journal of Urology & Nephrology: The journal covers the topics like epididymal cyst, microdot surgical vasectomy reversal, ultrasound, Renal failure, uro-oncology, Urogynecology, sepsis, prostate diseases, prostate cancer, etc: Lupine Publishers Journals.
Don't wanna be here? Send us removal request.
Text
Lupine Publishers | The Early Treatment of a Bodybuilder with Symptomatic Chronic Renal Failure with Intestinal Dialysis: A New Recommendation for Intestinal Dialysis Enhancement
Abstract
Background: Dietary therapy aiming primarily at reducing the generation and accumulation of urea through protein restriction is the most important non-dialytic therapeutic intervention in the management of chronic renal failure. The use of a urea lowering agent “acacia gum” with protein restriction has been increasingly used as a new form of dietary dialysis which has been increasingly known as intestinal dialysis. Just like in other forms of dialysis, the use of conservative dietary and pharmacological measures is also necessary in intestinal dialysis.
Patients and methods: The early treatment of a bodybuilder with symptomatic chronic renal failure with intestinal dialysis is described, and the relevant literatures were reviewed with the primary of identifying the evidence that can contribute to enhancing intestinal dialysis.
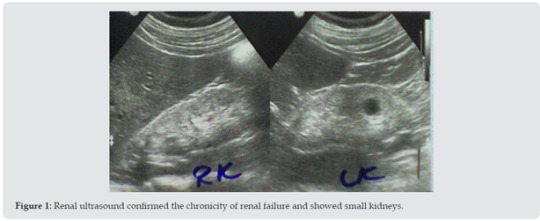
Results: At about the age of 50 years (March, 2022), a professional bodybuilder who presented with progressive symptomatic uremia associated with nausea, vomiting, pruritus, and mild anemia. His weight was about 100 Kg, and before his current illness he reported that his bench press single maximum repetition was 140Kg. On the 19th of March, blood urea level was 162 mg /dL and serum creatinine was 6.2 mg /dL. Renal ultrasound confirmed the chronicity of renal failure and showed small kidneys. The conservative dietary (Acacia gum supplementation plus very low protein diet) and pharmacological managements were prescribed according to the latest published intestinal dialysis guidelines and included oral iron and folic acid capsule, and calcium carbonate. After two weeks, the patient was asymptomatic and blood urea was lowered to 126.4 mg/dL, and the hemoglobin was increased to 11g/d.
Conclusion: This is just another case to demonstrate that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure. There is a convincing evidence to support that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement.
Keywords: Symptomatic uremia; Intestinal dialysis; Ketoanalogues of essential amino acids.
Introduction
Dietary therapy aiming primarily at reducing the generation and accumulation of urea through protein restriction is the most important non-dialytic therapeutic intervention in the management of chronic renal failure. The use of a urea lowering agent “acacia gum” with protein restriction has been increasingly used as a new form of dietary dialysis which has been increasingly known as intestinal dialysis. Just like in other forms of dialysis, the use of conservative dietary and pharmacological measures is also necessary in intestinal dialysis [1-14].
Patients and methods
The early treatment of a bodybuilder with symptomatic chronic renal failure with intestinal dialysis is described, and the relevant literatures were reviewed with the primary of identifying the evidence that can contribute to enhancing intestinal dialysis.
Results
At about the age of 50 years (March, 2022), a professional bodybuilder who presented with progressive symptomatic uremia associated with nausea, vomiting, pruritus, and mild anemia. He was not havening reduction in urine output, edema or hypertension. His weight was about 100 Kg, and before his current illness he reported that his bench press single maximum repetition was 140Kg. On the 19th of March, blood urea level was 162 mg / dL and serum creatinine was 6.2 mg /dL. Urinalysis showed 2 plus albuminuria and one plus amorphous urate. Blood calcium and serum electrolytes were within normal ranges, but he had mild hyperphosphatemia with serum phosphorus of 4.9 mg/dL (Normal range 2.4-4.4mg/dL). Hemoglobin was 10.7 mg/dL (Normal ranges: 11.5-16.5 g/dL). Liver function tests were normal (Total serum bilirubin 0.8 mg/dL, Aspartate aminotransferase (SGOT) 25 iu/L, alanine aminotransferase (SPOT) 21 iu/L, alkaline phosphatase 284 iu/L).
He reported history of episodes of hyperglycemia that in the case of bodybuilders is generally attributed to growth hormone administration in excessive doses. However, the patient was reluctant to provide details about the performance enhancing medications such as anabolic steroids and growth hormone, and he was not confirming or denying the use of such agent. He was simply saying that he was taking protein supplements. Renal ultrasound (Figure 1) confirmed the chronicity of renal failure and showed small kidneys (RK: 8 x 4, cortex 6 mm, LK: 8.2 x 4, cortex 6 mm). The kidneys had hyper-echoic texture with reduced cortical thickness and loss of the cortico-medullary differentiation. There were small cysts on both kidneys, not more than 1.5 cm in diameter. Abdominal ultrasound also showed small polyp in the gall bladder and mild enlargement of the prostate with a volume of 27 cm3 (Normally up to 25). The patient initially required oral prochorperazine 5 mg for two days control the nausea and vomiting, and oral antihistamine plus topical crotamiton 10% to control pruritus. The conservative dietary (Acacia gum supplementation plus very low protein diet) and pharmacological managements were prescribed according to the latest published intestinal dialysis guidelines and included oral iron and folic acid capsule, and calcium carbonate. He also received oral finasteride 5 mg daily for the prostatic enlargement. After two weeks, the patient was asymptomatic and blood urea was lowered to 126.4 mg/dL and the hemoglobin was increased to 11g/d. Ultrasound showed normal prostate size of 20 cm3. Literature review suggested that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement. Therefore, Ketosteril (Fresenius), was prescribed in a low initial dose of three tablets, and was ordered to be brought to the patient from Turkey.
Discussion
Until now, there is no evidence to support that high protein diet per se can cause chronic renal failure. However, nephrocalcinosis caused exogenous vitamin D intoxication was reported to cause renal failure in a bodybuilder athlete [15]. Therefore, an accurate causation of the chronic renal failure cannot be determined. Carrero et al (2020) emphasized the importance and benefits of fruits and vegetables in patients with chronic renal failure. The intake of fruits and vegetables is associated with a higher fiber intake which can cause a shift in the gut microbiota towards reduced production of uremic toxins. The intake of fruits and vegetables is also associated with lower intake phosphorus, and thus help in controlling hyperphosphataemia [16]. However, the latest published intestinal dialysis guidelines have already suggested intake of fruits and vegetables [17]. The use of Keto-analogues of essential amino acids in the management of chronic renal failure has been reported as early as the 1970s (Walser, 1978; Bauerdick and colleagues, 1978, Giovannetti et al, 1980) [18]. Bauerdick and colleagues (1978) reported the use of nitrogen-free hydroxy and keto precursers of amino acids in the treatment of patients with chronic renal failure with essential amino acid and a low-protein diet was associated with a more positive nitrogen balance [19]. In 1980, Giovannetti et al treated twenty patients with advanced chronic renal failure with a low protein diet (0.2 g/kg/day hour vegetable proteins) and essential aminoacids and ketoanalogues. They reported that treatment was associated with a favorable outcome [20]. In 1981, Barsotti et al emphasized that treatment of chronic renal failure a very low protein diet plus essential amino acids and ketoanalogues is not associated with reduction of creatinine clearance, while treatment with hemodialysis and free protein intake is associated with reduction of creatinine clearance. They treated thirty-one patients with a conventional low-protein diet, and treatment was associated with a linear reduction of creatinine clearance. A thirteen patient treated with hemodialysis experienced significantly accelerated decline of creatinine clearance. However, only one of a twelve patients treated with a very low protein diet supplemented plus essential amino acids and ketoanalogues, experienced continued a continued reduction in creatinine clearance [21].
Mitch and colleagues (1982) described the treatment of 9 patients who severe chronic renal failure (mean glomerular filtration rate 4.8 ml/min; mean serum creatinine 11.3 mg/dl). They were treated with protein restriction (22.5 g daily of mixed quality protein) plus essential amino acids and keto-analogues of essential amino acids including tyrosine, ornithine, and a high proportion of branched-chain ketoacids, and very little methionine. Phenylalanine and tryptophan were not provided. One month of treatment was associated with significant lowering of serum urea nitrogen. Hyperphosphatemia which was observed in three patients, improved. Treatment was not associated with side effects. The treatment precluded the need for dialysis in patients with severe chronic renal failure who would otherwise need dialysis [22]. In 1983, Barsotti et al described the treatment of 48 patients with chronic uraemia for a maximum of 36 months with low protein diet plus essential amino acids and keto-analogues. Ten patients experienced reduction of renal function and required dialysis.
Eight patients experienced difficulties in complying with treatment and also required dialysis. Three died for causes that are not directly related to renal failure. 27 patients continued with treatment without important reduction in renal function, and enjoyed satisfactory subjective and objective states without evidence of protein malnutrition or unwanted effects [23]. In 1985, Barsotti et al reported that the treatment of men who had uremia with a low protein diet plus essential amino acids and ketoanalogues was associated with restoration of testosterone levels in blood [24]. In 1985, Ciardella et al described the treatment of eighty-five patients with chronic renal failure with a vegetarian low-protein, low-phosphorus diet plus essential amino acids and ketoanalogues. Treatment was associated with marked reduction of serum triglycerides in the 61 men, but the reduction was not significant in woman. When the patients were later treated by maintenance hemodialysis without dietary restrictions, the experienced elevations in serum triglycerides levels which was attributed to the loss of the effect of the dietary restriction on uremic male hypogonadism [25].
Conclusion
This is just another case to demonstrate that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure. There is a convincing evidence to support that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement.
Conflict of interest
None.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers#articles#urology#submission#lupine journals#journal of urology & nephrology studies#open access journals#nephritis#nephrology#juns
0 notes
Text
Lupine Publishers | Briefly about The Ureter

Abstract
Every man has two kidneys. Each kidney has a ureter, which drains urine from a central collection point into the bladder. From the bladder, urine is excreted through the urethra from the body through the penis in men and the vulva in women. The most important role of the kidneys is to filter metabolic waste products and excess salt and water from the body and help remove them from the body. The kidneys also help regulate blood pressure and make red blood cells.
Keywords: Kidney; Obstruction; UTI; Injury
Introduction
The ureters are tubular structures responsible for the transportation of urine from the kidneys to the bladder. The length ranges from 22 to 30 cm, and they have a wall made up of multiple layers. The innermost layer is made up of transitional epithelium, surrounded by the lamina propria, which is a connective tissue layer. These two layers make up the mucosal lining. The next layer is made of smooth muscle that, as mentioned previously, is continuous with that of the calyces and the renal pelvis. However, one slight difference is that within the ureter, the smooth muscle layer is divided into an inner longitudinal and an outer circular layer. These muscular layers allow peristalsis of urine. The outermost layer is the adventitia, which is a thin layer enveloping the ureter, its blood vessels, and lymphatics. The ureter is often divided into three segments: the upper (proximal), middle, and lower (distal) segments. The upper segment starts from the renal pelvis to the upper border of the sacrum. The middle segment is the part between the upper border and lower border of the sacrum. The lower segment is from the lower border of the sacrum to the bladder.
Anatomy
Each ureter lies posterior to the renal artery and vein at the ureteropelvic junction [1]. They then descend anterior to the psoas major muscle and the ilioinguinal nerves, just anterior to the tips of the lumbar transverse vertebral processes. Approximately a third of the way down, the ureters are crossed by the gonadal vessels. They then cross the bifurcated common iliac arteries and run along the anterior border of the internal iliac artery toward the bladder, which it pierces in an oblique manner. It is this oblique entry of the ureter into the bladder, the intramural segment of the ureter that acts as a nonreturn valve preventing vesicoureteric reflux. This valve can be congenitally defective such as that seen in those with short intramural segments, or rendered ineffective as a result of injury, such as surgery or disease, all of which leads to reflux. Many congenital abnormalities of this oblique tunnel are seen in association with a duplex kidney and ureterocoele. In its lower third, the ureters pass posterior to the superior vesical branch of the internal iliac artery also called ‘umbilical artery’. On the right side, the ureter is related anteriorly to the second part of the duodenum, caecum, appendix, ascending colon, and colonic mesentery. The left ureter is closely related to the duodenojejunal flexure of Treitz, descending and sigmoid colon, and their mesenteries.
Obstruction
The diagnosis of obstruction cannot be made on the basis of haematological or biochemical tests [2]. There may be evidence of impaired renal function, anaemia of chronic disease, haematuria or bacteriuria in selected cases. Ultrasonography is a reliable means of screening for upper urinary tract dilatation. Ultrasound cannot distinguish a baggy, low-pressure, unobstructed system from a tense, high pressure, obstructed one, so that falsepositive scans are seen. A normal scan usually but not invariably rules out urinary tract obstruction. If obstruction is intermittent or in its very early stages, or if the pelvicalyceal system cannot dilate owing to compression of the renal substance, for example by tumour, the ultrasound scan may fail to detect the problem. Radionuclide studies may be helpful. If obstruction has resulted in prolongation of the time taken for urine to travel down the renal tubules and collecting system (obstructive nephropathy) this can be detected by nuclear medicine techniques and is diagnostic. Conversely, in the presence of a baggy, low-pressure, unobstructed renal pelvis and calyceal system, nephron transit time will be normal, but pelvic transit time prolonged. If doubt exists, frusemide may be administered; satisfactory ‘washout’ of radionuclide rules out obstruction and vice versa. Relative uptake of isotope may be normal or reduced on the side of the obstruction and peak activity of the isotope may be delayed. In general, absence of uptake of radiopharmaceutical indicates renal damage sufficiently severe to render correction of obstruction unprofitable. Isotope studies may thus provide a guide to the form of surgery to be undertaken. Antegrade pyelography and ureterography are extremely useful in defining the site and cause of obstruction and can be combined with drainage of the collecting system by percutaneous needle nephrostomy. The risk of introducing infection is less than with retrograde ureterography in which technique instrumentation of the bladder is followed by injection of contrast into the lower ureter or ureters. This technique is indicated if antegrade examination cannot be carried out for some reason or if there is a possibility of dealing with ureteric obstruction from below at the time of examination. Ureteral obstruction occurs in 2–10% of renal transplants and often manifests itself as painless impairment of graft function due to the lack of innervation of the engrafted kidney [3]. Hydronephrosis may be minimal or absent in early obstruction, whereas low-grade dilatation of the collecting system secondary to edema at the ureterovesical anastomosis may be seen early post-transplantation and does not necessarily indicate obstruction. A full bladder may also cause mild calyceal dilatation due to ureteral reflux and repeat ultrasound with an empty bladder should be carried out. Persistent or increasing hydronephrosis on repeat ultrasound examinations is highly suggestive of obstruction. Renal scan with furosemide washout may help support the diagnosis, but it does not provide anatomic details. Confirmation of the obstruction can be made by retrograde pyelogram but the ureteral orifice may be difficult to catheterize. Although invasive, percutaneous nephrostomy tube placement with antegrade nephrostogram is the most effective way to visualize the collecting system and can be of both diagnostic and therapeutic value.
Blood clots, technically poor reimplant, and ureteral sloughing are common causes of early acute obstruction after transplantation. Ureteral fibrosis secondary to either ischemia or rejection can cause intrinsic obstruction. The distal ureter close to the ureterovesical junction is particularly vulnerable to ischemic damage due to its remote location from the renal artery, hence compromised blood supply. Although uncommon, ureteral fibrosis associated with polyoma BK virus in the setting of renal transplantation has been well-described. Ureteral kinking, lymphocele, pelvic hematoma or abscess, and malignancy are potential causes of extrinsic obstruction. Calculi are uncommon causes of ureteral obstruction. Definitive treatment of ureteral obstruction due to ureteral strictures consists of either endourologic techniques or open surgery. Intrinsic ureteral scars can be treated effectively by endourologic techniques in an antegrade or retrograde approach. An indwelling stent may be placed to bypass the ureteral obstruction and removed cystoscopically after 2–6 weeks. An antegrade nephrostogram should be performed to confirm that the urinary tract is unobstructed prior to nephrostomy tube removal. Routine ureteral stent placement at the time of transplantation has been suggested to be associated with a lower incidence of early postoperative obstruction. Extrinsic strictures, strictures that are longer than 2 cm, or those that have failed endourologic incision, are less likely to be amenable to percutaneous techniques and are more likely to require surgical intervention. Obstructing calculi can be managed by endourologic techniques or by extracorporeal shock wave lithotripsy.
UTI
Urinary tract infections (UTIs) are the most common bacterial infection in the paediatric population [4]. The incidence is initially higher in boys, affecting up to 20.3% of uncircumcised boys and 5% of girls at the age of 1. There is a gradual shift, with UTIs affecting 3% of prepubertal girls and 1% of prepubertal boys. The National Institute for Health and Care Excellence (NICE) have defined a recurrent UTI as two or more episodes of pyelonephritis, or one episode of pyelonephritis plus one or more episodes of cystitis, or three or more episodes of cystitis. Diagnostic investigations include urinalysis, which may require suprapubic bladder aspiration or bladder catheterisation in infants. A urine culture and microscopy should be carried out if there is evidence of infection. The role of further imaging is to differentiate between an uncomplicated and complicated UTI, but should also be considered in those with haematuria. A UTI is complicated in the presence of an abnormal urinary tract including upper tract dilatation, atrophic or duplex kidneys, ureterocoele, posterior urethral valves, intestinal connections, and vesico-ureteric reflux (VUR). NICE guidelines recommend an urgent ultrasound of the urinary tract for all those with recurrent UTI under six months. For children six months and older, NICE in the UK recommends an ultrasound within six weeks of the latest infective episode. All children with recurrent UTIs should be referred to a paediatric specialist and have a dimercaptosuccinic acid (DMSA) scan within four to six months of an acute infection to evaluate for renal scarring. European Association of Urology (EAU) guidelines recommend a renal tract ultrasound in febrile UTIs if there is no clinical improvement, as an abnormal result is seen in 15% of these patients. Routinely repeating a urine culture in children treated with an antibiotic based on previous urine culture susceptibilities is not necessary [5]. Approximately 10%–30% of children develop at least one recurrent UTI, and the recurrence rate is highest within the first 3 to 6 months after a UTI. Renal scarring increases with an increasing number of febrile UTIs and with delayed treatment; therefore, parents should be counseled regarding the high risk of recurrent UTI and seek prompt evaluation for subsequent febrile illnesses in their child. Children who had a febrile UTI should routinely have their height, weight, and blood pressure monitored by their primary care provider. Children with significant bilateral renal scars or a reduction of renal function warrant long-term follow-up for the assessment of hypertension, renal function, and proteinuria.
Colic
Urolithiasis represents the commonest cause of acute ureteric colic, with calcium stones accounting for approximately 80% of cases [6]. Ureteric calculi have a prevalence of approximately 2–3% in Caucasian populations, with a lifetime risk of 10–12% in males and 5–6% in females. They are more common in developed countries, in men, in those with a positive family history, and in those with inadequate daily water intake. Ureteric colic typically presents with acute severe loin pain – which patients often describe as unrelenting despite a number of postural changes – and haematuria. Vomiting is often a feature of severe uncontrolled pain. Most patients with renal colic present because of severe uncontrolled pain and do not have signs of overt sepsis. Opiates are commonly given, although diclofenac sodium has been shown to be at least as effective for pain relief, particularly via rectal administration. Despite initial concerns, diclofenac sodium therapy has not been associated with renal toxicity in patients with preexisting normal renal function. Fever, tachycardia, tachypnoea and hypotension suggest sepsis secondary to an infected, obstructed kidney, which represents a life-threatening condition. Immediate management comprises prompt resuscitation, establishment of intravenous (IV) antibiotics, rapid diagnosis and decompression of the obstructed renal system, usually by ultrasound-guided percutaneous nephrostomy. Early involvement of urology and critical care services is essential in such patients. The management of ureteric calculi depends upon factors relating to the stone and the patient. Pre-eminent stone factors are size and site. It has been shown that 71–98% of stones <5 mm will pass spontaneously, whereas rates of spontaneous passage for stones >7 mm are low. Stone passage is also related to location in the ureter; 25% of proximal, 45% of mid and 75% of distal ureteric stones will pass spontaneously. In patients in whom stone passage is deemed likely, a trial of conservative management should be employed. Exceptions include patients with a functional or anatomical solitary kidney, bilateral ureteric obstruction, uncontrolled pain, or the presence of infection. Patients without contraindications should receive diclofenac sodium 50 mg tds, which has been shown to reduce the frequency of recurrent renal colic episodes. Recent evidence suggests that additional treatment with smooth muscle relaxants is associated with increased rates of stone passage over analgesics alone. A recent meta-analysis of studies using either nifedipine or tamsulosin, showed an approximate 65% greater chance of stone passage when such agents were used compared with equivalent controls. Intervention is generally reserved for large stones (>7 mm), conservative treatment failures and those with contraindications to a watchful waiting approach. Options include extracorporeal shockwave lithotripsy and retrograde ureteroscopic stonefragmentation.
A stone can traverse the ureter without symptoms, but passage usually produces pain and bleeding [7]. The pain begins gradually, usually in the flank, but increases over the next 20–60 min to become so severe that narcotics may be needed for its control. The pain may remain in the flank or spread downward and anteriorly toward the ipsilateral loin, testis, or vulva. A stone in the portion of the ureter within the bladder wall causes frequency, urgency, and dysuria that may be confused with urinary tract infection. The vast majority of ureteral stones <0.5 cm in diameter pass spontaneously. Helical computed tomography (CT) scanning without radiocontrast enhancement is now the standard radiologic procedure for diagnosis of nephrolithiasis. The advantages of CT include detection of uric acid stones in addition to the traditional radiopaque stones, no exposure to the risk of radiocontrast agents, and possible diagnosis of other causes of abdominal pain in a patient suspected of having renal colic from stones. Ultrasound is not as sensitive as CT in detecting renal or ureteral stones. Standard abdominal x-rays may be used to monitor patients for formation and growth of kidney stones, as they are less expensive and provide less radiation exposure than CT scans. Calcium, cystine, and struvite stones are all radiopaque on standard x-rays, whereas uric acid stones are radiolucent.
Ureteral Stone Disease
Nephrolithiasis occurs with an estimated overall prevalence of 5.2% and there is evidence that stone disease is on the rise [8]. However, many stones in the kidney go undetected because they cause no symptoms or obstruction. Conversely, ureteral stones rarely remain silent, and they have greater potential for causing pain and obstruction. As such, ureteral stones that fail to pass spontaneously require surgical intervention. Although the introduction of medical expulsive therapy (MET, the use of pharmacological agents to promote spontaneous stone passage) has changed the natural history of ureteral stone disease, not all ureteral stones respond to MET. Indications for surgical intervention to remove ureteral calculi include stones that are unlikely or fail to pass spontaneously with or without MET, stones that cause unremitting pain regardless of the likelihood of spontaneous passage, stones associated with persistent, high-grade obstruction, stones in patients with an anatomically or functionally solitary kidney or in those with renal insufficiency or stones in patients for whom their occupation or circumstances mandate prompt resolution (i.e. pilots, frequent travelers, etc.). Once the decision has been made to intervene surgically for a patient with a ureteral stone, treatment options include shock-wave lithotripspy, ureteroscopy, percutaneous antegrade ureteroscopy and open or laparoscopic ureterolithotomy. Although special cirumstances may dictate the application of percutaneous antegrade ureteroscopy or ureterolithotomy (large, impacted stones, stones in patients with urinary diversions or stones that fail less invasive approaches), the two most widely practiced treatment modalities for ureteral stones are shock-wave lithotripsy (SWL) and ureteroscopy (URS). Both are associated with high success rates and low morbidity. However, the optimal treatment for ureteral stones remains controversial because of passionate advocates on both sides of the controversy. Proponents of SWL cite the noninvasiveness, high patient satisfaction and ease of treatment, while URS advocates favor the short operative times, high success rates and short time interval to become stone free.
Cystoscopy
Cystoscopic examination of urethra and bladder should be systematic [9]. The female urethra is only 2.5–4 cm long. Urethral mucosa should be examined for strictures, diverticular opening, or polyps, and the bladder neck is visualised as scope enters and exits the bladder. Base and trigone of the bladder are initially inspected. Trigone lies proximal to the bladder neck; it is the triangular area bounded by the inter-ureteric ridge and the bladder neck at the base of the bladder. One of the most common features of the trigone is squamous metaplasia, present in up to 50% of the women. It is a benign feature with no malignant potential. In staging for cervical cancer, when imaging suggests stage 3 or 4 disease, cystoscopy is indicated. The bladder base and trigone appearance such as bullous edema, inflammatory changes, or infiltration has to be documented, and in case of infiltration, biopsy should be part of the evaluation. Ureteric orifices are slit-like openings easily identifiable by the presence of efflux on either side of the inter-ureteric ridge. The ureteral orifices location, number, nature of ureteric efflux (clear, blood stained), and any anatomical distortion is noted. In a woman with anterior vaginal wall prolapse or an underlying cervical mass, identification of trigone or ureteric orifices may be difficult. In such cases, placing a finger inside the vagina and elevating the bladder base with a finger will be helpful. Blood stained ureteric efflux denotes upper tract pathology and further assessment of the ureter and kidneys is indicated, either by ultrasound or a CT scan of the kidneys, ureters, and bladder (CT KUB). In intra-operative or postoperative ureteral integrity assessment, presence of just ureteric peristalsis does not rule out ureteral injury. Checking for ureteric efflux after administration of methylthionium chloride or indigo carmine (5ml) IV is effective in confirming ureteral patency.
Injury
Most ureteral injuries are iatrogenic in the course of pelvic surgery [10]. Ureteral injury may occur during transurethral bladder or prostate resection or ureteral manipulation for stone or tumor. Ureteral injury is rarely a consequence of penetrating trauma. Unintentional ureteral ligation during operation on adjacent organs may be asymptomatic, though hydronephrosis and loss of renal function results. Ureteral division leads to extravasation and urinoma. If the ureteral injury is not recognized at surgery, the patient may complain of flank and lower abdominal pain on the injured side. Ileus and pyelonephritis may develop. Later, urine may drain through the wound (or through the vagina following transvaginal surgery) or there may be increased output through a surgical drain. Wound drainage may be evaluated by comparing creatinine levels found in the drainage fluid with serum levels; urine exhibits very high creatinine levels when compared with serum. Intravenous administration of 5 mL of indigo carmine causes the urine to appear blue-green; therefore, drainage from an ureterocutaneous fistula becomes blue, compared to serous drainage. Anuria following pelvic surgery not responding to intravenous fluids may rarely signify bilateral ureteral ligation or injury. Peritoneal signs may occur if urine leaks into the peritoneal cavity.
Conclusion
The ureter starts from the renal pelvis, descends down the retroperitoneal space of the abdominal cavity and enters the pelvis, where it ends by pouring into the urinary bladder. Therefore, it distinguishes the abdominal and pelvic part. The boundary between these parts is the so-called terminal line. When entering the pelvic cavity, the ureter forms a border curve and in this narrowed part the kidney stone can be stopped. The curvature of the ureter is projected on the anterior abdominal wall in the area of the Hale topographic point. Urinary tracts pass through the bladder wall obliquely. When the bladder wall is stretched, the fold of its mucosa acts as a valve and prevents the return of urine to the ureters and the possible spread of infection from the bladder to the kidneys.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#nephrology#lupine journals#open access journals#journal of urology & nephrology studies#nephritis#submission#articles#juns#lupine publishers#urology
0 notes
Text
Lupine Publishers | Radiology; USG and Colour Doppler of Post Renal Transplant Complications

Abstract
Kidney transplant is the treatment of choice for patients with end-stage renal disease. Kidney transplant offers better quality of life. It is more cost effective than hemodialysis. Advances in surgical technique, along with improvement in organ preservation and immunosuppression have improved patient outcomes. Post-operative complications, however, can limit this success. Ultrasound and Doppler study is the primary imaging modality for evaluation of renal transplant, providing real –time information about complication in graft. A multimodality approach including CT scan, MRI or conventional angiography may be necessary in cases when sonography and Doppler are inconclusive to diagnose the etiologies of these complications. Radiologists play an integral role within the multidisciplinary team in care of transplant patient at every stage of the transplant process. Therefore, the radiologist should always be aware when evaluating the failing renal graft, whether the cause is renal or extrinsic. In this pictorial essay we tried to gather the most common complication of transplant kidney in different cases that occurred in our hospital, with an emphasis on Ultrasound and Doppler study.
Keywords: USG; Colour Doppler; Post renal transplantation; Complications
Introduction
The preferred modality for renal replacement is renal transplantation, and its superiority in prolonging the longevity of patients with end-stage renal disease is well established [1]. Kidney transplantation is typically classified as deceased-donor (formerly known as cadaveric) or living-donor transplantation depending on the source of the donor organ. Due to improvement in transplantation technology, advancement in immunosuppressant and graft preservation techniques the 1-year survival rates for grafts, are reported to be 80% for mismatched cadaveric renal grafts; 90% for nonidentical living related grafts; 95% for human lymphocyte antigen-identical grafts [2]. Radiologists play a major role at every stage of the transplant process with transplantation team. Ultrasonography with colour Doppler is the principal modality used for evaluation of renal allograft. USG is a relatively cheap, noninvasive, and non-nephrotoxic modality. It is applied for diagnostic and monitoring purposes in the post-transplant period. This pictorial essay describes USG and Doppler imaging appearances of the major complications that may occur in renal transplantation. All our images have been obtained from a single our center which is major transplantation center in India. All post renal transplant patients undergo a USG and comprehensive Doppler evaluation on post-operative day one. The sonographic examination algorithm includes gray-scale evaluation of the graft and spectral Doppler. Further imaging is based on clinical follow-up including daily monitoring of laboratory values. If clinical parameters are abnormal, repeat sonography is performed and depending on the results, CT, MRI, or angiography may be requested.
Surgical Technique
Surgical technique and location of placement of renal allograft depends on the variation in arterial and venous anatomy of donor. The transplanted kidney is usually placed extraperitoneally in the patient’s right iliac fossa (less commonly in left iliac fossa), with end-to-side or end to end anastomosis to the external iliac vasculature. The currently preferred method for restoring urinary drainage is ureteroneocystostomy, a procedure by which the ureter is implanted directly into the dome of the bladder (Figure 1).
Urologic Complications
The prevalence of urologic complications varied from 10% to 25% with a mortality rate ranging from 20% to 30%. Incidence rate is decreased range between 3% and 9% in the current era because of advancement in surgical techniques and frequent use of ureteral stents [3,4].
Urinary Obstruction
a) Incidence: 2%-5% of kidney transplant recipients.
b) Site of obstruction: Approx. 90% of stenoses occur in the distal third of the ureter due to its poor vascular supply.
c) Imaging appearance: US can easily confirm the diagnosis of hydronephrosis and dilated renal pelvis and thus determine the level of ureteral obstruction (Figure 2). When contents of pelvic calyceal system are echogenic and weakly shadowing, fungus balls should be considered, whereas low-level echoes suggest pyonephrosis (Figure 3).
Urine Leaks and Urinomas
a) Incidence: up to 6 % of renal transplant recipients [5]
b) Location: extraperitoneal or intraperitoneal, if intraperitoneal may present with ascites.
c) Imaging appearance: USG findings are nonspecific, well defined anechoic fluid collection with septa or without septation, adjacent to the lower pole of the transplant in most of the cases (Figure 4).
Drainage of fluid under ultrasound guidance and testing the fluid for creatinine helps to differentiate it from seromas or lymphoceles. High concentration of creatinine will be found in case of urinoma if we compare with serous fluid.
Calculous Disease
a) Incidence: 1% to 2 % of post-transplant cases develops clinically relevant stones as compared to general population [6]. As the kidney is denervated patient will not suffer typical renal colic.
b) Imaging appearance: Calculus appears as strongly reactive focus of variable size producing acoustic shadowing on USG and twinkling artifact on colour Doppler (Figure 5). Other rare urologic complications are ureteric necrosis and vesico-ureteric reflux.
Peritransplant Fluid Collections
Fluid collection in peritransplant region has been reported in up to 50 % of renal transplantation. They are urinomas, hematomas, lymphocele and abscess, the clinical relevance of these collection is largely determined by their size, location and possible growth. In immediate postoperative period, small hematomas or seroma are almost expected. Their size should be documented at baseline examination. Rarely do they lead to graft dysfunction or obstruction of collecting system. Urinomas and hematomas are most likely to develop immediately after transplantation, whereas lymphoceles generally develop after 4 to 8 weeks. The ultrasonography characteristics of peritransplant fluid collections, however, are entirely nonspecific, correlation with clinical findings helps to restrict differential diagnosis. Ultimately, diagnosis may be made only with percutaneous aspiration and then biochemical analysis. Differentiation between Peritransplant and subcapsular collection is important. A subcapsular collection likely to cause mass effect on parenchyma, usually cresenteric and show extension along the contour of kidney deep to the renal capsule
Hematoma
a. Incidence: Varies from 4 to 8 % [7]
b. Imaging appearance: Hematomas have a complex appearance. Hematomas appears echogenic in acute case and progressively become less echogenic with time (Figure 6). Chronic hematomas even appear anechoic, more closely resembling fluid and septation may develop later on.
Lymphocele
a. Incidence: Affecting up to 20% of the patients [8] It occurs after surgery owing to the surgical disruption of the normal lymphatic channels along the iliac vessels or around the hilum of the graft.
b. Imaging appearance: on Ultrasound it appears as anechoic bur may contain septation. They may become infected and gave more complex appearance (Figure 7).
Peritransplant abscesses
a) Imaging appearance: USG cannot always differentiate an abscess from other collection. Collection may show low level echoes and thick irregular wall. If gas is seen, an abscess is probable. In any pyrexia patient, any perinephric collection should be considered infected until proven otherwise through the appropriate imaging and guided diagnostic aspiration.
Parenchymal Complication / Graft Dysfunction
Diseases of the renal parenchyma are usually diffuse and often leads graft dysfunction. Differential diagnosis is difficult by imaging alone. USG is not sensitive or specific for evaluation; differential will be relying on biopsy. USG still has a central role in qualitative assessment of graft perfusion and to guide the biopsy.
Acute tubular necrosis (ATN)
Acute tubular necrosis is due to reversible ischemic damage to the renal tubular cells prior to engrafting and reperfusion injury.
i. Incidence: affects 20–60 % of cadaveric renal grafts.
ii. Time of onset: in the first 48 hours after transplantation.
iii. Imaging appearance: No specific imaging pattern for the diagnosis of ATN. The kidney may appear normal, in severe cases it looks enlarged, edematous and echo poor with loss of corticomedullary differentiation (Figure 8) and shows elevated RI (above 0.8).
Rejection
Rejection can be classified according to the period of appearance as hyper acute (occurring within minutes), acute (occurring within days to weeks), late acute (occurring after 3 months), or chronic (occurring months to years after transplantation). Classification of renal allograft rejection by Banff classification of allograft pathology is routinely followed nowadays. It is based on a combination of histopathologic features coupled with molecular, serologic, and clinical parameters.
Acute Rejection (AR)
i. Incidence: up to 40% of patients in the early transplant period [9].
ii. Imaging appearance: Graft enlargement due to edema, Decreased cortical echogenicity, swelling of the medullary pyramids, echogenic sinus fat, edematous wall of pelvic calyceal system, focal hypoechoic areas in parenchyma which may favors infarct and collection in perigraft region due to necrosis or hemorrhage. PI and RI elevated in both ATN as well as in AR, but AR has high values of it. In severe cases, Power Doppler shows reduced, absent or reversed diastolic flow with elevation of the RI (Figure 9).
Chronic Rejection
Chronic rejection occurs in case of insufficient immunosuppression given to recipient to control the residual antigraft lymphocytes and antibodies.
i. Imaging appearance: US appearance is not typical, ranging from normal to hyper echogenic, along with cortical thinning, reduced number of intrarenal vessels, and mild hydronephrosis (Figure 10). RI measurements are not reliable for this diagnosis. The diagnosis is made histologically.
Drug Nephrotoxicity
Calcineurin inhibitors are key immunosuppression agents administered to avoid acute rejection, but they are nephrotoxic.
A. Imaging appearance: USG may show completely normal results or nonspecific findings such as graft swelling, increased or decreased renal echogenicity and loss of cortico-medullary differentiation. Doppler study may show a RI increase of 0.80. Findings of USG and Doppler study should be correlated with the serum drug levels. USG and Doppler findings of ATN and AR is almost similar, but both can be differentiated by time course of the findings. Clinical & biochemical correlation and serial measurements of RI and Pulsatile index (PI) would be further helpful to monitor the patient.
Infections and abscesses
Incidence and time of onset: More than 80% of renal transplant recipients have at least 1 episode of infection during the first year of post transplantation.
I. Imaging appearance: USG appearance is quite variable. Focal pyelonephritis appear as a focal hyperechoic or hypoechoic area, but this finding is nonspecific because it can represent infarction or rejection also (Figure 11). Abscess has varied appearance on USG like- heterogeneous, hypoechoic or cystic. Urothelial thickening may be seen. In febrile post renal transplant patient low level echoes in dilated collecting system may favors pyonephrosis. Fungus ball appears as focal rounded, weakly shadowing or echogenic structure in dilated pelvic calyceal system. In emphysematous pyelonephritis, gas in the parenchyma of the renal graft produces an echogenic line with distal reverberation artifacts. Papillary necrosis has no typical sonographic findings, and it subsequently leads to ureteric obstruction.
End stage disease
Nonfunctional renal grafts are left in place in abdominal cavity. Gradually graft becomes small and can have fatty replacement, hydronephrosis, infarcts, hemorrhage or calcification.
Vascular Complications
Vascular complications in post renal transplantation have significant negative influence of graft survival. They are infrequent, occur in approximately 1%–2%; [10] but can cause sudden loss of renal allograft. Selective catheter angiography is the gold standard for diagnosis; however, it is invasive and may cause various complications. Hence it is not used as a screening tool but reserved for patients with inconclusive results on the noninvasive screening tests. Noninvasive imaging like ultrasound, Doppler, scintigraphy, CT and MR angiography plays major role to evaluate them.
Renal artery thrombosis (RAT)
Incidence: Ranges from 0.5% to 3.5 % [11]
Imaging appearance: No evidence of any arterial or venous flow noted on color, spectral and power Doppler study (Figure 12). Doppler sonography had 100% sensitivity and specificity for diagnosis and hardly any other imaging study is required for diagnosis [12].
Focal Renal Infarction
Imaging appearance: A segmental infarct appears as a poorly marginated wedge shaped hypoechoic mass or a hypoechoic mass with a well-defined echogenic wall without colour flow (Figure 13). If the infarction is global, the kidney will appear hypoechoic and be diffusely enlarged.
Renal Artery Stenosis (RAS)
Incidence: It has wide range varying from 1% to 23 % depending on the definition and diagnostic techniques used.
Site: usually at anastomotic site
Imaging appearance: On gray scale USG, there is lack of normal post-transplant hypertrophy. On color Doppler study appearance of focal color aliasing noted at stenotic segments. On spectral Doppler study, peak systolic velocity in main renal artery >300 cm/sec and Ratio of PSV in transplanted main renal artery and external iliac artery greater than or equal to 1.8 are highly suggestive of significant stenosis. Indirect criteria are low resistive index <0.56, Acceleration time >0.07 sec, Acceleration index <3 meter/ sec and Intrarenal tardus–parvus waveform (Figure 14).
Limitation: Results are strongly depends on the operator’s individual experience and skill.
Rate of restenosis is less than 10 %. Doppler ultrasonography is the procedure of choice to evaluate graft perfusion before and after revascularization The term pseudo transplant renal artery stenosis (TRAS) refers to thrombosis or stenosis of iliac artery or aorta proximal to transplant renal artery.
Renal vein thrombosis: (RVT)
Incidence: Ranges from 0.9% to 4.5 % [13]
Imaging Findings: Graft appears swollen and hypoechoic.
Doppler shows absent venous flow. Renal arterial Doppler spectrum shows absent or reversal of diastolic flow due to increased resistance (Figure 15). Reversal flow in renal artery is nonspecific as it also seen in severe rejection and in acute tubular necrosis, its combination with absent venous flow is the diagnosis of renal vein thrombosis. Partial thrombosis also can occur near anastomosis or within the transplanted kidney (Figure 16).
Extra parenchymal pseudo aneurysm
Incidence: Anastomotic pseudoaneurysm is a rare complication of renal transplantation occurring in 0.3%. [14]
Imaging findings : On gray scale ultrasound it appears as cystic lesion which shows color flow and to and fro spectral pattern on doppler study(Figure 17). Endovascular treatment with covered stent placement to exclude pseudoaneurysm can also be evaluated with USG and colour doppler (Figure 18).
Intra-parenchymal arteriovenous fistula and pseudoaneurysm
AVF occurs when both artery and vein are simultaneously lacerated, while pseudoaneurysm results when only artery is lacerated.
Incidence: 1-18% of the biopsies [15]
Time of onset: occur at time of biopsy. They depend on many factors – ultrasound guidance, needle caliber and imaging follow up.
Imaging appearance: colour Doppler study shows AVF as focal areas of disorganized flow adjoining the normal vasculature. Spectral waveforms show increased arterial and venous flow with high velocity and low resistance (Figure 19). Pseudoaneurysm appears as simple or complex cyst on B mode ultrasound and intracystic flow on colour Doppler mode (Figure 20).
Neoplasms after renal transplantation
Post renal transplantation patients are at higher risk of development of neoplasms. Urologic tumour are 4 to 5 times more common in post renal transplantation recipients than normal population with significant exposure to cyclophosphamide immunosuppressant agent.
Renal cell carcinoma
Etiology: by means of transplanted organ or de novo development by immunocompromised status, patients on hemodialysis in case of chronic renal failure develop acquire renal cystic disease
Prevalence: 90 % occurring in native kidney and 10 % in transplanted kidney [16]
Imaging appearance: lesion appear heterogeneous with vascularity, similar picture as seen in native kidney [17] (Figure 21).
Lymphomas
Incidence: 1 % of renal allograft recipients [18]
Time of onset: Post transplantation lymphoproliferative disorder is diagnosed at a median of 80 months after transplantation.
Imaging appearance: Lymphadenopathy at various sites but can also affect any solid organ or hollow viscera or transplant graft parenchyma itself. It appears as low or mixed reactive masses and tends to have a predilection for the renal hilum.
Recurrent Native disease
It depends on the primary disease before transplantation.
Imaging appearance: Imaging has no specific pattern in these situations apart from excluding the treatable cause of reduced renal function.
Abdominopelvic Complications
Renal graft is placed in extraperitoneal space via a peritoneal window in laparoscopic and robot assisted surgical techniques. So these cases are prone to same complications experienced by other surgical cases in whom peritoneum is exposed.
Renal Allograft Compartment Syndrome (RACS)
It is a rare syndrome, and it is under recognized cause of early transplant dysfunction or even loss. It may occur as a result of intracompartment hypertension and ensuring ischemia of renal graft [19]. Imaging appearance: absent or diffuse diminished cortical flow in transplant kidney at colour Doppler.
Fascial dehiscence and bowel or allograft evisceration
They tend to occur in perioperative period. Herniation of bowel through a transplant peritoneal defect may lead to compromise of intestine or transplant itself.
Limitations
The USG examination is examiner dependent and limited accessibility in obese patents impairs the evaluation and often leads misinterpretation. The RI index is also unspecific and influenced by many factors like site at which the RI is measures, increased intraabdominal pressure during forced inspiration and the pulse rate.
Summary
Kidney transplant is the treatment of choice for patients with end-stage renal disease. Improvements in surgical techniques and advanced immunosuppressive drugs have resulted in remarkable survival of patients and renal grafts. Still complications occur in both the early postoperative period and later. Kidney transplants follow up is common in radiology and sonography practice.
Ultrasonography and Doppler examination can accurately depict and characterize many of the potential complications of renal transplantation. It facilitates prompt and accurate diagnosis and thus guiding treatment.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers#open access journals#urology#nephrology#submission#journal of urology & nephrology studies#juns#lupine journals#nephritis#articles
0 notes
Text
Lupine Publishers | Evaluation of Buccal Mucosal Graft Urethroplasty for The Treatment of Female Urethral Strictures- A Single Centre Experience

Abstract
Introduction: Female urethral stricture is a highly under-reported and underdiagnosed condition encountered by the reconstructive urologist. Urethral dilatation is often performed with urethroplasty offered in select cases. In the present study, we describe our results in a series of women surgically treated for female urethral stricture disease using a suprameatal approach with a buccal mucosal graft dorsal on lay technique. Materials and Methods: All females diagnosed of urethral stricture who underwent buccal mucosal graft urethroplasty from January 2015 to January 2020 were evaluated retrospectively. Intraoperative and postoperative parameters were assessed. Results: A total of 14 female patients underwent buccal mucosal urethroplasty were evaluated. The mean age of the patients was 49.5 years ranging from 35 to 64 years. Mean preoperative maximum flow rate [Qmax] on uroflometry was 6.5 ml/second and the mean residual urine 156 ml. All patients underwent uneventful buccal mucosal graft dorsal on lay technique. At 3 months follow up, the mean Qmax was 23.2 ml/second with mean residual urine of 14 ml. A Self-reporting satisfaction scores using the Patient Global Impression of Improvement showed that seven patients scored 1 (very much better), four scored 2 (much better), two patients scored 3 (a little better), and one scored 4 (no change) none of the patients scored a 5 (worse).No recurrence was noted. Conclusion: Buccal mucosal graft urethroplasty is a feasible surgery for female urethral strictures with minimal short term complications
Keywords: Buccal Mucosal Graft Urethroplasty; Female Urethral Strictures
Introduction
Female urethral stricture is a highly under-reported and underdiagnosed condition encountered by the reconstructive urologist. The aetiology of urethral stricture is still unclear. Symptoms range from clinically insignificant to severe and debilitating voiding symptoms. Urethral dilatation has been overused for primary and chronic treatment. Urethroplasty with various grafts and flaps have been used with good results in recent times. In this present study, we describe our results in a series of women surgically treated for female urethral stricture disease using a suprameatal approach with a buccal mucosal graft dorsal on lay technique.
Patients and Methods
A total of 14 female patients with urethral stricture who underwent buccal mucosal graft urethroplasty from January 2015 to January 2020 were evaluated retrospectively. Patients were evaluated with a detailed history, physical examination including focused neurological evaluation, uroflometry, a micturating cystourethrogram and an ultrasound of abdomen and pelvis. Evaluation also included a gentle calibration of urethra with a 14 French catheter to assess the site of stricture. All patients had a history of poor stream of urine, 10 (71%) had a sense of incomplete voiding, 2 (14%) presented with recurrent urinary tract infection and 2 (14%) patients had terminal dribbling. Aetiology of the stricture was idiopathic in 9 (64%) of the patients, instrumentation in 3 (22%) and catheterization in 2 (14%). More than half, that is 8 (57%) of the patients had history of repeated urethral dilatation.
Surgical technique
Routine preoperative antibiotic prophylaxis was given and under general anaesthesia with nasal intubation, the surgery was performed in lithotomy position. An initial Urethroscopy was performed with a 6 French ureteroscope to assess the site of stricture, the proximity of the stricture to the bladder neck and any abnormalities in the bladder and trigone. A diluted mixture of 2% xylocaine and adrenaline was injected submucosally into the periurethral tissue using a 26 gauge needle for hydro dissection and haemostasis. An inverted ‘U’ shaped incision (Figure 1A) was given over the urethra exposing the dorsal part of the urethra. Sharp dissection was then done in order to separate the vulvar mucosa from the urethra (Figure 1B). Utmost care was taken to prevent damage to the clitoral cavernous tissue and the anterior portion of the striate sphincter. A full thickness urethrotomy was made using tenotomy scissors at 12 ‘O’ clock position over a guide wire and then extended proximally till normal healthy mucosa could be visualised. The urethra was then calibrated with 18 French catheter to rule out proximal stricture.
The buccal mucosal graft was then harvested. The Stenson’s duct was marked opposite the upper second molar tooth. Methylene blue was used to mark the graft area to be harvested. A diluted mixture of 2% xylocaine and adrenaline was injected submucosally using a 26 gauge needle for hydrodissection and hemostasis. The buccal mucosal graft was harvested based on the length of the stricture. Haemostasis was achieved with bipolar cautery and the raw area was packed with adrenaline soaked gauze. Buccal mucosa was allowed to heal by secondary intention. Defatting of the graft was performed and the graft was placed in a container with gentamycin and saline. An 18 French catheter was then introduced into the urethra and bulb was inflated. The buccal graft was then sutured to the apex at 11, 12 and 1 ‘0’ clock position (Figure 1C). The lateral margins of the urethra and buccal mucosa were sutured in a dorsal on lay fashion with 4-0 vicryl suture (Figure 1D). This augmented urethra was then quilted to the clitoral body to cover the new urethral roof (Figure 1E). Vulvar mucosa was then approximated with 4-0 vicryl suture. Patients were discharged with catheter after 2 days and were called for catheter removal after 3 weeks (Figures 2A-2D). Patients were followed up after 3 months with assessment of voiding symptoms, examination (Figure 3), uroflometry and a micturating cystourethrogram. The criteria of successful reconstruction were defined as postoperative maximum flow rate (Qmax) greater than 15ml/sec with minimal post void residue (<10% of pre-void). A Self-reporting satisfaction scores using the Patient Global Impression of Improvement was used for assessment of urinary symptoms at 3 months follow up. At subsequent follow ups, the patients were assessed with voiding symptoms and an uroflometry.
Results
A total of 14 female patients underwent buccal mucosal urethroplasty from January 2015 to January 2020. The mean age of the patients was 49.5 years ranging from 35 to 64 years. Mean preoperative maximum flow rate [Qmax] on uroflometry was 6.5ml/ second ranging from 4 to 7.2ml/second (Table 1). The mean residual urine was 156 ml. In all the patients, calibration with 14 Fr catheter was not possible preoperatively. The mean operative time was 96 minutes ranging from 84 minutes to 116 minutes. Mean stricture length was 1.4 centimetres ranging from 1 to 2.2 centimetres. The mean length of the harvested graft was 2.5 cms ranging from 2.0 to 3.5 cms. Our mean follows up period was 22 months ranging from 6 months to 45 months. None of the patients developed any evidence of graft necrosis post operatively. No wound infection was noted in our series. No donor site complication was noted in our series. Mean hospital stay was 2.5 days ranging from 2-4 days. At 3 months follow up, the mean Qmax was 23.2 ml/second with mean residual urine of 14ml. A Self-reporting satisfaction scores using the Patient Global Impression of Improvement was used which showed that that seven patients scored 1 (very much better), four scored 2 (much better), two patients scored 3 (a little better), and one scored 4 (no change) none of the patients scored a 5 (worse) (Table 2). None of the patients developed recurrence, incontinence, or sexual dysfunction during the course of our follow up.
Discussion
Female urethral stricture has been described for almost 200 years but is still a widely underdiagnosed condition [1]. Brannen described the history of female urethral stricture and said that it was first described by Liz Frank in 1824 and the first case of female urethral stricture was reported to the earl of London in 1828 [2]. The exact incidence of this entity remains unknown with very few case reports and retrospective case series reported in contemporary literature till date [3]. The aetiology for female urethral stricture has been attributed to idiopathic (49%), chronic irritation, Prior dilatation, catheterization, instrumentation (7%) and trauma (6%) [4-7]. Trauma may be in the form of obstetric injuries, blunt pelvic trauma, or even repeated vigorous coitus [2]. In our study, idiopathic stricture was the most common cause accounting for 64% of the cases. Patients usually present with long standing complaints of voiding and storage symptoms, recurrent urinary tract infections and sometimes upper urinary tract changes. Irritative voiding symptoms may be the presenting complaint in 35% of the patients [8]. Evaluation of the patients include a detailed history including history of stress and urge incontinence, local examination of the meatus, uroflometry and measurement of residual urine. Examination should also include gentle calibration with a 14 French catheter [9]. Radiological evaluation of the stricture with a micturating cystourethrogram helps visualise the stricture. Options for management of these strictures include urethral dilatation, urethroplasty with grafts (oral/vaginal) or flaps (vaginal/labial) [4]. Urethral dilatation alone has a dismal success rate of 47% at a mean follow up of 43 months [4]. The mean time to recurrence of stricture has been reported to be around 12 months [10]. Dilatation combined with daily intermittent self-catheterization is said to have a success rate of 57% [10,11]. Like in males, it appears that, if a single dilatation fails, then it is probable that further dilatations are likely to be palliative rather than curative [4]. Urethral reconstruction in women is different from that of men because of the shorter length of the urethra and that the female urethra is sphincter active. Hence any urethral surgery in the female carries a substantial risk of incontinence. Anastamotic urethroplasty has not been described till date. The various approaches to urethral reconstruction are ventral approach or a dorsal approach. We preferred the dorsal approach because of the good mechanical and vascular support provided by the clitoral and cavernosal tissue, it prevents the downward angulation of the urethral meatus, which may have a subsequent impact on the direction of the urinary stream and further, it spares the ventral aspect of urethra for further anti-incontinence surgery. The disadvantages being increased chances injury to the sphincter and neurovascular bundle which may lead to in continence or sexual dysfunction. A total of 15 studies with 115 patients described urethroplasty for female urethral strictures, with 6 studies describing flaps and 10 studies describing the use of free grafts for urethral augmentation [4]. A total of 6 studies have described the use of oral grafts for female urethral strictures [12-17]. The individual success rate with the use of oral mucosal graft was 94% with a mean follow up of 15 months [4]. There were no reported incidences of de novo incontinence. Oral mucosa is particularly helpful when vaginal atrophy and fibrosis precludes the use of a local flap. In our series, there were no cases with recurrence and none of the patients complained of urinary incontinence post op. With the use of vaginal or labial grafts, the success rate was reported to be around 80% at a mean follow up of 22 months [4]. Successful outcome with the use of vaginal flaps is reported to be around 91% at 32 month follow up. The main drawbacks of this study are the retrospective nature of study, lack of control group and short term follow up. A randomized study with a control group and long term follow up is needed to further validate this procedure.
Conclusion
Buccal mucosal graft urethroplasty is a feasible surgery for female urethral strictures with minimal short term complications.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine journals#urology#nephrology#juns#journal of urology & nephrology studies#open access journals#articles#submission#research#review#casereports
0 notes
Text
Lupine Publishers | Safety and Efficacy Assessment of Pcnl in The Pediatric Population: A Single Centre Experience

Abstract
Introduction and Objective: Renal stone disease in children is on the rise with increased incidence and better modalities to diagnose the disease. Hence there is a necessity to strategize the evaluation and treatment of children with kidney stones. Our study was conducted to assess stone distribution, stone burden, and efficacy of PCNL in pediatric age group. Methods: All paediatric patients with renal stone disease who subsequently underwent PCNL at our department from January 2017 to December 2020 were analysed. Results: 84 patients ranging 1-18 years were analysed. Pain abdomen was the most common presenting symptom (61.9%) followed by fever (19.04%). The mean stone size was 2.16cm with equal side distribution. Most stones were located in the lower calyx (38%). The mean operative time was 65 minutes. Exposure to radiation from C arm ranged from 1.6-8.3 minutes. Complete stone clearance was achieved in 90.47% with a mean post- drop in Hb value to 0.72 gm/dl. Mean duration of nephrostomy tube in situ was 2.4 days and with a mean hospital stay of 3.8 days. Calcium oxalate was the most common type of stone (48%) Conclusion: PCNL is safe and effective treatment for pediatric renal calculi with minimal morbidity and increased stone free rates irrespective of stone size. Proper patient selection, surgical skill and postoperative care contribute towards the success of the procedure
Keywords: Pediatric PCNL; Pediatric Renal Calculi; Renal Stone Disease
Introduction
The prevalence of renal stone disease in children ranges from 5 to 15%. Stone disease has a higher risk of recurrence in the pediatric age group making it crucial to identify the most effective method for complete stone removal to prevent recurrence from residual fragments. The optimal management of for pediatric stone disease is still evolving [1,2]. Currently, ESWL is the procedure of choice for treating most upper urinary tract calculi in the pediatric population. However, a higher incidence of metabolic and anatomical abnormalities in pediatric patients has led to increased recurrence. Moreover, ESWL has relatively less efficacy for stones >1.5cm. Surgical intervention should be preferred in such cases so as to minimize the need for retreatment [3-5]. Percutaneous nephrolithotomy (PCNL) is less invasive than open surgery which can be a good candidate for complex and large burden stones [6]. Several studies over time with different power and limitations have reported safety and efficacy of PCNL leading to its consideration as the treatment of choice for children with stone larger than 15mm [7-9]. The advent of newer, finer instruments and increase in experience of endourological techniques such as tubeless PCNL, mini-perc, ultra-mini perc and micro-perc has resulted in reducing the morbidity rate among patients without affecting the outcomes in terms of clearance [10-14]. Therefore, our study was conducted to assess the safety and efficacy of PCNL in the pediatric age group in terms of (1) renal stone distribution & stone burden (2) the outcomes of PCNL including stone clearance, operative time, hospital stay, haemoglobin changes and (3) the associated complications of PCNL.
Materials and Methods
A prospective study was conducted at our hospital from January 2017 to December 2020 after obtaining institutional ethical committee clearance. All pediatric patients posted for PCNL at MS Ramaiah Medical College were considered in the study. The patients compatible for the study were interviewed and after obtaining informed and written consent they were enrolled in the study. Inclusion Criteria: All the patients below the age of 18 years undergoing PCNL. Exclusion Criteria: Anatomic abnormalities of the kidney (horseshoe kidney/malrotated kidney); Bleeding disorders; deranged renal function. Patients were initially evaluated with a detailed medical history and a thorough clinical examination followed by a battery of investigations including CBC, RFT, Serum electrolytes, serum levels of calcium, phosphorus, alkaline phosphatase, uric acid, total protein, carbonate, albumin, parathyroid hormone (if there is hypercalcaemia), blood group & Rh typing, HbsAg, HIV, HCV, Urine: Routine & Microscopy, Urine: Culture & Sensitivity. For imaging –ultrasonography was used as a first study followed by spiral CT KUB if no stone was found. Intravenous pyelography was performed when a need arose to delineate the calyceal anatomy prior to percutaneous or open surgery. A sterile urine culture was confirmed before surgery. In patients with evidence of infection, antibiotics were given preoperatively to clear the infection prior to surgery. All patients received broad-spectrum antibiotics beginning 12 h prior to the procedure and these were continued until 5 days postoperatively. All PCNL are performed under general anaesthesia. The patient initially placed in lithotomy position and a ureteric access catheter was placed under fluoroscopic guidance. The patient was then turned prone. After initial puncture, the tract was dilated using metallic or Teflon dilators. Paediatric PCNL was performed using adult instruments and clearance assessed intraopeartively by fluoroscopy. Ureteric stents and nephrostomy tubes were placed in most patients at the end of the procedure. Baseline patient characteristics, intraoperative and post-operative data were collected and analysed. Perioperative complications were classified using the modified Clavien Dindo system. In case of a supra-costal puncture, a chest X-ray was obtained subsequently in the post op period. An x ray of kidney ureter bladder was taken at 48 hours after PCNL. If needed a re-look procedure was done. The patient was followed up with an ultrasound & serum creatinine at 3 months, DMSA scan after 6 months to know the functional status of kidney & amount of renal scarring. Statistical analysis was performed using SPSS 22. Categorical variables were presented as number and percentage (%), whereas continuous variables were presented as mean ± standard deviation and median (Table 1).
Results
84 patients ranging between 1-18 years of age were analysed with the mean age of study population of 11.04 years. Pain abdomen was the most common presenting symptom (61.9%) followed by fever (19.04%) with 4/84 having had prior surgical intervention for stone disease (Figure 1). The mean stone size was 2.16cm with equal side distribution. Most stones were located in the lower calyx (38%) followed by renal pelvis – 33%, middle calyx 17% and upper calyx 12% (Figure 2a). The total operative time ranged from 30 minutes to 120 minutes with a mean of 65 minutes. Exposure to radiation from C arm ranged from 1.6-8.3 minutes. Intraoperative location of stone, puncture and after clearance are shown in Figures 1a,1b,1c. Complete stone clearance was achieved in 90.47% with a mean post- drop in Hb value to 0.72 gm/dl. Mean duration of nephrostomy tube in situ was 2.4 days and with a mean hospital stay of 3.8 days. Intra-operative and post-operative complications in the study population are depicted in Table 2. Calcium oxalate was the most common type of stone (48%) (Figure 3).
Discussion
Although short wave lithotripsy (SWL) is considered the treatment of choice for symptomatic upper urinary tract calculi in children, but not a preferred option in patients with large stone burden, owing to higher rates of failure and residual stones. In such cases, PCNL with proven advantages can be safely advocated as a suitable treatment option in order to avoid numerous SWL sessions under anesthesia. Despite pediatric PCNL being described as early as 1985 by Woodside et al. [6], pediatric surgeons had their reservation in performing PCNL in children. This apprehension was due to the fear of parenchymal damage, early exposure to radiation and risk of major complications associated with the surgery. However, Dawaba et al. [9] proved the fear to be baseless by demonstrating that PCNL in paediatric population improved overall renal function without causing renal scarring. Similarly, Mor et al. reported no significant scarring or loss of renal function in radionuclide renal scans (19). He concluded that adult type tract dilation to 24Fr to 26Fr was not associated with significant renal function loss [19]. The size, number and site of tracts are not well defined in pediatric PCNL. While Gunes et al. reported a higher complication rate in children <7 years operated with adult sized instruments [17], Desai et al. observed that intraoperative bleeding during PCNL in children is related to the size and number of tracts and suggested the need for technical modifications in children [20]. Although this calls for reduction in tract size, it may have an effect on the clearance rates. In our study, 54(64.28%) underwent standard PCNL vs 30(35.71%) underwent Miniperc. We used amplatz sheath sizes in the range of 16F-28F. Size of tract dilatation was based on dilatation of pelvicalyceal system, the stone burden and no of punctures. Our clearance rates & transfusion rates were found to be similar in miniperc & standard PCNL. Our results are in concurrence with Bilen et al. who reported that smaller tracts did not significantly affect stone-free rates but achieved lower transfusion rates [21]. They concluded that a 20Fr tract was as effective as working with adult sized devices and did not significantly increase the operative time. (18) Provided the quality of the puncture and subsequent tract is high, there is no greater morbidity than that reported from miniperc. Large tracts and instruments can facilitate more rapid and complete stone clearance (Table 3).
Most of the stone burden was located in lower calyx (38%) in most of our cases with staghorn calculus noted in 4 patients. Single tract access was done in 72 patients with lower calyceal puncture being used mostly (43%) (Figure 2b). Multiple punctures were required in 12 cases (14.2%). We did not find any significant increase in complications following an upper calyceal puncture or with multiple punctures in our study which is comparable to Sedat Oner et al. who concluded that an upper pole approach did not prolong operative time or add to the complications, making it a good alternative. A surgeon who has reached competence at performing PCNL should therefore not hesitate to use a superior caliceal approach in pediatric patients if deemed appropriate for stone removal [22]. Our length of hospital stay duration of nephrostomy tube in situ is comparable to previously published data. 42.85% of our cases were tubeless, which is safe when performing uncomplicated PCNL [34]. Prior renal surgery on the same side didn’t have any impact on outcome of PCNL [35]. Aldaqadossi et al. have suggested that a previous open pyelolithotomy or nephrolithotomy does not affect the efficacy and morbidity of subsequent PCNL in pediatric patients [35]. We achieved a complete clearance rate of 90.47% which is similar to the published literature. Residual calculi noted in 8 cases were managed by ESWL. The complication rate during and after PCNL in paediatric patients varies widely in the literature. The difference in complication rates may be explained by the difference in stone burden location and experience of the surgical team. Our complication rate was 9.52% with fever being the most common. The lower incidence in complications could be attributed to the surgeon expertise at our center.
Limitations
Our study population was from single referral center, which may not be generalizable considering small sample size. Another limitation is the lack of comparative groups such as ESWL/RIRS while evaluating the efficacy of PCNL.
Conclusion
PCNL is safe and effective treatment for pediatric renal calculi with minimal morbidity and increased stone free rates irrespective of stone size. Proper patient selection, surgical skill and postoperative care contribute towards the success of the procedure and reduces the complications.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#journal of urology & nephrology studies#submission#open access journals#nephritis#lupine journals#lupine publishers#urology#articles#nephrology
0 notes
Text
Lupine Publishers | Reasons and Outcome of Patients after Permanent Transfer from Peritoneal Dialysis to Hemodialysis: A Review of 16 years of Experience in Senegal

Abstract
Introduction: Peritoneal dialysis (PD) and haemodialysis (HD) are two complementary and non-competitive renal replacement therapy (RRT). A patient can be transferred from one technique to the other. The objective of this study was to assess the reasons for transferring patients from PD to HD and to follow their outcome. Patients and Methods: This is a 16-year descriptive and analytical retrospective study (March 1, 2004 - August 31, 2020) conducted at the PD unit of the Aristide Le Dantec University Hospital in Dakar. Were included, patients on PD for at least 30 days, over 18 years of age and permanently transferred to HD. The probability of survival for any duration of post-transfer follow-up was estimated by the Kaplan-Meier method. Results: The analysis covered 98 out of 113 cases. The mean age of the patients was 45.2 ± 14.09 years at the initiation of PD and 47 ± 13.91 years at the time of transfer, with a sex ratio of 0.66. The mean duration in PD was 19.9 ± 17.25 months [range, 1.0-90.0 months]. The transfer to HD concerned 73.5% of patients in the first two years. The reasons for transfer were mainly associated with infection (82.7%), mechanical complications (23.5%), social reasons (12.2%) and inadequate dialysis (6.1%). It was programmed in 11.4% of cases and 6% of patients had a permanent approach. At the endpoint date, the mean duration in hemodialysis was 43.3 months with 42.8% of patients still in HD. There was a kidney transplant patient; a return to PD. Mortality was 34.6%. The mean HD survival was 126 months. There was a statistically significant relationship between infection as a reason for transfer and mortality (p = 0.047). Conclusion: The main reasons for transferring PD to hemodialysis identified in the literature are found in our context. This transfer must be anticipated to reduce morbidity and mortality.
Keywords: Peritoneal dialysis; Transfer of Patients; Peritonitis
Introduction
Peritoneal dialysis (PD) and haemodialysis (HD) are two complementary modalities of renal replacement therapy. There is an increasing use of PD in the world, with a prevalence multiplied by 2.5 between 1997 and 2008 [1]. Indeed, this technique has shown survival rates similar to HD [2] or even better [3]. The main limitations of PD are related to the failure of the technique, forcing patients to switch to HD [4]. In the 2010 ANZDATA (Australia and New Zealand Dialysis and Transplant) report, 20% of PD patients were definitively transferred to HD [5]. Similar rates have been reported in other countries [6]. According to the REIN registry, each year 10% of patients in PD are transferred to HD, mainly during the first two years of treatment [7]. The most common causes of technique failure are peritoneal infection, inadequate dialysis, catheter dysfunction or patient choice [8-10]. In addition, the risk of transfer to HD is relatively high during the first 6 months of the onset of PD [8,9]. Several risk factors for PD technique failure have been identified. They include advanced age, high permeability of the peritoneal membrane, reduced peritoneal ultrafiltration, malnutrition, diabetes, and increased body mass index [8-11]. In Senegal, there is little or no data available that has examined the reasons for transfer and the outcome of patients transferred from PD to HD. This is why we conducted this work in order to assess the reasons for transferring patients from PD to haemodialysis and to monitor their future on haemodialysis.
Patients and Methods
This was a descriptive, analytical and multicenter retrospective study over a period from March 01, 2004, to August 31, 2020. Were included, all patient in PD for at least 30 days, over 18 years of age and permanently transferred to HD. The data were collected on an operating sheet, from the medical files. Epidemiological data: existence of comorbidities at the start of PD and at the time of transfer to HD (the Charlson comorbidity score [12] was calculated retrospectively at the initiation of PD and at the start of treatment with HD); the stay in PD and entry into HD were analyzed. As of the August 31, 2020, point date, we have also collected the following parameters: a) Haemodialysis parameters: weekly number and duration of HD sessions, mean inter-dialytic weight gain over one-week, average dialysis dose over one week, type of vascular access: b) For the outcome of the patients: a. Duration in HD. b. Status: deceased, kidney transplant returned to PD, still in HD or lost to follow-up. c. If death: date / year, cause. d. If returned in DP: reasons.
Results
Ninety-eight (98) were included in the study. The mean age at initiation of PD was 45.17 ± 14.09 years. At the time of transfer the mean age was 47.00 ± 13.91 years. There was a female predominance with a sex ratio of 0.66. The initial nephropathy was undetermined in 29.6% of cases. Nephroangiosclerosis and chronic glomerulonephritis of undetermined origin accounted for 28.6% and 15.3% respectively (Table 1). Forty-nine (49) patients received hemodialysis before access to PD. At the initiation of PD, high blood pressure was the most common medical history (94.90%), followed by diabetes and a history of cardiovascular disease (8.16%). The mean Charlson’s index was 2.88 ± 1.31. The mean distance traveled to reach the PD unit was 68.62 ± 104.61 km. The mean length of stay in PD was 19.94 ± 17.25 months. Seventy-two (72) patients were transferred during the first two years of PD. The majority of patients had infectious complications (92.85%) (Table 2) and 59.18% of cases had mechanical complications (Table 3). The main reason for transfer was infection (82.7%), followed by mechanical causes (23.5%) and social reasons (12.2%) (Table 4). There was a statistically significant relationship between infection as a reason for transfer and mortality (p = 0.047). Transfer was program in 9 patients (11.4%). The vascular access at the first haemodialysis session was permanent in 6 patients. Twenty-seven (27) patients had a permanent vascular access after transfer. The mean time to have arterio-venous fistula was 6.46 ± 4.60 months. The mean duration on Haemodialysis was 43.29 ± 44.82 months.
Discussion
At the time of HD transfer, the mean age of transferred patients was 47.00 ± 13.91 years in our series, while it was 60.0 ± 14.2 years according to Chen et al. [13], and 68.6 ± 16.8 years according to Habib et al. [14]. Contrary to what is conventionally described in the literature, our transferred patients were younger. This can be explained by the reluctance of elderly patients to change modality, preferring to stay on PD rather than doing multi-weekly HD sessions. We can also assume that these older patients are transferred less frequently, as they die before an HD transfer is necessary. The mean PD duration was 19.94 ± 17.25 months, and the median was 15.50 months [1.0-90.0]. Habib et al. reported a mean duration of 25.9 ± 23.44 months, [14] and Panagoutsos et al. found a mean of 36 ± 16 months [15]. Ferreira et al. and Szeto et al. found a mean PD duration of 40.9 ± 26.3 months and 50.9 ± 41.5 months [16,17] respectively. This disparity between studies is related to the definition of PD technique failure adopted by each team. In some studies, technique failure was defined as a 30-day transfer to HD and others defined it as a transfer of 60 days or more. In the literature, the timing of transfer from PD to HD depends on the centers and definitions of the variables. The transfer at 6 months varied between 7% and 25%, while at 1 year it was between 21 and 44%. Eighty-eleven (91) patients developed infectious complications during their PD stay. The mean of infectious episodes was 3.36 episodes [1]. Eightynine (89) patients had peritoneal infections (97.80%). The mean time to onset of the first episode of peritoneal infection was 9.73 +/- 11.51 months [1]. In France in Le Maner’s series, 53.62% of patients transferred after 6 months of PD had presented one or more episodes of peritonitis [18]. While in the Béchade series, 2.60% of patients transferred early (during the first 6 months) had peritoneal infections [19]. In Australia, Lan et al. found that 50% of cases had peritoneal infections [4]. The occurrence of peritoneal infection within the first 6 months is considered a risk factor for transfer from PD to HD. This is explained by the structural modifications of the peritoneal membrane, which will result in the failure of the technique. The high rate of peritoneal infections in our study may be related to the tropical climate. The heat and humidity promote the loosening of the dressings and the proliferation of germs. Several teams, in Australia and Hong Kong, have shown that the rates of peritoneal infections vary with the seasons, and increase with temperature and humidity [20]. The main reason for transfer was infection, 76 (77.6%) patients were transferred due to peritoneal infection. In second place come the mechanical causes in 23.5% of the patients, and the social reasons come in third place in 12.2% of the cases then the inadequate dialysis in 6.1% of the cases. Severe undernutrition and sclerosis peritonitis accounted for only 1% each. Szeto et al. found that 71.1% of patients were transferred following peritoneal infection and catheter ablation while 28.9% of transfers were due to loss of UF and other medical causes [17]. In Panagoutsos’s serie , the causes of transfer were peritoneal infections (61%), loss of UF (27%), sclerosis peritonitis (9%) and social reasons (3%) [21]. The mean duration on haemodialysis in 53 patients was 43.29 ± 44.82 months. The majority of patients (42.8%) were still in HD, 34.6% of cases had died and one patient was transplanted (1%). According to Le Maner, 73 patients (62.4%) had died, 22 patients (18.8%) were transplanted, and 22 patients (18.8%) were still on hemodialysis [18]. In Szeto’s serie, 61.4% of patients had died, 18.3% were transplanted, 4.6% were transferred to another center and 15.7% were still in HD [17]. Thirty-four (34.6%) patients had died. Eight (61.5%) patients had died during the first 12 months of the transfer. Patient survival according to the Kaplan Meier method was on average 126.51 ± 11.66 months, and the median was 152 months. The 5-year survival was 77%. Szeto et al. found a 5-year survival of 39.9%. They also noted that mortality was high during the first 12 months. Analysis of survival in patients who remained alive after 12 first months showed a rate of 65.2% [17]. According to Habib et al. the mean survival was 79.2 months, and the median was 90 months [14]. While in Van Biesen’s serie, the median survival was 95.7 months [22].
Conclusion
The main reasons for transferring PD to haemodialysis identified in the literature are found in our context. This transfer must be anticipated to reduce morbidity and mortality.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers#lupine journals#articles#urology#nephrology#nephritis#open access journals#submission#journal of urology & nephrology studies
0 notes
Text
Lupine Publishers | A Rare Case of Penile Schwannomatosis Presenting with Painful Nocturnal Penile Tumescence

Abstract
Background: Penile schwannoma is a rare tumor. They commonly present as an asymptomatic, painless and slow growing mass. Other presentations include sexual dysfunction, most commonly dyspareunia, followed by erectile dysfunction, abnormal penile curvature or pain with ejaculation. Case presentation: A 26-year-old male presented atypically with painful nocturnal penile tumescence, along with multiple nodules over the dorsal penis. Excision of multiple penile tumors under general anaesthesia was performed and histopathologic examination revealed benign schwannoma. Conclusion: Our hypothesis is that the schwannoma lies along the axis of the dorsal penile nerve, and compression of this nerve occurs during his erection causing pain. However, there are limited presentations of painful erections in penile schwannomas, and we hope that future studies can help confirm this theory.
Keywords: Penile; schwannoma; painful tumescence; sexual dysfunction
Background
Schwannomas are a form of peripheral nerve tumors made up of neoplastic Schwann cells that typically occur as solitary, encapsulated masses. They can occur throughout the body, but more commonly arise on the head, neck, or flexor surfaces of limbs [1,2]. The tumors are sporadically associated with genetic syndromes such as schwannomatosis and neurofibromatosis or may be the result of therapeutic irradiation [3]. Schwannomas have a low annual incidence of 0.6 per 100,000 people [4]. These are rare and only 27 cases have been reported in literature since it was first described in 1968 [5]. Penile schwannomas are typically asymptomatic, painless and slow growing. Possible presentations include sexual dysfunction, most commonly dyspareunia, followed by erectile dysfunction, abnormal penile curvature, or pain with ejaculation. Our patient presented with painful nocturnal penile tumescence, which is not a well-known presentation of penile schwannomas. There is limited published literature on such cases and hence little is known about this condition (Figures 1&2).
Case Presentation
A 26-year-old man with a history of ankylosing spondylitis (HLA B27 gene) and previous circumcision first presented with a oneyear history of recurrent painful nocturnal erections. He had prior consultations with various urologists and did not respond to oral analgesia. The frequency of painful nocturnal erections increased from once per week, to thrice per week over the past year. Each episode of painful nocturnal tumescence lasted approximately five minutes and the patient was often awaken from sleep by the severe pain, which affected his sleep and quality of life (Figure 3). There was no history of priapism, sexual transmitted disease, or genital trauma. There were no persons with known neurofibromatosis in his family. On examination, four lumps could be palpated over the dorsum of the stretched penis. Two were superficial nodules on the distal shaft, with one deep nodule each at the mid shaft and base of the penis. The nodules were 0.5cm or less in diameter and firm in nature. The mid shaft nodule was tender on palpation and correlated with the site of painful nocturnal erections. The penis was otherwise unremarkable and there was neither penile curvature on erection nor any palpable lymph nodes in the femoral or inguinal areas. Nodules or café-au-lait spots were not present in the rest of the body (Figure 4). Over a five-year follow-up, patient developed worsening symptoms with the painful erections occurring twice every night from one episode a week. Physical examination and interval ultrasound imaging demonstrated an increase in the number of nodules from four to five with further growth of the existing nodules. Most noticeably, the right intracavernosal nodule increased from 4 mm to 7 mm in diameter. The patient decided for surgical excision of multiple penile nodules (Figures 5&6).
Investigations
Laboratory findings included normal blood cell counts, chemistries and urinalysis. Initial ultrasound penis showed multiple rounded heterogeneously echogenic nodules in the subcutaneous region of the dorsal penile shaft. The nodules show minimal central and peripheral vascularity. A small cystic lesion with internal echoes is also noted in the corpus cavernosum. The patient initially declined surgical intervention and opted for annual ultrasound imaging (Figure 7). Magnetic resonance imaging (MRI) of the penis performed prior to surgery showed multiple enhancing sub-centimetre nodules in the penile shaft, most of which were superficial. There was a nodule in the right corpus cavernosum, and another in the dorsal midline which disrupts the wall of both corpora cavernosa.
Treatment
Patient underwent excision of multiple penile tumors under general anaesthesia. A circumferential incision was made at the previous circumcision site. The penis was then degloved to its base and the layers dissected down to Buck’s fascia. There were five superficial tumors adherent to the tunica albuginea (two at right distal shaft, two at midshaft, one at base of penis). A deep-seated tumor was located at the right corporal mid shaft. The tumors measured approximately 1-1.5cm in diameter (Figure 8). The cut surface of the tumors was homogenously yellowish with noted feeding vessels. All tumors were excised, and histology was sent from all locations.
Outcome and Follow-Up
All specimens show similar morphology. The circumscribed and thinly encapsulated nodules were made up of Schwann cells arranged as a mixture of more cellular Antoni A and less cellular Antoni B areas. The more cellular Antoni A areas consists of Schwann cells arranged as short fascicles or parallel rows of nuclear pallisading (Verocay bodies). The less cellular Antoni B areas show a looser myxoid stroma. The nodules are associated with thickened and oedematous nerve fibres, and occasional more plexiform Schwannian areas are seen involving the nerve fibres (Figures 9&10). No high-grade nuclear atypia, increased mitosis or tumour necrosis is seen. On immunostaining, the lesion shows diffuse staining with S-100, which is indicative of Schwannoma. In the follow up consultations over a year after surgery, there was no further nightly painful erections and patient was able to sleep well. On examination there is a small nodularity at mid shaft which is likely due to scar tissue formation. However, the patient did experience difficulty maintaining erection due to discomfort over the surgical site. This was managed well with Viagra 25mg, with an improvement in International Index of Erectile Function score from 8/25 to 19/25. Patient declined genetic testing as he was not keen on childbearing.
Discussion
Schwannomas rarely present with penile pain. There have been postulations made regarding the correlation of symptoms to neuroanatomy. A literature review done by Huang et al. concluded that patients with penile root schwannomas are more prone to symptoms with discomfort or sexual dysfunction (4 of 6) compared with patients with penile shaft schwannoma (7 of 16) or glans schwannoma (2 of 7) [6]. Based on anatomy, penile schwannomas at the mid shaft or glans should originate from the dorsal nerve of the penis, which is the deepest division of the pudendal nerve. The pudendal nerve does pass through the penile root, but there is no clear branching or tracking of the nerve origin of the tumor [7]. Pain may occur in the region of the tumor and any nerve the tumor originates from, but pain may not be specific enough to discern the particular involved nerve. Neurologic deficits of sensory and motor function correspond to the nerve in which the tumor originates or which it is compressing, and as such will often be most useful in localizing the tumor [8,9]. This patient presented with painful nocturnal erections corresponding to the mid shaft schwannoma. Our hypothesis is that the mid shaft schwannoma lies along the axis of the dorsal penile nerve and pain could arise when the nerve is compressed by the schwannoma during full erection. During nocturnal tumescence, the cavernosus arteries dilate, leading to engorgement of the corpora cavernosa and increase of intracorporal pressure. This pushes the schwannoma towards the dorsal penile nerve leading to nerve irritation, compression and pain. Penile schwannomas normally occur at the dorsal penile shaft. However, there have been documented cases where the tumor has infiltrated the glans and prepuce [10]. In such cases, we have to consider other possible diagnoses including benign soft-tissue lesions such as lipoma, fibroma, leiomyoma, Peyronie’s disease, injection-related fibrosis, and rarely malignant sarcomas. Clinical history taking and clinical examination are important, but imaging can aid in narrowing the differentials by locating the plane of the lesion and delineating the mass. Ultrasound examination can demonstrate hypoechoic lesions, and doppler ultrasound can detect hypervascularity. CT scan is rarely used, and mostly performed to exclude metastasis. Schwannomas demonstrate typical MRI features of T1 isointensity to hypointensity, T2 hyperintensity, and postcontrast enhancement. Heterogeneous signal intensity and postcontrast enhancement are suggestive of internal hemorrhage and myxoid/cystic changes [11]. Otherwise, excision biopsy of the tumor would be the gold standard for final diagnosis. Treatment of penile schwannomas is symptomatic, focused primarily on pain management. Complete surgical excision is the recommended treatment for penile schwannomas, with low recurrence rates [1- 10]. This patient recovered well with no signs of recurrence one year postoperatively. Schwannomas of the penis are usually benign, but four malignant variants have been reported in literature. No cases of benign penile schwannoma have been reported to be associated with hereditary diseases [12]. Schwannomatosis is the third major form of neurofibromatosis, and is characterized by a predisposition for schwannomas, in the absence of schwannomas on both vestibular nerves. Its diagnosis is based on a criterion [13]. Most patients present in adulthood with multiple schwannomas and pain, and approximately 20 percent of patients have a family history of schwannomas or schwannomatosis [14]. So far, there have been no confirmed causes of penile schwannoma with schwannomatosis. There is no strong evidence about the correlation between schwannoma and erectile dysfunction [15]. This patient’s postoperative erectile dysfunction is likely due to pain surrounding the surgical wound site. Also, there were no surgical complications other than possible scar tissue formation on the penile shaft.
Conclusion
Schwannomas of the penis are extremely rare and typically present as a solitary, asymptomatic, painless and slow-growing tumor. The rarity in this case is that our patient presented with painful nighttime erections. Based on the penile neuroanatomy, penile schwannomas at the mid shaft should originate from the dorsal nerve of the penis. Our hypothesis is that the schwannoma lies along the axis of the dorsal penile nerve and compression of this nerve occurs during his erection causing pain. However, there are limited presentations of painful erections in penile schwannomas, and we hope that future studies can help confirm this theory.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
1 note
·
View note
Text
Lupine Publishers | The TUR Syndrome Re-Incarnating as ARDS after Saline use as Irrigating Fluid in Endoscopic Surgery

Abstract
Objective: To demonstrate the TUR syndrome characterized with hyponatraemia (HN) will no longer be seen after using saline as irrigating fluid in urology, but it has re-incarnated as the acute respiratory distress syndrome (ARDS) presenting with the same clinical picture of the multiple organ dysfunction syndrome (MODS). Material and Methods: A focused objective and relevant narrative review of other eminent authors’ work and mine are used here. Results: The TUR syndrome characterized with HN will no longer occur in urology after the use of saline as irrigating fluid in endoscopic surgery. It has reincarnated as ARDS presenting with the same MODS clinical picture. It is induced by VO caused by iv fluid infusions. This induces cardiovascular shock (VOS) that cause ARDS. The latter is already common in clinical practice due to the excessive us of iv fluids in the management of shock, acutely ill patients, and prolonged major surgery as iatrogenic complication of fluid therapy. The wrong Starling’s law dictates the current faulty rules on fluid management of shock that mislead physicians into giving too much fluid. The correct replacement is the hydrodynamics of the porous orifice (G) tube which should be the new scientific basis for fluid therapy in shock management. The currently available hypertonic sodium therapy of 5%NaCl and/ or 8.4%NaCo3 is lifesaving therapy for HN, the TUR syndrome and ARDS. Conclusion: The TUR syndrome may seem to have been eradicated in urology with the use of saline as irrigating fluid in endoscopic surgery. However, it has reincarnated as ARDS with the same clinical picture of MODS. It is an iatrogenic complication of fluid therapy dictated by the wrong Starling’s law for which the hydrodynamic of the G tube is the correct replacement that should be the new scientific basis for a new policy on fluid management of shock.
Keywords: The TUR syndrome; Endoscopic Surgery; ARDS; Shock; Fluid Therapy; Starling’s law, Capillary-ISF transfer
Introduction
My beginning with the transurethral resection of the prostate (TUR) syndrome started in 1981 after I attended post-mortem (PM) examinations on 3 patients who died after the TURP surgery. I was only an SHO in urology working for the late Mr. KC Perry and JP Ward at DGH in Eastbourne. At the PM examination it was clear and obvious to me that these patients died of internal drowning as result of massive volumetric overload (VO) of fluids used for resuscitation of a cardiovascular shock they suffered, and the fluid was retained in their bodies. When I asked the pathologist why doesn’t he mention that retained VO in his report? He replied: “because it offends treating physicians”? The word offends hit me right hard on my head like a hammer. My next question to myself was if it offends them why do physicians do it? This had led me to immediately replace the term fluid overload with the new and original Volumetric Overload (VO) after adding the cardiovascular hypotension Shock to it to become (VOS) that was introduced to avoid the word offends but it has proved to be a new scientific medical discovery. Another few questions such as: “What is misleading physicians into giving too much fluid during the resuscitation of shock? What shock is it? I communicated with Richard Harrison III (who may be late now) who is the originator of the hyponatraemic shock of the TUR syndrome and the use of 5%NaCl therapy in clinical practice for years during his retirement [1]. I reported later the true pioneer originators of this shock and the hypertonic sodium therapy (HST) were Danowski et al who induced it experimentally in dogs by massive 5%Glucose infusion [2]. Harrison advised me to “put the poison in the honey” that I could not accept. After the PM examination I suspected and incriminated Starling’s law being the scientific basis of fluid therapy in shock that dictates the wrong rules on fluid therapy for shock management documented in articles and books [3-7], for which the hydrodynamics of the porous orifice (G) tube is the correct replacement (Figures 1a&b) [8,9]. I felt so strongly about it that I wrote a letter to the late great professor of physiology Eric Neil and author of Sampson Wright Textbook of Physiology later in 1983 [10,11]. He nicely replied in handwritten letter as he was in retirement asking: Why and how may Starling’s law cause death of patients? The answer is there now after 40 years of hard scientific research and investigations [12]. The inflow pressure pushes fluid through the orifice. Creating fluid jet in the lumen of the G tube**. The fluid jet creates negative side pressure gradient causing suction maximal over the proximal part of the G tube near the inlet that sucks fluid into lumen. The side pressure gradient turns positive pushing fluid out of lumen over the distal part maximally near the outlet. Thus, the fluid around G tube inside C moves in magnetic field-like circulation (5) taking an opposite direction to lumen flow of G tube. The inflow pressure 1 and orifice 2 induce the negative side pressure creating the dynamic G-C circulation phenomenon that is rapid, autonomous, and efficient in moving fluid and particles out from the G tube lumen at 4, irrigating C at 5, then sucking it back again at 3, Maintaining net negative energy pressure inside chamber C. **Note the shape of the fluid jet inside the G tube (Cone shaped), having a diameter of the inlet on right hand side and the diameter of the exit at left hand side (G tube diameter). I lost the photo on which the fluid jet was drawn, using tea leaves of fine and coarse sizes that run in the centre of G tube leaving the outer zone near the wall of G tube clear. This may explain the finding in real capillary of the protein-free (and erythrocyte-free) sub-endothelial zone in the Glycocalyx paradigm. It was also noted that fine tea leaves exit the distal pores in small amount maintaining a higher concentration in the circulatory system than that in the C chamber- akin to plasma proteins.
What is the TUR Syndrome? And what is causing the “Understanding Gap”? Our prospective cohort study on the TUR syndrome was conducted in 1987-8, a letter to the editor of BJU was reported in 1988 [13], MD Thesis was accepted November 1988 [14], and the article reported in 1990 [15]. The TURP syndrome is a condition induced by gaining large volume of sodium-free fluid overloading the cardiovascular system and spelling over into the interstitial fluid (ISF) space of vital organs and subcutaneous. The fluid of 1.5%Glycine used as irrigating fluid gets absorbed, or rather infused through peri-prostatic veins, during the TURP surgery as well as all endoscopic surgeries performed under sodium-free fluid irrigation of any type such as Mannitol, Sorbitol, Glucose and Cytal. Also, intravenous (iv) infusion of 5% Glucose considerably and significantly contributes to it- as well as saline. What is more, excessive infusion of saline or any sodium-based fluid such as Saline, Hartman, Ringer, plasma, and plasma substitutes, and blood worsens it transferring the shock being treated from VOS 1 into VOS 2 [16] and causing ARDS 1 and 2 [17,18] with apparent correction of HN, and has high morbidity and mortality later. The TUR syndrome has a characteristic severe drop of serum sodium level causing acute dilutional hyponatraemia (HN) induced by VO 1 (Figures 2 & 3) with severe clinical symptoms affecting all vital organs causing the multiple organ dysfunction syndromes (MODS) (Table 1) or ARDS [17,18] with recognizable clinical picture but one system may predominate such as acute kidney injury (AKI). The HN of <120 mmol/l has 2 paradoxes and 2 nadirs that have eluded authorities and physicians on HN, and that has made the TUR syndrome most elusive and invisible making it though obvious it has remained invisible even to authorities on HN. Professors and consultant urologists who are such swift good resection experts have testified that the TUR syndrome does not exist as no fluid absorption occurs, with a negative prospective study of 100 patients [19]. Off course no such hyponatraemia occurs when the irrigating fluid is saline whatever the volume absorbed and infused. Another important reason that prevents massive 1.5% glycine absorption and the TUR syndrome is for the Urologist not to breach the prostate capsule and not to open the venous sinuses where the irrigating fluid is directly injected intravenously (iv) into the periprostatic veins. There was also another good swift urologist who reported >1000 consecutive TURP surgeries without seeing the TUR syndrome. The risk of VO during endoscopic surgery will continue to occur as long as there are registrars in training and even with the experienced consultants who occasionally and inadvertently breach the prostatic capsule and open the venous sinuses. However, the TUR syndrome due to 1.5% Glycine VO with its characteristic HN has an undoubted reality [13-15] and [20-22]. Our study reported 10% incidence of the TUR syndrome with one near death case that was saved [14] and a similar study done a year earlier in the same department reported 7% incidence of morbidity with 1% mortality [22]. Before the TUR syndrome disappears into oblivion and is totally replaced by ARDS a most comprehensive literature review on the subject was reported in 2018 after the wide use of saline as irrigating fluid in the TURP surgery [23]. Here a distinction between a physiological VO of <2 L infused in less than one hour that is extensively studied by Hahn in volunteers and patients is known as Volume Kinetic (VK) (20) and the pathological VO of 3.5-5 L gained in < 1 h that causes the TUR syndrome [15] is highlighted. This has been a cause of serious misunderstanding gap in the pathogenesis of the TUR syndrome. The physiological response of VK is remarkably different from the pathological response of VO which is paradoxical: VK elevates blood pressure and induces diuresis while VO causes hypotension with bradycardia and causes acute renal failure.
The TURP syndrome starts by presenting with cardiovascular hypotension shock to anaesthetists and surgeons in theatre [24,25] and at times by cardiac or cardiopulmonary arrest [26] and sudden death. By next morning the surviving patients present with coma, convulsion and bizarre paralysis to physicians, neurologists, and ICU specialists [15]. It has the characteristic serum hypo-osmolality. BUT other solute contents dilutions seem to be apparently spontaneously improving due to water shift into cells [Table 2, Figures 1 and 2]. The HN of <120 mmol/l causes cardiovascular hypotension shock. Volumetric overload (VO) is the most highly significant factor causing its patho-aetiology with a (p=0.0007). Osmolality was also significantly low (p=0.02) while all other serum solute changes including the most remarkable drop in serum sodium and huge elevation in serum glycine did not reach statistical significance in the multiple regression analysis, yet it did alone when pre- and post-operative levels are compared!? [Table 2 and 3]. This cardiovascular shock of VOS is easily confused with and mistaken for haemorrhagic or septicaemia shock and is wrongly treated with further massive volume expansion that usually kills the patient as happened in the 3 patients mentioned above!?
The toxic theory of the TUR syndrome and septic theory of ARDS.
Sepsis and septic shock in the pathogenesis of ARDS is as innocent as the wolf in Josef story [18], so is glycine in the aetiology of the TUR syndrome [15], particularly as correctly mentioned that the TUR syndrome occurs with Mannitol, Sorbitol, and Glucose. Professor Alan Arieff has clearly reported the morbidity and mortality of hyponatraemia (HN) of the TUR syndrome induced by 1.5%Glycine as well as the excessive 5%Glucose infused intravenously during prolonged surgery in healthy women [27]. That does not mean that I deny the toxicity of glycine and the seriousness of sepsis. I am just saying they are misleading like a mirage to someone thirsty and lost in the desert. While thinking about it please, try to attend the PM examination of some patients who died from the TUR syndrome and ARDS. Every anaesthetist should examine own practice when he embarks on Bolus Fluid Therapy (BFT) during anaesthetic induction and watch out how much fluid is given during prolonged major surgery. Review the scientific basis of fluid therapy in the management of septic and all other types of shock on which bases the current practice is implemented.
Fluid therapy Regimen and Iatrogenic complications
The TUR syndrome occurs because of combination of fluid absorption and direct iv infusion of the irrigating fluid when the prostatic capsule is breached, and venous sinuses are open. In clinical practice all ARDS cases occur as result of iv infusion of fluids. In our study 7 cases of capsule breaching occurred among the 10 TUR syndrome cases as observed by the surgeon. The iv infusion occurs with both the liberal regimen of Early Goal-Directed Therapy (EGDT) and Bolus fluid therapy (BFT) of the conservative regimen. Hahn is a professor and consultant of anaesthesia and intensive care. He is also a leader and world authority on fluid therapy and the editor of a book on the same subject. I would and have recommended him as the head of a committee to write the new guidelines on fluid therapy in shock management. He has my new book that will help him for >8 months now, please read it if you’ve not done so already. Like all anaesthetists, Intensive care therapists, surgeons, and physicians of the whole world who remain to practice the liberal fluid therapy regimen also well known as EGDT in the management of shock, don’t you? Go to any ICU near you and observe the swollen-up ARDS patients mostly with trunk oedema comparing their body weight on hospital admission with their current weight while suffering from ARDS. Try to attend the PM examination of the TURP patients and ARDS patients. Allow me to reproduce this section from my article later that is most recommended reading to all physicians interested in the subject of fluid therapy, the TUR syndrome, HN, VOS and ARDS [18].
The role of Starling’s law
Starling’s law [28,29] dictates the current faulty rules on fluid therapy in the management of shock. It thus misleads physicians into giving too much fluid during shock resuscitation [30]. More than 21 reasons were reported to show that Starling’s law is wrong [31], none of it can be denied or refuted. The correct replacement is the hydrodynamic of the porous orifice (G) tube [8,9] (Figure 1 a & b) that was built on capillary ultrastructure anatomy of having precapillary sphincter [32] and a porous wall [33] that allow the passage of plasma proteins-hence nullify the oncotic pressure. It follows that the extended Starling Principle is wrong and a misnomer [34,35] and all the equations are also wrong.
Two types of VO inducing VOS and causing ARDS of type 1 and 2
There are two types of VO: Type 1 induced by sodium-free fluid and Type 2 induced by sodium-based fluid. These in turn induce VOS 1 and VOS 2 which cause ARDS 1 and ARDS 2, respectively. The clinical picture is the same for both types (Table 1). Type 1 is characterized with HN of the TUR syndrome with which the cerebral neurological manifestations of coma, convulsions, and bizarre paralysis predominate while type 2 may have moderate hypoproteinemia if induced by crystalloids and none when plasma, plasma substitutes and blood are used. Type 2 may complicate Type 1 or may occur do novo. Manifestations of the multiple organ dysfunction syndrome (MODS) are the same and appear in every case, but one system may predominate. When Hahn sent me his article on Revised Starling Principle calling for revalidation [34] I immediately responded with an article: Revised Starling’s Principle (RSP): a misnomer as Starling’s law is proved wrong. I considered research on validating RSP is a total waste of money, time, and efforts.
Proof by eminent authors on the VO role in the aetiology of the TUR syndrome and ARDS
Professor Robert Hahn from Sweden has done lots of research infusing various types of fluid used in clinical practice to normal adult volunteers and patients, as well as animal research and clinical studies and reported >340 articles on the TURP syndrome alone (PubMed 2017) and 532 articles in total (PubMed search 2021): Here is what Robert Hahn said: in the abstract of an article reported in 2017 [36]:
Abstract [36]:
“Adverse effects of crystalloid fluids are related to their preferential distribution to the interstitium of the subcutis, the gut, and the lungs. The gastrointestinal recovery time is prolonged by 2 days when more than 2 liters is administered. Infusion of 6-7 liters during open abdominal surgery results in poor wound healing, pulmonary oedema, and pneumonia. There is also a risk of fatal postoperative pulmonary oedema that might develop several days after the surgery. Even larger amounts cause organ dysfunction by breaking up the interstitial matrix and allowing the formation of lacunae of fluid in the skin and central organs, such as the heart.” Thank you, Professor Hahn for a most impressive work indeed. New guidelines based on currently available evidence on fluid therapy for resuscitation of sepsis, septic shock, trauma patients, critically ill patients, ARDS and patients undergoing prolonged major surgery are badly needed. Professor Hahn is the expert witness on fluid therapy. Why does not Hahn believe his own results? Why doesn’t he make the most obvious conclusion based on what he said in the abstract above? What and how much more evidence and years that he needs to believe that the pathological VO of massive fluid infusions induces cardiovascular shock that is VOS of both types and causes ARDS? If my articles referenced here and the books [3- 7] particularly the one Hahn has now for 8 months and being held in the press awaiting his introduction, then allow me most sincerely and humbly to give you a helping hand to lift you up to where I stand and clearly see the picture on the real issues discussed here. Hahn does not need to do any more research studies. Just report a re-analysis of data from previously reported articles he has done and reported before, based on his previous published articles on the TUR syndrome and saline-based fluid infusions. Please, reexamine and re-analyse your own research work in a manner and method identical to your article reported here [20]. Please, Hahn don’t bother with equations that are hard to understand and are meaningless and perhaps misleading or even wrong. Do not use fancy sophisticated graphs that does not impress me. I would love, most sincerely and humbly, to give you a hand to get you out of the huge maze you have been lost inside it for >3 decades. All you need to do my friend now is to liberate yourself from the illusive and misleading concepts of the toxic/septic hypotheses of glycine and sepsis!? One must unlearn old bad habits to be able to receive and acquire the new correct ones.
Evidence for the VO Theory causing VOS and ARDS
“The prevalence of “liberal fluid infusion” in resuscitation of all types of shocks not only septic shock in clinical practice all over the world is attributed to an impactful article by Rivers et al, reported at The N Engl J Med 2001 [37]. Dr Rivers’ investigation reported EGDT in the treatment of severe sepsis and septic shock. In this singlecenter study published more than 20 years ago involving patients presenting to the emergency department with severe sepsis and septic shock, the conclusion was: “mortality was markedly lower among those who were treated according to a 6-hour protocol of EGDT, in which intravenous fluids, vasopressors, inotropes, and blood transfusions were adjusted to reach central hemodynamic targets, than among those receiving usual care” Usual care means conservative fluid regime. There is something grossly wrong with this conclusion, but I cannot tell what is it? Not yet. Let us see what other author investigators have said first. The EGDT of liberal fluid infusion has been termed “aggressive” by some authors. However, it has been adopted all over the world not only for the therapy of septic shock but also whenever fluid therapy is required for the management of all types of shocks. “In another article by Dr Rivers 11 years later in 2012 [38] he compared the liberal to the conservative approach concluding in his last statement: “In contrast to what is true in politics, in fluid management of acute lung injury, it is OK to be both liberal and conservative.” So, Dr Rivers says it is OK to have it both ways: “one for the ebb and one for the flow”! Sorry, sir, I disagree. It is not OK. It is not politics either. No, you cannot have it both ways. The right way is only one. The issue here is how much fluid should be infused during the ebb phase of shock and does it have a maximum limit? Replace the loss but do not overdo it. Since the cardiovascular system (CVS)’ maximum capacity of an adult is 7 L and the normal blood volume is 5 L, the maximum infused volume of fluid should be limited by the maximum capacitance of the CVS. What do you expect when you try to fit 10-15 L of fluid into a 7 L capacity container? Simple physics and common sense indicate that it must spell over if it is open system or burst if closed! The cardiovascular system is no exception. Dr Rivers should re-examine his own data and tell us where and why he went so grossly wrong.” The EGDT has spread like fire in a haystack, and it remains operative in current clinical practice all over the world that is why ARDS is so common yet remains under recognized and underestimated affecting and killing hundreds of thousands of patients per year.” Other authors have confirmed the significant role of VO of crystalloids in causing the morbidity and mortality of ARDS both in adult and children of trauma patients [39,40]. All authors have stopped short of recognizing VOS as Cause of ARDS or MODS morbidity and mortality. Quoting also from this article [18] I mention here the remarkable multicenter study by Rowan et al. [41] Like Hahn they reported results that demonstrate the massive VO retained in the body of surviving ARDS patients. After sending 3 emails to Rowan commending the authors on their results and asking about the dead patients retained fluid VO, none of the 40+ authors replied. “The PRISM Investigators reported its Trial by Rowan et al at NEJM 2017 [41] concluded: “In this meta-analysis of individual patient data, EGDT did not result in better outcomes than usual care and was associated with higher hospitalization costs across a broad range of patient and hospital characteristics.” Thank you, Dr Rowan and colleagues for the excellent research and report. This is good evidence-based medicine, but more is needed, from you, and you have the data to provide it. Based on this conclusion that agrees with other multicenter trials I wonder is time to say goodbye Dr Rivers? The aggressive and deleterious liberal approach of EGDT is no longer wanted. It should be abandoned immediately. Even when the nasty liberal approach goes away, hopefully soon, it remains bad enough with the conservative regime as it is now that must be sorted out! I wonder what Dr Rivers has to say about this, particularly as authors of 3 other huge prospective multicenter trials of The ProCESS/ARISE/ProMISe reported similar conclusion by Huang et al. [42]. So, Rowan gave the results of: The cumulative VO was -136 ml in the conservative-strategy group, as compared with 6992 ml in the liberal-strategy group (P<0.001). For patients who were in shock at baseline, the cumulative seven-day VO was 2904 ml in the conservative-strategy group and 10,138 ml in the liberal strategy group (P<0.001). For patients who were not in shock at baseline, the cumulative VO was −1576 ml in the conservative-strategy group and 5287 ml in the liberal-strategy group (P<0.001)”. “First, the negative sign (-) indicating negative fluid balance has appeared in the data above and is very important. It characterizes the nonsymptomatic patients among the conservative-strategy group. These patients should be used as the controls for the statistical analysis of the data. I have been waiting for 40 years to see these VO results. I am still waiting to see VO data with statistical significance in mortality patients. I plead with and urge the respected authors of major randomized Trials of FACCT, PRISM, ProCESS, ARISE, and ProMISe to come forward with these data, please,
Clinical picture of (VOS, The TUR syndrome, ARDS and MODS)
The clinical picture of ARDS is that of the multiple organ dysfunction syndrome (MODS) (Table 1) reported previously by Khadarow and Marshal in 2002 [43]. Another remarkable article was reported by Schrier in 2010 [44]. Demonstrating the link of the TUR syndrome with ARDS by having identical clinical picture with minor variations was reported by Ghanem as complications of VO covering the cardiovascular/hematological that appear first under general Anaesthesia with bradycardia [45], the cerebral/ neurological with coma appear first under spinal/epidural Anaesthesia and convulsions and bizarre paralysis predominate in the TUR syndrome, not in ARDS [46], the respiratory of ARDS and hepatic/gastrointestinal manifestations [47] and AKI predominate later were documented recently in individual specific reports. Excessive bleeding and leukocytosis in the absence of sepsis also occur.
Therapy of VOS, the TUR syndrome and ARDS [17] Prevention
Based on the above discussion, ARDS is an iatrogenic complication of fluid therapy in hospital, never in community, that is overlooked and underestimated. Being iatrogenic; means it is preventable. In order to prevent VOS and ARDS a limit to the maximum amount of fluid used during shock resuscitation or major surgery must be agreed upon. Professor Hahn [36] found that infusing 2 L of saline to human volunteers produces symptoms. Infusing >3 L is pathological. More than 5 L is associated with deleterious morbidity [38,39]. So, the maximum volume of fluids that can be infused safely to an adult patient is 3 L which is the daily fluid requirement, and no more fluid of any kind is given for 24 hours except replacing the actual loss that does not include urine loss. The patient should be put on a weighing scale every day from hospital admission till discharge or death. Any retained volume of fluid above his body weight on admission is pathological. On using CVP for monitoring fluid therapy, please refrain from persisting to elevate CVP to levels above 12 and up to 18-22 cm saline [48]. This is a major cause for inducing VO and VOS and ARDS during shock resuscitation, particularly septic shock [37]. Look up any physiology textbook to find out that the normal CVP is 0 and it swings between -7 and +7 cm saline which is the level that should be aimed at in monitoring fluid replacement in shock of sepsis, trauma, and bleeding, acutely ill and during major surgery. Elevating CVP is not synonymous with elevating arterial pressure. If hypotension develops later during ICU stay, inotropic drugs, hydrocortisone 200 mg and HST should be used. The latter restores the pre-capillary sphincter tone (peripheral resistance) so that the capillary works as normal G tube again [9], but no isotonic crystalloids or colloids infusions of above the daily fluid requirement should be given. If persistence with the current liberal regimen of Early Goal-Directed Therapy (EGDT) and conservative Bolus Fluid Therapy regime continues, then more reports on ARDS will continue. Future authors will be hopefully taking into consideration the mentioned above data concerning VO/Time, or the retained fluid VO at the time of inducing ARDS or death on reporting new trials or case reports.
Treatment of ARDS [6]
Hypertonic sodium therapy (HST) of 5%NaCl and/or 8.4%NaCo3 has truly proved lifesaving therapy for the TUR syndrome and acute dilution HN [17,18] as well as Secondary VOS 2 that complicates fluid therapy of VOS 1 causing ARDS. It works by inducing massive diuresis; being a potent suppressor of antidiuretic hormone. My experience in using it for treating established ARDS with sepsis and primary VOS 2 that causes ARDS is limited. However, evidence on HST suggests it will prove successful if given early, promptly, and adequately to ARDS patients while refraining from any further isotonic crystalloid or colloid fluid infusions using saline, Hydroxyethyle starch and/or plasma therapy- just give the normal daily fluid requirement and no more. After giving HST over one hour using the CVP catheter already inserted, the patient recovers from AKI and produces through a urinary catheter massive amount of urine of 4-5 L as you watch. This urine output should not be replaced. Just observe the patient recovering from his AKI, coma and ARDS and asks for a drink. This is done in addition to the cardiovascular, respiratory, and renal support on ICU. Patients with AKI on dialysis, the treating nephrologist should aim at and set the machine for inducing negative fluid balance. The HST of 5%NaCl and/or 8.4%NaCo3 is given in 200 ml doses over 10 minutes and repeated. I did not have to use more than 1000 ml during the successful treatment of 16 patients. Any other hypertonic sodium concentration is not recommended- I know Hahn tried 1.8%NaCl and it does not work. A dose of intravenous diuretic may be given but it does not work in a double or triple the normal dose. A dose of 200 mg of hydrocortisone is most useful. Antibiotic prophylactic therapy is given in appropriate and adequate doses to prevent sepsis and septic shock. No further fluid infusions of any kind of crystalloids, colloids and blood is given. The urinary loss should not be replaced as this represent a surplus in the body and must be discarded otherwise defeats the objective of treatment.
Addendum: Relevant articles on the history of the TUR syndrome and ARDS
This addendum is dedicated to important landmark articles on the history of the TUR syndrome and ARDS that could not be fitted directly on the above focused narrative review on how the TUR syndrome has been reincarnated into ARDS. It is optional reading for the interested reader, but it completes this review. The first part is dedicated to eminent authors on the TUR syndrome and ARDS whether directly or indirectly. The second part is a section on selfreferences by the author that report important issues that highlight aspects of the presentation.
A. Other Eminent Authors
Creevy was the first author to report the TUR syndrome as acute water Intoxication [49]. Ashbaugh et al were the first to report ARDS in the Lancet in 1967 [50]. Lessels et al. reported in a letter to the editor as the only article on death during prostatectomy [51]. Hendry was first to report that the osmotic pressure of various body fluid is the same as plasma [52]. Guyton and Coleman reported the negative pressure of the subcutaneous space of -7 cm water, a fact that cannot be explained by Starling’s law [53]. Calnan et al reported the negative pressure in lymphatic vessels [54]. Renkin was the first to call for reconsideration of Starling’s law [55]. The Coshran injuries Group, Finfer, Vincent and futier et al demonstrated that oncotic pressure does not work and the argument on albumin versus saline is obsolete [56-59].
B. Self-references
Articles 60 and 61 have educational and entertainment value. Articles 62 and 63 shows the relevance of my work on ARDS to Covid-19 pandemic ARDS. Article 64- 66 corrects other received misconceptions on capillary physiology to augment the discovery of the G tube hydrodynamics and its impact on the capillary- ISF transfer. Articles 67 and 68 report the two clinical studies on which the above article is based. Article 68 corrects some errors and misconceptions on fluid therapy. Article 70 is on preventing renal failure in the critically ill patients. Article 71 reports my Experience with cystoprostadenectomy with “prostatic capsule sparing” for orthotopic bladder replacement. Article 72 is on Features and Complications of Nephroptosis Causing the Loin Pain and Haematuria Syndrome. Article 73 reports “New Discoveries in Medicine and Physiology Originated in Urology”. Article 74 is on an Update on Ghanem’s new scientific discoveries in physics, Physiology, and Medicine, Article 75 is on Goodbye Starling’s law, hello G tube.
Conclusion
The TUR syndrome as defined and characterized with acute dilutional hyponatraemia will no longer be seen in urology after the use of saline as irrigating solution in endoscopic surgery. However, the ARDS will replace it with identical clinical picture of MODS that continue to occur with high morbidity and mortality that is underrecognized and underestimated. The ARDS is common in clinical practice and is induced by excessive sodium-based fluid infusion and is likely to occur in urology due to the added risk of irrigating fluid absorption and infusion through periprostatic veins. Neither the toxic theory nor the septic theory plays the significant assumed rule in the pathogenesis of the TUR syndrome and ARDS. Both are iatrogenic complications of fluid therapy, induced by VO of > 3 L in <1 h time and is severe at 7-10 L of retained fluid VO in surviving ARDS patients wile mortality occur with 12 L, and both have preventative and curative therapy of HST of 5%NaCl and/or 8.4%NaCo3.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
0 notes
Text
Lupine Publishers | Swathed Ureter, an Enigma in Diagnosis- A Pictorial Essay

Abstract
This pictorial essay is an educational article aiming to provide both textual and visual portrayals consisting of a collection of images and texts on an important issue by reviewing and extrinsic encasing pathology of the ureter to provide a guide to those who are involved in diagnostic intervention. While considering diseases of the urinary system, physicians mainly focus on the kidneys and the bladder. Only scant attention is paid to the ureters. Most of the UTO due to calculi are readily identifiable whereas many cases of ureteric exterior encasement are frequently missed from early detection even by experienced clinicians and radiologists. Failure in recognition of the encasement of ureters and its causes may lead to mistaken diagnosis with resultant inappropriate management. However, problems with the ureters can adversely affect the functioning of the kidneys and could even be lethal. In this article we focus only ‘encasement of ureters’ with a few common examples and salient signs that help in the diagnosis.
Keywords: Encasement of Ureters; Endometriosis; MRI; RPF; Obstructive Uropathy
Background
Urinary tract Obstruction (UTO) an alarming but common clinical condition affecting more women than men at any age, though common in 20 to 60 years of age having an overall incidence of hydronephrosis in 3.1% of autopsy. Over time, UTO results in irreversible loss of numerous nephrons leading to obstructive nephropathy and end-stage renal failure. If the obstruction of the ureter is partial and brief and if intervention is done at correct time, after relief of obstruction. complete recovery of renal function is possible. One has to be aware that UTO lasting more than 24 hours may cause irreversible loss of renal function. Radiology investigations may show UTO without ureteric dilation and dilation of ureter without UTO creating potential pitfall in radiologic diagnosis of UTO. Most of the UTO due to calculi are readily identifiable whereas many cases of ureteric exterior encasement are frequently missed from early detection even by experienced clinicians and radiologists. Failure in recognition of the encasement of ureters and causes may lead to mistaken diagnosis with resultant inappropriate management [1]. The most common benign cause of encasement of ureters is retroperitoneal fibrosis and the most frequent malignant causes are extension from an adjacent primary tumour such as sarcoma, lymphoma, (E.g.: sarcomas and lymphomas of uterus, ovaries, urinary bladder and prostate). Among benign conditions of swathed ureters, Extrinsic benign tumours, Retroperitoneal lymphadenopathy, Retroperitoneal abscess, Retroperitoneal fibrosis, Inflammatory abdominal aortic aneurysm or iliac artery aneurysm, and Endometriosis are significant. Chronic fibrosing conditions of the abdomen may involve multiple systems by their proliferative deep fibromatoses which form pseudotumor which cannot be differentiated from neoplastic conditions at imaging. Peri-ureteral inflammation (E.g.: peritonitis, salpingitis, and diverticulitis), multifocal idiopathic fibrosclerosis and schistosomiasis are some other known causes. Encasement may also be associated with blocked ureter [2-4]. Besides the inherent features of the disease causing the encasement of the ureters, in general, clinical features include recurrent fever, pain abdomen, oliguria, frequency, dysuria, haematuria nocturia, and hypertension. Patients may also present with fatigue, anorexia, weight loss, fever, hydroceles, scrotal pain, lower extremity oedema, and pulmonary embolism. Since ureters may be affected, various degrees of ureteral obstruction, hydronephrosis, and renal failure are also considered early and common clinical manifestations. In this article, we will review the four important diseases that cause ureteric encasement with some key imaging features for the diagnosis [5,6].
Imaging
On imaging ureteral pathology, plain abdominal radiography does not play a major role in imaging. Intravenous excretory urography (EUG or IVU) was once the study of choice for evaluating the renal collecting system and ureters; although now replaced by Computed Tomography urography (CTU), IVU is still performed in some centres. IVU has limited utility in patients with impaired renal function. In patients with renal insufficiency the risk for contrast-induced nephropathy from iodinated contrast media is high [7-10]. Systemic IV contrast should be avoided, and direct injection of a contrast medium can be performed with antegrade or retrograde pyelography. This allows evaluation of the collecting system and ureters and the opportunity for interventions such as stent placement. In cases where a detectable mass is not present, computed tomography and ultrasound, while helpful, are probably less sensitive and less specific than the retrograde ureterogram. Ultrasonography is not at all used to evaluate mid ureteral stricture or encasement; but is useful to diagnose if ureters are well distended in urinary obstruction due to a retroperitoneal mass and retroperitoneal fibrosis. However, overlying bowel often obscures visualization of the mid-ureter.
CT scan is the first choice to demonstrate ureters CT urography (CTU) is the major imaging modality for evaluating the ureters and secondary findings that help to narrow the differential diagnosis of the cause of the ureteral stricture and allows visualization of adjacent structures to differentiate an extrinsic pathology from an intrinsic process. For CTU, a three-scan CT protocol is used. After voiding completely all patients are asked to drink 800-1000 mL of water immediately prior to the examination [11]. The urinary system is imaged in both unenhanced and contrast enhanced CT (CECT) scan, by using multidetector helical CT scanner. On CECT, 100 mL of Non-ionic Low-osmolar contrast media (LOCM) is injected(2.5ml/sec); the kidneys are scanned 25-80 seconds after intravenous administration of the contrast for cortico-medullary phase, after 100 seconds for nephrographic phase, 8-10 minutes after contrasting medium injection for pyelographic phase, by using a maximum collimation of 1.0 mm. All three scans are performed with a tube voltage of 120 kVp and a tube current of 200 mAs.
Magnetic resonance urography (MRU) is very useful in patients with renal failure and or with Iodine contrast allergy. Absence of ionizing radiation, evaluation of paediatric patients and pregnant women, the high T2 signal intensity of urine in the dilated collecting system, are the advantages. Disadvantages of MRU are higher cost, motion artifacts from respiration and ureteral peristalsis, small field of view because of coil size nephrogenic systemic fibrosis secondary to gadolinium and bowel artifacts, However, when compared with CT, MRI offers better contrast resolution, but CT has higher spatial resolution [12-14]. Among Nuclear Medicine imaging in the setting of ureteral stricture and obstruction, diuretic renograms can be used to differentiate collecting system dilation from urinary obstruction. Radionuclide studies are not used for the detection of encasement of the ureters although positron emission tomography (PET) scan may show increased uptake. The role of PET in evaluating urothelial lesions is limited. Occasionally, ureteral obstruction may be identified in patients undergoing PET for other processes [15,16]. In general, on imaging, fibrosis displays hypoechogenic with acoustic shadowing at ultrasonography (US), hypovascularity at Doppler imaging, isoattenuating to muscle at computed tomography (CT), and isointensity at T1W1 and markedly low signal intensity at T2-weighted imaging. Due to reduced cellularity, the fibrous tissue typically does not show significant restriction at diffusion-weighted imaging. Contrastenhanced CT and MR will not be informative although delayed phase enhancement may be present. Other diseases mimicking Chronic fibrosing conditions such as mesenteric panniculitis, retractile mesenteritis, mesenteric carcinoid, Crohn disease with fibrofatty proliferation, and desmoid (mesenteric fibromatosis), carcinoid and carcinomatosis, mesenteric lipogenic liposarcoma will be the primary differential diagnoses [17].
IgG4-related sclerosing disorders
Retroperitoneal Fibrosis (RPF) is now considered an important abdominal component of the group of IgG4-related sclerosing disorders. It is an aggressive, rare fibro- proliferative process causing deposition of unencapsulated fibrous tissue masses in the retroperitoneal space usually centered at the lumbosacral junction. it is typically seen in men (2-3 times commoner than in women) between the 5th and 7th decades of life. Up to 15% of RPF patients have additional fibrotic processes in the mediastinum, thyroid (Riedel thyroiditis), biliary tree. sclerosing mesentery, and orbital tissue(pseudotumor). It leads to progressive encasement of the retroperitoneal structures, especially the ureters. and may cause hydronephrosis secondary to the extrinsic compression of the ureters [18]. RPF plaque may involve both ureters and may draw them medially towards the spine. This appearance on retrograde pyelogram resembles a narrow-waisted maiden (Figure 1a). Encasement of the ureter cause abrupt tapered narrowing of the ureteral lumen The dilated proximal ureter (the bullet) and the nondilated, encased distal ureter may appear as ‘The bullet and bodkin’ and this appearance can be caused by malignancy such as lymphoma, or rarely by RPF (Figure 1b). However, on the above imaging pictures, the ureteric encasement is not visually demonstrable. On CT, the area of fibrosis and encasement appears as a soft-tissue mass with variable contrast enhancement. The active and inactive stages of the disease can be differentiated on CT and MRI. In the retroperitoneum, RPF tissue surrounds the aorta (arrow) sparing posterior aspect (Figure 2a) indicating a benign stage. In contrast to the figure above, in malignant RPF, the RPF has mass effect with adjacent structures (Figure 2b below). Another noteworthy finding is the intensity difference of RPF mass on MRI imaging (Figure 3). A high intensity signal of the mass on T2WI is suggestive of early active RPF whereas a low intensity signal on T2WI is highly suggestive of benign RPF in a late inactive stage. On FDG PET/CT imaging, the fibrous tissue in the affected areas exhibit avid FDG uptake (Figure 4b). When the infiltrating tissue encompass both ureters and compromise the lumen, bilateral hydronephrosis will ensue regardless of the location of the mass in the abdomen or pelvis (Figure 5a-b). Abdominal pain and obstructive uropathy should, therefore, raise suspicion for retroperitoneal fibrosis and imaging should be obtained. On the other hand, new onset of hypertension, flank pain radiating to groin in a known case of RPF or malignancy should arise the suspicion of ureteral encasement and appropriate imaging should confirm [19]. Failure in recognition of the encasement of ureters and causes may lead to mistaken diagnosis with resultant inappropriate management.
Perianeurysmal Fibrosis
Reports of 56 previous cases showed striking male predominance. An abdominal mass may be palpable in 45% of cases. Fibrosis is often seen in association with atherosclerosis of the aorta (Figure 6), possibly due to fibrosis as an immune response to the leakage of ‘ceroid’ from the atheromatous plaque into the perivascular tissues. When perianeurysmal fibrosis occurs in association with an abdominal aortic aneurysm it may encase and produce ureteric obstruction and renal function impairment. The computerized tomography scan is able to provide accurate details of aortic aneurysm, intraluminal thrombosis, calcification, periaortic inflammation, entrapped ureters and hydronephrosis (Figure 6).
Neoplasm
Locally invasive neoplasm in the retroperitoneum and pelvis can involve ureters by three ways: 1. by malignant infiltration of their walls causing narrowing of their lumen (stricture) and affecting the peristalsis; 2. by external compression by the mass itself that may encroach, encompass and compress and displace the kidneys and ureters (Figure 7a) or by the adjacent aorto-caval and common iliac lymphadenopathy around abdominal ureter and internal and external iliac nodes around the pelvic ureter (carcinoma of prostate, uterus, cervix and or colon cause lymph node enlargement or organ enlargement); 3. by encircling and encasing the ureter as a whole. True hematogenous or lymphatic metastases to the ureter may occur from primary somewhere (Figure 7b). The primary may be in stomach, pancreas, lung, breast, neuroendocrine tumour) But it is exceedingly uncommon to find ureteral obstruction due to metastases to the ureter from distant primary tumors. Until now, only about 400 cases confirmed by post-mortem have been reported [20,21].
Endometriosis
Currently endometriosis is considered between benign and malignant status and is known as a “malignant” benign disease by experts. Its histopathologic, and molecular data suggest that endometriosis has malignant potential and is associated with ovarian cancer [22,24]. Although urinary tract endometriosis occurs in ~1% of women mainly in women of child-bearing age with pelvic endometriosis it may cause hydronephrosis by involving the ureters secondarily by encompassing and or compressing them. Any lapse or delayed diagnosis can lead to renal failure. Ultrasound, CT and MRI (Figure 8) may be revealed and or laparoscopy may reveal the encasement of ureters.
Conclusion
Both ureters are retroperitoneal throughout their course in the abdomen. An encompassing inflammation and or infiltrative malignant process usually cause circumferential wall thickening and an abrupt change in the diameter of the ureter resulting in dilated proximal ureter with a narrow or normal-calibred distal ureter. RPF is usually diagnosed through clinical presentation and imaging studies. The severity of RPF is emphasised by the fact that, in about 56%–100% of patients with idiopathic RPF, the fibroinflammatory tissue entraps the ureters and causes obstructive uropathy and subsequent renal failure. However, in any case biopsy should be considered to exclude malignancy. Any of the above discussed pathologies may involve the renal vessels and contribute to renal insufficiency or cause renovascular hypertension. New onset of hypertension, flank pain radiating to groin in a known case of RPF or malignancy should arise the suspicion of ureteral encasement and appropriate imaging should confirm. Failure in recognition of the encasement of ureters and its causes may lead to flawed diagnosis and irrecoverable damage to the kidneys. Early detection, corticosteroids, Radiation therapy, Retrograde ureteral stent and PCN placement, bilateral ureterolysis and resection of the aneurysm and other surgical interventions are some in the management algorithm to be chosen to broaden the treatment arena and provide encouraging results.
Acknowledgement and Disclaimer
The author would like to thank Taylor’s University, Malaysia for the time granted for reviewing work. I am also grateful for the insightful comments offered by the anonymous peer reviewers of the Journal of Urology & Nephrology studies. This paper would not have been possible without the exceptional support of my amazing partner A. Riaz Ahmed. His enthusiasm, contributions and the responses kept my work on track.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/index.php
0 notes
Text
Lupine Publishers | Lasers in Urology – what has Survived of our Research Starting 1970*

Short Communication
In 1970 I started to investigate the Laser-Technology for urologic surgery together with H. Müßiggang. First of all, it was to check the interacting of the different lasers with biological tissue. The result you can gather from the Figure 1 Decisive are the two factors: absorption and scattering of light into the tissue. The strong light absorption of the CO2 – laser leads to an excellent incision effect with low edema reaction. The light of the is mainly absorbed in the tissue by hemoglobin and pigment colorings and therefore suitable for the destruction of highly vascularized tumors or malformations. For achieving greater volume effects, - necessary for destruction of solid tumors, bilharzial bladder-lesions and inflamed areas in interstitial cystitis, - the Nd:YAG – laser was used by us since 1976 (Figure 2). Presupposed for the clinical application of lasers was the developing of a quartz glass fiber transmission system by Nath, a physicist from the Neuherberg Laser Labor (1973) and the developing of a special cystoscope insert, designed by my working-group (Staehler, Frank et al.) and constructed by the Storz Compagny/Tuttlingen/Germany (Figure 3). The next steps were the laser induced shock wave lithotripsy, developed between 1978 to 1986 (Munich/Lübeck) and the photodynamic procedures for early tumor diagnosis (Figures 5a & 5b).
Now is to ask – what survived?
The laser-lithotripsy, especially in ureter, and after a long fight, the photodynamic diagnosis for bladder tumors and Cis, are on routine. Also in tumors of external genitalia, - e. c. condylomata acuminate, hemangiomas, penile cancer, - the ND:YAG- and Diodelasers are well established. Established are also the vaporization and enucleation of prostate tumors by laser. In contrast to these indications, the Nd: YAG-laser-application in bladder-tumors seems to be forgotten. I think this method is head and shoulders above the common TUR-resection because it generates a total homogenous necrosis with closing the blood- and lymphatic vessels simultaneously, it means, no bleeding, no tumor-cellspreading. You can perform the operation under excellent viewing, so that it`s possible to destroy also any satellite-tumors and the tumor vessels (Figures 6a, b, c, d, e). Histological examinations of the tumor tissue after Nd:YAG-laser-application have never been problematic according to our pathologist Dr. Keiditsch. It depends on the fact, that during the coagulation the temperature didn`t exceed 100 C°, it means, the cell-structure remains unchanged. I observed 5 patients, who refused the radical cystectomy, only treated by ND:YAG –laser, between 10 and 40 years. They all remain tumor free. Our photodynamic therapy of localized prostate cancer, published 1993, is still in the early stages. It seems, nobody is interested on this procedure. Forgotten seems to be also our interstitial laser coagulation (ILC) of prostate adenomas and bladder-neck-stenoses, although by this procedure the retrograde ejaculation can be avoided in the most cases (Figures 7a-f).
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers group#lupine journals#urology#nephrology#kidney#open access journals#juns#submissions
0 notes
Text
Lupine Publishers | Chronic Kidney Disease, Data from MIKD

Abstract
Chronic kidney disease (CKD) is a leaping up public health matter. It has an augmented effect on cardiovascular diseases and affects every system in body. In developed countries the extent of prevalence is available due to renal registries. In middle or low socio economic countries the proportion of this evolving health issue is not known. CKD is rising and a major contributor is soaring number of diabetes mellitus worldwide. Other significant addition in this cohort of CKD is by rising numbers of obese and hypertensive patients. In order to curtail this rising health matter immediate and intense measures both at preventive and curative levels are required.
Aim: Due to lack of formal renal registry we wanted to see spectrum of chronic kidney disease (CKD) in our catchment area and at what CKD stage they present to tertiary care hospital.
Methods: All patients who presented to Emergency department of Multan Institute of kidney disease from 01 Sep 2017 till Sep 2019 data were evaluated. Record of Emergency department patients were taken from electronic system of our hospital. Some patients had multiple visits and we took first visits kidney function in our analysis. eGFR was calculated from serum creatinine with help of CKD-EPI equation.
Results: Total 4303 patients were included in study. Males were 60% and females 40%. Age range from 13 years to 96 year old. 945 patients were excluded as they were not falling in chronic kidney disease category. Remaining 3358 patients had chronic kidney disease. Sub-analysis according to CKD stage showed 66.17% patients presented at CKD stage V.
Conclusion: Kidney disease is rising globally. Countries where renal registries are established provide incidence of chronic kidney disease ranging from 10 to 15%. Still a lot of countries worldwide do not have established system of data collection so true incidence is not established. Our work is first of its kind reported from this area. Drastic preventive strategies are need of time from health budget planners.
Introduction
Multan Institute of kidney diseases is a tertiary care nephrology and urology hospital located in Multan (Largest city) in South Punjab, Pakistan. Catchment area include Multan division, Dera ghazi khan division and Bahawalpur division. According to last census the total population of these 3 divisions is just over 34 million. All patients who presented to Emergency department of our institute from 01 September 2017 till 01 September 2019 were taken from electronic medical record of hospital.
Inclusion & Exclusion Criteria
Total number of patients in our data are 4303. Patients from Age 13 year and above were taken in study. Less than age 13 were not included. Maximum age recorded was 96 year old. In case of multiple visits to Emergency department by same patient, only first presentation kidney functions were included in study and subsequent visits kidney function were excluded. 467 patients had normal kidney functions, normal urinalysis and no structural abnormality on renal imaging and were excluded from interpretation. 478 patients were found to have acute kidney injury as per acute kidney injury network (AKIN) classification. These patients were also excluded from analysis. Rest of the patients (n=3358) had chronic kidney disease [Table 1].
Kidney Function Measurement (eGFR)
Serum Creatinine was taken to measure estimated glomerular filtration rate (eGFR). Various methods of eGFR calculation are available, including the Cockcroft-Gault equation, MDRD (Modification of Diet in Renal Disease) Study equation, and the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. We took CKD-EPI for measuring eGFR. Patients were divided in CKD stages from 1 to 5.
CKD Stages and Prevalence
Total 3358 patients were identified with chronic kidney disease. Majority (n=2222) of patients presented when eGFR was <15mls/min for the first time to tertiary care facility. CKD stages with number of patients in each group are in following Table 2 [Figure 1].
Causes of CKD
We analyzed our data to look into etiologies of chronic kidney disease. It showed 30 % patients had diabetic kidney disease and same proportion were hypertensive nephropathy patients. Next in line was obstructive nephropathy with 23.85%. Glomerulonephritis was about 4.5% and roughly 10% patients cause was not established as they presented late. Inherited diseases were just over 1% and interstitial nephritis was just 0.47% [Table 3]. Among the patient cohort diabetic kidney disease was separated based on presence of diabetes mellitus and one of the criteria (proteinuria, normal size kidneys, diabetic retinopathy on fundoscopy or >5 years duration of diabetes mellitus). Nearly 30% of total patients had diabetic kidney disease and more than 2/3rd of them had CKD-5 on presentation. A detailed analysis of CKD stages of diabetic group is in following table [Table 4] [Figure 2].
Discussion
Chronic kidney disease is rising worldwide. It has big impact on health economics [1,2]. Developed countries have registries showing its prevalence which helps in health budget planning. Unfortunately under developed countries lack such planning. In Pakistan recently a national renal registry has started working and will give informative statistics regarding renal disease spectrum. This will help in health management planning and focusing on areas where rampant growing renal issues are focused.
Reviewing published data spectrum of renal disease is variable in different geographical areas [3, 4]. Ethnicity, dietary habits, use of herbal medicines, low birth weight & climate all have been postulated as contributing factors in prevalence of chronic kidney disease. According to a Meta-analysis, chronic kidney disease has prevalence of 11% to 13% globally [5]. In Pakistan studies published on chronic kidney disease prevalence are usually from tertiary hospital or region based [6]. Tertiary care centers are in urban cities so there is no good data from rural areas. South Punjab of Pakistan majority comprise of rural area. According to last census the population of south Punjab 3 divisions (Multan, Bahawalpur and Dera Ghazi Khan) is 34 million [7]. This is the catchment area served by Multan institute of kidney disease (MIKD). MIKD is an emerging kidney institute in this area. We analyzed all patients who presented to Emergency department of our institute. We analyzed presentation creatinine and used CKD-EPI Creatinine Equation for eGFR calculation as recommended by the National Kidney Foundation [8]. Chronic kidney disease in Pakistan is on rise and presentation to specialist center is late. Majority of patients present with CKD-stage 5 requiring dialysis. Our hospital’s 2 year record tells us that 66.17% patients who attended Emergency room were at CKD-5 and majority required renal replacement therapy. Diabetic kidney disease was alarmingly on 29.66 %. Further sub analysis of diabetic kidney disease patients revealed that majority patients (72%) were at CKD stage 5 when presented to hospital emergency department. We labelled diabetic kidney disease based on presence of one of the following criteria’s (established diabetic retinopathy and/or micro albuminuria and/or long duration (>5 years) of diabetes mellitus). Further stage of CKD was established based on CKD-EPI calculation. Diabetes mellitus is at tremendous rate in Pakistan. International diabetes federation data reveals that 17.1% rising adult population of Pakistan is now living with diabetes mellitus [9]. With such enormous rise in diabetics in last decade, the burden of diabetic nephropathy is on rise too. In rural areas a lot of people are undiagnosed and usually present to health facility with end organ damage. According to systematic analytic study done in 2016 prevalence of diabetes mellitus in Pakistan is more in males as compared to females and more common in urban areas [10]. The rising burden of diabetes is imputed to environmental and emotional changes. The main contributors are sedentary lifestyle including internet and TV usage, caloric rich diets leading to increasing obesity. Pakistan with very high numbers of prevalent diabetes needs a cost-effective population based approach for screening [11]. Obstruction leading to chronic kidney disease was immensely at 23.85% in our studied population. Obstructive nephropathy due to stone is highly prevalent in this area and reviewing published data the number is almost 2-3 times in comparison to other parts of world. Nephrolithiasis is a highly prevalent disease worldwide with rates ranging from 7 to 13% in North America, 5–9% in Europe, and 1–5% in Asia. Due to high rates of new and recurrent stones, management of stones is expensive and the disease has a high level of acute and chronic morbidity [12]. Epidemiologic studies have demonstrated that the stone risk incidence increases with Body Mass Index, through multiple pathways. Metabolic syndrome and diabetes are associated with an increased renal stones disease incidence [13, 14]. There is a known high incidence of stone disease in Pakistan as this country belongs to the so-called stone belt [15]. In summing up our chronic kidney disease burden we have identified Diabetic kidney disease, Hypertension and Kidney stones as our bulk. This helps in health budget planning and preventive strategies to cut down disease load. Lack of renal registry in Pakistan has been emphasized [16]. Recently at national level Pakistan renal data system (PKDRS) is established and will provide data from all parts of country in coming years [17]. Curtailing diabetes will have significant reduction in diabetic kidney disease and strategies have been proposed. Creating multidisciplinary team, primary preventive strategies and nationwide diabetic care programme are proposed steps to deal this impending pandemic [18].
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers#lupine journals#urology#nephrology#open access journals#articles#submission#lupine publishers group
0 notes
Text
Lupine Publishers | Lymphocele after Renal Transplantation: A Contemporary Review and a Modern Approach for Prevention and Treatment

Abstract
A lymphocele is a common finding after renal transplantation. The majority of patients are asymptomatic. However, once a lymphocele has become symptomatic, this condition has to be treated. Lymphoceles may originate either from the lymphatic system of the recipient or the transplanted kidney. The most sensible measures to prevent their occurrence therefore seems to be to restrict the transplant bed to the smallest permissible level with careful ligature of the lymphatic vessels in the area of the kidney hilum.
Therapy of a lymphocele after renal transplantation should commence with minimally invasive measures and continue with invasive procedures only if these are unsuccessful, namely, puncture and drainage then sclerotization, and then laparoscopic or open marsupialization.
Keywords: Lymohocele; kidney; transplantation; recipient
Introduction
Lymphocele is a well-known complication of renal transplantation occurring in 0,6% to 22% of the recipients [1- 4]. Lymphocele may require surgical intervention because of the complications they cause urinary obstruction, leg edema, deep vein thrombosis, pelvic discomfort, herniation, and lymph leakage through the wound [5]. There are many contributing factors to lymphocele occurrence after kidney transplantation. One of these is donor renal lymphatics. It has been proposed that meticulous ligation of severed lymphatics of the kidney graft in the back table especially in the laparoscopically procured kidneys may decrease the lymphatic complications after transplantation [6]. Although various methods of diagnosis, management, and prevention have been discussed in the literature, the primary focus has been on treatment and no review has summarized all issues together. The aim of this study was to summarize the current strategies for the prevention and management of lymphoceles.
Etiologies and surgery related factors
The development of lymphoceles after renal transplantation is well documented. The etiology of lymphoceles remains unclear, although they are present in all kidney transplant experiences [7]. The old controversy whether lymphocele is the result of lymph leakage from either the severed recipient iliac lymphatic vessels or the grafted kidney lymphatics seems to favor the latter [8]. A physiological review shows that lymphatic capillaries are more abundant in the kidney cortex compared to the medulla. They run along the intralobular, arcuate and interlobar arteries; not only beside theses arteries but also within their walls [9]. At the renal hilum, 2 to 5 lymphatic ducts are found in close proximity to the main vessels (renal artery and vein).
The well-known and commonly cited contributing factors for lymphocele formation include: the type of immunosuppression used [10], high dose steroid use, use of diuretics, extensive perivascular dissection of the iliac vessels, acute rejection episodes, delayed graft function, source of graft (cadaveric vs living related donor), the etiology of the patient’s renal failure such as adult polycystic kidney disease, re transplantation, and some pediatric population [2, 7, 11]. Concerning the pediatric population, in a retrospective single institution review of 241 pediatric kidney transplants performed from 2000 to 2013; Giuliani et al. showed that older age (≥11 yr), male gender, BMI percentile for age ≥95%, and multiple transplantations were Significant risk factors for lymphocele formation [12]. The formation of post-transplant lymphoceles obviously originates in the surgical transection of lymphatic ducts. As demonstrated by lymphangiography two sources of lymphatic leak have been proposed: injured lymphatics in recipient’s iliac space and injured lymphatics in the kidney graft [13,14].
A possible distinction between these two origins is feasible by analyzing their composition. In fact, reports showed higher levels of creatine kinase in lower limbs lymphatics vessels compared to renal lymphatics [15-17]. It was believed that the perivascular lymphatics dissection along the iliac vessels was a determining factor for lymphocele development, and that lymphocele could be prevented by ligation of these vessels. Despite many reports showing absence of lymphocele after an accurate ligation of the iliac lymphatics [18], things are still unclear. Many studies were published concerning the influence of some surgical aspects in decreasing the lymphocele incidence. Indeed, one prospective study suggested a cephalad implantation of the renal graft using vascular anastomoses on the common iliac vessels to minimize lymphocele incidence, but this technique has not yet gained wide exposure [11]. The same concept was reevaluated in another study. This time, a significant reduction of the incidence of lymphocele from 8.5% to 2.1% was noted in 140 patients operated with the new technique versus 140 patients in the control group operated with the standard method [14].
Another retrospective study done by Saidi et al, evaluated the impact of laparoscopic living donor nephrectomy on lymphatic complications after kidney transplantation. They concluded that the incidence of prolonged lymphatic leak is higher in recipients who received kidney grafts procured laparoscopically. These observations may indicate that the major source of persistent lymphatic leakage is lymphatics of the allograft rather than severed recipient lymphatics. More meticulous ligation of severed lymphatics of the kidney graft in the back table, especially in the laparoscopically procured kidneys, may decrease the lymphatic complications after kidney transplantation [13]. To our date, many researchers are still questioning whether surgical preparation of the kidney with accurate ligature of the hilar lymphatic vessels would effectively reduce its incidence. Hence, a clear answer is reported in our study favoring lymphatic vessels ligation over non preparation of the kidney graft on lymphocele incidence. Indeed, acute rejection rates dropped significantly from 15 to 6.3%, and incidence of symptomatic lymphocele decreased from 17.5% to 0%.
Diagnosis and Clinical Aspects
Ultrasound is currently the preferred method for diagnosis of lymphoceles after the renal transplantation. In complicated cases, radioisotope imaging, computed tomography and magnetic resonance imaging are additional methodologies commonly used [5]. Lymphoceles may lead to deterioration of renal function and the patient with a lymphocele may be inappropriately treated for allograft rejection. Other clinical findings associated with lymphoceles in renal allograft recipients include lower abdominal swelling or mass, edema over the allograft or of the ipsilateral leg, hypertension, drainage from the incision, enlarged allograft, fever without an obvious source of infections, urinary frequency, ipsilateral ileo femoral thrombo phlebitis, and weight gain [4].
Prevention
Prevention of lymphocele formation primarily involves the best method for controlling perivascular lymphatic leaks. A study comparing surgical ties to ultrasonic devices in the surgical dissection technique for control of lymphatics failed to show a statistical advantage to either technique when groups were compared based on patient age, gender, graft source, or repeat transplant [19]. Berardinelli et al. demonstrated the effectiveness of a synthetic polyethylenglycol(PEG) sealant to prevent lymphocele formation after kidney transplantation [20].
Treatment
Lymphoceles are usually asymptomatic and diagnosed incidentally by ultrasound. In most cases, lymphocele disappear spontaneously without any need for a treatment. Several important factors can guide our choice of treatment: severity of the symptoms, lesion size, potential post-therapeutic complications, and the clinical condition of the patient. For the conservative treatment of posttransplant lymphoceles, percutaneous needle aspiration, continuous drainage over a period of time via various kinds of catheters, and sclerotherapy with various agents have been proposed [21].
Aspiration
Ultrasound-guided aspiration can be used as a diagnostic tool or treatment. to both diagnose and treat a lymphocele. It can be used as the initial treatment modality to relieve urinary obstruction, recover kidney function, and prevent emergency situations. Although simple, safe, and economical, a repeated treatment may be necessary with a low a low risk of infection in each aspiration. A systematic review by Lucewicz et al. [4] looking at over 20 studies, reported that simple aspiration alone has a recurrence rate ranging between 10% and 95% [ 22].
External drain placement
A lymphocele can also be treated by external drainage by placing a drain. However, this procedure takes a long time and can cause problems related to major fluid loss and secondary infection (particularly in immunosuppressed transplant recipients). External drainage has an efficacy of 50% and a recurrence rate of 20%–60% [23].
Sclerotherapy
The instillation of a sclerosing agent is another treatment approach. These include povidone iodine, fibrin glue, 95% ethanol, fibrinogen, bovine protease inhibitor, human thrombin, calcium chloride, gentamy sodium tetradecyl sulphate and tetracycline]. The sclerosing agent has been instilled and kept in situ for varying periods ranging from 5 min to 24 h [24,25]. Tasar et al., reported a mean therapy duration of 17 days and a mean alcohol volume of 30 cm3 per session. Out of 18 cases, there was one recurrence, one graft loss, and ten minor complications including local discomfort and low- grade fever. The authors concluded that this method of sclerotherapy was safe and cost effective [25]. Another analysis of 30 lymphocele patients demonstrated that alcohol injection was a safe and cost-effective treatment, with a success rate of 94%. The authors reported two cases of recurrence and all complications were minor, including catheter-induced infections and catheter displacements [26]. Povidone iodine has been used also as a sclerotherapy agent with a failure rate of less than11%, but it takes 20–30 days for leaking to cease and iodine induced acute kidney failure may occur [27]. Limited success has been reported using tetracycline as a sclerosing agent [28].
Instillation of sclerosing agents improves the rate of success of percutaneous management; however, it may cause a dense scar around the renal transplant with potential problems in the longterm [21]. Continuous drainage as well as repeated instilling of sclerosants could be done if needed, by placing a percutaneous drain. However, the main problem encountered during repeated installation of sclerosants is the risk of introducing infection. Furthermore, several case reports have reported direct graft injury and graft loss as a result of sclerosant installation [25]. Hence, with the cost of repetition, it is worthwhile emphasizing that external drainage or sclerosing therapy are not correct options. Post-transplant lymphoceles have also been treated with a combination of percutaneous aspiration and sclerotherapy. Although this reduced the recurrence rate, recurrences were still reported in 20% of cases [22].
-Surgery: Byron et al. [29] first described open surgical internal drainage in 1966, and these techniques have successfully been used in many patients; however, they still reflect an invasive procedure. The operative strategy is to perform a peritoneal fenestration through a laparotomy, minilaparotomy, or via a laparoscopic approach. Some authors suggest the use of an omentum flap to decrease the risk of lymphocele relapse, but others do not [30]. Open surgical drainage of lymphocele is required in the presence of infection (external drainage) or where laparoscopic fenestration is not possible (internal drainage to the peritoneum). The open procedure is safe and 100% effective because the lymphocele can be localized accurately. However, the recurrence rate is still 15%. This may be attributed to the high rate of lymph vessel injuries incurred during the open method. The recurrence rate of the laparoscopic method is lower (0%– 10%) because the rate of lymph vessel injuries is lower. Also, the hospitalization period is shorter in this method [31]. In a meta-analysis, Lucewicz et al reported that 12% of laparoscopic operations had to be converted to open surgery, due to technical difficulty in reaching the lymphocele, peritoneal adhesions, thick, impenetrable lymphocele capsule and injury to abdominal viscus [21]. Indeed, it would be helpful in some cases to use an intra-operative ultrasound can avoid organ injury during laparoscopy. Schips et al reported a technique by which the lymphocele was laparoscopically fenestrated under diaphanoscopic guidance and the lymphocele cavity was dilated through the injection of a sterile fluid. Using this approach, the authors were able to determine the exact site of the incision by detecting the light of the cystoscope [32]. Laparoscopic fenestration can cause intestinal herniation into the peritoneal window leading sometimes to strangulation requiring urgent intervention. However, in this era of laparoscopy, open drainage is only of historical importance. in addition, the effectiveness of the laparoscopic approach along with its, low recurrence rate, and low complication rate make it the treatment of choice when other methods fail [28].
Conclusions
Lymphoceles are common and well-known complications that occur in up to 26% of kidney transplant recipients. The cause of lymphocele formation is unclear, but it is believed to result from transection of the lymphatic vessels accompanying the external iliac vessels during transplantation surgery and subsequent lymph accumulation in a nonepithelialized cavity in the extra-peritoneal plane adjacent to the transplanted kidney. In order to prevent the formation of a lymphocele, preparative steps should be kept to the necessary minimum, and lymph vessels in the vicinity of the kidney hilus carefully ligatured. Therapy of a lymphocele after renal transplantation should commence with minimally invasive measures and continue with invasive procedures only if these are unsuccessful, namely, puncture and drainage then sclerotization, and then laparoscopic or open marsupialization.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupinepublishers#lupine journals#journal of urology & nephrology studies#renal replacement therapy#diabetic nephropathy#Dialysis#hemofiltration#submissions#articles#open access journals
0 notes
Text
Lupine Publishers | Efficacy and Safety of Prolonged Alfuzosin Treatment in Patients with Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia: 7 Years of Observation

Abstract
Introduction: Amongst all of the medications prescribed to BPH patients undergoing conservative therapy, α1-blockers were used in 80% of cases. The question of optimal treatment length is one that has been constantly asked during the last years. The current study will encompass the data we have collected of a small study group of BPH patients treated with 10 mg of Alfuzosin daily, over a period of more than 7 years.
Material and Methods: From 2009 to 2011, 41 patients with mean age 67.7 years began medical therapy taking 10 mg of Alfuzosin per day. The mean treatment length was 7.6 years as of today. 16 patients (39%) with prostates exceeding 60 cm3 were additionally prescribed 5-ARIs.
Results: Overall, a positive dynamic was found in 85.4% of patients. Not a single patient chose to discontinue treatment. A very high level of satisfaction was reported by 88% of our patients. A statistically significant (P < 0.05) decrease in IPSS scores by 8.5 ± 6.1 (47.5%) was found. Mean QoL indices decreased from 3.7 ± 1.1 to 2.3 ± 1.1 over the period of observation. Mean Qmax values increased from 9.7 ± 0.52 mL/s to 14 ± 0.60 mL/s (an increase of 44.3%).
Conclusions: This study has demonstrated a high level of safety and efficacy when using Alfuzosin to treat voiding dysfunction in patients suffering from LUTS/BPH. Our study resolves the issue surrounding a prolonged course of α1-blockers, which, due to its widespread use, remains the gold standard for medical treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia.
Keywords: LUTS, BPH, Alfuzosin, BOO
Introduction
It is well known that the prevalence of voiding dysfunction and patient age are in direct proportion and that prevalence is particularly high in patients over 50 years of age. Typical complaints associated with voiding dysfunction are termed lower urinary tract symptoms (LUTS) and have a wide variety of underlying causes. The most common aetiologies of LUTS are prostatitis, urethral strictures, cystitis, bladder stones, prostate cancer, and benign prostatic hyperplasia (BPH).
BPH is usually managed with surgical intervention, pharmacotherapy, or a strategy of watchful waiting, and numerous factors affect the decision of which method should be employed. Symptom severity is one of the most important subjective factors and is measured using the international prostate symptom score (IPSS) questionnaire. The nature of the symptoms (whether they fall under the category of obstructive or irritative) also influences the final decision. The primary objective factor can be considered to be the quality of voiding as measured by uroflowmetry, the interpretation of which should consist not only of the peak flow rate (Qmax), but also flow time, time to Qmax and many other indicators. Postvoid residual urine is an equally important value because it helps identify poor bladder contraction ability (detrusor underactivity) in the case of progressive intravesical obstruction. There are also those factors, no less important than both uroflowmetry and IPSS, which relate to the potential risk of BPH progression, and thus make their impact on the choice of treatment. Whilst the size of the prostate itself cannot be ignored, it is by no means a marker for surgical intervention when considered by itself, but rather a hallmark for potential future complications such as acute urinary retention (AUR) amongst other things. In recent years, prostate-specific antigen (PSA) levels have also become associated with BPH progression. Today, PSA levels are not only used to screen for prostate cancer, but also to reflect cellular proliferation rates of the prostate. Thus, high PSA levels increase the risk of rapid BPH progression. [1] The sum of the factors mentioned above influences the ultimate decision of which type of treatment should be employed in a specific patient.
Modern standards of medical practice dictate that pharmacotherapy is recommended in BPH patients with moderate LUTS without upper urinary tract complications, patients without obvious surgical indications, and patients who either decline surgical intervention, or are unable to commit to surgery in the nearby future. Watchful waiting is acceptable if a patient exhibits mild LUTS (IPSS ≤ 8), or if the patient’s symptoms do not significantly impact their quality of life (QoL). Patients who fall under this category should be made aware of the necessity to maintain an appropriate lifestyle as well as the importance of regular blood, urine, PSA, uroflowmetry and ultrasound testing. Surgical intervention is needed if a patient exhibits severe symptoms with upper urinary tract complications, or if there are sufficient reasons to suspect that medication will be ineffective[2,8].
In order to better illustrate modern tendencies in the treatment of BPH, we turn our attention to the TRIUMPH study. The TRIUMPH study recorded the treatment and outcomes of 2351 newly presenting LUTS/BPH patients in 6 European countries over a 1-year follow-up period. Out of the entire study population, 23.8% were managed with watchful waiting, 72.5% were prescribed medication, and only 2.7% underwent surgical treatment. These statistics accurately reflect the rise in medical management of LUTS as well as the decreasing need for surgical intervention during the past decade. It should be interesting to note that amongst all of the medications prescribed to BPH patients undergoing conservative therapy, α1-adrenergic receptor antagonists (α1-blockers) were used in 80% of cases [3].
The efficacy of α1-blockers in patients with BPH has been well documented in a large quantity of double-blind, placebo-controlled studies.[4,5,6,7] If it is possible to consider transurethral resection of the prostate (TURP) the method of choice for surgical intervention in BPH patients, then α1-blockers will fall under the same criterion for the medical management of BPH. It is important to note that the European Association of Urology considers α1-blockers the first line of therapy for the management of voiding dysfunction in patients suffering from BPH[8].
Whereas the rationale behind α1-blockers does not arouse suspicion in the majority of specialists, the question of optimal treatment length is one that has been constantly asked during the last 6 years. Solutions offered to address this problem range from a life-long course of medication, to one that only lasts a few months. There is no one correct way to approach this matter, but it is the duty of every qualified specialist to incorporate certain principles when making such a decision. Personal experience and knowledge gained from a wide variety of international studies should encompass the core of such precepts. It is arguable that the lack of a formulated strategy that applies to such clinical trials is hindering the formation of a unified point of view. If we turn to medical literature, we find that despite an abundance of studies indicating the effectiveness of α1-blockers in BPH patients, the studies lack the duration necessary to examine long-term effects and are mostly comprised of 3-6 month trials with only a few mentioning 1-3 year-long results. This has led many to assume that a 3-month course (being the most prominent study length) of α1-blockers is sufficient, whilst the actual reasoning behind the short length of most studies lies in the knowledge that the results achieved during that period of time will continue over the remaining course of treatment, however long it may be. Confirmation of this can only be found in a few currently available publications that demonstrate long-term results. Most articles cite results that were obtained using studies based on principles of Good Clinical Practice (GCP) in which the categories of patient inclusion/exclusion were so rigid, they created unrealistic expectations for the wide variety of patients that are actually treated. Only a few trials provide us with results that are based on a realistic study population. Such trials include those of Roehrborn C. et al., Elhilali M. et al. 2006 and Vallancien G. with Emberton M. et al. 2008[9,10,11].
During the early 21st century, the suitability of α1-blockers was no longer debated in the Russian Federation, whilst the question of long-term use was left unanswered even for us. Even now, considering the ageing population and the necessity of prolonged medical treatment, many specialists are hesitant to prescribe α1-blockers for more than a few years without consulting medical literature about the safety and efficacy of such a treatment.
Materialand Methods
The current study will encompass the data we have collected of a small study group of BPH patients treated with 10 mg of Alfuzosin daily, over a period of more than 7 years. We must mention that the data provided isn’t final, in the sense that patients are still undergoing treatment to this day. We had not originally planned for this to be a study with a specific design, but retrospective aspects make it similar to various non-comparative, real-life surveillance studies. Our goal is to introduce the reader to the results of a longterm course of α1-blockers that, in our opinion, are a safe and effective method of long-term medical therapy in patients suffering from BPH. From 2000 to 2002, 41 patients with mean age 67.7 years (range 51– 83) began medical therapy. The mean treatment length was 7.6 years as of today. We would like to mention that this group of patients wasn’t specifically chosen as a study group at the time, nor were they the only patients that had been prescribed Alfuzosin. Our detailed observation of the isolated group in question is partially due to chance, and partially due to loss to follow-up. Patients began treatment taking 5 mg of Alfuzosin twice daily. From January 2005 onwards, all patients take 10 mg of Alfuzosin once daily at night due to numerous complaints of increased voiding problems during this time. Prostate volumes fluctuated between 25 cm3 and 100 cm3, and 16 patients (39%) with prostates exceeding 60 cm3 were additionally prescribed 5-alpha-reductase inhibitors (5-ARIs). During the early stages of treatment these patients were prescribed Finasteride, but during the last 2 years many have switched to Dutasteride in accordance with our recommendations.
Indications
Patients with LUTS/BPH
Contraindications
Patients with surgical indications Patients known to have insufficient results from taking α1- blockers in the past Patients with postural hypotension Patients taking alternative medication for LUTS with good results Patients with unstable angina (pectoris) Patients with life-threatening comorbidities
The mean duration of morbidity at the time of patient inclusion in the trial was 1.9±1.1 years. During ultrasound testing, 31.2% of patients had postvoid residual urine measuring 82 ± 41.7 mL. Mean PSA levels of all patients before the trial were 2.9 ± 2.8 ng/mL. The initial data of the patients included in this trial are summarized in Table 1. Out of the 41 patients, 14 (34.1%) had hypertension 11 of which (26.8%) were receiving some form of hypotensive therapy, and 5 patients had suffered a myocardial infarction before inclusion into the trial. The efficacy of the treatment was analysed using IPSS scores as well as QoL indices. Additional factors included Qmax and prostate volume in patients who were receiving combination therapy with 5-ARIs. The safety of the treatment was analyzed based on recorded incidents of adverse cardiac side effects (mainly hypotensive) during the trial. Vital-sign dynamics were also analyzed. Follow-ups were conducted biannually during the first 2 years, and no less than annually thereafter. Two-sided hypothesis tests were conducted with a significance P-value cut-off of 0.05. Dynamics of safety and efficacy variables were analysed before and after the trial (absolute and relative change was considered). A paired Student’s t-test was used if statistics followed a normal distribution; a Wilkinson’s test was used if they did not.
Results
Not a single patient chose to discontinue treatment, notwithstanding the long period of observation. We must mention, however, that several patients stopped being prescribed Alfuzosin during the early stages of the trial but were obviously not considered in the study group. Subjective assessments of patient satisfaction were carried out using a questionnaire specifically tailored to this publication and not used in our routine clinical practice. A very high level of satisfaction was reported by 88% of our patients, whilst the remaining 12% consisted of those actively taking a large quantity of medication for various comorbidities, and patients with diabetes. Many of the aforementioned patients were prescribed medication after they began treatment with Alfuzosin. Therefore, these facts could be taken into consideration by general practitioners who have patients undergoing treatment for LUTS.
Mean IPSS scores were 17.9 ± 5.3 before the treatment, and 9.4 ± 5.2 after 7.6 years. A statistically significant (P < 0.05) decrease in IPSS scores by 8.5 ± 6.1 (47.5%) was found. A decrease in IPSS scores by > 50% was noted in 28 patients (68.3%). Overall, a positive dynamic was found in 85.4% of patients. IPSS score dynamics are summarized in Table 2. Dynamics of irritative (questions 1, 3, 5 and 6 on the IPSS questionnaire) and obstructive (questions 2, 4 and 7 on the IPSS questionnaire) symptoms were also analyzed. Mean irritative IPSS scores decreased from 6.3 ± 2.9 to 3.7 ± 2.4 (33.7% lower) whilst mean obstructive IPSS scores decreased from 9.6 ± 4 to 5.5 ± 3.7 (35.7% lower). Incidents of nocturia decreased from 2.4 ± 1.1 to 1.5 ± 1.1. Diagram 1 illustrates a major decrease in the percentage of patients with moderate to severe voiding dysfunction symptoms as well as a six-fold increase of patients with mild symptoms. All of the statistics mentioned above were statistically significant (Figure 1). QoL dynamics are presented in diagram 2. Mean QoL indices decreased from 3.7 ± 1.1 to 2.3 ± 1.1 over the period of observation. The mean decrease in QoL indices was 32.3% (reflecting an increase in QoL). All changes were statistically significant. Overall, 72.7% of patients had an increase in their QoL (Figure 2).
It is important to note that the minority of patients who did not demonstrate clear improvements in symptom severity either underwent surgery or continued to take Alfuzosin. The latter group either demonstrated a minimal but satisfactory improvement or was unable to undergo surgery for various reasons. We believe that it is of utmost importance to understand that the current publication presents a comparative analysis before and after Alfuzosin treatment, and whilst all of the patients had demonstrated an improvement during various periods in the trial, the dynamics of IPSS scores and changes in QoL were not registered at those intervals. It is also important to highlight the role of the detrusor in BPH symptoms, something that was originally demonstrated by Russian authors O.B.Loran and E.L.Vishnevskiy in 1998. The contraction capability, level of energy metabolism, and nature of the biochemical events of the detrusor influence voiding quality no less than the level of intravesical obstruction.[13] This could explain treatment ineffectiveness in certain patients.
Changes in Qmax over the course of the study were considered as a secondary measurement of treatment effectiveness and were analysed during every follow-up, making this particular variable very intriguing. Mean Qmax values increased from 9.7 ± 0.52 mL/s to 14 ± 0.60 mL/s (an increase of 44.3%). Diagram 3 illustrates Qmax dynamics throughout the entire length of the study. One can conclude that early treatment results remained constant throughout the observation period (Figure 3).
During the period of observation, 5 patients underwent TURP procedures. The rationale behind TURP in one patient was due to an episode of AUR provoked by alcohol ingestion. After 3 days of catheterisation, the patient did not show any signs of improvement in voiding ability and was subsequently operated. The other 4 patients exhibited worsening LUTS as shown by an increase in their IPSS scores. During ultrasound and transrectal ultrasound testing, a median lobe was detected in all 4 patients. All operations performed did not deviate from a standard TURP procedure. The median prostate volume of the 16 patients receiving combination therapy had decreased. The value fell from 74.1 cm3 before treatment to 50.1 cm3 after 7.6 years of therapy (a decrease of 32.4%). Throughout the trial, PSA levels of all patients were measured annually. Median PSA levels were 2.9 ng/mL before the trial and 3.2 ng/mL afterwards, demonstrating no significant variance. PSA levels of patients undergoing combination therapy were considered after the second year of treatment and were doubled. PSA levels of over 4 ng/mL requiring transrectal ultrasound-guided multifocal prostatic biopsies were noted in 3 patients aged 68–74 years. None of the subsequent histological analyses revealed the presence of cancerous cells.
The safety of this treatment was assessed based on the frequency and type of adverse side effects recorded during the trial. In total, 9 patients (22%) exhibited adverse effects. The most common findings were dizziness (2 patients/4.9%) and asthenia (2 patients/4.9%). Another 3 patients experienced dyspepsia, shortness of breath and headaches. Out of the 16-patient subgroup receiving combination therapy, 2 patients complained of decreased libido (possibly attributed to 5-ARIs). One of these patients had their sexual drive normalise after 1 year of therapy, whilst the other retained a low libido throughout the treatment. Considering the relatively small study population involved, a true understanding of the statistical significance of these adverse effects as well as their immediate connection to the treatment in question is not possible. However, the fact that most of the aforementioned adverse effects were present as isolated episodes in patients during the first week of therapy demonstrates a high level of treatment safety.
Discussion
The current study has demonstrated a high level of efficacy with minimal adverse side effects in the treatment of lower urinary tract symptoms (LUTS) arising from benign prostatic hyperplasia (BPH) with α1-adrenergic receptor antagonist (α1-blocker) Alfuzosin. The effectiveness of the treatment was determined using subjective and objective criteria. A 45.7% decrease in LUTS severity as measured by the International Prostate Symptom Score (IPSS) questionnaire was found in 85.4% of the entire study population. Mean Quality of Life (QoL) indices measured using the QoL scale increased by 32.2% and mean peak flow rate (Qmax) values increased by 44.3%. The fact that the positive results achieved during the early months of therapy had stabilised and showed no signs of regression during the entire study period is quite remarkable. Such results verify the notion that a prolonged course of α1-blockers provides consistent long-term results. The high level of patient satisfaction with treatment results and the information that patients themselves have provided us with, mark not only the fact that patients are not bothered by the need to take medication daily, but also (and most importantly) the great sense of confidence patients feel regarding the stability of the results they have achieved, leading to a positive outlook on their future treatment.
Several observational studies indicate results very similar to ours. In the ALFORTI study, 311 patients on Alfuzosin were surveyed over a period of 9 months. A decrease was shown in mean IPSS scores and QoL indices measuring 45.6% and 36.4%, respectively. Adverse effects (most likely due to the medication in question) occurred in 4.4% of the study population.[12] A similar study published by the same authors analyzed the safety and efficacy of Alfuzosin. A decrease in mean IPSS scores and QoL indices was shown and measured 32% and 27.8%, respectively[14]. Combination therapy in the form of α1-blockers and 5-alphareductase inhibitors (5-ARIs) is currently the recommended form of treatment in patients with LUTS and an increased prostate volume according to the European Association of Urology.[12] The safety and efficacy of this treatment has been confirmed by a considerable decrease in LUTS severity and prostate volume (a mean decrease of 32.4%) in our 16 patient subgroup. Similar results have also been demonstrated by such well-known trials as MTOPS, ALTESS and COMBAT[1,2,8,9].
When the information provided by the various authors is analysed, it becomes apparent that Alfuzosin attains a similar degree of effectiveness in every single case. The fact that the results gained from a continuous course of therapy remain consistent throughout prolonged treatment is extremely important, but perhaps the most interesting observation made during our trial is that the considerable results are not only long-lasting, but are achieved and made stable during the first month of treatment. A study conducted in 2008 by Vallancien G. et al. is one of the few studies (similar to ours in design, but not in duration) that analysed the data collected in a real-life surveillance study format regarding the effectiveness of Alfuzosin during a 3-year course of therapy. The results were as follows: mean IPSS scores decreased by 33.4%, mean QoL indices by 40.7%, and nocturia severity by 25.5%. Adverse side effects (likely related to vascular dilation) were registered in 4.5% of cases, and surgical intervention was required in 5.7% of cases. These results are clearly similar to ours. [10] Similar findings were also recorded earlier during the ALFONE trial published by Elhilali M. et al. in 2006[11].
Whilst several adverse cardiac effects were noted, the analysis of adverse side effects in our trial is far from thorough, which is explained by the lack of the patients enrolled in the trial leading to insufficient statistical integrity. A study involving a multidisciplinary approach could aid the understanding of various guarantees that we may be able to give our patients in the future. These days, patients mostly consist of elderly men whose age and comorbidities make the use of pharmacotherapy ideal for managing the symptoms associated with BPH. Perhaps tomorrow, the necessity of prescribing Alfuzosin for over 10 years will increase considering the continually regressing number of patients who choose surgical intervention for BPH management; those that wish to retain a good quality of life whilst remaining ‘real men’ for many years to come. Today, when patients inquire about the potential length of their treatment, they are told that they may take Alfuzosin for as long as it is required.
Conclusions
This study has demonstrated a high level of safety and efficacy when using Alfuzosin to treat voiding dysfunction in patients suffering from LUTS/BPH. Furthermore, it is important to mention that many researchers have come to the same conclusion. Our study, whilst potentially lacking in statistical magnitude, resolves the issue surrounding a prolonged course of Alfuzosin, which, due to its widespread use, remains the gold standard for medical treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/
#lupine publishers#journal of urology & nephrology studies#journals#kidney disease#nephritis#submission#opena access journals#articles
0 notes
Text
Lupine Publishers | Journal of Urology & Nephrology Studies is wishing you a very Happy Thanksgiving Day!!!✨✨

0 notes
Text
Lupine Publishers | Robot-Assisted Radical Prostatectomy our Technique Description
Abstract
Objective: To describe step-by-step technique in Robot Assisted Radical Prostatectomy of our transperitoneal posterioranterior technique for prostatic dissection with preservation of the endopelvic fascia, preservation of the puboprostatic ligaments and dorsal venous complex.
Materials and Methods: Description of our surgical technique over 80 patients who underwent RARP and the characteristics group from 2016 to 2019 excluding the rest of cases who went to different surgical approach and technique for robot-assisted radical prostatectomy.
Results: The mean age was 63 years old, 7% of patients were overweight and 7.5% had obesity. The mean pre-operative prostate volume was 42.620 cc, mean prostatic specific antigen (PSA) of 10.414.8 ng/dl. The mean console time was 198±47. The surgical margins were positive in 13.75% of the patients. Complications were recorded in the peri-operative period, five (6.2%) Clavien-Dindo I and six (7.5%) Clavien-Dindo II.
Conclusions: After 8 years of experience in our center we have modified our technique of robot assisted radical prostatectomy, improving our results, following different worldwide concepts in the prostatic dissection. Even if necessary, to increase the number of cases we have find an easier way to reproduce with acceptable results.
Keywords: Robotic Radical Prostatectomy, Prostate Dissection, Transperitoneal Approach
Introduction
In the past year’s robot assisted laparoscopic surgery is becoming more easily available in Latin America, in México was introduce by our department in Mexico, City in 2013 we accomplished the first robot assisted radical prostatectomy. At the beginning we started performing an anterior technique, which is, in most centers the standard of treatment for localized prostate cancer. The technique varies depending on the place of learning or according to initial proctoring and preferences. Twenty years ago, was introduced the laparoscopic approach, at that time was usual the retrograde dissection starting with the apex. Guillonneau et al., described the mixed technique modifying the Mountsouris technique where they used and antegrade and retrograde approaches in 7 standardized steps. [1]After in 2003 following several years of evolution in the robotic radical prostatectomy technique the Frankfurt group published a case series of their robot-assisted technique using ascending and descending techniques for prostate approach [2]. In 2012 Asimakopuolus et al., described their intra fascial dissection of the neurovascular bundle [3]. Several approaches since that time have been created. In this article we describe our technique, which has been modified since our initial experience from 2011, after learning the lateral anterior approach in France, and then modifying to a mixed technique in between anterior and the Bocciardi approach[4].
Materials and Methods
Description of our surgical technique over 80 patients who underwent RARP and the characteristics group from 2016 to 2019 excluding the rest of cases who went to different surgical approach and technique for robot-assisted radical prostatectomy at the same time.
Technique step-by-step
We include a single surgeon experience over 80 cases from January 2016 up to December 2019 of Robotic Assisted Radical Prostatectomy, following the next description of the technique.
Port placement and Docking
The trocars placement starts once needle Veress insufflation of the cavity is performed. We use a four-arm X da Vinci robotic system (Intuitive Surgical, Sunnyvale, CA, USA). First robotic trocar is placed one centimeter higher to the umbilicus, two da Vinci X ports on the left side, first one, four cm to the left of the camera trocar and the second one, four more centimeters according to the anterior axillar line. On the right side, one more robotic port (8 mm) four centimeters from the camera port in a horizontal line. Two more accessory ports for the assistant are placed, one 12 mm port in a triangle between the camera port and first robotic port on the right, and a 5 mm port down and 3 centimeters upper the iliac crest (Figure 1).
TransperitonealPosterior Dissection
Sigmoid dissection is performed to allow enough space in the rectovesical area. Upper traction is done by the prograsp instrument in the middle line in between bladder fatty tissue and the line of the rectum. We follow the vas deferens reflection to start the perineotomy through the pouch of Douglas. Reaching the vas deferens we transected, we follow them laterally for each side preparing the seminal vesicles, as the description of Montsouris technique. The next step is to dissect the full posterior base of the prostate, opening the Denonvilliers fascia reaching the apex of the prostate area where the urethra can be visualized, once we finish the medial space in between the prostate and the rectum, we start the nerves bundles preservation. By manipulating the right vesicle with the fourth arm, we expose the angle of the vesicle tip and the base of the prostate. The inferior and superior portions of the lateral face of the prostate are dissected. Then we reproduce the same on the left seminal vesicle, up traction to expose the base of the prostate and we perform the nerve sparing on an antegrade way, if necessary we place 5 mm clips coming from one of the assistant ports to avoid the bleeding from the capsular arteries going through the prostate and to control the prostatic pedicles. We can reproduce different degrees of preservation, intrafascial, inter or extrafascial [5,6] (Figure 2). Once posterior base and lateral walls of the prostate are finished, we tract the seminal vesicles and perform a forward an up dissection in direction of the bladder neck, leaving the most anterior prostate bases (left and right) the closer to the start of the bladder neck, at this time we release the seminal vesicles and we move to the lymph node dissection, once accomplished the approach goes anteriorly.
Transperitoneal Anterior Dissection
We go traditionally anteriorly to create the Retzius Space with a parietal peritoneum incision, down the level of Cooper Ligament; we identify all the anterior prostate suspension structures by removing the fatty tissue that surrounds it. The bladder at this time has been pull up by the forth arm, we perform and incision at the lowest medial level of the puboprostatic ligament without opening the endopelvic fascia but very near to the lateral prostate capsule, we do respect the maximum length of the puboprostatic ligaments [7]. The endopelvic fascia is preserved, we go laterally to the prostate capsule from the initial incision up to the level of the bladder neck anteriorly and laterally, because of the previous down to up dissection and nerve sparing form the posterior dissection we can easily visualized the nerves already spared. The same steps are reproduced in the right side, sparing the endopelvic fascia, and the maximum length of puboprostatic ligament, going down till the bladder neck shape appears (Figure 3). Once both sides accomplished, we do a close traction by the fourth arm Prograsp and decreased the bladder catheter balloon to 5 cc. A U inverted incision on the anterior wall of the bladder is done, a very spare bladder neck is accomplished by cutting the posterior bladder neck area, following deeper to a fully access to the previously dissected seminal vesicles, this step allows a very well neck sparing technique [8].
Lateral Prostate and Apex
Next step is to move the prostate lateral dissection toward the apex, going close and down to the dorsal venous complex, without cutting it or suturing it, we follow the angle going down to the level of the urethra respecting the anatomical position of the plexus over the urethra, the plexus stays at the level of the respected puboprostatic ligaments and rounded endopelvic fascia. We correctly identified the urethra diameter and transected with the maximum length possible. The Denonvilliers fascia bellow properly dissected avoids posterior reconstruction. For the urethral – bladder anastomosis a van Velthoven technique is perform using a 3-0 V-Loc [9]. Finally we use the same V-Loc suture from each side of the anterior line of suture to recreate a suspension-like hammock stitches; this is accomplished by using the end tip of the suture from the lateral portion of the neck bladder to the previous position of the puboprostatic ligament, with this we enhance hypothetically, better continence. A Foley 18 fr catheter is placed with 15 cc inside, finalizing the procedure. Prostate is removed through the camera incision port.
Results and Discussion
The patients were aged between 44 and 86 years old (mean: 63 years old). The most common co morbidity was high blood pressure (n=17), seven percent of patients were overweight and 7.5% had obesity. The mean pre-operative prostate volume was 42.620 cc (range: 9.3-115), mean prostatic specific antigen (PSA) of 10.414.8 ng/dL (range: 0.9-131) and mean positive cores per biopsy were 4.93 (range: 1-12). The mean console time was 19847 minutes (range 120-400) and intraoperative blood loss was 368263 (range 50-1300). The uretro-vesical anastomosis was performed with the van Velthoven technique in 80 patients (100%). In 17 (21.3%) patients a closed suction drainage was placed. [10] Five (6%) patients required blood transfusion, and none required conversion to an open approach [11] (Table 1). No major complications were recorded in the peri-operative period, five (6.2%) Clavien-Dindo I and six (7.5%) Clavien-Dindo II.
The RARP Gleason score were 6 in 36 (45%), 7 in 26 (32.5%), 8 in 7 (8.8%), 9 in 9 (11.3%) and 10 in 2 (2.5%) patients. The T clinical stage and D’Amico risk group is described in Table 2. Pelvic lymph node dissection was performed as follows: 9 (11%) standard/ obturator, 23 (28%) extended and 11 (13%) super extended. The surgical margins were positive in 11 (13.75%) patients. The most common positive surgical margin was at the level of prostatic apex. [12]We found a positive Pearson correlation between RARP Gleason score and positive surgical margins (r=0.539, p=0.01). The mean hospital stay were 3 days (2-7 days), and the urethral catheter was removed in a mean period of 51 day (5-10 days) [13,14].
Respecting, as other authors, the puboprostatatic ligaments and the santorini complex as well as the endopevic fascia we can spare much more the prostate fossa, avoiding too much invasion on the pelvic structures. [15] Our final stich for an anterior suspension, keeps part of the anatomy form the bladder to the ligaments. As demostrated by Galfano et al., we bealive that starting the radical prostatectomy through the pouch of Douglas is an easier way to improve later during the procedure a most precise definition to the bladder neck as also a better definition of the anterior anatomical structures, and maybe is also a proper start if we want to fully perform a robotic retzis sparing radical prostatectomy [16].
Conclusions
We decide after four years of performing transperitoneal anterior dissection approach and base in different worldwide leaders and techniques for robotic radical prostatectomy, that some of the steps in the learning curve could be challenging, we started posteiror dissection with good overall outcomes and a good reproducible technique. Due to hight evolution in the technique we decide to follow steps to simplify the bladder neck approach. With posterior dissection, we can reproduce, from the vesicle tip and going laterally up to the body of the prostate a very fine neurovascular bundle dissection. Going thought the Retzius space with the previous posterior dissection gives a clear anatomical landmark to define a proper bladder neck sparing and to approach the Santorini plexus from behind and below avoiding anatomical damage and excessive blood loss. Different techniques have been described for robot assisted radical prostatectomy, and the preference and expertise of surgeon allows making different possibilities to the surgical robotic approach.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/
#lupine publishers#lupine group#open access journals#nephrology#urology#renal system#journal of urology & nephrology studies#lupine journals#openaccess#submissions#manuscripts#articles
0 notes
Text
Lupine Publishers | Relationship of Oxidative Stress and Paradoxic Vasaconstrictions of Cavernous Arteries

Introduction
Oxidative stress is one of the most common pathological processes, the essence of which is an imbalance in the state of the pro- and antioxidant systems of the blood, organs and tissues. This indicates the need for its direct pathogenetic correction, which can be carried out by means of specific and non-specific antioxidant therapy. Oxidative stress (OS) is a state in which the amount of free radicals generated in the body significantly exceeds the activity of endogenous antioxidant systems that ensure their elimination [1,2].The imbalance between the synthesis and elimination of reactive oxygen forms, affects the homeostasis of cellular oxidative stress, plays an important role in the development of a number of cardiovascular diseases (CVD) in the pathogenesis, including arterial hypertension, hypercholesterolemia, atherosclerosis, diabetes mellitus and heart failure [3-5]. Reactive oxygen species (ROS) are initially normal components of cellular metabolism and perform essential regulatory and metabolic functions in the body. Free radical reactions are necessary for the formation of many vital enzymes, as well as for the normal function of the immune system and its components. Sharp fluctuations in their concentration in cells and tissues can cause many pathological conditions in the body. The formation of OS, due to increased formation of ROS, especially superoxide anion (O-2) and insufficient mechanisms of antioxidant protection indicates the development of functional and structural disorders of the cardiovascular system. The main characteristic of CVD disorder in the vascular wall is endothelial dysfunction. In vascular cells, a superoxide anion is formed from ROS, which is inactivated by superoxide dismutase (SOD). Endothelial dysfunction is caused not by a decrease in nitric oxide (NO) production, but by excessive O-2 formation, which leads to oxidative inactivation of NO and a decrease in its bioavailability. In addition, O-2, directly or through the product of its interaction with NO (peroxynitrite), is capable of initiating peroxide damage to the cellular and matrix elements of the vessel wall, leading to disruption of the interaction of the endothelium with cellular elements and blood lipoproteins. These data indicate that the production of superoxide radicals and other forms of oxygen, uncontrolled by physiological antioxidant enzymes, can be considered as a potential source of vascular dysfunctions, a frequent manifestation of which is endothelial dysfunction.It has been established that both stable high and periodically increased glucose levels induce the development of OS, which, in turn, stimulates apoptosis of endotheliocytes [6]. Direct determination of the level of oxidants under in vivo conditions is practically impossible, since these are extremely reactive and, therefore, short-lived compounds. Ideal OS markers are oxidation products of lipids, carbohydrates, proteins, and nucleic acids, whose lifespan ranges from several hours to several weeks.8-iso-PgF2a (8-isoprostane) is considered to be one of the most specific biological markers that allow one to assess the level of free radical production with a sufficient degree of accuracy, reliability and reproduction of research results. 8-isoprostane is a metabolic product in the reactions of peroxidation of arachidonic acid, isomeric prostaglandin F2 and its amount is directly proportional to the level of formed free radicals [7].OS is one of the links in the formation of pathogenetic changes in the body [8]. An increase in OS and a decrease in antioxidant protection lead to mitochondrial DNA damage and depletion of adenosine triphosphate [9]. Today, the role of oxidative stress in the development of endothelial dysfunction has been proven [10]. Molecules formed by oxidation can serve as biomarkers. Their analysis is used to quantify oxidative stress in humans.Biomarkers of mitochondrial dysfunction and oxidative stress are:-8-isoprostane, malonic dialdehyde; O-tyrosine, 3-chlorothyrosine, 3-nitrotyrosine; protein oxidation products (AOPP);8-hydroxyguanosine (8-OHG); 8-hydroxy-2’-deoxyguanosine (8-OHdG); cellular mtDNA (its number and the presence of mutated variants with deletions); endogenous antioxidants (glutathione, cysteine, uric acid, ubiquinol) [11]. Comprehensive assessment of OS, expensive research, infrequent use and relatively long-term implementation, makes this research method limited. It should be noted that not every medical institution can allow the use of the OS assessment research method. The need to find a method for evaluating OS that differs in prostate availability has become relevant at the present time. In connection with the above, it is of interest to use, as an estimate and a potential regulator of the balance between the synthesis and elimination of ROS, infrared radiation of the far range (IRR FR), which includes electromagnetic oscillations with wavelengths from 1 to 10 mm [12]. One of the main properties of IRR FR is the dependence of the results of exposure on the phase of biological development and on the initial state of the object: the IRR FR practically does not affect the normal functioning of a healthy organism [12-14], and in the event of a pathology, it can regulate its functioning within the limits inherent in this biological kind [15,16]. ROS have a molecular spectrum of radiation and absorption of IRR FR (wavelength 10-2-10-4 m; frequency 1011-1013 Hz) [17]. It was found that the reactivity of molecules excited by an IRR FR quantum will be an order of magnitude higher than when excited by an extremely high frequency electromagnetic radiation quantum [18].
Purpose of research
To assess OS and the relationship with paradoxical vasoconstriction of the cavernous arteries.
Materials and Methods
17 men with CVD and cardiovascular risk factors aged 35 to 65 years (mean age 51.3 ± 1.33 years) were examined in a hospital. The control group consisted of 7 men without CVD and risk factors. Blood for biochemical, enzyme-linked immunosorbent assay was taken in the morning on an empty stomach, the next day after the patient was admitted to the hospital, 12 hours after eating. Blood sampling was performed from the cubital vein. The criteria for excluding patients from the study were concomitant inflammatory endocrine and other diseases that could affect the activity of oxidative processes. Overweight was assessed using the body mass index (Quetelet index), which is defined as the ratio of body weight (kg) to the square of height (m). According to modern data, 8-isoprostane (8-PI) is considered to be one of the most specific markers that allow, with a sufficient degree of accuracy, reliability and reproducibility of research results, to assess the level of production of free radicals in the body in a wide variety of pathologies. It is a metabolic product in the reactions of peroxidation of arachidonic acid, isomeric to prostaglandin F2α (PGF 2α). It belongs to the family of eicosanoids, the formation of which occurs during non-enzymatic (free radical) oxidation of phospholipids of cell membranes [9,10]. Its content in blood serum was determined by the enzyme immunoassay using the 8isoprostane ELISA kit from USBiological (USA). The data obtained were expressed in pg/ml. All men underwent a comprehensive examination, which included the collection of a general medical and sexological history, a general examination, a study of hormonal status, lipid spectrum and blood glucose. All patients underwent a questionnaire survey the International Index of Erectile Function (IIEF-5).In addition, all men underwent a study of the endothelial function of the cavernous arteries, using the method of ultrasound examination of changes in diameter after exposure to narrow-spectrum (far range) IR emitters.
The Percentage Increase in the Diameter of the Cavernous Arteries (PIDCA) was calculated by the formula: Formula 1. PIDCA = 100% x (D 2 –D 1) \D1. Where D1 is the average diameter of both cavernous arteries before irradiation with an IR emitter. D2 - the average diameter of both cavernous arteries after irradiation. The threshold value of PIDCA for identification of arteriogenic ED from other forms of ED was 30%. PIDCA <0% was regarded as paradoxical vasoconstriction.
Results and Discussion
The content of 8-isoprostane and the endothelial function of the cavernous arteries in the examined men of 2 groups are presented in Table 1. As you can see from the Table 1, the patients of the main group showed an increase in the content of 8-isoprostane in the blood serum by 14.1 times as compared with the control group. PIDCA characterizing endothelial function was within the normal range in men of the control group. In patients of the main group, endothelial dysfunction was revealed, and in 82.3% (n=14) cases, paradoxical vasoconstriction of the cavernous arteries was recorded (PIDCA <0%). Only in 3 patients (17.6%) the endothelial function of the cavernous arteries was recorded as positive. However, these indicators were below normal, which indicated the presence of endothelial dysfunction.
Correlation analysis between the OS biomarker (8-isoprostane) and paradoxical vasoconstriction of the cavernous arteries in patients with CVD and cardiovascular risk factors showed a negative relationship (r = -0.365; p = 0.043). Of the surveyed men in the main group, 23.5% were overweight; 76.4% were obese. Type II diabetes mellitus affected 5 (29.4%) men; hypercholesterolemia/dyslipidemia was detected in 94.1% (16) patients; active smokers were 7 (41.2%) patients. When analyzing the IIEF-5 questionnaires of the main group, it was found that the severity of ED was as follows: mild in 5 (29.4%) men, moderate in 10 (58.8%) patients, and severe in 2 (11.8%). Thus, the data obtained indicate an increase in the activity of 8-IP in the blood serum, which is interpreted as the formation of oxidative stress in CVD and cardiovascular risk factors (obesity, smoking). These indicators correlate with literature data [19-21]. The study of the 8-IP level is the gold standard for determining the OS activity in persons with CVD, as well as in patients with diabetes mellitus, obesity, hypercholesterolemia, and smokers [22].OS forms the development of endothelial dysfunction, the manifestation of which can be a predictor of dangerous vascular disease, and, therefore, can be used as a screening assessment for men after 35 years. The manifestation of endothelial dysfunction can be both disturbances in the normal blood flow in the penis (decrease or disappearance of spontaneous and adequate erections), and disturbances in the coronary circulation system. Violation of blood flow can manifest itself as erectile dysfunction, and as atherosclerotic coronary artery disease. Erectile dysfunction is a manifestation of endothelial dysfunction without intermediate stages, while atherosclerosis of the coronary arteries most often develops asymptomatically for a long time and manifests as acute coronary syndrome and ischemic heart disease. The OS, being a systemic process, first of all subjects the vascular endothelium to functional changes, spreading from the “periphery to the center”, that is, from the capillaries to the large main or organ vessels. The mechanism of paradoxical vasoconstriction of the cavernous arteries can be explained as follows. Under the influence of IRR FR, with a frequency corresponding to the molecular spectra of emission and absorption of NO and ROS, phagocytes (monocytes) of blood and endotheliocytes are activated. This leads to the release of NO and ROS, which increases the oxidative stress of the endothelium. In addition, excess NO can bind with superoxide radicals to form the strongest oxidizing agent, peroxynitrite. This leads to endothelial dysfunction, ATP depletion, and increased production of the vasoconstrictor endothelin 1.
Conclusion
Paradoxical vasoconstriction of the cavernous arteries is a manifestation of systemic oxidative stress at the level of regional circulation.The use of long-range infrared radiation in the diagnosis of endothelial dysfunction of the cavernous arteries can also be used to determine the severity of oxidative stress. The use of IRR FR in diagnostics of the system OS has unconditional advantages - these are non-invasiveness, simplicity and speed of execution, the possibility of repeated use, and most importantly, it is economically uncomplicated and beneficial.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/
#lupinepublishers#open access journals#nephrology#urology#Journal of Urology & Nephrology Studies#lupine publishers group#articles#research#submission#lupinejournals
0 notes
Text
Lupine Publishers | Private Health Insurance is a Favourable Prognostic Risk Factor in the Outcomes of Mortality Post Radical Cystectomy

Abstract
Objectives: There are well recognised differences in health outcomes generally in society due to a range of socio-economic factors. We have compared survival outcomes in patients undergoing radical cystectomy for aggressive bladder cancer in the national health service (NHS) and compared this with those with private health insurance (PHI).
Methods: This is retrospective study of 225 NHS and 32 PHI patients operated over 14 years by one surgeon. ASA scores were compared to approximate comorbidity status.
Results: There were significantly worse outcomes for all cause and disease specific mortality in the NHS group. There was no difference in ASA scores.
Conclusions: NHS patients fare worse than those with PHI. The reasons for this difference remain unclear. PHI patients have a more extensive lymphadenectomy and have fewer overall complications.
Keywords: NHS private insurance outcomes radical cystectomy bladder cancer
Introduction
We studied the outcome of patients with private health insurance (PHI) compared to national health service (NHS) patients. These patients had the same operation for muscle invasive cancer of the bladder, and we looked to see whether there were significant differences in both all-cause mortality and disease specific mortality. We have attempted to see whether we can propose any explanatory cause for such differences. There are barriers in society that delay detection and the treatment of cancers for those people with various social and economic disadvantages [1]. This imbalance creates health disparities such as differences in survival [2]. There are racial, gender, ethnic and socioeconomic components which are reflected in their insurance status [3]. Previous studies, mostly in the United States, although with some English and Danish studies, have looked at the effect of insurance status on outcomes of treatment for various cancers, including urological cancers of the prostate, kidney and bladder [4]. In the USA, the uninsured and those on Medicaid, have a worse stage at presentation [3-7]and a lower all cause survival than those with private insurance [2,1,8]. Worse disease specific mortality has also been shown to be an independent risk factor with worse outcomes for lower socio-economic status groups [9]. This finding, however, was not confirmed by a British study specifically looking at social deprivation [10]. Definitions of what constitutes adverse social groups are complex and may affect robustness of data [11]. A shift in classification of groups, pathological definitions and coding of cause of death may explain the lack of improvement in survival but not socioeconomic inequalities [11]. Many factors, including socio-demographic and clinical entities, form a complex network influencing survival. In the USA Insurance and socioeconomic status are predictors of mortality [1,8]. Uninsured patients are often treated at low volume centres[3]. Race and gender can also affect survival [12,13] as can communal poverty [14,15]. It has been suggested that poorer health generally, as demonstrated by more comorbidities and unhealthy lifestyle behaviours account for this phenomenon [2,15]. The affordable care act in the USA is an attempt to address the problem of the uninsured presenting with more advanced disease and their subsequent lower survival rates [16]. In addition, a negative association between income and prevalence of physicians for bladder and renal cancer mortality was found [17]. Those on Medicaid were less likely to receive definitive treatment [18]. Similar effects of socioeconomic status have been found in England [19]and Denmark [20]. These studies have all looked at the effect of all the range of treatments, both curative and palliative, for all types of cancer, including low- and high-grade disease which is of particular importance when discussing bladder cancer. For differences in outcomes after specific surgical treatments, research has focused on radical prostatectomy. Insurance status is associated with stage and cancer control [21]. Higher surgery rates and lower radiotherapy rates were observed in private patients who were more affluent [22]. We have shown previously that insurance status is a protective risk factor with radical prostatectomy, but wealth itself is not protective which may be due to different adverse lifestyle behaviour[23]. There are few published studies pertaining to insurance status and survival after a specific treatment for bladder cancer, particularly radical cystectomy [24].
Effect of comorbidity and ASA score
Greater comorbidities as assessed by the ASA score, increase the likelihood of complications after radical cystectomy [25]. Patients with an ASA score of three or more have a greater complication rate [25]. Severe comorbidity is also associated with increased mortality [26,27]. The ASA score has been used to predict outcomes [28] and should be used for counselling and risk stratification [29,30]. In younger patients the ASA score can be useful despite EAU guidelines suggesting otherwise [31,32]. Neglect of comorbidities can cause misleading impressions of results [33] but the ASA score can be useful at to ascertain complication risk particularly with the type of urinary diversion and the time taken to do more complex surgery [34]. Comorbidity assessed using the Charlson score is useful for younger and older patients to predict toxicity of treatment and outcomes [35,36]. All-cause mortality has a stronger relation to comorbidity than disease specific mortality does [37].
Urinarydiversion
In general, the neobladder has a higher complication rate and longer stay in hospital [38] although it may be offered to most patients [39-41]. However, in practice, it still tends to be reserved for younger and fitter patients and thus may serve as a proxy for generally better health [42].
Methods
This is a retrospective study. The 257 radical cystectomy with bilateral lymphadenectomy (with ileal conduit or neobladder) operations were performed by one surgeon in both NHS and the private sector in the same county of the UK, operated from 1999 to 2013 and followed up to the present day. There were 225 patients from the NHS and 32 patients with private health insurance. These were sequential patients who underwent potentially curative operative and neoadjuvant/adjuvant treatments. We compared various patient characteristics from health records using Iqutopia for operation details, ICE, Bostwick laboratories and Masterlab for pathology and PACS for imaging. Medical statistics were done using Medcalc software for logistic regression analysis to determine significant predictors of mortality and progression. Fishers, Mann-Whitney, t tests and log rank tests to compare Kaplan Meier. P values less than 0.05 were considered significant. The ASA score was documented by the attending anaesthetist and retrieved from Iqutopia where available.
Results
(Table 1-4) (Graph1-6)
There is a significant difference between the ages of the two populations with NHS patients being older by four years. Private patients had a greater degree of nodal dissection and fewer complications. However, there was no difference in tumour volume, positive surgical margin status, advanced stage, carcinoma in situ, nodal involvement, nodal extracapsular extension. Further, there was no difference in rates of neo adjuvant or adjuvant treatment or the use of a neobladder. Logistic regression showed private health status to have highly significant negative coefficients which are protective in all cause and disease specific mortality as well as lower progression rates. All-cause mortality was associated with negative coefficients for neobladder and PHI. Locally advanced stage was predictive of a deleterious outcome. Disease specific mortality also showed adverse coefficients for additional treatments (adjuvant and neoadjuvant), complications and nodal density involvement. Progression was adversely affected by additional treatment and increasing stage. The ASA score was available in 169 of the 257 patients (66%). There was no difference in the distributions of healthy and unhealthy patients as measured by this score. (ASA1 and 2 were summated). Log rank tests showed significant differences between the two groups for mortality.
Discussion
This study has supported generally what is known about bladder cancer and the negative impact of lower socio-economic status. However former studies have looked at bladder cancer as a whole, which includes low grade and non-muscle invasive disease as well as advanced muscle invasive disease, inoperable cases, cases treated with radiotherapy and palliative cases all grouped together. This study looks at a subsection of these, those deemed appropriate for radical cystectomy. We have seen significant differences in both all-cause mortality and disease specific mortality. These differences have not been previously demonstrated. There are many gross and subtle differences that are difficult to detect with such a heterogenous disease in a heterogenous population. We propose that looking at a subgroup like this will decrease some of the obfuscating factors, by limiting geographical variation and the operation being performed by one surgeon only.
All-causemortality
We have detected a significant difference in all-cause mortality (Graph 5) with NHS patients (Graph 2)faring far worse (log rank P = 0.025) and with an odds ratio 0.24 in favour of PP (Graph 1, Table 2). Overall survival at one year is 0.75 for NHS and 0.84 for PHI, and at five years it is 0.45 for NHS and 0.74 for PHI. Similarly, at ten years for NHS patients it is 0.34 compared to 0.53 for PP (Table 3). Numerous explanations have been proposed for the worse prognosis overall for bladder cancer in the uninsured [1- 12]. Overall worse health is the proposed principal reason for a worse all cause survival. We have tried to reflect potentially worse comorbidities with the ASA score as an approximation to overall health. There was, surprisingly, no significant difference detected between the two groups (P = 0.56) (Table 4). Private patients were four years younger on average and any chronic disease may not have reached a critical point to effect outcome (P =0.026) (Table 1). This was not a significant factor on regression analysis. We do see that having a neobladder is a significant (P = 0.0154) protective (negative coefficient -0.8) factor, perhaps standing as a proxy for overall health [38,39], but there was no significant difference in the use of this between the two groups (P=0.16) (Table 1). Locally advanced stage 3 and 4 were both significant as expected [43, 44] with increasingly worse odds ratios (Table 2). The five-year gold standard for radical cystectomy is about 50% [43-49], and our population in the NHS reflects this. The patients with PHI fare much better with a 5-year survival of 0.74.
Diseasespecificmortality
We found a significant protective coefficient and odds ratio of 0.33 in favour of private patients (Table2). There was a DSM of 0.87, 0.84 and 0.75 for PHI at one, five and ten years (Graph 3). This was superior to NHS patients with DSM of 0.82, 0.81 and 0.58 (Table 3, Graph 4). This compares favourably with other series (49). The log rank test comparing the two was significant (Graph 6, Table5). Interestingly, the NHS mass of tumour was twice that of PHI, yet there was no significant difference in tumour volume which one might expect if there were a longer time to diagnosis in NHS patients (11cc v 23cc P=0.18). Further, we did not detect a worse stage (NHS 56% localised, PHI 62% localised P=0.84) or a significantly greater positive surgical margin rate in NHS patients (14% NHS v 6% PHI P = 0.27). However, the trend was worse with all of these parameters for NHS patients. There was no difference in rates of CIS between the two. The use of neobladder was, once again, a protective risk factor (Table 2) which was used in 38% of PHI patients compared to 18% in NHS patients, however this did not reach significance (P=0.16) (Table 1). Additional treatments, neo-adjuvant and adjuvant therapies, were a significant detrimental risk factor (Table 2) but with no significant difference between the two groups (NHS 17% and PHI 13% P=0.79). The role of lymphadenectomy revealed no difference in severity of extracapsular invasion (Table 1)(P=1.0) or in nodal involvement with 19% of NHS patients having involved metastatic deposits within at least one node, compared to 22% of PHI patients (P=0.811). However, there was a significant difference in the number of nodes retrieved at lymphadenectomy, with an average of 9 for NHS and 13 for PHI patients (P=0.006). Node density is a significant predictor of DSM with an odds ratio of 9.67 (Table2). Complications, of all types, are a significant adverse predictor for DSM. With a significantly worse rate in NHS, 40% compared to PHI patients, 13% (P=0.002).
Progression
This also revealed PHI status to be protective and worse outcomes were associated with need for additional treatment and advancing stage. Time to diagnosis and time to treatment from diagnosis may be a factor, although studies are conflicting [50,51]. There is no lead time bias (perceived increased survival with no effect on course of cancer) as there is no screening protocol.
Conclusion
American studies have shown that insurance is important and that even Medicaid patients do worse than private patients. We concur that in the UK the NHS does not provide equal outcomes compared with PHI when comparing radical cystectomy. There may be social infrastructure issues involving hospital building capacity, education, prevention, detection and treatment. Assistance to patients to access and navigate healthcare systems and workplace policies to encourage overall better health is needed. National policies have been implemented to tackle this complex problem. The NHS cancer plan 2000 in the United Kingdom (http.//www.doh. gov.uk/cancer/pdfs/cancerplan.pdf) aims to reduce inequalities in survival between rich and poor with a 62-day target to treatment. The affordable care act in the USA highlights benefits of extending health coverage to the uninsured [1]. We have found significant differences in outcomes for all cause and disease specific mortality, yet the cause is elusive. We have shown a direct comparison of NHS and PHI to reveal these outcomes which can remain hidden in American studies as the SEER datebase does not include uninsured patients by definition. There is no difference in tumour volume, carcinoma in situ, advanced stage or not, nodal involvement, extracapsular involvement of nodes, rates of adjuvant and neo-adjuvant treatment. There are, however, differences in complication rates and extent of lymphadenectomy with favourable features pertaining to PHI. We need to look for other ways to measure overall health that include medical and lifestyle with socioeconomic components.
Limitations
The PHI cohort is small. We have not measured the time to diagnosis and to treatment. We have not been able to document the Charlson comorbidity score. We have not documented types of complications.
For More Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here:
https://lupinepublishers.com/urology-nephrology-journal/
#lupinepublishers#lupinejournals#journal of urology & nephrology studies#nephrotic syndrome#renal artery stenosis#nephrology#urology#lupine publishers llc#lupine publishers group
0 notes