I’m a year 12 student in the Uk applying for medical school next year currently working towards my gold YSA award. This blog is just cool science stuff i research on my way
Don't wanna be here? Send us removal request.
Text
Chemical Weapons
Chemical weapons are pretty well known for being horrible, and for a good reason. From sarin gas to chlorine, chemical weapons are atrocious; however it is important to know how and why they work. In this post we will look at some common chemical weapons and how they effect the body.
Pepper Spray
Pepper spray, the least extreme of our list contains a natural chemical you probably have heard of: capsaicin. Capsaicin is the chemical in chillies that makes them spicy. Now that doesn’t sound too bad but in pepper spray the capsaicin is much more concentrated. ‘Spiciness’ is measured in Scoville Heat Units (SHU): for context a jalapeno pepper is about 8000 SHU and pepper spray can be between 500,000 and 5,000,000 SHU. As you can see it’s a lot more dangerous than your average chili !

Capsaicin actually works by stimulating the cells used to detect warmth. These cells produce mucus and the reaction can cause temporary blindness (lasting 15-30 minutes), throat swelling and intense discomfort. Luckily there are no long lasting effects when pepper spray is used which makes it popular with law enforcement.
Fun Fact: The use of peppers as a weapon actually dates back to the Mayans as they’d burn chillies and use the smoke as an early chemical weapon!
Tear Gas
CS Tear Gas (again sometimes used by law enforcement) uses a different chemical, namely 2-chlorobenzalmalononitrile (CS).

This is a white solid that is usually dispersed as nano-particles in smoke. A smoke mixture ignite propelling the power through the air. The CS is an irritant: it causes a severe burning sensation and your body kicks in to expel the chemical. If it gets in the eyes lots of tears are produced, and if it gets in the respiratory system lots of mucus is produced (this reaction is what gives the tear gas its name). This reaction is to flush the chemical out of your system, similar to vomiting when you have food poisoning.
Most of the time, this is only temporary damage, however in some cases, especially with small children and elderly prolonged effects can be seen. Under the Geneva convention, tear gas is banned in warfare, however in domestic uses it is still allowed...
Chlorine
Chlorine gas is a particularly nasty chemical weapon used notably by the Syrian government in recent conflicts. One of the simplest chemical weapons with the formula Cl2 it can create devastating effects.

Chlorine can get into the respiratory system and cause fluid in the lungs causing the victim to asphyxiate. If that’s not bad enough it will also produce hydrochloric acid and hypochlorite (bleach) when it reacts with moisture in the air which causes chemical burns and possibly blindness.
Mustard Gas
Mustard gas is a dark yellow gas with a signature smell of garlic. This is an atrocious weapon used mainly in WW1 and caused death by asphyxiation. Mustard gas is a blistering agent and will burn your skin and most importantly your lungs. The lungs form blisters that block the airways stopping airflow and eventually killing the victim.
Mustard gas contains sulfur and chlorine, the active groups that alkynate the body. Alkylation is when an alkyl group (containing a double bonded oxygen) is transferred on to your molecules damaging your cells and even your DNA.

It has a number of properties that make it such a horrible but effective weapon. Firstly it is difficult to detect, the only way really is through the smell - despite the colour the gas will settle quickly and be invisible. If a mustard gas attack has happened it can linger for days as the relatively melting point means it can solidify and stick around. It also has a distinct latency period only showing symptoms 24-48 hours after the exposure, meaning often the soldiers didn’t know they were getting a deadly dose.
Sarin Gas
Sarin gas is perhaps the deadliest chemical weapon, only a tiny amount can cause paralysis and death. Unlike the previous four chemicals that cause respiratory issues, sarin gas is a nerve agent that stops the brain getting signals to the muscles.

Sarin gas has a similar structure to an important neurotransmitter called acetylcholine. Now normally the acetylcholine transfers across sending the signal and is then broken down by an enzyme called acetylcholinesterase; this tells the body to stop contracting that specific molecule. Now sarin confuses these enzymes and they try to break down the sarin gas but can’t. This means the signal stays on and the muscles are unable to relax. Paralysis sets in and you will become unable to breathe as that action is controlled by muscles in your chest.
Sarin is particularly lethal as it is odourless, colourless, flavourless and highly soluble. This means it can be used as an efficient poison as it can be mixed into food and drink.
Now you’ve become acquainted with some deadly chemical weapons I hope you have an appreciation of their chemistry, and just how damaging they can be.
PS. Don’t try this at home !
2 notes
·
View notes
Text
The Chemistry of Coffee
How does coffee work?
Caffeine is a very important substance, especially for students like me. It keeps the body awake and energised, even when you’ve had very little sleep.
To understand how caffeine works, first we have to look at a completely different molecule that is essential for your body’s functioning: adenosine. Adenosine is made of two main parts: a base called adenine (you might know adenine as one of the DNA 4 bases A,T,C and G) and a sugar called ribose (a part that also makes up RNA).
Adenosine is incredibly important as when it’s combined with phosphate ions - it makes a molecule called ATP (adenosine triphosphate) that provides all your cells with energy. It is produced through aerobic respiration and allows us to move, digest food, see, and everything else your body needs to do. Now when adenosine is on its own, without its three phosphate molecules, it actually does the opposite and signals to your body to sleep so it can replenish its supply of ATP and provide the body with more energy. Adenosine connects with a receptor called an A1 receptor which, in turn, tells the brain it’s time to rest.
Here’s where caffeine comes in. Caffeine has a very similar molecular structure to adenosine and will actually take adenosine’s place in the A1 receptor.

This stops the adenosine being recognised and the stops the signals telling the brain to sleep. Interestingly, the largest collection of A1 receptors is found on the heart, and when it senses the adenosine, it tells the heart to slow down as the body prepares to sleep. However when caffeine interferes with this mechanism, the heart rate will actually increase and that is one of the main effects of caffeine. Eventually the caffeine is digested and broken down and when this happens, a large amount of adenosine will have built up ready to signal to the brain. This means once the caffeine breaks down, your brain has loads and loads of signals telling it you need to sleep. This effect provides the crash you often feel after drinking coffee.
Other chemicals in coffee:
Caffeine isn’t the only chemical in coffee that has a physiological effect. It also contains a high concentration of a family of compounds called phenols - incredibly important compounds for your well-being .
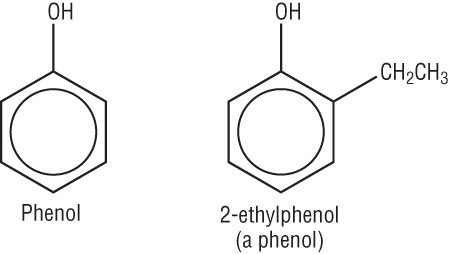
These chemicals are structured as benzene rings that have an alcohol group attached to them and act as important antioxidants - one of the reasons some people think coffee is really healthy and important for your body.
Oxygen is crucial. It allows us to efficiently respire and convert glucose to ATP which provides energy for your body’s active processes. Without oxygen, we wouldn’t be able to move, digest food and all our inner processes would grind to a halt. Despite oxygen’s many uses, it’s quite a dangerous molecule. When substances are oxidised they can become less useful and sometimes form quite violent reactions. For example, when fuels are burnt, they are just reacting with oxygen very exothermically. Scientists actually suspect this is why people die of old age, the oxygen slowly damages their body. This is why antioxidants like our phenols are so important, they remove the excess oxygen in your system and can even reverse some of the oxidising effects you might experience. The phenols react with the oxygen forming a carbonyl group and takes out a molecule of oxygen that could damage a protein or cause a cell to die.
0 notes
Text
The maths of rainbows
So I think it’s common knowledge that rainbows appear when the sun shines and it’s raining but why does this phenomenon actually happen? I remember being taught as a kid it all has to do with reflection of light and which somehow, as if by magic, splits the light into all the colours but I think we can do better than that. Well have no fear, all of your science stresses will be answered very shortly.
Well, firstly, let’s look at what we already know. Rainbows all have to do with reflection and refraction. I’m sure you’ll recall from GCSE physics that when light reflects off a surface, the angle of incidence is equal to the angle of reflection. You will probably also remember that when light refracts, the angle of refraction is less than the angle of incidence. Now we can build on all of that with a new law: Snell’s law (a great name by the way). Snell’s law states that the sines of the angles of refraction will be proportional based on the material the light is moving in to. I know that’s quite overwhelming but let’s try and work an example out to make this easier:
Using Snell’s law: n1sin(x1) = n2sin(x2): n is the refractive index of the materials, this is just a number for example air’s is one that relates to its density; x is just the angles that the light enters the material and leaves the material. Let’s work through an example to apply this law.
Let’s say water enters a raindrop at 30 degrees. (The refractive index of air is 1 and the index of water is 1.333.)
So let’s substitute in what we know:
1 x sin(30) = 1.333 x sin(x)
We can work out the value of x from this, and the angle of the light when it enters the raindrop:
As sin(30) = 0.5
0.375 = sin(x)
X = 22
As we can see, it’s actually pretty easy to work out the angles of refraction and it’s this rule that holds the key to how, where and why rainbows form.
So as the beam of light reaches the raindrop, it can do two things, it will both reflect and refract. Some of the light will reflect off the raindrop but some of it will enter. This light that is transferred through the raindrop will refract when it enters and move along until it reaches the boundary of the raindrop where it will both reflect and refract again. Now remember white light is made of all wavelengths and colours of light: so as this process repeats; on the fourth reflection the wavelengths of the parts making up white light have started to separate and so when this light is refracted out of the raindrop, the different wavelengths of light diffract and spread forming the rainbow.

Up until the 4th time, the light beam is still refracted into white light, the wavelengths take a few reflections and refraction in the raindrop to separate out. This means there is consistency which allows the cumulative effect of the light inside many, many raindrops to produce the rainbow. The fact the fourth reflection is the one the rainbow forms from means that the rainbow will always be exactly 42 degrees from the observer.
Now you may be wondering what happens after that though, surely it will keep going forever until the whole sky is filled with colour? Unfortunately not. Remember that everytime the light beam hits the surface some is split off and the light intensity is decreased. This means not much happens after the fourth reflection. However, on rare occasions, you can actually see the result of the 5th reflection - this is what forms a double rainbow.
Now it’s time for the segment: Fun facts about rainbows :)
There is a region in between a rainbow and the double rainbow called Alexander’s Band where the sky appears darker than normal
In a double rainbow colours are reversed, this can create the colour fuchsia (which doesn’t actually exist) it’s the overlapping of red and purple wavelengths
Because there are no definite boundaries between the colours in rainbows, each culture defines the number differently - Isaac Newton defined our 7 colour ROYGBIV rainbow but the Chinese see 5, renaissance artists thought there 4 and the ancient Greek philosophers debated between 1, 2 and 3
An old European belief was that anyone who passed under a rainbow would change into the opposite sex
A moonbow is possible - light reflected from the moon going through the process sunlight goes through however humans are only able to see it as white as the light is low at night.
I hope this post has explained some of how rainbows form and why they occur like they do. I got most of this information from a paper given to me by a lecturer who is studying the mathematics of light, waves and how that applies to natural phenomena.
Thanks for reading,
Jack
1 note
·
View note