Don't wanna be here? Send us removal request.
Photo
How do you read a Gas Chromatography and what does it tell you?
This article will explain to you one questions about Gas Chromatography. How to read Gas Chromatography?
Gas chromatography (GC)
Gas chromatography (GC) is an analytical technique used to separate and analyze samples that can be vaporized without thermal decomposition. Sometimes gas chromatography is known as gas-liquid partition chromatography (GLPC) or vapor-phase chromatography (VPC). Technically, GPLC is the most correct term, since the separation of components in this type of chromatography relies on differences in behavior between a flowing mobile gas phase and a stationary liquid phase.
The instrument that performs gas chromatography is called a gas chromatograph. The resulting graph that shows the data is called a gas chromatogram.
Much information can be gained from the chromatogram on the health of the GC or GC-MS system as well as the data required to perform qualitative or quantitative analysis.
How to read Gas Chromatography?
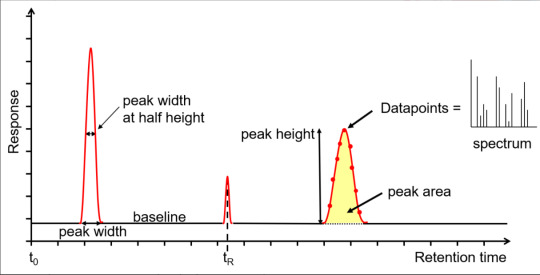
The x-axis is the retention time, taken from the time the sample was injected into the GC (t0) to the end of the GC run. Each analyte peak has a retention time measured from the apex of the peak, for example tR. The y-axis is the measured response of the analyte peak in the detector. The baseline shows the signal from the detector when no analyte is eluting from the column, or it is below the detection limit. The baseline response is a mix of electrical noise (usually low) and chemical noise, such as impurities in the carrier gas, column stationary phase bleed and system contamination. Hence, if the baseline is higher than it should be, it is an indication of a problem or that maintenance is required. Various measurements can be taken from the peak, such as width at the baseline, width at half height, total height and area. The latter two are proportional to the concentration, however it is the area that is used for quantitation as it is less affected by band broadening. The measurements can be used to calculate the extent of band broadening, the spread of the analyte molecules on the column. Narrower, sharper peaks give better sensitivity (signal to noise ratio) and better resolution (peak separation). The peaks shown are Gaussian, however peak tailing (the right side of the peak is wider) indicates activity or a dead volume in the system, whereas a peak fronting (the left side of the peak is wider) indicates the column is overloaded. Accurate measurements are affected by the number of data points across a peak, with an ideal number being 15-25. Too few, makes the peak look like a child’s join-the-dots drawing, affecting peak area, resolution and, with GC-MS, deconvolution. Too many reduces the signal to noise, reducing sensitivity. For GC-MS data, each data point is a mass spectrum, the third dimension of data.
Drawell Scientific specializes in manufacturing laboratory equipment and scientific instruments. The main products include a wide range of Chromatogaphy. If you have any questions about the spectrometer, you can contact us and our engineers will help you answer your questions.
0 notes
Text
What you need to know about Gas Chromatography?
What is gas chromatography?
Gas chromatography (GC) is an analytical technique used to separate the chemical components of a sample mixture and then detect them to determine their presence or absence and/or how much is present. These chemical components are usually organic molecules or gases. For GC to be successful in their analysis, these components need to be volatile, usually with a molecular weight below 1250 Da, and thermally stable so they don’t degrade in the GC system. GC is a widely used technique across most industries: for quality control in the manufacture of many products from cars to chemicals to pharmaceuticals; for research purposes from the analysis of meteorites to natural products; and for safety from environmental to food to forensics. Gas chromatographs are frequently hyphenated to mass spectrometers (GC-MS) to enable the identification of the chemical components.
How Gas Chromatography Works
First, a liquid sample is prepared. The sample is mixed with a solvent and is injected into the gas chromatograph. Typically the sample size is small -- in the microliters range. Although the sample starts out as a liquid, it is vaporized into the gas phase. An inert carrier gas is also flowing through the chromatograph. This gas shouldn't react with any components of the mixture. Common carrier gases include argon, helium, and sometimes hydrogen. The sample and carrier gas are heated and enter a long tube, which is typically coiled to keep the size of the chromatograph manageable. The tube may be open (called tubular or capillary) or filled with a divided inert support material (a packed column). The tube is long to allow for a better separation of components. At the end of the tube is the detector, which records the amount of sample hitting it. In some cases, the sample may be recovered at the end of the column, too. The signals from the detector are used to produce a graph, the chromatogram, which shows the amount of sample reaching the detector on the y-axis and generally how quickly it reached the detector on the x-axis (depending on what exactly the detector detects).
The chromatogram shows a series of peaks. The size of the peaks is directly proportional to the amount of each component, although it can't be used to quantify the number of molecules in a sample. Usually, the first peak is from the inert carrier gas and the next peak is the solvent used to make the sample. Subsequent peaks represent compounds in a mixture. In order to identify the peaks on a gas chromatogram, the graph needs to be compared to a chromatogram from a standard (known) mixture, to see where the peaks occur.
At this point, you may be wondering why the components of the mixture separate while they are pushed along the tube. The inside of the tube is coated with a thin layer of liquid (the stationary phase). Gas or vapor in the interior of the tube (the vapor phase) moves along more quickly than molecules that interact with the liquid phase. Compounds that interact better with the gas phase tend to have lower boiling points (are volatile) and low molecular weights, while compounds that prefer the stationary phase tend to have higher boiling points or are heavier. Other factors that affect the rate at which a compound progresses down the column (called the elution time) include polarity and the temperature of the column. Because temperature is so important, it is usually controlled within tenths of a degree and is selected based on the boiling point of the mixture.
How to choose an excellent Gas Chromatography producer?
Via Drawell International Technology Limited is a leading company of manufacturing and selling laboratory equipment and scientific instruments, founded in year 1999 in China, then has become an joint venture company after being invested by US company since 2014. We produce and provide high-quality Gas Chromatography products, including GC1290 Gas Chromatography (LCD Touch Screen), GC1120 Gas Chromatography, etc.
0 notes
Text
What are the Types of a Spectrophotometer?
Spectrophotometry is a scientific technique used to measure the intensity of light either transmitted through or reflected from gas, liquid or solid samples. In transmission, which is directly related to absorbance per Beer's law, spectrophotometry is the technique that measures how much a substance absorbs a beam of light passing through it. By measuring the intensity at each wavelength, a spectrum is created and this can tell us much information about the sample through which the light passed.
In reflectance, spectrophotometry measures the amount of light that is reflected from an opaque specimen. Again a spectrum is created by measuring the intensity at each wavelength of light. All this can be done with a spectrophotometer.
The is an optical instrument for measuring the intensity of light relative to wavelength. Electromagnetic energy, collected from the sample, enters the device through the aperture (yellow line) and is separated into its component wavelengths by the holographic grating. Simply put, the grating acts to separate each color from the white light. The separated light is then focused onto a CCD array detector where the intensity of each wavelength (or each color if in the visible region) is then measured by a pixel of the array. The CCD is then read-off to a computer and the result is a spectrum which displays the intensity of each wavelength of light.
Types of Spectrophotometers
Spectrophotometers are either single-beam or double-beam. Single-beam spectrophotometers measure the light intensity before and after the sample is introduced, while double-beam spectrophotometers compare the intensity of light between the reference light path and the sample that’s being measured. Double-beam models are more accurate because they are not as sensitive to light source fluctuations, but single-beam options have a higher range and are more compact.
Infrared Spectrometer
Infrared spectrometers, sometimes referred to as IR spectrometers, measure vibrations in the interatomic bonds within the sample being tested. When the sample is exposed to infrared wavelengths, the vibrations are measured at different frequencies. This spectrometer can also measure the number of absorbing molecules.
Infrared spectrometers can identify and study chemicals in gas, solid, or liquid form. It is useful for forensic analysis, organic and inorganic chemistry, microelectronics, manufacturing, art history, and various other applications.
Raman Spectrometer
Raman spectrometers are most often used in chemistry to provide the structural fingerprint to identify molecules. This type of spectroscopy relies on inelastic scattering of photons. It uses a source of monochromatic light, typically from a laser. Generally, it’s in the visible light, near-infrared, or near-ultraviolet spectrum, though it’s also possible to use x-rays. The laser interacts with excitations within the sample, which shifts the energy either up or down. That shift provides information about the vibrational modes, similar to the information infrared spectroscopy offers.
UV-Vis Spectrometer
UV-Visible spectroscopy exposes the sample to ultraviolet light, which excites the electrons upon absorbance of the light energy. The absorbance is measured based on how excited the electrons become. This type of spectroscopy is commonly used to research the chemical bonding of molecules in the sample material.
Near IR spectroscopy is based on the absorption of electromagnetic radiation at wavelengths from 780 to 2,400 nanometers. The light interacts with the sample and then the detector measures the transmittance and absorbance. Near IR spectroscopy has a wide range of applications, including, neonatal research, blood sugar, functional neuroimaging, urology, ergonomics, atmospheric chemistry, and more.
X-Ray Spectrometer
X-Ray spectrometers excite the inner electrons of the sample. When the excited electrons fall into the empty space generated as a result of energy absorption, x-rays are procured.
Regardless of the type, make, or model of spectrophotometer your lab requires, Drawell Scientific can help you get what you need. Of course, you can also consult our engineers first, and we will do our best to help you answer your questions.
0 notes
Text
What is a Spectrometer?
In the broadest sense a spectrometer is any instrument that is used to measure the variation of a physical characteristic over a given range; i.e. a spectrum. This could be a mass-to-charge ratio spectrum in the case of a mass spectrometer, the variation of nuclear resonant frequencies in an NMR spectrometer or the change in the absorption and emission of light with wavelength in an optical spectrometer.
The most ubiquitous type of spectrometer used for research are optical spectrometers; and when someone simply says “spectrometer”, without an additional qualifier, they are usually referring to an optical spectrometer and this diverse family of spectrometers is the focus of this article.There are many types of spectrometers designed to measure different types of spectra and to yield different types of information about their material samples.
For example, a spectrophotometer measures ultraviolet, visible and near infrared light that is either reflected from or transmitted through a sample. A Raman spectrometer measures the light scattered from a sample via Raman scattering. A fluorescence spectrometer measures the spectra of light emitted from a sample that has absorbed light at a different wavelength. All these different instruments give us different types of information about samples.
Nano Spectrophotometer
How Does a Spectrophotometer Work?
Let’s first break down all the parts of the instrument, as this makes it easier to understand how everything works together.
Light source: This is what provides the wavelengths of light at great intensity. The range spans from near-infrared to inside the ultraviolet range, and it includes the visible light spectrum.
Prism: Also known as the diffraction grating, this is what separates the light source into specific parts of the spectrum. When the variable wavelength selector is adjusted, the prism’s position changes so that different wavelengths of light are directed toward the sample compartment that contains the object or sample being analyzed.
Variable wavelength selector: This component is on the outside of the instrument and allows the light to be filtered so that it only transmits light at a certain wavelength or range of wavelengths.
Sample compartment: Here is where you’ll find the transparent tube, also known as a cuvette, that holds the sample you want to analyze, known as the analyte. The wavelengths you select with the selector pass through the analyte, which are then detected by the photodetector.
Photodetector: Light that passes the sample being analyzed hits the photodetector, which is made of semiconducting material. Electrons in the material are excited proportionally to the wavelength that strikes the photodetector. Increasing the light intensity produces additional electrons, so the signal processor receives a higher current.
Display: This component displays the transmittance of the sample. Many models also display the sample’s absorbance, too.
An important part of the entire instrument is the entrance slit because the size of that slit determines the amount of light that can enter and be measured. This affects not only the speed of the spectrometer’s engine but also the optical resolution. The optical resolution is expressed as the full width at half maximum. Smaller slit sizes translate to a better resolution. The slit can be adjusted to allow for more or less light to enter the spectrometer.
The basic components of a spectrophotometer are the light source, a sample holder, a device to separate the light into its component wavelengths and a detector.
The system focuses electromagnetic energy from the light source onto the material sample. Depending upon the system configuration, light is either reflected off the sample or transmitted through it. After the light is collected from the sample, it is focused onto the entrance slit of a monochromator. The monochromator is used to separate the light by wavelength using a dispersing element, most commonly an optical grating. That light is then focused onto a charge coupled device (CCD), which is made up of thousands of individual detectors, so that the intensity of light at each wavelength may be measured. The CCD is then read-off to a computer and the result is a spectrum which displays the intensity of each wavelength of light.
Drawell Scientific specializes in manufacturing laboratory equipment and scientific instruments. The main products include a wide range of spectrometers. If you have any questions about the spectrometer, you can contact us and our engineers will help you answer your questions.
0 notes