#Alumina Refinery
Explore tagged Tumblr posts
Text
Nuclear Power and Particle Acceleration unlocked.
I've been making plenty of mistakes up til this point. But now it's time to step up my game and start making MISTAKES!
#inb4 I somehow irradiate the entire fucking map#It's time for spicy science#And considering that I just today forgot the mk2 pipe limit#And was confused why my refineries were having trouble with the 720 alumina solution I wanted them to run...#I'm definitely ready for this#Satisfactory
2 notes
·
View notes
Text
New filter captures and recycles aluminum from manufacturing waste
New Post has been published on https://sunalei.org/news/new-filter-captures-and-recycles-aluminum-from-manufacturing-waste/
New filter captures and recycles aluminum from manufacturing waste

Used in everything from soda cans and foil wrap to circuit boards and rocket boosters, aluminum is the second-most-produced metal in the world after steel. By the end of this decade, demand is projected to drive up aluminum production by 40 percent worldwide. This steep rise will magnify aluminum’s environmental impacts, including any pollutants that are released with its manufacturing waste.
MIT engineers have developed a new nanofiltration process to curb the hazardous waste generated from aluminum production. Nanofiltration could potentially be used to process the waste from an aluminum plant and retrieve any aluminum ions that would otherwise have escaped in the effluent stream. The captured aluminum could then be upcycled and added to the bulk of the produced aluminum, increasing yield while simultaneously reducing waste.
The researchers demonstrated the membrane’s performance in lab-scale experiments using a novel membrane to filter various solutions that were similar in content to the waste streams produced by aluminum plants. They found that the membrane selectively captured more than 99 percent of aluminum ions in these solutions.
If scaled up and implemented in existing production facilities, the membrane technology could reduce the amount of wasted aluminum and improve the environmental quality of the waste that plants generate.
“This membrane technology not only cuts down on hazardous waste but also enables a circular economy for aluminum by reducing the need for new mining,” says John Lienhard, the Abdul Latif Jameel Professor of Water in the Department of Mechanical Engineering, and director of the Abdul Latif Jameel Water and Food Systems Lab (J-WAFS) at MIT. “This offers a promising solution to address environmental concerns while meeting the growing demand for aluminum.”
Lienhard and his colleagues report their results in a study appearing today in the journal ACS Sustainable Chemistry and Engineering. The study’s co-authors include MIT mechanical engineering undergraduates Trent Lee and Vinn Nguyen, and Zi Hao Foo SM ’21, PhD ’24, who is a postdoc at the University of California at Berkeley.
A recycling niche
Lienhard’s group at MIT develops membrane and filtration technologies for desalinating seawater and remediating various sources of wastewater. In looking for new areas to apply their work, the team found an unexplored opportunity in aluminum and, in particular, the wastewater generated from the metal’s production.
As part of aluminum’s production, metal-rich ore, called bauxite, is first mined from open pits, then put through a series of chemical reactions to separate the aluminum from the rest of the mined rock. These reactions ultimately produce aluminum oxide, in a powdery form called alumina. Much of this alumina is then shipped to refineries, where the powder is poured into electrolysis vats containing a molten mineral called cryolite. When a strong electric current is applied, cryolite breaks alumina’s chemical bonds, separating aluminum and oxygen atoms. The pure aluminum then settles in liquid form to the bottom of the vat, where it can be collected and cast into various forms.
Cryolite electrolyte acts as a solvent, facilitating the separation of alumina during the molten salt electrolysis process. Over time, the cryolite accumulates impurities such as sodium, lithium, and potassium ions — gradually reducing its effectiveness in dissolving alumina. At a certain point, the concentration of these impurities reaches a critical level, at which the electrolyte must be replaced with fresh cryolite to main process efficiency. The spent cryolite, a viscous sludge containing residual aluminum ions and impurities, is then transported away for disposal.
“We learned that for a traditional aluminum plant, something like 2,800 tons of aluminum are wasted per year,” says lead author Trent Lee. “We were looking at ways that the industry can be more efficient, and we found cryolite waste hadn’t been well-researched in terms of recycling some of its waste products.”
A charged kick
In their new work, the researchers aimed to develop a membrane process to filter cryolite waste and recover aluminum ions that inevitably make it into the waste stream. Specifically, the team looked to capture aluminum while letting through all other ions, especially sodium, which builds up significantly in the cryolite over time.
The team reasoned that if they could selectively capture aluminum from cryolite waste, the aluminum could be poured back into the electrolysis vat without adding excessive sodium that would further slow the electrolysis process.
The researchers’ new design is an adaptation of membranes used in conventional water treatment plants. These membranes are typically made from a thin sheet of polymer material that is perforated by tiny, nanometer-scale pores, the size of which is tuned to let through specific ions and molecules.
The surface of conventional membranes carries a natural, negative charge. As a result, the membranes repel any ions that carry the same negative charge, while they attract positively charged ions to flow through.
In collaboration with the Japanese membrane company Nitto Denko, the MIT team sought to examine the efficacy of commercially available membranes that could filter through most positively charged ions in cryolite wastewater while repelling and capturing aluminum ions. However, aluminum ions also carry a positive charge, of +3, where sodium and the other cations carry a lesser positive charge of +1.
Motivated by the group’s recent work investigating membranes for recovering lithium from salt lakes and spent batteries, the team tested a novel Nitto Denko membrane with a thin, positively charged coating covering the membrane. The coating’s charge is just positive enough to strongly repel and retain aluminum while allowing less positively charged ions to flow through.
“The aluminum is the most positively charged of the ions, so most of it is kicked away from the membrane,” Foo explains.
The team tested the membrane’s performance by passing through solutions with various balances of ions, similar to what can be found in cryolite waste. They observed that the membrane consistently captured 99.5 percent of aluminum ions while allowing through sodium and the other cations. They also varied the pH of the solutions, and found the membrane maintained its performance even after sitting in highly acidic solution for several weeks.
“A lot of this cryolite waste stream comes at different levels of acidity,” Foo says. “And we found the membrane works really well, even within the harsh conditions that we would expect.”
The new experimental membrane is about the size of a playing card. To treat cryolite waste in an industrial-scale aluminum production plant, the researchers envision a scaled-up version of the membrane, similar to what is used in many desalination plants, where a long membrane is rolled up in a spiral configuration, through which water flows.
“This paper shows the viability of membranes for innovations in circular economies,” Lee says. “This membrane provides the dual benefit of upcycling aluminum while reducing hazardous waste.”
0 notes
Text
New filter captures and recycles aluminum from manufacturing waste
New Post has been published on https://thedigitalinsider.com/new-filter-captures-and-recycles-aluminum-from-manufacturing-waste/
New filter captures and recycles aluminum from manufacturing waste


Used in everything from soda cans and foil wrap to circuit boards and rocket boosters, aluminum is the second-most-produced metal in the world after steel. By the end of this decade, demand is projected to drive up aluminum production by 40 percent worldwide. This steep rise will magnify aluminum’s environmental impacts, including any pollutants that are released with its manufacturing waste.
MIT engineers have developed a new nanofiltration process to curb the hazardous waste generated from aluminum production. Nanofiltration could potentially be used to process the waste from an aluminum plant and retrieve any aluminum ions that would otherwise have escaped in the effluent stream. The captured aluminum could then be upcycled and added to the bulk of the produced aluminum, increasing yield while simultaneously reducing waste.
The researchers demonstrated the membrane’s performance in lab-scale experiments using a novel membrane to filter various solutions that were similar in content to the waste streams produced by aluminum plants. They found that the membrane selectively captured more than 99 percent of aluminum ions in these solutions.
If scaled up and implemented in existing production facilities, the membrane technology could reduce the amount of wasted aluminum and improve the environmental quality of the waste that plants generate.
“This membrane technology not only cuts down on hazardous waste but also enables a circular economy for aluminum by reducing the need for new mining,” says John Lienhard, the Abdul Latif Jameel Professor of Water in the Department of Mechanical Engineering, and director of the Abdul Latif Jameel Water and Food Systems Lab (J-WAFS) at MIT. “This offers a promising solution to address environmental concerns while meeting the growing demand for aluminum.”
Lienhard and his colleagues report their results in a study appearing today in the journal ACS Sustainable Chemistry and Engineering. The study’s co-authors include MIT mechanical engineering undergraduates Trent Lee and Vinn Nguyen, and Zi Hao Foo SM ’21, PhD ’24, who is a postdoc at the University of California at Berkeley.
A recycling niche
Lienhard’s group at MIT develops membrane and filtration technologies for desalinating seawater and remediating various sources of wastewater. In looking for new areas to apply their work, the team found an unexplored opportunity in aluminum and, in particular, the wastewater generated from the metal’s production.
As part of aluminum’s production, metal-rich ore, called bauxite, is first mined from open pits, then put through a series of chemical reactions to separate the aluminum from the rest of the mined rock. These reactions ultimately produce aluminum oxide, in a powdery form called alumina. Much of this alumina is then shipped to refineries, where the powder is poured into electrolysis vats containing a molten mineral called cryolite. When a strong electric current is applied, cryolite breaks alumina’s chemical bonds, separating aluminum and oxygen atoms. The pure aluminum then settles in liquid form to the bottom of the vat, where it can be collected and cast into various forms.
Cryolite electrolyte acts as a solvent, facilitating the separation of alumina during the molten salt electrolysis process. Over time, the cryolite accumulates impurities such as sodium, lithium, and potassium ions — gradually reducing its effectiveness in dissolving alumina. At a certain point, the concentration of these impurities reaches a critical level, at which the electrolyte must be replaced with fresh cryolite to main process efficiency. The spent cryolite, a viscous sludge containing residual aluminum ions and impurities, is then transported away for disposal.
“We learned that for a traditional aluminum plant, something like 2,800 tons of aluminum are wasted per year,” says lead author Trent Lee. “We were looking at ways that the industry can be more efficient, and we found cryolite waste hadn’t been well-researched in terms of recycling some of its waste products.”
A charged kick
In their new work, the researchers aimed to develop a membrane process to filter cryolite waste and recover aluminum ions that inevitably make it into the waste stream. Specifically, the team looked to capture aluminum while letting through all other ions, especially sodium, which builds up significantly in the cryolite over time.
The team reasoned that if they could selectively capture aluminum from cryolite waste, the aluminum could be poured back into the electrolysis vat without adding excessive sodium that would further slow the electrolysis process.
The researchers’ new design is an adaptation of membranes used in conventional water treatment plants. These membranes are typically made from a thin sheet of polymer material that is perforated by tiny, nanometer-scale pores, the size of which is tuned to let through specific ions and molecules.
The surface of conventional membranes carries a natural, negative charge. As a result, the membranes repel any ions that carry the same negative charge, while they attract positively charged ions to flow through.
In collaboration with the Japanese membrane company Nitto Denko, the MIT team sought to examine the efficacy of commercially available membranes that could filter through most positively charged ions in cryolite wastewater while repelling and capturing aluminum ions. However, aluminum ions also carry a positive charge, of +3, where sodium and the other cations carry a lesser positive charge of +1.
Motivated by the group’s recent work investigating membranes for recovering lithium from salt lakes and spent batteries, the team tested a novel Nitto Denko membrane with a thin, positively charged coating covering the membrane. The coating’s charge is just positive enough to strongly repel and retain aluminum while allowing less positively charged ions to flow through.
“The aluminum is the most positively charged of the ions, so most of it is kicked away from the membrane,” Foo explains.
The team tested the membrane’s performance by passing through solutions with various balances of ions, similar to what can be found in cryolite waste. They observed that the membrane consistently captured 99.5 percent of aluminum ions while allowing through sodium and the other cations. They also varied the pH of the solutions, and found the membrane maintained its performance even after sitting in highly acidic solution for several weeks.
“A lot of this cryolite waste stream comes at different levels of acidity,” Foo says. “And we found the membrane works really well, even within the harsh conditions that we would expect.”
The new experimental membrane is about the size of a playing card. To treat cryolite waste in an industrial-scale aluminum production plant, the researchers envision a scaled-up version of the membrane, similar to what is used in many desalination plants, where a long membrane is rolled up in a spiral configuration, through which water flows.
“This paper shows the viability of membranes for innovations in circular economies,” Lee says. “This membrane provides the dual benefit of upcycling aluminum while reducing hazardous waste.”
#aluminum#atoms#author#batteries#boards#california#Capture#chemical#chemical bonds#chemical reactions#chemistry#circular economy#Cleaner industry#Collaboration#content#Desalination#Design#economy#efficiency#electrolysis#electrolyte#engineering#engineers#Environmental#experimental#Facilities#filter#Food#form#Forms
0 notes
Text
Understanding Pressure Swing Adsorption (PSA) Technology | Gas Processing
In the field of industrial gas separation, Pressure Swing Adsorption (PSA) is a highly effective and widely used technology. At Gas Processing, we specialize in providing advanced PSA systems that help businesses across various industries optimize gas separation and purification processes. In this blog, we will explore the fundamentals of Pressure Swing Adsorption, its applications, and how it can benefit your operations.
What is Pressure Swing Adsorption (PSA)?
Pressure Swing Adsorption (PSA) is a gas separation process that utilizes the principle of adsorption to separate different gases from a mixture. The process works based on the fact that gases can be selectively adsorbed onto solid materials (adsorbents) at different pressures. When the pressure is reduced, the adsorbed gases are released and can be collected for further use.
In simpler terms, PSA technology involves alternating between high and low pressures to separate and purify gases such as oxygen, nitrogen, hydrogen, carbon dioxide, and others. This cycle of pressure variation is why the process is referred to as "pressure swing."
How Does PSA Work?
The PSA process typically involves two main stages:
Adsorption Stage: In this stage, a gas mixture is passed through a bed of adsorbent material, typically made of zeolites, activated carbon, or alumina. At high pressure, some components of the gas mixture (such as nitrogen or carbon dioxide) are adsorbed onto the adsorbent material, while the remaining gases (like oxygen or hydrogen) pass through the bed.
Desorption Stage: Once the adsorbent material becomes saturated with gas, the pressure is reduced, causing the adsorbed gases to be released. This is known as desorption. The released gases are then collected and stored for further use.
The process alternates between these two stages in a cyclic manner, allowing for continuous gas separation and production. Multiple adsorption columns are often used in tandem, ensuring that one is always in the adsorption phase while another is in desorption.
Key Applications of PSA Technology
PSA technology has a broad range of applications across industries that require gas separation or purification. Some of the key applications include:
Oxygen Generation: PSA is widely used in oxygen generators for medical, industrial, and environmental applications. The process separates oxygen from air, providing a high-purity oxygen stream for various uses.
Nitrogen Generation: PSA is also employed in nitrogen generators, where it separates nitrogen from the air, producing high-purity nitrogen for applications in the chemical, food, and pharmaceutical industries.
Hydrogen Production: PSA technology is used to purify hydrogen in refineries, chemical plants, and fuel cells. It separates hydrogen from other gases like methane, carbon monoxide, and carbon dioxide.
Carbon Dioxide Removal: In industries such as natural gas processing, PSA is used to remove carbon dioxide (CO2) from gas streams, helping reduce emissions and improve product quality.
Air Separation: PSA is used for the separation of air into its constituent components (oxygen, nitrogen, and argon) for applications in medical and industrial settings.
Advantages of PSA Technology
PSA offers several benefits that make it an attractive choice for gas separation processes:
High Efficiency: PSA is highly efficient in separating gases, producing high-purity outputs with minimal energy consumption. The process can achieve purity levels of up to 99.99% for gases like oxygen or nitrogen.
Cost-Effective: Compared to other separation technologies, PSA is relatively cost-effective in terms of both capital investment and operational costs. Its use of solid adsorbents reduces the need for expensive and bulky equipment.
Flexibility: PSA systems can be customized to meet the specific requirements of different industries, providing flexibility in terms of gas separation and purity levels.
Low Operating Costs: PSA systems typically have low energy consumption, especially when compared to cryogenic air separation or membrane-based technologies.
Compact Design: PSA units are compact and easy to integrate into existing systems, making them a popular choice for both large-scale and small-scale operations.
Why Choose Gas Processing for PSA Systems?
At Gas Processing, we specialize in the design, installation, and maintenance of PSA systems tailored to your specific needs. Here’s why we are the preferred choice for businesses seeking PSA solutions:
Expertise: With years of experience in gas processing technologies, we have the knowledge and skills to design and implement effective PSA systems for a wide range of applications.
Customized Solutions: We understand that each business has unique requirements. Our PSA systems are designed and optimized to meet your specific gas separation and purification needs.
Quality and Reliability: We use only the highest quality materials and components in our PSA systems, ensuring durability, reliability, and long-term performance.
Comprehensive Support: From initial consultation and system design to installation and ongoing maintenance, we provide comprehensive support throughout the lifecycle of your PSA system.
Final Thoughts
Pressure Swing Adsorption (PSA) is a proven and efficient technology for gas separation and purification, offering significant benefits in terms of purity, cost-effectiveness, and energy efficiency. Whether you need to generate oxygen, nitrogen, or hydrogen, or remove unwanted gases like carbon dioxide, PSA can provide a reliable solution.
If you're looking for high-quality PSA systems and expert solutions for your business, Gas Processing is here to help. Contact us today to learn more about how our PSA technology can optimize your gas separation processes and contribute to your operational success.
0 notes
Text
Caustic soda is the common name for sodium hydroxide (NaOH). Caustic soda is available in solid, flake, or powder form. Caustic soda is obtained commercially as white solids and as solutions of various concentrations in water. It is used as a raw material to form chemicals that is used in the alumina, paper, textiles, dyes, refinery, and other industries, pure caustic soda is used for making candles or soap and Impure caustic soda is used in drain cleaner. It is used to make illegal drugs, it's harder to buy large quantities than in the past. The growth of the market is driven by a number of reasons such as rising demand for Alumina in the Industry. In Asia-Pacific, China and India, these countries are the major markets for pulp and paper production, while in Europe, Sweden and Finland accounted for approximately 60% of the European pulp production in 2022. In North America, there are about 110 pulp mills in the United States and Canada together, which used over 3 million metric ton of caustic soda every year.
0 notes
Text
Bauxite and Alumina: 2024’s Commodity Boom Stars 🌟
1️⃣ Supply Chain Chaos: What’s Fueling the Frenzy?
Guinea, Brazil, and Australia struggle with bauxite supply disruptions.
Key setbacks include flooding, port blasts, and operational halts.
2️⃣ Price Spikes You Can’t Ignore 📈
Alumina prices jump 70%, reaching $780/ton in China.
Aluminium follows with a 7% YTD increase, rippling across industries.
3️⃣ China’s Dual Role: Game-Changer and Market Driver 🇨🇳
Record 142M tons of bauxite imports in 2023.
China flips to net alumina exporter amid surging global demand.
4️⃣ The Ripple Effect on Smelters and Beyond 🌊
Alumina now accounts for 50% of aluminium production costs.
Russian giant Rusal slashes 250,000 tons of aluminium output.
5️⃣ Relief on the Horizon (But Not Yet) 🌅
China: 13M tons of new refining capacity by 2025.
India: Vedanta’s 6M-ton refinery expected by 2026.
Indonesia: Expansion in West Kalimantan to double refining capacity.
6️⃣ Winners and Losers: Industry Insights 💼
Winners: Metro Mining’s stock skyrockets by 190% YTD.
Losers: Aluminium-dependent sectors face margin squeezes and higher costs.
7️⃣ Lessons of 2024: Resilience in Focus 🚀
Global supply chain vulnerabilities exposed.
Strategic foresight needed to navigate the volatile market.
📣 Follow for more insights on the commodities shaping our world!
Visit - https://www.skrillnetwork.com/bauxite-and-alumina-the-unexpected-stars-of-2024s-commodity-boom
0 notes
Text
Why Choose Alumina Balls in Dubai for Your Industrial Operations?
Alumina Balls are a critical component in various industrial processes due to their unique properties, which make them ideal for applications in the chemical, petrochemical, and manufacturing industries. In Dubai, where industries are rapidly expanding, the demand for high-performance materials like Alumina Balls is growing. In this article, we will discuss why choosing Alumina Balls in Dubai can benefit your industrial operations.
What Are Alumina Balls?
Alumina Balls are ceramic balls made from alumina oxide (Al₂O₃). These balls are known for their hardness, wear resistance, and high-temperature stability, which make them suitable for a wide range of industrial applications. They are commonly used as grinding media, catalyst supports, and filtration media, among other things.
Applications of Alumina Balls in Dubai
Catalyst Support in Chemical Processing In Dubai’s thriving chemical industry, Alumina Balls are used as catalyst support in various chemical reactions. These balls provide a stable surface for catalysts to bond to, ensuring that chemical reactions proceed efficiently. They also help in evenly distributing catalysts, leading to more consistent results.
Grinding Media in Cement and Mining Alumina Balls are used extensively in grinding mills for grinding raw materials, cement, and other minerals. The high hardness of alumina ensures that these balls do not wear down easily, allowing them to last longer and reduce operational costs.
Filtration and Separation Alumina Balls are used in industrial filtration systems for separating solids from liquids and gases. Their low porosity and resistance to chemical reactions make them ideal for use in water treatment plants, where they can help improve the purity of water by removing contaminants.
Petrochemical Applications The petrochemical industry in Dubai also relies heavily on Alumina Balls for catalytic cracking and refining processes. These balls provide stability and support for the catalyst bed in reactors, helping improve the efficiency of the refining process.
Key Benefits of Alumina Balls in Industrial Operations
High Durability and Resistance to Wear One of the primary advantages of Alumina Balls is their exceptional durability. These balls can withstand high impact and abrasive conditions, making them an ideal choice for grinding and mechanical processes in industries such as cement and mining.
Thermal Stability Alumina Balls can tolerate high temperatures, which is essential in industries where heat is a significant factor. Whether it’s in chemical reactors or high-temperature grinding mills, Alumina Balls maintain their integrity under extreme conditions.
Chemical Resistance These ceramic balls are highly resistant to corrosion and chemical reactions. This makes them suitable for use in aggressive environments, such as petrochemical refineries and water treatment plants, where they come into contact with harsh chemicals and solvents.
Cost-Effectiveness Although the initial cost of Alumina Balls may be higher compared to other materials, their long-lasting nature makes them a cost-effective investment in the long term. Their ability to withstand wear and reduce maintenance requirements can significantly lower operational costs.
Improved Efficiency In applications like catalyst support and grinding, Alumina Balls help improve the overall efficiency of the process. By providing a stable surface for catalysts or reducing the wear and tear on grinding mills, these balls enhance operational performance and productivity.
Why SKJ Overseas is the Preferred Supplier of Alumina Balls in Dubai
At SKJ Overseas, we pride ourselves on offering premium quality Alumina Balls designed for superior performance. Our products are engineered to meet the demanding needs of industries in Dubai, including chemical processing, water treatment, petrochemicals, and more. Our Alumina Balls are manufactured with precision, ensuring high reliability and consistency.
With years of experience in the industry, SKJ Overseas is a trusted supplier that understands the unique challenges faced by businesses in Dubai. We offer customized solutions to meet the specific needs of our clients, ensuring that they get the most value from our products.
For more information or to place an order for Alumina Balls, contact us at:
SKJ Overseas Phone: +97155 849 6348 Address: IFZA Business Park - Premises Number 44631-001 - DDP - Dubai Silicon Oasis - Dubai - United Arab Emirates
#High Alumina Ceramic Balls#Alumina Balls in Dubai#Inert Ceramic Balls#Ceramic Saddles Manufacturer#Ceramic Saddles Supplier
0 notes
Text
Discover the Fascinating Journey of Aluminium Production?
While aluminium is the Earth's most abundant metal, it doesn't exist naturally in its pure form. Instead, aluminium readily combines with other metals to create compounds. Unlike iron, which can be isolated through straightforward furnace melting of its compounds, aluminium's production process is considerably more intricate, demanding substantial electrical power. Consequently, aluminium smelting facilities are strategically located near clean energy sources, typically hydroelectric power plants, to minimise environmental impact. But let's begin at the outset.
Let’s begin with the process.
Bauxite Mining
The aluminium production process consists of three main stages. First, bauxites, which are rich in aluminium, are extracted from the Earth. Second, these bauxites are refined into alumina or aluminium oxide. In the third stage, pure aluminium is produced through electrolytic reduction, where aluminium oxide is broken down into its components using electrical current. Approximately 4-5 tonnes of bauxite are processed into 2 tonnes of alumina, from which about 1 tonne of aluminium can be derived.
While various minerals can be used to extract aluminium, bauxite is the most common and preferred source. Bauxite is primarily composed of aluminium oxide, often mixed with other minerals. Bauxite is considered high quality if it contains over 50% aluminium oxide. These bauxite deposits exhibit a wide range of characteristics; they can be solid, dense, or crumbly and their colours can vary from brick red, flaming red, or brown due to iron oxide to grey or white in cases of low iron content. Bauxites with hues like yellow, dark green and multi-coloured varieties with bluish, purple, red and black streaks can be found.
Around 90% of global bauxite resources are situated in tropical and subtropical regions, with the majority, 73%, concentrated in just five countries: Guinea, Brazil, Jamaica, Australia and India. Guinea boasts the largest bauxite supply, totalling 5.3 billion tonnes, accounting for 28.4% of the global supply. Guinean bauxites are renowned for their exceptional quality, characterised by minimal impurities and their proximity to the surface simplifies the mining process.
Alumina Production
The subsequent production process involves converting bauxite into alumina, also known as aluminium oxide (Al2O3), a white powder. The predominant method for producing alumina from bauxite is the Bayer process, a century-old technique still widely employed today, with approximately 90% of global alumina refineries utilising this method. The Bayer process proves highly efficient but necessitates high-quality bauxite with relatively low impurities, mainly silicon.
The fundamental principle of the Bayer process is as follows: the crystallised aluminium hydrate present in bauxite readily dissolves in concentrated caustic soda (NaOH) at elevated temperatures. Aluminium hydrate crystallises upon cooling and subsequent solution concentration, while the other elements present in the bauxite (referred to as ballast) either remain undissolved or recrystallise and settle at the bottom long before aluminium hydrate crystallises. Consequently, after dissolving aluminium hydrate in caustic soda, the ballast can be effortlessly separated and removed, resulting in a byproduct known as red mud.
The Final Process
Following bauxite mining and alumina production, the final stage involves electrolytic reduction to create aluminium. The heart of an aluminium smelter, the reduction area, differs markedly from traditional steelworks. It consists of expansive rectangular buildings, some exceeding a kilometre in length, housing numerous reduction cells or pots connected to power sources via massive cables.
Operating at constant voltages between 4 and 6 volts, with amperages reaching 300 to 400 KA or more, electric current powers the highly automated production process, requiring only a minimal workforce. Within each reduction cell, aluminium is produced from alumina through an electrolytic reduction in a 950°C molten cryolite bath, with the cell's bottom acting as the cathode and large cryolite-carbon blocks serving as anodes.
An automated alumina feeding system introduces fresh alumina into the cell every thirty minutes. Electric current breaks down aluminium-oxygen bonds, accumulating aluminium at the cell's base, forming a 10-15 cm layer. At the same time, oxygen combines with carbon in the anode blocks, creating carbon dioxide. Aluminium is extracted from the cell using specialised vacuum buckets two to four times daily. A hole is punched in the surface cryolite crust, allowing a pipe to draw in liquid aluminium. On average, each reduction cell yields about 1 tonne of metal, while a vacuum bucket can hold up to 4 tonnes of molten aluminium before transport to the casthouse.
The aluminium production process emits 280,000 cubic metres of gases per tonne of aluminium produced, necessitating gas removal systems in every reduction cell. These systems direct emitted gases to a gas treatment plant, where modern dry gas treatment employs alumina to filter out toxic fluoride compounds, creating a closed-loop system. Due to the substantial electrical power required for aluminium reduction, using eco-friendly renewable sources is crucial.
Hydroelectric power plants are the primary choice, delivering ample power without environmental pollution. For example, in Russia, 95% of aluminium smelters rely on hydroelectric power. However, regions still dependent on coal-fired generation, such as China, see 93% of aluminium production powered by coal plants, resulting in significantly higher carbon dioxide emissions—21.6 tonnes per tonne of aluminium produced, compared to just 4 tonnes with hydroelectric power.

0 notes
Text
William Mason: Geopolitical Risks Support the Aluminium Market

The aluminium market has performed exceptionally well in 2024, primarily benefiting from geopolitical risks and improved demand. As a critical industrial metal, aluminium prices have continued to rise throughout 2024, driven by tight supply and rising costs. William Mason points out that the global alumina supply has fallen by over 2 million tons, leading to a market shortfall of 470,000 tons. This situation has increased the cost of aluminium production, which accounts for 25%-30% of total costs.
In addition, while supply tightness may ease in the second half of 2024 and into 2025, the short-term supply deficit will continue to support aluminium prices. Aluminium futures rose for the third consecutive month in May and have increased by over 10% year-to-date. Energy issues have significantly impacted alumina supply, with low hydroelectric power generation limiting output. Several refineries in Australia, India, and China have reduced production. Meanwhile, investments in power grids and the growth in electric vehicle production have boosted aluminium demand, pushing prices to a high of $2,700 per ton.
William Mason emphasizes that while some supply issues may be temporary, the combination of rising production costs, geopolitical risks, and strong demand provides a solid foundation for the aluminium market. Alcoa has cut production at its Kwinana alumina refinery in Western Australia, and Rio Tinto has declared force majeure on alumina deliveries from its Queensland refinery due to natural gas supply shortages. These factors combined will keep the aluminium market strong for the foreseeable future.
Future Outlook for the Global Aluminium Market
Looking ahead, William Mason believes the aluminium market will continue to be influenced by a series of factors. First, global alumina supply issues will persist, supporting aluminium prices. Second, geopolitical risks and rising production costs will continue to exert pressure on the market. Despite this, the growth of electric vehicles and power grid investments will provide strong momentum for aluminium demand.
William Mason points out that with the increase in aluminium demand, the market will face a more complex situation. He advises investors to closely monitor global supply chain dynamics and geopolitical risks when considering investments in the aluminium market. Additionally, given the rise in production costs, aluminium prices are likely to remain at high levels for some time.
William Mason emphasizes that investors should remain cautious and closely watch market changes. He believes that despite the challenges facing the aluminium market, strong demand and tight supply provide good investment opportunities. As market conditions become clearer, investors will be better positioned to capitalize on the potential of the aluminium market.
Investment Opportunities and Risk Management
In the current complex market environment, William Mason advises investors to adopt active risk management strategies. He notes that while the aluminium market outlook is positive, investors should be wary of potential market volatility and uncertainty. To mitigate risks, investors can diversify their investment portfolios to ensure stable returns amidst market fluctuations.
Furthermore, William Mason suggests that investors focus on long-term market trends rather than short-term fluctuations. By thoroughly analyzing market dynamics, investors can better predict future market directions and seize investment opportunities. He also stresses the importance of flexibility, urging investors to adjust their investment strategies promptly to adapt to market changes.
To better seize market opportunities, investors can use stock trading apps. These apps provide convenient and reliable online trading platforms, helping investors stay informed about market trends and make informed investment decisions.
0 notes
Text
Case study of Caustic Soda: A Deep Dive into the Technologies and Applications

Caustic soda, also known as sodium hydroxide (NaOH), is a chemical powerhouse with a surprisingly wide range of applications. From its role in manufacturing aluminum to keeping our homes clean, caustic soda's high reactivity and strong base properties make it a vital ingredient in countless processes.
This blog post will delve into the fascinating world of caustic soda, exploring its various forms, production methods, and the key sectors that rely on its unique properties. We'll uncover how caustic soda helps make everything from soap and detergents to textiles and paper products, and even plays a crucial role in water treatment and alumina production.
Introduction
Caustic Soda, or sodium hydroxide, serves as a fundamental ingredient extensively utilized across a broad spectrum of industries, either in its pure form or as a supplementary agent. It is available commercially in two primary forms: firstly, as a diluted solution, commonly referred to as lye, with concentrations typically ranging between 30-32% and 48-50%; secondly, in its solid state, presented as flakes, prills (pearls), or granules.
Caustic soda pearls, also known as sodium hydroxide pearls, beadles, or soda grains, are a type of caustic soda designed specifically for filtration applications. These odorless, spherical beads are about 0.7mm in diameter and can withstand temperatures up to 80 degrees Celsius. Their shape and size make them ideal for use in mash filters and vessels within the filtration process.
Caustic soda flakes, another form of sodium hydroxide, are created by boiling down a caustic soda solution until all the water evaporates. The remaining solid is then crushed into flakes, typically measuring between 0.8mm and 1.2mm in diameter and having a characteristic white color.
These flakes are industrial workhorses, playing a vital role in various sectors.
Textile engineering: Caustic soda flakes help treat fabrics during processing.
Soap and detergent manufacturing: They are a key ingredient in the production of many soaps and detergents.
Paper and pulp industries: Caustic soda flakes contribute to the process of creating paper products.
Alumina refineries: They are essential for extracting alumina from bauxite ore, a critical step in aluminum production.
Caustic Soda Liquid: Unlike its white counterparts, caustic soda liquid is a transparent solution, also known as caustic soda 50%, sodium hydroxide in an aqueous solution, or simply lye solution.
Manufacturing Processes
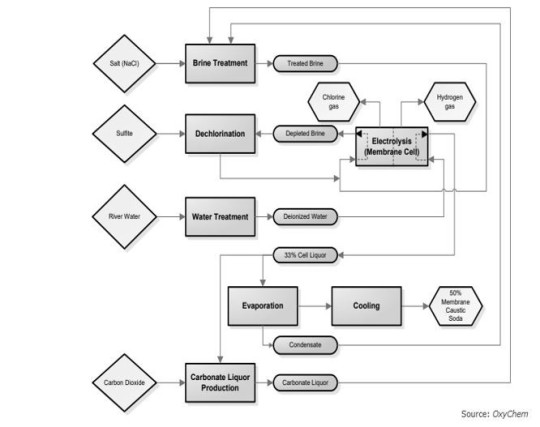
Membrane Cell Process
The membrane cell technique for producing Sodium Hydroxide (NaOH) typically yields approximately 13 percent NaOH. This method employs a specialized membrane to selectively separate Chlorine and Sodium ions. The membrane allows Sodium ions to pass through while retaining Chlorine gas and the salt (brine) solution in a separate compartment. These Sodium ions then react with purified water, akin to the mercury cell method, resulting in the production of Caustic Soda (NaOH). Evaporation is utilized to attain a nominal 50 weight percent solution. The distinctive diffusion properties of the membranes and the decreased evaporation volume in this process lead to less extensive targeting of salt concentrations.
Let’s discuss this process in further detail:
Each chlorine production cell features two electrical contact points: the anode and the cathode, which are divided by an ion-exchange membrane. This membrane selectively permits the passage of sodium ions and a minimal amount of water, guiding them towards the negatively charged cathode.
At the cathode, water undergoes electrolysis, resulting in the formation of hydrogen gas, which is released as bubbles and collected. The remaining caustic solution exits the cell at approximately 30% concentration before often undergoing further concentration to reach a 50% concentration outside the cell.
On the opposite side of the membrane, chlorine gas is produced at the anode, causing the "spent" brine to be replenished with additional solid salt before undergoing purification using an ion exchanger. The chlorine gas typically contains traces of oxygen and often requires purification through liquefaction and evaporation processes.
This method boasts the lowest consumption of electric energy among the three processes, with the steam required for caustic concentration being relatively minimal (less than one tonne per tonne of caustic soda).
Diaphragm Cell Process
The diaphragm cell method employs an asbestos separator to efficiently segregate sodium hydroxide and chlorine, which are the primary outputs of the reactions taking place within the cell. This process generates a liquid referred to as 'Cell liquor,' a diluted alkali solution containing approximately 12 to 14 percent sodium hydroxide by weight, along with a consistent quantity of salt. To attain a 50 percent sodium hydroxide solution, the resulting alkali solution undergoes evaporation to increase the concentration of NaOH. Moreover, this procedure facilitates the extraction of excess salt for recycling back into the cycle.
The sodium hydroxide produced through this technique is recognized under various designations such as Diaphragm cell-grade caustic soda, commercial grade, technical grade, and technical diaphragm. Another grade of caustic soda, known as sublime grade, can be obtained through further concentration of the 50% solution by evaporation, followed by dilution to reduce the salt content in the solution. The process of elevating the alkali concentration from diaphragm cells necessitates considerable heat for water evaporation and concentration enhancement. Despite the substantial heat requirement, diaphragm cells can prove to be more economically viable in comparison to other processes, particularly when steam costs are low, and they involve lower construction expenses.
Major Applications of Caustic Soda
Soaps & Detergents
Caustic soda finds application in the production of soaps and various detergents, many of which are utilized in household and commercial settings, including products like oven cleaners and chlorine bleach.
Aluminium
Caustic soda is employed in the extraction of alumina from bauxite. Alumina, in turn, serves as a key component in the manufacturing of aluminum and a diverse array of items such as foil, cans, and airplane components. Within the realm of building and construction, aluminum finds application in materials utilized for embellishing building exteriors and crafting window frames.
Paper & Pulp
In numerous paper-making procedures, wood undergoes treatment with a solution comprising sodium sulfide and sodium hydroxide. This process yields pure cellulose, which forms the foundation for producing paper sheets. Caustic soda plays a crucial role in the pulp and paper industry by facilitating the removal of impurities such as lignin, oleoresin, and waxes from the raw wood.
Water Treatment
In the procedure of water purification, the addition of caustic soda serves a multifaceted purpose. By adjusting the pH of water, caustic soda plays a pivotal role in mitigating the corrosive nature of water, thereby safeguarding the integrity of infrastructure and plumbing systems through the reduction of corrosive effects on pipes and fittings. Furthermore, this pH adjustment process aids in the precipitation and removal of toxic metals that may be dissolved in the water, contributing to the enhancement of water quality and safety standards.
Market Outlook
The global caustic soda market is poised for significant growth, driven by a substantial demand for alumina across various industries. Caustic soda, also known as sodium hydroxide, plays a pivotal role in the extraction of alumina, a prevalent oxide of aluminum, from naturally occurring mineral deposits. Its application extends across a diverse range of sectors including automotive, construction, and consumer goods like soda cans and food packaging. Alumina, derived through the utilization of caustic soda, finds extensive use in the automotive industry owing to its ability to enhance performance, fuel efficiency, and environmental friendliness without compromising safety or durability in vehicles. Moreover, caustic soda, in its various forms such as lye and flakes, serves as a crucial ingredient in the manufacturing processes of soaps, cleaners, and detergents. Sodium hydroxide flakes exhibit exceptional properties in dissolving oils, grease, and protein-based deposits, making them indispensable in the saponification process for converting vegetable oils into soap. Additionally, they contribute to the production of anionic surfactants, vital components in numerous detergent and cleaning formulations.
Caustic Soda Main Players
Top players operating in the Global Caustic Soda market are Olin Corporation., Formosa Plastics Corporation USA, Dow Chemical, Shintech, Westlake Chemical, Kem One,Covestro AG, Oxychem, Xinjiang Zhongtai Chemical Co., Ltd., INOVYN, Grasim Industries Limited, Tosoh Corporation, Dongying Jingling Chemical, and Others.
Conclusion:
Caustic soda plays a vital role in various sectors, such as in the Bayer's process, where it is utilized for extracting alumina from bauxite ore. In this process, a concentrated solution of caustic soda dissolves alumina to create sodium aluminate, from which alumina is subsequently separated through a reverse reaction. In the chemical industry, caustic soda serves as a crucial raw material, functioning as an intermediate, reactant, pH regulator, and aiding in acidic waste treatment. The escalating demand for caustic soda is attributed to the heightened demand for caustic soda spans various applications including chemicals, alumina, soaps & detergents, and pulp & paper products.
0 notes
Text
#Silicon carbide #alumina, #boron #tungsten #graphite #metal #sealing rings #mechanical seals #pump rings #sleeves #shaft #bush #bearing # bulletproof ceramics #hard body armor plates #armor ceramics #bulletproof helmets # bulletproof vests #pumps #magnetic #shielded #multi-stage #submerged #pipeline #slurry #food #sanitary #rotor #pump accessories #LED #semiconductors #photovoltaic #heat exchange #mining #metallurgy #pharmaceuticals Aviation #aerospace #military #pharmaceutical #chemical #water #distillery #refinery #paper mill #power plantv#pump #mechanical seal factory #electromechanical
0 notes
Text
Alumina Refinery Manufacturing Plant Setup
An alumina refinery represents a pivotal link in the broader chain of aluminum production. Integral to this process is the transformation of bauxite, an aluminum ore found primarily in tropical and subtropical regions, into aluminum oxide, or alumina, a white or nearly colorless crystalline substance.
0 notes
Text
Alumina Refinery Manufacturing Plant Setup
The alumina refinery project report provides detailed insights into project economics, including capital investments, project funding, operating expenses, income and expenditure projections, fixed costs vs. variable costs, direct and indirect costs, expected ROI and net present value (NPV), profit and loss account, financial analysis, etc.
0 notes
Text
Progress: It doesn’t happen “just so”!
Maybe we aren’t as smart as we think! Over half a century ago, in the year 1968 I left Trinidad for the first time. I was hired by a company in the US Virgin Islands and was sent a plane ticket from Piaco International Airport in Trinidad to St Croix.There was something of a construction boom taking place; both the Harvey Alumina plant where I was employed and the nearby Hess Oil refinery were…
View On WordPress
0 notes
Text
Life as a Senior NCO for a Head of Household that can't Provide
People wonder sometimes what I do. And it's complicated to simplify in just a few words. Especially in a world that is environmentally aware.
But, to make this as short as possible, sometimes society is unable to produce, for whatever reason, and my business is to over compensate for loss in production. Unless a "warrant" is issued, that states the economy "can't" produce.
And so, my business is to over produce what the economy can't.
0 notes
Text
Rising gas prices plus Ukraine-Russia geopolitical crisis threaten job security at Limerick alumina refinery
The Ukrainian-Russian geopolitical conflict and its economic fallout are threatening the future viability of Rusal-owned West Limerick, according to the company's directors and auditors.
The company has posted a net loss of more than 363 million euros since gas prices rose in the year before the Ukrainian invasion.
Now, because of the conflict, those prices have risen again, putting the group under intense pressure. Because of the document, nearly 500 employees at Europe's largest bauxite refinery, which produces 30 percent of the country's alumina, which is mainly used in aircraft, are again concerned.
Limerick Alumina Refining paid more than €46 million in wages to local workers in a report filed with the Companies Registry Office.
Aughinish's parent company has pledged continued support until December 31, 2023, and the directors said they "reasonably expected" the company would remain trading.
Niall Collins, a local TD who holds regular meetings with Aughinish management, said: "They told us, and no one refuted it, that Aughinish was the most efficient of the Rusal global groups. It was involved in the process of making a commodity that was in huge global demand. .”
Cllr Stephen Keary, former mayor and mayor of the area where the local alumina refinery is located, said: "I am very concerned about reading the reports on its viability."
Aluminum World Report
“This is a fantastic employer for West Limerick and North Kerry. If anything happens to it, it will be the death knell for the area. Many of the people who work there have a specific skill set and don’t have the opportunity to redeploy to Similar facilities," Cllr Keary said.
Dee Ryan, chief executive of Limerick Chamber, said: "If anything happens, which we hope doesn't happen, people in the area will have a hard time finding equivalent jobs nearby."
Adtech Metallurgical Materials Co.,Ltd is a Sino-foreign joint venture integrating R&D, production, operation and service of metallurgical materials. With strong technical force and perfect production and operation system, it has passed ISO 9001 quality system certification and ISO14001 environmental assessment certification. It has established long-term cooperative partnerships with more than a dozen countries and regions. The main products of adtech :

porous ceramic filter, cff filter. ceramic foundry filter . porous ceramic plate . ceramic filter plate . deep bed filter. deep bed filter aluminium.
ceramic foam filter . metal foam filter. alumina foam. metal filtration.
degassing aluminum,rotary degassing, inline degasser .online degasser . nitrogen degassing . degassing equipment . rotary degassing . rotary degasser,. aluminium degassing machine. rotary degassing aluminum .degassing machine. degassing unit .
boron nitride coating ,deslagging , granular flux,bn coating,tundish powder, refining flux, aluminum casting Flux.casting flux .aluminum degassing flux . refining agent . deslagging agent . cover flux for aluminium, cover flux, flux for aluminum casting . boron nitride paint . covering agent .
launder system . electric launder system .
tundish nozzle, tap out cone, tap out cone, caster tip , tap cone, etc.
To learn more, please follow website: https://www.alalloycasting.com/
Contact: [email protected]
#Aluminum#AluminumFiltration#AluminumDegassing#AluminumIngot#AluminumFactory#AluminumProcessing#porousceramicfilter#degassingunit
0 notes