#Early Phase Clinical Trial Outsourcings Market Size
Text
Early Phase Clinical Trial Outsourcings Market Scope & Growth Projection till 2032
Early Phase Clinical Trial Outsourcings Market provides in-depth analysis of the market state of Early Phase Clinical Trial Outsourcings manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Early Phase Clinical Trial Outsourcings in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Early Phase Clinical Trial Outsourcings Market Report:
The report offers a comprehensive and broad perspective on the global Early Phase Clinical Trial Outsourcings Market.
The market statistics represented in different Early Phase Clinical Trial Outsourcings segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Early Phase Clinical Trial Outsourcings are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Early Phase Clinical Trial Outsourcings.
Major stakeholders, key companies Early Phase Clinical Trial Outsourcings, investment feasibility and new market entrants study is offered.
Development scope of Early Phase Clinical Trial Outsourcings in each market segment is covered in this report. The macro and micro-economic factors affecting the Early Phase Clinical Trial Outsourcings Market
Advancement is elaborated in this report. The upstream and downstream components of Early Phase Clinical Trial Outsourcings and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/early-phase-clinical-trial-outsourcings-market-101449
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Global Early Phase Clinical Trial Outsourcings Market Share
Smart Microwave Oven Market Forecast
Shot Peening Market Size
Spectrometer Market Growth Rate
Operating Room Management Market Analysis
Indoor Location-based Services (LBS) Market Share
Digital Refractometers Market Growth
Electric Trucks Market
Global CMP Pad Regulator Market Size
Global Advanced Metering Infrastructure (AMI) Market Growth
RFID Printer Market Forecast
Global Laser Communication Terminal Market Share
Concrete Waterproofing Admixture Market Growth Rate
Thermal Treatment Air Filtration Systems Market Size
5G Security Market Share
Single Lead ECG Equipment Market Analysis
Personal Cloud Market
Fiber Coupled Superluminescent Light Emitting Diodes (SLED) Market Growth
Global Digital Talent Acquisition Market Growth
Global Hydraulic Dock Leveler Market Size
Global Robotic Process Automation Market Share
Carpet Manufacturing Machines Market Forecast
EV Charging Cables Market Size
Lighters Market Growth Rate
Reduced Iron Powder Market Analysis
Medical Audiometers Market Share
Peptide CDMO Market Growth
Intelligent Flow Meter Market
Global Tax Management Software Market Size
Global Aqua Feed Market Growth
Refurbished Medical Devices Market Forecast
Global Civil Engineering Design Software Market Share
Invisible Orthodontics Market Growth Rate
Self-Tanning Products Market Size
Laser Communications Terminals (LCTs) Market Share
Flower Pots and Planters Market Analysis
Battery Energy Storage Systems (BESS) Market
Potassium Bicarbonate Market Growth
Optical Transceivers Market Size
Digital Twin Technology Market Share
#Early Phase Clinical Trial Outsourcings Market Size#Early Phase Clinical Trial Outsourcings Market Share#Early Phase Clinical Trial Outsourcings Market Trends#Early Phase Clinical Trial Outsourcings Market Industry#Early Phase Clinical Trial Outsourcings Market Growth
0 notes
Text
Clinical Research Organisation In India – Understanding The Field Of Research
Clinical research plays a pivotal role in advancing medical science and bringing new drugs and therapies to market. In recent years, India has emerged as a key player in the global clinical research landscape. This growth is complemented by the dynamic and ever-expanding Indian pharmaceutical market.
Clinical Research Organizations act as intermediaries between pharmaceutical and biotech companies and healthcare regulatory agencies. Their primary objective is to conduct clinical trials efficiently, ensuring the safety and efficacy of new drugs and treatments.
Key features of CROs in India:
Diverse Service Offerings:
Indian CROs provide a wide range of services, including clinical trial management, data management, regulatory affairs, and bioanalytical services.
Many clinical research organisation in India have expertise in conducting early-phase clinical trials, making them attractive partners for global pharmaceutical companies.
Cost-Effective Solutions:
One of the main reasons for the surge in outsourcing clinical trials to India is the cost advantage.
Conducting trials in India is often more economical compared to developed countries, without compromising on the quality of research.
Skilled Workforce:
India boasts a pool of highly qualified and skilled healthcare professionals, including doctors, nurses, and clinical research experts.
English proficiency is widespread, facilitating effective communication with global sponsors.
Regulatory Environment:
The regulatory environment for clinical trials in India has improved significantly in recent years.
The approval process has become more streamlined, encouraging global pharmaceutical companies to choose India as a preferred destination for clinical research.
Indian Pharmaceutical Market Insights:
The pharmaceutical market in India is a dynamic and rapidly evolving landscape. Several factors contribute to its growth and prominence on the global stage.

Market Size and Growth:
India is among the largest pharmaceutical markets globally, with a growing population and increasing healthcare needs.
The pharmaceutical market has witnessed consistent growth, driven by rising incomes, healthcare awareness, and government initiatives.
Generic Drug Dominance:
The Indian pharmaceutical market is known for its strong focus on generic drug manufacturing according to the Indian Pharmaceutical Market Insights.
Generic drugs account for a significant share of the market, making healthcare more accessible and affordable.
Innovation and R&D:
Pharmaceutical companies in India are increasingly investing in research and development (R&D) to bring innovative drugs to market according to Indian Pharmaceutical Market Insights.
Collaboration with global pharmaceutical companies and partnerships with research institutions contribute to the innovation ecosystem.
Government Initiatives:
The Indian government has implemented various initiatives to promote the pharmaceutical sector, including the 'Make in India' campaign.
Incentives for research, development, and manufacturing have attracted both domestic and international players.
The synergy between clinical research organisation in India and the dynamic pharmaceutical market creates a unique ecosystem that fosters innovation and advancements in medical science. As the pharmaceutical landscape continues to evolve, India's role in global clinical research and drug development is likely to expand, contributing to improved healthcare outcomes worldwide.
#Clinical Research Organisation in India#Clinical Research Organisation#Pharmaceutical Company Strategy
0 notes
Text
Contract Research Organizations Market Revenue - Due To Increase In Huge Demand In This Sector


The global Contract Research Organizations market was valued at US$ xx million in the year 2019. The market is estimated to be valued at US$ xx million in the year 2020, and is expected to reach US$ xx million by the year 2027, with an estimated CAGR of xx% during the forecast period (2021-2027). The research study also includes exhaustive information about market dynamics such as drivers, restraints, opportunities, technological advancements, future trends, supply chain analysis, patent landscape, pricing analysis, regulatory and reimbursement framework for precise market estimations and forecasts.
Additionally, the business intelligence study encompasses the COVID-19 scenario as follows:
· Long-Term, Mid-Term, and Short-Term Impact of COVID-19
· Pre- and Post-COVID-19 Scenario
· Disruptions in the Supply Chain
Get Free PDF Brochure Of This Report @ https://www.fostermarketresearch.com/product/industry/10/166/Pdf%20Brochure/
Competitive Insights
Leading and emerging companies of the global Contract Research Organizations market were identified based on their current offerings. Further, the Contract Research Organizations industry is one of the most competitive industries, with the leading players actively competing against each other to gain a greater share in the industry. Some of the major companies in the Contract Research Organizations market are: PRA Health Sciences, Cereno Scientific AB, ICON plc, IQVIA, PAREXEL, LabCorp, PPD, Syneos Health, WuXi AppTech, Medpace, Charles River, and SGS, among others.
The competitive landscape of the Contract Research Organizations industryexhibits an inclination towards emerging strategies including product launches, partnerships, collaborations and contracts, acquisitions, funding, and other developments to achieve the objectives faster.
Our reports fill up the gaps and provide you with all the detailed analysis of competitive companies involving:
· Analyzing competitive strategies and techniques
· Competitive positioning of key players
· PORTER’s and SWOT analysis for competitive risk analysis
· Competitive Share Analysis
· Financial analysis of players to determine their withstand capacity
· Analyzing their sales path and product study
For More Information Of This Report @ https://www.fostermarketresearch.com/product/industry/10/166/
Key Segments Covered in the global Contract Research Organizations market report are:
· By Service Type: (Drug Discovery, Preclinical studies, Early Phase I – Iia, Phase IIa – III, Phase IIIb – IV, Drug Development, Medical Coding, and Writing, Monitoring, Clinical Data Management, Bio-statistics, Site Management, Protocol Development, Biomarker Discovery)
· By Application: (Infectious Disease, Oncology, Metabolic Disorders, Cardiovascular Diseases, Central Nervous System Diseases, Immunological Disorders, Respiratory Disorders, and Others), By Size of CROs (Small Size, Medium Size, and Large Size)
· By End User (Research Institute, Pharma and Biotech Companies, and Others )
Regional Coverage:
The global Contract Research Organizations market segregates into five regions including North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa. North America followed by Europe held the major share of the global market (in terms of value) in 2020. However, Asia-Pacific region exhibit highest CAGR (%) during the forecast period (2021-2027). Our research study further sub-divides regions into countries as follows:
· North America - the U.S. and Canada
· Europe - Germany, the U.K., France, Spain, Italy, Russia, and Rest-of-Europe
· Asia-Pacific - China, India, Japan, South Korea, Australia, ASEAN, and Rest-of-Asia-Pacific
· Latin America - Mexico, Brazil, and Rest-of-Latin America
· Middle East and Africa - Gulf Cooperation Council (GCC) Countries, South Africa, Israel, and Rest-of-Middle East and Africa
Market Dynamics:
Rapidly growing pharmaceuticals and biotechnology companies are investing in research organizations to maintain a position in the competitive space. The rising requirements for clinical trials of medical equipment, expanding capabilities of research organizations using technology, and increasing demand for outsourcing services in developing countries are prospecting the growth for the contract research organization market. Additionally, favorable regulatory environments in the developed countries in North America and the European region are providing a positive outlook for the market. The clinical development for coronavirus is positively impacting the CRO’s growth. The outsourced research has gained momentum for the development of drugs and vaccines in the COVID-19 situation. For instance, in April 2020, Covance Inc., a CRO took the clinical trials for developing a vaccine against the coronavirus.
Buy Now This Business Strategic Report To Improve Your Profits @ https://www.fostermarketresearch.com/product/buy/10/166/
About Foster Market Research:
Foster Market Research is a global market intelligence and advisory firm engaged in providing data-driven research extract from rigorous analysis, to the clients to make critical business decisions and execute them successfully. Foster connects over various distribution channels and numerous markets for great understanding of the trends and market to deliver our clients with accurate data.
Our focus is on providing market research that delivers a positive impact on your business. We work continuously to provide our clients with the most accurate analytics data and research reports without any delay so as to improve their business strategies and provide them with rich customer experience.
Contact Us:
1701 Royal Lane,
#1306, Dallas
Tx-75229
Phone: +1 469 4981929
Email: [email protected]
1 note
·
View note
Text
Clinical Trial Imaging Services Market Procurement Intelligence | New Opportunity, Outlook And Top Players 2025
Imaging plays an important role in modern medicine. Modern imaging techniques including X-rays, ultrasound, CT scans, and MRI shows structures inside the patient’s body in considerable detail. To improve decision making, increase efficiency and potentially reduce trial costs, sponsors need to work with an imaging partner that can provide strategic advice on biomarkers in early phase development and demonstrate how medical imaging endpoints can provide objective evidence of a drug’s safety and efficacy.
The core clinical imaging solution include protocol design, site selection, site training and support, electronic image transfer and de-identification, certified radiologic technologist image QC, independent blinded image review, clinical data management and biostatistics, preparation for audits, and regulatory and compliance decisions.
Read report summary or request a free sample copy of the report “Clinical Trial Imaging Services Market Procurement Intelligence, Supplier Intelligence, Supplier Ranking, Pricing & Cost Structure Intelligence, Best Practices, Engagement Model, Low & Best Cost Country, Day One Analysis Report, 2020 – 2025”
Imaging is currently being utilized in the following three areas:
Primary Study: Pharmaceutical companies are exploring innovative technologies that can bring new drugs to market sooner for unmet needs
Early-phase Clinical Trial: Imaging is a natural fit for early phase trials since they are more dynamic and involve more scientific work than later-phase trials
Late-phase Clinical Trial: As a product moves through clinical trials, the imaging focus shifts toward efficient trial management streamlined operational workflow for easy regulatory approval
Demand Outlook
The rise in clinical trials due to the surge in prevalence of chronic conditions, which require novel treatment option is driving the growth of the market. Project and data management is dominating the service type share in the market. It is also expected to witness the highest growth rate over the forecasted, as sponsors are outsourcing owing to high associated costs.
North America accounted for the major share of the clinical trial imaging services market and is expected to continue this trend owing to the surge in use of imaging as an endpoint in clinical trials which leads to sponsors outsourcing these services to save cost. Furthermore, the other major factor, which is the presence of major players such as Parexel International, ERT Clinical, Biospective, Inc. Intrinsic Imaging LLC leads to easy availability of clinical trial imaging service, which in turn boosts the growth of the market in the region.
Cost Drivers
High rise in cost for technological advancements and change in regulatory needs are few factors that compel pharmaceutical and biotechnology companies to outsource imaging services. Moreover, pharmaceutical companies are repeatedly engaged in the development of new therapeutics, which can be immensely expensive leading to a need to outsource.
Browse our other Reports:
Conveyor Belts Market Procurement Intelligence, Supplier Intelligence, Supplier Ranking, Pricing & Cost Structure Intelligence, Best Practices, Engagement Model, Low & Best Cost Country, Day One Analysis Report, 2020 – 2025
Employee Benefit Services Market Procurement Intelligence, Supplier Intelligence, Supplier Ranking, Pricing & Cost Structure Intelligence, Best Practices, Engagement Model, Low & Best Cost Country, Day One Analysis Report, 2020 – 2025
About us:
A smart and effective supply chain is essential for growth in any organization. Pipeline division at Grand View Research provides detailed insights on every aspect of the supply chain which helps in efficient procurement decisions.
Our services include (not limited to):
· Market Intelligence involving - market size and forecast, growth factors, and driving trends
· Price and Cost Intelligence - pricing models adopted for the category, the total cost of ownership
· Supplier Intelligence - rich insight on supplier landscape, and identifies suppliers who are dominating, emerging, lounging, and specializing
· Sourcing / Procurement Intelligence - best practices followed in the industry, identifying standard KPIs and SLAs, peer analysis, negotiation strategies to be utilized with the suppliers, and best-suited countries for sourcing to minimize supply chain disruption
Our market/procurement Intelligence reports include in-depth and actionable insights that help clients in understanding the different aspects of the supply chain and take more effective decisions.
Find More information @ https://www.grandviewresearch.com/pipeline
Contact Information:
Sherry James
Corporate Sales Specialist, USA
Grand View Research, Inc.
Phone: 1-415-349-0058
Toll Free: 1-888-202-9519
Email: [email protected]
Web: https://www.grandviewresearch.com
Follow Us: LinkedIn | Twitter
#Clinical Trial Imaging Services Market Procurement Intelligence#Clinical Trial Imaging Services Market#Clinical Trial Imaging Services
1 note
·
View note
Text
Ambulatory Surgical Centers Market worth USD 7.2 billion : Growing demand for cloud-based solution
According to the new market research report “Ambulatory Surgical Centers Market by Product (EHR, Practice Management, Telehealth, Healthcare Analytics, PHM, Supply Chain Management, RCM, Surgical planning, Quality Management), Specialty Type (Single, Multi-specialty) – Global Forecast to 2025″, published by MarketsandMarkets™, the Ambulatory Surgical Centers Market is projected to reach USD 7.2 billion by 2025 from 2.1 billion in 2020, at a CAGR of 27.6% during the forecast period.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=182183086
A majority of this growth is attributed to the growing need to curtail escalating healthcare costs, shift from inpatient to outpatient surgical procedures, growing demand for IT solutions such as mhealth, telehealth, and remote patient monitoring for better management, and the need to improve the quality of healthcare while maintaining the operational efficiency of healthcare organizations.
By-products and services, the healthcare providers segment accounted for the largest share of the Ambulatory Surgical Centers market in 2019
Based on the products and services, the clinical solutions segment accounted for the largest share of the Ambulatory Surgical Centers market in 2019. There is a high demand for clinical solutions due to the increasing demand for improved patient safety and patient care, the need to manage complex patients’ data from different medical devices and information systems, and the need for integrated healthcare systems. In this report, the clinical solutions segment is further segmented into EHR, population health management solutions, medical image analysis systems, e-prescribing solutions, practice management software, surgical planning software, telehealth solutions, healthcare integration solutions, and other.
Browse in-depth TOC on “Ambulatory Surgical Centers Market“
112 – Tables
36 – Figures
176 – Pages
By components, the services segment accounted for the largest share of the Ambulatory Surgical Centers market in 2019
Based on components, the Ambulatory Surgical Centers market is segmented into services, software, and hardware. In 2019, the services segment accounted for the largest share of the Ambulatory Surgical Centers market. This is attributed to the need for integration and interoperability of software, the development of complex software, the growing demand for consulting and outsourcing of various healthcare processes such as revenue cycle management and EHR management.
By specialty type, the multi-specialty segment accounted for the largest share of the Ambulatory Surgical Centers market in 2019
Based on specialty type, the Ambulatory Surgical Centers market is segmented into single and multi-specialty. In 2019, the multi-specialty segment accounted for the largest share of the Ambulatory Surgical Centers market. The large share of this segment can be attributed to the large number of surgical procedures performed in these facilities and the availability of reimbursement for these procedures.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=182183086
“North America to hold the largest regional market share in 2019.”
North America accounted for the largest share of the Ambulatory Surgical Centers market, followed by Europe. The large share of this region can be attributed to the high adoption of Ambulatory Surgical Centers for reducing the soaring healthcare costs, increasing volume of surgical procedures performed, and the presence of major market players, such as Epic Systems Corporation (US), Cerner Corporation (US), McKesson Corporation (US), Philips Healthcare (Netherlands), and Allscripts Healthcare Solutions, Inc. (US).
The prominent players in this market are Cerner Corporation (US), McKesson Corporation (US), Allscripts Healthcare Solutions, Inc. (US), GE Healthcare (US), Philips Healthcare (Netherlands), athenahealth, Inc. (US), Optum (US), Epic Systems Corporation (US), Medical Information Technology, Inc. (MEDITECH) (US), eClinicalWorks (US), athenahealth, Inc. (US), Advanced Data Systems Corporation (US), NextGen Healthcare (US), CureMD (US), HST Pathways (US), and Surgical Information Systems (US).
Browse Adjacent Markets: Healthcare IT Market Research Reports & Consulting
Browse Related Reports:
Healthcare IT Market by Product (EHR, RIS, PACS, VNA, CPOE, HIE, Telehealth, Healthcare Analytics, Population Health Management, Supply Chain Management, CRM, Fraud Management, Claims Management) End User (Provider, Payer) – Global Forecast to 2024 https://www.marketsandmarkets.com/Market-Reports/healthcare-it-252.html
eClinical Solutions Market by Clinical Trial Phases, Product (CTMS, eCOA, Analytics, RTMS, eTMF, Safety, CDMS, EDC), Delivery Mode (On-Demand, On-premise, Cloud-based), End User (CROs, Hospitals, Pharma/Biopharma Companies) – Global Forecast to 2022 https://www.marketsandmarkets.com/Market-Reports/eclinical-solutions-market-553.html
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the “Growth Engagement Model – GEM”. The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write “Attack, avoid and defend” strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, “Knowledge Store” connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Research Insight: https://www.marketsandmarkets.com/ResearchInsight/ambulatory-surgical-center-market.asp
Visit Our Website: https://www.marketsandmarkets.com
Content Source: https://www.marketsandmarkets.com/PressReleases/ambulatory-surgical-center.asp
0 notes
Text
Healthcare Contract Research Outsourcing Market Revenue Share, Growth Factors, Trends, Analysis & Forecast, 2021–2030
Market Overview:
The Global Healthcare Contract Research Outsourcing Market held USD 36.9 billion in 2020 and is to grow with a CAGR of 6.4% from 2020-2030. Healthcare and pharmaceutical companies outsource not only drug production but also their clinical trials. With rising privatization of clinical trials, the outsourcing to developed countries is on the increase. Companies in pharmaceutical sector have begun outsourcing R&D activities which are complex and require regular monitoring. Healthcare CROs functions and manages the processes of bringing new products to the market according to the customer’s timeline provided. Emerging economies like Japan, India and China are chosen to outsource business. Many healthcare contract research organizations are now forming partnerships to expand their market reach by providing services across a wide market space and strengthening the relationship between client and contractor. IQVIA for instance formed an alliance with Box in 2018. This partnership is intended to assist the company in offering cloud-based content management solutions for healthcare and life science companies. Key players are in the process of purchasing other contract research organizations, in addition to partnerships, to achieve leverage.
Market Dynamics and Factors:
Increasing investment in R&D programs, growing demand for outsourcing activities due to time and cost constraints in the healthcare sector are key factors expected to drive the healthcare contract research outsourcing market growth in the years to come. Contract research outsourcing collaborations offer state-of-the-art services and therefore government organizations prefer to assign projects to contract research organizations (CROs), thus facilitating market demand. Increasing pressure on drug producers in terms of clinical data management, regulatory environments, and stringent safety standards is expected to drive demand within the healthcare sector for contract research organizations. Models including transactional relationships, Multi-FSP, and alliances are widespread and are widely adopted by drug producers.
Request for Sample with Complete TOC and Figures & Graphs @https://www.trendsmarketresearch.com/report/sample/13669
Market Segmentation:
Based on the type, the healthcare contract research outsourcing industry is segmented into drug discovery, pre-clinical and clinical. Drug discovery has been further segmented into target validation, lead optimization and lead identification. Clinical has been further segmented into phase I, phase II, phase III and phase IV trial services. On the basis of services, the market is segmented into project management, regulatory/medical affairs, investigator payments, data management, clinical monitoring, quality management/ assurance, medical writing, bio-statistics, and others. Geographical breakdown and analysis of each of the aforementioned segments includes regions comprising North America, Europe, Asia-Pacific, and RoW.
Geographic Analysis:
Direct Purchase this Market Research Report Now @https://www.trendsmarketresearch.com/checkout/13669/Single
North America dominates the healthcare contract research outsourcing market share with the large demand clinical trials in the region. The region is also a hub of advanced healthcare infrastructure. In addition, growing government support for R&D activities through grants and funds to research institutes and companies has driven this regional market. Additionally, increasing government funding for R&D activities has guided this regional market through grants and funds to research institutes and companies. For example, in January 2015, the U.S. government announced a USD 215.0 million investment for the precision medicine initiative. The investment is for the NIH, the National Institute for Cancer (NCI) and the FDA. Asia Pacific held the largest share of the CRO healthcare market in 2019 and is expected to grow rapidly over the forecast period due to the availability of diverse populations, high prevalence of chronic conditions, easy recruitment and retention of patients and the establishment of regulations according to accepted standards.
Competitive Scenario:
Get Discount On The Purchase Of This Report @https://www.trendsmarketresearch.com/report/discount/13669
Global healthcare contract research outsourcing market growth is witnessing fierce competition. In terms of healthcare contract research outsourcing market share, certain players gather majority of market share. Key companies are also expanding their manufacturing facilities to cater to growing demand for this drug globally. Key players enhancing the global Healthcare Contract Research Outsourcing market size include Zydus Cadila, Bayer, Laurus Labs, Sun Pharma, Sanofi, Ipca Laboratories Ltd, Novartis AG, Teva Pharmaceutical Industries Ltd and McW Healthcare. Charles River Laboratories International, Inc., for example, purchased WIL Research for close to USD 585 million in 2016. This acquisition is probable to support enhance company foothold in the healthcare sector, particularly in the early stages of drug discovery business.
0 notes
Text
Contract Research Organization Market to grow at a Significant Rate During the Forecast Period | TechSci Research
Growing expenses for research and development is driving the growth of Global Contact Research Organization Market during 2022-2026.

According to TechSci Research report, “Global Contract Research Organization Market By Service (Early Phase Development Services, Clinical Research Services, Laboratory Services, Consulting Services and Data Management Services) By Size of CROs (Small Size, Medium Size, Large Size) By Application (Oncology, Central Nervous System, Cardiovascular, Respiratory, Infectious Diseases, Autoimmune Disorders, Others) By End User (Pharmaceutical and Biopharmaceutical Companies, Medical Device Companies, Academic Institutes) By Region, Competition Forecast & Opportunities, 2026”, the global contract research organization market is anticipated to show growth with a double digit CAGR in the upcoming five years. The market has shown a consistent growth in the future, but the recent wake of enhanced and rapid research work is expected to boost the growth in forecast period, 2022-2026. Government aid, humanitarians support toward the research and developments of effective drugs, effective treatment plans and their performances on the diseases by conducting human trials are also supporting the growth of the global contract research organization market positively.
Contract research organizations (CROs) are organizational systems that help pharmaceutical and biopharmaceutical companies with their drug trials, data management, and performs long term and strategic role in developmental processes. These organizations are rapidly increasing, and the market is growing on the need to finding cure for terminal and unknown diseases. Moreover, colleges and universities are actively promoting higher studies and doctoral research by the students which is boosting the growth of global contract research organization market in next five years.
Due to the onset of COVID-19 and urgency to find the cure, prevention, and containment of the virus drove the market growth to rise exponentially in 2020. Countries across the world were cumulatively putting in their effort to find the vaccine for the disease that caused millions of deaths in the span of few months. By the middle of the year, vaccines were already in for various phases of trial that boosted the growth of CROs multiple fold. The economy was adversely affected in many countries but a complete governmental focus and support from health industry itself resulted in the growth of the contract research organization market.
Browse over XX market data Figures spread through 110 Pages and an in-depth TOC on "Global Contract research organization Market"
https://www.techsciresearch.com/report/contract-research-organization-market/7311.html
The global contract research organization market is segmented on the basis of service, size of CROs, application, end user, and regional distribution. Based on application, the market is further fragmented into oncology, central nervous system, cardiovascular, respiratory, infectious diseases, autoimmune disorders, and others. Oncology application is anticipated for the largest share in the market, owing to the rising cases of cancer and the evolving nature of the carcinogenic cells. Research has suggested that cancerous cells have the tendency to grow on their own and sometimes the reason for the cancers are because of the DNA alterations. Extensive studies and research are regularly being conducted thereby requirement of the CROs for the trials and data management and record maintenance is driving the growth of the market in the next five years.
North America region is anticipated toward the largest market in the world, owing to its excellence in the efficient and high standard pharmaceutical industry. Healthcare industry in the countries like USA, Canada, and Mexico are considered best, thereby guaranteeing the better and effective drugs, effective treatment, and thus requires regular clinical trials for the new developing drugs. Moreover, rapid growth in the region’s biosimilar and biologics market is also driving the growth of the market.
Some of the major competitors in the market are Abbott Laboratories, Agilent Technologies, Inc., PerkinElmer Inc., Fluidigm Corporation, Illumina, Inc., GE Healthcare, Bio-Rad Laboratories Inc., Cepheid Inc., Thermo Fisher Scientific, Inc., F. Hoffmann-La Roche AG, bioMérieux SA, Horiba, Ltd., Qiagen N.V., Randox Laboratories Ltd., OriGene Technologies, Inc., Dynamic Biosensors GmbH, General Electric Company, Genalyte, Inc., Hologic, Inc., Arbor Biosciences, among others. The companies are focusing on extensive research and developments activities to stay competitive in the market. Other competitive strategies include formation of alliances and partnerships.
Download Sample Report @ https://www.techsciresearch.com/sample-report.aspx?cid=7311
Customers can also request for 10% free customization on this report.
“Outbreak of pandemic, and regular research has enhanced the role of pharmaceutical and biopharmaceutical companies in the healthcare industry. The companies are in continuous need of outsourcing for early phase development, clinical and laboratory testing of the developed drugs there by creating maximum demand for CROs, Since the method boosts the profit margins, aids in avoiding high expenses, and thus reduces the time to conduct the whole developing and testing process and validation procedure of product, the market is very promising for the future endeavors. New investors can create a partnership with the pharmaceutical companies for an effective business in the future,” said Mr. Karan Chechi, Research Director with TechSci Research, a research based Global management consulting firm.
“Global Contract Research Organization Market By Service (Early Phase Development Services, Clinical Research Services, Laboratory Services, Consulting Services and Data Management Services) By Size of CROs (Small Size, Medium Size, Large Size) By Application (Oncology, Central Nervous System, Cardiovascular, Respiratory, Infectious Diseases, Autoimmune Disorders, Others) By End User (Pharmaceutical and Biopharmaceutical Companies, Medical Device Companies, Academic Institutes) By Region, Competition Forecast & Opportunities, 2026”, has evaluated the future growth potential of Global contract research organization and provides statistics & information on market size, structure and future market growth. The report intends to provide cutting-edge market intelligence and help decision makers take sound investment decisions. Besides, the report also identifies and analyzes the emerging trends along with essential drivers, challenges, and opportunities in Global contract research organization.
Browse Related Reports:
Global Next-generation Sequencing Market By Product (Consumables, Platforms and Services), By Technology (Sequencing by Synthesis, Ion Semiconductor Sequencing, Sequencing by Ligation, Single Molecule Real Time Sequencing and Other Technologies), By End User (Academic & Clinical Research Centers, Pharmaceutical & Biotechnology Companies, Hospitals & Clinics and Others), By Application (Biomarkers & Cancer, Diagnostics, Reproductive Health, Personalized Medicine, Agriculture & Animal Research and Other Applications), By Region, Competition, Forecast & Opportunities, 2025
https://www.techsciresearch.com/report/next-generation-sequencing-market/4508.html
Global CAR-T Cell Therapy Market By Indication (Diffused Large B-cell Lymphoma (DLBCL), Acute Lymphoblastic Leukemia (ALL), Multiple Myeloma (MM), Chronic Lymphocytic Leukemia (CLL), Follicular Lymphoma (FL), Others), By Targeted Antigen(CD 19, CD22, BCMA (B-Cell Maturation Antigen), HER1, HER2, Meso, EGFRvlll, Others), By Product Type (Yescarta (Axicabtagene Ciloleucel), Kymriah (Tisagenlecleucel), JCAR017 (Lisocabtagene Maraleucel), bb2121), By End User (Hospitals, Cancer Treatment Centers, Others), By Region, Forecast & Opportunities, 2025
https://www.techsciresearch.com/report/car-t-cell-therapy-market/5168.html
Contact
Mr. Ken Mathews
708 Third Avenue,
Manhattan, NY,
New York – 10017
Tel: +1-646-360-1656
Email: [email protected]
Website: https://www.techsciresearch.com/
For More Market Research Blogs Visit: https://techsciblog.com/
#TechSci#Market Research Reports#Healthcare#Global Contact Research Organization Market#Contact Research Organization Market#Contact Research Organization Market Size#Contact Research Organization Market Share#Contact Research Organization Market Trend#Contact Research Organization Market Forecast
0 notes
Text
Contract Research Organization Market to grow at a Significant Rate During the Forecast Period | TechSci Research
Growing expenses for research and development is driving the growth of Global Contact Research Organization Market during 2022-2026.

According to TechSci Research report, “Global Contract Research Organization Market By Service (Early Phase Development Services, Clinical Research Services, Laboratory Services, Consulting Services and Data Management Services) By Size of CROs (Small Size, Medium Size, Large Size) By Application (Oncology, Central Nervous System, Cardiovascular, Respiratory, Infectious Diseases, Autoimmune Disorders, Others) By End User (Pharmaceutical and Biopharmaceutical Companies, Medical Device Companies, Academic Institutes) By Region, Competition Forecast & Opportunities, 2026”, the global contract research organization market is anticipated to show growth with a double digit CAGR in the upcoming five years. The market has shown a consistent growth in the future, but the recent wake of enhanced and rapid research work is expected to boost the growth in forecast period, 2022-2026. Government aid, humanitarians support toward the research and developments of effective drugs, effective treatment plans and their performances on the diseases by conducting human trials are also supporting the growth of the global contract research organization market positively.
Contract research organizations (CROs) are organizational systems that help pharmaceutical and biopharmaceutical companies with their drug trials, data management, and performs long term and strategic role in developmental processes. These organizations are rapidly increasing, and the market is growing on the need to finding cure for terminal and unknown diseases. Moreover, colleges and universities are actively promoting higher studies and doctoral research by the students which is boosting the growth of global contract research organization market in next five years.
Due to the onset of COVID-19 and urgency to find the cure, prevention, and containment of the virus drove the market growth to rise exponentially in 2020. Countries across the world were cumulatively putting in their effort to find the vaccine for the disease that caused millions of deaths in the span of few months. By the middle of the year, vaccines were already in for various phases of trial that boosted the growth of CROs multiple fold. The economy was adversely affected in many countries but a complete governmental focus and support from health industry itself resulted in the growth of the contract research organization market.
Browse over XX market data Figures spread through 110 Pages and an in-depth TOC on "Global Contract research organization Market"
https://www.techsciresearch.com/report/contract-research-organization-market/7311.html
The global contract research organization market is segmented on the basis of service, size of CROs, application, end user, and regional distribution. Based on application, the market is further fragmented into oncology, central nervous system, cardiovascular, respiratory, infectious diseases, autoimmune disorders, and others. Oncology application is anticipated for the largest share in the market, owing to the rising cases of cancer and the evolving nature of the carcinogenic cells. Research has suggested that cancerous cells have the tendency to grow on their own and sometimes the reason for the cancers are because of the DNA alterations. Extensive studies and research are regularly being conducted thereby requirement of the CROs for the trials and data management and record maintenance is driving the growth of the market in the next five years.
North America region is anticipated toward the largest market in the world, owing to its excellence in the efficient and high standard pharmaceutical industry. Healthcare industry in the countries like USA, Canada, and Mexico are considered best, thereby guaranteeing the better and effective drugs, effective treatment, and thus requires regular clinical trials for the new developing drugs. Moreover, rapid growth in the region’s biosimilar and biologics market is also driving the growth of the market.
Some of the major competitors in the market are Abbott Laboratories, Agilent Technologies, Inc., PerkinElmer Inc., Fluidigm Corporation, Illumina, Inc., GE Healthcare, Bio-Rad Laboratories Inc., Cepheid Inc., Thermo Fisher Scientific, Inc., F. Hoffmann-La Roche AG, bioMérieux SA, Horiba, Ltd., Qiagen N.V., Randox Laboratories Ltd., OriGene Technologies, Inc., Dynamic Biosensors GmbH, General Electric Company, Genalyte, Inc., Hologic, Inc., Arbor Biosciences, among others. The companies are focusing on extensive research and developments activities to stay competitive in the market. Other competitive strategies include formation of alliances and partnerships.
Download Sample Report @ https://www.techsciresearch.com/sample-report.aspx?cid=7311
Customers can also request for 10% free customization on this report.
“Outbreak of pandemic, and regular research has enhanced the role of pharmaceutical and biopharmaceutical companies in the healthcare industry. The companies are in continuous need of outsourcing for early phase development, clinical and laboratory testing of the developed drugs there by creating maximum demand for CROs, Since the method boosts the profit margins, aids in avoiding high expenses, and thus reduces the time to conduct the whole developing and testing process and validation procedure of product, the market is very promising for the future endeavors. New investors can create a partnership with the pharmaceutical companies for an effective business in the future,” said Mr. Karan Chechi, Research Director with TechSci Research, a research based Global management consulting firm.
“Global Contract Research Organization Market By Service (Early Phase Development Services, Clinical Research Services, Laboratory Services, Consulting Services and Data Management Services) By Size of CROs (Small Size, Medium Size, Large Size) By Application (Oncology, Central Nervous System, Cardiovascular, Respiratory, Infectious Diseases, Autoimmune Disorders, Others) By End User (Pharmaceutical and Biopharmaceutical Companies, Medical Device Companies, Academic Institutes) By Region, Competition Forecast & Opportunities, 2026”, has evaluated the future growth potential of Global contract research organization and provides statistics & information on market size, structure and future market growth. The report intends to provide cutting-edge market intelligence and help decision makers take sound investment decisions. Besides, the report also identifies and analyzes the emerging trends along with essential drivers, challenges, and opportunities in Global contract research organization.
Browse Related Reports:
Global Next-generation Sequencing Market By Product (Consumables, Platforms and Services), By Technology (Sequencing by Synthesis, Ion Semiconductor Sequencing, Sequencing by Ligation, Single Molecule Real Time Sequencing and Other Technologies), By End User (Academic & Clinical Research Centers, Pharmaceutical & Biotechnology Companies, Hospitals & Clinics and Others), By Application (Biomarkers & Cancer, Diagnostics, Reproductive Health, Personalized Medicine, Agriculture & Animal Research and Other Applications), By Region, Competition, Forecast & Opportunities, 2025
https://www.techsciresearch.com/report/next-generation-sequencing-market/4508.html
Global CAR-T Cell Therapy Market By Indication (Diffused Large B-cell Lymphoma (DLBCL), Acute Lymphoblastic Leukemia (ALL), Multiple Myeloma (MM), Chronic Lymphocytic Leukemia (CLL), Follicular Lymphoma (FL), Others), By Targeted Antigen(CD 19, CD22, BCMA (B-Cell Maturation Antigen), HER1, HER2, Meso, EGFRvlll, Others), By Product Type (Yescarta (Axicabtagene Ciloleucel), Kymriah (Tisagenlecleucel), JCAR017 (Lisocabtagene Maraleucel), bb2121), By End User (Hospitals, Cancer Treatment Centers, Others), By Region, Forecast & Opportunities, 2025
https://www.techsciresearch.com/report/car-t-cell-therapy-market/5168.html
Contact
Mr. Ken Mathews
708 Third Avenue,
Manhattan, NY,
New York – 10017
Tel: +1-646-360-1656
Email: [email protected]
Website: https://www.techsciresearch.com/
For More Market Research Blogs Visit: https://techsciblog.com/
#TechSci#Market Research Reports#Healthcare#Global Contact Research Organization Market#Contact Research Organization Market#Contact Research Organization Market Size#Contact Research Organization Market Share#Contact Research Organization Market Trend#Contact Research Organization Market Forecast
0 notes
Text
Major Key Players of preclinical CRO market & Industry share 2021–2027
A research study conducted by Global Market Insights, Inc., suggests that preclinical CRO market is likely to exceed the $7.8 billion mark by 2027, in terms of revenue. Rise in number of drug development activities and growing R&D expenditure in the healthcare sector is expected to drive the preclinical CRO market growth over the forthcoming years.
Ongoing preclinical trials in oncology and the increased number of investigational compounds will pave the way for the growth of preclinical CRO business size in the coming years. Additionally, growing concerns of the government regarding the efficacy, drug safety along with the preclinical CROs will further augment business landscape.
Request for sample of this research report @ https://www.gminsights.com/request-sample/detail/2860
Some of the major companies functioning in the preclinical CRO market include Envigo, Laboratory Corporation of America Holdings, Charles River Laboratories, and Pharmaceutical Product Development among others. Industry players are taking part in strategic initiatives like new/innovative product launches, mergers, acquisitions, and business expansion. For instance, in 2018, Charles River Laboratories acquired MPI Research, a non-clinical research organization that provides comprehensive testing services. This strategy enabled the company to expand early-stage contract research offerings for medical device and biopharmaceutical markets.
As per service type, the preclinical CRO industry is classified into DMPK and bioanalysis studies, toxicology testing, and others. The toxicology testing service segment accounted for USD 1.7 billion of the total market revenue. Toxicity studies include testing of toxicity outline of a drug by sensing its effect on functionality or on organ structure. These studies are important in the preclinical phase, as they recognize and portray all the toxicities related to a drug to anticipate unfavorable events. These benefits could boost the adoption of toxicology testing services in preclinical trial phase.
The preclinical CRO segment is bifurcated into government & academic institutes, medical device companies, biopharmaceutical companies, and others based on end-use. The government and academic institutes will show lucrative growth of 5.6% between 2021 to 2027 owing to the growing number of outsourcing research activities to academic institutes.
The Preclinical CRO market of Asia Pacific is expected to grow at a significant pace of 8.6% during the forecast timeframe. Increasing prevalence of chronic diseases such as cancer, diabetes, and cardiac diseases is the reason for high growth rate in the region.
Browse key industry insights spread across 104 pages with 113 market data tables & 14 figures & charts from the report, “Preclinical CRO Market Size By Service (Bioanalysis & DMPK Studies, Toxicology Testing), By End-use (Biopharmaceutical Companies, Government & Academic Institutes, Medical Device Companies), Industry Analysis Report, Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2021 – 2027” in detail along with the table of contents:
https://www.gminsights.com/industry-analysis/preclinical-cro-market
About Global Market Insights:
Global Market Insights, Inc., headquartered in Delaware, U.S., is a global market research and consulting service provider; offering syndicated and custom research reports along with growth consulting services. Our business intelligence and industry research reports offer clients with penetrative insights and actionable market data specially designed and presented to aid strategic decision making. These exhaustive reports are designed via a proprietary research methodology and are available for key industries such as chemicals, advanced materials, technology, renewable energy and biotechnology.
Contact Us:
Arun Hegde
Corporate Sales, USA
Global Market Insights, Inc.
Phone:1-302-846-7766
Toll Free: 1-888-689-0688
Email: [email protected]
Web: https://www.gminsights.com/
0 notes
Text
Contract Pharmaceutical Manufacturing Market Analysis (2020-2027)
Pharmaceutical companies are now pushing hard to reduce overall manufacturing and research costs by outsourcing various processes related to research, development and manufacturing. Furthermore, patent expiration and generic drug competition, continue to fuel demand for pharmaceutical contract manufacturing organizations in the market.
Rising number of Food and Drug Administration (FDA) approvals and clinical trials are supporting growth of biopharmaceutical industry, which in turn is fueling growth of the contract pharmaceutical manufacturing market. For instance, according to ClinicalTrials.gov, from September 2008 to October 2018, there were around 288,064 clinical trial studies registered. In addition, there is a significant increase over the past few years with registered clinical studies of 205,428, 233,234, and 262,429 in 2015, 2016, and 2017, respectively.
Furthermore, stringent regulatory policies for clinical research studies and manufacturing make entire drug manufacturing process more complex, as it requires more resources to develop new drugs and biologics. These processes require expertise in broad scientific disciplines of preclinical, clinical, ancillary clinical in chemistry, packaging, manufacturing, project management, and regulatory affairs, which are provided by the CROs and CMOs. This is considered as a major reason for pharmaceutical companies to outsource clinical trials and manufacturing processes.
Market Dynamics
Contract manufacturing organizations (CMOs) provide wide range of manufacturing services, which include contract packaging, quality testing, and development service to pharmaceutical and biotechnology industries. Biopharmaceutical companies prefer CMOs due to the complexity involved in manufacturing process of biomolecules, as it consists of different shape, size, and behavior with significantly complex process than pharmaceutical drugs. Furthermore, by outsourcing manufacturing capabilities from contract manufacturing organization, allows recruiter firms to focus more on the research and development (R&D), product development, and marketing aspects.
Pharmaceutical market is increasingly concentered with generic products and is highly competitive with various companies located in Asia Pacific, which are entering into the developed markets such as the U.S., Germany, France, and the U.K. Rising number of patents expiring in the near future serves to be a major opportunity for generic drugs manufacturers to prosper in the market.
Furthermore, mergers and acquisitions between generic drug manufacturers, with major players focusing on enhancing their product portfolio through inorganic strategies, will support the addition of generic drug portfolio into company’s offering and thus, generating demand for CMOs to fulfil it.
For instance, in August 2016, Teva Pharmaceuticals Industries strengthened its position in the generic drugs market through acquisition of the generic segment of Allergan, plc for US$ 40.5 billion. This resulted in significant growth in revenue contribution of its generic drug segment, pegged at US$ 9.5 billion in 2016.
Key features of the study:
· This report provides in-depth analysis of contract pharmaceutical manufacturing market, its market size (US$ Million) and Compound Annual Growth Rate (CAGR %) for the forecast period (2018 – 2026), considering 2017, as the base year.
· It elucidates potential revenue opportunity across different segments and explains attractive investment proposition matrix for this market
· This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, regional outlook, and competitive strategy adopted by the leading players
· It profiles leading players in the global contract pharmaceutical manufacturing market based on the following parameters – company overview, financial performance, product portfolio, geographical presence, distribution strategies, key developments and strategies.
· Key companies covered as a part of this study includes Accenture plc, Cognizant Technology Solutions, ATOS SE, Catalent, Inc., Covance, Inc., Boehringer Ingelheim GmbH, Genpact Limited, Lonza Group, PAREXEL International Corporation, Quintiles Transnational Corporation, Abbvie, Inc., Baxter International Inc., Dr. Reddy’s Laboratories Ltd., Aurobindo Pharma, Pfizer, Inc., The Almac Group, Teva Pharmaceutical Industries Ltd. and Piramal Enterprises Ltd.
· Insights from this report would allow marketers and the management authorities of the companies to make informed decision regarding their future type upgradation, market expansion, and marketing tactics.
· The global contract pharmaceutical manufacturing market report caters to various stakeholders in this industry including investors, researchers, contract pharmaceutical manufacturers, distributors, new entrants, and financial analysts.
· Stakeholders would have ease in decision making through the various strategy matrices used in analyzing the contract pharmaceutical manufacturing market.
Detailed Segmentation:
· Global contract pharmaceutical manufacturing market, By Service Type:
o Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
o Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
· Global Contract Pharmaceutical Manufacturing Market, By Molecule Type:
o Small Molecules
o Large Molecules
· Global Contract Pharmaceutical Manufacturing Market, By Geography:
o North America
§ By Country:
§ U.S.
§ Canada
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
o Europe
§ By Country:
§ U.K.
§ Germany
§ Italy
§ Spain
§ France
§ Russia
§ Rest of Europe
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
o Asia Pacific
§ By Country:
§ China
§ India
§ Japan
§ ASEAN
§ Australia
§ South Korea
§ Rest of Asia Pacific
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
o Latin America
§ By Country:
§ Brazil
§ Mexico
§ Argentina
§ Rest of Latin America
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
o Middle East
§ By Country:
§ GCC
§ Israel
§ Rest of Middle East
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
o Africa
§ By Country:
§ North Africa
§ Central Africa
§ South Africa
§ By Service Type:
§ Contract Manufacturing Organization (CMO)
§ API Manufacturing
§ Final dosage form manufacturing
§ Packaging
§ Contract Research Organization (CRO)
§ Drug Discovery
§ Preclinical studies
§ Early Phase I - IIa
§ Phase IIa - III
§ Phase IIIb - IV
§ Medical coding and writing
§ Monitoring
§ Clinical Data Management
§ Bio-statistics
§ Site management
§ Protocol development
§ By Molecule Type:
§ Small Molecules
§ Large Molecules
· Company Profiles
o Accenture plc*
§ Company Overview
§ Product Portfolio
§ Financial Performance
§ Key Strategies
§ Recent Developments
o Cognizant Technology Solutions
o ATOS SE
o Catalent, Inc.
o Covance, Inc.
o Boehringer Ingelheim GmbH
o Genpact Limited
o Lonza Group
o PAREXEL International Corporation
o Quintiles Transnational Corporation
o Abbvie, Inc.
o Baxter International Inc.
o Dr. Reddy’s Laboratories Ltd.
o Aurobindo Pharma
o Pfizer, Inc.
o The Almac Group
o Teva Pharmaceutical Industries Ltd.
o Piramal Enterprises Ltd.
Request sample copy here :
https://www.coherentmarketinsights.com/insight/request-sample/2397
Request PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/2397
Click here to buy: https://www.coherentmarketinsights.com/insight/buy-now/2397
CMI Services: https://www.coherentmarketinsights.com/services
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
· Customized Market Research Services
· Industry Analysis Services
· Business Consulting Services
· Market Intelligence Services
· Long term Engagement Model
· Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email : [email protected]
Reference/Source: https://www.coherentmarketinsights.com/market-insight/contract-pharmaceutical-manufacturing-market-2397
0 notes
Text
Contract Research Organization (CRO) Market Size, High Demand, Manufacturers, Revenue, Growth Drivers,Traders and Product Scope till
Contract Research Organization (CRO) services (CRO) are used in the biotechnology, pharmaceutical and medical device industries. Also, Contract Research Organization (CRO) (CRO) offers various services such as biologic assay development, biopharmaceutical development, preclinical research, Clinical Research, Clinical Trail Management, and Pharmacovigilance. Contract research organizations are mainly designed to minimize cost for companies developing new drugs.
Rise in research and development activities which are expected to propel the growth of global Contract Research Organization (CRO) (CRO) market growth. Furthermore, high demand for outsourcing analytical testing and clinical trial services which are expected to boost the growth of global Contract Research Organization (CRO) (CRO) market. In addition to that, increase in regulatory pressure on contract research services will have the positive impact on global Contract Research Organization (CRO) (CRO) market growth.
Get Sample Copy of this Report @ https://qualiketresearch.com/request-sample/Contract-Research-Organization-CRO-Market/request-sample
However, lack of skilled professional is the major restraining factor which is expected to hamper the global Contract Research Organization (CRO) (CRO) market growth. Also, aligning of personnel expertise for specific project will affect the global Contract Research Organization (CRO) (CRO) market growth.
Global Contract Research Organization (CRO) Market Segmentation
Global Contract Research Organization (CRO) Market is segmented into type such as Clinical Research Services, Early Phase Development Services, Laboratory Services, and Consulting services, by application such as Oncology, Infectious Diseases, Central Nervous System Disorders, Respiratory Disorders, Cardiovascular Diseases, Diabetes, and Others. Further, Global Contract Research Organization (CRO) Market is segmented into end user such as Medical Device Companies, Pharmaceutical & Biotechnological Companies, and Academic Institutes.
Global Contract Research Organization (CRO) Market Key Players
Various key players are discussed in this report such as IQVIA, PAREXEL,PPD, ICON PLC, Syneos Health,Charles River Laboratories,SGS, LabCorp, WuXI Pharmatech, MPI Research, and PRA Health Sciences.
Global Contract Research Organization (CRO) Market Taxonomy
By Types
Clinical Research Services
Early Phase Development Services,
Laboratory Services
Consulting services
By Application
Oncology
Infectious Diseases
Central Nervous System Disorders
Respiratory Disorders
Cardiovascular Diseases
Diabetes
Others
By End User
Medical Device Companies
Pharmaceutical & Biotechnological Companies
Academic Institutes
By Region
North America
Latin America
Europe
Asia Pacific
Middle East & Africa
Browse Full Research Report @ https://qualiketresearch.com/reports-details/Contract-Research-Organization-CRO-Market
About Us
QualiKet Research is a leading Market Research and Competitive Intelligence partner helping leaders across the world to develop robust strategy and stay ahead for evolution by providing actionable insights about ever changing market scenario, competition and customers. QualiKet Research is dedicated to enhancing the ability of faster decision making by providing timely and scalable intelligence. We use different intelligence tools to come up with evidence that showcases the threats and opportunities which helps our clients outperform their competition.
0 notes
Text
Contract Research Organization (CRO) Market Size, Analysis and Forecast Report to 2027
Latest published report on the Contract Research Organization (CRO) market, found on the Qualiket Research website revealed a great deal about various market dynamics. These driving factors influence the market from a very miniscule level to its holistic standard and can traverse limitations to assist the market achieve a significant growth rate over the analysis period of 2020-2027.
Request Sample Copy of this Report @ https://qualiketresearch.com/request-sample/Contract-Research-Organization-CRO-Market/request-sample
Contract Research Organization (CRO) services (CRO) are used in the biotechnology, pharmaceutical and medical device industries. Also, Contract Research Organization (CRO) (CRO) offers various services such as biologic assay development, biopharmaceutical development, preclinical research, Clinical Research, Clinical Trail Management, and Pharmacovigilance. Contract research organizations are mainly designed to minimize cost for companies developing new drugs.
Market Drivers
Rise in research and development activities which are expected to propel the growth of global Contract Research Organization (CRO) (CRO) market growth. Furthermore, high demand for outsourcing analytical testing and clinical trial services which are expected to boost the growth of global Contract Research Organization (CRO) (CRO) market. In addition to that, increase in regulatory pressure on contract research services will have the positive impact on global Contract Research Organization (CRO) (CRO) market growth.
Market Restraints
However, lack of skilled professional is the major restraining factor which is expected to hamper the global Contract Research Organization (CRO) (CRO) market growth. Also, aligning of personnel expertise for specific project will affect the global Contract Research Organization (CRO) (CRO) market growth.
Global Contract Research Organization (CRO) Market Key Players
Various key players are discussed in this report such as IQVIA, PAREXEL, PPD, ICON PLC, Syneos Health,Charles River Laboratories,SGS, LabCorp, WuXI Pharmatech, MPI Research, and PRA Health Sciences.
Global Contract Research Organization (CRO) Market Taxonomy
By Types
Clinical Research Services
Early Phase Development Services,
Laboratory Services
Consulting services
By Application
Oncology
Infectious Diseases
Central Nervous System Disorders
Respiratory Disorders
Cardiovascular Diseases
Diabetes
Others
By End User
Medical Device Companies
Pharmaceutical Biotechnological Companies
Academic Institutes
By Region
North America
Latin America
Europe
Asia Pacific
Middle East Africa
Get discount on this report @ https://qualiketresearch.com/request-sample/Contract-Research-Organization-CRO-Market/ask-for-discount
About Us:-
QualiKet Research is a leading Market Research and Competitive Intelligence partner helping leaders across the world to develop robust strategy and stay ahead for evolution by providing actionable insights about ever changing market scenario, competition and customers. QualiKet Research is dedicated to enhancing the ability of faster decision making by providing timely and scalable intelligence. We use different intelligence tools to come up with evidence that showcases the threats and opportunities which helps our clients outperform their competition.
Contact Person:-
Vishal Thakur
Research Support Specialist
QualiKet Research
6060 N Central Expy #500, TX 75204, U.S.A
Email: [email protected]
Website: https://qualiketresearch.com
0 notes
Text
Healthcare Contract Research Outsourcing Market Revenue Growth, New Launches, Regional Share Analysis & Forecast Till 2030
Market Overview:
The Global Healthcare Contract Research Outsourcing Market held USD 36.9 billion in 2020 and is to grow with a CAGR of 6.4% from 2020-2030. Healthcare and pharmaceutical companies outsource not only drug production but also their clinical trials. With rising privatization of clinical trials, the outsourcing to developed countries is on the increase.
Companies in pharmaceutical sector have begun outsourcing R&D activities which are complex and require regular monitoring. Healthcare CROs functions and manages the processes of bringing new products to the market according to the customer's timeline provided. Emerging economies like Japan, India and China are chosen to outsource business.
Many healthcare contract research organizations are now forming partnerships to expand their market reach by providing services across a wide market space and strengthening the relationship between client and contractor. IQVIA for instance formed an alliance with Box in 2018. This partnership is intended to assist the company in offering cloud-based content management solutions for healthcare and life science companies. Key players are in the process of purchasing other contract research organizations, in addition to partnerships, to achieve leverage.
Market Dynamics and Factors:
Increasing investment in R&D programs, growing demand for outsourcing activities due to time and cost constraints in the healthcare sector are key factors expected to drive the healthcare contract research outsourcing market growth in the years to come. Contract research outsourcing collaborations offer state-of-the-art services and therefore government organizations prefer to assign projects to contract research organizations (CROs), thus facilitating market demand. Increasing pressure on drug producers in terms of clinical data management, regulatory environments, and stringent safety standards is expected to drive demand within the healthcare sector for contract research organizations. Models including transactional relationships, Multi-FSP, and alliances are widespread and are widely adopted by drug producers.
Request for Sample with Complete TOC and Figures & Graphs @https://www.trendsmarketresearch.com/report/sample/13669
Market Segmentation:
Based on the type, the healthcare contract research outsourcing industry is segmented into drug discovery, pre-clinical and clinical. Drug discovery has been further segmented into target validation, lead optimization and lead identification. Clinical has been further segmented into phase I, phase II, phase III and phase IV trial services. On the basis of services, the market is segmented into project management, regulatory/medical affairs, investigator payments, data management, clinical monitoring, quality management/ assurance, medical writing, bio-statistics, and others. Geographical breakdown and analysis of each of the aforementioned segments includes regions comprising North America, Europe, Asia-Pacific, and RoW.
Geographic Analysis:
Direct Purchase this Market Research Report Now @https://www.trendsmarketresearch.com/checkout/13669/Single
North America dominates the healthcare contract research outsourcing market share with the large demand clinical trials in the region. The region is also a hub of advanced healthcare infrastructure. In addition, growing government support for R&D activities through grants and funds to research institutes and companies has driven this regional market. Additionally, increasing government funding for R&D activities has guided this regional market through grants and funds to research institutes and companies. For example, in January 2015, the U.S. government announced a USD 215.0 million investment for the precision medicine initiative. The investment is for the NIH, the National Institute for Cancer (NCI) and the FDA. Asia Pacific held the largest share of the CRO healthcare market in 2019 and is expected to grow rapidly over the forecast period due to the availability of diverse populations, high prevalence of chronic conditions, easy recruitment and retention of patients and the establishment of regulations according to accepted standards.
Competitive Scenario:
Get Discount On The Purchase Of This Report @https://www.trendsmarketresearch.com/report/discount/13669
Global healthcare contract research outsourcing market growth is witnessing fierce competition. In terms of healthcare contract research outsourcing market share, certain players gather majority of market share. Key companies are also expanding their manufacturing facilities to cater to growing demand for this drug globally. Key players enhancing the global Healthcare Contract Research Outsourcing market size include Zydus Cadila, Bayer, Laurus Labs, Sun Pharma, Sanofi, Ipca Laboratories Ltd, Novartis AG, Teva Pharmaceutical Industries Ltd and McW Healthcare. Charles River Laboratories International, Inc., for example, purchased WIL Research for close to USD 585 million in 2016. This acquisition is probable to support enhance company foothold in the healthcare sector, particularly in the early stages of drug discovery business.
0 notes
Text
By 2027, U.S. Contract Research Organization (CROs) Market To Surpass US$ 25,049.0 Mn
From 2018 to 2027, U.S. Contract Research Organization (CROs) Market To show CAGR of 9.4%
Request for Sample PDF Copy:
https://www.coherentmarketinsights.com/insight/request-pdf/3179
Description:
The U.S. Contract Research Organization (CROs) Market, by Service Type (Drug Discovery, Preclinical studies, Early Phase I – IIa, Phase IIa – III, Phase IIIb – IV, Drug Development, Medical Coding and Writing, Monitoring, Clinical Data Management, Bio-statistics, Site Management, Protocol Development, and Biomarker Discovery), by Therapeutic Application (Oncology, Cardiovascular Diseases, Central Nervous System Diseases, Infectious Diseases, Metabolic Disorders, Immunological Disorders, Respiratory Disorders, and Others), and by Size of CRO (Small Size, Medium Size, and Large Size), is estimated to be valued at US$ 12,174.4 million in 2019, and is expected to exhibit a CAGR of 9.4%, during the forecast period (2019-2027), as highlighted in a new report published by Coherent Market Insights.
Globally, there are several healthcare IT companies, which develop and modify clinical trial management software. There is availability of customized software to manage clinical trials data according to client requirement and study protocol. For instance, according to the study conducted by the University of Minnesota and University of Michigan in 2018, all the companies that conduct clinical trials use OnCore clinical trial management system, which offers functional features such as Clinical Research Management, Study Setup, eCRFs, Financials, Visit Tracking & Data Capture, Study Data Management, and Revenue Management. This is expected to be one of the important factors expected to drive growth of the clinical trial management market over the forecast period.
Request Customized Report Here:https://www.coherentmarketinsights.com/insight/request-customization/3179
Browse 09 Market Data Tables and 27 Figures spread through 301 Pages and in-depth TOC on " U.S. Contract Research Organization (CROs) Market, by Service Type (Drug Discovery, Preclinical studies, Early Phase I – IIa, Phase IIa – III, Phase IIIb – IV, Drug Development, Medical Coding and Writing, Monitoring, Clinical Data Management, Bio-statistics, Site Management, and Protocol Development, and Biomarker Discovery), by Therapeutic Application (Oncology, Cardiovascular Diseases, Central Nervous System Diseases, Infectious Diseases, Metabolic Disorders, Immunological Disorders, Respiratory Disorders, and Others), and by Size of CRO (Small Size, Medium Size, and Large Size), - Global Forecast to 2027"
To know the latest trends and insights related to U.S. Contract Research Organization (CROs) Market, click the link below: https://www.coherentmarketinsights.com/market-insight/us-contract-research-organizations-market-3179
Growth of the U.S. contract research organization (CROs) market is largely driven by increased research & development expenditure and increasing outsourcing. This has attributed to shift in the industry focusing towards development of therapeutics for rare diseases, as more biopharmaceutical manufacturers have increased focus towards rare diseases. Therefore, increasing research and development expenditure in the healthcare sector is a major factor that is expected to drive growth of the U.S. contract research organization (CROs) market during the forecast period.
For instance, according to the Pharmaceutical Research and Manufacturers of America (PhRMA), 2018, overall research and development spending of pharmaceutical and biotechnology companies was around US$ 62.2 billion in 2018 compared to US$ 55.8 billion in 2017, which was majority of all biopharmaceutical R&D spending in the U.S. This increasing research and development expenditure has led to an increase in number of drug approvals in the recent past. For instance, according to the U.S. FDA reports, around 59 novel drugs were approved in 2018 as compared to 46 drugs in 2017. Adoption of inorganic strategies such as acquisitions and collaborations by major players in CRO market is expected to drive the U.S. contract research organization (CROs) market.
For instance, in March 2018, contract research organizations (CROs): ICON plc, Medpace, Pharmaceutical Product Development, LLC (PPD), PRA Health Sciences, Syneos Health, UBC, and Veeva Systems entered into a collaboration to introduce Align Clinical CRO: a new industry standards group for sponsors and CROs to work together during clinical trials. Moreover, in June 2018, LabCorp acquired Sciformix Corporation, a scientific process outsourcing company focused on pharmacovigilance and regulatory solutions for biopharmaceutical and medical device clients.
Key Takeaways of the U.S. Contract Research Organization (CROs) Market:
The U.S. contract research organization (CROs) market is expected to exhibit a CAGR of 9.4% during the forecast period (2019-2027), owing to increasing demand for specialized clinical trial services and increasing number of clinical trials is expected to drive the Early Phase I – IIa segment growth during the forecast period. For instance, in 2018, LabCorp introduced a dedicated offering for biotech, medical device, and diagnostics companies. The new service offering comprises development services, including non-clinical and first-in-human studies, Phase I development, central laboratory services, and regulatory and market access consulting.
Technological advancement in the field of clinical research to improve efficiency and efficacy of clinical trials is expected to drive the monitoring segment growth in the U.S. contract research organization (CROs) market during the forecast period. For instance, in February 2019, IQVIA Holdings Inc. launched IQVIA RIM Smart, an integrated, cloud-based, end-to-end regulatory information management solution for the management of a product portfolio’s complete regulatory lifecycle. The system is expected to increase speed, efficiency, and improve compliance performance.
Major players operating in the U.S. contract research organization (CROs) market include Laboratory Corporation of America Holdings (Covance), IQVIA, Paraxel International Corporation, Syneos Health, PRA Health Sciences, Charles River Laboratoires International Inc. (CRL), Pharmaceutical Product Development (PPD), ICON Public Limited Corporation, Wuxi Apptec, Medpace Holdings, Inc, Medidata Solutions, Inc., Theorem Clinical Research, Pharmaron, Envigo, Clinipace, CMIC Holdings Co., Ltd, EPS International, Synteract, CROMSOURCE, Pharm-Olam, Linical Accelovance, Accumedix, Inc., AlcheraBio LLC, Amarex Clinical Research LLC, Arianne Corporation, Absolute Research Solutions, BioClinica Inc., BioPharma Services Inc., Neurovasc Preclinical Services, Inc., PSI, WCCT Global Inc., RHO, Inc., CATO Research LLC, Spaulding Clinical Research, Celerion, Clindatrix, Inc., Comparative Biosciences, Inc., Axiom Real-Time Metrics, CPC Clinical Research, Axis Clinicals LLC, DP Clinical, Egeen Inc., Grayline, Cmed, Integrium, LLC, MedTrials, Novum, Shin Nippon Biomedical Laboratories, Ltd., KPS Life, Pinnacle Research Group, LLC Promedica International., Alta Science, Prometrika, LLC, 4clinics, and Premier Research.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes players from across all business verticals in over 150 countries worldwide. We do offer wide range of services such as Industry analysis, Consulting services, Market Intelligence, Customized research services and much more. We have expertise in many fields such as healthcare, chemicals and materials, Automation, semiconductors, electronics, energy, food and beverage, packaging and many more. Visit our website to know more.
Buy Report Here:
https://www.coherentmarketinsights.com/insight/buy-now/3179
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave.
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
10 Tips to Get Your Medical Device to Market
The procedure to bring medical devices in the market can be complicated, requiring a clear understanding of the medical device software, market analysis, fulfilling the regulatory compliance of the Food and Drug administration (FDA) which include myriads of regulations.

Here are the top 10 tips in the process that medical device manufacturers should consider.
1. Understanding of the product overview
Though it seems obvious, but it is important for medical device manufacturers to have a clear understanding of what class of device has been created. Every medical device software has a different testing requirements, cost and timelines and upon completion will have a different pool of requirements for regulatory, testing, validation and aspects of clearance or approval procedure. Every medical device has unique product specification and require different aspects of mechanical testing. Hence, a clear understanding of the medical device is necessary.
2. Project timeline and cost involved
A 360-degree understanding of the project timeline before even beginning the project is essential which includes estimates for each individual phase and not just overall project time phase. Developing Software as a medical device (SaMD) and software in a medical device (SiMD) requires working among different departments and involves various teams. Thus, defining a timeline is important for managing the resources efficiently and creating an accurate budgetary estimate. Funding also plays an important role in achieving milestones, so manufacturers need to be careful and require thorough planning to keep all project budgets in check in the beginning.
3. Creating a prototype
Before manufacturing the final product into the market, manufacturers consider creating a prototype to troubleshoot problems and improve certain features of the medical device. A prototype of medical device gives a more realistic representation of the end product. Once the team is satisfied with the prototype, it is easy to picture the real product. This working prototype can be helpful in doing basic feasibility studies. The purpose of these studies is to undermine whether you should move forward with pursuing a full-fledged project.
4. Classification of medical devices and understanding the applicable controls
The first and foremost step to market a medical device by medical device companies is to determine how the FDA has classified the medical device. Generally, medical devices are categorized into three classes (I, II, III) which is based on the degree of risk they possess on people’s lives. As the class of medical device move from class I to class II to class III, the regulatory control is subject to become strict.
5. Selection and preparation for the right premarket submission
There is no single approach to the marketplace. The medical device manufacturers should select and arrange premarket submission, required for specific products classification. Most medical devices need to have an appropriate submission type within the product classification which can be obtained from the public product classification database.
6. Adherence to applicable regulatory compliance
Fulfilling the regulatory compliance is crucial for medical devices to market as it involves lives. It also provides FDA the insight about the effectiveness and safety of the medical device. So, it becomes pertinent for medical device companies to manufacturer medical devices in accordance with the good manufacturing practises and label their devices in accordance with the labelling regulations without adulteration or misbranding.
7. Early stage deliberations
There are many costs that are overshowed by medical device manufacturers during the early process of the product. To bring a medical device into the market, considering every cost associated with manufacturing equipment, medical device testing, certifications with regulatory bodies, product registrations, clinical trials and much more is important. Before planning to build a manufacturing medical device, a robust and exhaustive plan can help in the success of the project.
8. Hire the right team
A dedicated and experienced team is important for medical device companies to market a full functional medical device to the market. Selecting a proper team or outsourcing resources can play an important role for medical device manufacturers. A capable and well managed team ensures that the medical device software meets the regulatory requirements specified in ISO 13485 and enable the release of safe software to the market. While choosing the right time, make sure they have extensive experience in testing standalone, web, mobile and embedded software for medical devices on various platform. They are capable of meeting the ever-growing demand for regression testing of medical software through test automation framework.
9. Implementing robust Testing & Quality management system
A medical device software and application testing requires several different types of testing including functional, regression, safety, performance etc. Before marketing a medical device, it is important to set up a framework of robust testing mechanism to ensure everything in the medical device is at place. Before bringing a product into the market, certain criteria under the Quality Management needs to be taken care. the rule of 1 size fits all doesn’t apply in medical devices. There are multiple regulatory bodies who expect to follow certain regulations. These criteria for medical devices can be fulfilled by establishing a QMS to comply with their expectations.
10. Risk Management
While designing or creating a medical device, patient safety is of paramount importance. Thus, most engineers are likely to think about mitigating risks with the initiation of the device. Creating a device that can save lives and improve the quality of life for end users is a challenge and comes with a lot of responsibility. So, the medical device design should always consider the risk outlined in the ISO 14971 on risk management and plan medical devices accordingly.
Conclusion
Bringing a robust medical device to the market could be complicated and stressful for a news developer. However, by implementing a strategic planning from the beginning can eliminate changes of uncertainty and ensure a smooth process while improving chances of bringing your product to the market.
MicroGenesis, with around 20+ years of experience in the medical device companies can help you with its medical device software solutions. MicroGenesis is a leading software solutions provider for medical devices and applications. They provide a single window of services in Development, Testing and Regulatory Compliance for medical software including Software as a Medical Device (SaMD) and Software for a Medical Device (SiMD).
0 notes
Text
Pharmacovigilance Market
Pharmacovigilance Market was valued US$ XX Bn in 2018 and is expected to reach US$ XX Bn by 2026, at CAGR of XX% during forecast period of 2019 to 2026.
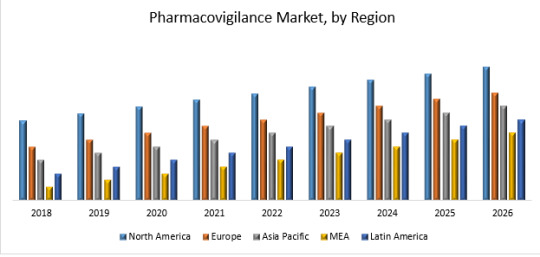
Pharmacovigilance Market Drivers and Restrains:
Pharmacovigilance is a scientific discipline, which is conducted in real time to ensure that early identification of safety signals. The pharmacovigilance markets are driven by factors such as increasing consumption of drugs, rising adoption of outsourcing services, rise in the cases of adverse drug reactions and drug toxicity would boost demand for pharmacovigilance activities. However, high risk associated with data security in pharmacovigilance outsourcing and lack of availability of skilled labors are restraining the market growth at global level. Inadequate pharmacovigilance system for herbal products in Asia Pacific might hamper the market growth. Leading pharma companies in urbanized countries are focusing on outsourcing PV service in an attempt to reduce cost and to minimize operational expenses are anticipated to provide an opportunity for contact research organizations in emerging regions to gain more revenue share.
Pharmacovigilance Market Segmentation Analysis:
Based on the clinical trial phase, the pharmacovigilance market has been segmented into Pre-clinical, Phase I, Phase II, Phase III and Phase IV. The phase IV segment held the dominant market share in 2018 owing to safety worries relating to marketed products, growing necessity of designing systems to compare safety profiles of homologous medicinal products, and growth in public health awareness campaigns. The rising awareness about the importance of phase 4 clinical trials is likely to boost this segment’s growth in the years ahead.
Based on the service provider, the pharmacovigilance market has been segmented into in-house and contract outsourcing. The contract outsourcing service (CRO) provider segment is expected to grow at the largest CAGR of XX% during the. The CROs provide specialized services, enable better monitoring and reporting of drug effects at a significantly low cost. Thus, many manufacturers are increasingly outsourcing their pharmacovigilance associated work to major industry players in developing countries.
Pharmacovigilance Market Regional Analysis:
Geographically, the pharmacovigilance market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America held the largest share of the Pharmacovigilance market in 2018 due to increased death rates because of adverse drug reactions (ADRs) and rise in patient fears regarding safety of medicinal products. According to the Centers for Disease Control and Prevention (CDC), around XX deaths caused by ADRs in the U.S., which are counted among the top ten leading causes of death in the country. The market in Asia Pacific is expected to expand at a high CAGR of XX% during the forecast period. Stringent regulations for reporting adverse drug reactions coupled with rising demand for pharmacovigilance outsourcing around the nation will bolster the market growth. Lower labor cost as well as attractive market place for conducting clinical trials will positively impact the regional market growth.
A report covers the recent development in market for the market i.e. Accenture Company entered in a cooperative agreement with BioCelebrate to develop a platform for aggregating and analyzing clinical information for improved drug emerging efficiency, thus enhancing its R&D capabilities.
Pharmacovigilance Market Competitive landscape
Major Key players operating in this market are Accenture, IBM Corporation, Wipro, Cognizant, and Capgemini. Manufacturers in the Pharmacovigilance are focusing on competitive pricing as the strategy to capture significant market share. Moreover, strategic mergers and acquisitions and technological innovations are also the key focus areas of the manufacturers.
The objective of the report is to present a comprehensive analysis of Pharmacovigilance Market including all the stakeholders of the industry. The past and current status of the industry with forecasted market size and trends are presented in the report with the analysis of complicated data in simple language. The report covers all aspects of the industry with a dedicated study of key players that includes market leaders, followers and new entrants by region. PORTER, SVOR, PESTEL analysis with the potential impact of micro-economic factors by region on the market are presented in the report. External as well as internal factors that are supposed to affect the business positively or negatively have been analyzed, which will give a clear futuristic view of the industry to the decision-makers. The report also helps in understanding Pharmacovigilance Market dynamics, structure by analyzing the market segments and project the Pharmacovigilance Market size. Clear representation of competitive analysis of key players By Type, Price, Financial position, Product portfolio, Growth strategies, and regional presence in the Pharmacovigilance Market make the report investor’s guide.
For more information visit:https://www.maximizemarketresearch.com/market-report/pharmacovigilance-market/39235/
Scope of the Pharmacovigilance Market:
Pharmacovigilance Market, by Clinical Trial Phase:
• Pre-clinical
• Phase I
• Phase II
• Phase III
• Phase IV
Pharmacovigilance Market, by Type:
• Spontaneous Reporting
• Intensified ADR Reporting
• Targeted Spontaneous Reporting
• Cohort Event Monitoring
• EHR Mining
Pharmacovigilance Market, by Service Provider:
• In-house
• Contract Outsourcing
Pharmacovigilance Market, by End User:
• Hospitals
• Research Organizations
• Industrial
Pharmacovigilance Market, by Region:
• Asia Pacific
• North America
• Europe
• Latin America
• Middle East Africa
Pharmacovigilance Market, Major Players:
• Accenture
• Clinquest Group B.V.
• Cognizant
• Laboratory Corporation of America Holdings
• IBM Corporation,
• ArisGlobal
• ICON plc.
• Capgemini
• ITClinical
• iMEDGlobal Corporation
• Foresight Group International AG
• TAKE Solutions Ltd.
• PAREXEL International Corporation
• BioClinica
• Wipro Ltd.
• United BioSource Corporation.
• Bristol- Myers Squibb
• F. Hoffmann-La Roche
• Pharmaceutical Clinical Trial Phase Development
• Pfizer
• Novartis International
• GlaxoSmithKline
• iGATE Corporation
• inVentiv Health
This Report Is Submitted By : Maximize Market Research Company
Customization of the report:
Maximize Market Research provides free personalized of reports as per your demand. This report can be personalized to meet your requirements. Get in touch with us and our sales team will guarantee provide you to get a report that suits your necessities.
About Maximize Market Research:
Maximize Market Research provides B2B and B2C research on 20,000 high growth emerging opportunities & technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics & Communications, Internet of Things, Food and Beverages, Aerospace and Defense and other manufacturing sectors.
Contact info:
Name: Lumawant Godage
Organization Address: MAXIMIZE MARKET RESEARCH PVT. LTD.
Email: [email protected]
Address : Pune, Maharashtra 411051, India.
Contact: +919607195908
0 notes