#Bactec Blood Culture Test
Explore tagged Tumblr posts
Text
Lab Tour
hi y'all, I said I would do this, so here's a short walkthrough of the instruments in the lab I work in ^_^

This is our coagulation study machine! It runs tests for PT, PTT, and D-dimer. (One of my coworkers is doing a QC study which is why you see that note on there.)


These are the hematology analyzers! The 690 runs with cassettes (you can see them in front) and so you can put multiple samples on the machine and walk away. The 520 is our smaller back-up machine. It's very cute :) but when the 690 is down it's a pain bc the 520 can only run 1 sample at a time.


These are our chemistry analyzers. The AU is what we call the "big" analyzer. It does all the metabolic panels, toxicology, and A1Cs. The Access is the "little" analyzer. It takes care of cardiac biomarkers, thyroid hormones, and other assorted tests not covered by the AU.


Here are the micro machines! On the left is the Cepheid. It's a PCR machine. We run a variety of tests on it, like the "Fourplex" (RSV, COVID, Influenza A and B), Strep A, Strep B, Chlamydia/Gonorrhea, etc. And on the right is our Bactec, for blood cultures.
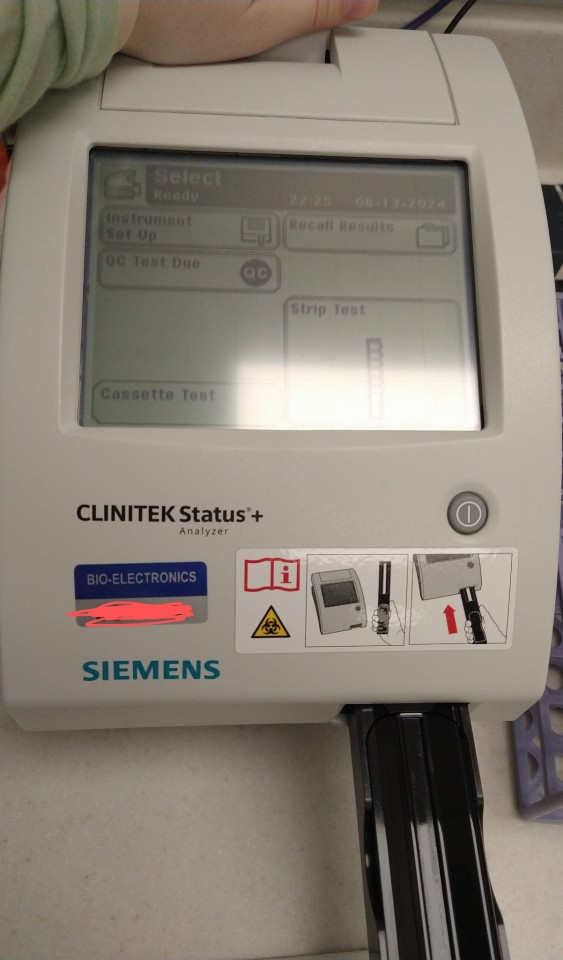
This is the Clinitek! It's a urine strip reader. Basically, we take urine, we dip a test strip in it, blot the strip, and put it on the machine. Depending on the results, we then look at the urine under a microscope. For example, if nitrates and leukocytes (WBCs) are present, we would look at the urine microscopically. There, if we saw bacteria, we could basically confirm a UTI.

Finally, this is the Blood Bank centrifuge/incubator. We use gel cards for all our blood banking. The incubator is for IgG antibody screens. Our blood banking is done manually (some bigger hospitals have machines to do it, which is cool, but I do enjoy the manual method).
Aaaand yeah! That's basically it! I did leave out the centrifuges (2 for blood and 1 for urine) but those aren't that exciting lol. Thanks for coming with meeeee :)
8 notes
·
View notes
Link
The BD BACTEC™ Automated Blood Culture System utilizes fluorescent technology in detecting the growth of or- ganisms in the blood culture bottles. When microorganisms are present in the cultured vials, they metabolize nutrients in the culture medium, releasing carbon dioxide into the medi- um.
0 notes
Text
Sepsis Diagnostics Market Growth Drivers & Opportunities | MarketsandMarkets
The demand for sepsis diagnostic products is expected to grow mainly due to factors such as the increasing public-private funding for sepsis diagnostic research activities, the growing burden of infectious diseases, the rising number of sepsis incidences, and growing government initiatives for creating sepsis awareness.
Sepsis is a very difficult condition to diagnose and the risk of mortality increases by 7.6% with a delay of even 1 minute in antibiotic administration in septic shock patients with hypertension. Thus, increasing the need for the rapid diagnosis of sepsis to reduce the delay of antibiotic therapy among patients with sepsis. Many sepsis diagnostic manufacturers are expanding their product offering in point of care technology rapidly detecting the sepsis, reducing the overall turnaround time of diagnosis.
The BACTEC Plus, BacT/Alert, and BACTEC FX blood culturing instruments manufactured by BD Company (US) is an automated microbial detection system offering a rapid diagnosis of sepsis in the turnaround time of three hours.
According to the new market research report "Sepsis Diagnostics Market by Technology (Microbiology, PCR, Sequencing, Biomarkers), Product (Reagents, Assay, Instruments, Software), Test Type (Lab, POC), Pathogen (Bacterial, Viral, Fungal), End User (Hospital, Pathology Lab) - Global Forecast to 2026", published by MarketsandMarkets™, the global market is expected to reach USD 771 million by 2026 from USD 503 million in 2021, at a CAGR of 8.9% from 2021 to 2026.
Browse in-depth TOC on "Sepsis Diagnostics Market" 201 – Tables 47 – Figures 236 – Pages
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=92673155
The demand for sepsis diagnostic products is expected to grow mainly due to factors such as the rising prevalence of sepsis across the globe. Factors such as the increasing geriatric population, a growing number of surgical procedures, a high incidence of hospital-acquired infections, and the commercialization and availability of a wide variety of approved sepsis diagnostic devices are expected to drive market growth.
The COVID-19 pandemic had a positive effect on the sales of diagnostics products for sepsis. The global spread of covid-19 and the emerging cases of sepsis among covid-19 patients are likely to increase the demand for rapid diagnosis, accelerating the utilization of instruments, reagents, assay kits for detection of sepsis. Lockdowns resulting from the pandemic caused people to delay undergoing health checkups, affecting the number of tests performed and reagent sales.
The shortage of skilled professionals is a major concern worldwide; only 50% of patients with severe sepsis transported by the EMS system have a paramedic. The lack of trained paramedics affects each phase of patient caremdash;sepsis awareness, screening patients for the presence of sepsis, and making an appropriate.
The current COVID-19 pandemic has highlighted the risk faced by older adults, who are more susceptible to complications, including acute respiratory distress syndrome, usually due to pneumonia, which increases the risk of developing sepsis. Thus, increasing the need for early diagnosis of sepsis among patients with covid-19 infections.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=92673155
Based on technology, the biomarkers segment is expected to register the highest growth rate during the forecast period
Based on technology, the global sepsis diagnostics market is segmented into microbiology, molecular diagnostics, immunoassay, flow cytometry, microfluidics, and biomarkers. The biomarkers segment is expected to grow at the highest CAGR during the forecast period. The growth of this segment can be attributed to various advantages offered by this technique in the diagnosis of sepsis and the growing need for early disease diagnosis.
North America accounted for the largest share of the sepsis diagnostics market
Geographically, the sepsis diagnostic market is segmented into North America, Europe, Asia Pacific, and the Rest of the World. North America is estimated to account for the largest share of the market. This can be attributed to the growing prevalence of sepsis, increasing incidence of hospital-acquired infections, fast adoption of high-end sepsis diagnostics devices, and rising government support for sepsis-related research.
0 notes
Text
Impact of COVID-19 on Automated Cell Cultures in Healthcare Industry

COVID-19 Impact on Automated Cell Cultures in Healthcare Industry
The economy and businesses across the world have been influenced greatly because of the COVID-19 pandemic. The COVID-19 has spread globally in unprecedented ways due to its high infectious and contagious nature and lack of vaccine. The World Health Organization (WHO) declared COVID-19 as a pandemic due to its increased spread across the globe. According to the situation report of 7th June 2021 by WHO, 174 million corona cases had been reported globally and 3.7 million patients died due to the coronavirus. On a slightly positive note, a total of 157 million people have recovered, and 1.9 million vaccine doses have been administered.
Cell culture refers to removing cells from an animal or plant and their subsequent growth in a favorable artificial environment. Automated cell culture systems are instruments that mechanically carry out the steps involved in growing and maintaining a cell culture useful in any lab that works with cell biology, cell signaling, protein expression, or drug discovery. An automated cell culture system helps to grow cell cultures while saving labor time and reducing errors. Increasing demand for cell culture technology in vaccine production and wide acceptance of cell culture techniques in various applications accelerate the market growth. However, the high cost of automated cell culture systems and lack of skilled and certified professionals are expected to obstruct the market growth.
COVID-19 pandemic created problems for many drug, clinical, medical equipment, and device manufacturing companies, including market players that provided automated cell culture products & services. Though, different kind of policies has been adopted by different companies across the globe for carrying forward the manufacturing processes.
PRICE IMPACT
COVID-19 claimed a considerable number of lives worldwide, which is a concern in countries with high patient co-payments and an appreciable number of families going into poverty when members become ill. Consequently, there is a need to review prices and availability of pharmaceutical products during the COVID-19 pandemic to provide future direction.
The pandemic has impacted on utilization and prices of pertinent medicines and products but moderated by increased scrutiny. Key stakeholder groups can play a role in enhancing evidence-based approaches and reducing inappropriate purchasing in the future.
For instance;
• The Price of BACTEC FX-40 Automated Blood Culture System manufactured by BD is USD 10,608.08
• The price of Gibco HEPES, a reagent used in cell culture manufactured by Thermo Fisher Scientific, is USD 30.90
Product cost is the major setback for the market as it is expected to decrease the demand due to high costs. As the instruments and consumables related products such as cell counters, reagents, and buffers have high prices, the cost of automated cell cultures can impact the market.
IMPACT ON DEMAND
Today, most biotechnology products are primarily dependent on the mass culturing of cell lines. Cell cultures have found applications in diverse areas and serve as a model system for numerous research efforts. An increase in funding from the government for cell-based research is significantly triggering the growth of the market. In addition, cell culture techniques are widely used as an alternative to current egg-based strategies for developing cell-based vaccines. Thus, cell culture technology has been widely used in developing U.S. -licensed vaccines such as vaccines against rubella, smallpox, chickenpox, hepatitis, rotavirus, and polio.
For instance,
• Over the last month and a half, the Centre for Cellular and Molecular Biology has established stable cultures of COVID-19 causing coronavirus. The lab's ability to culture the virus enables CCMB to develop vaccines and test potential drugs to fight COVID-19.
As observed, because of the current COVID-19 situation, the demand for automated cell culture services gradually increased due to the wide acceptance of cell culture techniques in various applications and growth in the biotechnology sector. This shows that various initiatives taken by organizations and market players are helping to tackle this COVID-19 situation as products are made available, and the situation is likely to get better in the future.
IMPACT ON SUPPLY CHAIN
Lockdown policies in different countries have resulted in the closing of product providers, decreasing walk-in customers to prevent the spread of the disease. This led to a significant delay in the supply of the product. Also, during the lockdown period, the market for online delivery of products has seen a rise, which is normal considering the situation. The companies dealing in the automated cell culture worldwide have taken strategic steps to supply products to people across the world properly.
The Coronavirus Disease 2019 (COVID-19) pandemic triggered unprecedented increased demand for some clinical devices, as well as significant disruptions to global medical and clinical device manufacturing and supply chain operations. The FDA is monitoring the medical and clinical product supply chain and working closely with manufacturers and other stakeholders to evaluate the risk of disruptions and prevent or reduce their impact on patients, health care providers, and the public's health at large.
For instance,
· On March 27th 2020, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) was signed into law. The CARES Act added Section 506J to the Federal Food, Drug, and Cosmetic Act (FD&C Act). It provided the FDA—for the first time—with new authority intended to help prevent or mitigate negative public health impacts of medical device supply chain disruptions “during, or in advance of, a public health emergency declared by the Secretary under section 319 of the Public Health Service (PHS) Act.”
The FDA issued an immediately in effect guidance, Notifying CDRH of a Permanent Discontinuance or Interruption in Manufacturing of a Device Under Section 506J of the FD&C Act During the COVID-19 Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff. This guidance is intended to assist manufacturers in providing the FDA timely, informative notifications about changes in the production of certain medical devices that will help the Agency prevent or mitigate shortages of such devices during the COVID-19 public health emergency.
· In April 2020, according to Indian Drug Manufacturers Association (IDMA), Vice-Chairman, T Sathish, the pharma sector in the country has been hit by a shortage of workforce, packaging materials, and transport the COVID-19 lockdown. This has led to a shortage of product packaging materials and has made matters worse for the pharmaceutical companies. He urged the government to permit the supporting industry to function to ensure that the supply chain was not disrupted.
This shows that even if the COVID-19 situation disrupts the supply chain of products, the initiatives taken by the government and different companies create hope for the proper supply and use of products available.
STRATEGIC DECISIONS FOR MANUFACTURERS
COVID-19 might have hampered the supply and use of products, but it also allows companies to improve their business by different means. As a pharmaceutical company always does, it tends to find newer and better ways to treat a disease; the same is true with automated cell culture services and products. Always bringing out better solutions in the market will increase their business efficiency.
For instance,
· In 2020, in the U.S., the companies with direct exposure to COVID-19 outbreak were taking several actions, including transporting available inventory to areas away from quarantine zones and near ports where it can be accessed for shipping, buying ahead to procure inventory and raw material that are in short supply in impacted areas, activating pre-approved raw material substitutions in places where the primary supplier is impacted. However, a secondary supplier is not updating customers about delays and adjusting customer allocations to optimize profits on near-term revenue or to meet contractual terms and shaping demand by offering a discount on the available inventory in cases where supply may be short for late winter-early spring fulfillment optimizing near-term revenue.
This signifies that despite this COVID-19 situation, the companies make different strategic decisions that will make their business grow at least back to pre-pandemic levels.
CONCLUSION
Pandemic has taken a toll on every aspect of life, including the global economy. With the significant downfalls in many sectors, a collaborative effort of government, industry players, and consumers can win the fight against COVID-19. The first wave had already inflicted severe blows to the population and economy. The currently experiencing the second wave and expected the third wave is likely to be more disastrous to the masses and healthcare markets.
As this pandemic situation has resulted in many restrictions in different places around the world, the market players dealing in automated cell culture services and products were still able to manage their stock. Different companies were finding out their way to deal with this pandemic situation. The government and companies around the globe are working together and have issued advice for those who are undergoing treatment during these unprecedented times during COVID-19 lockdown around the world.
The supply chain was destroyed, but several steps were taken by the government and companies, which will help them get their needed output for the products. By increasing the material price, the companies can maintain their overall revenue.
Thus, different companies dealing in automated cell culture services and products are finding out their ways to deal with this pandemic situation.
#Automated Cell Cultures Market by Type#Automated Cell Cultures Market Forecast#Automated Cell Cultures Market Future Innovation
0 notes
Photo





Many people think that textbooks can teach a student everything they need to know, through thoughts and words. However, in my opinion the best way to learn is not just from merely reading books, nor surfing at the computer, not even from the lectures, but from hands on experiences. They would say that nothing beats experience, as the perfect learning tool. They say that words and thoughts are often forgotten a few months or weeks after learning. But a person never forgets a real life experience. And that makes internship a great example wherein it puts you in that environment already and gives all sorts of experience that we can use to our advantage.
Partaking in the internship program and having been assigned in one of the prestigious hospitals in our country was very fulfilling and got me motivated to do my best. The experience of working as an intern at San Lazaro Hospital is very overwhelming and I’m hoping that these experiences would be enough to mold me as I advanced into my career. This internship program at least gives me a taste of the real world and a glimpse of what lies ahead after I graduate.
I was assigned at Microbiology Section, my very first post. I admit that I wasn’t prepared since Micro was my weakness of all the major subjects I’ve taken up. But as time goes by, I’ve learned to appreciate Micro. In my 20 day duty in Micro Laboratory, it gives me the chance to reflect upon what I have learned from school and have applied it in the laboratory in a theoretical manner. And now I was able to physically see and experience the concepts that I have learned from just merely reading books and lectures like seeing real different bacteria cultures growing from culture media, reading and interpreting gram stain results and identifying live organisms. Moreover, I was also able to gain some very important insights from working with senior interns, co-interns and the MT staffs in reality. I’m looking forward that I would be able to utilize this experience as a guide to correct my errors and discovering more skills that might be of use in my future career.
Another thing that the internship can give you is an idea of how is the job of the MT performed on a daily basis; this idea also includes the practical training and the special handling of the medical equipments, tests, and machines used in the laboratory. As an intern, we were also taught how to operate machines in the laboratory. In Micro lab these are some of the equipment and tests I’ve encountered; BD BacTec Machine, Vitek, Gaspak, Gram stain etc. The BD BacTec Machine is an automated Blood culture system that utilizes fluorescent technology in detecting the growth of organisms in the blood culture bottles. The Vitek 2 is an automated microbiology system utilizing growth-based technology. The systems accommodate colorimetric reagent cards that are incubated and interpreted automatically. The Gaspak is a method used in the production of an anaerobic environment for the growth of anaerobic microorganism. Lastly, the most common test request we handled is the gram stain. This test differentiates the bacteria into Gram Positive and Gram Negative Bacteria, which helps in the classification and differentiations of microorganisms.
Being in an internship allows me to gain training in my field of choice and gaining experience in my field will give me a lot of different strengths as a potential professional. In this regard, I had the opportunity to identify my skills, especially in phlebotomy. In San Lazaro Hospital, our phlebotomy skills are certainly being honed since we were assigned at warding from time to time. We were already accustomed at extracting various patients at any circumstances and situations. And I would say that it was the particular skill that I have greatest improvement so far. Some skills that I have acquired during my duty were; I was able to process specimens properly in accordance to the standard laboratory procedure, ability to accomplish routine laboratory examination of patient’s specimens with high accuracy and I can also thrive under pressure and willing to work for long hours and I even timed-out late because there’s still so many tasks to finished. These skills had made me flexible in doing such tasks in the workplace. For me, the particular skill that needs further improvement is my computer skills, especially in the printing of results.
Being happy with where you work and whom you are working with is probably one of the most important considerations in the workplace. After all, who wants to go to a job that they hate? I surely don't, and I am sure that the saying, "working in a job that you love does not feel like work" is actually true because you met new people and welcoming them into your life is very rewarding and you’ll felt the sense of belonging. But honestly in my first day of duty I had this feeling of edginess and nervousness as I was anticipating my first day of work with a group of people I have never met, at a place I was unfamiliar with. But as I further got to know all the people around me known as my co-interns and the staffs I seemed to slowly descend from my state of anxiousness. I learned through experience how to interact and communicate with patients. But at first I was also hesitant to interact with patients most especially during warding at PAV 10 with TB patients. I was just observing from time to time how my other co interns interact with some patients and eventually I’ve also learned how to deal with these various patients.
0 notes
Text
The Global Sepsis Diagnostics Market is Projected To Reach $771 Million By 2026
[236 Pages Report] The global Sepsis Diagnostics Market size is expected to reach USD 771 million by 2026 from USD 503 million in 2021, at a CAGR of 8.9%. The demand for sepsis diagnostic products is expected to grow mainly due to factors such as the increasing public-private funding for sepsis diagnostic research activities, the growing burden of infectious diseases, the rising number of sepsis incidences, and growing government initiatives for creating sepsis awareness.
The global spread of covid-19 and the emerging cases of sepsis among covid-19 patients are likely to increase the demand for rapid diagnosis, accelerating the utilization of instruments, reagents, & assay kits for detection of sepsis. Lockdowns resulting from the pandemic caused people to delay undergoing health checkups, affecting the number of tests performed and reagent sales.
The current COVID-19 pandemic has highlighted the risk faced by older adults, who are more susceptible to complications, including acute respiratory distress syndrome, usually due to pneumonia, which increases the risk of developing sepsis. Thus, increasing the need for early diagnosis of sepsis among patients with covid-19 infections.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=92673155
Sepsis is a very difficult condition to diagnose and the risk of mortality increases by 7.6% with a delay of even 1 minute in antibiotic administration in septic shock patients with hypertension.
Thus, increasing the need for the rapid diagnosis of sepsis to reduce the delay of antibiotic therapy among patients with sepsis. Many sepsis diagnostic manufacturers are expanding their product offering in point of care technology rapidly detecting the sepsis, reducing the overall turnaround time of diagnosis.
The BACTEC Plus, BacT/Alert, and BACTEC FX blood culturing instruments manufactured by BD Company (US) is an automated microbial detection system offering a rapid diagnosis of sepsis in the turnaround time of three hours.
1 note
·
View note
Text
Updated Market Research Analysis For #Sepsis #Diagnostics
The demand for sepsis diagnostic products is expected to grow mainly due to factors such as the increasing public-private funding for sepsis diagnostic research activities, the growing burden of infectious diseases, the rising number of sepsis incidences, and growing government initiatives for creating sepsis awareness.
According to a new market research report, "Sepsis Diagnostics Market by Technology (Microbiology, PCR, Sequencing, Biomarkers), Product (Reagents, Assay, Instruments, Software), Test Type (Lab, POC), Pathogen (Bacterial, Viral, Fungal), End User (Hospital, Pathology Lab) - Global Forecast to 2026", size is expected to reach USD 771 million by 2026 from USD 503 million in 2021, at a CAGR of 8.9% from 2021 to 2026.
Moreover, these factors have prompted market players to improve and strengthen their current manufacturing and distribution capabilities especially in emerging markets, which are expected to witness the highest growth.
The COVID-19 pandemic had a moderately positive effect on the sales of diagnostics products for sepsis. The global spread of covid-19 and the emerging cases of sepsis among covid-19 patients are likely to increase the demand for rapid diagnosis, accelerating the utilization of instruments, reagents, & assay kits for detection of sepsis.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=92673155
Sepsis is a very difficult condition to diagnose and the risk of mortality increases by 7.6% with a delay of even 1 minute in antibiotic administration in septic shock patients with hypertension.
Many sepsis diagnostic manufacturers are expanding their product offering in point of care technology rapidly detecting the sepsis, reducing the overall turnaround time of diagnosis. . The BACTEC Plus, BacT/Alert, and BACTEC FX blood culturing instruments manufactured by BD Company (US) is an automated microbial detection system offering a rapid diagnosis of sepsis in the turnaround time of three hours.
Sepsis is a life-threatening condition that needs to be diagnosed and treated at the earliest with the help of skilled healthcare professionals. The shortage of skilled professionals is a major concern worldwide; only 50% of patients with severe sepsis transported by the EMS system have a paramedic. The lack of trained paramedics affects each phase of patient care—sepsis awareness, screening patients for the presence of sepsis, and making an appropriate
The sepsis diagnostics market, by technology, the market is segmented into blood culture, immunoassays, molecular diagnostics, flow cytometry, microfluidics, and biomarkers.
Read Full Press Release @ https://www.prnewswire.com/news-releases/sepsis-diagnostics-market-worth-771-million-by-2026--exclusive-report-by-marketsandmarkets-301475547.html
0 notes