#B.1.1.7
Explore tagged Tumblr posts
Text
Um estudo feito com mais de 20 mil profissionais da saúde do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), que foram vacinados contra a covid-19 com a vacina CoronaVac, mostrou a eficiência do imunizante em comparação ao restante da população que não tomou a vacina. A CoronaVac é fabricada pelo Instituto Butantan e pela farmacêutica chinesa Sinovac e vem sendo aplicada no país por meio do Programa Nacional de Imunizações (PNI). Segundo o estudo, após os profissionais da saúde terem tomado as duas doses do imunizante, a taxa de eficiência da CoronaVac encontrada foi de 50,7% duas semanas após tomar a segunda dose, e de 73,8% a partir de cinco semanas da aplicação da segunda dose. A pesquisa levou em conta os casos sintomáticos de funcionários e comparou esses resultados com o que é observado no restante da população da cidade de São Paulo. Hoje (7), mais cedo, um outro estudo, feito com profissionais da área da saúde de Manaus, já havia demonstrado que a CoronaVac é também eficiente com relação à variante P.1, que surgiu na capital amazonense. Segundo os pesquisadores, o número de casos de covid-19 registrado entre os profissionais da saúde do Hospital das Clínicas que tomaram a vacina não acompanhou o crescimento exponencial dos casos que estão ocorrendo entre o restante da população. No ano passado, antes da vacina estar disponível, as ocorrências de covid-19 entre os profissionais da área de saúde do Hospital das Clínicas cresciam no mesmo nível do restante da população. Com o início da vacinação entre os profissionais da saúde, essa tendência mudou. Na terceira semana do mês de janeiro deste ano, quanto teve início a vacinação para os profissionais da saúde do estado de São Paulo, a capital paulista havia registrado 16,2 mil novos casos de covid, enquanto que, no Hospital das Clínicas, maior complexo hospitalar da América Latina, ocorreram 51 casos. Já na última semana do mês de março, foram registrados 23,9 mil novos casos entre a população paulistana e 46 no Hospital das Clínicas. Representação do número de casos semanais de COVID-19 na cidade de São Paulo (SP) e entre profissionais de saúde do HCFMUSP. As setas marcam a administração das 2 doses de CoronaVac no hospital. - Divulgação/HCFMUSP Se essa mesma tendência de crescimento observada na população paulistana se repetisse no Hospital das Clínicas, os pesquisadores calculam que haveria 175 casos entre os profissionais de saúde do complexo em março. No entanto, o que foi observado é que, com a vacinação, esse número de infecções pelo novo coronavírus foi 73,8% menor entre os imunizados. Os profissionais da saúde desse complexo hospitalar, que reúne oito institutos, foram vacinados entre os dias 18 e 21 de janeiro, com a primeira dose, e entre 14 e 16 de fevereiro, com a segunda dose. “Nesse estudo, falamos em efetividade da vacina porque é uma aplicação na vida real, diferente do que é realizado nos ensaios clínicos, que avaliam a eficácia em condições específicas e consideradas ideais. Esse estudo com os funcionários do HC, que vacinou um número grande de pessoas, é fundamental porque corrobora os resultados obtidos nos estudos clínicos do Butantan”, disse Anna Sara Levin, chefe da Divisão de Moléstias Infecciosas e Parasitárias do HCFMUSP. O estudo feito entre os profissionais do hospital também avaliou a ocorrência de variantes do coronavírus. Dentre 142 amostras analisadas aleatoriamente, 67 foram identificadas como variantes, das quais 57 do Amazonas (P1), 5 do Reino Unido (B.1.1.7) e outras 5 que não puderam ser identificadas pelos métodos utilizados no estudo.
0 notes
Text
Deciphering the Complexities of COVID-19 Variants
Introduction:
The global COVID-19 pandemic has posed an unprecedented challenge to humanity. As the virus continues its relentless spread, it constantly evolves through mutations, giving rise to an array of variants. In this in-depth journey, we will embark on a thorough exploration of the intricate realm of COVID-19 variants to equip you with the indispensable knowledge you need.
1. Unraveling the Intricate World of COVID-19 Variants:
COVID-19 variants are akin to unique adaptations of the SARS-CoV-2 virus, each sculpted by genetic mutations. These genetic transformations can lead to substantial changes in the virus's characteristics, influencing its transmissibility, disease severity, and resistance to immunity. Think of these variants as distinct "iterations" of the same virus, each bearing its genetic signature.
2. An In-Depth Exploration of Prominent COVID-19 Variants:
Alpha (B.1.1.7): First detected in the United Kingdom, the Alpha variant gained worldwide attention due to its heightened transmissibility. However, it didn't necessarily translate into more severe illness or increased fatality rates.
Beta (B.1.351): Originating in South Africa, the Beta variant raised concerns about its potential resistance to immunity, including vaccine-induced immunity. Researchers maintained a vigilant watch over its behavior.
Delta (B.1.617.2): The Delta variant, initially identified in India, has played a pivotal role in the pandemic. Its extraordinary transmissibility led to surges in cases worldwide, resulting in increased hospitalizations and posing challenges to containment efforts.
Omicron (B.1.1.529): Omicron made global headlines due to its numerous mutations in the spike protein, the primary target of most COVID-19 vaccines. Scientists are actively researching its transmissibility, severity, and vaccine efficacy, given its potential risk.
3. Understanding the Genesis of Variants:
Why Do They Emerge? Variants are an inherent facet of a virus's life cycle. As the virus replicates and spreads, genetic changes occur. While many of these changes are random, some provide advantages to the virus. For instance, mutations that enhance transmissibility help the virus spread more efficiently from person to person, ultimately increasing its prevalence.
4. Assessing the Impact of Variants on Vaccines:
A major concern regarding COVID-19 variants revolves around their impact on vaccine effectiveness. Vaccine manufacturers and researchers vigilantly monitor these variants. While some variants may marginally reduce vaccine effectiveness, it is crucial to understand that vaccines continue to offer robust protection against severe illness, hospitalization, and death. Even when a variant affects vaccine efficacy, vaccines remain potent in mitigating the virus's impact.
In response to the emergence of variants, booster shots have been recommended to enhance immunity, especially against newer and more challenging variants like Delta and Omicron. These booster doses bolster the body's immune response, providing additional layers of protection.
5. The Pivotal Role of Public Health Measures:
Irrespective of the variants that emerge, public health measures remain crucial for controlling the spread of COVID-19. These measures encompass:
Mask-Wearing: Consistently don masks in crowded or indoor settings, especially in regions with high transmission rates.
Social Distancing: Maintain physical distance from others, particularly during close social interactions.
Hand Hygiene: Practice regular handwashing with soap and water or use hand sanitizers.
Vaccination: If eligible, get vaccinated and adhere to guidance on booster shots when provided.
Testing and Isolation: Undergo testing if you display symptoms or have been exposed to a COVID-19-positive individual. Prompt isolation upon receiving a positive result is essential to curb further transmission.
These measures not only safeguard individual health but also act as barriers against the emergence of new variants.
Summary:
COVID-19 variants are an intrinsic part of the virus's evolution. Scientists diligently explore their characteristics and potential impact on public health. In this ever-evolving landscape, vaccination and adherence to public health measures remain our most unwavering allies in the battle against the pandemic. Staying informed and heeding guidelines from reputable health authorities, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), are essential actions to safeguard ourselves and our communities.
0 notes
Text
Unmasking the Complexity of COVID-19 Variants
Introduction:
The global COVID-19 pandemic has posed an unprecedented challenge to humanity. As the virus continues its relentless spread, it constantly evolves through mutations, giving rise to an array of variants. In this in-depth journey, we will embark on a thorough exploration of the intricate realm of COVID-19 variants to equip you with the indispensable knowledge you need.
1. Unraveling the Intricate World of COVID-19 Variants:

COVID-19 variants are akin to unique adaptations of the SARS-CoV-2 virus, each sculpted by genetic mutations. These genetic transformations can lead to substantial changes in the virus's characteristics, influencing its transmissibility, disease severity, and resistance to immunity. Think of these variants as distinct "iterations" of the same virus, each bearing its genetic signature.
2. An In-Depth Exploration of Prominent COVID-19 Variants:
Alpha (B.1.1.7): First detected in the United Kingdom, the Alpha variant gained worldwide attention due to its heightened transmissibility. However, it didn't necessarily translate into more severe illness or increased fatality rates.
Beta (B.1.351): Originating in South Africa, the Beta variant raised concerns about its potential resistance to immunity, including vaccine-induced immunity. Researchers maintained a vigilant watch over its behavior.
Delta (B.1.617.2): The Delta variant, initially identified in India, has played a pivotal role in the pandemic. Its extraordinary transmissibility led to surges in cases worldwide, resulting in increased hospitalizations and posing challenges to containment efforts.
Omicron (B.1.1.529): Omicron made global headlines due to its numerous mutations in the spike protein, the primary target of most COVID-19 vaccines. Scientists are actively researching its transmissibility, severity, and vaccine efficacy, given its potential risk.
3. Understanding the Genesis of Variants:
Why Do They Emerge? Variants are an inherent facet of a virus's life cycle. As the virus replicates and spreads, genetic changes occur. While many of these changes are random, some provide advantages to the virus. For instance, mutations that enhance transmissibility help the virus spread more efficiently from person to person, ultimately increasing its prevalence.
4. Assessing the Impact of Variants on Vaccines:

A major concern regarding COVID-19 variants revolves around their impact on vaccine effectiveness. Vaccine manufacturers and researchers vigilantly monitor these variants. While some variants may marginally reduce vaccine effectiveness, it is crucial to understand that vaccines continue to offer robust protection against severe illness, hospitalization, and death. Even when a variant affects vaccine efficacy, vaccines remain potent in mitigating the virus's impact.
In response to the emergence of variants, booster shots have been recommended to enhance immunity, especially against newer and more challenging variants like Delta and Omicron. These booster doses bolster the body's immune response, providing additional layers of protection.
5. The Pivotal Role of Public Health Measures:
Irrespective of the variants that emerge, public health measures remain crucial for controlling the spread of COVID-19. These measures encompass:
Mask-Wearing: Consistently don masks in crowded or indoor settings, especially in regions with high transmission rates.
Social Distancing: Maintain physical distance from others, particularly during close social interactions.
Hand Hygiene: Practice regular handwashing with soap and water or use hand sanitizers.
Vaccination: If eligible, get vaccinated and adhere to guidance on booster shots when provided.
Testing and Isolation: Undergo testing if you display symptoms or have been exposed to a COVID-19-positive individual. Prompt isolation upon receiving a positive result is essential to curb further transmission.
These measures not only safeguard individual health but also act as barriers against the emergence of new variants.
Summary:
COVID-19 variants are an intrinsic part of the virus's evolution. Scientists diligently explore their characteristics and potential impact on public health. In this ever-evolving landscape, vaccination and adherence to public health measures remain our most unwavering allies in the battle against the pandemic. Staying informed and heeding guidelines from reputable health authorities, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), are essential actions to safeguard ourselves and our communities.
0 notes
Text
Demystifying the Complexities of COVID-19 Mutations: A Comprehensive Exploration
Introduction:
The global COVID-19 pandemic has posed an unprecedented challenge to humanity. As the virus continues its relentless spread, it constantly evolves through mutations, giving rise to an array of variants. In this in-depth journey, we will embark on a thorough exploration of the intricate realm of COVID-19 variants to equip you with the indispensable knowledge you need.
1. Unraveling the Intricate World of COVID-19 Variants:
COVID-19 variants are akin to unique adaptations of the SARS-CoV-2 virus, each sculpted by genetic mutations. These genetic transformations can lead to substantial changes in the virus's characteristics, influencing its transmissibility, disease severity, and resistance to immunity. Think of these variants as distinct "iterations" of the same virus, each bearing its genetic signature.
2. An In-Depth Exploration of Prominent COVID-19 Variants:
Alpha (B.1.1.7): First detected in the United Kingdom, the Alpha variant gained worldwide attention due to its heightened transmissibility. However, it didn't necessarily translate into more severe illness or increased fatality rates.
Beta (B.1.351): Originating in South Africa, the Beta variant raised concerns about its potential resistance to immunity, including vaccine-induced immunity. Researchers maintained a vigilant watch over its behavior.
Delta (B.1.617.2): The Delta variant, initially identified in India, has played a pivotal role in the pandemic. Its extraordinary transmissibility led to surges in cases worldwide, resulting in increased hospitalizations and posing challenges to containment efforts.
Omicron (B.1.1.529): Omicron made global headlines due to its numerous mutations in the spike protein, the primary target of most COVID-19 vaccines. Scientists are actively researching its transmissibility, severity, and vaccine efficacy, given its potential risk.
3. Understanding the Genesis of Variants:
Why Do They Emerge? Variants are an inherent facet of a virus's life cycle. As the virus replicates and spreads, genetic changes occur. While many of these changes are random, some provide advantages to the virus. For instance, mutations that enhance transmissibility help the virus spread more efficiently from person to person, ultimately increasing its prevalence.
4. Assessing the Impact of Variants on Vaccines:

A major concern regarding COVID-19 variants revolves around their impact on vaccine effectiveness. Vaccine manufacturers and researchers vigilantly monitor these variants. While some variants may marginally reduce vaccine effectiveness, it is crucial to understand that vaccines continue to offer robust protection against severe illness, hospitalization, and death. Even when a variant affects vaccine efficacy, vaccines remain potent in mitigating the virus's impact.
In response to the emergence of variants, booster shots have been recommended to enhance immunity, especially against newer and more challenging variants like Delta and Omicron. These booster doses bolster the body's immune response, providing additional layers of protection.
5. The Pivotal Role of Public Health Measures:
Irrespective of the variants that emerge, public health measures remain crucial for controlling the spread of COVID-19. These measures encompass:
Mask-Wearing: Consistently don masks in crowded or indoor settings, especially in regions with high transmission rates.
Social Distancing: Maintain physical distance from others, particularly during close social interactions.
Hand Hygiene: Practice regular handwashing with soap and water or use hand sanitizers.
Vaccination: If eligible, get vaccinated and adhere to guidance on booster shots when provided.
Testing and Isolation: Undergo testing if you display symptoms or have been exposed to a COVID-19-positive individual. Prompt isolation upon receiving a positive result is essential to curb further transmission.
These measures not only safeguard individual health but also act as barriers against the emergence of new variants.
Summary:
COVID-19 variants are an intrinsic part of the virus's evolution. Scientists diligently explore their characteristics and potential impact on public health. In this ever-evolving landscape, vaccination and adherence to public health measures remain our most unwavering allies in the battle against the pandemic. Staying informed and heeding guidelines from reputable health authorities, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), are essential actions to safeguard ourselves and our communities.
0 notes
Text
📆 Apr 2021 📰 How Novel Coronavirus Variants Could Complicate Our COVID-19 Vaccination Drive ✍️ Dr Vipin Vashishtha
Some of my peers working in the bigger COVID-19 hospitals in metropolitan cities have also observed that those who received vaccines and tested positive for COVID-19 within two weeks are both outnumbering those who haven’t been vaccinated and are running a more severe course of the disease. India’s health ministry hasn’t shared any such data thus far, however.
Could these apprehensions be true? Let’s find out.
Speculation that vaccines could paradoxically increase the risk of infections possibly originated following the 2009 influenza A (H1N1pdm09) pandemic, when 4 Canadian studies suggested that getting the seasonal influenza vaccine increased the risk of laboratory-confirmed infections. This led to 5 additional studies, each of which substantiated these initial findings.
One proposed mechanism for this phenomenon is called original antigenic sin. It was first used to describe how one’s first exposure to the influenza virus shapes the outcome of subsequent exposures to antigenically related strains. (The antigen is the part of the object that provokes the immune response – like the novel coronavirus’s spike protein.) This specific immune phenomenon explains the failure of the immune system to generate an immune response against related antigens.
When an individual is infected by an evolved strain with a new dominant antigen, slightly different from the original strain against which the person has been vaccinated, the immune system produces antibodies against the original strain. This happens through high-affinity memory B-cells that inhibit activation of naïve B-cells, resulting in a weak immune response against the newer strain. So the risk of infection paradoxically increased in vaccinated individuals compared to unvaccinated individuals.

This phenomenon could be at work when different variants of the SARS-CoV-2 virus are in play. As you may know, most existing COVID-19 vaccines are based on the wild strain that was circulating around the world last year. However, towards the end of 2020, many variants with mutations in the spike protein have emerged. These new variants have replaced their predecessors in most countries. In India as well, one or two key variants (B.1.1.7 and B.1.617) are feared to be circulating around the country.
So a vaccine may mount a strong immune response against the original epitopes contained in the ‘original’ strain due to preformed B-cells. (“The antibody recognises a particular part of the virus, called the epitope, and forms a bond with it using a part called the paratope” – source.) These preformed committed B-cells outnumber naïve B cells and prevent them from mounting a strong response that is specific to the epitopes contained in the new mutant.
As a result, the immune system becomes unable to mount a faster, stronger secondary response. The implication is that when the epitope varies slightly, then the immune system relies on the memories of the previous infection (with the wild variant) rather than mount another primary or secondary response to the new epitopes present in the new variant (B.1.617 or B.1.1.7).
As a result, the immunological response may be inadequate against the new variant – because the immune system depends on a memory instead of launching into a fresh response.
0 notes
Text
既感染ハムスターにおけるSARS-CoV-2オミクロン変異体への再感染の影響
SARS-CoV-2の変異は多様であるため、以前の変異型に感染した個体が再感染する可能性があり、このリスクはB.1.1.529オミクロン変異型の出現によってさらに高まっている。
本研究では、ハムスターを用いたin vivo感染モデルを用いて、SARS-CoV-2に過去に感染した個体がオミクロン変異体に再感染する可能性を評価し、またそのような感染に関連する病態を検討した。
最初に、シリアンハムスターに系統A、B.1.1.7、B.1.351、B.1.617.2、またはオミクロンの亜種であるBA.1株を接種し、5週間後にBA.1株に再感染させた。その後、BA.1株に過去に感染した人を対象に、オミクロンの亜変異体(BA.1およびBA.2)に再感染させた場合の影響を調べた。ウイルスの感染と複製は上気道と下気道の両方で抑制されたが、再感染後、ほとんどのハムスターの気道でウイルス関連RNAが検出された。ウイルスの複製は上気道よりも下気道でより強く抑制された。変種BA.1に一次感染したハムスターの上気道では一貫したアミノ酸置換が観察されたが、同じ変種に再感染したハムスターでは多様な変異が出現した。���理組織学的には、いずれの再感染群においても急性肺炎や疾患の増強は認められず、さらに再感染動物の気道における炎症性サイトカインやケモカインの発現は軽度上昇するにとどまった。これらの知見は、SARS-CoV-2の新しい変異体による再感染のリスクを理解する上で重要である。
0 notes
Text
The Untold Story of SARS-CoV-2 Spike Variants Revealed: How Their Receptor Binding Domain Impacts Virus Interactions with Human Cells
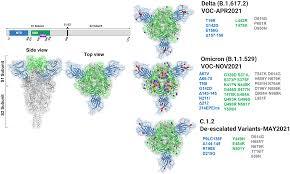
The Untold Story of SARS-CoV-2 Spike Variants Revealed: How Their Receptor Binding Domain Impacts Virus Interactions with Human Cells The Untold Story of SARS-CoV-2 Spike Variants Revealed: How Their Receptor Binding Domain Impacts Virus Interactions with Human Cells The spike protein of SARS-CoV-2 is a crucial determinant of infection and a key target for vaccines and therapeutics. Recent studies have identified several spike variants with mutations in the receptor binding domain (RBD) that are associated with changes in virus infectivity and immune evasion. This article aims to provide an overview of the untold story of SARS-CoV-2 spike variants and how their RBD impacts virus interactions with human cells. What is the RBD of SARS-CoV-2 Spike Protein? The spike protein of SARS-CoV-2 is a trimeric glycoprotein that mediates virus entry into host cells by binding to the ACE2 receptor on the cell surface. The RBD of the spike protein is a small domain that interacts specifically with the ACE2 receptor and is critical for virus attachment and entry. The RBD consists of a core and a receptor-binding motif (RBM), which is a flexible loop that undergoes conformational changes during ACE2 binding. What are the Spike Variants and their RBD Mutations? Several spike variants of SARS-CoV-2 have been identified, including the UK variant B.1.1.7, the South Africa variant B.1.351, the Brazil variant P.1, and the California variant B.1.427/B.1.429. These variants have mutations in the RBD that affect virus infectivity and immune evasion. For example, the UK variant has a mutation N501Y, which is thought to increase virus transmissibility by enhancing ACE2 binding. How Do Spike Variants Affect Virus Infectivity and Immune Evasion? Spike variants with RBD mutations can affect virus infectivity and immune evasion in several ways. First, mutations can increase or decrease ACE2 binding affinity, which affects virus entry into host cells. Second, mutations can alter the conformation of the RBD and affect antibody recognition and neutralization. Third, mutations can lead to the accumulation of multiple mutations in the RBD that enhance immune evasion and reduce vaccine efficacy. These effects have important implications for the development of vaccines and therapeutics against SARS-CoV-2. How Do Spike Variants Impact Vaccine Efficacy? Spike variants with RBD mutations can impact vaccine efficacy by reducing the neutralizing activity of antibodies generated by vaccination. For example, the South Africa variant B.1.351 has multiple mutations in the RBD that reduce the neutralizing activity of antibodies by up to 10-fold. This has prompted the development of new vaccine strategies that target multiple regions of the spike protein, rather than just the RBD. What Are the Implications of Spike Variants for Public Health? Spike variants of SARS-CoV-2 with RBD mutations have important implications for public health. These variants can increase virus transmissibility, reduce vaccine efficacy, and affect the severity of disease. To address these challenges, it is essential to continue monitoring SARS-CoV-2 variants and develop new vaccine and therapeutic strategies that are effective against multiple strains of the virus. How Does Research on Spike Variants Advance Our Understanding of SARS-CoV-2? Research on spike variants of SARS-CoV-2 advances our understanding of the virus by identifying key determinants of virus infectivity and immune evasion. This knowledge can inform the development of vaccines and therapeutics that are effective against multiple strains of the virus. Additionally, research on spike variants can help us understand the evolutionary dynamics of SARS-CoV-2 and the emergence of new variants in the future. What Are the Future Directions for Research on SARS-CoV-2 Spike Variants? Future research on SARS-CoV-2 spike variants should focus on several key areas. First, it is important to continue monitoring the emergence and spread of new variants and their impact on virus transmissibility, vaccine efficacy, and disease severity. Second, it is necessary to develop new vaccine and therapeutic strategies that are effective against multiple strains of the virus. Third, it is essential to understand the mechanisms of immune evasion and develop new strategies for overcoming this challenge. Conclusion In summary, the untold story of SARS-CoV-2 spike variants has revealed the importance of the RBD in virus infectivity and immune evasion. Spike variants with RBD mutations can impact virus transmissibility, reduce vaccine efficacy, and affect disease severity. To address these challenges, it is essential to continue monitoring variants and develop new vaccine and therapeutic strategies that are effective against multiple strains of the virus. FAQs: 1. How do RBD mutations affect SARS-CoV-2 infection? 2. What is the impact of spike variants on vaccine efficacy? 3. How does antibody recognition and neutralization play a role in RBD mutations? 4. Can vaccines be effective against multiple strains of SARS-CoV-2? 5. What are the future directions for research on SARS-CoV-2 spike variants? #TECH Read the full article
0 notes
Text
Global loss of cellular m6A #RNA methylation following infection with different SARS-CoV-2 variants [RESEARCH]
Insights into host–virus interactions during SARS-CoV-2 infection are needed to understand COVID-19 pathogenesis and may help to guide the design of novel antiviral therapeutics. N6-Methyladenosine modification (m6A), one of the most abundant cellular #RNA modifications, regulates key processes in #RNA metabolism during stress response. Gene expression profiles observed postinfection with different SARS-CoV-2 variants show changes in the expression of genes related to #RNA catabolism, including m6A readers and erasers. We found that infection with SARS-CoV-2 variants causes a loss of m6A in cellular #RNAs, whereas m6A is detected abundantly in viral #RNA. METTL3, the m6A methyltransferase, shows an unusual cytoplasmic localization postinfection. The B.1.351 variant has a less-pronounced effect on METTL3 localization and loss of m6A than did the B.1 and B.1.1.7 variants. We also observed a loss of m6A upon SARS-CoV-2 infection in air/liquid interface cultures of human airway epithelia, confirming that m6A loss is characteristic of SARS-CoV-2-infected cells. Further, transcripts with m6A modification are preferentially down-regulated postinfection. Inhibition of the export protein XPO1 results in the restoration of METTL3 localization, recovery of m6A on cellular #RNA, and increased #mRNA expression. Stress granule formation, which is compromised by SARS-CoV-2 infection, is restored by XPO1 inhibition and accompanied by a reduced viral infection in vitro. Together, our study elucidates how SARS-CoV-2 inhibits the stress response and perturbs cellular gene expression in an m6A-dependent manner. http://genome.cshlp.org/cgi/content/short/33/3/299?rss=1&utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
A sudden alteration that turned the world upside down
by Eeya Baguio

It started of an unknown virus that exploded like a bomb out of nowhere. No expectancy, no signals, nothing at all. Affecting people one by one, all across the world. The COVID-19 virus.
As the days gone by, turned into weeks, months, and unexpectedly, years. It has evolved into a more alarming one. Thousands of deaths being reported, lockdowns every now and then, necessities being sold out to every stores, it was a sudden alteration that turned the world upside down. Thus, given its evolution, different variants were created, in which some are very much alarming.
Alpha variant (B.1.1.7): first identified in the UK in late 2020, it spreads more easily than earlier variants and may be associated with a higher risk of hospitalization and death.
Beta variant (B.1.351): first identified in South Africa in late 2020, it may be associated with reduced effectiveness of some vaccines.
Gamma variant (P.1): first identified in Brazil in late 2020, it is similar to the Beta variant and may also be associated with reduced vaccine effectiveness.
Delta variant (B.1.617.2): first identified in India in late 2020, it is more transmissible than earlier variants and has been associated with increased hospitalizations and deaths.
Omicron variant (B.1.1.529): first identified in South Africa in late 2021, it has a large number of mutations and is still being studied, but early evidence suggests that it may be more transmissible than other variants.
It's worth noting that viruses constantly mutate, and new variants of COVID-19 are likely to emerge in the future.
References:
https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html
https://www.nih.gov/news-events/nih-research-matters/variant-covid-19-spreading-faster-india
https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-mutations-strains-and-variants
0 notes
Text
A Covid UK Variant is a Disease Caused by a Genetic Variation of the COVID Gene
A covid uk variant is a disease caused by a genetic variation of the COVID gene. This version of COVID is found in people in the United Kingdom. It causes symptoms that include a severe cough, fever, and difficulty breathing.
BA.2 sub-lineage of Omicron
The BA.2 sub-lineage of Omicron is gaining steam across Europe. It has been designated as a variant of concern by the WHO technical advisory group, which assessed data on reinfection and diagnostics. This is a relatively new variant and it is difficult to predict its future lineage.
The UK Health Security Agency (UKHSA) said it was investigating the possibility that the BA.2 variant could be more transmissible than the original Omicron strain. There is no evidence of a difference in the effectiveness of vaccination against the BA.2 variant, but the UKHSA is conducting further detailed studies.
Dr Tom Peacock, of the UKHSA, has warned against complacency. He says there is a "clear pattern of growth" in multiple countries that suggests the BA.2 strain is more transmissible than the original Omicron.
Dr Peacock said that although the cases are small, they provide an opportunity for scientists to study the properties of the variant. "The nature of viruses is to evolve, so we will have to see if any changes in the variant's growth rate will affect the level of transmission and the potential for more severe disease," he said.
Several mAbs have shown some effectiveness against the BA.2 variant, but Sotrovimab is less effective. Although the number of new variants is increasing, the number of test-positive cases is low. Therefore, the CDC continues to monitor the emergence of new lineages.
Transmissibility of B.1.1.7
A new study has explored the transmission of the novel alpha variant of the coronavirus (B.1.1.7) in England. The study was conducted over a one-year period that coincided with the emergence of the variant.
The variant is more transmissible than the previous variants of B.1.1.7, and has driven a significant change in the trajectory of the virus. It has increased overall reproduction numbers by 1.35 times. This increase is comparable to estimates from previous studies.
Researchers at Helix, a provider of COVID-19 testing, collaborated with Scripps Research. They analyzed PCR tests on swabs of patient specimens. These tests can measure viral load, which is the number of virus particles in the patient's throat. Despite the higher R number, there was no statistically significant difference between the prevalence of the B.1.1.7 variant and the other variants.
Dr. Britta Jewell, who leads the New and Emerging Respiratory Virus Threats Advisory Group for the British Health Authority, said that the variant has increased transmission, but did not affect the disease itself. She noted that this is an area of unpredictability.
However, the study did identify several lines of evidence that indicate that the B.1.1.7 variant is more transmissible than other strains. For example, the E484K mutation, which is found in beta and gamma variants, helps the virus evade antibodies.
Similarly, the variant is more infectious than the non-volatile lineages of B.1.1.7. Moreover, the risk of death from the variant is 1.6 times that of the previous covid variants.
Symptoms of COVID uk variant
COVID-19 symptoms are usually similar to other illnesses, but may vary depending on your immune status and vaccination status. This is why it's important to keep an eye out for symptom changes. You should also wear a mask if you're in a crowded area. When you're feeling better, you can resume your normal activities.
COVID-19 is not new to the UK, but the number of confirmed cases has grown. The latest cases were identified in England, with a total of 10 cases confirmed. The UK has a particularly high number of cases amongst older adults, making them at risk for a severe illness if they aren't up to date with their vaccinations.
There are multiple variants of COVID-19 and the newer ones are thought to be milder. In addition, the virus is constantly mutating. Some of the newest variants are thought to be the same as the previous versions, although they've been given different names.
Although the Omicron COVID-19 variant isn't known to cause breakthrough infections, it is associated with more symptoms than the Delta variant. These include a hoarse voice and a change in sense of taste. Symptoms typically occur 2 to 14 days after you first encounter the virus. However, hospitalisations can take weeks to arrive, a time during which you might not know that you have been exposed to the virus.
A PCR test is the only way to accurately measure the Omicron COVID-19 variant's effects. However, as many people in the UK are not eligible for free NHS testing, you may need to pay for it.
Intensity of ICU admissions with BA.2
A recent study has found that the intensity of ICU admissions with BA.2 covid variant has been declining over time. This could be due to an increased vaccine coverage against Omicron. But scientists warn against complacency.
There are dozens of different gene changes that the BA.2 virus has compared to the original Omicron strain. The virus is more capable of causing cells to stick together, making it easier for it to spread through the body. In addition, it has an ability to copy itself in cells more rapidly. These abilities could explain why it is less likely to be detected on PCR tests.
However, there are still questions about whether the variants are contributing to an increase in severe illness. Scientists at the US Centers for Disease Control and Prevention estimate that about 4% of Covid-19-infected people have been infected with the BA.2. While the number of cases of this variant is relatively small, they are growing fast.
As a result, the US Centers for Disease Control and Prevention is monitoring the outbreak closely. ECDC is encouraging countries to remain vigilant for signs of the emergence of the variants.
UK Health Security Agency (UKHSA) has published a technical briefing on the emergence of the variant. Data for Scotland was not included in the briefing. Currently, the UKHSA has confirmed 1,072 genomically confirmed cases of BA.2. It is estimated that a small proportion of the population, mainly elderly, remain at risk of developing a severe illness.
S-gene drop-out detected on six specimens in five patients
S-gene drop-out is a common variant finding in patients with a new coronavirus. This is an indicator of the presence of Omicron. However, the presence of Omicron does not necessarily mean severe illness. In fact, the proportion of patients with this variant is relatively low. Nevertheless, it is important to recognize that there is an increasing number of people testing positive for this variant.
UKHSA is examining all available biological evidence, including genome sequencing, to determine the properties of the new variant. As a result, the designation of the variant will change. These changes will be reflected in future technical briefings.
The original Delta variant remains dominant in the UK. It is the variant responsible for over 99% of COVID-19 cases. However, there is a growing number of cases with BA.4 and BA.5 variants, which are immune escapes. They are increasing in number internationally.
A new variant has been identified in the United States. The variant has a higher reproduction rate than the preexisting variants. Its mutations in the spike protein gene can affect its behavior when exposed to vaccines and transmissibility.
The CDC continues to investigate new lineages of the virus. It has also designated a number of other variants. Meanwhile, health officials in the UK continue to monitor the status of the existing variants.
The rapid identification of the influenza virus variant is testament to the increased capacity for genomic sequencing developed during the pandemic. However, it is important to note that it takes several days for the sequencing process to complete.
Intensity of ICU admissions with SARS-CoV-2 VOC 202012/01
A new study provides some insight into the relative severity of the two SARS-CoV-2 variants omicron and delta. It also indicates that the incidence of these two variants is declining. The results have implications for healthcare capacity planning.
The study was conducted using a high-quality database called QResearch. It is a national database on the UK health care system based on primary care records from 1350 practices in England.
The purpose of the study was to compare the prevalence and clinical features of the two variants. This was done by comparing data on 609,352 cases of the two variants. In particular, the study examined whether the omicron or the delta variant had a larger impact on the proportion of cases that were hospitalized after a SARS-CoV-2 infection.
The relative risk (RR) of hospitalization was evaluated in different age groups. For those aged under 80, the RR was 0.37 (95% confidence interval: 0.30 to 0.46) and the age group from ages 80 to 99 had a RR of 0.64. However, it is important to note that the relative risk of hospitalization is not the only indicator of the quality of medical care.
Compared to the other omicron-related data, the Alpha variant of SARS-CoV-2 was not significantly associated with increased mortality or ICU admissions. That is not to say that the Alpha variant isn't a threat. Some studies have suggested that the Alpha variant has a higher mortality rate than the wild-type variant.
0 notes
Text
O 13º Boletim Epidemiológico Covid-19, divulgado hoje (2) pela prefeitura do Rio de Janeiro, mostra que desde o início da pandemia, no ano passado, o município registra 227,79 mil casos da doença, com 20,68 mil óbitos. Em 2021, foram 35,32 mil casos confirmados, dos quais 7,34 mil casos graves e 2,77 óbitos. A taxa de letalidade atinge 7,8%, inferior à de 2020, de 9,3%. A taxa de incidência atinge 530,3 por 100 mil habitantes e a taxa de mortalidade é de 41,6 por 100 mil habitantes. Com base em dados do Ministério da Saúde, o boletim mostra que a média móvel de casos segue em tendência de alta, o mesmo acontecendo com a média móvel dos óbitos. A boa notícia é que a curva dos atendimentos de casos de síndrome gripal e síndrome respiratória aguda grave já começa a apresentar queda na média móvel dos últimos sete dias. De acordo com o boletim, nesta semana foram identificados nove casos de novas variantes do novo coronavírus no Rio, seis deles em moradores do município. No total, já são 192 casos na cidade, sendo 151 de moradores, dos quais 143 são da variante brasileira (P.1) e oito da britânica (B.1.1.7). Dos moradores infectados pelas novas cepas, 16 morreram, três permanecem internados e 132 foram considerados curados. A rede municipal de saúde tem 717 leitos ocupados. A capacidade, entretanto, é um pouco maior, atingindo 824 leitos. As ações de fiscalização realizadas até as 13h de ontem (1º), integrando equipes da Secretaria Municipal de Ordem Pública (Seop), com equipes do Instituto de Vigilância Sanitária (Ivisa-Rio), Guarda Municipal e Defesa Civil, totalizaram 886 inspeções, 591 infrações sanitárias, 63 bairros e 183 interdições. Vacinação Esta semana, foram abertos mais cinco postos de vacinação na cidade: no Jockey Club Brasileiro (Gávea), no Hotel Fairmont (Copacabana), no Museu da Justiça (centro), na Cidade das Artes (Barra da Tijuca) e no Museu do Amanhã (centro), visando a dar mais opções para a população. No próximo dia 6, será aberto um novo local, na quadra do bloco Cacique de Ramos, em Olaria, às 8h. Já foram vacinadas 804,69 mil pessoas no Rio de Janeiro com a primeira dose da vacina contra covid-19, o que equivale a 11,9% da população. O percentual de idosos a partir de 60 anos já vacinados com a primeira dose alcança 53,7%. Com a segunda dose, foram vacinadas 227,46 mil, o que totaliza 1,032 milhão. Amanhã (3), os postos de vacinação atenderão pessoas com 67 anos ou mais nos seguintes horários: clínicas da familia e centros municipais de Saúde, das 8h às 17h; Museu da República (Catete) e Paróquia Nossa Senhora do Rosário (Leme), das 8h às 15h; quartéis do Corpo de Bombeiros de Humaitá, Copacabana e Barra da Tijuca (Busca e Salvamento, das 8h ao meio-dia; drive-thru na Cidade Universitária da Universidade Federal do Rio de Janeiro -UFRJ (Ilha do Fundão), Parque Madureira, Parque Olímpico (Barra) e Sambódromo (Santo Cristo), das 8h às 15h; drive-thru no Engenhão (Engenho de Dentro), das 8h às 14h. Sputnik Durante a apresentação do boletim epidemiológico, o prefeito do Rio, Eduardo Paes, disse que assinou ontem (1°) à noite memorando de entendimento com o consórcio de governadores do Nordeste para aquisição de 8 milhões de doses para a cidade do Rio de Janeiro da vacina russa Sputnik V. O imunizante está em análise na Agência Nacional de Vigilância Sanitária (Anvisa). “Ontem, estive em contato com representantes do Consórcio do Nordeste para a compra de 8 milhões de doses da Sputnik para a cidade do Rio. Esse é mais um esforço para que possamos acelerar o processo de vacinação na cidade. Queremos que o Rio seja marcado como exemplo de recuperação dessa doença”, disse Paes. O prefeito não quis entrar em detalhes em relação à vacina russa, alegando questão de confidencialidade. O processo de negociação está em curso e até que o contrato seja assinado, ele não pode fornecer mais dados.
0 notes
Text
SARS-CoV-2 vaccine-breakthrough infections (VBIs) by Omicron (B.1.1.529) variant and consequences in structural and functional impact
Preliminary report; We examined the effects of mutations on domains (NID, RBM, and SD2) found at the interfaces of spike domains Omicron B.1.1529, Delta/B.1.1529, Alpha/B.1.1.7, VUM B.1.526, B.1.575.2, and B.1.1214 (formerly VOI Iota). We tested the affinity of Omicron for hACE2 and found that the wild and mutant spike proteins were using atomistic molecular dynamics simulations. According to binding free energies calculated during mutagenesis, hACE2 bound Omicron spike more strongly than SARS-CoV-2 wild strain. T95I, D614G, and E484K are three substitutions that significantly contribute to the RBD, corresponding to hACE2 binding energies and a doubling of Omicron spike proteins' electrostatic potential. Omicron appears to bind hACE2 with greater affinity, increasing its infectivity and transmissibility. The spike virus was designed to strengthen antibody immune evasion through binding while boosting receptor binding by enhancing IgG and IgM antibodies that stimulate human {beta}-cell, as opposed to the wild strain, which has more vital stimulation of both antibodies. https://www.biorxiv.org/content/10.1101/2022.12.12.520021v1?rss=1%22&utm_source=dlvr.it&utm_medium=tumblr Read more ↓
0 notes
Text
Inside the B.1.1.7 Coronavirus Variant
At the heart of each coronavirus is its genome, a twisted strand of nearly 30,000 “letters” of RNA. These genetic instructions force infected human cells to assemble up to 29 kinds of proteins that help the coronavirus multiply and spread.

As viruses replicate, small copying errors known as mutations naturally arise in their genomes. A lineage of coronaviruses will typically accumulate one or two random mutations each month.
Some mutations have no effect on the coronavirus proteins made by the infected cell. Other mutations might alter a protein’s shape by changing or deleting one of its amino acids, the building blocks that link together to form the protein.
Through the process of natural selection, neutral or slightly beneficial mutations may be passed down from generation to generation, while harmful mutations are more likely to die out.
Mutations In the B.1.1.7 Lineage
A coronavirus variant first reported in Britain has 17 recent mutations that change or delete amino acids in viral proteins.
The variant was named Variant of Concern 202012/01 by Public Health England, and is part of the B.1.1.7 lineage of coronaviruses.

Notable mutations in the B.1.1.7 lineage are listed below. Six other mutations, not shown in the diagram above, do not change an amino acid.
Eight Spike Mutations
Researchers are most concerned about the eight B.1.1.7 mutations that change the shape of the coronavirus spike, which the virus uses to attach to cells and slip inside.
Each spike is a group of three intertwined proteins:

Building one of these spike proteins typically takes 1,273 amino acids, which can be written as letters:

Spike proteins in the B.1.1.7 lineage have two deletions and six substitutions in this sequence of amino acids.

Written as letters, a B.1.1.7 spike protein looks like this:

These mutations alter the shape of the spike protein by changing how the amino acids fold together into a complex shape.
The Spike N501Y Mutation
Scientists suspect that one mutation, called N501Y, is very important in making B.1.1.7 coronaviruses more contagious. The mutation’s name refers to the nature of its change: the 501st amino acid in the spike protein switched from N (asparagine) to Y (tyrosine).

The N501Y mutation changes an amino acid near the top of each spike protein, where it makes contact with a special receptor on human cells.

Because spike proteins form sets of three, the mutation appears in three places on the spike tip:

In a typical coronavirus, the tip of the spike protein is like an ill-fitting puzzle piece. It can latch onto human cells, but the fit is so loose that the virus often falls away and fails to infect the cell.
The N501Y mutation seems to refine the shape of the puzzle piece, allowing a tighter fit and increasing the chance of a successful infection.

Researchers think the N501Y mutation has evolved independently in many different coronaviruses lineages. In addition to the B.1.1.7 lineage, it has been identified in variants from Australia, Brazil, Denmark, Japan, the Netherlands, South Africa, Wales, Illinois, Louisiana, Ohio and Texas.
In addition to N501Y, the B.1.1.7 has 16 other mutations that might benefit the virus in other ways. It’s also possible that they might be neutral mutations, which have no effect one way or the other. They may simply be passed down from generation to generation like old baggage. Scientists are running experiments to find out which is the case for each mutation.

One mysterious mutation in the B.1.1.7 lineage deletes the 69th and 70th amino acids in the spike protein. Experiments have shown that this deletion enables the coronavirus to infect cells more successfully. It’s possible that it changes the shape of the spike protein in a way that makes it harder for antibodies to attach.

Researchers call this a recurrent deletion region because the same part of the genome has been repeatedly deleted in different lineages of coronaviruses. The H69–V70 deletion also occurred in a variant that infected millions of mink in Denmark and other countries. Scientists are beginning to identify a number of these regions, which may play an important role in the virus’s future evolution.

In another recurrent deletion region, a number of coronavirus lineages are missing either the 144th or 145th amino acid in the spike protein. The name of the mutation comes from the two tyrosines (Y) that are normally in those positions in the protein.
Like the H69–V70 deletion, Y144/145 occurs on the edge of the spike tip. It may also make it harder for antibodies to stick to the coronavirus.


This mutation changes an amino acid from P to H on the stem of the coronavirus spike:

When spike proteins are assembled on the surface of a coronavirus, they’re not yet ready to attach to a cell. A human enzyme must first cut apart a section of the spike stem. The P681H mutation may make it easier for the enzyme to reach the site where it needs to make its cut.
Like N501Y, the P681H mutation has arisen in other coronavirus lineages besides B.1.1.7. But it’s rare for one lineage to carry both mutations.

ORF8 is a small protein whose function remains mysterious. In one experiment, scientists deleted the protein and found that the coronavirus could still spread. That suggests that ORF8 is not essential to replication, but it might still give some competitive edge over mutants that have lost the protein.
ORF8 is typically only 121 amino acids long:

But a B.1.1.7 mutation changes the 27th amino acid from Q to a genetic Stop sign:

Researchers assume that this ORF8 stump cannot function. But if losing the protein leaves B.1.1.7 at a disadvantage, it’s possible that the advantages of another mutation like N501Y might make up for the loss.

Detection and Spread
B.1.1.7 first came to light in the United Kingdom in late November. Researchers looked back at earlier samples and found that the first evidence dates back to Sept. 20, in a sample taken from a patient near London.
The B.1.1.7 lineage has now been detected in over 50 countries, including the United States. Britain has responded to the surge of B.1.1.7 with stringent lockdowns, and other countries have tried to prevent its spread with travel restrictions.

B.1.1.7 is estimated to be roughly 50 percent more transmissible than other variants. Federal health officials warn that it may become the dominant variant in the United States by March. It is no more deadly than other forms of the coronavirus. But because it can cause so many more infections, it may lead to many more deaths.

B.1.1.7 has been detected in at least 14 states, but the United States has no national surveillance program for determining the full extent of its spread.
How Did the Variant Evolve?
A number of researchers suspect that B.1.1.7 gained many of its mutations within a single person. People with weakened immune systems can remain infected with replicating coronaviruses for several months, allowing the virus to accumulate many extra mutations.
When these patients are treated with convalescent plasma, which contains coronavirus antibodies, natural selection may favor viruses with mutations that let them escape the attack. Once the B.1.1.7 lineage evolved its battery of mutations, it may have been able to spread faster from person to person.
Other Mutations in Circulation
One of the first mutations that raised concerns among scientists is known as D614G. It emerged in China early in the pandemic and may have helped the virus spread more easily. In many countries, the D614G lineage came to dominate the population of coronaviruses. B.1.1.7 descends from the D614G lineage.

A more recent variant detected in South Africa quickly spread to several other countries. It is known as 501Y.V2 and is part of the B.1.351 lineage. This variant has eight mutations that change amino acids in the spike protein. Among these mutations is N501Y, which helps the spike latch on more tightly to human cells.

None of these variants are expected to help the coronavirus evade the many coronavirus vaccines in clinical trials around the world. Antibodies generated by the Pfizer-BioNTech vaccine were able to lock on to coronavirus spikes that have the N501Y spike mutation, preventing the virus from infecting cells in the lab.
Experts stress that it would likely take many years, and many more mutations, for the virus to evolve enough to avoid current vaccines.
Sources: Andrew Rambaut et al., Virological; Andrew Ward, Scripps Research; Trevor Bedford, nextstrain.org; Paul Duprex, University of Pittsburgh School of Medicine; Houriiyah Tegally et al., medRxiv; Nature; Centers for Disease Control and Prevention; Global Report Investigating Novel Coronavirus Haplotypes. Spike models from Ward Lab, Scripps Research. Spike-receptor model by Cong Lab, Chinese Academy of Sciences. ORF8 model by the Yang Zhang Research Group, University of Michigan. Cahill-Keyes map projection by Gene Keyes. By Jonathan Corum and Carl Zimmer (The New York Times).
#science#medicine#virology#molecular biology#B.1.1.7#public health#COVID-19#academia#coronavirus mutations#infectious diseases#medblr
260 notes
·
View notes
Text
Unraveling the Intricacies of COVID-19 Variants
Introduction:
The COVID-19 pandemic has presented us with a global challenge of unparalleled proportions. As the virus continues its relentless spread, it undergoes a subtle transformation, giving rise to various mutations and thereby creating different versions of itself, known as variants. In this in-depth exploration, we will dive deep into the complex world of COVID-19 variants to unravel what you need to know about them.
The Essence of COVID-19 Variants

COVID-19 variants are akin to distinct versions of the SARS-CoV-2 virus, each having undergone genetic mutations. These genetic alterations can result in noteworthy changes in the virus’s characteristics, including how easily it spreads, the severity of the disease it causes, and its resistance to immunity. Think of these variants as unique “strains” of the same virus, each possessing its own genetic signature.
2. A Glimpse at Common COVID-19 Variants
Alpha (B.1.1.7): First spotted in the United Kingdom, the Alpha variant quickly seized global attention due to its heightened transmissibility. However, it was not necessarily more severe in terms of causing severe illness or fatalities.
Beta (B.1.351): Originating in South Africa, the Beta variant raised concerns regarding its potential resistance to immunity, including immunity induced by vaccines. Researchers kept a vigilant eye on its behavior.
Delta (B.1.617.2): The Delta variant, initially identified in India, has been a game-changer in the pandemic. With its exceptional transmissibility, it has caused surges in cases worldwide, leading to increased hospitalizations and posing challenges to containment efforts.
Omicron (B.1.1.529): Omicron made headlines globally due to its myriad mutations in the spike protein, the primary target of most COVID-19 vaccines. Scientists are actively engaged in the study of its transmissibility, severity, and vaccine efficacy, as it poses a potential risk.
3. The Genesis of Variants: Why Do They Occur?
Variants emerge as a natural facet of a virus’s life cycle. As the virus replicates and spreads, genetic changes can manifest. These changes are often random, but some provide advantages to the virus. For instance, mutations that enhance transmissibility can assist the virus in spreading more efficiently from person to person, consequently increasing its prevalence.

4. The Influence of Variants on Vaccines
A major concern regarding COVID-19 variants revolves around their impact on vaccine effectiveness. Vaccine manufacturers and researchers maintain vigilant monitoring of these variants. While some variants may slightly reduce vaccine effectiveness, it is imperative to understand that vaccines continue to offer robust protection against severe illness, hospitalization, and death. It is crucial to reiterate that, even when a variant partially diminishes vaccine efficacy, vaccines retain their potency in mitigating the virus’s impact.
In response to the emergence of variants, booster shots have been recommended to enhance immunity, particularly against newer and more challenging variants such as Delta and Omicron. These booster doses fortify the body’s immune response, providing additional layers of protection.
5. The Perseverance of Public Health Measures
Irrespective of the variants that come and go, public health measures remain a linchpin in controlling the spread of COVID-19. These measures encompass:
Mask-Wearing: Continuously don masks in crowded or indoor settings, particularly in regions with elevated transmission rates. Social Distancing: Uphold physical distancing from others, especially when engaged in close social interactions. Hand Hygiene: Maintain consistent handwashing with soap and water or employ hand sanitizers. Vaccination: If eligible, seek vaccination and heed the advice on booster shots when provided. Testing and Isolation: Undergo testing when displaying symptoms or after exposure to a COVID-19-positive individual. Prompt isolation upon a positive result is essential in curbing further transmission. These measures not only safeguard individual health but also act as bulwarks against the emergence of novel variants.
Summary:
COVID-19 variants are an inherent element of the virus’s evolution. Scientists diligently probe their characteristics and potential impact on public health. In this dynamic landscape, vaccination and adherence to public health measures continue to be our foremost allies in combating the pandemic. Staying informed and adhering to guidelines issued by esteemed health authorities, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), constitute essential actions to safeguard ourselves and our communities.
The panorama of COVID-19 and its variants remains in constant flux. It is imperative to rely on trustworthy sources and remain attuned to the latest information. By remaining informed and adopting responsible actions, we collectively contribute to the containment of COVID-19 and its variants.
Remember, our collective efforts wield a profound impact and are pivotal in surmounting this global challenge. Stay safe, and remain vigilant in your commitment to adhering to public health guidelines.
0 notes
Photo
Mutation in SARS-CoV-2 Variant Does Not Affect Vaccine: Study
An engineered coronavirus with the N501Y mutation—one of many mutations present in the emerging B.1.1.7 and 501.V2 variants of the coronavirus—is neutralized by the sera of COVID-19 vaccine recipients.
Serum samples from 20 individuals who received the Pfizer-BioNTech vaccine against SARS-CoV-2 thwarted a version of the coronavirus with the so-called N501Y mutation, according to a preprint posted to bioRxiv yesterday (January 7). This mutation is one of many sequence changes present in the B.1.1.7 and 501.V2 variants of SARS-CoV-2 that were first detected in the UK and South Africa, respectively, and are now rapidly spreading around the world.
“There’s no reason to think the vaccines won’t work just as well on these strains,” Frederic Bushman of the University of Pennsylvania who tracks how the virus mutates and was not involved in the work, tells the Associated Press. But he adds that the study only examined one mutation and the B.1.1.7 and 501.V2 variants have many more mutations that were not tested.
N501Y resides within the coronavirus’s spike protein that enables entry into host cells. Scientists at the University of Texas Medical Branch at Galveston had already engineered a version of SARS-CoV-2 with the N501Y mutation to study in mice when the new variants emerged, The Washington Post reports. The researchers collaborated with scientists at Pfizer to expose serum—an antibody-containing component of blood—from vaccine recipients to the engineered virus, and found no differences in neutralization between the N501Y virus and virus with the original Y501 sequence.
According to Reuters, Pfizer had challenged its vaccine against 15 other mutations previously, finding them all to be inconsequential. “So we’ve now tested 16 different mutations, and none of them have really had any significant impact. That’s the good news,” Philip Dormitzer, Pfizer’s vice president and chief scientific officer of viral vaccines, tells Reuters. “That doesn’t mean that the 17th won’t.”
In particular, scientists have expressed concern about a mutation in 501.V2 called E484K, which is next to be tested, Dormitzer tells the AP.
Coauthor Pei-Yong Shi of UTMB tells the Post he expects to receive a viral variant next week to study in the lab. Moderna, AstraZeneca, and other vaccine makers are also in the process of challenging their vaccines with the B.1.1.7 and 501.V2 variants. Bushman tells the AP he expects similarly positive results. “A mutation will change one little place, but it’s not going to disrupt binding to all of them.”
Nevertheless, vaccine developers have not ruled out the possibility that a variant could evolve that would require reformulating vaccines. “These data don’t suggest a need for a change, but the mutations are hitting close enough to home that we need to be prepared,” Dormitzer tells STAT.
By Kerry Grens (The-Scientist). Image: A patient cell infected with SARS-CoV-2, NIAID
88 notes
·
View notes
Text

Mutations may happen randomly, but the rate at which they occur depends on the virus. The enzymes that copy DNA viruses, called DNA polymerases, can proofread and fix errors in the resulting strings of genetic letters, leaving few mutations in each generation of copies.
But RNA viruses, like SARS-CoV-2, are the evolutionary gamblers of the microscopic world. The RNA polymerase that copies the virus’s genes generally lacks proofreading skills, which makes RNA viruses prone to high mutation rates—up to a million times greater than the DNA-containing cells of their hosts.
Coronaviruses have a slightly lower mutation rate than many other RNA viruses because they can do some light genetic proofreading. “But it’s not enough that it prevents these mutations from accumulating,” says virologist Louis Mansky, the director for the Institute for Molecular Virology at the University of Minnesota.
The true mutation rate of a virus is difficult to measure though. “Most of those mutations are going to be lethal to the virus, and you’ll never see them in the actively growing, evolving virus population,” Mansky says.
Instead, genetic surveys of sick people can help determine what’s known as the fixation rate, which is a measure of how often accumulated mutations become “fixed” within a viral population. Unlike mutation rate, this is measured over a period of time. So the more a virus spreads, the more opportunities it has to replicate, the higher its fixation rate will be, and the more the virus will evolve, Duffy says.
For SARS-CoV-2, scientists estimate that one mutation becomes established in the population every 11 days or so. But this process may not always happen at a steady pace.
In December 2020, the variant B.1.1.7 caught scientists’ attention when its 23 mutations seemed to suddenly crop up as the virus rampaged through Kent, England. Some scientists speculate that a chronically ill patient provided more opportunities for replication and mutation, and the use of therapies such as convalescent plasma may have pressured the virus to evolve.
Mutations drive evolution, but they are not the only way that a virus can change over time. Some viruses, like influenza, have other ways to increase their diversity.
Influenza is made up of eight genetic segments, which can be rearranged—a process called reassortment—if multiple viruses infect a single cell to replicate at the same time. As the viral progeny are packaged into their protein capsules, the RNA segments from the parent viruses can be mixed and matched like viral Legos. This process can cause rapid shifts in the viral function. For example, reassortments of flu strains circulating in pigs, birds, and humans led to the 2009 H1N1 flu pandemic.
Unlike influenza, however, coronaviruses possess no physical segmentation to undergo reassortment. Coronaviruses can experience some shifts in function through a process known as recombination, when segments of one viral genome are spliced onto another by the enzyme making the viral copy. But researchers are still working to determine how important this process is for SARS-CoV-2’s evolution.
0 notes