#22-9376
Explore tagged Tumblr posts
Photo

GTARDO.DE Plattenroller, Tragkraft 200 kg, Luft, Rad-Ø 260x85 mm, Maße 320x320 mm 81,00 € https://gtardo.de/plattenroller-tragkraft-200kg-luft-rad-260x85mm-masse-320x320mm-aufstellbreite-60mm-22-9376
#Transport#Transportgeräte#Karren#Tragkraft 200kg#Rad-Ø 260x85 mm#Luft#Plattenkarren#Plattenroller#Maße 320x320 mm Tragkraft 200 kg#Maße 320x320 mm#Aufstellbreite 60 mm#22-9376
0 notes
Text
Result TotoMacau (22:00) 19 Juni 2021
Result : 9376
Shio : Anjing
Totomacaupools
Selamat Kepada Para Pemenang di Pasaran TotoMacau.
Jangan Menyerah dan Semoga Selalu Beruntung
http://masterlotre.me/
Master Lotre

0 notes
Text
Cervical Cancer
Intro
Cervical Cancer
Cervical cancer is a cancer from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early no symptoms are seen. Later symptoms may include abnormal vaginal bleeding, pelvic pain, or pain during sexual intercourse.
Cervical cancer typically develops from precancerous changes over 10 to 20 years. About 90% of cervical cancer cases are squamous cell carcinomas, 10% are adenocarcinoma, and a small number are other types. Diagnosis is typically by cervical screening followed by a biopsy. Medical imaging is then done to determine whether or not the cancer has spread.
HPV vaccines protect against between two and seven high-risk strains of this family of viruses. Regular Pap smears are also used as a mechanism to detect cancer. Other methods of prevention include: having few or no sexual partners and the use of condoms. Cervical cancer screening using the Pap smear or acetic acid can identify precancerous changes which when treated can prevent the development of cancer. Treatment of cervical cancer may consist of some combination of surgery, chemotherapy, and radiotherapy.
Causes
HPV
Smoking
Oral contraceptives
Multiple Pregnancies
Stages
1A, 1B, 2A, 2B, 3B, 4A, 4B.
Treatment
Microinvasive cancer (stage 1A) may be treated by hysterectomy (removal of the whole uterus including part of the vagina). For stage 1A2, the lymph nodes are removed, as well. Alternatives include local surgical procedures such as a loop electrical excision procedure or cone biopsy. For 1A1 disease, a cone biopsy (cervical conization) is also used.
If a cone biopsy does not produce clear margins, one more possible treatment option for women who want to preserve their fertility is a trachelectomy. This attempts to remove the cancer while preserving the ovaries and uterus. It is a viable option for those in stage 1 cervical cancer which has not spread. Due to the possible risk of cancer spread to the lymph nodes in stage 1b cancers and some stage 1a cancers, the surgeon may also need to remove some lymph nodes from around the uterus for evaluation.
A radical trachelectomy can be performed abdominally or vaginally. A radical abdominal trachelectomy with lymphadenectomy complications are uncommon. Recurrence in the residual cervix is very rare if the cancer has been cleared with the trachelectomy.
Early stages (IB1 and IIA less than 4 cm) can be treated with radical hysterectomy with removal of the lymph nodes or radiation therapy. Radiation therapy is given as external beam radiotherapy to the pelvis and brachytherapy (internal radiation). Women treated with surgery who have high-risk features found on pathologic examination are given radiation therapy with or without chemotherapy to reduce the risk of relapse.
Larger early-stage tumors (1B2 and 2A) may be treated with radiation therapy and cisplatin-based chemotherapy, hysterectomy (which then usually requires adjuvant radiation therapy), or cisplatin chemotherapy followed by hysterectomy.
Advanced-stage tumors (2B-4A) are treated with radiation therapy and cisplatin-based chemotherapy. Hycamtin and cisplatin, for women with late-stage (4B) cervical cancer treatment. Combination treatment has significant risk of neutropenia, anemia, and thrombocytopenia side effects.
References
“Cervical Cancer Treatment (PDQ®)”. NCI. 2014-03-14. Retrieved 24 June 2014.
“Defining Cancer”. National Cancer Institute.
Tarney, CM; Han, J (2014). “Postcoital bleeding: a review on etiology, diagnosis, and management.”. Obstetrics and Gynecology International.
Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology (8th ed.). Saunders Elsevier.
Kufe, Donald (2009). Holland-Frei cancer medicine. (8th ed.). New York: McGraw-Hill Medical.
World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.12.
Dunne, EF; Park, IU (Dec 2013). “HPV and HPV-associated diseases.”. Infectious Disease Clinics of North America. 27 (4): 765–78.
“Cervical Cancer Treatment (PDQ®)”. National Cancer Institute. 2014-03-14.
“FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV”. U.S. Food and Drug Administration. 10 December 2014.
“Human Papillomavirus (HPV) Vaccines”. National Cancer Institute. 2011-12-29.
Tran, NP; Hung, CF; Roden, R; Wu, TC (2014). “Control of HPV infection and related cancer through vaccination.”. Recent Results in Cancer Research. 193: 149–71.
“Cervical Cancer Prevention (PDQ®)”. National Cancer Institute. 2014-02-27.
World Health Organization (February 2014). “Fact sheet No. 297: Cancer”.
“SEER Stat Fact Sheets: Cervix Uteri Cancer”. NCI.
World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.1.
Baalbergen, Astrid; Veenstra, Yerney; Stalpers, Lukas; Baalbergen, Astrid (2013). “Primary surgery versus primary radiotherapy with or without chemotherapy for early adenocarcinoma of the uterine cervix”. Reviews.
Erstad, Shannon (2007-01-12). “Cone biopsy (conization) for abnormal cervical cell changes”.
Jones WB, Mercer GO, Lewis JL, Rubin SC, Hoskins WJ (1993). “Early invasive carcinoma of the cervix”. Gynecol. Oncol. 51 (1): 26–32.
Dolson, Laura (2001). “Trachelectomy”.
Burnett AF (2006). “Radical trachelectomy with laparoscopic lymphadenectomy: review of oncologic and obstetrical outcomes”. Curr. Opin. Obstet. Gynecol. 18 (1): 8–13.
Cibula D, Ungár L, Svárovský J, Zivný J, Freitag P (2005). “[Abdominal radical trachelectomy–technique and experience]”. Ceska Gynekol (in Czech). 70 (2): 117–22.
Plante M, Renaud MC, Hoskins IA, Roy M (2005). “Vaginal radical trachelectomy: a valuable fertility-preserving option in the management of early-stage cervical cancer. A series of 50 pregnancies and review of the literature”. Gynecol. Oncol. 98 (1): 3–10.
Roy M, Plante M, Renaud MC, Têtu B (1996). “Vaginal radical hysterectomy versus abdominal radical hysterectomy in the treatment of early-stage cervical cancer”. Gynecol. Oncol. 62 (3): 336–9.
Dargent D, Martin X, Sacchetoni A, Mathevet P (2000). “Laparoscopic vaginal radical trachelectomy: a treatment to preserve the fertility of cervical carcinoma patients”. Cancer. 88 (8): 1877–82.
Schlaerth JB, Spirtos NM, Schlaerth AC (2003). “Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer”. Am. J. Obstet. Gynecol. 188 (1): 29–34.
Waggoner, Steven E (2003). “Cervical Cancer”. The Lancet. 361 (9376):
“FDA Approves First Drug Treatment for Late-Stage Cervical Cancer”. U.S. Food and Drug Administration. 2006-06-15.
Sardain, H; Lavoue, V; Redpath, M; Bertheuil, N; Foucher, F; Levêque, J (August 2015). “Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review.”. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 41 (8): 975–85.
1 note
·
View note
Photo

original url http://www.geocities.com/SouthBeach/Boardwalk/9376/ last modified 2001-02-22 00:26:05
7 notes
·
View notes
Note
I'm writing a story about wizards who terraform planets via magic. How might a moon/moons affect their work, especially if they are moving continental plates around and creating oceans? Would there be any reason they would want to redesign the moon's orbit? If they did that, how would the planet be affected while they moved the moon?
The planetary engineers of Magrathea had to figure out that when they built the Earth. They got pretty good at it, so I guess planetary engineering isn’t that difficult to do it.

The effect of a moon on its planet depends on three things: the distance the moon orbits, the size of the moon, and the ratio of the moon’s mass to its planet’s mass.
The closer the moon orbits its planet, the more effect it will have.
The more massive the moon is, the more effect it will have.
The larger the moon’s mass is in comparison to it’s planet, the more effect it will have.
A moon will affect its planet mostly through gravity. The larger and closer the moon is, the greater its gravitational effects will be. Earth’s Moon is pretty big - both in actual terms and in comparison to Earth (the Moon masses 1.2% of the mass of the Earth). The moon orbits 385,000 km from Earth.
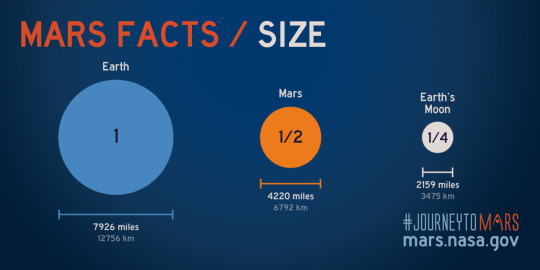
In comparison, Mars’ largest moon, Phobos, is only 22 km in diameter and masses less than 0.0000016% the mass of Mars. Even though Phobos orbits 9376 km from Mars, it’s so little that you can say it’s gravitational effect on Mars is zero and not be very wrong.

A moon will affect its planet by pulling on the side closest to it. On Earth, this causes ocean tides, but also flexes the crust and affects earthquakes and volcanoes. If your moon is smaller or farther away, it will raise much lower - or effectively no - tides.
I wrote about what might happen if the Moon was instantly removed from Earth then returned to it. If your people are moving continents, they’d be aware of these possible problems, and should be able to handle them with just a little bit of planning.
However, if you have the ability to move continents around, chances are you have enough power that you can handle any complications moving your moon might cause. If they get in the way, pop them into a higher orbit for a while, then return them to where they were.
Or, maybe they want to improve the planet by moving the moons because they want a brighter or dimmer night, or maybe they’ll move the moon so close that it’ll break up and form rings around the planet.
Cause rings are awesome, and will always look pretty.
18 notes
·
View notes
Text
RESULTADO JOGO DO BICHO - 25/07/2019 QUINTA 18:20 HORAS - (LOOK-GOIAS)
RESULTADO JOGO DO BICHO – 25/07/2019 QUINTA 18:20 HORAS – (LOOK-GOIAS)
1• 2475 19 Pavão 🦆 2• 4764 16 Leão 🦔 3• 9376 19 Pavão 🦆 4• 8287 22 Tigre 🐅 5• 1356 14 Gato 🐈 Super(5): 25 26 03 19 20
View On WordPress
0 notes
Link
CURE GBM BRAIN CANCER NEWS Recent FDA Announcements Herald Advances in Personalized Approaches Against Cancer December 22, 2017 Arthur N. Brodsky, Ph.D. While we’ve known for decades that genetic mutations fuel cancer development and progression, only recently have doctors been able to begin translating that insight into improved treatment approaches and clinical benefits for patients. Since November, the U.S. Food and Drug Administration (FDA) has authorized the use of two tumor-profiling tools—both relying on next-generation genome sequencing—that should help spur even more advances in this area. On December 1, 2017, the FDA approved the use of FoundationOne’s CDx genomic test (F1CDx), which enables doctors to determine if patients’ tumors possess any mutations among 324 clinically relevant genes. The test can also detect abnormal expression of non-mutated genes. In this way, F1CDx can serve as a companion diagnostic tool that guides doctors’ treatment decisions for their patients. For example, if a doctor finds that a patient’s lung tumor has a mutated version of EGFR (epidermal growth factor receptor), it suggests that an FDA-approved targeted therapy such as erlotinib (Tarceva®) or osimertinib (Tarisso®) might be effective. Similarly, a breast cancer patient whose tumor overexpresses HER2 (human epidermal growth factor receptor 2) would likely be a good candidate for treatment with the HER2-targeting antibody trastuzumab (Herceptin®). In all, F1CDx can help pair patients with five different cancer types—lung, breast, melanoma, colorectal, and ovarian—with 15 different FDA-approved therapies. Another significant aspect of F1CDx is its ability to determine if a tumor’s genome is characterized by microsatellite instability (MSI-hi). In May, a checkpoint immunotherapy—pembrolizumab (Keytruda®)—became the first treatment of any type to be approved for patients with tumors that possessed an unstable, MSI-hi genome, no matter where those tumors are located. Another checkpoint immunotherapy—nivolumab (Opdivo®)—is approved for colorectal cancer patients with MSI-hi tumors. Furthermore, F1CDx can quantify a tumor’s mutational burden—essentially tell the doctor how many mutations it has overall. When treating patients with immunotherapy, it’s been shown that patients whose tumors are heavily mutated are much more likely to respond successfully to checkpoint immunotherapy. In addition to pembrolizumab and nivolumab, four other checkpoint immunotherapies—ipilimumab (Yervoy®), atezolizumab (Tecentriq®), avelumab (Bavencio®), durvalumab (Imfiniz®)—have been approved for patients with several types of advanced, metastatic cancers. On November 15, 2017, the FDA authorized the use of Memorial Sloan Kettering Cancer Center’s MSK-IMPACTTM, which also provides doctors with the ability to characterize the mutations that a patient’s tumor possesses. (In this case, MSK-IMPACT analyzes 468 different cancer-associated mutations or alterations, compared to F1CDx’s 324.) Currently, MSK-IMPACT is only available for use at Memorial Sloan Kettering Cancer Center; however, the FDA has also taken steps to facilitate the development of additional, similar tools elsewhere. They announced “a new, streamlined regulatory process” that aims to make it easier, in terms of both time and cost, for other hospitals and medical institutions to develop their own tools to aid doctors and patients. In addition to pairing patients with the currently approved treatments most likely to help them, these two tumor-profiling tools will aid investigations into new treatments that are being evaluated in clinical trials. Contact Us Cancer Research Institute | National Headquarters 29 Broadway, 4th Floor | New York, NY 10006-3111 (800) 992-2623 (212) 832-9376 Staff Directory

0 notes
Photo

GTARDO.DE Plattenroller, Tragkraft 200 kg, Luft, Rad-Ø 260x85 mm, Maße 320x320 mm 81,00 € https://gtardo.de/plattenroller-tragkraft-200kg-luft-rad-260x85mm-masse-320x320mm-aufstellbreite-60mm-22-9376
#Transport#Transportgeräte#Karren#Tragkraft 200kg#Rad-Ø 260x85 mm#Luft#Plattenkarren#Plattenroller#Maße 320x320 mm Tragkraft 200 kg#Maße 320x320 mm#Aufstellbreite 60 mm#22-9376
0 notes
Text
Poesía para ser dicha
Poesía para ser dicha
Con “Poesía para ser dicha”, biblioteca celebra 79 años de historia
Con un guión elaborado por el periodista y escritor Daniel Samper Pizano, se presentará en Pereira, el martes 22 de agosto, a partir de las 6 de la tarde, el montaje poético teatral “Poesía para ser dicha”, durante el cual los actores Humberto Dorado, Carmenza Gómez y Luis Fernando Múnera, compartirán poemas que hacen parte de la…
View On WordPress
0 notes
Text
乃木坂46 17thシングル店舗別特典まとめ
【CDショップチェーン別先着特典決定!!】3/22発売 乃木坂46 17thシングル「タイトル未定」 2017.02.06 http://www.nogizaka46.com/news/2017/02/cd322-46-17th.php 3/22発売となる乃木坂46の17thシングル{初回仕様限定盤Type A(SRCL-9370~9371)、初回仕様限定盤Type B(SRCL-9372~9373)、初回仕様限定盤Type C(SRCL-9374~9375)、初回仕様限定盤Type D(SRCL-9376~9377)、通常盤(SRCL-9378)}を下記CDショップチェーンにてご購入いただいた方に、1枚お買い上げにつき1枚、先着で下記オリジナル特典を差し上げます。 ★予約者優先 先着オリジナル特典★ ※Type-Aのリンクを付記 Amazon.co.jp ポストカード(Type…
View On WordPress
0 notes
Photo

GTARDO.DE Plattenroller, Tragkraft 200 kg, Luft, Rad-Ø 260x85 mm, Maße 320x320 mm 81,00 € https://gtardo.de/plattenroller-tragkraft-200kg-luft-rad-260x85mm-masse-320x320mm-aufstellbreite-60mm-22-9376
#Transport#Transportgeräte#Karren#Tragkraft 200kg#Rad-Ø 260x85 mm#Luft#Plattenkarren#Plattenroller#Maße 320x320 mm Tragkraft 200 kg#Maße 320x320 mm#Aufstellbreite 60 mm#22-9376
0 notes
Text
Look Loterias das 18 Horas – 25/07/2019 – (Goiás) } Jogo do Bicho
Look Loterias das 18 Horas – 25/07/2019 – (Goiás) } Jogo do Bicho
1º 2475 19 – PAVÃO 2º 4764 16 – LEÃO 3º 9376 19 – PAVÃO 4º 8287 22 – TIGRE 5º 1356 14 – GATO Soma: 26258
View On WordPress
0 notes
Text
RESULTADO JOGO DO BICHO - 22/05/2018 TERÇA FEIRA EXTRAÇÃO DAS 19:15 HORAS [ASSU (NATAL-RN)]
RESULTADO JOGO DO BICHO – 22/05/2018 TERÇA FEIRA EXTRAÇÃO DAS 19:15 HORAS [ASSU (NATAL-RN)]
22/05/2018 TERÇA FEIRA EXTRAÇÃO DAS 19:15 HORAS ASSU (NATAL-RN) 1• 3324 06 Cabra 🐐 2• 7429 08 Camelo 🐪 3• 3623 06 Cabra 🐐 4• 6679 20 Peru 🦃 5• 1495 24 Veado 🦌 6• 5065 17 Macaco 🐒 7• 2332 08 Camelo 🐪 8• 9827 07 Carneiro 🐑 9• 9376 19 Pavão 🦆 10• 1196 24 Veado 🦌
View On WordPress
0 notes