study blog?? FIU graphic design BFA senior, starting BS in biology next year. bad at studying consistently so I’m processing all the material as I learn it here.
Don't wanna be here? Send us removal request.
Text
CHAPTER 2.1-2.4 NOTES 2.1 atom: the smallest quantity of matter that retains the properties of matter element: substance that cannot be broken down further into simpler substances
atoms were first proposed as an idea by Democritus (philosopher) in fifth century BC * originally thought to be indivisible but can actually be further divided into subatomic particles * number of each subatomic particles as well as their arrangements and individual natures are what determine the properties of atoms and therefore the properties of the matter they form
2.2 like charges repel and opposite charges attract (Coulomb's law)

cathode ray: negatively charged plate, electrical current sent through, cathode ray goes out from the negative plate to the positive plate, then to specific points on the screen at the end of the cathode based on if there is an electrical or magnetic field around it or the two fields cancel each other out or there is no field at all. electrons discovered using this because the negatively charged plate repelled the cathode ray, indicating the presence of negatively charged particles that were repelled by the negative charge of the first plate.
Millikan used charged oil particles to determine the mass of an electron based on the amount of electric field needed to suspend a particle between two charged plates. He used the charge to mass ratio, already calculated by another scientist, and the charge of just one individual electron to determine the mass of an electron. He deduced the charge of an individual electron based off the fact that every charge he measured was a multiple of -1.6022 x 10^-19 C(coulomb), which we now know is the charge of an individual electron.
radioactivity: spontaneous emission of particles or radiation from unstable nuclei
three types of radiation: *alpha rays: made of positively charged particles (alpha particles). repelled by positively charged plate and attracted to negative. *beta rays: made of negatively charged particles (beta particles). repelled by negatively charged plate and attracted to positive. *gamma rays: no charge, higher energy, unaffected by magnetic or electrical fields.
protons(positively charged subatomic particles) are contained in the nucleus in a concentrated core. atoms are mostly empty space, so most alpha particles can pass through with out being repelled by the positively charged nucleus. however, when the alpha particles do get close to the nucleus they experience a repulsive force, making them not able to pass straight through and are thrown off course. if they travel directly at the nucleus they may be repelled so much their direction is completely reversed.
protons have same magnitude of charge as electrons, just in the opposite direction (positive not negative), and almost 2000x the mass of electrons. nucleus is very tiny but tons of mass- occupies very little volume (atom = stadium, nucleus = marble).
use picometers (1 x 10^-12 m) for most atomic dimensions
2.3 all atoms except most common form of hydrogen (has one proton no neutrons) have both protons and neutrons (nucleons).

isotope: atoms of an element that have the same atomic number (number of protons) but a different mass number (protons and neutrons). therefore isotope is determined by number of neutrons and identified by the element's mass number.
chemical properties of an element are determined mostly by number of protons and electrons, not neutrons, so what isotope an element is doesn't have very much impact on its chemical properties.
2.4
density: mass/volume (use g/cm^3 as units) volume of the nucleus can be calculated from known radius using formula for volume of a sphere(V = 4/3 π r^3). use g/cm^3 in calculations; convert other units to cm and g beforehand.
Atomic mass unit (amu) is equivalent to 1.67 x 10^-27 kg
all those protons in one tightly packed nucleus should repel each other. great force is required to maintain a stable nucleus. short-range attraction (proton-proton, neutron-neutron, proton-neutron) accomplishes this (in this case, strong nuclear force specifically). if the short-range attraction is stronger, the nucleus remains stable; if the coulombic repulsion is stronger, the nucleus deteriorates and emits particles or radiation. the electrons are kept from flying out of the orbit of the atom by their attraction to the positive protons. the electron's velocity and attraction towards the nucleus help it maintain its orbit.
nuclear stability determined by neutron to proton ratio. the higher the atomic number, the more neutrons needed to counteract the protons' repulsion.
2, 8, 20, 50, 82, and 126 neutrons or protons are most likely to be stable (contain the most stable isotopes compared to other elements). also more stable nuclei with even number of both protons and neutrons. atomic number higher than 82 are always radioactive (unstable). technetium and promethium are ALL radioactive, every isotope. ion: atom that gains or loses electrons, giving it a net charge. anion: negatively charged ion cation: positively charged ion

atomic mass isn't a whole number because it's the weighted average of the masses of each of the element's isotopes (remember each isotope has a different mass) in the percentages they were found in in the sample used (each isotope's mass is multiplied by the percentage in which it was found in the sample). ex. sample is 99% Carbon-12 and 1% Carbon-13; C-12(0.99) is added to C-13(0.01). average atomic mass which would be shown where the decimal is in the figure above = 12.01. *the isotope whose mass is closest to the average atomic mass is the most abundant. FUNDAMENTAL PARTICLES
0 notes
Text
CHAPTER 1 NOTES atom: most basic form of stable matter molecule: particle made up of multiple atoms matter: takes up space and has volume *four phases: plasma (charged particles caused by gaining or losing electrons), solid (atoms packed together in fixed positions), liquid (no defined shape), and gas (no defined shape or volume). "-genous" = phase (i.e. of matter) "homo" = same "hetero" = different
two types of solids: *crystalline solid: components (atoms/molecules/compounds, etc.) arranged in repeating and regular array (crystal lattice). clearly defined melting point and defined edges and faces. examples: minerals, salts, metal, etc. *amorphous solid: relatively random arrangement of components, less well-defined melting point. examples: rubber, wax, chocolate, glass, skin, etc... MOST ORGANIC MATTER. deposition: gas changes phase directly into solid, skipping the liquid phase sublimation: solid changes phase directly into gas, skipping the liquid phase (think dry ice)
compressibility: parameter used to calculate how much a given volume of matter decreases when placed under pressure. mostly applies to gases; solids and liquids do not generally experience significant volume changes when placed under pressure.
cold air sinks, warm air rises (cold air is denser, warm air has more energy & atoms move around and are farther apart). 1 cm^3 = 1 mL
to convert Celsius to Kelvin add 273.15 to the Celsius temperature: Kelvin = Celsius + 273.15
intensive property of matter: does not depend on the amount of the substance but on the identity of the substance itself (ex. boiling point, melting point, density, temperature, conductivity, chemical properties, conductivity, specific heat capacity, some physcal properties etc.). can often be used to identify a substance. extensive property: depends on the amount of the substance (number of moles, mass, weight, volume, length/width, area, internal energy, heat capacity, entropy, enthropy, etc.).
if a property depends on an extensive/intensive property, that property is going to be the same type as the property it depends on (extensive/intensive).
ratio of two extensive properties can be an intensive property [m/V = ρ (density)].
0 notes
Text
oh trust me i am watching the Phacus
"What the fuck?"
no, "Watch the Phacus".
Take a 30 second scrolling break to watch these little algae swim.
16K notes
·
View notes
Text
me when cryptic animals
Moss-and-Lichen Katydid: these katydids are covered in cryptic markings and textures that allow them to blend in with their mossy, lichen-covered habitat, and their wings even mimic the appearance of a twig

The katydids of this genus (Anaphidna) can be found in the rainforests of Central and South America. They have cryptic features that mimic the mossy, lichen-covered environments in which they live -- their bodies are covered in green, brown, and white markings that are accented by bumpy, moss-like features, and their long, slender wings are usually held upward at a 45-degree angle in order to mimic the shape of a twig.

As this article notes:
These katydids fly well and probably live in the canopy, perhaps on trunks and mossy branches, where the camouflage should be particularly effective.

The moss-and-lichen mimic katydids of the group treated here are easily recognized by their long and slender wings held upward at an almost 45-degree angle. They live in rainforests of central and northern South America, with one species ranging southward to subtropical forest in NE Argentina.

Sources & More Info:
Journal of Orthoptera Research: The Group Paraphidniae, with Three New Species from Guatemala and Ecuador
iNaturalist: Genus Anaphidna
Zoosystematica Rossica: Review of the Neotropical Genus Paraphidnia
Orthoptera Species File: Anaphidna
2K notes
·
View notes
Text
to clarify they’re not trade secrets they’re just the most unhinged sequences of words known to man
taking the mnemonics im using to memorize info for this class to the grave
3 notes
·
View notes
Text
taking the mnemonics im using to memorize info for this class to the grave
3 notes
·
View notes
Text





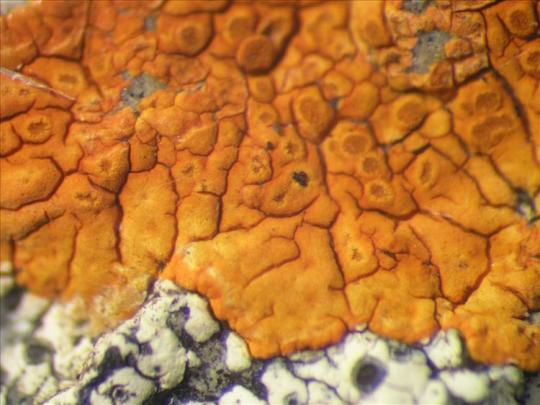
Caloplaca cinnabarina
There are more than 500 species of Caloplaca lichen described thus far in the world. To put that into perspective for you, there are approximately 174 species of ducks, geese and swans (Anatidae) worldwide. So when I say that the name C. cinnabarina has been grossly misused for Caloplaca ID, I think you can understand both the scope of the error, as well as how impossible it is to know and tell the difference between all the species of Caloplaca and give lichen IDers a break. I even deleted a picture I was gonna use for this post from a prominent lichenologist because the picture just didn't really match the description I was seeing. No shade because this shit is hard! C. cinnabarina has an areolate (tile-like) thallus made up of angular areoles (tile-like subsections) and short marginal lobes (only in larger, older specimens) growing in rounded patches on siliceous rock. It has a distinctly deep orange to red orange thallus, and immersed apothecia with darker orange discs.
images: source
info: source | source | source
232 notes
·
View notes
Note


some tiny little guys have established themselves on my car mirrors- first noticed them as little specks this summer, they’re getting to be a respectable size
I love that some lichens are like, highly sensitive habitat specialists that only survive in idyllic, pristine conditions, and other lichens are just like, fuck it. Car mirror.
4K notes
·
View notes
Text
GEN CHEM I: WEEK ONE

first week of spring in the books! besides regular syllabus review, it was a lot of basic math and units review, as well as some basics like what elements vs compounds are, the definitions of mass, density, volume, etc., and significant figures (new concept to me). first lab is tomorrow... but I'm already kinda behind on just my lecture homework and study material for this class so we'll see if I can keep up with the lab too LOL
hoping to post my concept map(s) from chapter one at some point today or tomorrow, and maybe my notes dump at some point this week.
0 notes
Text
@edwardspoonhands i am humbly requesting that you hyperfixate on tumblr to the point of dropping twitter again so i can think about redeleting elon's gross little app. set me free king ����
0 notes
Text
“Pitcher Plant”
I dislike the term “pitcher plant”. It reeks of outdated ignorance and describes a vast number of species from around the world, many of which are not closely related to each other.
As a botanist, and an evil one at that, I prefer to be precise with my language. You too can become an educated scientist and terrific snob by using the correct terms for each variety of “pitcher plant”. If you require education on the matter, allow me to inform you.
There are three families of “pitcher plant”: Sarraceniaceae, Nepenthaceae, and Cephalotaceae. Sarraceniaceae has 3 genera — namely Sarracenia, Heliamphora, and Darlingtonia. Nepenthaceae has a single genus (Nepenthes), and Cephalotaceae has a single species. An entire family with only one species. Ugh.
Now, they look quite distinct from each other, so here are some photos and facts.

This species belongs to Sarracenia, the North American or trumpet pitcher plants. Note the height and slender shape.

This is also a Sarracenia. Note the lack of height and squat shape. Most Sarracenia species look like one of these two — they are quite easy to identify. They are found in boggy, temperate areas around North America and reach a height of up to 4 feet tall.

This is a stunning example of a Nepenthes (tropical pitcher plants) species. These are what you likely think of when someone mentions “pitcher plants”. Beautiful, found in warm, humid regions of the world. They are climbing vines and pitchers can reach over a foot tall (this is species-dependent).

This is an example of Heliamphora, the sun pitchers. They can be found in South America. While still belonging to the family Sarraceniaceae, they are not as tall as Sarracenia, but still quite graceful. If you have a mind for Greek, you may wonder if the “heli” in Heliamphora is for sun (from “helios”). It is not. The name Heliamphora instead comes from “helos”, meaning marsh. The name “sun pitcher” is misleading and comes from a misunderstanding — these plants would be more accurately called “marsh pitchers”.

I have a passionate love-hate relationship with Cephalotus follicularis. Cephalotus is a monotypic genus (a genus with only one species) and of course it is Australian. They look similar to Nepenthes but are unrelated and much smaller — the plants reach just shy of 8 inches tall.
There are also the cobra lilies, Darlingtonia, which belong to Sarraceniaceae. Those are arguably similar enough to Sarracenia that they do not need to be discussed here. Darlingtonia is another monotypic genus within Sarraceniaceae.
Now you have absolutely no excuse. You have been informed on the major genera of “pitcher plants” and should weaponize this knowledge as you see fit.
The brilliant and brave may also wish to weaponize the plants themselves. Kindly send me updates if you do. I am ever so curious…
2K notes
·
View notes
Text
the hardest part of coming back to tumblr for school is missing my glory days (70 followers and 10 moots who religiously followed my lafayette x reader x thomas jefferson modern!au fanfic series)
0 notes
Text
girl sees lichen on her feed. girl whispers “hell yeah” to herself. rinse, reblog, repeat

3 notes
·
View notes
Text
i love him i love him i love him i love him i love him
Round 2.5 - Tardigrada - Eutardigrada

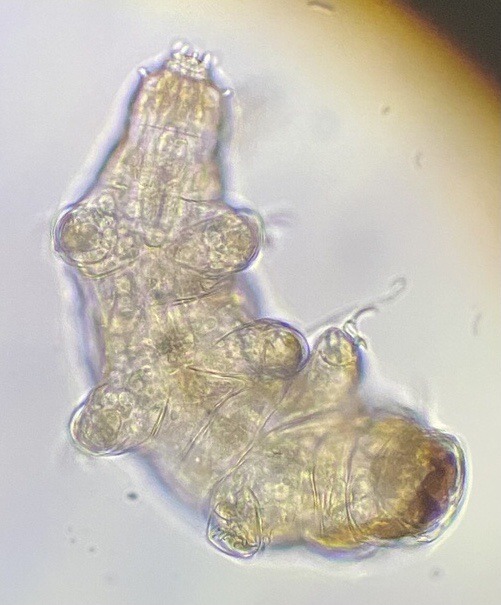

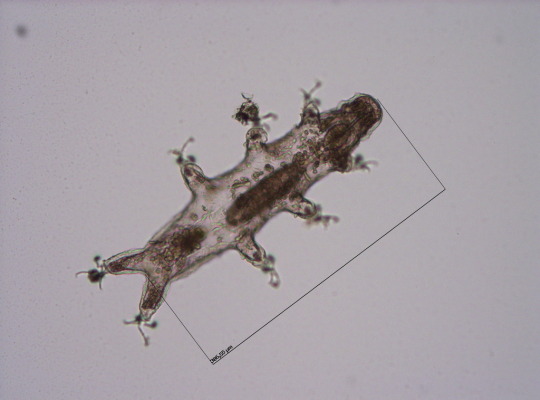
(Sources - 1, 2, 3, 4)
One of two classes of tardigrades, Eutardigrada (“Smooth-bodied Tardigrades”) consists of the orders Apochela and Parachela.
When you picture a tardigrade, it is probably a eutardigrade. Eutardigrada contains the most species of the two tardigrade classes. Most of them are terrestrial, though some are adapted to a freshwater or marine habitat. As opposed to heterotardigrades, eutardigrades are smooth and sausage-shaped, and lack external sensory organs. Their claws are combined into double claws or aligned in two rows.
Most of the scientific study on the survivability of tardigrades has been done on eutardigrades. They are known for their extreme resilience, being able to survive extreme temperatures and pressures, air deprivation, radiation, dehydration, starvation, and even exposure to outer space. However, they prefer to just live in mosses, lichens, and sediments, munching away on plant cells, algae, bacteria, or small invertebrates (including smaller tardigrade species; that’s not cannibalism btw, plenty of chordates eat other chordate species!) They are not considered extremophiles though, as they are not adapted to exploit extreme environments, only to endure them. They do this by going dormant in harsh environments, for up to 30 years, only to rehydrate and continue living when conditions are safer!
Members of Eutardigrada have been around since at least the Middle Cretaceous. The oldest known living species are Beorn leggi and Aerobius dactylus, which have existed since the Late Cretaceous!
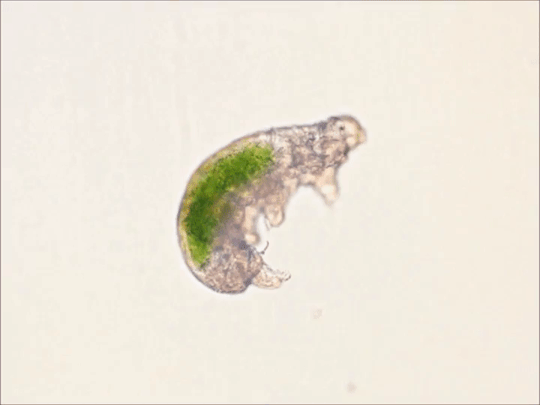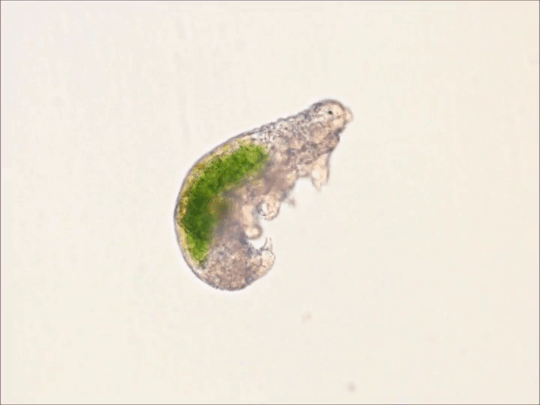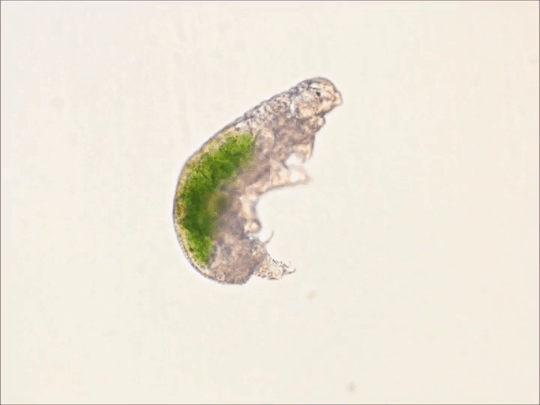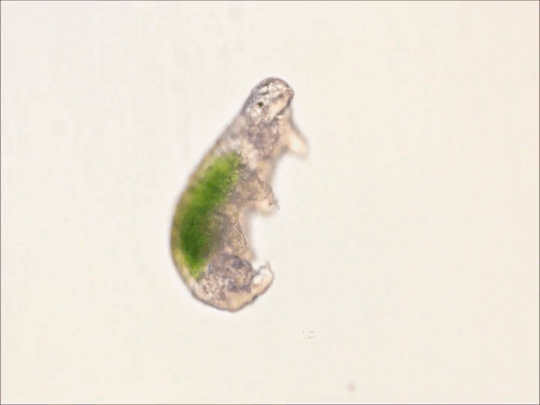
76 notes
·
View notes
Text
What are the Key Mechanical Properties of Solids?
Mechanical properties of solids refer to the characteristics that define how a material responds to external forces, such as stress, strain, and deformation. These properties include elasticity, plasticity, hardness, toughness, strength, and ductility, among others. They are fundamental in determining a material’s suitability for various applications, influencing its behaviour under different conditions.
The significance of mechanical properties spans numerous fields. In engineering, these properties ensure the safety and durability of structures like bridges, buildings, and dams. In construction, they guide the selection of materials for specific purposes, such as load-bearing or flexibility. Material science relies heavily on these properties to develop innovative materials that meet evolving industrial demands.
This article aims to delve into the science behind mechanical properties, their key characteristics, real-world applications, and their indispensable role in innovation and progress.
The Science Behind Mechanical Properties
Stress and Strain: Fundamentals
Stress is the force per unit area exerted on a material. It can be mathematically expressed as:
where is the applied force and is the cross-sectional area of the material. Strain, on the other hand, measures the deformation caused by stress. It is defined as the ratio of the change in length to the original length: where is the change in length and is the original length.
Types of Stress and Strain
Tensile Stress and Strain: Occurs when forces act to stretch a material. Examples include the stretching of rubber bands and cables.
Compressive Stress and Strain: Results from forces that compress or squeeze a material. Concrete in buildings often experiences compressive stress.
Shear Stress and Strain: Arises when forces are applied parallel to a surface. Scissors cutting paper is an example of shear stress in action.
Hooke’s Law and Elasticity
Elasticity is the ability of a material to return to its original shape and size after the removal of stress. Hooke’s Law describes this behaviour:
where is the Young’s modulus, a measure of a material’s stiffness. Elasticity is crucial for applications requiring temporary deformation, such as springs.
Plasticity and Permanent Deformation
When stress exceeds a material’s elastic limit, it undergoes plastic deformation, leading to permanent changes in shape or size. Metals like aluminium and copper exhibit plasticity, making them ideal for moulding and shaping processes.
Key Mechanical Properties
1. Young’s Modulus (Stiffness)
Represents a material’s resistance to deformation under tensile stress.
Example: Steel has a high Young’s modulus, making it ideal for construction.
2. Shear Modulus and Bulk Modulus
Shear Modulus measures resistance to shape changes under shear stress.
Bulk Modulus evaluates a material’s response to uniform compression.
Applications include hydraulic systems and pressure vessels.
3. Poisson’s Ratio
Describes the ratio of lateral strain to longitudinal strain. Materials with low Poisson ratios, like cork, are used for sealing applications.
4. Strength
Includes tensile, compressive, and shear strength. Determines how much load a material can bear without failure.
Example: Carbon fiber composites are used in aerospace for their high strength-to-weight ratio.
5. Ductility and Malleability
Ductility is the ability to be stretched into a wire. Copper’s ductility makes it suitable for electrical wiring.
Malleability is the ability to be hammered into sheets. Gold’s malleability is utilized in jewellery making.
6. Brittleness and Toughness
Brittleness: Materials like glass break without significant deformation.
Toughness: Measures a material’s ability to absorb energy before fracturing. Rubber is an example of a tough material.
7. Hardness
Resistance to indentation or scratching. Hardness tests include the Mohs scale and the Brinell test.
Example: Diamond, the hardest known material, is used in cutting tools.
Applications in Engineering and Design
Civil Engineering
Bridges: Steel and reinforced concrete combine high strength and ductility to withstand loads.
Buildings: Materials like concrete and glass are chosen based on compressive strength and aesthetic appeal.
Aerospace and Automotive Industries
Lightweight yet strong materials like titanium alloys ensure efficiency and safety in aircraft.
Automotive components use materials with high impact resistance, such as advanced polymers.
Manufacturing Tools and Machinery
Machine tools rely on hard materials like tungsten carbide for durability and precision.
Conveyor belts require toughness and flexibility to handle wear and tear.
Product Design and Safety Analysis
Consumer goods, such as smartphones, use materials that balance hardness and toughness.
Safety-critical systems, like helmets and airbags, are designed with energy-absorbing materials.
Material Science and Advancements
Developing New Materials
Nanomaterials and composites offer superior strength and lightweight properties.
Innovations like graphene enhance conductivity and mechanical strength.
Sustainability and Recycling
Recyclable materials, such as aluminium, reduce environmental impact.
Bio-based polymers provide eco-friendly alternatives for packaging and construction.
Future Trends
Smart materials, such as shape-memory alloys, adapt to environmental changes.
Research focuses on enhancing the durability of materials under extreme conditions.
The mechanical properties of solids are the backbone of modern engineering, construction, and material science. They ensure the safety, functionality, and efficiency of structures and products. By understanding and leveraging these properties, we can drive innovation, create sustainable solutions, and meet the challenges of an ever-evolving world.
As we continue to explore new materials and techniques, the importance of mechanical properties will remain at the forefront of progress, shaping a future of resilience and ingenuity.
For more simplified explanations like the one above, visit the physics blogs on the Tutoroot website. Elevate your learning with Tutoroot’s personalised Physics online tuition. Begin your journey with a FREE DEMO session and discover the advantages of online tuitions.
2 notes
·
View notes
Text
hell yeah
Here's a big project I've been working on for a few weeks: a phylogenetic tree of everything in Minecraft! It would take ages to explain everything here, so if you want an explaination of any inclusions, exclusions, categorisations or Latin names PLEASE PLEASE PUHLEASE ask me I would love to answer any questions :3
Here's the slides I used to make it since i'm aware the text on the image there is pretty much unreadable.
Reblogs appreciated!
Edit: there are some problems with the image on here aside from the quality, so please check the slides for a slightly more accurate version! Also, if you have a question check the notes first! Odds are someone else has asked already.
10K notes
·
View notes