#Biventricular Pacing Treatment
Explore tagged Tumblr posts
Text
AI Robot in Heart Treatment Market Set for Explosive Growth

Advance Market Analytics published a new research publication on "AI Robot in Heart Treatment Market Insights, to 2028" with 232 pages and enriched with self-explained Tables and charts in presentable format. In the Study you will find new evolving Trends, Drivers, Restraints, Opportunities generated by targeting market associated stakeholders. The growth of the AI Robot in Heart Treatment market was mainly driven by the increasing R&D spending across the world.
Get Free Exclusive PDF Sample Copy of This Research @ https://www.advancemarketanalytics.com/sample-report/186484-global-ai-robot-in-heart-treatment--market The AI Robot in Heart Treatment Market report covers extensive analysis of the key market players, along with their business overview, expansion plans, and strategies. The key players studied in the report include: Heartlander Surgical (United States), Intuitive Surgical (United States), Medrobotics Corporation (United States), CMR Surgical Limited (United Kingdom), Corindus Vascular Robotics (United States), Hansen Medical (United States) Definition: AI robots are rapidly growing in popularity in the medical to perform various clinical tasks and surgeries. Growing preference towards minimally invasive surgery and emerging trends of remote surgery across the healthcare sector will accelerate the growth of AI robots in heart treatment. Most surgical robots have a camera that captures real-time images and videos and sends them to the monitor in the surgical room to guide doctors during surgery. The following fragment talks about the AI Robot in Heart Treatment market types, applications, End-Users, Deployment model etc. A thorough analysis of AI Robot in Heart Treatment Market Segmentation: by Type (Rehabilitation Robots, Surgical Robots), Application (Cardiac Ablation, Myocardial Regeneration, Biventricular Pacing Lead Placement, Valve Repair, Removal of Cardiac Tumors, Others), Dimensional Type (2D, 3D), End-user (Electrophysiologists, Interventional Cardiologists, Cardiothoracic Surgeons, Government Hospitals, Rehabilitation Centers, Others) AI Robot in Heart Treatment Market Drivers:
Surging Demand for AI Robots in the Heart Surgery to Reduce the Surgery Timing and Patient Exposure to Contrast Agent and Radiation
High Growth of AI and ML in the Surgical Robots to Perform Complicated Surgeries and Improve the Experience
AI Robot in Heart Treatment Market Trends:
Increased Focus on the Technological Advancements of Robotics by the Market Players
AI Robot in Heart Treatment Market Growth Opportunities:
Increasing Number of Cardiac Patients Across the Globe Due to Change in Lifestyle
Evolution of 5G Network Technology and Increased Adoption of Advanced Technologies like AI, ML, and Robotics in the Developing Countries
As the AI Robot in Heart Treatment market is becoming increasingly competitive, it has become imperative for businesses to keep a constant watch on their competitor strategies and other changing trends in the AI Robot in Heart Treatment market. Scope of AI Robot in Heart Treatment market intelligence has proliferated to include comprehensive analysis and analytics that can help revamp business models and projections to suit current business requirements. We help our customers settle on more intelligent choices to accomplish quick business development. Our strength lies in the unbeaten diversity of our global market research teams, innovative research methodologies, and unique perspective that merge seamlessly to offer customized solutions for your every business requirement. Have Any Questions Regarding Global AI Robot in Heart Treatment Market Report, Ask Our Experts@ https://www.advancemarketanalytics.com/enquiry-before-buy/186484-global-ai-robot-in-heart-treatment--market Strategic Points Covered in Table of Content of Global AI Robot in Heart Treatment Market:
Chapter 1: Introduction, market driving force product Objective of Study and Research Scope the AI Robot in Heart Treatment market
Chapter 2: Exclusive Summary and the basic information of the AI Robot in Heart Treatment Market.
Chapter 3: Displaying the Market Dynamics- Drivers, Trends and Challenges & Opportunities of the AI Robot in Heart Treatment
Chapter 4: Presenting the AI Robot in Heart Treatment Market Factor Analysis, Porters Five Forces, Supply/Value Chain, PESTEL analysis, Market Entropy, Patent/Trademark Analysis.
Chapter 5: Displaying the by Type, End User and Region/Country 2018-2022
Chapter 6: Evaluating the leading manufacturers of the AI Robot in Heart Treatment market which consists of its Competitive Landscape, Peer Group Analysis, BCG Matrix & Company Profile
Chapter 7: To evaluate the market by segments, by countries and by Manufacturers/Company with revenue share and sales by key countries in these various regions (2023-2028)
Chapter 8 & 9: Displaying the Appendix, Methodology and Data Source
Finally, AI Robot in Heart Treatment Market is a valuable source of guidance for individuals and companies. Read Detailed Index of full Research Study at @ https://www.advancemarketanalytics.com/reports/186484-global-ai-robot-in-heart-treatment--market What benefits does AMA research study is going to provide?
Latest industry influencing trends and development scenario
Open up New Markets
To Seize powerful market opportunities
Key decision in planning and to further expand market share
Identify Key Business Segments, Market proposition & Gap Analysis
Assisting in allocating marketing investments
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Middle East, Africa, Europe or LATAM, Southeast Asia. Contact US : Craig Francis (PR & Marketing Manager) AMA Research & Media LLP Unit No. 429, Parsonage Road Edison, NJ New Jersey USA – 08837 Phone: +1 201 565 3262, +44 161 818 8166 [email protected]
#Global AI Robot in Heart Treatment Market#AI Robot in Heart Treatment Market Demand#AI Robot in Heart Treatment Market Trends#AI Robot in Heart Treatment Market Analysis#AI Robot in Heart Treatment Market Growth#AI Robot in Heart Treatment Market Share#AI Robot in Heart Treatment Market Forecast#AI Robot in Heart Treatment Market Challenges
0 notes
Text
Permanent Pacemaker Implantation: PART - 1
A pacemaker is also named a cardiac pacing device.
Pacemakers can be specified as devices that are placed in your body by surgery to support the electrical system in your heart. It is generally used to stabilize irregular heart rhythms and prevent life dangering or disrupting odds. Single-chamber pacemakers use a single wire connected to one chamber of your heart, whereas Dual-chamber pacemakers use two wires.
A pulse generator is a small metal case that contains an electronic circuit with a small computer and battery that regulates the pulses sent to the heart.
A lead is an insulated wire that connects to the pulse generator at one end and is placed in one of the heart chambers at the other end. Leads are most often placed through large veins in the chest that lead directly to the heart. An electrode at the tip of the lead contacts the heart wall. The leads send electrical impulses to the heart. It also senses the heart's electrical activity and sends that information back to the pulse generator. Pacing leads are placed in the atrium (superior atrium), ventricle (inferior atrium), or both, depending on the medical condition.
A new type of pacemaker called a biventricular pacemaker is now used to treat certain types of heart failure. Heart failure can cause the two ventricles to malfunction. Ventricular dyssynchrony is a general term used to describe this abnormal pumping pattern. When this happens, less blood is pumped out of the heart. A biventricular pacemaker stimulates both ventricles simultaneously to increase the volume of blood pumped out of the heart. This type of treatment is called cardiac resynchronization therapy (CRT).
After your pacemaker is placed, you will have regular appointments to make sure your pacemaker is working properly. Your doctor uses a special computer called a programmer to check your pacemaker's activity and adjust settings as needed.
Accounting for complete experience and practice, Dr. Aritra Konar is a Consultant Interventional Cardiologist at Apollo Gleneagles Hospital, Kolkata. He has been deemed one of the best cardiologists and heart surgeons in Kolkata for his expertise lies in Coronary (femoral & radial routes) angiography, Coronary angioplasty (including primary angioplasty), Peripheral angiography and angioplasty, Permanent pacemaker, ICD, CRT implantation, BMV, BPV, Right heart catheterization and so on, in procedural skills. His guidance is trusted and so are his skills.
0 notes
Text
Dr Ramji Mehrotra | What is cardiac resynchronization therapy?

Cardiac resynchronization therapy (CRT) is a specialized medical treatment designed to improve the function of the heart in individuals with certain types of heart failure. Also known as biventricular pacing, CRT involves the implantation of a device that helps coordinate the contractions of the heart's ventricles, thereby enhancing its pumping efficiency. This therapy has proven to be a valuable option for individuals who experience heart failure symptoms despite optimal medical management.
Heart failure can occur due to various underlying conditions, such as coronary artery disease, hypertension, or cardiomyopathy. As a result, individuals with heart failure may experience symptoms like fatigue, shortness of breath, fluid retention, and reduced exercise capacity.
According to Dr. Ramji Mehrotra, one of India’s most popular cardiac surgeons, CRT is primarily used to treat a specific subset of heart failure patients with "dyssynchrony". Dyssynchrony refers to a lack of coordination in the contraction of the heart's ventricles, the lower chambers responsible for pumping blood to the body. In these individuals, the right and left ventricles do not contract simultaneously, leading to inefficient pumping and reduced blood flow.
CRT involves the implantation of a small device known as a cardiac resynchronization therapy device (CRT-D) or pacemaker (CRT-P) under the skin, typically below the collarbone. This device is equipped with leads (thin insulated wires) that are threaded through veins and positioned in specific regions of the heart.
Once in place, the CRT device delivers precisely timed electrical impulses to the right and left ventricles. These impulses synchronize the contractions of the ventricles, ensuring that they pump together, which can significantly improve the heart's efficiency and overall function.
Not all heart failure patients are suitable candidates for CRT. Typically, it is recommended for individuals who meet specific criteria:
Reduced Ejection Fraction: CRT is usually reserved for individuals with reduced left ventricular ejection fraction (LVEF), which indicates a weakened heart muscle.
Symptoms Despite Medications: Candidates often continue to experience heart failure symptoms despite receiving optimal medical therapy.
Specific ECG Patterns: Certain ECG (electrocardiogram) patterns, such as left bundle branch block, may indicate the presence of dyssynchrony and make individuals suitable candidates for CRT.
Dr Ramji Mehrotra says that the primary goal of CRT is to improve the patient's quality of life by reducing symptoms and enhancing their ability to perform daily activities. Some of the key benefits include:
Improved Heart Function: CRT helps the heart pump more efficiently, increasing the amount of blood circulated with each beat.
Symptom Relief: Many patients experience a reduction in symptoms like fatigue, shortness of breath, and swelling.
Reduced Hospitalizations: CRT can lower the risk of hospitalizations related to heart failure exacerbations.
Prolonged Life: In some cases, CRT may extend the lifespan of individuals with heart failure.
It is crucial for individuals with heart failure to take their doctor’s opinion in determining whether CRT is an appropriate treatment option for them.
#dr ramji mehrotra#best heart surgeon in india#best cardiac surgeon in delhi#best cardiac surgeons in india#best cardiac surgeon in india#cardiac surgeon in delhi#beating heart surgeons in delhi#best heart surgeon in delhi#health & fitness#heart
0 notes
Text
Biventricular Pacing Treatment in India
Cardiac Resynchronization Therapy (CRT), also referred to as biventricular pacemaker, uses a special type of pacemaker that is designed to help the ventricles contract more easily. It keeps ventricles left and right together, cranking through the leads through small electrical pulses. This treatment has been shown to enhance the risk factors for heart disease and overall quality of life in some patients with severe symptoms that are not controlled by medication.
Leads are tiny wires inserted through a vein into the right ventricle and then into the coronary sinus vein to accelerated the rate or control the left ventricle. Usually lead is also implanted in the left atrium. This helps your heartbeat more reasonably. Conventional pacemakers are used to treat the slow rhythms of the heart. Pacemakers restrict the right atrium and the right ventricle to maintain a good heart rate and keep the atrium and ventricle working around each other. It's called AV synchrony. Biventricular pacemakers add the third result to assist the left ventricle has a standard contraction when it doesn't function properly either.
People with the condition who have a poor ejection fraction (<35%) are at risk for rapid, irregular sometimes and existence heart rhythms. Ejection fraction is an indicator of how much blood is stoked out of the left ventricle of the heart. The CRT may be suitable for people who have:
· Have severe or moderately severe symptoms of heart failure
· Are taking medicines to treat heart failure
· Have the electrical activation of the heart delayed (such as intraventricular conduction delay or bundle branch block)
· Have a background of cardiac arrest or are at risk of cardiac arrest
Together, you and your doctor will determine whether this treatment is right for you. People will receive an instruction sheet describing how to start preparing for the procedure. Here's an overview of the instructions.
In most cases, the implant procedure is performed in a special room in the Electrophysiology Lab. The procedure is performed in a surgical suite when the epicardial implant approach is used.
Monitors During the proceedings
· Defibrillator/pacemaker/cardioverter
· Electrocardiogram or EKG
· Blood pressure monitor
· Oximeter monitor
· Fluoroscopy
The CRT device may be implanted using an endocardial or epicardial method.
1. With the endocardial (transvenous) approach, a local anesthetic (pain-relieving medicine) is injected to numb the region and you will be fully conscious during the procedure.
2. Small incisions are made in the chest where the lead and the device are implanted. When an endocardial approach is used, the recovery time of the hospital is generally 24 hours.
3. Endocardial technology is technically challenging. In some cases, this technique may not be successful because of the size, shape, or location of the vein (s). If the endocardial approach cannot be used or is unsuccessful, the epicardial approach will be used.
4. The epicardial approach may also be used to place the CRT if you are already undergoing surgery to treat another heart condition.
With the epicardial (surgical) approach, local anesthesia is given to make you sleep during the process. Leads are steered to the heart with the help of a fluoroscopic machine.
The hospital's recovery time is generally 3 to 5 days. Although recovery with an epicardial approach is greater than that of a transvenous approach, minimally invasive techniques allow for shorter hospital stays and faster recovery time. Your doctor will decide on the implant procedure for you, based on your condition.
The first 48 hours after the procedure, you may feel discomfort at the pacemaker implant site. Your doctor will tell you what medications you can take to relieve your pain. Tell your doctor or nurse if your side effects are continuous or extreme.
CRT improves symptoms of heart failure in about 50 percent of patients who have been treated with a maximum of drugs but still have intense or moderately severe symptoms of heart failure. CRT improves survival, quality of life, heart function, right to exercise, and helps reduce hospitalization in selected patients with severe or moderately severe heart problems.
We offer compassionate, personalized, and outstanding care at Healing Touristry. As a medical tourism service provider, our expertise is not limited to combining you with the best physicians and hospitals, but to ensuring your overall well-being in a country that may be opposed to yours. A success rate of more than 90% is proof of our commitment to providing the most advanced treatment for both routine and critical illnesses.
At Healing Touristry, we can provide you with an expert pool of Biventricular Pacing Treatment by Cardiologists and you can also access the best Biventricular Pacing Treatment Hospital in India from our elaborate list of Hospitals. Healing Touristry is widely recognized by both NRIs and foreign nationals as one of the best in medical treatment, hygiene, and safety, as well as in the safety and privacy of patients.
#Biventricular Pacing Treatment in India#Biventricular Pacing Treatment in Delhi#Biventricular Pacing Treatment#Biventricular Pacing#Biventricular Pacing Treatment Hospitals in India#Biventricular Pacing Treatment Doctors in India#Healing touristry
0 notes
Link
Biventricular Pacing Treatment in India. in addition, the heart failure patient may or may not need this type of pacemaker to treat slow heart rhythms and may or may not need an internal defibrillator (implantable cardioverter defibrillator, or ICD), which is designed to treat people at risk for sudden cardiac death or cardiac arrests.

#Biventricular Pacing Treatment in India#Biventricular Pacing Treatment in Delhi#Best Hospital for Biventricular Pacing Treatment#Best Doctors for Biventricular Pacing Treatment
0 notes
Text
Notes from 10/17 HF lecture:
HF with preserved ejection fraction and HF with reduced ejection fraction = diastolic and systolic heart failure, respectively.
Ischemia = most common cause of HF.
Systolic HF: Peripartum cardiomyopathy causes HF. Takotsubo cardiomyopathy, alcoholic and cocaine-induced cardiomyopathy cause systolic heart failure. Systolic HF can be idiopathic.
Diastolic HF is due to infiltrative diseases, pericarditis.
Orthopnea, PND (Paroxysmal Nocturnal Dyspnea), pink frothy sputum, edema, fatigue, headache, presyncope/syncope, progressive dyspnea, CP = HF.
MI = most common cause of HF. Always get EKG first. Then get cardiac enzymes, BMP, echo, chemistry (for Na, Mg, K). See if pt has renal failure. Check BMP and ProBNP (honestly not sure if she was saying "BNP" or "BMP"). Need to differentiate COPD exacerbation from HF because treatments differ (steroids are given for COPD but would worsen HF).
Tx: lasix (furosemide), morphine (venodilates), O2, NTG, ACEIs (if pt HTN).
Check I&Os. Pt must have negative balance to know if the lasix is working. Need pt’s daily weights (must lose 2 lbs a day in order to be diuresing adequately). If pt is better, you can remove Foley catheter and move him to floors; switch to oral lasix. On floor, ask same questions you asked when the pt was in the CCU (any CP, SOB, etc.) D/C Foley ASAP to avoid multi-drug resistant UTI, which would be your fault, would cause comorbidities for pt, and would not be covered by the insurance company.
NYHA HF classification:
Class I = no dyspnea on normal activity Class II = dyspnea with ordinary physical activity Class III = dyspnea with less than normal daily activities Class IV = dyspnea at rest
Stages of HF (A = no structural heart disease but has risk factors; B = structural heart disease but has no overt HF sxs, C = structural heart disease +... I didn't get it all).
ACEIs needed, unless pt has angioedema, B/L renal Artery stenosis, pregnancy, hyperkalemia greater than 5.5). Relative C/I for ACEIs = cough, acute renal failure (if CKD is stable, you can give ACEIs). Aldosterone antagonists, beta blockers, furosemide, ASA, statins, NTG (hydralazine + NTG work like ACEIs). Stage 3 = digoxin. AICD if EF less than 35%, has meaningful life survival more than 1 year, 40 days post MI. Biventricular pace maker also given to synchronize beating of LV and RV.
If QRS is wide, you need biventricular pacemaker.
For stage D, palliative care/hospice is given.
Ischemia as MCC of HF and ACEIs = most important things to remember from the lecture.
3 notes
·
View notes
Text
Pacemakers Market Size, Share, Trends And Forecast 2030

The global pacemakers market size is anticipated to reach USD 5.94 billion by 2030, according to a new report by Grand View Research, Inc. The market is expected to expand at a lucrative CAGR of 3.4% from 2022 to 2030. This growth is owing to various factors such as technological advancements and the increasing prevalence of cardiovascular devices. Furthermore, the growing sedentary lifestyle coupled with the rising geriatric population is also anticipated to fuel the market growth during the projected period. During the COVID-19 pandemic, the market experienced a decline in revenues. This is owing to the postponement and cancellation of surgeries. According to the National Center for Biotechnology Information (NCBI), there was around a 73% reduction in de-novo pacemaker implantation during the initial months of the pandemic, which heavily impacted the market.
The increasing prevalence of cardiovascular diseases is a key growth driver for the market. As per the CDC in 2017, CVDs account for about 800,000 deaths in the U.S alone. Moreover, coronary heart disease accounts for the highest number of deaths, followed by stroke and heart failure. As per the British Heart Foundation Centre in 2018, nearly 7.4 million individuals are living with circulatory and heart diseases in the U.K. More than 43,000 individuals under 75 years of age die due to cardiac diseases every year in the U.K. To curb the rising prevalence of CVDs is government bodies and key market players are channelizing revenues to offer a potential treatment. This is anticipated by the influx of advanced products in this market space.
As per the CDC, 2020, more than 15% of U.S. adults are physically inactive that shows the prevalence of adult physical inactivity. Technological developments are quickly renovating the pacemaker market. Key players are focusing on expanding their current portfolio as in January 2020, BIOTRONIK launched an injectable cardiac monitor, BIOMONITOR III in Japan. It is intended to measure irregular heart rhythms with increased clarity. The injectable cardiac monitor also documents the unexplained syncope. Moreover, in November 2021, Abbott revealed the latest data for its Aveir leadless pacemaker for the treatment of patients with abnormal heart rhythms. The data demonstrated that the product if approved would provide various benefits during the treatment of slow heart rhythms.
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/pacemaker-market
Pacemakers Market Report Highlights
The market was valued at USD 4.38 billion in 2021 and is expected to witness a CAGR of 3.4% during the forecast period
The MRI compatible pacemakers segment is expected to witness the highest CAGR from 2022 to 2030 owing to a higher patient preference rate as 75% of the patients with pacemakers are likely to get MRI during their lifetimes
The atrial fibrillation segment held the largest revenue share in 2021 owing to the heightened prevalence of growing with age. As per the National Center for Biotechnology Information, 2020, nearly 6 to 12 million people globally are expected to suffer from atrial fibrillation in the US alone by 2050
The biventricular pacemakers segment is expected to grow fast from 2022 to 2030. It is also known as Cardiac Resynchronization Therapy (CRT). An increase in the prevalence of heart failure is one of the major factors driving the demand for biventricular pacemakers
Key companies are adopting new strategies to attain a competitive edge. Product development, collaborations, partnerships, mergers and acquisitions, and regional expansion are a few of them
Key Companies & Market Share Insights
Key companies undertook various strategic initiatives such as product launches, mergers and acquisitions, and partnerships and collaborations to gain more penetration. For instance, in June 2021, Medtronic launched the Micra AV, a self-contained, miniaturized pacemaker for delivering advanced pacing technology for atrioventricular (AV) block patients through a minimally invasive approach. Some of the prominent players in the pacemakers market include: OSYPKA MEDICAL, Boston Scientific Corporation, Zoll Medical Corporation an Asahi Kasei Group Company, Medtronic, BIOTRONIK SE & Co. KG, MicroPort Scientific Corporation, MEDICO S.R.L., Shree Pacetronix Ltd., Abbott, OSCOR Inc, Lepu Medical Technology(Beijing)Co. Ltd.
Request Free Sample Report: https://www.grandviewresearch.com/industry-analysis/pacemaker-market
0 notes
Text
Iris Publishers-Anaesthesia & Surgery Open Access Journal | Ventricular Assist Devices Insertion, Overview and Anesthetic Considerations

Authored by: Nabil A Mageed
Abstract
Ventricular assist devices (VAD) represent a revolution for the management of severe heart failure. Their insertion requires the use of cardiopulmonary bypass. They are used either permanently for long term treatment of refractory heart failure or temporally as a bridge until cardiac transplantation and until cardiac recovery from reversible cardiomyopathy. Insertion of VAD is a risky surgery with high incidence of complication such as bleeding, cardiac tamponade, renal failure and device failure. Anesthetic management of patients with heart failure undergoing VAD insertion requires full review of the patient critical condition, understanding VAD physiology, extensive hemodynamic monitoring and a harmony between cardiac anesthesia and surgical teams. Marinating and protection the right ventricular function is highly important for the continuation of VAD function. The aim of this review is to put new insights on anesthetic management of VAD insertion and to show their different types and their physiology.
Keywords: Heart Failure; Ventricular assist devices; Anesthesia; Insertion
Introduction
Heart failure (HF) is a chronic disease with progressive deterioration occurring over years or decades. The increase in the incidence of HF can be attributed to high survival after cardiac insults like myocardial infarction, improvement of drug treatment of circulatory disorders, and population aging [1]. Although the great advances in medical therapy and management, HF is still a leading cause of high incidence of hospitalization, readmission and mortality [2].
Stages of Heart Failure
The American College of Cardiology and American Heart Association have designed a conceptual classification to help understanding the disease progression in four stages. Stage A represents a patient who are at high risk for HF but has no organic heart disease or symptoms of HF, for example, patients with hypertension, atherosclerosis disease, or diabetes mellitus. Stage B includes patients with organic heart disease but without overt clinical presentation, such as myocardial infarction and left ventricular remodeling. Stage C are those patients who have organic heart disease with previous or current symptoms of HF, such as shortness of breath and fatigue. Stage D represents patients with end-stage HF and sever symptoms at rest despite maximal medical therapy [3,4].
In stage D, different management approach should be employed including end-of-life care, heart transplant, permanent circulatory mechanical support, or drugs [5]. Medical treatment for chronic heart failure includes angiotensin-converting enzyme inhibitors (ACEIs), beta blockers, proper use of diuretics to relieve volume overload, and digoxin which may help to improve resistant symptoms and reduce hospital, anesthesia and surgical readmission rates. For all patients with wide QRS more than 150 milliseconds, biventricular pacing should be considered and for some patients with QRS of 120 to 150 milliseconds according the condition.
More recently, sinus-node inhibitor ivabradine, and LCZ696, which combines angiotensin II inhibition with a neprilysin inhibitor, have been demonstrated to hold promise for HF patients [6-9]. However for patients with end-stage heart failure the only effective treatment is surgical intervention. Heart transplant is the gold standard for treatment of end-stage HF. The left ventricular assist device is an increasingly used option for patients in Stage D HF to prolong life in lieu of heart transplant. Ventricular assist devices (VADs) are mechanical systems that reduce the workload of the heart, permitting the ventricle to rest, whilst maintaining cardiac output and perfusion of vital organs. They have subsequently gained popularity in both acute and chronic heart failure as potentially lifesaving treatment modalities [10].
Development of INTERMACS
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) was designed to facilitate communication with other colleagues regarding any patient with failing response to optimal medical therapy and needs further discussion of possible implantation of mechanical circulatory support devices (MCDs). Also is used for adjustment of pre-operative risk and clarification of target populations for future devices. INTERMACS has been acquiring data on most patients with implanted MCDs under the sponsorship of the National Heart, Lung, and Blood Institute (NHLBI) [11]. These profiles should facilitate collection of outcome data, help to address the varied needs of advanced heart failure patients and hoped to increase clarity of clinical profiling which will illuminate the progress of other surgery, other devices and all treatments for the advanced stages of heart failure [12,13].
Table 1:INTERMACS profile.
History and classification
First-generation devices
These mimic normal cardiac function and circulation by providing pulsatile blood flow at physiological rates. The energy source used to eject blood from the pumping chamber may be pneumatic, hydraulic, or by mechanical pusher plate [14,15]. These mechanisms produce audible pump operation. Durability beyond 2 years is rare and operations for device exchange carry high risk (Figure 1).
Second-generation devices
Flow is continuous and non-pulsatile, which reduces the pump size and the need for external venting. The term second generation describes rotary pumps, typically with axial blood flow, which have an internal rotor within the blood path that is suspended by contact bearings. These bearings are generally mechanical (ball, ruby, or fluid design) [16,17]. Spinning of the internal rotor and flow generation is by magnetic coupling of the rotor magnet to an external rotor (Figure 2).
Third-generation devices
These rotary pumps have non-contact bearings and utilize centrifugal blood flow, incorporating either magnetic or hydrodynamic levitation of an internal impeller. Impeller rotation achieves blood flow through magnetic coupling to the pump motor [18,19]. Greater blood flow can be achieved around the impeller, which reduces thrombus formation and the intensity of antithrombotic therapy required (Figure 3).
The following selected definitions are important to the understanding of how mechanical support devices operate:
RPM: the revolutions per minute (RPMs), which determine pump flow.
Flow: the continuous flow from the LVAD is created by a spinning impeller, which generates forward flow. The device flow = Rotor speed/(Pump inflow – Pump outflow).
Pump power: LVAD pump power is a measure of the current and voltage applied to the motor and varies directly with pump speed and flow.
Pulsatility index: (PI) corresponds to the magnitude of flow pulse through the pump [20,21].
Indications for a Left Ventricular Assist Device
Strong indications: Bridge to transplant, destination or bridge to recovery [22]. All must apply
a. NYHA IV for 60–90 days.
b. Maximal tolerated medical therapy and CRT/ICD if indicated.
c. Chronic inotrope dependence.
d. LVEF 25%, PCWP 20 mm Hg.
Moderate indications: More often destination than bridge to transplant or recovery [23]. All must apply
a. NYHA IV for 30 days.
b. Maximal tolerated medical therapy and CRT/ICD if indicated.
c. Intermittent inotrope dependence.
d. LVEF 25%.
Indication to enable heart transplant. Either must apply
a. PVR 5 Woods units, secondary to chronic HF and expected to reverse after LVAD.
b. GFR, 25–30 mL/min/1.73 m2, secondary to chronic HF and likely to improve after LVAD.
Contraindications for a Left Ventricular Assist Device
Some may be relative, especially as technology improves [24].
a. Acute cardiogenic shock or arrest with uncertain neurologic status.
b. Irreversible contraindication to heart transplant if destination or recovery is not the aim.
c. Non-systolic HF.
d. Co-existing illness with life expectancy < 2 years.
e. Terminal severe comorbidity, e.g. metastatic or advanced cancer, severe liver disease or severe lung disease.
f. Active uncontrolled systemic infection or significant risk of infection.
g. Active severe bleeding.
h. Right HF not secondary to left HF.
i. Moderate or severe aortic insufficiency that will not be corrected.
j. Anatomical considerations such as hypertrophic cardiomyopathy, large ventricular septal defect.
k. Psychosocial limitations, e.g. inability to comply with medical regimen or device and driveline maintenance or inability of patient or companion to maintain LVAD operation and interpret alarms [25].
Anesthesia for VAD Insertion
Preoperative considerations
Patient optimization targets platelet count >150 x 103 mm-1, serum albumin >33 gm litre-1, normal liver enzyme levels, estimated glomerular filtration rate > 50 ml kg-1 min-1, hematocrit >34%, mean pulmonary artery pressures <25 mm Hg, and low inotropic support. Neurological assessment is documented, via transient cessation of sedation if the patient is intubated. Premedication is avoided to prevent cardiac or respiratory depression [26].
Monitoring and vascular access
Standard cardiac monitoring, including five-channel electrocardiography, is used. Existing I.V. access should be changed if there is an infection risk and lines sent for culture. Central venous access is mandatory, both for pulmonary artery catheterization and fluid resuscitation, with meticulous attention to sterility. Mixed venous oxygen saturation assesses the adequacy of cardiac output, aiming for >65%. Transoesophageal echocardiography (TEE) is used throughout the perioperative and immediate postoperative period. Assessment of coagulation via thromboelastography (TEG) provides a baseline to compare with after operation [6].
Induction and maintenance
Induction must prevent haemodynamic decompensation from reduced preload and contractility and is best performed inside the operating theatre with titrated increments of induction agents and inotropic support. Inhalation or I.V. anesthesia is suitable, but nitrous oxide best avoided. External defibrillation pads are attached. Antibiotic prophylaxis is customized to the institution’s pathogenic flora and includes broad-spectrum gram positive bacterial and anti-fungal cover. Body warming devices are used to maintain normothermia.
Surgical access is commonly via median sternotomy, but left thoracotomy with lung isolation via a double-lumen endotracheal tube is an option. Bleeding is common so aprotinin anti-fibrinolysis and cell salvage are routinely used. Full heparinization is required, irrespective of preoperative coagulation [27].
Perioperative period
TEE is used to exclude valve lesions, shunts and intracardiac thrombi as discussed above. CPB is generally but not universally used for placement of inflow cannulae within the ventricle, but duration can be minimized by tunnelling the percutaneous lead and completing the outflow cannula anastomosis before instituting bypass.
Inflow cannulae are directed posteriorly towards the mitral valve to prevent obstruction. Before VAD activation, all air should be removed, and the heart filled with blood. Deairing is via backflow of blood from the aorta to a Luer lock connection on the outflow cap and by elevating the LV apex. The VAD is initiated at low speed with a cross-clamp on the outflow cannula and a needle vent. CPB flow is then reduced followed by removal of the graft cross-clamp. The LV should be full and off CPB before the pump speeds are increased to avoid entraining air into the system, which is further minimized by flooding the surgical field with saline or carbon dioxide [6].
Weaning from CPB
Atelectatic lung is expanded before weaning bypass to prevent Valsalva-induced hypotension. CPB weaning concerns are from RV failure, pulmonary hypertension, systemic vasodilatation, and bleeding. TEE is vital for deairing, RV assessment, and to check cannulae positions and flow [28]. RV dysfunction can be difficult to predict, thus monitoring and prophylaxis against right heart failure must be instituted in all cases before separation from bypass, using inhaled nitric oxide, I.V. nitrates and phosphodiesterase inhibitors. RV dysfunction results in chamber dilatation and leftward ventricular septal deviation on TEE, predicting imminent failure. Atrial or AV sequential pacing is useful to maintain adequate cardiac output. Protamine must be used to reverse heparinization but can worsen RV function and cause pulmonary hypertension [29].
Potential Complications Associated with LVAD
Thrombosis
Patients with LVADs have an increased risk of thrombosis because the LVAD is foreign material in constant contact with the bloodstream. Patients are maintained on anticoagulation (usually warfarin) and antiplatelet therapy (usually aspirin). Patients who develop a thrombus may present with signs of HF. In the case of thrombosis, additional medical treatment such as heparin, glycoprotein 2b/3a antagonists, or tissue plasminogen activator may be necessary [30,31].
Infection
The current LVAD design is not a completely closed system as there is an open driveline exit. Trauma to this percutaneous exit site is a common cause for infection. The patient’s condition can rapidly progress to sepsis and/or shock. Treatment involves establishing hemodynamic stability. Blood culture and imaging tests are necessary to evaluate for internal abscess or vegetation in or around the device. The patient should be treated with appropriate antibiotics and may require long term suppressive antibiotic therapy [32,33].
Gastrointestinal bleeding
The use of anticoagulation and antiplatelet therapy increases risk of bleeding, particularly in the gastrointestinal tract and brain. Also, chronic anticoagulation can lead to platelet dysfunction or acquired Von Willebrand disease. In severe cases, patients will demonstrate hypotension, hypovolemic shock, and rectal bleeding [34,35].
Stroke
Patients with LVADs are at increased risk for both ischemic and hemorrhagic stroke. Acute ischemic strokes result from thromboembolic events due to pump thrombosis, subtherapeutic anticoagulation, or a prothrombotic state associated with activation of the immune system. Alternatively, patients on longterm anticoagulation are at increased risk for hemorrhagic stroke. Hemorrhagic stroke can occur in these patients due to hemorrhagic transformation of an ischemic stroke, over anticoagulation, or infection [36].
Right HF
After LVAD implantation, there is increased output from the left ventricle that can lead to right ventricle failure (RVF). An echocardiogram and laboratory testing for liver enzymes are indicated if RVF is suspected. It can be treated with diuretics and modification of LVAD parameters. In severe cases, mechanical support of the right ventricle may be required with a biventricular assist device [37,38].
Ventricular dysrhythmias
LVAD-induced ventricular dysrhythmias can occur if the patient has a preload deficiency with decreased filling of the left ventricle due to hypovolemia. With diminished left ventricular filling, the inflow cannula can become drawn down into the cardiac ventricular tissue. The cannula’s contact with the cardiac tissue can irritate the area and trigger a ventricular dysrhythmia. These dysrhythmias tend to be short and are repeated until the hypovolemia is corrected. Ventricular dysrhythmias can lead to cardiac arrest. Advanced cardiac life support measures that include chest compressions can be used in patients with LVADs [39].
The future
Design improvements in current VAD systems include totally implantable devices with transcutaneous energy transmission systems, which eliminate the need for a driveline insertion site and also designs allowing more acceptable side-effect profiles for thromboembolism, infection, and device failure.
In addition, there are two total artificial hearts in clinical use, which involve explantation of the native heart and replacement with the device. These are the CardioWest total artificial heart (Syncardia Inc.) and AbioCor (Abiomed Corporation).
The latter organization also produces the Impella, one of a group of minimally invasive, catheter-based assist devices, which is inserted via the femoral artery, the tip of which crosses the aortic valve to rest in the LV and drive forward flow (Figure 4).
Finally, with more devices in production, there is a need for regulation. At present, all devices are regulated in accordance with rules set out by the INTERMACS controlling use in the USA.
Conclusion
Perioperative anesthetic management of patients undergoing VAD implantation represent a challenging mission for the cardiac anesthesia team. This require full cooperation between surgical and anesthetic teams. Extensive hemodynamic monitoring and cardiopulmonary bypass support are essential.
Acknowledgement
None.
Conflict of Interest
No conflict of interest
Read More…Full Text
For More articles in Anaesthesia & Surgery Open Access Journal please click on:
For more open access journals in Iris Publishers
1 note
·
View note
Text
What is Pacemaker?
A pacemaker is an implantable device which is positioned typically underneath your left collarbone. It consists of a battery with a computer circuit connected to one or more pacing electrodes (electrical wires) which attach to your heart.
Types of Pacemakers
Single-chamber pacemaker
This type of pacemaker has one lead that connects the pulse generator to one chamber of your heart. For most people, we use the single-chamber pacemaker to control heartbeat pacing by connecting the lead to your right ventricle (lower heart chamber). Depending on your symptoms and the type of pacing you need, we connect the lead to your right atrium (upper heart chamber) to stimulate the pacing in that chamber. This pacemaker helps the two chambers work together, contracting and relaxing in the proper rhythm. The contractions allow blood to flow properly from the right atrium into the right ventricle. Depending on the pacing needs of your heart, a dual-chamber device may be an appropriate option for you.
Dual-chamber pacemaker
With two leads, this device connects to both chambers on the right side of your heart, the right atrium and the right ventricle. The doctor programs the dual-chamber pacemaker to regulate the pace of contractions of both chambers.
Biventricular pacemaker
This pacemaker, also known as a cardiac re synchronization therapy (CRT) device, has three leads connected to the right atrium and both ventricles. We use the bi ventricular pacemaker to treat people with arrhythmia caused by advanced heart failure. For many people with heart failure, the left and right ventricles do not pump at the same time. Our doctors program the bi ventricular pacemaker to coordinate the contractions of the ventricles, so that they both pump together. Coordinating the ventricles’ contractions helps your heart pump blood more efficiently and can relieve your heart failure symptoms. The treatment is known as cardiac re synchronization therapy because it re synchronizes the ventricles’ pumping action.
1 note
·
View note
Text
Cardiogenic Shock Following Explanation of a Biventricular Pacemaker by Line Lisbeth O*

Abstract
Due to this unfortunate case about irreversible cardiogenic shock after explantation of a CRT, in a patient with unexplained severe infection and with high-risk ischemic cardiomyopathy, it is recommended to disable the LV-function and observe the patient and the EF before deciding to explant a CRT.
Keywords: CRT Explantation; Cardiogenic Shock; LV-Pacing Disabling; Device Infection; Case Report
Abbreviations: ACS= Acute Coronary Syndrome; CRT= Cardiac Resynchronization Therapy; LV= Left Ventricle; EF= Ejection Fraction; COPD= Chronic Obstructive Pulmonary Disease; LM= Left Main Artery; LAD= Left Anterior Descendent Artery; CX= Circumflexus Artery; PCI= Percutaneous Coronary Intervention; CAGB= Coronary Artery Bypass Grafting
Introduction
Cardiogenic shock in patients with chronic systolic heart failure may result from progressive decline in the ejection fraction or acute deterioration precipitated by cardiac or non-cardiac causes such as acute coronary syndrome (ACS), arrhythmias, sepsis [1-3]. This is the first case to be presented where cardiogenic shock developed within a few hours following removal of a cardiac resynchronization therapy device (CRT). Device infection as well as left ventricular (LV) pacing lead extraction are expected to increase [4-12]. In order to avoid an unfortunate outcome as in the present case it is essential to attempt to identify predictors of difficulties and complications. Prior to proceeding with withdrawal of biventricular pacing and lead extraction the consequences should be evaluated [6,7,10].
Case Report
This 70-year-old male had been a heavy smoker since his youth and suffered from chronic obstructive pulmonary disease (COPD). About 30 years previously, he was diagnosed with arterial hypertension and type 2 diabetes mellitus. He had problems with the peripheral circulation, and 10 years earlier necrosis had started to affect his toes and feet. He was treated with percutaneous transluminal angioplasty of the stenotic femoral arteries, in addition to amputation of the right forefoot and the left crus. In his last year, ischemic and infected wounds again developed on his right foot. He also had psoriatic affection of the skin. One year previously, while on vacation abroad, he suffered a myocardial infarction. Coronary angiography (CAG) showed stenosis of the left main artery (LM), the left anterior descendent artery (LAD), and the proximal circumflexus artery (CX), and he was treated with percutaneous coronary intervention (PCI) and stents. EF 25% and reduced right ventricular function. Sinus rhythm and left bundle branch block 174 ms. (Figure 1).
The patient received treatment with an angiotensin-converting enzyme inhibitor, beta-blocker, diuretics, statin, and salicylate and was scheduled implantation with a CRT-D. Before this could be realized, however, he was admitted with ACS with EF dropping to 10% and protracted cardiogenic shock with multiorgan dysfunction. Restenosis was revealed in the stented areas and treated with balloon angioplasty. He slowly recuperated.
About two months later, he suffered another ACS and in addition to severe in-stent restenosis, corresponding to severe stenosis in the distal LM, the ostial LAD, and the proximal CX, a stenosis in the proximal right coronary artery (RCA) was also detected (Figure 2). He was in a poor state and performing PCI or coronary artery bypass grafting (CAGB) posed a very high risk; instead medical treatment was recommended. His condition remained unstable and characterized by angina pectoris, dyspnea and problems with comorbidities. Due to the poor prognosis, a limited level of treatment was decided in consultation with the patient and his family. This decision was re-evaluated on an ongoing basis.
Implantation of a CRT-D was postponed due to septicemia with Staphylococcus aureus, which was cultivated from the blood and from the wound on his right foot. He was administered antibiotic therapy. Three weeks later, all signs of infection had disappeared. However, the leg wound was not completely closed, but the biochemical screenings values were normal. The CRT-D was implanted without complications (an antibacterial envelope was not used). The patient improved, his shortness of breath subsided from NYHA III to NYHA II, and the QRS-duration diminished to 146 ms. (Figure 3).
As the next step, CABG was planned, but had to be postponed due to recidivating septicemia and infection without a recognized focus. Staphylococcus aureus, Staphylococcus epidermidis, and Corynebacterium were cultivated from the blood. Transesophageal echocardiography revealed discrete changes related to a pace lead, no typical vegetations and no valvular involvement. Positron emission tomography-computerized tomography (PET-CT) showed a change in the same area. Specialists were called upon to evaluate the patient, and it was finally concluded that the infection was probably related to the pacemaker, and system extraction was mandated. Percutaneous explantation was performed, 6 weeks after the implantation, by an experienced operator, with simple traction of the leads, and without procedural complications; a few hours later, however, he began to develop shock with falling bloodpressure, and his temperature rose to 38.5. Monitoring echocardiography showed a drop in ejection fraction (EF) from 20% to 5% despite intensive care.He exhibited no pericardial effusion. His condition deteriorated rapidly, and implantation of a new “rescue” CRT was not considered a possibility. In addition, revascularization could have been a solution, but with a very high risk and it was rejected due to his poor condition and the comorbidities. Extracorporeal membrane oxygenation (ECMO) and impella were not an option due to the peripheral vascular disease.He did not respond to vasopressor therapy (norepinephrine) and died within 17 hours of surgery.
Discussion
From a short-term and a long-term perspective, increased mortality is seen in patients who have had their CRT explanted without a new one being implanted [7]. Besides, various surgical complications in connection with the explantation may be fatal [8,12]. However, until now, the occurrence of cardiogenic shock a few hours after explantation has not been described in the literature, which is actually surprising since the condition ought to be expected due to the loss of hemodynamic support and the resultant increased dyssynchrony, hypokinesia, and mitral insufficiency [7,13,14]. In this case, the removal of the CRT precipitated acute malignant cardiogenic shock. There are several likely explanations, because due to end stage cardiomyopathy any perturbation (a procedure, co-illness, infection, blood loss, etc.) could have send him down the road to shock. Shock due to sepsis syndrome was unlikely because no bacteria were cultivated, neither from the leads, nor from the generator pocket. Besides, the patient was administered broad-spectrum antibiotics. Cardiogenic shock is associated with high mortality due to the serious underlying cardiac disease and several risk factors that may trigger an additional impairment of cardiac contractility. Reversible causes of cardiogenic shock should be treated immediately in order to increase the patient’s chances of survival. ACS is the most frequent cause of cardiogenic shock and early revascularization is the most important treatment strategy in cardiogenic shock because it is potentially lifesaving [1-3], as when this patient suffered a cardiogenic shock the first time. Unfortunately, the coronary stenosis recurred and CAG revealed high risk, central, and severe three-vessel coronary artery disease including LM-stenosis, placing the patient at high risk for recurrent shock because even a minor change in blood pressure and hemodynamics could lead to a death spiral.
When neither the precipitating factor, nor the underlying disease is treatable, the prognosis is very poor, and the treatment for cardiogenic shock may only protract the foreseeable course of events and inevitable death [1]. This patient had a number of risk factors predisposing to device infection: Diabetes mellitus, COPD, heart failure, skin disorder, and pre-procedural fever. Device-type is an independent risk factor with CRT being most exposed to infection [4-6]. Device infection carries an ominous prognosis and despite risk of fatal complications due to device removal, the recommended treatment consist of early, complete system extraction, prolonged intravenous antibiotic therapy, and biventricular device reimplantation [4,6-8,10]. In this case, however, minor surgery and closed irrigation with antibiotics [15] or protracted antibiotic treatment possibly had been preferable to explantation because, as became evident, the patient depended with his life upon pacing of the left ventricle [6,10]. Had this been discovered in due course, and before removing the CRT, the LV-pacing could had been disabled and stopped and the patient’s reactions observed before a decision to operate or not was taken. Unfortunately, this was not the case.
Conclusion
A high-risk patient with severe ischemic cardiomyopathy had an infected CRT explanted. A few hours later, he developed irreversible cardiogenic shock. It is suggested to disable LV-pacing and monitor clinical response and EF before deciding whether to remove a CRT.
Funding: The author received no specific funding for this work.
Conflicts of Interest: I have no conflicts to disclose.
For more information about Article :
https://ijclinmedcasereports.com/ijcmcr-cr-id-00101/ https://ijclinmedcasereports.com/pdf/IJCMCR-CR-00101.pdf
#CRT Explantation#Cardiogenic Shock#LV-Pacing Disabling#Device Infection#Coronary Artery Bypass Grafting#Line Lisbeth O*#clinical studies
0 notes
Text
Cardiac Assist Devices Market is Booming Worldwide Scrutinized in New Research
Cardiac Assist Devices (CAD) are a type of mechanical pumps that work along with the heart to improve its pumping efficiency and maintain optimum blood flow throughout the body. Cardiac assist devices are used to treat end stage heart failure and are determined by the needs of the individual. These are designed to take over the function of the heart when it becomes weak and help it function normal. Cardiac assist devices are of three types: ventricular assist devices, intra-aortic balloon pumps (IABPs), and total artificial heart. Ventricular assist device is implanted in the patient's chest and consists of an electric motor and a driveline. It pumps blood through the heart to the entire body and has the required force to distribute sufficient blood throughout the body. Intra-aortic balloon pump is a long skinny balloon that controls the flow of blood through the aorta, which is the largest blood vessel. The device expands and contacts according to blood flow. It gets smaller when the heart pumps, so that blood can flow out to the rest of the body. It gets bigger when the heart relaxes in order to keep more blood in the heart.
Read Report Overview: https://www.transparencymarketresearch.com/cardiac-assist-devices.html
Rise in prevalence of cardiovascular diseases, increase in novel treatments, affordability of medical devices, high adoption and acceptance of cardiac assist devices in developing markets, and innovation and development in medical devices drive the cardiac assist devices market. Moreover, the global aging population and lack of donors for heart transplantation are boosting the implantation of cardiac assist devices in patients with severe heart complications. However, steep competition among existing players, complications and severity involved in the treatment, and lack of awareness among the rural population in developing and underdeveloped economies are likely to restrain the cardiac assist devices market.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1810
The global cardiac assist devices market can be classified based on product type, modality, application, and region. In terms of product type, the global cardiac assist devices market can be segmented into ventricular assist devices, intra-aortic balloon pumps (IABPs), and total artificial heart. Further ventricular assist devices can be divided into left ventricular assist devices (LVADs), right ventricular assist devices (RVADs), and biventricular assist devices (BIVADs). The ventricular assist devices segment is likely to hold a major share of the cardiac assist devices market due to high acceptability of the product among patients. Moreover, the device does not restrict the movement of patients who have it implanted in their heart. By modality, the global cardiac assist devices market can be segmented into transcutaneous and implantable. Application of cardiac assist devices can be classified into bridge-to-transplant (BTT) therapy, destination therapy, bridge-to-recovery (BTR) therapy, and bridge-to-candidacy (BTC) therapy.
Request for Analysis of COVID19 Impact on Cardiac Assist Devices Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=1810
Based on region, the global cardiac assist devices market can be bifurcated into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. In 2017, North America dominated the global cardiac assist devices market due to high prevalence of cardiovascular diseases and presence of key players in the region. Asia Pacific and Latin America are emerging markets and are anticipated to expand at a considerable pace during the forecast period due to increase in incidence of heart failure, expansion of the health care industry, and increase in government investment to develop health care infrastructure in these regions. The cardiac assist devices market in countries such as China, India, and Brazil is projected to expand at a significant growth rate during the forecast period due to increase in awareness among people about heart diseases and their treatment.
Major players operating in the cardiac assist devices market include Abiomed, Berlin Heart, CardiacAssist, Inc., Cardiobridge GmbH, Carmat, HeartWare, Jarvik Heart, Inc., XENIOS AG, MiTiHeart Corporation, and SynCardia Systems, LLC.
Pre-book Cardiac Assist Devices Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=1810<ype=S
The report offers a comprehensive evaluation of the market. It does so via in-depth qualitative insights, historical data, and verifiable projections about market size. The projections featured in the report have been derived using proven research methodologies and assumptions. By doing so, the research report serves as a repository of analysis and information for every facet of the market, including but not limited to: Regional markets, technology, types, and applications.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/rising-patient-pool-and-increasing-healthcare-expenditure-to-invite-favorable-growth-prospects-for-gout-therapeutic-market-during-2019-2027-tmr-301177001.html
https://www.prnewswire.co.uk/news-releases/single-use-cystoscope-market-to-reach-valuation-of-us-150-mn-by-2031-growing-acceptance-of-patient-ready-instruments-in-urology-to-bolster-demand-tmr-study-818277477.html
https://www.prnewswire.com/news-releases/growing-cases-of-spinal-disorders-to-pain-strokes-of-growth-across-bone-graft-substitutes-market-between-2020-and-2030-tmr-301182424.html
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through adhoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
TMR believes that unison of solutions for clients-specific problems with right methodology of research is the key to help enterprises reach right decision.”
Contact Transparency Market Research State Tower, 90 State Street, Suite 700, Albany NY - 12207 United States USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com/
1 note
·
View note
Text
What are the different kinds of Cardiac Pacemakers?
What are the different kinds of Cardiac Pacemakers?
📷
A fit heart strokes at a steady beat as controlled by the present disorder of the form such as whether it is in gesture or break, or if it is ill or in a demanding state. The heart is gestured through a sequence of electrical instincts that is sent by a bunch of cells called the sinoatrial (SA) bulge which is the form's usual pacemaker. In circumstances where the heart is incapable to uphold a stable beat, a small titanium expedient that purposes as an imitation Cardiac Pacemaker is located under the casing in a patient's ribcage. This pacemaker, found with Cardiac Pacemaker dealers which is motorized by a cordless, generates effortless electrical instincts faking those of the AV bulge that sign the heart to beat with an even beat so that blood is driven properly through the form.
Kinds of Cardiac Pacemakers
Normal Pacemaker: The heart already has a stepping scheme that signs the precise pace of pounding and this is the AV bulge. In a fit body, the heart takes instructions from this and uphold even blood flow and pressure. In case of a heart anxiety that does not allow the AV bulge to purpose competently, an artificial pacemaker manufactured by Cardiac Pacemaker manufacturers is used.
Reproduction Pacemaker: These are of dissimilar kinds and can be categorized majorly as perpetual and provisional pacemakers.
Enduring pacemaker: A perpetual pacemaker can be committed to either a solitary heart cavity, two cavities, or even three heart cavities, and are correspondingly called single cavity pacemakers, dual cavity pacemaker and triple cavity (biventricular pacemaker). The last one orchestrates both subordinate cavities of the heart, and the lead cords attach the cardiac pacemaker to both of them as well as an upper cavity, and so the biventricular pacemaker terminology is normally used for it. Contingent on the sternness of disorder, and which heart atria or ventricle are most pretentious, a medic would commend a pacemaker operation using one of these plans.
A Perpetual pacemaker is a proposition presented for long-lasting circumstances in circumstances where heart fitness is often believed irreparable due to obstruction, or arrhythmia is recurring notwithstanding existence variations or treatment. Bradyarrhythmia is the more shared anxiety where enduring pacemaker implantation is suggested and the inset of the expedient occurs just below the dermatological fat, while the lead cords attach to the heart muscles to be enthused.
Today, leadless pacemaker is also obtainable and becoming more suggested by medics. The cardiac pacemaker dangers related with addition of this expedient is characteristically very little, since the pacemaker is very minor and the imbedding does not necessitate cutting near the ribcage area. It is typically strapped through a slight cut through the great femoral vein that attaches to the heart and implanted into the right patio.
Provisional Pacemaker: A provisional pacemaker is always introduced under medicinal surveillance and the cardiac pacemaker process occurs under measured medical circumstances when the patient's obligation for an imitation pacemaker is assumed to be provisional and the dysrhythmia is to be fixed soon after. A provisional pacemaker may also be entrenched before an enduring one is delivered.
Tran’s vein pacemakers are the more usually used expedients, and a patient rests under surveillance in a hospice until the time the provisional pacemaker is in the form, after which the tube is drawn out. Transcutaneous striding expedients are also baptized exterior pacemakers, and are located under the skin and transports electrical instincts to make the heart shrink faster. These Cardiac Pacemakers were advanced since the upper heart cavities are close to the gullet, making it a sensible location for provisional pacemaker expedients.
If you want to find the top Cardiac Pacemaker suppliers in India, please log onto Ozahub.
1 note
·
View note
Text
Dilated Cardiomyopathy (DCM) Market Trends, Market Size, Share, Treatment | DCM Market Report 2030
Cardiomyopathy is a general term that refers to the disorders of the cardiac muscle that cause mechanical or electrical dysfunction resulting in dilated, hypertrophic or restrictive pathophysiology. In Cardiomyopathy, the walls of the heart chambers become stretched, thickened or stiff; this affects the heart's ability to pump blood around the body.
DelveInsight’s ‘Dilated Cardiomyopathy (DCM) - Market Insights, Epidemiology and Market Forecast —2030’ report delivers an in-depth understanding of the Dilated Cardiomyopathy, historical and forecasted epidemiology as well as the Dilated Cardiomyopathy market trends in the United States, EU5 (Germany, France, Italy, Spain, and United Kingdom), and Japan.
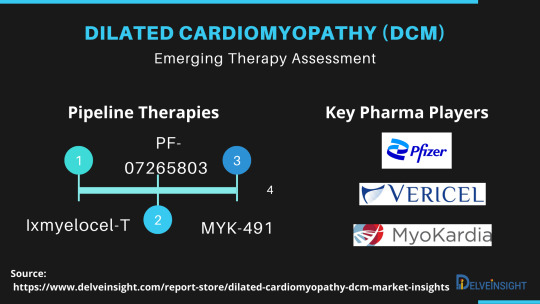
The Dilated Cardiomyopathy market report provides current treatment practices, emerging drugs, Dilated Cardiomyopathy market share of the individual therapies, current and forecasted Dilated Cardiomyopathy market size from 2017 to 2030 segmented by seven major markets.
Dilated Cardiomyopathy Overview
Dilated Cardiomyopathy (DCM) is characterized by left ventricular or biventricular dilation and impaired contraction, which lessens the heart effectiveness at pumping blood that is not explained by abnormal loading conditions like hypertension, valvular heart disease or coronary artery disease. It is a non-ischemic heart muscle disease with structural and functional myocardial abnormalities.
The World Health Organization (WHO) defines DCM as a severe cardiac disorder in which structural or functional abnormalities of the heart muscle can lead to substantial morbidity and mortality owing to complications such as heart failure and arrhythmia.
Request for a detailed sample copy of the report: https://www.delveinsight.com/sample-request/dilated-cardiomyopathy-dcm-market-insights
Dilated Cardiomyopathy Diagnosis
The diagnosis of DCM is mainly based on the person's symptoms, the results of a physical examination, and additional tests. Diagnosis usually includes blood test for common viruses which can cause DCM and when doctors suspect infection as an underlying cause. Additionally, electrocardiography (ECG) may detect abnormalities in the electrical activity of the heart. However, these abnormalities are not sufficient evidence for diagnosis usually.
Dilated Cardiomyopathy Treatment
Currently, the treatment pattern of DCM is mainly dependent on pharmacological therapy, pacing therapy, surgical options, and Corlanor (ivabradine).
The pharmacological therapies consist of diuretics, inotropic agents, afterload reducing agents, beta-blockers, anticoagulation medications, anti-arrhythmia medications.
Dilated Cardiomyopathy Epidemiology
The Dilated Cardiomyopathy epidemiology division provides the insights about historical and current Dilated Cardiomyopathy patient pool and forecasted trend for each seven major countries.
Key Findings
• The total prevalent cases of Dilated Cardiomyopathy in the 7MM were found to be 2,486,633 in 2017 which is expected to grow during the study period, i.e., 2017–2030.
• As per the DelveInsight analysis, the total diagnosed cases of Dilated Cardiomyopathy in the 7MM were 846,615 cases in 2017 which is expected to grow during the study period, i.e., 2017–2030.
Dilated Cardiomyopathy Approved Drugs
Corlanor (ivabradine): Amgen
Dilated Cardiomyopathy Emerging Drugs
PF-07265803/ARRY-371797/ARRY-797: Pfizer
Ixmyelocel-T: Vericel
BC007: Berlin Cures GmbH
Ifetroban: Cumberland Pharmaceuticals
Danicamtiv/MYK-491: MyoKardia
CAP-1002: Capricor Therapeutics
Click here and get access to a free sample copy of our DCM Market Report.
Dilated Cardiomyopathy Market Outlook
Besides treating any recognizable and reversible underlying causes, the management and treatment of DCM are in concordance with the standard heart failure guidelines. Currently, the treatment pattern of DCM is mainly dependent on pharmacological therapy, pacing therapy, surgical options, and Corlanor (ivabradine).
Key Findings
According to DelveInsight’s estimates, the total Dilated Cardiomyopathy therapeutic market in seven major markets was found to be USD 244 million in 2017 which is expected to increase during the study period 2017–30.
Related Reports
Dilated Cardiomyopathy (DCM) - Epidemiology Forecast to 2030
Dilated Cardiomyopathy - Pipeline Insights, 2021
Visit our Insightful Press Release @https://icrowdnewswire.com/2021/05/03/rich-insights-into-end-stage-renal-disease-esrd-market-epidemiology-segmentation-pipeline-therapies-and-major-key-players-involved/
About DelveInsight:
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences providing comprehensive end-to-end solutions to improve performance.
Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
0 notes
Text
Global Swimwear Industry - Industry Analysis, Size, Share, Growth, Trends and Forecast 2020-2025
A new market study, “ Global Swimwear Industry - Industry Analysis, Size, Share, Growth, Trends and Forecast 2020-2025 ” has been featured on WiseGuyReports.
The global cardiac pacemaker market is expected to grow at a CAGR of XX% to reach a market value of USD XX million by 2026. Cardiac pacemaker is a battery operated device which senses the heart beating rate. In case of any abnormalities, the device sends signals to the heart making it beat at the correct pace. Majorly pacemakers have two components, a generator, which contains the battery and the information to control the heartbeat, and leads. Leads are the wires connecting the heart and the generator to carry the electrical messages. The generator is implanted under the skin by making a cut on the left side of the chest and using X-rays, leads are put through the cut. Global Cardiac Pacemaker Market – Market Dynamics The global market for the cardiac pacemaker market is primarily driven by the rising prevalence of Cardiovascular Diseases (CVD), growing geriatric population, and technological advances in the cardiovascular field. According to the World Health Organization (WHO), CVD was the major number one cause of death globally in 2017. In 2016, around 17.9 million people are estimated for death from CVDs which represents 31% of deaths worldwide. Moreover, 85% of the deaths from CVD are due to heart attack and stroke. Individuals with high risk of CVD may show high blood pressure and obese conditions. According to WHO, in 2015, around 20.1% women and 24.1% men in adults (above 18 years) suffered from a high prevalence of raised blood worldwide. The number of adults with high blood pressure increased from 594 million in 1975 to 1.13 billion in 2015. High costs of equipment and patient concerns post implantation are the a few major restraining factors for Cardiac Pacemaker market growth. While the new emerging market players are designing the modern implantable pacemakers, which can be an opportunity for Cardiac pacemakers market. Global Cardiac Pacemaker Market – Segment Analysis Based on product type, implantable pacemakers is expected to hold the largest market share in cardiac pacemakers market owing to its effectiveness due of multiple leads which allow for normal functioning of heart and ease of further advancements. Implantable pacemakers are again categorized into a single chamber, dual chamber, and biventricular pacemakers. The U.S. Government has implemented reimbursement plans for certain implantable pacemakers. For instance, the cost for a single chamber pacemaker is 10,000 USD, and approximately 7,500 USD is reimbursed as a result of the Medicare plan in the U.S. Hospital segment is expected to account for the largest share in the cardiac pacemakers market based on end-users primarily due to the higher number of patients undergoing treatments in hospitals than in clinics; implementation of government initiatives of reimbursements and increasing number of hospitals in emerging Asian countries.
Also Read : https://www.einpresswire.com/article/501908744/sar-market-2019-global-key-players-trends-share-industry-size-segmentation-opportunities-forecast-to-2026
The Ambulatory Surgical Centers (ASC) segment is expected to show high growth rate in the forecast period owing to the growing adoption of ASCs in developed countries, and economic constraints are expected to boost the segment growth over the coming years.
According to the U.S. Department of Health & Human Services, in 2017, around 19.3 million hospital visits are noted which included both inpatient and ambulatory services. More than half of these visits (54.9%) are noted at hospital owned ambulatory settings and rest 45.1 % were inpatient services.
According to National Health Service UK In 2018 stated that the number of emergency admissions in NHS increase by almost 42% in a decade with an average 3.2% per annum due to rise in sicker patients
Global Cardiac Pacemaker Market– Geographical Analysis
North America holds the highest market share in cardiac pacemaker market globally owing to the well-developed infrastructure, good reimbursement policies, high awareness among people, and high income of the region have augmented the market growth in this region.
The Asia Pacific is expected to show growth in the forecast period owing to the anticipated government policies to establish a free and open economy and the high deaths in the region.
According to WHO, over three-quarters of CVD deaths take place in low- and middle-income countries. Around 17.9 million people has died from CVDs in 2016, representing 31% of all global deaths. Of these deaths, 85% are due to heart attack and stroke.
The population in low- and middle-income countries don't usually have many benefits from integrated primary health care, and hence there are lesser chances for early detection when compared to people in high-income countries.
Global Cardiac Pacemaker Market– Competitive Analysis
Key players are adopting strategies such as mergers and acquisitions, partnerships, and regional expansion to stand out as strong competitors in the market. New product launches along with an increased focus on R&D are other ways the leading players improve their market presence. The key players in the market are Medtronic Plc., St. Jude Medical, Biotronik, Abbott Laboratories, Boston Scientific, Vivatron, Sorin Group, Lepu Medical, Shree Pacetronix, and Medico S.P.A
On 30th April 2019, Biotronik announced the launch of the Acticor device family which includes Acticor DX and CRT-DX devices. In USA, patients are being treated with the new implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
On 1st January 2018, Medtronic Plc. Launched its MyCareLink Heart mobile application to support the portfolio of pacemakers by providing the comfort of communication directly with patients' smart devices.
On 8th May 2017, Biotronik launches its first FDA approved CRM (Cardiac rhythm management) Devices with MRI auto detect Technology. The MRI AutoDetect technology makes sure that patients with CRM devices can have safe MRI scans and thereby minimize programming burdens for hospitals.
Why Purchase the Report?
• Visualize the composition of the global cardiac pacemaker market across each indication, in terms of product type, applications and end users, highlighting the key commercial assets and players.
• Identify commercial opportunities in global cardiac pacemaker market by analyzing trends and co-development deals.
• Excel data sheet with thousands of data points of the global cardiac pacemaker market - level 4/5 segmentation.
• PDF report with the most relevant analysis cogently put together after exhaustive qualitative interviews and in-depth market study.
• Product mapping in excel for the key products of all major market players
Target Audience:Product mapping in excel for the critical Global Soft Tissue Sarcoma products of all major market players
FOR MORE DETAILS: https://www.wiseguyreports.com/reports/3792131-global-sar-market-2018-2025
About Us:
Wise Guy Reports is part of the Wise Guy Research Consultants Pvt. Ltd. and offers premium progressive statistical surveying, market research reports, analysis & forecast data for industries and governments around the globe.
Contact Us:
NORAH TRENT
Ph: +162-825-80070 (US)
Ph: +44 2035002763 (UK)
0 notes
Text
Implantable Cardiac Pacemaker Market - Global Forecast to 2026

Implantable cardiac pacemaker is a small device implanted in the chest or abdomen for the treatment of abnormal heart rhythms, arrhythmia, heart block, and atrial fibrillation. Implantable cardiac pacemaker is evolved from battery-powered transistorized wearable pacemakers to current leadless pacemaker, an entire pacemaker which is placed within cardiac chambers. Cardiac pacemakers are used to treat abnormal electrical signaling in the heart, which causes arrhythmias. Implantable cardiac pacemaker use low-energy electrical signals to speed up the slow heartbeat, help to control an abnormal and fast heart rhythm, and prevents long QT syndrome, a disorder of the heart’s electrical activity, which causes sudden, uncontrollable, and dangerous arrhythmias.
Download PDF Brochure Of This Research Report @ https://www.coherentmarketinsights.com/insight/request-pdf/1663
Market Dynamics
Market players are engaged in developing novel pacemakers with ability to control the abnormal heart rhythm, monitor and record patient’s heart's electrical activity, blood temperature, and breathing rate. Technological advances in the field of implantable cardiac pacemakers along with increasing prevalence of cardiovascular disease is projected to drive the growth of implantable cardiac pacemaker market over the forecast period.
Introduction of Advanced Pacemaker Technologies is Expected to Drive Growth of the Implantable Cardiac Pacemaker Market
Adoption of advanced technologies in cardiac pacemakers such as MRI safe-pacemakers, pacemakers with improved battery life, delay the progression of persistent Atrial Fibrillation (AF) in patients suffering from bradycardia and wireless automated technologies. This advancements are gaining significant traction of various market players of implantable cardiac pacemakers with affordable costs, which in turn are expected to drive growth of implantable cardiac pacemakers market. For instance, in February 2017, Abbott received the Food and Drug Administration (FDA) approval for its MR-conditional labeling for both of its products i.e. Assurity MRI pacemaker and the Tendril MRI pacing lead. Furthermore, introduction of advance technology such as wireless and automated pacemaker monitoring pacemakers are gaining significant traction among healthcare providers.
For instance, in 2016, Biotronik launched CardioMessenger Smart, portable monitoring device in the U.S. This system is about the size of a modern smartphone used to keep pacemaker, Implantable Cardioverter Defibrillator (ICD) and Insertable Cardiac Monitor (ICM) patients connected to their physician remotely. This CardioMessenger Smart helps to ensure patient compliance and the consistent transmission of data necessary for physicians to identify and prevent potential cardiac events. Thus, such wireless technologies are gaining traction of vendors, which is expected to drive the growth of the implantable cardiac pacemaker market
Increasing Prevalence of Cardiovascular Disease is Expected to Drive Growth of Implantable Cardiac Pacemaker Market
Increasing incidence of cardiovascular diseases is expected to boost growth of the implantable cardiac pacemakers market over the forecast period. According to the Centers for Disease Control and Prevention (CDC), August 2017 data findings, Atrial Fibrillation (AFib or AF) is most common type of heart arrhythmia, which affected an estimated 2.7 to 6.1 million people in the U.S. and the number is expected to increase in near future. Furthermore, Atrial Fibrillation cases increase with age and it is more prominent in men than women. Moreover, increasing number of geriatric population across the various regions worldwide along with high prevalence of heart disease is expected to propel demand for implantable cardiac pacemakers in the near future.
However, implantable pacemakers associated infections in cardiac patients is hindering growth of the implantable cardiac pacemaker market. Pacemaker surgery is associated with swelling, bruising, bleeding, or infection of the area where the pacemaker was placed leading to blood vessel or nerve damage and adverse reaction of medicine used during the pacemaker implantation surgery, which may reduce the adoption of implantable cardiac implants in patients.
For More Information @ https://www.coherentmarketinsights.com/market-insight/implantable-cardiac-pacemaker-market-1663
Global Key Players:
Some of the key players operating in implantable cardiac pacemaker market include Medtronic plc, BIOTRONIK, Inc., Boston Scientific Corporation, St. Jude Medical, Vitatron Holding B.V., Shree Pacetronix Ltd., MEDICO S.p.A., Lepu Medical Technology Co Ltd., LivaNova PLC, and Qinming Medical.
Detailed Segmentation:
Global Implantable Cardiac Pacemaker Market, By Product Type:
Single Chamber Pacemaker
Dual Chamber Pacemaker
Biventricular Pacemaker
Global Implantable Cardiac Pacemaker Market, By Application:
Heart Block
Arrhythmia
Atrial fibrillation
Long QT Syndrome
Bradycardia
Tachycardia
Others
Global Implantable Cardiac Pacemaker Market, By End User:
Hospitals
Emergency Care Centers
Others
Global Implantable Cardiac Pacemaker Market, By Region:
North America
Europe
Asia Pacific
Middle East
Latin America
Africa
Request For Customization @ https://www.coherentmarketinsights.com/insight/request-customization/1663
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes