#Chemokine
Explore tagged Tumblr posts
Photo
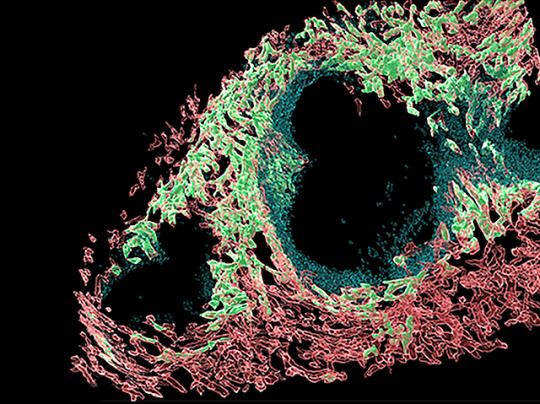
Directing Traffic
Chemical messengers called chemokines are the traffic police of your body, telling cells on the move where to go via a chemokine concentration gradient. Atypical chemokine receptors (ACKRs) on certain cells help create these gradients by binding and engulfing specific chemokines. Three called GPR182, ACKR3 and ACKR4 are located in lymph and blood vessels, and research suggests may be found together in certain microenvironments within organs. However, there’s no comprehensive map of where they are. Researchers now genetically engineer mice with fluorescently-tagged GPR182, ACKR3, ACKR4 and ACKR-specific chemokines to locate them. Fluorescence microscopy revealed unique and shared distribution patterns of these ACKRs in a variety of organs, including the spleen (pictured, ACKR4 in green, GPR182 in red). Meanwhile, fluorescently-tagged chemokines revealed distinct activity zones for ACKR4 and GPR182 in the liver. These mouse models, therefore, provide a useful tool to probe ACKRs in different organs and microenvironments.
Written by Lux Fatimathas
Image from work by Serena Melgrati and colleagues
Institute for Research in Biomedicine, Università della Svizzera italiana, Bellinzona, Switzerland
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in PLOS Biology, May 2023
You can also follow BPoD on Instagram, Twitter and Facebook
6 notes
·
View notes
Link
0 notes
Text
Endothelial inflammation in COVID-19 - Published Nov 28, 2024
The vascular endothelium forms a crucial interface between tissues and the blood stream and maintains normal blood flow (1). In its homeostatic state, the endothelium resists blood clotting, vasoconstriction, and inflammation and maintains selective barrier functions. This tightly regulated suite of properties can shift rapidly to unleash a series of functions vital to stanch blood loss from wounds or mobilize innate and adaptive immune defenses to repair injury and fight pathogenic microorganisms. But these defensive actions of endothelial cells can, if overexuberant, aggravate disease. Infection with severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) has highlighted how altered endothelial functions contribute to multiorgan health effects during the acute phase of COVID-19 and potentially to the longer-term consequences associated with Long Covid.
The resting endothelial cell has multiple mechanisms that resist thrombosis (blood clotting) and favor fibrinolysis (clot clearance), including anticoagulant heparan sulfate proteoglycans (1) (see the figure). Nitric oxide and prostacyclin produced by the endothelium combat platelet aggregation and promote vasodilation. The cell surface protein thrombomodulin binds thrombin and paradoxically confers anticoagulant properties on this usually procoagulant molecule by enabling thrombin to activate protein C. This protein, in turn, proteolytically inactivates coagulation factors Va and VIIIa. The resting endothelium further promotes fibrinolysis by expressing plasminogen activators on its surface.
Endothelial function can quickly switch from the homeostatic state to a potentially pathogenic defense posture (see the figure). In response to proinflammatory cytokines, released in response to viral sepsis, the endothelium can produce tissue factor, a potent procoagulant, and plasminogen activator inhibitor–1 (PAI-1), an inhibitor of blood clot breakdown. Many endothelial cells release von Willebrand factor, a key mediator of thrombus formation. Proinflammatory cytokines also stimulate endothelial cells to increase their own production of cytokines and to express adhesion molecules that bind leukocytes as well as chemoattractant chemokines that beckon the adherent inflammatory cells to traverse the endothelium.
Pathogens such as SARS-CoV-2, which presumably first encounter respiratory epithelial cells, elicit an initial burst of cytokine release, including the proinflammatory interleukin-1 (IL-1) and interferons. Local phagocytes and the neighboring extensive pulmonary endothelial cell network amplify this cytokine release. The initial wave of IL-1 can induce the production of large amounts of IL-6 by many cell types. IL-6, in turn, instigates the acute phase response in hepatocytes, augmenting the production of fibrinogen (the immediate precursor of blood clots) and stimulating local endothelial production of PAI-1.
In diseases such as COVID-19, the early onset of fever indicates that IL-1 is acting on the hypothalamic thermoregulatory center. Thus, fever provides a clinical indicator of the systemic cytokine storm that can flip homeostatic functions of the endothelium to properties that promote thrombus accumulation and inflammation in many organs, even in early phases of the disease (2). In COVID-19 and other forms of severe sepsis, the endothelial barrier can become leaky, resulting in extravasation of fluid and blood constituents, notably into the lung’s gas exchange spaces (3). Instead of the low leukocyte trafficking in the homeostatic state, the inflamed endothelium expresses adhesion molecules for white blood cells, and local bursts of chemoattractants can direct the migration of inflammatory cells into tissues such as the lung, impairing oxygenation or impairing gas exchange. Inflammation can further cause vasoconstriction. In the presence of superoxide anion that is produced by activated leukocytes, the endogenous endothelial dilator nitric oxide can form the highly pro-oxidant species peroxynitrite, thereby propagating tissue damage. The potent vasoconstrictor endothelin produced by endothelial cells can also promote inflammation.
By disrupting normal endothelial functions, thrombosis can complicate many severe infections, including SARS-CoV-2 infection. The early and consistent elevation of D-dimer, a product of fibrin breakdown, and the correlation of its plasma concentrations to poor outcomes in COVID-19, support a systemic and ongoing activation of coagulation during the disease. COVID-19 can drive increased clot accumulation through changes to the endothelial cell surface and contents of the blood. In addition, activated polymorphonuclear leukocytes (also known as granulocytes) can undergo a specialized form of cell death that forms neutrophil extracellular traps (NETs). NETs at the endothelial surface can promote thrombosis by entrapping platelets and fibrin and activating the contact coagulation pathway.
During the early stage of the COVID-19 pandemic, cases of ischemic strokes were attributed to the obstruction of arteries that feed the brain. This complication represents an example of thrombosis of large arteries (4). In addition, microscopic postmortem examinations of the heart, kidney, and lungs in individuals who succumbed to COVID-19 have revealed widespread thrombosis in microvessels and evidence of NET formation (5, 6). These findings indicate that thrombosis contributes to multiorgan system failure in this disease by impairing local blood flow. Myocardial injury in COVID-19, affecting up to a third of hospitalized patients, can arise from such microvascular blood clots and can include vasomotor disorders that affect smaller arteries, increased oxygen demand owing to fever and tachycardia (rapid heartbeat), decreased coronary artery blood flow, and even direct infection of cardiomyocytes by SARS-CoV-2 (7). Venous thrombosis can also complicate COVID-19 (8) and is evident in up to 20% of patients. Clots in veins can travel to pulmonary arteries and provoke pulmonary embolism (9). Both microvascular blood clots and emboli in larger pulmonary arteries also contribute to the morbidity of COVID-19. Given the frequency of pneumonia in SARS-CoV-2 infection, the decreased gas exchange owing to concomitant pulmonary thromboembolic disease can contribute to worsened clinical outcomes.
Considerable interest in the long-term consequences of SARS-CoV-2 infection has emerged (10). Resolution and healing of acute inflammation can cause fibrosis in organs, including the lungs, kidneys, and heart. Magnetic resonance imaging indicates edema and fibrosis in many individuals recovering from COVID-19. Studies show impaired myocardial blood flow capacity persisting for more than 4 months in more than 40% of individuals who have recovered from COVID-19 compared with contemporaneous controls (11). Derangements in autonomic cardiovascular control resembling postural orthostatic tachycardia syndrome (POTS), which is characterized by inappropriate rapid heart action upon standing, can also affect people with Long Covid.
Endothelial function in health and disease In its resting state, the vascular endothelium promotes homeostasis through multiple mechanisms that resist blood clotting, vasoconstriction, and inflammation. Injury or infection [and their associated damage- and pathogen-associated molecular patterns ( DAMPs and PAMPs)] can trigger a rapid shift to an activated state to stem blood loss and mobilize immune defenses. Overactivation can lead to endothelial dysfunction and aggravate disease, as observed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.

Some individuals who have had COVID-19 report cognitive impairment, commonly referred to as “brain fog.” According to recent mouse studies (12), inflammatory activation within the central nervous system, mediated by the cytokine chemokine 11, may lead to central nervous system abnormalities that can impair cognition. Long-term consequences may accrue from multiple acute infectious processes caused by viruses or bacteria. These observations raise concerns about continued cardiovascular complications, such as impaired cardiovascular reserve and the persistence of microscopic fibrotic areas in the heart muscle that could become substrates for arrythmias in the long term.
What are the therapeutic implications of systemic inflammatory activation and dysregulated coagulation and thrombosis in SARS-CoV-2 infection? Given the evidence for a high prevalence of thromboembolic disease in vessels of all types, anticoagulant and/or antiplatelet therapy might be expected to improve outcomes. Yet, the current clinical evidence suggests the contrary: Antiplatelet therapy (with aspirin or a class of drugs known as P2Y12 inhibitors) confers no net benefit while augmenting bleeding risk (13). Likewise, anticoagulant administration has not improved outcomes and is also associated with increased bleeding risk in acute patients. Bleeding risks counterbalance the potential beneficial effects of these interventions. Thus, current practice guidelines recommend “prophylactic” or low-dose anticoagulation with heparin products only for hospitalized patients.
Because proinflammatory cytokines likely mediate much of the systemic inflammation in advanced COVID-19, many studies have evaluated anticytokine therapies. Glucocorticoids, such as dexamethasone, have proven useful in treating acute COVID-19. Neutralizing IL-6 has also received considerable attention (14). Indeed, the case of anti–IL-6 receptor therapy with the monoclonal antibody tocilizumab provides an instructive example of the confusion stemming from the rush to validate treatments for advanced COVID-19 (15). Despite glimmers of efficacy in retrospective observational or nonrandomized evaluations, the prospective randomized trials of tocilizumab have provided mixed results. Current guidance recommends anti–IL-6 therapy or baricitinib—an inhibitor of Janus kinase 1 and 2 implicated in COVID-19–associated inflammation—only in severely ill COVID-19 patients. Anti-inflammatory interventions may impair host defenses, providing a possible explanation for the lack of clear benefit of some such therapies.
The complications of SARS-CoV-2 infection highlight the key role of endothelial functions in health and disease. Unfortunately, the rush to respond to the public health emergency led to a proliferation of observational and nonrandomized studies that sowed more confusion than illumination. This experience should inform responses to future public health emergencies, encouraging a more concerted fashion. Coordinated public health messaging and deployment of proven preventive measures would limit the need for expensive advanced therapies with mixed results regarding efficacy and would lessen potential adverse effects when we confront the next pandemic.
Acknowledgments P.L. acknowledges support from the National Heart, Lung, and Blood Institute (1R01HL134892 and 1R01HL163099-01); the American Heart Association (18CSA34080399); the RRM Charitable Fund; and the Simard Fund.
References and Notes 1 M. A. Gimbrone Jr., G. García-Cardeña, Circ. Res. 118, 620 (2016).
2 P. Libby, T. Lüscher, Eur. Heart J. 41, 3038 (2020).
3 S. Xiong et al., J. Clin. Invest. 130, 3684 (2020).
4 S. Nannoni et al., Int. J. Stroke 16, 137 (2021).
5 M. Ackermann et al., N. Engl. J. Med. 383, 120 (2020).
6 J. E. Johnson et al., Am. J. Pathol. 192, 112 (2022).
7 P. Libby, J. Am. Coll. Cardiol. Basic Transl. Sci. 5, 537 (2020).
8 A. Di Minno et al., Semin. Thromb. Hemost. 46, 763 (2020).
9 Ò. Miró et al., Eur. Heart J. 42, 3127 (2021).
10 T. J. Gluckman et al., J. Am. Coll. Cardiol. 79, 1717 (2022).
11 B. Weber et al., J. Am. Heart Assoc. 11, e022010 (2022).
12 V. Venkataramani, F. Winkler, N. Engl. J. Med. 387, 1813 (2022).
13 J. M. Connors, P. M. Ridker, JAMA 327, 1234 (2022).
14 J. B. Parr, JAMA Intern. Med. 181, 12 (2021).
15 E. J. Rubin et al., N. Engl. J. Med. 384, 1564 (2021).
#mask up#wear a mask#public health#pandemic#wear a respirator#covid#covid 19#coronavirus#still coviding#sars cov 2
11 notes
·
View notes
Text
The human immune system is based on cells that communicate with each other via signaling molecules known as cytokines and chemokines. One of these signaling molecules is the protein MIF (macrophage migration inhibitory factor). It plays an important role in the regulation of various immune reactions by binding to suitable receptors of various cell types in a ternary complex, thereby activating certain signaling pathways in these cells. Surprisingly, there are plant proteins that are very similar to the human MIF protein in the sequence of their individual building blocks (amino acids) and these are referred to as MDL proteins. A team led by Jürgen Bernhagen from the Institute for Stroke and Dementia Research (ISD) at University of Munich Hospital and Professor Ralph Panstruga from the Unit of Plant Molecular Cell Biology at RWTH Aachen University in collaboration with a research group led by Professor Elias Lolis from Yale University in the U.S., has now shown that MIF and MDL proteins are also astonishingly similar in their spatial structure. Lead author Lukas Spiller and the team also found that the plant MDL proteins bind to the receptors of the MIF protein, alone or in complexes with the human MIF protein, and are thus able to activate immune-relevant signaling pathways—in some cases more efficiently than the human MIF protein alone.
Continue Reading.
57 notes
·
View notes
Text
Human Cell Tournament Round 1
Propaganda!


ILC2 cells, or type 2 innate lymphoid cells are a type of innate lymphoid cell. Not to be confused with the ILC. They are derived from common lymphoid progenitor and belong to the lymphoid lineage. These cells lack antigen specific B or T cell receptor because of the lack of recombination activating gene. ILC2s produce type 2 cytokines (e.g. IL-4, IL-5, IL-9, IL-13) and are involved in responses to helminths, allergens, some viruses, such as influenza virus and cancer. ILC2s play the crucial role of secreting type 2 cytokines in response to large extracellular parasites. They express characteristic surface markers and receptors for chemokines, which are involved in distribution of lymphoid cells to specific organ sites. ILC2s are activated upon respiratory virus infections in mice and humans. For instance, during Influenza A virus infection, which induces IL-33 production, ILC2s are activated and drive airway hyper-responsiveness. [image credit]
The Y chromosome is one of two sex chromosomes in therian mammals and other organisms. Along with the X chromosome, it is part of the XY sex-determination system, in which the Y is the sex-determining chromosome because the presence of the Y chromosome causes offspring produced in sexual reproduction to be of male sex. In mammals, the Y chromosome contains the SRY gene, which triggers development of male gonads. The Y chromosome is passed only from male parents to male offspring. Most therian mammals have only one pair of sex chromosomes in each cell. Males have one Y chromosome and one X chromosome, while females have two X chromosomes. In mammals, the Y chromosome contains a gene, SRY, which triggers embryonic development as a male. The Y chromosomes of humans and other mammals also contain other genes needed for normal sperm production.
#lymphoid cells#y chromosome#poll#polls#tumblr poll#tumblr polls#tournament poll#wikipedia#cells of the human body#science tournament#biochemistry
10 notes
·
View notes
Text
Note Cards (February 2024)
2nd Law and Acceleration
3' Untranslated Region
3rd Cuneiform Facet Shape
4th Rib and Age
18-Aldocorticosterone
30S Initiation Factors
Actions of Adductor Magnus
Age and Cranial Sutures
Ancylostoma duodenale Pathogenesis
Anthropological Linguistics
Brachialis OIA
Breeding Isolates
Causes of Negative Nitrogen Balance
Chemokines
Components of Hill Plots
Derivatives of Oxaloacetate
Echinococcosis
Endocrinology
Femoral Popliteal Surface
Fibularis Brevis
Flexor Digiti Minimi Brevis OIA
H. erectus at Ceprano Site
IgE
Ilex verticillata Names
Intermediate Filament
LCL vs MCL
Malate Dehydrogenase 1
Nail Matrix
Neanderthal Metabolism
Obturator Nerve Muscles
Parts of Epiphyses
Peptide Bond Structure
Primary vs Secondary Metabolites
sanguino-
Selective Pressures
Siding Metacarpal 3
Skull of Arago 21
Steps of Whole-Genome Shotgun Sequencing
Strongyloides stercoralis
Structure of α-Helix
T. Dale Stewart
T. trichiuria Appearance
Talus - Plantar View
Taphonomy
Teres Minor
Transcriptional Fusion
Trichuris trichiuria Pathogenesis
Vena Cava Inferior
venulo-
Zygomatic - Lateral View
.
Patreon
#studyblr#notes#studying#masterlist#study masterlist#master list#study master list#studyblr resources#study resources#learning#learning resources#school#school resources#free learning#science#academics#academia#learning science#mcat resources#mcat notes#mcat studyblr#mcat masterlist#resource masterlist#scienceblr#medblr
16 notes
·
View notes
Note
Wren your egg lore is so interesting
Thank you!! Without spoiling too much, I'll tell you a bit about how it is the 'egg' spores infect their host, scientifically speaking. (from discord) Basically, premature spores get inside an open wound and into the bloodstream. From there they release proteins that are similar in structure to a certain immune cell, allowing them to stay undetected by the immune system. These spores also have immunosupressing effects. The spores make their way into the central nervous system via the bloodstream. SEM spores in addition to already being similar in shape and structure to helper T-cells, which are critical to the process of activating the immune system against threats. The spores are disguised as Helper T-cells, and even produce their own chemical signals altering other immune cells. From there, they circulate throughout the body undetected by other immune cells due to the proteins and chemical signals the spores produce. Once into the bloodstream, the disguised spores make their way into the right and left common carotid arteries, which are located in the neck. As the spores travel deeper into the internal carotid arteries, these disguised cells end up at the cerebral capillary wall and penetrate it through extravasation. This includes producing a special Exoenzyme signal to essentially trick the endothelial cells into opening up- and allowing the spores into the brain tissue. From there, they use chemokines to navigate to the cerebral cortex.
#thanks for the ask!#the wren calls#mcyt#mcytblr#mcyt worldbuilding#dream smp#dsmp#dsmpblr#dsmp lore#tw medical#tw fungi#egg arc#egg lore#eggpire#speculative biology#speculative immunology#the fungus among us chronicles
34 notes
·
View notes
Text
Immune cells invading the tumour microenvironment secrete pro-inflammatory cytokines give the cancer cells exactly what they want... chemokines that induce motility, growth factors that induce growth, proliferation, and epithelial to mesenchyme transition allowing the cancer to invade the extracellular matrix and into blood circulation to establish metastasis at a secondary site. OMG stop you're making it worse. You're making the cancer stronger
16 notes
·
View notes
Text
Pneumonia In Children And Adults

Introduction
Pneumonia stands as a prevalent respiratory infection, exerting a significant burden on global public health. Its impact extends beyond mere morbidity, contributing to substantial healthcare costs and socioeconomic consequences. This discussion aims to elucidate the general nature of pneumonia, encompassing its pathophysiology, clinical presentation, diagnostic modalities, treatment strategies, complications, and preventive measures. By indulging into these factors, we aim to provide a better understanding of pneumonia’s complexity and underscore the importance of timely recognition and management.
Pathophysiology

Pneumonia ensues from the infiltration of infectious agents, including bacteria, viruses, fungi, and less commonly, parasites, into the lower respiratory tract. Upon inhalation or aspiration of these pathogens, they gain access to the alveoli, where they incite an inflammatory response. This inflammatory cascade triggers the release of pro-inflammatory cytokines and chemokines, recruiting immune cells to the site of infection. Neutrophils, macrophages, and lymphocytes converge to eradicate the invading pathogens, leading to the characteristic consolidation and exudate formation within the affected lung tissue. As the infection progresses, alveolar edema, impaired gas exchange, and parenchymal damage ensue, culminating in the clinical manifestations of pneumonia.
Clinical Presentation

The clinical presentation of pneumonia encompasses a spectrum of symptoms, ranging from mild respiratory complaints to life-threatening respiratory failure. Common symptoms include cough, productive sputum production, fever, chills, pleuritic chest pain, dyspnea, tachypnea, and systemic manifestations such as malaise and fatigue. The severity of symptoms varies depending on factors such as the underlying pathogen, the extent of lung involvement, the host’s immune status, and comorbidities. In pediatric populations, pneumonia may present with nonspecific symptoms such as feeding difficulties, lethargy, and irritability, posing diagnostic challenges. Conversely, elderly individuals may exhibit atypical presentations characterized by confusion, hypothermia, and exacerbations of underlying chronic conditions.
Diagnostic Modalities

The diagnosis of pneumonia hinges on a comprehensive clinical assessment, augmented by various diagnostic modalities to confirm the presence of pulmonary infection and reveal its etiology. A thorough history and physical examination provide invaluable insights into the patient’s symptomatology, risk factors, and clinical trajectory. Symptomatic findings such as crackles, wheezes, and diminished breath sounds may aid in localizing the site of infection and assessing disease severity. Radiographic imaging, notably chest X-rays and computed tomography (CT) scans, serves as the cornerstone of pneumonia diagnosis, revealing characteristic radiographic findings such as airspace opacities, lobar consolidation, and interstitial infiltrates. Laboratory investigations, including complete blood count (CBC), C-reactive protein (CRP), and procalcitonin levels, may corroborate the clinical suspicion of pneumonia and guide therapeutic decisions. Additionally, microbiological testing of respiratory specimens through techniques such as sputum culture, blood cultures, and polymerase chain reaction (PCR) assays facilitates pathogen identification and antimicrobial susceptibility testing, thereby informing targeted therapy.
Treatment Strategies

The management of pneumonia hinges on prompt initiation of empiric antimicrobial therapy tailored to the likely causative pathogen(s) and disease severity. Antibiotics represent the mainstay of treatment for bacterial pneumonia, with the choice of agent dictated by factors such as local antimicrobial resistance patterns, patient age, comorbidities, and recent antibiotic exposure. Commonly prescribed antibiotics include beta-lactam agents (e.g., penicillins, cephalosporins), macrolides, fluoroquinolones, and combination regimens for severe or healthcare-associated infections. Conversely, viral pneumonia necessitates supportive care measures, given the limited efficacy of antiviral agents in most cases. Influenza-associated pneumonia may benefit from neuraminidase inhibitors such as oseltamivir, while respiratory syncytial virus (RSV) pneumonia may warrant ribavirin therapy in select cases. Adjunctive therapies such as oxygen supplementation, bronchodilators, and corticosteroids may mitigate respiratory distress and improve clinical outcomes, particularly in severe or hypoxemic patients. The duration of antimicrobial therapy varies depending on factors such as the causative pathogen, clinical response, radiographic resolution, and the presence of complications. Close monitoring of clinical parameters and serial imaging studies guide the decision-making process, enabling clinicians to tailor therapy to individual patient needs.
Complications

Pneumonia harbors the potential for various complications, ranging from mild to life-threatening sequelae, necessitating vigilant monitoring and timely intervention. Common complications include pleural effusion, empyema, lung abscess, respiratory failure, septic shock, and acute respiratory distress syndrome (ARDS). Pleural effusion denotes the accumulation of fluid within the pleural space, secondary to inflammation or impaired lymphatic drainage, manifesting as dyspnea, pleuritic chest pain, and dullness to percussion on physical examination. Empyema represents a purulent collection within the pleural cavity, often complicating bacterial pneumonia and necessitating drainage via thoracentesis or chest tube placement. Lung abscesses manifest as circumscribed cavities containing necrotic debris and pus within the lung parenchyma, triggered by persistent fever, productive cough, and hemoptysis. Respiratory failure ensues from impaired gas exchange and alveolar hypoventilation, caused by worsening hypoxemia, hypercapnia, and respiratory acidosis, necessitating mechanical ventilation and intensive care support. Septic shock represents a life-threatening complication of severe pneumonia, characterized by systemic inflammatory response syndrome (SIRS) and end-organ dysfunction, requiring aggressive fluid resuscitation, vasopressor therapy, and broad-spectrum antibiotics. ARDS denotes a severe form of acute lung injury, characterized by diffuse alveolar damage, refractory hypoxemia, and bilateral infiltrates on chest imaging, necessitating lung-protective ventilation and supportive care in the intensive care unit (ICU). The occurrence of complications portends a poor prognosis and underscores the need for early recognition and intervention to mitigate adverse outcomes.
Preventive Measures

Preventing pneumonia entails a broad approach encompassing vaccination, infection control measures, and health promotion strategies aimed at reducing the risk of respiratory infections and their sequelae. Vaccination stands as a cornerstone of pneumonia prevention, targeting common bacterial and viral pathogens implicated in pneumonia pathogenesis. Vaccines such as the pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23) confer protection against Streptococcus pneumoniae, the leading bacterial cause of pneumonia, particularly in high-risk populations such as young children, older adults, and immunocompromised individuals. Influenza vaccination remains paramount in mitigating influenza-associated pneumonia and reducing disease transmission, underscoring the importance of annual vaccination campaigns targeting vulnerable populations. Additionally, adherence to infection control measures, including hand hygiene, respiratory etiquette, and environmental sanitation, plays a pivotal role in reducing the spread of respiratory pathogens in healthcare settings and the community at large. Health promotion efforts aimed at smoking cessation, optimizing nutrition, and addressing underlying comorbidities such as chronic obstructive pulmonary disease (COPD), asthma, and immunodeficiency bolster immune resilience and mitigate pneumonia risk. Furthermore, early identification and management of predisposing factors such as malnutrition, homelessness, and overcrowded living conditions attenuate pneumonia susceptibility and enhance overall health outcomes.
Conclusion
In conclusion, pneumonia emerges as a formidable respiratory infection, posing significant challenges to global public health. Its diverse etiology, clinical manifestations, diagnostic modalities, treatment modalities, complications, and preventive measures underscore the nature of pneumonia management. Timely recognition and intervention are imperative in mitigating the morbidity and mortality associated with pneumonia, necessitating a collaborative approach among healthcare providers, public health authorities, and policymakers. By fostering a comprehensive understanding of pneumonia’s manifest and implementing evidence-based strategies, we can strive towards reducing its burden and improving patient outcomes. Through ongoing research, education, and advocacy efforts, we can envision a future where pneumonia-related morbidity and mortality are substantially diminished, paving the way for enhanced respiratory health and well-being worldwide.
In managing pneumonia, compassion, empathy, and a holistic approach are essential alongside clinical expertise. Striving for excellence in knowledge and practice allows us to enhance respiratory medicine and patient outcomes.
As we address pneumonia and broader cardiovascular health complexities, let’s remain committed to optimal patient care. Together, we can impact lives positively and foster a healthier future.
Medical students encounter significant academic challenges during their studies, balancing coursework, clinical rotations, research, and personal commitments. Expert Academic Assignment Help offers tailored assistance to meet their needs, providing study materials, tutoring, assignment help, and exam preparation. Beyond academics, it fosters a supportive environment for mentorship and guidance. In essence, Expert Academic Assignment Help is a valuable resource for medical students, empowering them to excel academically and develop into competent healthcare professionals. Contact us at [email protected] for professional assistance
#assignment help#medical students#healthcare#nursing school#nursing student#medicine#medical help#academic assignments#university student#medical university#university life#university#student#student life#study blog#study inspiration#studyblr community#studyblr#study motivation#medication#medical student#medical school#medicare#writing#writers on tumblr#writerscommunity#writeblr#online writing#academic writing
2 notes
·
View notes
Text
Does Inflammation Help Healing

The immune system's normal reaction to damage, infection, or foreign substances is inflammation. It's a complex process that includes the creation of inflammatory mediators like chemokines and cytokines in addition to the activation of various immune cells. Despite the fact that inflammation is frequently associated with discomfort, swelling, and redness, it is a crucial step in the healing process.
2 notes
·
View notes
Text
“Melanoma Growth-Stimulating Activity”, Victor McKusick, Mendelian Inheritance in Man, 1966. (MGSA).
Here I present: “Melanoma Growth-Stimulating Activity”, Victor McKusick, Mendelian Inheritance in Man’, 1966. (MGSA). INTRODUCTION. Melanoma growth stimulatory activity (MGSA) is an acid and heat stable, auto-stimulatory growth factor which was first isolated from culture medium conditioned by the Hs294T-human melanoma cell line. Chemokine (CXC motif) ligand-1 (CXCL1) is a small peptide belonging…
0 notes
Text
Reference archived on our website
Abstract
Objective COVID-19 induces the development of autoimmune diseases, including SLE, which are characterised by inflammation, autoantibodies and thrombosis. However, the effects of COVID-19 on SLE remain unclear.
Methods We investigated the effects of COVID-19 on SLE development and progression in three animal models. Plasmids encoding SARS-CoV-2 spike protein and ACE2 receptor were injected into R848-induced BALB/C lupus mice, R848-induced IL-1 receptor antagonist knockout (KO) lupus mice and MRL/lpr mice. Serum levels of albumin and autoantibodies, lymphocyte phenotypes and tissue histology were evaluated.
Results In R848-induced BALB/C lupus mice, the SARS-CoV-2 spike protein increased autoantibody and albumin levels compared with vehicle and mock treatments. These mice also exhibited splenomegaly, which was further exacerbated by the spike protein. Flow cytometric analysis revealed elevated T helper 1 cell counts, and histological analysis indicated increased levels of the fibrosis marker protein α-smooth muscle actin. In KO mice, the spike protein induced splenomegaly, severe kidney damage and pronounced lung fibrosis. In the MRL/lpr group, spike protein increased the serum levels of autoantibodies, albumin and the thrombosis marker chemokine (C-X-C motif) ligand 4.
Conclusion COVID-19 accelerated the development and progression of lupus by inducing autoantibody production, fibrosis and thrombosis.
#long covid#lupus#covid#covidー19#mask up#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator#covid isn't over#covid conscious#covid is airborne#covid pandemic#covid19
16 notes
·
View notes
Text
0 notes
Text
C-C motif chemokine receptor-2 blockade ameliorates pulmonary hypertension in rats and synergizes with a pulmonary vasodilator

https://doi.org/10.1093/cvr/cvae244
0 notes
Text
How Nature's Morphine Conolidine Provides Safe, Non-Opioid Relief
Dubbed as "Nature's morphine," Conolidine is gaining recognition for its powerful pain-relieving properties. Unlike traditional painkillers, it targets the atypical chemokine receptor (ACKR3), a unique receptor that prevents natural opioid peptides in your body from binding to classical opioid receptors. By modulating ACKR3, Conolidine boosts the availability of these natural pain-relievers, delivering effective analgesia without the risks associated with opioids.
Why Choose Conolidine for Back Pain?
Back pain can disrupt your life, making simple tasks feel like a challenge. Many turn to Conolidine for back pain because it provides relief without the side effects linked to opioids. It doesn’t affect the μ-opioid receptor or alter dopamine levels, which means there’s no risk of addiction or the highs typical of many painkillers.
Traditional medications often come with a list of problems like nausea, dependency, and tolerance. Conolidine, however, offers a natural, non-addictive alternative that works with your body’s natural systems. For anyone dealing with chronic or acute back pain, this makes it a game-changer.
How to Use Conolidine for Back Pain
Using Conolidine for back pain is quick and easy. Just place your daily serving under your tongue, let it sit for 60 seconds, then swallow. During this time, the potent alkaloids absorb into your bloodstream, providing fast relief that lasts for hours and gets even better with consistent use.
Conolidine vs. Traditional Painkillers
Traditional painkillers like opioids can cause significant complications over time, including dependency and reduced effectiveness. On the other hand, Conolidine stands out because it provides pain relief without these challenges. It’s non-opioid, doesn’t disrupt your natural dopamine levels, and is safe for long-term use.
If you're looking for a natural option to tackle pain—whether it’s back pain or something chronic—consider giving Conolidine a try.
Take control of your pain with Conolidine and experience the difference today! Try today!
0 notes
Text
CXCR4 Antagonist Market: Unveiling Key Trends, Growth Drivers, and Industry Challenges in 2024 - UnivDatos
According to a new report by UnivDatos Market Insights, The CXCR4 Antagonist Market was valued at approximately USD 507.04 million in 2023 and is expected to grow at a substantial CAGR of around 12.37% during the forecast period (2024-2032). This growth is due to several factors mainly, the need for better treatment solutions for diseases that are on the rise including cancer and HIV. The availability of individualized treatments, improved drug delivery systems, and the use of artificial intelligence in developing drugs, will also drive the market further. Furthermore, the increasing interest in the production of orphan drugs, as well as combination therapies signals a new trend towards more focused treatments that allow for higher effectiveness of treatment for particular patient populations. North America remains the largest buyer of the equipment, while Asia-Pacific is expected to show the highest growth over the forecast period as a result of a growing healthcare expenditure and a larger population of patients.
Request To Download Sample of This Strategic Report - https://univdatos.com/get-a-free-sample-form-php/?product_id=68257&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
For instance, on September 2023, BioLineRx Ltd., a commercial-stage biopharmaceutical company focused on certain cancers and rare diseases, announced that the U.S. Food and Drug Administration (FDA) has approved APHEXDA™ (motixafortide) in combination with filgrastim (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma. APHEXDA is administered by injection, for subcutaneous use.
Increasing Prevalence of Cancer
Rising cancer incidence and prevalence enhances the global demand for CXCR4 antagonist market. According to World Health Organization, approximately 19 million patients are diagnosed with cancer every year and this figure is predicted to increase by approximately 70% within the next two decades. This situation requires the application of reasonable therapeutic approaches in combating different types of cancer, such as breast, lung, and prostate cancers, that have been showing high incidences in recent years. CXCR4, which is a chemokine receptor with high-level expression on tumours, plays a significant role in cancer progression and metastasis. It promotes the movement of cancer cells to other parts of the body, thus making it a major consideration for treatment. The mechanism of action of CXCR4 antagonists lies in preventing the binding of this receptor to its ligand CXCL12 depriving cancer cells to undertake processes that precipitate metastasis. This mechanism places these antagonists as potential candidate agents in oncology treatments. For example, the highly investigated compound, Plerixafor which is part of the CXCR4 antagonist family, has demonstrated abilities to suppress the metastatic potential of tumours, besides increasing the efficiency of other treatments such as chemotherapy and immunotherapy. In addition, drug-resistant cancer cases are on the rise, consequently pushing the demand for new treatments. A number of patients develop the ability to resist the action of traditional treatments; therefore, the utilization of an antagonizing target, such as CXCR4 antagonists, may be effective in overcoming this problem.
Ask for Report Customization - https://univdatos.com/get-a-free-sample-form-php/?product_id=68257&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
According to the report, the Asia-Pacific region is expected to be the fastest-growing region in the forecast period
The CXCR4 antagonist market in the Asia-Pacific (APAC) market is expected to experience significant growth owing to increasing cancer and HIV incidence that requires adequate treatment. Moreover, the increasing interest in treatment for stem cell mobilization and the development of new drugs are some other factors investing in the market growth of CXCR4 antagonists. The trends in precision medicine and other targeted therapies are also very important as more APAC countries transition to patient-centric systems of healthcare delivery. In addition, greater clinical trial transparency and favorable regulatory conditions are now motivating pharma to continue the development of novel CXCR4 antagonists for expanded use in other therapeutic indications. For instance, in November 2021, Abbisko Therapeutics Co. Ltd., which is a China-based pharmaceutical company Joined hands with X4 Pharmaceuticals, Inc. for the development of CXCR4 antagonists.
0 notes