes bone formation in part via the mTOR signaling pathway.
PMID: Mol Med Rep. 2019 Dec ;20(6):5197-5207. Epub 2019 Oct 16. PMID: 31638215 Abstract Title: Kaempferol promotes bone formation in part via the mTOR signaling pathway. Abstract: Previous research indicates that kaempferol (Kae) promotes osteogenesis, but its underlying mechanism of action remains unclear. The present study hypothesized that the osteogenic effects of Kae were mediated through mammalian target of rapamycin (mTOR). To validate this hypothesis, bone marrow mesenchymal stem cells (BMSCs) from ovariectomized (OVX) rats were differentiated into osteoblasts. The bone mineral density and bone microarchitecture of the OVX rats was measured in vivo, while osteogenesis was evaluated in vitro via Alizarin Red S staining and alkaline phosphatase activity measurements in cultured BMSCs. The levels of phosphorylated eukaryotic translation initiation factor 4E‑binding protein 1 (p‑4E/BP1) and phosphorylated ribosomal protein S6 kinase B1(p‑S6K), and the expression of Runt‑related transcription factor 2 and Osterix, were concurrently quantified by western blot analysis. The data suggested that Kae prevented OVX‑induced osteoporosis in rats by promoting osteoblastogenesis. Furthermore, treatment with Kae in rat BMSCs enhancedmineralization, elevated ALP activity, increased the expression levels of Runx‑2 and Osterix and increased the levels of p‑S6K and decreased the levels of p‑4E/BP1 and, consistent with its ability to promote osteoblast differentiation. In contrast, treatment with rapamycin, an mTOR inhibitor,produced the opposite phenotype. Taken together, these data suggested that the protective effects of Kae in BMSCs and in the OVX rat model resulted from the induction of osteogenesis via mTOR signaling, or at least partially via the regulation of downstream effectors of the mTOR pathway.
read more
0 notes
Text
Disruption of the Rbm38-eIF4E Complex with a Synthetic Peptide Pep8 Increases p53 Expression
Rbm38 is a p53 target and an RNA-binding protein known to suppress p53 translation by preventing eukaryotic translation initiation factor 4E (eIF4E) from binding to p53 mRNA. In this study, we show that synthetic peptides corresponding to the binding interface between Rbm38 and eIF4E, including an 8 amino acid peptide (Pep8) derived from Rbm38, are effective in relieving Rbm38-mediated repression of p53. Molecular simulations showed that Ser-6 in Pep8 forms a hydrogen bond with Asp-202 in eIF4E. Substitution of Ser-6 with Lys, but not with Asp, enhanced the ability of Pep8 to inhibit the Rbm38-eIF4E complex. Importantly, Pep8 alone or together with a low dose of doxorubicin potently induced p53 expression and suppressed colony and tumor sphere formation and xenograft tumors in Rbm38- and p53-dependent manners. Together, we conclude that modulating the Rbm38-eIF4E complex may be explored as a therapeutic strategy for cancers that carry wild-type p53.Significance:Disruption of the Rbm38-eIF4E complex via synthetic peptides induces wild-type p53 expression, suppresses tumor growth and progression, and may serve as a novel cancer therapeutic strategy. http://bit.ly/2TSQakg
0 notes
Text
PLK1 regulates spindle association of phosphorylated eukaryotic translation initiation factor 4E binding protein, and spindle function in mouse oocytes.
Pubmed: http://dlvr.it/PdKD6W
0 notes
Text
4EBP2 Antibody
4EBP2 Antibody Catalog number: B2020487 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 25 ug Molecular Weight or Concentration: N/A Supplied as: Liquid Applications: molecular tool for various biochemical applications Storage: 2-8°C Keywords: Eukaryotic translation initiation factor 4E-binding protein 2, eIF4E-binding protein 2 Grade: Biotechnology grade. All products are…
0 notes
Text
IJMS, Vol. 20, Pages 991: Sensory Neuropathy Affects Cardiac #miRNA Expression Network Targeting IGF-1, SLC2a-12, EIF-4e, and ULK-2 #mRNAs
Background: Here we examined myocardial #microRNA (#miRNA) expression profile in a sensory neuropathy model with cardiac diastolic dysfunction and aimed to identify key #mRNA molecular targets of the differentially expressed #miRNAs that may contribute to cardiac dysfunction. Methods: Male Wistar rats were treated with vehicle or capsaicin for 3 days to induce systemic sensory neuropathy. Seven days later, diastolic dysfunction was detected by echocardiography, and #miRNAs were isolated from the whole ventricles. Results: Out of 711 known #miRNAs measured by #miRNA microarray, the expression of 257 #miRNAs was detected in the heart. As compared to vehicle-treated hearts, miR-344b, miR-466b, miR-98, let-7a, miR-1, miR-206, and miR-34b were downregulated, while miR-181a was upregulated as validated also by quantitative real time polymerase chain reaction (qRT-PCR). By an in silico network analysis, we identified common #mRNA targets (insulin-like growth factor 1 (IGF-1), solute carrier family 2 facilitated glucose transporter member 12 (SLC2a-12), eukaryotic translation initiation factor 4e (EIF-4e), and Unc-51 like autophagy activating kinase 2 (ULK-2)) targeted by at least three altered #miRNAs. Predicted upregulation of these #mRNA targets were validated by qRT-PCR. Conclusion: This is the first demonstration that sensory neuropathy affects cardiac #miRNA expression network targeting IGF-1, SLC2a-12, EIF-4e, and ULK-2, which may contribute to cardiac diastolic dysfunction. These results further support the need for unbiased omics approach followed by in silico prediction and validation of molecular targets to reveal novel pathomechanisms. http://bit.ly/2EuQOPA
0 notes
Text
FebA: A gene for eukaryotic translation initiation factor 4E-binding protein (4E-BP) in Dictyostelium discoide
http://dlvr.it/N8V0bp
0 notes
Text
eIF4E Human Recombinant
eIF4E Human Recombinant Catalog number: B2017244 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 100 µg Molecular Weight or Concentration: 27.19 kDa Supplied as: Solution Applications: a molecular tool for various biochemical applications Storage: -80°C Keywords: Eukaryotic Translation Initiation Factor 4E, mRNA Cap-binding Protein Grade: Biotechnology grade. All products are…
0 notes
Text
Human 4E-BP1 Protein
Human 4E-BP1 Protein Catalog number: B2015566 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 10 ug Molecular Weight or Concentration: 14.7 kDa (138 aa) Supplied as: Solution Applications: a molecular tool for various biochemical applications Storage: -20°C Keywords: Eukaryotic Translation Initiation Factor 4E-Binding Protein 1, 4E-BP1, eIF4E-Binding Protein 1, Phosphorylated…

View On WordPress
0 notes
Text
4EGI-1 represses cap-dependent translation and regulates genome-wide translation in malignant pleural mesothelioma
Summary
Deregulation of cap-dependent translation has been implicated in the malignant transformation of numerous human tissues. 4EGI-1, a novel small-molecule inhibitor of cap-dependent translation, disrupts formation of the eukaryotic initiation factor 4F (eIF4F) complex. The effects of 4EGI-1-mediated inhibition of translation initiation in malignant pleural mesothelioma (MPM) were examined. 4EGI-1 preferentially inhibited cell viability and induced apoptosis in MPM cells compared to normal mesothelial (LP9) cells. This effect was associated with hypophosphorylation of 4E���binding protein 1 (4E–BP1) and decreased protein levels of the cancer-related genes, c-myc and osteopontin. 4EGI-1 showed enhanced cytotoxicity in combination with pemetrexed or gemcitabine. Translatome-wide polysome microarray analysis revealed a large cohort of genes that were translationally regulated upon treatment with 4EGI-1. The 4EGI-1-regulated translatome was negatively correlated to a previously published translatome regulated by eIF4E overexpression in human mammary epithelial cells, which is in agreement with the notion that 4EGI-1 inhibits the eIF4F complex. These data indicate that inhibition of the eIF4F complex by 4EGI-1 or similar translation inhibitors could be a strategy for treating mesothelioma. Genome wide translational profiling identified a large cohort of promising target genes that should be further evaluated for their potential significance in the treatment of MPM.
http://ift.tt/2m4OMyy
0 notes
Text
Inhibition of eIF4E cooperates with chemotherapy and immunotherapy in renal cell carcinoma
Abstract
Purpose
Although overexpression of the eukaryotic translation initiation factor 4E (eIF4E) is detected in patients with renal cell carcinoma (RCC) and associated with poor prognosis, the possible roles of eIF4E in RCC have not been revealed.
Methods
The effects of eIF4E inhibition on cell growth, migration, survival, chemo-/immunotherapy and eIF4E pathways via pharmacological inhibitor and genetic siRNA knockdown were analyzed in RCC cells.
Results
In this work, we demonstrate that eIF4E is critically involved in multiple biological functions of RCC. We firstly inhibited eIF4E activity by ribavirin in two cell lines (Caki-1 and ACHN) representing RCC metastasis models. We demonstrated that ribavirin inhibited proliferation and migration and induced apoptosis in RCC in a dose-dependent manner. We further confirmed that the inhibitory effects of ribavirin were attributed to its ability in inhibiting eIF4E-regulated protein translation and activity. eIF4E inhibition using siRNA knockdown mimicked ribavirin’s effector in RCC cells. Importantly, eIF4E inhibition by both ribavirin and siRNA knockdown significantly sensitized RCC response to chemo- and immunotherapeutic agents in vitro as well as in vivo.
Conclusions
Our findings clearly demonstrate the roles of eIF4E in RCC growth, survival, metastasis and resistance. Ribavirin is an antiviral drug, and its clinical efficacy is currently being investigated in the treatment of various cancers. Our findings support and provide a preclinical evidence for clinical trial for the combination of ribavirin with chemo-/immunotherapy in RCC.
http://ift.tt/2hp5HqZ
0 notes
Text
Inhibition of eIF4E cooperates with chemotherapy and immunotherapy in renal cell carcinoma
Abstract
Purpose
Although overexpression of the eukaryotic translation initiation factor 4E (eIF4E) is detected in patients with renal cell carcinoma (RCC) and associated with poor prognosis, the possible roles of eIF4E in RCC have not been revealed.
Methods
The effects of eIF4E inhibition on cell growth, migration, survival, chemo-/immunotherapy and eIF4E pathways via pharmacological inhibitor and genetic siRNA knockdown were analyzed in RCC cells.
Results
In this work, we demonstrate that eIF4E is critically involved in multiple biological functions of RCC. We firstly inhibited eIF4E activity by ribavirin in two cell lines (Caki-1 and ACHN) representing RCC metastasis models. We demonstrated that ribavirin inhibited proliferation and migration and induced apoptosis in RCC in a dose-dependent manner. We further confirmed that the inhibitory effects of ribavirin were attributed to its ability in inhibiting eIF4E-regulated protein translation and activity. eIF4E inhibition using siRNA knockdown mimicked ribavirin’s effector in RCC cells. Importantly, eIF4E inhibition by both ribavirin and siRNA knockdown significantly sensitized RCC response to chemo- and immunotherapeutic agents in vitro as well as in vivo.
Conclusions
Our findings clearly demonstrate the roles of eIF4E in RCC growth, survival, metastasis and resistance. Ribavirin is an antiviral drug, and its clinical efficacy is currently being investigated in the treatment of various cancers. Our findings support and provide a preclinical evidence for clinical trial for the combination of ribavirin with chemo-/immunotherapy in RCC.
http://ift.tt/2hp5HqZ
0 notes