#vipivotide tetraxetan
Explore tagged Tumblr posts
Text
Prostate Cancer Drug Shortage Leaves Some With Uncertainty#Prostate #Cancer #Drug #Shortage #Leaves #Uncertainty
A radioligand treatment approved for certain men with metastatic castration-resistant prostate cancer (CRPC) is in short supply because of manufacturing and delivery issues, according to the US Food and Drug Administration (FDA). The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up…

View On WordPress
0 notes
Text
Trends | Future and Growth for Prostate Health Market
Prostate health is an important concern for men. The prostate is a small gland that is part of the male reproductive system. It is located just below the bladder and in front of the rectum. Its primary function is to produce fluid that transports sperm during ejaculation. Prostate health is important for a number of reasons.
An enlarged prostate can cause discomfort and can lead to urinary problems such as frequent urination, difficulty urinating, or a weak urine stream. An enlarged prostate can also increase the risk of prostate cancer. Other risks for prostate cancer include age, family history, and ethnicity.
There are a number of steps men can take to promote prostate health and reduce their risk of prostate cancer. Eating a balanced diet and engaging in regular physical activity can help keep the prostate healthy. Additionally, men should have a yearly prostate exam to check for any signs of prostate cancer.
According to a research report "Prostate Health Market by Disease Indication (Prostate Cancer, PARP Inhibitors, Cytotoxic Drug, Benign Prostate Hyperplasia (BPH), Tamsulosin, 5 Alpha Reductase, Prostatitis, OTC, Prescription (Rx), & Region (NA, Europe, APAC) - Global Forecasts to 2026" published by MarketsandMarkets, the global prostate health market is projected to reach USD 48.9 billion by 2026 from USD 31.8 billion in 2021, at a CAGR of 9.0% during the forecast period.
Request for assumptions & how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=107055093
Market Growth Drivers
Growing prevalence of prostate cancer and BPH
Market Growth Opportunities
Emerging economies (such as China, India, Brazil, and Mexico) are projected to offer significant growth opportunitie
Market Challenges
Low awareness regarding prostate health among men
Industry Trends
This represents an annual growth of about 7% and a total increase of 58% from 2010. Obesity and Benign Prostatic Hyperplasia (BPH) are closely associated.
Finasteride and Dutasteride (among other 5-ARIs) convert testosterone to dihydrotestosterone. These drugs also decrease the synthesis of several neuroactive steroids, but the modulation of the neuroendocrine stress response may lead to depression. Other side effects of 5-ARIs are gynecomastia, impotence, and reduced libido and ejaculate volume.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107055093
Some of the prominent players operating in the prostate health market are Eli Lilly and Company (US), Pfizer Inc. (US), Merck & Co., Inc. (US), GlaxoSmithKline plc. (UK), Abbott (US), and Astellas Pharma Inc. (Japan), and other players.
Research Developments
In March 2022, Lantheus Holdings, Inc. (US) collaborated with Novartis AG (Switzerland) to include PYLARIFY (piflufolastat F18) in prostate cancer clinical trials with Pluvicto (lutetium Lu 177 vipivotide tetraxetan) to support prostate cancer clinical development.
In March 2022, Novartis AG (Switzerland) received approval for PluvictoTM (lutetium Lu 177 vipivotide tetraxetan) (formerly referred to as 177Lu-PSMA-617) by FDA for the treatment of adult patients with a certain type of advanced cancer called prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC).
In January 2021, Pharex Tamsulosin for the treatment of benign prostatic hyperplasia was introduced by PHAREX Health Corporation (Philippines) in collaboration with the Philippine Urological Association (PUA).
In October 2020, Israeli medical device manufacturer Butterfly Medical raised USD 7 million in a Series B round to develop an anatomically shaped nitinol implant positioned in the prostatic urethra to relieve BPH symptoms
Content Source: 1. https://www.prnewswire.com/news-releases/prostate-health-market-worth-48-9-billion-by-2026--exclusive-report-by-marketsandmarkets-301443961.html
1 note
·
View note
Text
Lutetium Lu 177 vipivotide tetraxetan
Lutetium Lu 177 vipivotide tetraxetan
Lutetium Lu 177 vipivotide tetraxetan FDA APPROVED 2022/3/23, Pluvicto To treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer following other therapies FormulaC49H65N9O16. Lu. 3HCAS1703749-62-5Mol weight1214.0819 Antineoplastic, Radioactive agent DiseaseProstate cancer (PSMA positive) ルテチウム(177Lu)ビピボチドテトラキセタン; UNII-G6UF363ECX, WHO…
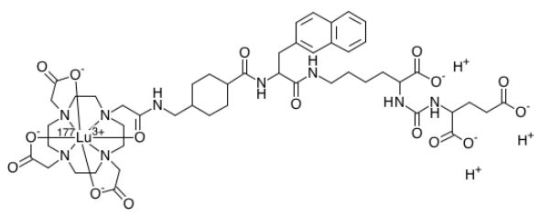
View On WordPress
#APPROVALS 2022#ルテチウム(177Lu)ビピボチドテトラキセタン#FDA 2022#Lutetium Lu 177 vipivotide tetraxetan#PROSTRATE CANCER
0 notes
Text


PSMA-targeted PET and F18-fluciclovine PET are utilized primarily in the setting of biochemical recurrence of prostate cancer.
In 2016, FDA approved F18-fluciclovine, an amino acid analog that localizes to prostate cancer metastases via amino acid transporters.
In 2021, FDA approved F18-piflufolastat which binds the membrane protein "prostate-specific membrane antigen" (PSMA). In 2022, they added approval for Ga-68-PSMA-11 PET. PSMA is a misnomer because it is not prostate-specific and may find utility in imaging multiple types of cancer. PSMA PET performs well for bone metastases (similar detection rate to NaF PET) as well as nodal and visceral metastases; in one study it was shown to detect metastases at lower PSA values than fluciclovine PET. In addition, PSMA PET has the advantage of allowing patients with PSMA-positive disease to receive PSMA-directed radioligand therapy, approved by the FDA in March 2022 (Lu-177 vipivotide tetraxetan). As such, most centers are abandoning fluciclovine PET in favor of PSMA PET.
Image labeled "B" depicts an F18-piflufolastat PET scan showing pelvic and rib metastases. The second image depicts an F18-fluciclovine PET scan showing pelvic nodal metastasis and an incidental sigmoid colon cancer. Note the absence of significant bladder activity in fluciclovine PET, potentially offering superior detection of disease near the bladder.
References:
Lawhn-Heath et al. Radiology 2021, 299, 248-260.
Baruch et al. PET Clin. 2017, 12, 145-157.
1 note
·
View note
Text
Shortage of Prostate Cancer Drug; Win for DBT Mammo; Double Lung Transplant#Shortage #Prostate #Cancer #Drug #Win #DBT #Mammo #Double #Lung #Transplant
Lutetium Lu 177 vipivotide tetraxetan (Pluvicto) is currently in shortage, according to the FDA, leaving some prostate cancer patients with limited options; the drug’s manufacturer Novartis said it’s working to increase production. Meanwhile, the FDA expanded the indication for Illuccix for use in selecting patients for lutetium Lu 177 vipivotide tetraxetan, Telix announced. Multiple cancer drugs…

View On WordPress
0 notes
Text
FDA Approves Pluvicto (177Lu-PSMA-617)
Novartis and Advanced Accelerator Applications, the radioligand business of Novartis, has announced that their investigational treatment, Pluvicto™, was approved by the FDA. Pluvicto, lutetium Lu 177 vipivotide tetraxetan (formerly referred to as 177Lu-PSMA-617) is the first approved targeted radioligand therapy for the treatment of men with progressive, PSMA‑positive metastatic castration-resistant prostate cancer.
Specifically, the approval is only for the treatment of men with advanced prostate cancer that is metastatic (spread outside the prostate gland), castrate resistant (no longer responding to primary hormone therapy), and be prostate-specific membrane antigen (PSMA) positive. The approval also includes the requirement that the men should have already been treated with other anticancer treatments (androgen receptor pathway inhibition and taxane-based chemotherapy).
To confirm that the prostate cancer is PSMA positive, meaning that the tumor expresses the target, PSMA, along with the approval of Pluvicto, the FDA also approved a complementary diagnostic imaging agent, Locametz. Locametz is a radiolabeling method using gallium-68 to confirm and identify PSMA-positive lesions. Not all prostate cancer expresses PSMA. Since Pluvicto uses PSMA as a target, it will only be effective for those PSMA positive cancers.
The approval was based on pivotal Phase III VISION trial, where men with pre-treated PSMA-positive mCRPC who received Pluvicto plus standard of care had a statistically significant reduction in risk of death over those just treated with the standard of care (SOC).
The most common adverse events (all grades) in the Pluvicto arm of the study were fatigue (43%), dry mouth (39%), nausea (35%), anemia (low red blood cell counts) (32%), decreased appetite (21%), and constipation (20%).
Novartis also is running two additional Phase III studies evaluating Pluvicto in earlier stages of treatment for men with metastatic prostate cancer.
0 notes