#Antibody Drug Conjugate Market scope
Explore tagged Tumblr posts
Text
Antibody Drug Conjugate Market Analysis, Size, Share, and Forecast 2031
#Antibody Drug Conjugate Market#Antibody Drug Conjugate Market research#Antibody Drug Conjugate Market scope
0 notes
Text
🌟 𝐓𝐫𝐚𝐧𝐬𝐟𝐨𝐫𝐦𝐢𝐧𝐠 𝐂𝐚𝐧𝐜𝐞𝐫 𝐓𝐫𝐞𝐚𝐭𝐦𝐞𝐧𝐭: 𝐓𝐡𝐞 𝐑𝐢𝐬𝐞 𝐨𝐟 𝐀𝐧𝐭𝐢𝐛𝐨𝐝𝐲 𝐃𝐫𝐮𝐠 𝐂𝐨𝐧𝐣𝐮𝐠𝐚𝐭𝐞𝐬 (𝐀𝐃𝐂𝐬) 🌟-IndustryARC™
The Antibody Drug Conjugate Market size is estimated to reach $15275 million by 2030, growing at a CAGR of 14.20% during the forecast period 2024-2030.
👉 𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞
𝐇𝐞𝐫𝐞 𝐚𝐫𝐞 𝐬𝐨𝐦𝐞 𝐤𝐞𝐲 𝐟𝐢𝐧𝐝𝐢𝐧𝐠𝐬 𝐟𝐫𝐨𝐦 𝐭𝐡𝐞 𝐫𝐞𝐩𝐨𝐫𝐭
𝐀𝐝𝐯𝐚𝐧𝐜𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐓𝐚𝐫𝐠𝐞𝐭𝐞𝐝 𝐓𝐡𝐞𝐫𝐚𝐩𝐲: ADCs represent a targeted approach to cancer therapy, allowing for selective delivery of cytotoxic drugs to cancer cells, reducing damage to healthy cells and enhancing treatment effectiveness. This shift towards targeted therapies is a major trend in oncology.
𝐆𝐫𝐨𝐰𝐢𝐧𝐠 𝐏𝐢𝐩𝐞𝐥𝐢𝐧𝐞 𝐨𝐟 𝐀𝐃𝐂𝐬: Pharmaceutical companies are actively developing ADCs, with a growing number of ADC candidates in clinical trials. Increased R&D investments and strategic collaborations are fueling the expansion of ADC pipelines, particularly for solid tumors and hematologic cancers.
𝐈𝐧𝐜𝐫𝐞𝐚𝐬𝐢𝐧𝐠 𝐅𝐃𝐀 𝐀𝐩𝐩𝐫𝐨𝐯𝐚𝐥𝐬 𝐚𝐧𝐝 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐒𝐮𝐩𝐩𝐨𝐫𝐭: Regulatory bodies are accelerating approvals for ADCs due to their effectiveness and safety profile in cancer treatment. Recent approvals of ADCs, such as those targeting breast and bladder cancers, have driven further interest and investment.
𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐢𝐜𝐚𝐥 𝐈𝐦𝐩𝐫𝐨𝐯𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐋𝐢𝐧𝐤𝐞𝐫 𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐲: Innovations in linker technology, which attaches the antibody to the drug payload, are enhancing ADC stability and precision. Advanced linkers improve the therapeutic index, enabling more controlled drug release and minimizing off-target effects.
𝐅𝐨𝐜𝐮𝐬 𝐨𝐧 𝐍𝐨𝐯𝐞𝐥 𝐏𝐚𝐲𝐥𝐨𝐚𝐝𝐬: ADCs are moving beyond traditional cytotoxic agents, incorporating novel payloads such as immune modulators and DNA-damaging agents. These novel payloads expand ADC applications and offer enhanced potency against resistant cancer cells.
𝐄𝐱𝐩𝐚𝐧𝐬𝐢𝐨𝐧 𝐢𝐧𝐭𝐨 𝐍𝐨𝐧-𝐎𝐧𝐜𝐨𝐥𝐨𝐠𝐲 𝐀𝐩𝐩𝐥𝐢����𝐚𝐭𝐢𝐨𝐧𝐬: While oncology remains the primary focus, ADCs are increasingly being explored for autoimmune and infectious diseases. This diversification presents new opportunities and broadens the market’s scope beyond cancer.
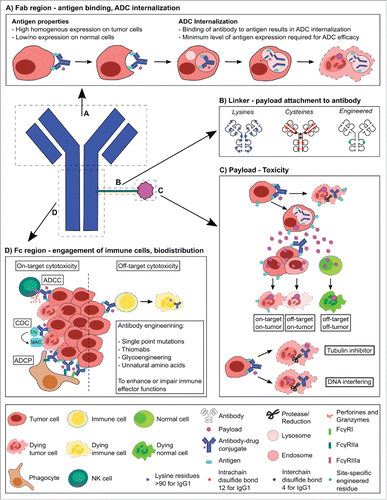
#antibodydrugconjugates#oncology#cancertherapy#targetedtherapy#precisionmedicine#cancerresearch#biotechnology#drugdevelopment#pharma#cancerimmunotherapy#biopharma#noveltherapeutics#tumortargeting#personalizedmedicine
0 notes
Text
Global Immunotoxin Market Analysis 2024: Size Forecast and Growth Prospects
The immunotoxin global market report 2024from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Immunotoxin Market, 2024report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size - The immunotoxin market size has grown strongly in recent years. It will grow from $55.94 billion in 2023 to $60.97 billion in 2024 at a compound annual growth rate (CAGR) of 9.0%. The growth in the historic period can be attributed to the discovery of target antigens, advancements in monoclonal antibody technology, preclinical efficacy studies, clinical trials and regulatory approval, and identification of resistance mechanisms.
The immunotoxin market size is expected to see strong growth in the next few years. It will grow to $86.29 billion in 2028 at a compound annual growth rate (CAGR) of 9.1%. The growth in the forecast period can be attributed to the increasing incidence of cancer, rising demand for natural and organic products, expansion of the cosmetic industry, expansion of R and D financing, and improving healthcare infrastructure. Major trends in the forecast period include precision medicine approach, enhanced targeting strategies, multifunctional immunotoxins, combination therapies, and advancements in monoclonal antibody technology.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/immunotoxin-global-market-report
Scope Of Immunotoxin MarketThe Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Immunotoxin Market Overview
Market Drivers -The increasing incidence of breast cancer is expected to propel the growth of the immunotoxin market going forward. Breast cancer refers to a type of cancer that forms in the cells of the breast. The rising incidence of breast cancer is primarily due to improved screening, lifestyle changes, and increased life expectancy. Immunotoxins help in breast cancer by targeting and killing cancer cells while sparing healthy cells, reducing side effects associated with traditional chemotherapy. For instance, in 2023 according to the American Cancer Society, a US-based non-profit organization, there were 300,590 new cases of breast cancer reported, showing an increase compared to the 290,560 cases reported in 2022. Therefore, an increasing incidence of breast cancer is driving the growth of the immunotoxin market.
Market Trends - Major companies operating in the immunotoxin market are focused on developing antibody-drug conjugates (ADCs) to enhance the specificity and efficacy of cancer treatment. ADCs are biopharmaceutical medicines that are intended to be used as targeted cancer treatments. Antibody-drug conjugates are made up of an antibody coupled to a cytotoxic medication, enabling targeted drug delivery to cancer cells that express a specific antigen. For instance, in April 2024, Pfizer Inc., a US-based pharmaceutical company, and Genmab A/S, a Denmark-based biotechnology company, received full U.S. Food and Drug Administration (FDA) approval for TIVDAK, a first antibody-drug conjugate designed to treat patients with metastatic or recurrent cervical cancer. The toxic payload of TIVDAK is monomethyl auristatin E, a microtubule-disrupting chemical that causes cell death. This novel mechanism leads to its efficiency in cancer treatment. TIVDAK showed statistically significant increases in overall survival, making it a promising alternative for individuals with recurrent or metastatic cervical cancer.
The immunotoxin market covered in this report is segmented –
1) By Type: Anthrax Based Toxins, Diphtheria Toxin (DT) And Derivatives, Pseudomonas Exotoxin (PE) And Derivatives, Ribosome Inactivating Proteins Based Immunotoxins, Ribonucleases-Based Immunotoxins, Other Types 2) By Application: Solid Tumors, Leukemias, Other Applications 3) By End User: Hospitals And Clinics, Cancer And Radiation Therapy Centers, Research Labs, Other End-Users
Get an inside scoop of the immunotoxin market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=15828&type=smp
Regional Insights - North America was the largest region in the immunotoxin market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the immunotoxin market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Key Companies - Major companies operating in the immunotoxin market are Pfizer Inc., Roche Holding AG, AbbVie Inc., Bayer AG, Bristol Myers Squibb, Thermo Fisher Scientific Inc., AstraZeneca plc, Merck KGaA, BioNTech SE, Ipsen Pharma, Seagen Inc., Orion Corporation, GenScript, Cytek Biosciences, MacroGenics Inc., Sutro Biopharma Inc., Sorrento Therapeutics Inc., Innate Pharma Inc., Cayman Chemical, ImmunoGen Inc., Mersana Therapeutics, Molecular Templates Inc., Celldex Therapeutics, CytImmune Sciences Inc., Enzo Life Sciences Inc.
Table of Contents 1. Executive Summary 2. Immunotoxin Market Report Structure 3. Immunotoxin Market Trends And Strategies 4. Immunotoxin Market – Macro Economic Scenario 5. Immunotoxin Market Size And Growth ….. 27. Immunotoxin Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected]
Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Bioengineered Protein Drugs Market: Trends Fuel Advanced Remedies

As per IDF Diabetes Atlas 10th edition, around 537 million adults are diagnosed with diabetes globally; this number is anticipated to surge to 643 million by 2030. Type 1 diabetes is among the most common autoimmune diseases, followed by lupus, rheumatoid arthritis, and psoriasis. According to the National Stem Cell Foundation, nearly 4% of the world’s population is distressed by at least 1 out of 80+ diseases. Such high prevalence has increased the adoption of bioengineered protein medications due to high affinity and low toxicity. Based on our estimates, the global bioengineered protein drugs market is predicted to witness progress with a CAGR of 7.7% during the forecast period 2023-2030.
The burden of diseases due to the rising aging population has elevated the demand for advanced medications. As per the World Population Prospects 2019, 1 in 6 people will be over 65 globally by 2050. This has prompted advancements in biotechnology and protein engineering, resulting in the availability of various drugs, including monoclonal antibodies, peptide hormones, vaccines, and fusion proteins.
Let’s look deeper into the key trends prompting developments in drug delivery systems, leading to the bioengineered protein drugs market’s expansion.
· Rising Chronic Cases Amplifies Recombinant Proteins’ Application
Over the past decade, the incidence rate of chronic diseases has soared due to unhealthy lifestyles. According to WHO, more than 15 million people between 30-69 years decease from chronic conditions annually. Cardiovascular disease accounts for most non-communicable disease mortality, followed by cancers, diabetes, etc. This has led to a significant shift in the pharmaceutical industry from chemical drugs to protein drug development. For instance, between 2021 and 2022, the US FDA approved 19 mAbs, 6 peptide hormones, 1 fusion protein, 4 therapeutic enzymes, etc.
Protein drugs using recombinant DNA technology have gained significant traction recently, leading to various launches. For instance, in February 2023, Researchers at the Indian Institute of Technology (IIT) Guwahati developed a ‘Recombinant Protein Toolbox’ consisting of six proteins that convert skin cells into heart cells, especially cardiomyocytes. This toolbox regenerates damaged heart tissues using DNA technology.
· Manufacturing Advancements Widen Drug Scope
Earlier, protein drugs were obtained from humans or other resources. For example, vaccines were formulated using egg cultures. However, recombinant DNA technology has enabled manufacturers to produce bioengineered protein drugs like vaccines, monoclonal antibodies, and blood factors using genetically modified organisms. To elucidate, blood products can be manufactured using DNA technology in bacterial expression or mammalian cell culture systems. The prominence of protein engineering platform technologies is mainly owing to their ability to enhance medications’ functionality, purity, and circulating half-life. Protein conjugation and derivatization are among the major approaches that elevate circulating half-life.
Advancements in protein engineering have led to further R&D investments in protein-based drug manufacturing. For instance, in March 2023, Eli Lilly and Company invested $1.5 billion to expand its manufacturing footprint with new facilities in North Carolina, Indiana, and Ireland. Its facility in Ireland is set to be the most technically advanced manufacturing site for existing monoclonal antibodies, producing existing and pipeline clinical products by 2026. Hence, drug manufacturing and delivery advancements create opportunities for the bioengineered protein drugs market.
Growth Projections: R&D Expands Treatment Possibilities
Considering the rising demand for effective therapies and high unmet needs, healthcare companies are surpassing research boundaries to improve treatments. This has led the pharmaceutical industry to move towards novel molecular formats to deliver breakthrough therapies. Moreover, rare diseases with few or no treatment drugs have influenced players to expand research capabilities and launch new drugs. Since numerous factors drive many complex conditions, constraining one target may not prosper in achieving efficiency. In such a scenario, developing multi-specific antibody formats treats multi-layered diseases by engaging two or more targets with one molecule. Therefore, rising research and development activities are expected to boost the bioengineered protein drugs market.
FAQs:
Q1) Which region holds the highest share in the bioengineered protein drugs market?
North America holds the highest share of the global market.
Q2) Which are the major bioengineered protein drug types?
Monoclonal antibodies, peptide hormones, vaccines, blood factors & peptide antibiotics, fusion proteins, cytokines, and therapeutic enzymes are major drug types.
0 notes
Text
Trends Influencing the Future of Aptamers Market
Market Overview –
Aptamers market size is expected to reach USD 2.3 billion by 2022. The Aptamers market industry is expected to increase from USD 2.74 billion in 2023 to USD 11.42 billion in 2032, with a compound annual growth rate (CAGR) of 19.50% during the forecast period (2023-2032).
The Aptamers Market is expanding rapidly, driven by the increasing interest in aptamer therapeutics for various medical applications. Aptamers are single-stranded DNA or RNA molecules that bind to specific targets, offering potential advantages over traditional therapeutics. The market offers a range of aptamer-based drugs and diagnostics, promising innovative solutions for treating diseases and improving patient outcomes.
The aptamers market focuses on a class of molecules known as aptamers, which are short, single-stranded DNA or RNA sequences that bind to specific target molecules with high affinity and specificity. Aptamers are used in various applications, including diagnostics, therapeutics, drug delivery, and research tools.
Market growth is driven by several factors, including the increasing demand for personalized medicine, growing investments in pharmaceutical research and development, and advancements in aptamer technology. As researchers and healthcare providers seek innovative solutions for disease diagnosis and treatment, there is a growing interest in aptamers due to their unique properties, such as high specificity, low immunogenicity, and ease of synthesis.
Technological advancements and innovations in aptamer selection, optimization, and conjugation techniques are shaping the market, offering new opportunities for aptamer-based diagnostics and therapeutics. From cell-SELEX and high-throughput screening methods to aptamer-drug conjugates and targeted delivery systems, these advancements expand the scope of aptamer applications across various biomedical fields.
Moreover, the COVID-19 pandemic has highlighted the potential of aptamers in diagnostics and therapeutics, as researchers rapidly develop aptamer-based assays for virus detection and aptamer-based therapies for COVID-19 treatment. Aptamers offer several advantages over traditional antibodies, including faster development timelines, lower production costs, and greater stability, making them valuable tools in combating emerging infectious diseases.
However, challenges such as limited commercialization of aptamer-based products, regulatory hurdles, and competition from alternative technologies pose obstacles to market growth. Addressing these challenges requires collaboration between aptamer developers, pharmaceutical companies, regulatory agencies, and healthcare providers to validate aptamer-based products, establish standardized protocols, and overcome market barriers.
Segmentation –
The Global Aptamers Market has been segmented based on Type, Application, And End-User.
Based on Type, the market is segmented into Nucleic Acid and Peptide Aptamers. The dominance of the Nucleic Acid category in the Aptamers market can be attributed to the widespread use of nucleic acid-based aptamers, such as DNA and RNA aptamers, due to their high affinity, specificity, and versatility for targeting diverse biomolecules in various applications.
Based on the Application, the market is segmented into Diagnostics and Therapeutics Development. The Diagnostics category primarily focuses on the accurate and timely detection, identification, and monitoring of diseases, conditions, or health parameters through various tests, techniques, and tools.
Based on the Technology, the market is segmented into Selex, X-aptamer, and MARAS techniques. The SELEX category primarily focuses on the systematic evolution of ligands by the exponential enrichment (SELEX) process, which is used to select aptamers with high affinity and specificity for specific target molecules.
Based on the End-User, the market is segmented into Biotechnology and Pharmaceutical Companies. The Biotechnology sector that dominates Aptamers primarily focuses on harnessing the unique properties of aptamers for various applications, including targeted drug delivery, diagnostics, biomarker discovery, and therapeutics development.
Regional Analysis –
The Aptamers Market showcases distinct regional trends influenced by factors such as research funding, technological advancements, and regulatory frameworks. North America leads the market, with the United States at the forefront of aptamer research and development. Advanced biotechnology infrastructure, coupled with substantial investments in R&D, contribute to market dominance in this region.
Europe follows suit, with countries like the UK, Germany, and Switzerland making significant strides in aptamer-based therapeutics and diagnostics. In the Asia Pacific region, increasing focus on precision medicine and growing investments in healthcare innovation propel market growth, particularly in countries like China, Japan, and South Korea. Latin America and the Middle East & Africa regions also show potential for market expansion, albeit with challenges related to infrastructure and regulatory hurdles. Overall, the regional analysis underscores the importance of collaborative efforts between industry stakeholders and policymakers to realize the full potential of aptamer technologies in diverse healthcare settings.
Key Players –
Aptamers companies include SomaLogic Operating Co., Inc., Aptamer Group Inc, Aptagen LLC, Aptamer Sciences Inc., Base Pair Biotechnologies Inc., Aptus Biotech SL, TriLink Biotechnologies, AstraZeneca, AM Biotechnologies, Aptadel Therapeutics, and Ophthotech Corporation.
Related Reports –
Diabetic Ulcer Treatment
Medical Tourniquets
Forensic Swab
Ascites
For more information visit at MarketResearchFuture
#Aptamers Market#Aptamers Market Size#Aptamers Market Share#Aptamers Market Trends#Aptamers Market Growth
1 note
·
View note
Text
Global Monoclonal Antibodies Market Size Expected to Reach $158.3 Bn by 2030 | iDataAcumen
The report "Global Monoclonal Antibodies Market - Global Forecast to 2030", is approximated to be USD 158.3 Billion in 2023, and it is projected to reach USD 359.2 Billion by 2030, at a CAGR of 11.6%.
In the dynamic landscape of healthcare, the rise of monoclonal antibodies (mAbs) has sparked a revolution, offering new avenues of treatment for a myriad of diseases. From cancer to autoimmune disorders, these remarkable biopharmaceuticals are transforming the way we approach medical care, driving growth and innovation in the Monoclonal Antibodies Market.
One of the key dynamics shaping the Monoclonal Antibodies Market is the increasing prevalence of chronic and complex diseases. As the global burden of conditions like cancer, rheumatoid arthritis, and multiple sclerosis continues to rise, there is a growing demand for targeted and effective treatment options. Monoclonal antibodies, with their ability to specifically target diseased cells while sparing healthy tissue, are uniquely positioned to meet this need, driving market growth in the process.
Moreover, advancements in biotechnology and genetic engineering have fueled the development of novel monoclonal antibodies with enhanced efficacy and reduced side effects. From antibody-drug conjugates to bispecific antibodies, these next-generation therapies are expanding the scope of possibilities in the Monoclonal Antibodies Market, opening doors to new indications and treatment modalities.
Furthermore, the increasing adoption of personalized medicine is driving the demand for monoclonal antibodies tailored to individual patient profiles. With the advent of precision medicine and molecular diagnostics, healthcare providers can now identify patients who are most likely to benefit from mAb therapy, optimizing treatment outcomes and reducing healthcare costs in the process.
Ask for Sample Copy: https://www.idataacumen.com/request-sample/global-monoclonal-antibodies-market
In conclusion, the Monoclonal Antibodies Market stands at the forefront of healthcare innovation, propelled by a convergence of factors including the rising prevalence of chronic diseases, advancements in biotechnology, and the shift towards personalized medicine. As we continue to harness the power of monoclonal antibodies to tackle some of the most pressing health challenges of our time, the future holds boundless potential for growth, discovery, and improved patient outcomes.

#monoclonalantibodies#healthcareinnovation#medicaladvancements#medicalbreakthroughs#betterpatientoutcomes#chronicdiseases#targetedtherapies#marketdemand#biotechnology#geneticengineering#nextgenerationtherapies#precisionmedicine#personalizedtherapies#optimizedtreatment
0 notes
Text
#Antibody Drug Conjugate Market#Antibody Drug Conjugate Market Research#Antibody Drug Conjugate Market Scope
0 notes
Text
High Potency API’s Market Manufacturers, Suppliers, Vendors Sales, Revenue, Market Share 2022
The reports also help in understanding the High Potency API’s Market Value dynamic, and structure by analyzing the market segments and projecting the High Potency API’s Market Value. Clear representation of competitive analysis of key players by Design, price, financial position, product portfolio, growth strategies, and regional presence in the High Potency API’s Market Value makes the report investor's guide.
High Potency API’s Market Overview:
This High Potency API’s Market industry research provided a comprehensive analysis of the worldwide High Potency API’s Market, taking into account all critical variables such as growth factors, limitations, market advancements, top investment pockets, future prospects, and trends. The research begins by emphasizing the important trends and possibilities that may develop in the near future and have a favorable influence on overall industry growth.
The High Potency API’s Market was valued at US$ 19.60 Bn. in 2021. Global High Potency API’s Market size is expected to grow at a CAGR of 8.6% through the forecast period.
Request a Free PDF Sample Report @
Market Scope:
High Potency API’s Market Research Report analyzed the current state of the definitions, classifications, applications, and industry chain structure. The analysis provides unbiased professional commentary on the present market scenario, prior market performance, production and consumption rates, demand and supply ratios, and income generation forecasts for the projected period. The High Potency API’s Market study also gives information on the leading businesses functioning in the High Potency API’s Market industry's strategic ambitions and company growth strategies. Mergers and acquisitions, government and corporate transactions, partnerships and collaborations, joint ventures, brand promotions, and product launches are among the methods evaluated in the research. To summarise what has been said thus far,
The High Potency API’s Market report presents insights into each of the leading High Potency API’s Market end users along with annual forecasts to 2027. The report provides revenue forecasts with sales and growth rate of the global High Potency API’s Market. Forecasts are also provided for the market's product, application, and geographic segments. Forecasts are produced to help people understand the industry's future outlook and potential.
Segmentation:
Based on Type, Innovative drugs accounted for the greatest revenue share of more than 70.0 % in 2021, due to increased R&D initiatives for novel drug development and favorable government regulations. The increased focus on tailored and precision medicines to treat specific patient ailments, such as Antibody Drug Conjugates (ADCs), is pushing the creation of innovative APIs, making this sector a high-impact key driver of the market.
Purchase Inquiry:
Key Players:
The research includes the most recent news and industry developments regarding High Potency API’s Market expansions, acquisitions, growth strategies, joint ventures and collaborations, product launches, market expansions, and so on. Among the main companies in the High Potency API’s Market, the sector is
• Lonza • BASE SE • Cordenpharma • Dr, Reddy’s Lab • Carbogen Amcis • Pfizer • Sun Pharmaceuticals • Teva Pharmaceuticals • Albany Molecular • Sanofi SA • Merck & Co. • Novartis AG • F. Hoffmann LA Roche • Bristrol Myers Squibb • Boehringer Ingelheim • Cipla • Eli Lily & Company • Abbvie Inc
Regional Analysis:
The primary goal of this study is to assist the user in understanding the market in terms of definition, segmentation, market potential, significant trends, and the problems that the industry is experiencing across ten key regions.
COVID-19 Impact Analysis on High Potency API’s Market:
The research details the overall impact of COVID-19 on the Health Insurance Market by providing a micro- and macroeconomic analysis. The precise study focuses on market share and size, which clearly depicts the impact that the pandemic has had and is anticipated to have on the global Health Insurance Market in the future years.
Want your Report customized?
Key Questions answered in the High Potency API’s Market Report are:
What is the function of the High Potency API’s Market?
What is the predicted revenue generation of the High Potency API’s Market?
At what growth rate is the High Potency API’s Market evolving?
Who are the major market giants operating in the High Potency API’s Market?
About Us:
Maximize Market Research provides B2B and B2C research on 12000 high-growth emerging opportunities & technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics & Communications, Internet of Things, Food and Beverages, Aerospace and Defense, and other manufacturing sectors .
Contact Us:
MAXIMIZE MARKET RESEARCH PVT. LTD
3rd Floor, Navale IT Park Phase 2,
Pune Banglore Highway,
Narhe, Pune, Maharashtra 411041, India.
High Potency API’s Market Size, Share, Analysis, Growth, Trends, Drivers, Opportunity
0 notes
Text
Antibody Drug Conjugate Market Analysis, Size, Share, and Forecast 2031
#Antibody Drug Conjugate Market#Antibody Drug Conjugate Market scope#Antibody Drug Conjugate Market size
0 notes
Link
0 notes
Link
0 notes
Text
Head and Neck Cancer Drugs/Therapeutics Market Emerging Revenue Driving Factors, Future Demands & Top Players
The global Head and Neck Cancer Drugs/Therapeutics Market research report provides complete insights on industry scope, trends, regional estimates, key application, competitive landscape and financial performance of prominent players. It also offers ready data-driven answers to several industry-level questions. This study enables numerous opportunities for the market players to invest in research and development.
Market Overview:
The global Head and Neck Cancer Drugs/Therapeutics Market was valued at USD 752.5 million in 2016 and is estimated to grow significantly in the coming years owing to the development of cost-effective and innovative Head and Neck Cancer treatments. Cancers of squamous cells present in the linings of larynx, nose, salivary glands, throat, lips, and mouth cause the Head and Neck Cancers. The Head and Neck Cancer is amongst the common type of cancers. The past records reveal that every year a massive number of Head and Neck Cancers are detected globally with more than half accounting to death annually. The increasing number of Head and Neck Cancer patients and the demand for cost-efficient treatment options are helping the Head and Neck Cancer Drugs/Therapeutics Market to grow with a CAGR of 9.4%.
Key Players:
Bristol-Myers Squibb
Merck
Sanofi
Eli Lilly
Request free sample to get a complete analysis of top-performing companies @ https://www.millioninsights.com/industry-reports/head-neck-cancer-drugs-therapeutics-market/request-sample
Growth Drivers:
The most preferred therapy performed by the surgeons is the surgical therapy combined with the radiation therapy. Moreover, the present treatment options available for Head and Neck Cancer results in less chances of survival rate. The latest developments in advanced radiotherapy and chemotherapy has helped to preserve some functions of the face. The treatment for Head and Neck Cancer is a significant challenge for the surgeons since the choice of treatment differs from patient to patient and tumor location. Generally, the Head and Neck Cancers are operated with the surgery, but the complicated facial structures and functions restricts the surgical operations. However, more focus is now being given to the technological developments of new targeted molecules like gene therapy, antibody drug conjugates, monoclonal antibodies, etc. This is expected to widen the market opportunities in near future, which will fuel up the market growth.
The market categorized on the grounds of treatment type, disease indication, end user and geography. On the grounds of treatment type, the market is distributed into Chemotherapy, Radiation Therapy, External Radiation Therapy, Internal Radiation Therapy, Surgery and Targeted Therapy. Surgery, bring the most preferred therapy choice is projected to lead the Head and Neck Cancer Drugs/Therapeutics Market in the forecast period. To prevent the reoccurrence of the cancer, the Radiation therapy is used which is expected to contribute largely to the market after the surgery. Furthermore, Chemotherapy is an assistant treatment to other therapies and therefore is expected to contribute little share in the Head and Neck Cancer Drugs/Therapeutics Market.
On the grounds of disease indication, the market is distributed into Laryngeal Cancer, Lip and Oral Cavity Cancer, Nasopharyngeal Cancer, Oropharyngeal Cancer, Salivary Gland Cancer and etc. The Lip and Oral Cavity cancer is predicted to rise globally due to growing number of people affected by lip and oral cancer. On the grounds of end user, the Head and Neck Cancer Drugs/Therapeutics Market is distributed into Hospitals, Specialty Clinics and Ambulatory Surgical Centers. Hospital is expected to contribute a comparatively larger share.
Class Outlook:
PD Inhibitors
EGFR Inhibitors
Microtubule Inhibitors
Regional Outlook:
Geographically, the market is segmented as North America, Latin America, Europe, Asia Pacific and Middle East & Africa. The Head and Neck Cancer Drugs/Therapeutics Market share from North America will continue to dominate due to high prevalence high smoking rate and HPV-induced cancers. After this, Europe is expected to contribute second largest market share.
Browse Related Category Research Reports @ https://blog.naver.com/tomclark
0 notes
Text
Global Biologics Outsourcing Market Analysis, Historic Data and forecast 2017-2022
Summary – A new market study, titled "Global Biologics Outsourcing Market: Insights, Trends & Forecast (2019-2023)" has been featured on WiseGuyReports.
The global biologics outsourcing market is estimated to reach US$26.4 billion in 2023, growing at a CAGR of 16.77% for the period spanning from 2019 to 2023. The factors such as increasing aging population, higher healthcare spending, rising incidences of chronic conditions, increasing R&D spending on biologics drugs and growing focus on biologics are expected to drive the market. However, growth of the industry will be challenged by high capital requirement, high technical requirement, regulatory issues and ability to capture customers from established competitors. A few notable trends include new drug development and increasing demand for antibody conjugates.
Also read – https://wiseguyreports.wordpress.com/2020/07/30/impact-of-covid-19-outbreak-on-biologics-outsourcing-market-2020/
The global biologics outsourcing market is broadly segmented into two types which are instruments and kits & reagents. The use of antibodies for treating various chronic diseases such as asthma and diabetes is increasing investment in research activities which has stimulated the demand for biologics outsourcing market globally.
The fastest growing regional market is China owing to the Chinese government which has issued many regulations and policies to support the development of the biologics outsourcing market in China. Marketing Authorization Holder or MAH, Pilot Program for drug approval process allows biologics applicants with products that are manufactured in China to outsource the manufacturing process to third party biologics outsourcing service providers. The U.S. and Asia are highly established premium markets that contribute to significant shares in the global market.
Scope of the report:
• The report provides a comprehensive analysis of the global biologics outsourcing market, segmented into two types which are instruments and kits & reagents.
• The major regional markets have been analyzed, with the coverage of China.
• The market dynamics such as growth drivers, market trends and challenges are analyzed in-depth.
• The competitive landscape of the market, along with the company profiles of leading players (Lonza Group, Wuxi Biologics, Boehringer Inhgelheim, Catalent Inc., Abzena and Thermo Fisher) are also presented in detail.
Key Target Audience:
• Pharmaceutical Biotech Manufacturers
• Individual Researchers
• CROs/CDMOs/CMOs
• Academic Institutes
• Investment Banks
• Government Bodies & Regulating Authorities
For more details - https://www.wiseguyreports.com/reports/5266279-global-biologics-outsourcing-market-insights-trends-forecast-2019-2023
About Us:
Wise Guy Reports is part of the Wise Guy Research Consultants Pvt. Ltd. and offers premium progressive statistical surveying, market research reports, analysis & forecast data for industries and governments around the globe.
Contact Us:
NORAH TRENT
Ph: +162-825-80070 (US)
Ph: +44 2035002763 (UK)
0 notes
Text
Cervical Cancer Market is Expected to Increase With a CAGR of 16.9% for the Study Period of 2017-2030 in 7MM: DelveInsight
Cervical Cancer Market is Expected to Increase With a CAGR of 16.9% for the Study Period of 2017-2030 in 7MM: DelveInsight
LAS VEGAS, US (DelveInsight)-- The coming decade is expected to witness a rise in targeted therapies in the pipeline and a better drug delivery approach that shall give the cervical cancer market a boost. An increase in cervical cancer incidence is also a major reason behind the accelerated momentum of the cervical cancer market size growth. Further, increased healthcare spending, better diagnosis, and heightened R&D to facilitate a better understanding and increased awareness of the disease are some other driving factors.
View report: https://www.delveinsight.com/report-store/cervical-cancer-market
Some of the key insights from cervical cancer market report:
The incidence of cervical cancer in 7MM (US, UK, Germany, Italy, Spain, France & Japan) in 2020 is expected to be 43,712.
In the 7MM, the USA is the major contributor to the cervical cancer market revenue in 2020, and the region is expected to hold its position in the upcoming years.
Key companies propelling the market size include Regeneron, AstraZeneca, Lovance, Zeria Pharmaceutical, Seattle Genetics/Genmab, Agenus Bio, Roche, Akeso Biopharma and Vaccibody/Roche, among others.
Launch of cervical cancer pipeline therapies, including Cemiplimab (Regeneron), Durvalumab (AstraZeneca), LN-145 (Iovance), Z-100 (Zeria Pharmaceutical), Tisotumab vedotin (Seattle Genetics/Genmab) in the next decade is expected to propel the market growth.
Get a cervical cancer sample copy or download a cervical cancer infograph: www.delveinsight.com/sample-request/cervical-cancer-market
View report: https://www.delveinsight.com/report-store/cervical-cancer-market
Cervical cancer is a cause of infection from Human papillomavirus (HPV), which can be prevented. Due to its slow progression, the precancerous cells can be detected through proper screening with a Pap smear test and HPV test; and can be prevented through HPV vaccination (Cervarix and Gardasil 9). However, a lack of awareness and misconceptions hinder screening and its early diagnosis.
DelveInsight estimated that cervical cancer is most often diagnosed in women between the ages of 35 and 44, with the average age at diagnosis being 50. The late diagnosis in women is a consequence of a lack of vaccination and screening in women in the early stages of their lives.
For cancer, which is preventable in the first place, the mortality rate due to advanced cervical cancer is relatively high. However, the rate varies with the different stages of cancer, geographies, ethnicities, and age-groups.
To know more about Cervical Cancer Incidence, Diagnosis and Mortality Visit: https://www.delveinsight.com/report-store/cervical-cancer-market
View report: https://www.delveinsight.com/report-store/cervical-cancer-market
The cervical cancer epidemiology section in the report provides historical and forecasted analysis upto 2030 segmented by:
Incident cases of Cervical Cancer
Stage-specific Incidence of Cervical Cancer
Histopathologic Types of Cervical Cancer
Age-specific causes of Cervical Cancer
The present treatment market for cervical cancer is mainly bifurcated into therapeutics and preventive. The preventive cervical cancer treatment market comprises two candidates, namely Cervarix (Merck) and Gardasil (GlaxoSmithKline).
For advanced, metastatic cervical cancer, the available treatments in the market constitute traditional chemotherapies, targeted monoclonal antibodies, biosimilars, and immunotherapies. Targeted therapies such as Avastin have revolutionized the treatment landscape for cervical cancer, which are often given in combination with chemotherapy. Avastin has enjoyed market exclusivity and has been a top-earner of Roche. However, after its patent expiry in 2019 in the US, it faced rigorous competition from Amgen's Mvasi, Pfizer's Zirabev, and Samsung's Aybintio. The sales of the therapy have declined in the US due to the approvals of copycats, which are priced considerably lower than the original one. The sales of the drug will decline in the European market in January 2022 due to a similar fate.
View report: https://www.delveinsight.com/report-store/cervical-cancer-market
The cervical cancer market also homes Merck's star, Keytruda, which got approval in 2018 for advanced cervical cancer for PD-L1 positive patients. Keytruda is the only approved immunotherapy in the cervical cancer market landscape; hence it enjoys the monopoly in the domain. However, sooner it will be vying for dominance as Regeneron and Sanofi's contender Libtayo is proceeding towards the finish line. Furthermore, to minimize the effect of inevitable patent expiry, Merck is testing a combination of Keytruda with Chemoradiotherapy for patients with high-risk locally advanced cervical cancer (NCT04221945). Enrolment is ongoing for Phase III, and the study is expected to be completed by the end of 2024.
Recently, at ASCO 2020, Genmab demonstrated the positive benefit-to-risk profile of its drug Tisotumab vedotin, a first-in-class antibody-drug conjugate directed against tissue factor (TF). Now the company plans to investigate the drug in combination with other cancer drugs, namely Bevacizumab, Pembrolizumab, and Carboplatin in a Phase Ib/II trial to find out the correct dosage in recurrent or metastatic cervical cancer (NCT03786081).
The Cervical cancer market has several treatment drugs in the late-stage as well as early phase of development in the pipeline. The expected launch of pipeline therapies in the foreseeable future shall significantly impact the Cervical cancer market. A better understanding of the HPV and its interaction with the host has given rich insights into the therapeutic domain.
Further, the launch of prophylactic as well as therapeutic vaccines, will transform the cervical cancer treatment landscape. Prophylactic cancer vaccines have been successful in dampening the disease burden; however, they cannot help the patients with established tumors, which Scientists believe can be tackled by the use of therapeutic vaccines. However, the neoantigens that are the basis of the therapeutic cancer vaccine are unique to patients thus, it requires the development of personalized therapies. Among all the candidates in the distant pipeline, only one to reach Phase III is VGX-3100, a candidate of Inovio Pharmaceuticals.
Also, research is underway to identify therapeutic targets and develop curative therapy to treat cervical cancer. Scientists are investigating the potential of biomarkers among cervical cancer patients that shall define the screening and diagnosis of cancer to a greater extent. A combination of multiple potential biomarkers, including serum proteins, microRNAs, ctDNA mutations, and others, can significantly increase the sensitivity of the screening and detection of cervical cancers.
View report: https://www.delveinsight.com/report-store/cervical-cancer-market
Although cervical cancer market growth may face obstructions in the face of lack of awareness among common people, delayed diagnosis, high cost of treatment, and palliative treatment options for metastatic cancer. Nevertheless, the renewed interest in targeted therapies, personalized medicine, and rising R&D that has facilitated the identification of actionable biomarkers offer a promising and flourishing environment to the global cervical cancer market.
Scope of the Report
Geography Covered: 7MM - The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), Japan.
Study Period: 3-year historical and 11-year forecasted analysis (2017-2030).
Markets Segmentation: By Geographies, By Therapies (Forecasted + Historical).
Companies Covered: Regeneron, AstraZeneca, Iovance, Zeria Pharmaceutical, Seattle Genetics/Genmab, Agenus Bio, Roche, Akeso Biopharma, and Vaccibody/Roche among others.
Analysis: Comparative and conjoint analysis of emerging therapies, Attribute Analysis,
Case Studies
KOL's Views
Analyst's View
Request for Webex demo: https://www.delveinsight.com/report-store/cervical-cancer-market
Table of Contents
1
Key Insights
2
Executive Summary of Cervical Cancer
3
SWOT Analysis of Cervical Cancer
4
Cervical Cancer Epidemiology Overview at a Glance
5
Cervical Cancer Market Overview at a Glance
6
Disease Background and Overview: Cervical Cancer
7
Epidemiology and Patient Population
8
Country Wise-Epidemiology of Cervical Cancer
9
Cervical Cancer Treatment and Management
10
Unmet Needs in the Cervical Cancer Market
11
Cervical Cancer Marketed Therapies
12
Cervical Cancer Emerging Therapies
13
Cervical Cancer: Other Promising Therapies
14
Cervical Cancer 7 Major Market Analysis
15
7MM Cervical Cancer Country-Wise Analysis
16
Cervical Cancer Market Drivers
17
Cervical Cancer Market Barriers
18
Appendix
19
DelveInsight Capabilities
20
Disclaimer
21
About DelveInsight
*The TOC is illustrative, request for a detailed TOC.
Related Report
Cervical Intraepithelial Neoplasia Market Insight, Epidemiology and Market Forecast -2030
Cervical Intraepithelial Neoplasia (CIN) or cervical dysplasia is a precancerous condition characterized by an unusual growth of the cells on the surface lining of the cervix or endocervical canal.
The report proffers a detailed section of the indication overview, signs symptoms, with a major focus on CIN epidemiology in the 7MM, present treatment modalities, and emerging therapies in the pipeline. The report lays down an in-depth understanding of the market landscape, key collaborations, partnerships, R&D development, and driving forces as well as restrains in the market growth.
Human Papillomavirus (HPV) Associated Disorders- Market Insight, Epidemiology and Market Forecast -2030
Human Papillomavirus virus (HPV) is a cause of a significant burden on global healthcare. HPV infections, if neglected, can results in several diseases, including anogenital warts and recurrent respiratory papillomatosis and several forms of cancers.
The report provides rich insights into the indication, its etiology, pathogenesis, manifestation with an exclusive focus on historical as well as forecasted epidemiology and market trends. The report brings a comprehensive analysis of the market landscape, recent happenings, breakthroughs, key partnerships, present and emerging treatments, market barriers, and drivers.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing end to end comprehensive solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Contact Us:
Shruti Thakur [email protected] +1(919)321-6187
www.delveinsight.com
0 notes