#Bharat Biotech Vaccine
Explore tagged Tumblr posts
Text
Bharat Biotech and University of Sydney Sign MoU to Advance Academic and Vaccine Research Collaboration
Hyderabad, India, and Sydney, Australia – 28th November 2023 – Bharat Biotech International Limited, a world leading vaccines & bio-therapeutics manufacturer and the University of Sydney Infectious Diseases Institute (Sydney ID) today announced a Memorandum of Understanding (MoU) to advance vaccine research initiatives, strengthen academic-industry partnerships and augment global efforts to…

View On WordPress
0 notes
Text
0 notes
Text
Odisha CM showing infrastructures built by Patnaik govt to visiting Singapore President: BJD
Bhubaneswar: The Opposition Biju Janata Dal Friday slammed BJP government in Odisha for attempting to take mileage from the Singapore President’s visit to the World Skill Centre and a vaccine manufacturing plant in the state. BJD spokesperson Lenin Mohanty welcoming the visit of the Singapore President to Odisha, said both the World Skill Centre and Bharat Biotech plant were built during the…
0 notes
Text
[ad_1] Singapore President Tharman Shanmugaratnam arrived in New Delhi on Tuesday for a significant State Visit, coinciding with the 60th anniversary of diplomatic relations between India and Singapore. The visit, which runs from January 14-18, aims to deepen cooperation between the two nations across various sectors, including trade, technology, and cultural exchange. #WATCH | Delhi: President Tharman Shanmugaratnam of Singapore arrives in India on a State Visit. He was received by MoS Jitin Prasada at the airport. pic.twitter.com/ylDYEXxtF3 — NewsMobile (@NewsMobileIndia) January 14, 2025 Upon his arrival, President Tharman was greeted with warmth and enthusiasm by Jitin Prasada, India’s Minister of State for Commerce and Industry. The Ministry of External Affairs (MEA) described the visit as a “special celebration�� of six decades of enduring partnership. MEA spokesperson Randhir Jaiswal shared the moment on social media, highlighting the President’s arrival as a key milestone in India-Singapore relations. A special celebration of 60th anniversary of #IndiaSingapore diplomatic relations. President @Tharman_S of Singapore arrives in New Delhi on a State Visit. Warmly received by MoS @JitinPrasada at the airport. pic.twitter.com/tPmvJDQHOM — Randhir Jaiswal (@MEAIndia) January 14, 2025 President Tharman’s agenda in the capital includes a ceremonial welcome at Rashtrapati Bhawan on January 16. He will meet with Indian President Droupadi Murmu, who is hosting a state banquet in his honor. Prime Minister Narendra Modi is also scheduled to engage with the Singaporean leader to discuss bilateral cooperation and shared global challenges. Additionally, External Affairs Minister S. Jaishankar and other senior dignitaries are expected to hold discussions with President Tharman, further strengthening the ties between the two nations. In a significant move to highlight Singapore’s contribution to India’s development, President Tharman will travel to Odisha from January 17-18. While in Bhubaneswar, he will visit the World Skills Centre, a state-of-the-art facility established with the support of Singapore’s Institute of Technical Education Education Services (ITEES) and funding from the Asian Development Bank. This collaboration underscores the shared commitment to enhancing global competitiveness through skill development. The President’s visit to Odisha will also include a tour of Bharat Biotech’s vaccine manufacturing facility, a key player in India’s healthcare innovation. Additionally, he will explore sites reflecting the region’s rich cultural heritage, further emphasizing the cultural connections between the two nations. Click here for Latest Fact Checked News On NewsMobile WhatsApp Channel For viral videos and Latest trends subscribe to NewsMobile YouTube Channel and Follow us on Instagram [ad_2] Source link
0 notes
Text
[ad_1] Singapore President Tharman Shanmugaratnam arrived in New Delhi on Tuesday for a significant State Visit, coinciding with the 60th anniversary of diplomatic relations between India and Singapore. The visit, which runs from January 14-18, aims to deepen cooperation between the two nations across various sectors, including trade, technology, and cultural exchange. #WATCH | Delhi: President Tharman Shanmugaratnam of Singapore arrives in India on a State Visit. He was received by MoS Jitin Prasada at the airport. pic.twitter.com/ylDYEXxtF3 — NewsMobile (@NewsMobileIndia) January 14, 2025 Upon his arrival, President Tharman was greeted with warmth and enthusiasm by Jitin Prasada, India’s Minister of State for Commerce and Industry. The Ministry of External Affairs (MEA) described the visit as a “special celebration” of six decades of enduring partnership. MEA spokesperson Randhir Jaiswal shared the moment on social media, highlighting the President’s arrival as a key milestone in India-Singapore relations. A special celebration of 60th anniversary of #IndiaSingapore diplomatic relations. President @Tharman_S of Singapore arrives in New Delhi on a State Visit. Warmly received by MoS @JitinPrasada at the airport. pic.twitter.com/tPmvJDQHOM — Randhir Jaiswal (@MEAIndia) January 14, 2025 President Tharman’s agenda in the capital includes a ceremonial welcome at Rashtrapati Bhawan on January 16. He will meet with Indian President Droupadi Murmu, who is hosting a state banquet in his honor. Prime Minister Narendra Modi is also scheduled to engage with the Singaporean leader to discuss bilateral cooperation and shared global challenges. Additionally, External Affairs Minister S. Jaishankar and other senior dignitaries are expected to hold discussions with President Tharman, further strengthening the ties between the two nations. In a significant move to highlight Singapore’s contribution to India’s development, President Tharman will travel to Odisha from January 17-18. While in Bhubaneswar, he will visit the World Skills Centre, a state-of-the-art facility established with the support of Singapore’s Institute of Technical Education Education Services (ITEES) and funding from the Asian Development Bank. This collaboration underscores the shared commitment to enhancing global competitiveness through skill development. The President’s visit to Odisha will also include a tour of Bharat Biotech’s vaccine manufacturing facility, a key player in India’s healthcare innovation. Additionally, he will explore sites reflecting the region’s rich cultural heritage, further emphasizing the cultural connections between the two nations. Click here for Latest Fact Checked News On NewsMobile WhatsApp Channel For viral videos and Latest trends subscribe to NewsMobile YouTube Channel and Follow us on Instagram [ad_2] Source link
0 notes
Text
The report published by Kearney in collaboration with the Confederation of Indian Industry titled “Taking India’s life sciences industry to the global stage”, reported that “India’s vaccines industry can grow from $2 billion to $4 billion or even $5 billion”. This has been made possible by a number of factors prominent being the inclusion of vaccines in the product portfolios of several domestic and global pharmaceutical companies. With the pandemic kicking in 2019 end, the entire world came together to build an effective vaccine to fight against the deadly coronavirus.
This report speaks a lot about the Indian biosimilars and vaccines industries and how it is competing globally and scopes for India in the global biosimilars and vaccines industry. It lays down the challenges faced by the Indian pharmaceutical industry and how we can overcome these. It lays down abundant solutions to the challenges faced and how India can ace the Global Pharma sector and command its worthy position both in terms of volume and value.
Indian Vaccine Industry
The global Pharmaceutical industry has made vaccines their priority. India commands a massive 40% share of the global vaccine industry by volume. India has been playing a pivotal role in combating deadly diseases for years and now the coronavirus pandemic. India is all set to grow to hold a more significant place in the global pharma industry in the coming years. With the introduction of newer and better technologies such as gene therapies, Ai-based informatics, etc has opened boundless opportunities to conduct research and experimentation and develop innovative and effective medications.
India already possesses a strong local pharma industry, fundamentally strong technical capabilities, and rich scientific acumen, that has helped us emerge as an innovational hub of the world. India is the global supplier of drugs and medicines and everybody prefers India for its low-cost manufacturing processes and affordable drugs.
However, Indian Pharmaceutical Industry still lags behind in terms of the value of our market share through being the third-largest volume producer. India faces several challenges that need to be overcome. COVID pandemic offers a great opportunity for India to take a step ahead to bag the top position both in terms of volume and value.
Massive Scope for Global Vaccine supply
There is abundant scope for the export of COVID-19 vaccines globally to countries unable to manufacture vaccines on their own. Moreover, there are several diseases globally that need vaccination, and these infectious diseases amount to more than 70% of the world population. Many countries rely on India for the supply of vaccine doses to combat various infectious diseases. Our own nation has a huge demand for vaccines owing to the huge population count and prevalence of abundant diseases such as diphtheria, tetanus, hepatitis B, measles, mumps, rubella, etc.
India has long been involved in supplying the highly required vaccines to several developing nations at the most affordable prices. So now also the world relied on India for COVID vaccine and India proved to be the biggest exporter of Covid vaccines globally. India itself faced a shortage of vaccines initially due to the heavy burden on the limited resources besides having huge commitments globally. Only two vaccine manufacturers i.e. Serum Institute of India (SII) and Bharat Biotech were operational in the beginning. With limited manufacturing resources, it became difficult to meet both domestic and global vaccine requirements.
This shows the scope for vaccine manufacturing in India provided we develop the required infrastructure and support our pharma medicine developers with the latest and advanced technologies to conduct their research. India requires a favorable statutory and political environment to lead the world with its pharmaceutical products. To be the global leader in terms of value, India needs to be more research-oriented to develop unique formulations to treat health conditions and obtaining Intellectual Property Rights. This is how India can occupy a major pharma share both in terms of volume as well as value.
#IndiaVaccineIndustry#VaccineMarketGrowth#IndianPharma#HealthcareIndia#BiotechInnovation#VaccineDevelopment#IndiaHealthcare#PharmaIndustry#GlobalHealth#India2026#VaccineEconomy#PharmaGrowthIndia#MedicalInnovation#IndiaVaccines#BiotechGrowth
1 note
·
View note
Text
10th Annual Biotech Industry Conclave on Biotechnology for Humanity: Innovations Shaping a Better World
KIIT School of Biotechnology organized the 10th Biotech Industrial Conclave on “Biotechnology for Humanity: Innovations Shaping a Better World” during 23rd–24thAugust 2024. The conclave, spanning over two days, had enlightening talks, discussion and spirited interactions between experts from the biotech industry, academia, and students to foster collaboration and innovation in the field. During the inaugural session, Dr. Achyuta Samanta, Founder, KIIT & KISS, welcomed all the dignitaries and stressed upon development of strong collaboration between KIIT and biotechnology industry.

Padmashri Dr. Krishna Ella, Founder & Executive Chairman, Bharat Biotech International Limited echoed the need for close interaction between premier educational institutes and the biotech industry for inclusive growth. Dr. Ella, in his keynote address, shared his personal journey in entrepreneurship and innovation, highlighting the challenges and rewards of building a successful biotech company. He emphasized the importance of practical learning experiences for PhD students and the role of the Ella Foundation in supporting their entrepreneurial endeavors. He also stressed upon the need for vaccine development for neglected diseases.
Dr. Shilpa Gadgil, Vice President, Enzene Biosciences Ltd.; Dr. Priyabrata Pattnaik, Dy. Managing Director, Indian Immunologicals Ltd.; Raghavendra P. Rao, Principal Scientist, The Himalaya Drug Company; Dr. Ranendra N. Saha, Chief Scientific Advisor, Biophore; Dr. Sreedharala Venkata Nookaraju, Executive Vice President, Aizant Drug Research Soln. Lt;. and Dr. Prabuddha Kundu, Co-Founder & MD, Premas Biotech discussed about emerging trends in complex therapeutic biomolecules like monoclonal antibodies, novel vaccines, complex protein production processes. Mr. Dushyant Deshmukh, GM-HR, Glenmark Pharmaceuticals; Mr. Hemant Nikam, Sr Director and Head HR, Eisai Pharmaceuticals India; and Mr. Pritesh Bhatia, Associate Director HR, Jubilant Biosys Ltd. discussed about the emerging trends in human resource requirements and how students should prepare themselves to become successful biotechnology professionals.
Mr. Lalit Sisaudia, Vice President of Nutaste Group and Ms. Rosina Panda, Business Manager, Intertek spoke about food safety and the need for precision to address global food security challenges.
Dr. Emili Banerjee, Senior Genetic Counselor, Neuberg Diagnostics, Dr. Raghavendra Goud, Executive Director at PharmNXT Biotech and Mr. Ashish Dubey, Co-Founder and COO, Redcliffe Labs discussed the opportunities in molecular diagnostics in healthcare and how AI driven processes can accelerate disease detection and make healthcare accessible in the remote regions of the country. In the last session, five alumni turned entrepreneurs Mr. Pritam Dhalla, Founder, Larkai Healthcare; Mr. Akashdeep Dan, Co-Founder of Bogmalo Foods and Hospitality; Mr. Prateek Mahapatra, Director of Grow Green Consortium Pvt. Ltd.; Ms. Varsha Biswal, CEO, Trupti Dairy; and Dr. Shailesh Samal, CEO, Inflanova AB shared the memories in KSBT and how it helped them to excel in their areas of passion.
Dr. Mrutyunjay Suar, DG-Research & Innovation, and CEO, KIIT-TBI discussed about start-up innovation system in KIIT and role of Bhubaneswar City Knowledge Cluster in developing fruitful collaboration in the biotechnology sector. Dr. Jnyana Ranjan Mohanty, Registrar and Dr. Srinivas Patnaik, Dean KSBT described the academic initiatives taken for skill development of the students. Dr. Rahul Modak, Convener of the conclave thanked all the dignitaries and participants for their active participation and contribution in the conclave.
0 notes
Text
Indian Company Makes Oral Vaccine To Meet Global Shortage

HYDERABAD, (REUTERS) – India’s Bharat Biotech said on August 27 its oral cholera vaccine cleared a late-stage trial and that it plans a global launch, aiming to make up to 200 million doses a year amid an expanding outbreak of the disease and treatment shortages.
India’s drug regulator has approved the vaccine, called Hillchol, and Bharat Biotech will apply for the World Health Organization’s pre-qualification to supply to major buyers like the U.N. children’s agency UNICEF, the company said.
Bharat Biotech’s vaccine will compete with South Korea’s EuBiologics Co, which is the only WHO-approved producer of cholera vaccines.
0 notes
Text
Bharat Biotech Unveils Oral Vaccine to Tackle Global Cholera Threat
On Tuesday, Bharat Biotech introduced an oral cholera vaccine aimed at combating the persistent global threat of cholera, particularly in areas with poor sanitation. http://dlvr.it/TChpn2
0 notes
Text
Current affairs - 30 August 2024
1.Oral Cholera Vaccine (Hillchol) The Hindu Background: Bharat Biotech, in collaboration with Hilleman Labs, has launched Hillchol (BBV131), an oral vaccine for cholera. Global Demand: The vaccine aims to address the global shortage of Oral Cholera Vaccines (OCVs), with a demand exceeding 100 million doses per year. Dosage: Hillchol is administered in two oral doses. Clinical Trials: Phase…

View On WordPress
0 notes
Text
Oral Cholera Vaccine Launched
India's Bharat Biotech said on Tuesday its oral cholera vaccine cleared a late-stage trial and that it plans a global launch, aiming to make up to 200 million doses a year amid an expanding outbreak of the disease and treatment shortages.
India's drug regulator has approved the vaccine, called Hillchol, and Bharat Biotech will apply for the World Health Organization's pre-qualification to supply to major buyers like the U.N. children's agency UNICEF, the company said.
https://www.reuters.com/business/healthcare-pharmaceuticals/indias-bharat-biotech-says-oral-cholera-vaccine-phase-3-trials-proves-safety-2024-08-27/
0 notes
Text
Job opportunity for Production department at Bharat biotech
Bharat Biotech International Limited is a versatile biotechnology firm that focuses on research, development, and production of vaccines and biotherapeutics. The company is dedicated to creating advanced vaccines and bio-therapeutics through groundbreaking and cooperative research efforts. BBIL’s cutting-edge manufacturing facility stands as the largest in the Asia-Pacific region. Join Bharat…

View On WordPress
0 notes
Text
[ad_1] Singapore’s President Tharman Shanmugaratnam will visit India on January 15, marking a significant milestone in the 60th anniversary of diplomatic ties between the two nations. Diplomatic sources told ANI that this visit is expected to elevate India-Singapore relations to new heights, with a focus on nontraditional areas of cooperation such as energy, industrial parks, and skills. This year marks the 60th anniversary of diplomatic ties between the two countries, and ties are expected to reach a new high. A series of events and engagements are planned to mark this special year. Singapore’s President Tharman Shanmugaratnam will visit New Delhi on January 15. Later this year, Prime Minister Lawrence Wong is expected to visit India. A few months later, the next round of the India-Singapore ministerial roundtable is also slated to take place. According to diplomatic sources, the relationship got a major boost last year after the roundtable, followed by Prime Minister Modi’s visit to Singapore last year. The India-Singapore relationship was upgraded to a strategic partnership during Prime Minister Modi’s visit to Singapore in 2015. After Modi visited Singapore in September 2024, the Indian High Commission in Singapore noted that India-Singapore relations were elevated to a Comprehensive Strategic Partnership. Singapore is the second country after Australia to share this level of relationship. Diplomatic sources told ANI that Singapore wants to mark the upgrading of the relationship with a series of high-level visits and increased cooperation. India and Singapore will also unveil the logo for the 60th year of diplomatic ties soon. During President Tharman Shanmugaratnam’s visit, Singapore will also confer the highest honour of citizenship to the former director of the Confederation of Indian Industries Tarun Dass. This honour was earlier bestowed upon the late Ratan Tata. During the President’s visit, the focus will be on nontraditional areas of cooperation, such as semiconductors, energy, industrial parks, and skills. According to diplomatic sources, two semiconductor and skill development pacts are also in the pipeline. In addition to meeting Indian leadership, including Prime Minister Modi and President Murmu, the Singaporean President will meet top economists, academics, and NITI AAYOG officials. Southern states like Karnataka and Tamil Nadu have remained the main attractions for investments from Singapore. Singapore wants to diversify its investments into new states, with Odisha being a key focus area. The President will visit Odisha and hold talks with the Chief Minister to explore new avenues of cooperation. With this view, Singapore’s President will visit Odisha during his visit to India and explore new avenues of cooperation. He will also hold talks with Odisha’s Chief Minister. The Indian government focuses on diversifying areas of investment, and Singapore is also looking for new engines of growth. Talks on improved connectivity will also be held during the visit. The Singaporean President is also expected to tour the World Skills Centre in Bhubaneshwar. Notably, the centre was developed with the help of Singapore. The diplomatic sources said he is also likely to tour the vaccine manufacturer, Bharat Biotech and UNESCO sites. India wants to have a first-mover advantage. Singapore will be making significant investments in India’s northeast via Assam, with fresh fruits from the state being exported to Singapore via cargo flight. According to sources, Singapore will be working very closely with the Tata group for semiconductors. Last year, India and Singapore signed a pact on a semiconductor ecosystem partnership. Interestingly, Singapore is one of India’s biggest contributors to foreign direct investment. Last year, Singapore invested USD 12 billion, which is expected to exceed further this year, the diplomatic sources said. [ad_2] Source link
0 notes
Text
[ad_1] Singapore’s President Tharman Shanmugaratnam will visit India on January 15, marking a significant milestone in the 60th anniversary of diplomatic ties between the two nations. Diplomatic sources told ANI that this visit is expected to elevate India-Singapore relations to new heights, with a focus on nontraditional areas of cooperation such as energy, industrial parks, and skills. This year marks the 60th anniversary of diplomatic ties between the two countries, and ties are expected to reach a new high. A series of events and engagements are planned to mark this special year. Singapore’s President Tharman Shanmugaratnam will visit New Delhi on January 15. Later this year, Prime Minister Lawrence Wong is expected to visit India. A few months later, the next round of the India-Singapore ministerial roundtable is also slated to take place. According to diplomatic sources, the relationship got a major boost last year after the roundtable, followed by Prime Minister Modi’s visit to Singapore last year. The India-Singapore relationship was upgraded to a strategic partnership during Prime Minister Modi’s visit to Singapore in 2015. After Modi visited Singapore in September 2024, the Indian High Commission in Singapore noted that India-Singapore relations were elevated to a Comprehensive Strategic Partnership. Singapore is the second country after Australia to share this level of relationship. Diplomatic sources told ANI that Singapore wants to mark the upgrading of the relationship with a series of high-level visits and increased cooperation. India and Singapore will also unveil the logo for the 60th year of diplomatic ties soon. During President Tharman Shanmugaratnam’s visit, Singapore will also confer the highest honour of citizenship to the former director of the Confederation of Indian Industries Tarun Dass. This honour was earlier bestowed upon the late Ratan Tata. During the President’s visit, the focus will be on nontraditional areas of cooperation, such as semiconductors, energy, industrial parks, and skills. According to diplomatic sources, two semiconductor and skill development pacts are also in the pipeline. In addition to meeting Indian leadership, including Prime Minister Modi and President Murmu, the Singaporean President will meet top economists, academics, and NITI AAYOG officials. Southern states like Karnataka and Tamil Nadu have remained the main attractions for investments from Singapore. Singapore wants to diversify its investments into new states, with Odisha being a key focus area. The President will visit Odisha and hold talks with the Chief Minister to explore new avenues of cooperation. With this view, Singapore’s President will visit Odisha during his visit to India and explore new avenues of cooperation. He will also hold talks with Odisha’s Chief Minister. The Indian government focuses on diversifying areas of investment, and Singapore is also looking for new engines of growth. Talks on improved connectivity will also be held during the visit. The Singaporean President is also expected to tour the World Skills Centre in Bhubaneshwar. Notably, the centre was developed with the help of Singapore. The diplomatic sources said he is also likely to tour the vaccine manufacturer, Bharat Biotech and UNESCO sites. India wants to have a first-mover advantage. Singapore will be making significant investments in India’s northeast via Assam, with fresh fruits from the state being exported to Singapore via cargo flight. According to sources, Singapore will be working very closely with the Tata group for semiconductors. Last year, India and Singapore signed a pact on a semiconductor ecosystem partnership. Interestingly, Singapore is one of India’s biggest contributors to foreign direct investment. Last year, Singapore invested USD 12 billion, which is expected to exceed further this year, the diplomatic sources said. [ad_2] Source link
0 notes
Text
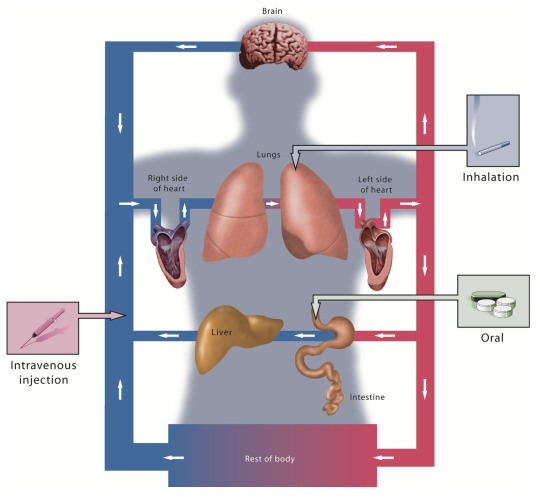
India Specialty Drug Distribution Market generated a revenue of US$ 2,045.35 million in 2022 and is estimated to reach a valuation of US$ 5,997.7 million by 2031 at a CAGR of 12.93% during the forecast period 2023–2031.
Key Players in the Market Report
Aark Pharmaceuticals
Astellas Pharma India Pvt. Ltd.
Aurobindo Pharmaceuticals
Arlak Biotech
Bharat Serums and Vaccines Limited
Biotic Healthcare
Cipla
D.Vijay Pharma
Dr. Reddy’s Laboratories Ltd
Divis Laboratories
Feron Healthcare
Gaia Pharmaceutical Trade
Glenmark Pharmaceuticals
GNova Biotech
Ikris Pharma
IMS Medi
Jay-Pharma
Lupin
Meher Distributors Pvt. Ltd.
Merck & Co., Inc.
Novartis AG
Novacare
Pax Healthcare
Prime Health
Sanify Healthcare
Serum Institute of India Pvt. Ltd.
Sun Pharmaceutical Industries Ltd.
Servocare Lifesciences
Shubham Pharmaceutical
Swisschem Healthcare
Torrent Pharmaceuticals
Vardhman Health Specialities Pvt. Ltd.
Zydus Lifesciences Limited
Intas Pharmaceuticals Ltd.
3S Corporation
Other Prominent Players
0 notes
Link
0 notes