#Apis and Intermediates Manufacturing Company
Explore tagged Tumblr posts
Text
As a growing organization, we collaborate and work in unison with a large number of reputed and like-minded partners and clients who can nourish our aspirations. We commit to quality and superior standards in all areas of our operations. It has wide range of products and compounds for Pharma, Agro, Fine and Specialty Chemicals, CRO and CDMO sectors.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#cro ind#cro lab#cro industry
0 notes
Text
api intermediates manufacturers
Book drug, the capital of India, produces a wide range of intermediate products exporters. We produce high quality intermediate products at affordable prices. A leading international supplier of active pharmaceutical ingredients with the industry's most extensive portfolio of specialized international manufacturing locations. Synthetic and natural are further categorized into innovative and generic. The services produced and sold there can also be considered intermediate goods if they are used as inputs in the production process of other goods. Salt is an intermediate product, and companies incorporate it into many food and non-food final products. Wheat is an intermediate product because companies process it as part of another product, usually a food or grocery product.
2 notes
·
View notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
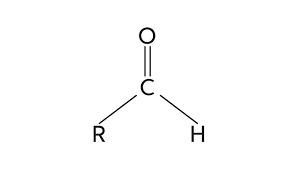
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
Cinchonine: A Powerful Alkaloid for Malaria Treatment
Understanding Cinchonine and Its Importance
Cinchonine is a natural alkaloid derived from the bark of the Cinchona tree. It has been widely studied for its anti-malarial properties and is used in pharmaceutical formulations to combat malaria. As an essential component in malaria treatment drugs, cinchonine plays a crucial role in the healthcare industry.
At Prism Industries Pvt. Ltd., we specialize in producing high-quality cinchonine for pharmaceutical applications, ensuring the best standards of purity and efficacy. Our expertise in the API pharmaceutical company sector makes us a trusted name in anti-malarial drugs manufacturing.
Cinchonine Uses: How It Helps in Medicine
The medicinal applications of cinchonine are extensive. Some of the major cinchonine uses include:
Malaria treatment: Cinchonine is a key ingredient in the formulation of best tablets for malaria, helping in effective treatment.
Anti-inflammatory properties: It is known for its ability to reduce inflammation and pain.
Fever reduction: Similar to quinine, cinchonine helps in lowering high fevers caused by infectious diseases.
Cardiac health: Research suggests it has potential benefits in cardiovascular treatments.
Pharmaceutical intermediate: It is widely used in drug synthesis in the API manufacturing industry.
Best Anti-Malaria Solutions: How Cinchonine Works
Cinchonine works by interfering with the life cycle of the malaria parasite Plasmodium, which is transmitted through mosquito bites. It prevents the parasite from multiplying in the bloodstream, reducing the symptoms and ultimately curing the infection. This mechanism makes cinchonine one of the best anti-malaria solutions available in pharmaceutical formulations.
Best Tablet for Malaria: Why Cinchonine-Based Drugs Are Effective
The market for best tablets for malaria is growing as malaria remains a major health concern worldwide. Some of the most trusted malaria treatment drugs contain cinchonine as a primary or complementary ingredient. Cinchonine-based anti-malarial drugs are preferred because they:
Have proven efficacy against malaria.
Offer a natural alternative to synthetic drugs.
Show fewer side effects compared to chemical-based formulations.
Are widely used in traditional and modern medicine.
Malaria Treatment Drugs: The Role of Cinchonine in Modern Medicine
Several malaria treatment drugs incorporate cinchonine to enhance their effectiveness. At Prism Industries Pvt. Ltd., we ensure that our API pharmaceutical company standards meet the highest global regulatory requirements for drug manufacturing. Some of the commonly used anti-malarial drugs that include cinchonine or its derivatives are:
Cinchona alkaloid-based formulations: Traditional remedies used for centuries.
Combination therapy drugs: Where cinchonine is used alongside other anti-malarial agents.
Herbal supplements: Containing natural extracts with anti-malarial properties.
API Pharmaceutical Company: Excellence in Cinchonine Production
As a leading API pharmaceutical company, Prism Industries Pvt. Ltd. is committed to delivering top-quality cinchonine for pharmaceutical applications. Our API manufacturing industry expertise ensures that we produce cinchonine in compliance with international quality and safety standards. We focus on:
Purity & Quality: Ensuring pharmaceutical-grade cinchonine.
Research & Development: Innovating to enhance drug efficacy.
Regulatory Compliance: Meeting FDA, WHO, and GMP guidelines.
Global Supply Chain: Catering to the needs of pharmaceutical companies worldwide.
Why Choose Prism Industries Pvt. Ltd. for Cinchonine?
At Prism Industries Pvt. Ltd., we take pride in being a trusted name in the API manufacturing industry. Our commitment to excellence and innovation makes us the preferred choice for pharmaceutical companies looking for high-quality cinchonine. With our advanced manufacturing processes and stringent quality control, we ensure that our products meet the highest industry standards.
Conclusion
The use of cinchonine in anti-malarial drugs has been proven effective over centuries. As a key player in the API pharmaceutical company sector, Prism Industries Pvt. Ltd. continues to provide high-purity cinchonine to pharmaceutical manufacturers globally. Whether for malaria treatment drugs or best tablets for malaria, our cinchonine ensures the best therapeutic outcomes.
For high-quality cinchonine and API pharmaceutical company solutions, trust Prism Industries Pvt. Ltd. – your reliable partner in cinchonine manufacturing.
#cinchonine#malaria_treatment_drugs#anti-malarial_drugs#active_pharmaceutical_ingredients#active_pharma_ingredients
0 notes
Text
Understanding Pharmaceutical Chemicals and Their Uses in Healthcare Industries

Pharmaceutical chemicals play a crucial role in the medical and healthcare industries. These chemicals are used in drug formulation, research, and the development of life-saving medicines. But what exactly are pharmaceutical chemicals, and how are they used?
What Are Pharmaceutical Chemicals and Their Uses?
Pharmaceutical chemicals are specialized compounds used in the production of medicines, vaccines, and other healthcare products. These chemicals include active pharmaceutical ingredients (APIs), excipients, intermediates, and raw materials essential for drug synthesis.
Common Uses of Pharmaceutical Chemicals:
Active Ingredients: Essential for the therapeutic effect of medicines.
Excipients: Used to stabilize and enhance drug formulations.
Intermediates: Chemical compounds used during drug manufacturing.
Preservatives: Extend the shelf life of pharmaceutical products.
From antibiotics and painkillers to vaccines and vitamin supplements, pharmaceutical chemicals contribute significantly to modern healthcare solutions.
Top Pharmaceutical Chemical Manufacturers
When it comes to reliable pharmaceutical chemical manufacturers, several companies lead the industry in producing high-quality raw materials for medicine production. These manufacturers focus on quality control, compliance with international standards, and innovative research.
Leading Pharmaceutical Chemical Manufacturers:
Akshat Rasayan – A trusted name in manufacturing and supplying pharmaceutical chemicals.
Sun Pharmaceutical Industries Ltd. – One of the largest pharmaceutical companies globally.
Dr. Reddy’s Laboratories – Known for high-quality APIs and generic medicines.
Aarti Industries Ltd. – Produces specialty chemicals and pharmaceutical ingredients.
Lupin Ltd. – Focuses on research-driven pharmaceutical manufacturing.
These manufacturers provide essential chemical compounds that enable pharmaceutical companies to produce effective medicines and healthcare products.
Pharmaceutical Chemical Dealers in Delhi

Delhi, being a hub for the pharmaceutical industry, has several reputed dealers offering high-quality pharmaceutical chemicals to manufacturers, research laboratories, and hospitals.
Top Pharmaceutical Chemical Dealers in Delhi:
Akshat Rasayan – A leading supplier of premium pharmaceutical chemicals.
Shree Chem Pharma – Offers a wide range of chemical raw materials.
Galaxy Chemicals – Specializes in high-purity pharmaceutical compounds.
Nexus Chemicals – Supplies APIs and intermediates to major pharmaceutical companies.
Sai Pharma Distributors – Deals in bulk and specialty pharmaceutical chemicals.
These dealers ensure a steady supply of essential chemicals for pharmaceutical production and research.
Reliable Pharmaceutical Chemical Suppliers
Pharmaceutical chemical suppliers play a vital role in the healthcare industry by ensuring the consistent availability of high-quality raw materials. A reliable supplier maintains strict quality control, complies with regulatory standards, and provides timely deliveries.
Key Considerations When Choosing a Supplier:
Quality Assurance: Ensure compliance with GMP and ISO standards.
Product Range: Availability of diverse chemical compounds.
Reputation: Check customer reviews and industry experience.
Delivery Network: Nationwide or international shipping capabilities.
By partnering with a reputable pharmaceutical chemical supplier, manufacturers can maintain consistent production quality and efficiency.
Conclusion
Pharmaceutical chemicals are essential for the production of life-saving medicines, and choosing the right manufacturers, dealers, and suppliers is crucial for quality and efficiency. Whether you are looking for pharmaceutical chemical dealers in Delhi, top pharmaceutical chemical manufacturers, or reliable pharmaceutical chemical suppliers, it is essential to select industry leaders who maintain high standards and regulatory compliance.
If you are searching for the top chemical powder wholesalers in Delhi, ensure they offer high-quality, cost-effective solutions to meet your pharmaceutical needs.
For premium pharmaceutical chemicals, explore Akshat Rasayan, a trusted name in the industry, known for its high-quality chemical products and excellent customer service.
0 notes
Text
n-Propanol Prices, News, Trend, Graph, Chart, Monitor and Forecast
n-Propanol prices are influenced by a range of factors, including raw material costs, supply chain disruptions, demand from various end-use industries, and regional economic conditions. This solvent, also known as propan-1-ol, plays a crucial role in industries such as pharmaceuticals, cosmetics, coatings, and chemical manufacturing. The pricing dynamics of n-Propanol are closely tied to the cost of propylene, its primary feedstock, which is derived from crude oil and natural gas. Any fluctuations in crude oil prices directly impact propylene costs, subsequently affecting the market value of n-Propanol. Geopolitical tensions, trade policies, and environmental regulations further add to the price volatility of this chemical compound.
Global demand for n-Propanol is another key factor shaping market prices. The pharmaceutical sector, which utilizes n-Propanol as an intermediate in drug formulations and as a solvent for active pharmaceutical ingredients (APIs), significantly influences its demand. During the COVID-19 pandemic, the demand for sanitizers and disinfectants, where n-Propanol serves as an effective ingredient, surged, leading to sharp price increases. However, as the pandemic subsided, the market stabilized, and prices began to normalize. In contrast, the coatings and paints industry continues to be a steady consumer of n-Propanol, as it is widely used as a solvent in formulations requiring fast evaporation rates and high solubility.
Get Real time Prices for n-Propanol: https://www.chemanalyst.com/Pricing-data/n-propanol-1182
The supply side of the n-Propanol market is also a determining factor in price trends. Production capacity expansions, plant shutdowns, maintenance activities, and unplanned outages at major manufacturing facilities can create supply constraints, leading to temporary price hikes. Additionally, transportation and logistics challenges, such as rising freight costs and container shortages, influence the final price of n-Propanol in different regions. Manufacturers and suppliers in North America, Europe, and Asia closely monitor these factors to ensure competitive pricing while maintaining profitability.
Regional price variations exist due to differences in production capacities, availability of raw materials, and local demand. In North America, the presence of well-established chemical manufacturing plants helps maintain a relatively stable supply of n-Propanol, but fluctuations in crude oil and propylene costs can still create price volatility. In Europe, stringent environmental regulations and energy costs add an additional layer of complexity to price movements. Meanwhile, in Asia, particularly in China and India, rapid industrialization and increasing demand from pharmaceuticals and coatings industries drive market growth. China, being a major chemical producer, plays a significant role in determining global price trends through its production output and trade policies.
Market players adopt various strategies to mitigate price fluctuations and remain competitive. Long-term supply agreements with propylene suppliers help stabilize costs, while diversification of sourcing locations reduces the risk of supply chain disruptions. Companies also invest in process optimizations and technological advancements to enhance production efficiency, thereby reducing overall manufacturing costs. Strategic inventory management allows businesses to navigate price volatility effectively and ensure a consistent supply to customers.
Economic conditions and currency fluctuations also impact n-Propanol prices in international markets. Exchange rate movements affect import and export costs, influencing regional price disparities. A stronger local currency may reduce import costs, making n-Propanol more affordable in specific regions, while currency depreciation can lead to higher prices due to increased procurement expenses. Additionally, inflation and changes in interest rates affect production costs, labor wages, and overall operating expenses, which ultimately contribute to price adjustments.
Sustainability trends and regulatory compliance are becoming increasingly important in shaping the n-Propanol market. Governments and environmental agencies worldwide are enforcing stricter regulations on chemical manufacturing, emissions control, and waste management. Compliance with these regulations often requires additional investments in cleaner production technologies, which can impact production costs and, subsequently, market prices. The push toward green chemistry and the development of bio-based alternatives are also influencing the long-term outlook of the n-Propanol market, as companies explore sustainable production methods to meet regulatory requirements and consumer preferences.
Trade policies, tariffs, and geopolitical developments further add to the complexity of n-Propanol price fluctuations. The imposition of trade restrictions, import duties, or sanctions on key exporting countries can disrupt supply chains and create regional imbalances in availability and pricing. For instance, trade tensions between major economies can lead to shifts in sourcing strategies, with companies seeking alternative suppliers to avoid additional costs. Free trade agreements, on the other hand, can facilitate smoother cross-border transactions, potentially stabilizing prices in specific regions.
The future price trends of n-Propanol will be shaped by ongoing market developments, technological advancements, and evolving consumer demands. Analysts closely monitor factors such as feedstock availability, industrial production levels, and emerging applications of n-Propanol to provide accurate market forecasts. Digitalization and data analytics are playing a growing role in price forecasting, helping businesses make informed decisions and optimize procurement strategies. Companies that adopt a proactive approach to market changes, invest in sustainable production, and maintain flexibility in supply chain management are likely to navigate price fluctuations more effectively and sustain long-term growth.
Overall, the n-Propanol market is dynamic, with multiple interconnected factors influencing price movements. From raw material costs and production capacity to demand patterns and regulatory policies, every aspect plays a role in determining the final market price. Businesses operating in this sector must stay updated on global market trends, leverage strategic partnerships, and embrace innovation to remain competitive in an evolving economic landscape. As industries continue to expand and sustainability initiatives gain momentum, the n-Propanol market is expected to witness steady growth with periodic price adjustments based on supply-demand dynamics and macroeconomic conditions.
Get Real time Prices for n-Propanol: https://www.chemanalyst.com/Pricing-data/n-propanol-1182
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#N-Propanol News#n-Propanol Price Monitor#N-Propanol Database#N-Propanol Price Chart#India#united kingdom#united states#Germany#business#research#chemicals#Technology#Market Research#Canada#Japan#China
0 notes
Text
The Global Azathioprine API Market: Trends, Challenges, and Growth Opportunities

Azathioprine, an important immunosuppressive drug, is widely used in organ transplantation, autoimmune diseases, and inflammatory conditions such as rheumatoid arthritis and Crohn’s disease. The global Azathioprine Active Pharmaceutical Ingredient (API) market has seen steady growth due to increasing demand for immunosuppressants, advancements in pharmaceutical manufacturing, and expanding healthcare infrastructure.
However, this market also faces several challenges, including regulatory hurdles, raw material shortages, and pricing pressures. At the same time, new technological advancements, sustainability initiatives, and increasing generic drug approvals present growth opportunities for API manufacturers.
This article explores the current trends, challenges, and future opportunities in the global Azathioprine API market.
Market Trends in Azathioprine API Manufacturing
1. Rising Demand for Immunosuppressants
The increasing prevalence of autoimmune diseases and organ transplant procedures has significantly boosted demand for Azathioprine-based formulations. With more cases of rheumatoid arthritis, lupus, and Crohn’s disease being diagnosed globally, pharmaceutical companies are scaling up production to meet the demand.
2. Growth of Generic Drug Manufacturing
The expiration of patents and cost-effective generic alternatives have led to higher demand for Azathioprine API. Many Indian and Chinese pharmaceutical companies have entered the market, offering high-quality APIs at competitive prices.
3. Regulatory Push for High-Quality API Production
Stringent regulations imposed by the US FDA, European Medicines Agency (EMA), and WHO-GMP have pushed API manufacturers to enhance production quality and adopt Good Manufacturing Practices (GMP). Leading players are investing in:
Advanced synthesis technologies
Automated quality control systems
Sustainable production methods
4. Increased API Outsourcing to Emerging Markets
Major pharmaceutical companies in North America and Europe are outsourcing API production to India and China due to:
Lower manufacturing costs
Availability of skilled labor
Strong production capabilities
India, in particular, is becoming a global hub for API manufacturing, with companies like Sun Pharma, Dr. Reddy’s, and Aurobindo Pharma leading the market.
Challenges Facing the Azathioprine API Market
1. Stringent Regulatory Requirements
The production of Azathioprine API must comply with Good Manufacturing Practices (GMP), US FDA approvals, and European Union standards. Any failure to meet these requirements can result in:
Product recalls
Import bans
Market reputation damage
Compliance requires continuous investment in quality control, audits, and process validation, increasing operational costs.
2. Fluctuations in Raw Material Availability
The supply chain for pharmaceutical raw materials is highly sensitive to disruptions caused by:
Geopolitical conflicts (such as trade tensions between China and the US)
COVID-19-related lockdowns and restrictions
Shortages of key starting materials (KSMs) and intermediates
Raw material price fluctuations affect profit margins for API manufacturers, making pricing unpredictable.
3. Competition from Low-Cost API Producers
While demand for Azathioprine is rising, API manufacturers face price pressures from:
Chinese manufacturers offering APIs at lower costs
Generic drug manufacturers pushing for competitive pricing
Strict price controls in regulated markets (such as India’s National Pharmaceutical Pricing Authority - NPPA)
To stay competitive, companies must optimize production processes, improve supply chain efficiency, and explore alternative raw material sources.
4. Environmental Concerns and Sustainability Regulations
The pharmaceutical industry is under increasing scrutiny for environmental impact, particularly regarding:
Chemical waste disposal
Emission of hazardous byproducts
Water and energy consumption
Governments are pushing for eco-friendly production methods, compelling API manufacturers to invest in green chemistry and sustainable waste management solutions.
Growth Opportunities in the Azathioprine API Market
1. Expansion into Emerging Markets
The Asia-Pacific and Latin American regions offer lucrative opportunities for Azathioprine API manufacturers. Factors driving growth in these regions include:
Expanding healthcare infrastructure
Rising affordability of immunosuppressant drugs
Government support for generic drug production
Local API producers in India, China, and Brazil are partnering with global pharmaceutical companies to expand their market presence.
2. Investment in Advanced Manufacturing Technologies
To enhance efficiency, consistency, and cost-effectiveness, API manufacturers are investing in:
Continuous manufacturing for faster production
Automated quality control using AI-driven analytics
High-purity synthesis methods to improve drug safety
Companies that adopt cutting-edge production techniques will have a competitive edge in regulated markets.
3. Diversification into High-Margin APIs
Many Azathioprine API manufacturers are expanding their product portfolios to include other high-demand oncology and immunosuppressive APIs. This strategy helps reduce dependency on a single product and improves profit margins.
4. Adoption of Sustainable API Manufacturing Practices
Regulatory authorities are encouraging eco-friendly pharmaceutical production. API manufacturers can benefit from:
Green chemistry innovations to reduce waste
Water and energy conservation methods
Adoption of bio-based solvents for synthesis
Implementing sustainable production practices not only ensures regulatory compliance but also enhances brand reputation among global buyers.
5. Strategic Collaborations and Mergers
Many API manufacturers are entering into:
Joint ventures with pharmaceutical companies
Long-term supply agreements with drug manufacturers
Mergers & acquisitions to expand production capacity
Such partnerships ensure a steady demand for APIs and improve global market positioning.
Conclusion
The global Azathioprine API market is experiencing steady growth, driven by increasing demand for immunosuppressants, regulatory compliance improvements, and API outsourcing trends. However, challenges such as regulatory hurdles, raw material shortages, and environmental concerns require strategic management.
Despite these obstacles, growth opportunities abound, particularly in emerging markets, advanced manufacturing technologies, and sustainability initiatives. Manufacturers that embrace innovation, optimize production costs, and expand globally will be well-positioned to lead the Azathioprine API market in the coming years.
0 notes
Text
Zinc Reagent Manufacturers in India
In the rapidly evolving field of chemical manufacturing, the demand for high-quality reagents plays a critical role in driving innovation and ensuring accuracy in research and industrial applications. Among these, zinc reagents stand out due to their diverse applications across industries such as pharmaceuticals, agriculture, electronics, and materials science. Symax Labs, a trusted name in the chemical industry, has established itself as one of the leading zinc reagent manufacturers in India, delivering products that meet stringent quality standards.
Why Zinc Reagents Are Essential
Zinc reagents are indispensable in numerous chemical reactions and industrial processes. They are widely used in organic synthesis, catalysis, and material fabrication. In the pharmaceutical industry, zinc-based compounds contribute to the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Their role in biochemical assays and diagnostic applications further highlights their versatility and importance.
Symax Labs: Commitment to Quality and Innovation
At Symax Labs, quality and innovation are at the heart of every product. The company employs advanced manufacturing techniques and rigorous quality control processes to ensure the production of high-purity zinc reagents. By adhering to international quality standards, Symax Labs guarantees that their products meet the diverse requirements of their global clientele.
High Purity and Consistency: Symax Labs ensures that every batch of zinc reagents maintains exceptional purity levels, which is crucial for reproducibility in scientific research and industrial applications.
Customized Solutions: Understanding that different industries have unique needs, Symax Labs offers customized zinc reagent formulations tailored to specific applications.
Sustainable Practices: The company is committed to eco-friendly manufacturing processes, minimizing environmental impact while maintaining product excellence.
Applications of Symax Labs’ Zinc Reagents
Symax Labs’ zinc reagents are utilized in a variety of sectors:
Pharmaceutical Industry: For the synthesis of APIs, intermediates, and in the development of new drug formulations.
Agriculture: As micronutrients in fertilizers, enhancing plant growth and crop yield.
Electronics: In the fabrication of semiconductors and other electronic components.
Materials Science: For the development of advanced materials and coatings.
Why Choose Symax Labs?
With a reputation built on reliability, innovation, and customer satisfaction, Symax Labs stands out as a preferred partner for zinc reagent requirements. The company's dedication to research and development ensures that they stay ahead of industry trends, offering cutting-edge solutions to meet the evolving demands of their customers.
Experienced Team: A team of skilled chemists and researchers dedicated to continuous improvement and product development.
Global Reach: Serving clients not just in India, but across international markets, ensuring timely delivery and support.
Customer-Centric Approach: Prioritizing customer needs with personalized service and technical assistance.
Contact Symax Labs
If you are looking for high-quality zinc reagents that deliver consistent performance across various applications, Symax Labs is your go-to manufacturer. For more information on their product range and services, visit their website or contact their customer support team.
0 notes
Text

Polymer product testing Medical device testing
Interested in this topic? Click here to try in-depth search Introduction to pharmaceutical intermediates Pharmaceutical intermediates are chemical products produced during the synthesis process of chemical APIs. They are advanced intermediates used to synthesize chemical drugs and are usually regarded as pharmaceutical raw materials. Under the regulations of the State Food and Drug Administration, they do not need to be approved for production and apply for batch numbers in accordance with pharmaceutical rules1. Pharmaceutical intermediates play a vital role in the synthesis of drugs because the synthesis of drugs has a strong dependence on intermediates3.
Smart recommendation What are the pharmaceutical journals? - Regular (Zhi-Wan-Wei) includes good journals Advertisement What are the pharmaceutical journals? 15 years of regular journal publishing experience, (10000+ regular national and provincial cn journals are optional for professional title evaluation), journals are easy to pass, intermediate and advanced articles can be published urgently, the research and doctoral team is dedicated to help, the publication is stable, and the whole process saves time and worry View details Classification and application pharma intermediates can be divided into primary pharmaceutical intermediates and advanced pharmaceutical intermediates. Advanced pharmaceutical intermediates often only need one or two steps of synthesis process to make APIs. In addition, pharmaceutical intermediates include two types of products: general and customized. According to the different stages of outsourcing services, the customized business model of intermediates can generally be divided into CRO (contract research and development outsourcing) and CMO (contract manufacturing outsourcing). With the continuous development of the pharmaceutical industry, the CDMO (contract research and development outsourcing) model has also emerged. This model requires manufacturers to participate in the customer's research and development process, provide customers with process improvements or optimizations, achieve high-quality large-scale production, and reduce production costs1.
Industry characteristics and development The pharmaceutical intermediates industry is an important link in the pharmaceutical industry chain. Since pharmaceutical intermediates are mainly used in the production of pharmaceutical raw materials, the pharmaceutical intermediates industry is directly related to the pharmaceutical industry. With the deepening of social division of labor and the advancement of production technology, the pharmaceutical industry has transferred some pharmaceutical intermediates to chemical companies for production. Pharmaceutical intermediates are fine chemical products, and the production of pharmaceutical intermediates has now become a major industry in the international chemical industry. China and India have become the world's major pharmaceutical intermediates research and development and production bases
0 notes
Text
Sigachi Industries Expands R&D Capabilities with a State-of-the-Art Center in Hyderabad
Sigachi Industries Limited, a key player in the pharmaceutical sector, has announced a significant step in its journey toward innovation and excellence. The company is establishing a state-of-the-art Research and Development (R&D) facility in Hyderabad, India. This initiative, backed by an investment of up to USD 1 million, is aimed at centralizing critical API developments and analytical efforts under one roof, reinforcing the company's commitment to technological advancement and efficiency.
With this expansion, Sigachi seeks to enhance its R&D capabilities, ensuring the seamless integration of synthesis and analytical processes. The facility will play a crucial role in optimizing API production while maintaining compliance with stringent global regulatory standards. The company is set to fuel six additional Certificate of Suitability (CEP) filings in the next six months, strengthening its pipeline and market positioning.
The new R&D center is designed to support the development of both existing and new molecules, providing a foundation for long-term growth and sustained profitability. A dedicated team of 15-20 skilled scientists will lead innovative research, focusing on regulated markets and advancing pharmaceutical solutions that meet international benchmarks. By streamlining its pharmaceutical product portfolio, Sigachi aims to prioritize high-value APIs and intermediates, allowing for greater synergies in manufacturing and regulatory filings.
Amit Raj Sinha, Managing Director and CEO of Sigachi Industries Limited, expressed enthusiasm about the expansion, emphasizing the company’s ongoing commitment to innovation and R&D investments. He highlighted that Sigachi has made substantial progress in delivering Active Pharmaceutical Ingredients (APIs) to the pharmaceutical market. The increased focus on R&D aligns with the company’s strategic vision of excellence, reinforcing its long-term growth objectives and strengthening its overall pipeline.
Sigachi Industries Limited has built a strong reputation in the pharmaceutical industry, specializing in APIs, intermediates, excipients, vitamin and mineral nutrient blends, and operations and maintenance (O&M) services. With over 35 years of experience, the company serves customers across 65+ countries, leveraging cutting-edge technology and global expertise to develop high-value pharmaceutical solutions. Operating from multiple facilities in Telangana, Gujarat, and Karnataka, and with subsidiaries in the Middle East and the U.S., Sigachi remains committed to expanding access to reliable and high-quality pharmaceutical ingredients.
This strategic investment in Hyderabad underscores Sigachi’s vision of continuous innovation, operational efficiency, and market leadership. As the company strengthens its research and development framework, it paves the way for greater advancements in the pharmaceutical sector, ensuring the delivery of superior healthcare solutions worldwide.
0 notes
Text
Contract Manufacturing : Reliable and High-Quality Production Solutions
OctaneX Labs offers contract manufacturing services to pharmaceutical companies seeking reliable and high-quality production solutions. Our state-of-the-art manufacturing facilities adhere to stringent quality control measures and regulatory guidelines, ensuring the highest standards of safety and efficacy.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services
0 notes
Text
Global Market Trends for Tofacitinib API: Demand, Supply, and Growth Prospects

The Tofacitinib Active Pharmaceutical Ingredient (API) market has seen significant growth in recent years, driven by increasing demand for immunosuppressive drugs to treat autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. As the pharmaceutical industry continues to expand, the role of Tofacitinib API manufacturers in supplying high-quality active ingredients is becoming more crucial.
This article explores the global market trends for Tofacitinib API, focusing on demand, supply chain challenges, and future growth opportunities.
1. Rising Demand for Tofacitinib API
Increased Prevalence of Autoimmune Diseases
The demand for Tofacitinib API is primarily driven by the rising incidence of autoimmune disorders worldwide. Conditions such as rheumatoid arthritis (RA), ulcerative colitis, and psoriatic arthritis have seen a steady increase, creating a higher demand for JAK inhibitors like Tofacitinib.
According to WHO, the prevalence of rheumatoid arthritis affects approximately 1% of the global population.
The global ulcerative colitis market is projected to grow significantly, further increasing demand for Tofacitinib-based medications.
As patients and healthcare providers seek effective and targeted therapies, the market for Tofacitinib API manufacturers is expected to expand accordingly.
Shift Towards Generic and Biosimilar Drugs
With Tofacitinib’s patent expiration in various countries, pharmaceutical companies are focusing on generic drug development to make this treatment more accessible. Generic versions of Tofacitinib-based medications are expected to fuel growth in emerging markets such as India, China, and Brazil, where affordability is a key concern.
2. Supply Chain and Manufacturing Challenges
Raw Material Sourcing and Price Fluctuations
The production of Tofacitinib API requires high-purity raw materials, and supply chain disruptions can impact production costs and availability. Factors such as:
Limited availability of key intermediates.
Fluctuations in raw material prices, particularly in China and India, where a large portion of API manufacturing occurs.
Stringent environmental regulations affecting production plants in China, leading to temporary closures and supply shortages.
Regulatory Compliance and Quality Standards
Tofacitinib API manufacturers must meet strict GMP (Good Manufacturing Practices) and regulatory approvals from:
US FDA (Food and Drug Administration)
EMA (European Medicines Agency)
PMDA (Japan Pharmaceuticals and Medical Devices Agency)
Failure to comply with these standards can delay production, impact global supply, and restrict market access. Manufacturers investing in high-quality production facilities and compliance mechanisms will have a competitive advantage.
3. Growth Prospects and Future Opportunities
Expansion in Emerging Markets
Emerging economies in Asia, Latin America, and the Middle East are showing strong potential for Tofacitinib-based treatments due to:
Improved healthcare infrastructure and rising healthcare expenditures.
Growing middle-class population with increased access to rheumatology and immunology treatments.
Government initiatives promoting the use of cost-effective generic medicines.
This geographical expansion is expected to drive further growth in the Tofacitinib API market over the next decade.
Advancements in API Manufacturing
Innovation in green chemistry and advanced synthesis methods is transforming API production. Sustainable and cost-efficient manufacturing processes are now a key focus for pharmaceutical companies to:
Reduce solvent waste and environmental impact.
Improve yield efficiency and reduce production costs.
Enhance API purity to meet global regulatory standards.
Companies investing in sustainable API manufacturing will gain long-term market stability and global approval.
Increased R&D for Combination Therapies
Research into combination therapies using Tofacitinib with other JAK inhibitors, biologics, or immunosuppressants is gaining attention.
Combination drugs may enhance efficacy, reduce side effects, and offer personalized treatment solutions.
Pharmaceutical companies are expanding clinical trials to explore new therapeutic areas, potentially increasing Tofacitinib API demand.
Conclusion
The global Tofacitinib API market is on a strong growth trajectory, fueled by rising demand for autoimmune disease treatments, increasing generic drug production, and expanding emerging markets. However, supply chain challenges, regulatory hurdles, and production cost fluctuations remain key challenges.
For Tofacitinib API manufacturers, investing in quality assurance, compliance, and sustainable production methods will be crucial to maintaining a competitive edge in the global pharmaceutical market. As research advances and healthcare accessibility improves, the future of Tofacitinib API manufacturing looks promising with continued expansion and innovation.
0 notes
Text
Sucroferric Oxyhydroxide Exporters
Sucroferric oxyhydroxide is an innovative phosphate binder used primarily in the management of hyperphosphatemia in patients with chronic kidney disease. As a crucial pharmaceutical product, its production and export require stringent quality standards and adherence to global regulatory norms. Among the key players in the industry, Kreative Organics stands out as a trusted exporter of sucroferric oxyhydroxide, catering to the diverse needs of pharmaceutical companies worldwide.
Why Choose Kreative Organics?
Uncompromising Quality Standards: Kreative Organics is committed to delivering high-quality sucroferric oxyhydroxide. The company’s production processes comply with stringent Good Manufacturing Practices (GMP), ensuring consistent quality in every batch. Rigorous quality control measures are in place to meet global pharmacopoeial standards.
State-of-the-Art Manufacturing Facilities: The company’s advanced manufacturing facilities are equipped with cutting-edge technology to produce sucroferric oxyhydroxide efficiently and sustainably. These facilities adhere to international regulatory requirements, ensuring compliance and product reliability.
Expertise and Experience: With years of experience in the pharmaceutical industry, Kreative Organics has developed unparalleled expertise in producing and exporting pharmaceutical intermediates and APIs like sucroferric oxyhydroxide. This experience translates into a deep understanding of global market demands and client expectations.
Global Reach: Kreative Organics serves a broad network of clients across the globe. The company’s robust export framework ensures timely delivery and seamless logistics, enabling clients to rely on them as a trusted partner.
Applications of Sucroferric Oxyhydroxide
Sucroferric oxyhydroxide plays a pivotal role in healthcare by managing phosphate levels in patients with chronic kidney disease (CKD). By binding dietary phosphate in the gastrointestinal tract, it reduces phosphate absorption, helping patients maintain healthy phosphate levels and improve their quality of life.
Commitment to Sustainability
Kreative Organics is dedicated to sustainable and eco-friendly practices in manufacturing and export operations. The company prioritizes reducing its environmental impact through efficient resource utilization and adherence to sustainable practices.
Partner with Kreative Organics
For pharmaceutical companies seeking a reliable exporter of sucroferric oxyhydroxide, Kreative Organics is the ideal choice. The company’s unwavering focus on quality, compliance, and customer satisfaction has cemented its reputation as a trusted global partner.
Contact Kreative Organics today to learn more about their sucroferric oxyhydroxide offerings and how they can meet your pharmaceutical needs.
0 notes
Text
A Leading Manufacturer of Ritonavir Intermediates & API in India
Vineet Labs is a prominent player in the pharmaceutical industry, renowned for its expertise in manufacturing high-quality Active Pharmaceutical Ingredients (APIs) and intermediates. With a strong focus on innovation and quality, Vineet Labs has established itself as a leading supplier of Ritonavir intermediates and API in India.
Ritonavir: A Critical Antiretroviral Drug
Ritonavir is a crucial antiretroviral medication used in the treatment of HIV/AIDS. It belongs to a class of drugs known as protease inhibitors, which work by blocking the enzyme protease, essential for the replication of the HIV virus.
Vineet Labs' Role in Ritonavir Production
Vineet Labs plays a vital role in the production of Ritonavir by manufacturing key intermediates required for the synthesis of the final API. These intermediates are crucial building blocks in the complex chemical synthesis process of Ritonavir.
Key Strengths of Vineet Labs:
State-of-the-art Manufacturing Facilities: Vineet Labs boasts modern and well-equipped manufacturing facilities adhering to stringent quality standards like GMP (Good Manufacturing Practices). Focus on Quality and Compliance: The company prioritizes quality and compliance in all its operations, ensuring that all products meet the highest regulatory standards. Experienced Team: Vineet Labs has a team of highly skilled and experienced professionals with expertise in pharmaceutical chemistry and manufacturing. Strong R&D Capabilities: Continuous research and development efforts enable Vineet Labs to optimize manufacturing processes, improve product quality, and develop innovative solutions. Customer Focus: The company maintains strong customer relationships, providing excellent service and support to its clients globally. Contribution to Global Healthcare
By efficiently manufacturing Ritonavir intermediates and API, Vineet Labs contributes significantly to the global fight against HIV/AIDS. Access to affordable and high-quality antiretroviral medications is crucial for effective treatment and improved patient outcomes.
Conclusion
Vineet Labs is a key player in the Indian pharmaceutical industry, making significant contributions to the production of essential medicines like Ritonavir. With its commitment to quality, innovation, and customer satisfaction, Vineet Labs continues to play a vital role in improving global healthcare access.
0 notes
Text

Top API Pharma Database Company in India
India’s pharmaceutical industry is a global leader, recognized for its cost-effective manufacturing and innovation in Active Pharmaceutical Ingredients (APIs). To navigate this dynamic landscape, companies rely on comprehensive databases that provide detailed insights into APIs, intermediates, and chemical formulations. This blog explores the top API pharma database companies in India, highlighting their roles and capabilities, while incorporating key industry-related keywords.
#Cosmetics Database#Biotech Database#Polymer Database#Veterinary Database#CDMO Database#Dyes Database
1 note
·
View note