#cdsco import license process
Explore tagged Tumblr posts
Text
#Online CDSCO registration process in India#medical device registration approval process in India#AYUSH license registration process#apply for drugs license in India#import license from the CDSCO
0 notes
Text
CDSCO Certification & Registration | Import License & Process | Central Drugs Standard Control Organisation : Eikomp
CDSCO, Central Drugs Standard Control Organisation, CDSCO Certification, CDSCO Registration, CDSCO Certificate, cdsco license,cdsco registration process, cdsco import license.
#CDSCO#Central Drugs Standard Control Organisation#CDSCO Certification#CDSCO Registration#CDSCO Certificate#cdsco license#cdsco registration process#cdsco import license.
0 notes
Text
Unlocking Compliance: Essential Guide to the Non-Conviction Certificate for Medical Devices in India
In India, medical device registration is essential for manufacturers, importers, and distributors aiming to ensure regulatory compliance and public safety. Governed by the Central Drugs Standard Control Organization (CDSCO) under the Medical Device Rules 2017, the process requires various approvals, including CDSCO medical device registration, product approvals, import licenses, and the Non-Conviction Certificate (NCC). Among these, the NCC is a crucial document that reinforces a company’s compliance record. This guide delves into the Non-Conviction Certificate, highlighting its importance, eligibility, and the steps involved in obtaining it.
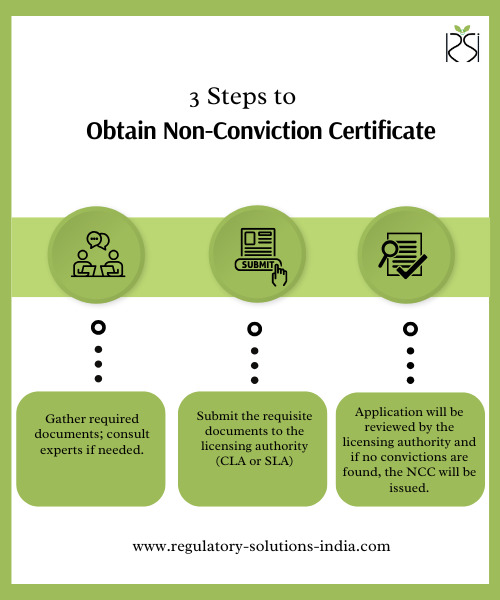
What is a Non-Conviction Certificate (NCC)?
A Non-Conviction Certificate (NCC) is issued by the CDSCO to Indian medical device companies. This certificate attests that a company has not been convicted of offenses related to safety, quality standards, or product malfunctions associated with its devices. Often essential in international tenders and product registration in foreign markets, the NCC demonstrates a company's adherence to high regulatory standards. This certification is vital in the medical device regulatory services landscape in India, underscoring the company’s commitment to ethical practices.
Importance of the Non-Conviction Certificate (NCC)
The Non-Conviction Certificate has numerous advantages for medical device companies, including:
- Building Trust: Demonstrates a company’s dedication to safety, quality, and regulatory compliance. - Unlocking Business Opportunities: Required in many tenders and pre-qualification applications, especially in CDSCO medical device registration. - Maintaining Market Reputation: Strengthens a company’s credibility and reputation in the medical devices India sector.
Eligibility Criteria for the Non-Conviction Certificate
To be eligible for the NCC, a company must: - Hold a valid medical device registration India license from the Central Licensing Authority (CLA) or State Licensing Authority (SLA). - Maintain a clean record with no prior convictions or violations under the Drugs and Cosmetics Act 1940 and related Rules.
Required Documentation for the Non-Conviction Certificate
Obtaining an NCC involves compiling the following documents: 1. A cover letter stating the purpose of the application. 2. A copy of the company's valid medical device registration or import license. 3. A list of products for which the NCC is being requested. 4. Prescribed government fees. 5. A Legal Undertaking on a ₹100 registered, notarized stamp paper, confirming no convictions related to product malfunctions or compliance violations.
Who Issues the Non-Conviction Certificate?
The licensing authority that initially issued the manufacturing or import license (either the CLA or SLA) is responsible for granting the NCC.
Validity of the Non-Conviction Certificate
The NCC is valid for one year from issuance or until the company's manufacturing license expires, whichever occurs first.
Steps to Obtain the Non-Conviction Certificate
1. Submit the application with the necessary documents to the relevant licensing authority. 2. For a smooth application process, consider engaging a regulatory consultant in India who specializes in medical device regulatory consultancy.
Benefits of Hiring a Regulatory Consultant for NCC Application
Engaging a medical device regulatory consultant in India can ease the application process, given their expertise in regulatory consultancy services. Consultants bring several advantages: - Requirement Clarity: Ensures the applicant meets eligibility criteria and provides complete documentation. - Efficient Navigation of Procedures: Streamlines applications and reduces potential delays. - Expert Advice: Consultants bring in-depth knowledge, particularly beneficial for medical device registration India requirements. - Peace of Mind: Consultants manage the regulatory process, allowing companies to focus on core operations.
Consult Regulatory Solutions India for Your NCC Needs
Regulatory Solutions India (RSI) offers expert regulatory consultancy services for medical devices and IVDs in India, providing end-to-end support for compliance, including CDSCO consultancy services. With RSI's guidance, you can ensure compliance with regulatory standards, obtain the Non-Conviction Certificate, and gain a competitive edge in India’s regulated markets. ContactRSI today to discuss your specific requirements. Our team of regulatory consultants is here to assist with your CDSCO medical device registration and NCC needs, making the compliance journey smoother and more efficient.
0 notes
Text
Challenges Faced by Nilotinib API Manufacturers in India: Regulatory, Sourcing, and Market Competition

India has long been recognized as a hub for pharmaceutical manufacturing, particularly in the production of Active Pharmaceutical Ingredients (APIs). One of the significant APIs produced in India is Nilotinib, a critical component used in the treatment of chronic myeloid leukemia (CML). While the Indian pharmaceutical sector is thriving, manufacturers of Nilotinib API face a unique set of challenges that span regulatory compliance, raw material sourcing, and intense market competition.
This article delves into these key challenges and explores how Nilotinib API manufacturers in India are navigating this complex landscape.
1. Regulatory Challenges: Meeting Stringent Global Standards
One of the most pressing challenges faced by Nilotinib API manufacturers in India is adhering to stringent global regulatory requirements. The production of Nilotinib, like any API, must meet high standards of quality, safety, and efficacy, set by regulatory bodies such as the United States Food and Drug Administration (FDA), the European Medicines Agency (EMA), and India’s own Central Drugs Standard Control Organization (CDSCO).
However, the complexity of these regulations can pose significant hurdles for manufacturers, especially for those looking to export Nilotinib API to international markets. Some of the major regulatory challenges include:
Quality Control and Documentation: Regulatory bodies require exhaustive documentation for every step of the API manufacturing process, from raw material sourcing to production and final testing. Failure to comply with these documentation requirements can lead to costly delays, product recalls, or even the suspension of production licenses.
Good Manufacturing Practice (GMP) Compliance: Indian API manufacturers must ensure that their facilities comply with GMP standards, which are crucial for maintaining product safety and efficacy. Regular audits from international regulators are common, and even minor deviations from GMP guidelines can result in the loss of market access.
Navigating Multiple Regulatory Frameworks: For manufacturers that export to various regions, staying compliant with different regulatory frameworks is a challenging task. Each regulatory authority may have its own specific guidelines, and meeting these varying requirements adds an additional layer of complexity.
Despite these challenges, Indian Nilotinib API manufacturers are investing in advanced quality control systems and automated production techniques to maintain compliance and avoid costly regulatory setbacks.
2. Sourcing of Raw Materials: Dependency on Imports
Another significant challenge for Nilotinib API manufacturers in India is the sourcing of raw materials, particularly key starting materials (KSMs) and intermediates that are crucial for Nilotinib production. A considerable portion of these raw materials is imported, primarily from China, making Indian manufacturers highly dependent on international supply chains.
This reliance on imports brings several challenges:
Supply Chain Disruptions: Geopolitical tensions, trade restrictions, and events like the COVID-19 pandemic have highlighted the vulnerability of global supply chains. Any disruptions in the availability of raw materials can lead to production delays and increased costs for Indian manufacturers.
Rising Costs: The cost of importing raw materials has been steadily increasing due to factors such as fluctuating currency exchange rates, inflation, and rising shipping costs. These increased input costs make it difficult for Indian manufacturers to maintain competitive pricing for Nilotinib API, especially when margins are already tight due to intense market competition.
Quality Assurance: Ensuring the quality and purity of imported raw materials is critical. If the KSMs or intermediates are not up to standard, it can result in subpar batches of Nilotinib API, leading to potential regulatory issues or product recalls.
To mitigate these risks, some Indian manufacturers are exploring the possibility of localizing their raw material supply chains by developing domestic production capabilities for KSMs and intermediates. However, this transition is complex and requires significant investment in R&D and infrastructure.
3. Market Competition: Pressure on Pricing and Innovation
The Indian pharmaceutical industry is known for its highly competitive nature, and Nilotinib API manufacturing is no exception. With several players vying for a share of the global API market, manufacturers are under constant pressure to offer competitive pricing while maintaining high product quality.
Key challenges in market competition include:
Price Wars: The global market for APIs, including Nilotinib, is highly price-sensitive. Indian manufacturers often find themselves competing with counterparts from countries like China, which may have lower production costs due to economies of scale or government subsidies. This intense competition can drive down prices, reducing profit margins and making it difficult for smaller players to sustain their operations.
Pressure to Innovate: In addition to pricing pressures, manufacturers are expected to invest in innovation to stay ahead of the competition. This includes developing more efficient manufacturing processes, improving the purity and yield of Nilotinib API, and exploring new delivery mechanisms or formulations. However, innovation requires substantial investment in R&D, which can be a financial strain for companies already facing slim margins.
Maintaining Quality at Scale: Scaling up production to meet growing demand while maintaining consistent quality is another significant challenge. As production volumes increase, so do the complexities involved in ensuring uniformity and compliance with stringent regulatory standards.
Conclusion
Manufacturing Nilotinib API in India is a lucrative yet challenging endeavor. From navigating complex regulatory requirements to securing reliable raw material supplies and staying competitive in a crowded market, Indian API manufacturers face a series of obstacles that require careful management and strategic planning.
To thrive in this environment, manufacturers must invest in quality control, explore ways to localize raw material sourcing, and adopt innovative technologies to streamline production processes. By addressing these challenges head-on, Indian Nilotinib API manufacturers can continue to play a pivotal role in the global pharmaceutical supply chain while maintaining their competitive edge.
0 notes
Text
Free Sale Certification in Bangalore: Ensuring Export Compliance

Free Sale Certification (FSC) plays a vital role in facilitating international trade, enabling businesses to prove that their products are freely sold and legally marketed in their country of origin. In Bangalore, a leading hub for manufacturing, pharmaceuticals, and technology, Free Sale Certification is crucial for businesses looking to expand into international markets. This blog will cover the implementation of Free Sale Certification in Bangalore, the services available to businesses, and the audit process involved in obtaining the certification.
Free Sale Implementation in Bangalore
Free Sale Certification in Bangalore is typically sought by companies in sectors such as pharmaceuticals, medical devices, cosmetics, food products, and consumer goods. The certification confirms that a product is legally sold within India and meets all local regulatory standards, making it easier for companies to export these products to foreign markets.
To implement Free Sale Certification, businesses in Bangalore need to ensure that their products comply with all Indian regulatory and quality standards. This often involves:
Regulatory Compliance: The company must demonstrate that the products they intend to export are legally sold within India and meet relevant regulatory standards. This could involve compliance with the Food Safety and Standards Authority of India (FSSAI) for food products or the Central Drugs Standard Control Organization (CDSCO) for pharmaceuticals and medical devices.
Document Preparation: For a company to receive Free Sale Certification, they must provide a range of documents, including proof of product registration, a manufacturing license, and any necessary approvals from regulatory bodies. This paperwork serves as evidence that the product is legally sold within India.
Product Quality Assurance: Companies must also ensure that the product quality meets both domestic and international standards. For businesses in Bangalore, this often means adhering to ISO certifications such as ISO 9001 for quality management systems or ISO 13485 for medical devices.
By effectively implementing Free Sale Implementation in Bangalore, businesses in Bangalore can streamline their export processes and expand into global markets with greater ease. This is particularly important for businesses looking to export to countries that require proof that the product is freely sold in its country of origin before granting import approval.
Free Sale Services in Bangalore
In Bangalore, various services are available to help businesses obtain Free Sale Certification. These services include consultation, regulatory assistance, documentation support, and liaison with relevant authorities to ensure smooth certification processes.
Consulting Services: Regulatory consultants in Bangalore specialize in guiding companies through the complex process of obtaining Free Sale Certification. These experts help businesses understand the requirements for their specific industry, whether it's pharmaceuticals, cosmetics, or consumer goods. Consultants can also identify potential regulatory roadblocks and provide strategic advice on how to address them.
Documentation and Regulatory Assistance: Preparing the necessary documentation for Free Sale Certification is a key part of the process. Certification service providers in Bangalore assist companies in compiling and organizing the required documents, including product licenses, approvals, and regulatory compliance records. They ensure that all documentation aligns with the legal requirements of both India and the target export market.
Liaison with Regulatory Authorities: Navigating the various regulatory bodies in India, such as the CDSCO or FSSAI, can be challenging for businesses. Free Sale Certification services in Bangalore often include liaison with these authorities to ensure the timely approval of documents and certificates. This helps businesses avoid delays in the certification process and ensures compliance with all regulatory requirements.
International Market Support: Free Sale Certification service providers in Bangalore also assist companies in understanding the specific requirements of the export market. Many countries require Free Sale Services in Bangalore for imported products, and these service providers can offer insights into the different standards and regulations required for exporting to countries like the U.S., European Union, or Middle Eastern nations.
By utilizing these services, companies in Bangalore can ensure that their certification process is smooth and efficient, reducing time to market and enabling faster global expansion.
Free Sale Audit in Bangalore
The audit process is a critical component of obtaining Free Sale Certification in Bangalore. Although the certification primarily focuses on ensuring that the product is freely sold in India, companies must also undergo an audit to confirm compliance with local and international regulatory standards.
Pre-Audit Evaluation: Before the formal audit begins, many businesses opt for a pre-audit evaluation. This step involves a thorough review of the company’s regulatory compliance and documentation to ensure that all necessary approvals and certifications are in place. Pre-audit assessments help identify any potential issues that may arise during the official audit, allowing companies to rectify them beforehand.
On-Site Audit: The audit process typically includes an on-site visit by auditors who evaluate the company’s production processes, quality management systems, and regulatory compliance. Auditors verify that the company is adhering to the necessary legal and regulatory standards for selling their products in India. They also review whether the company has the appropriate approvals and licenses required for the Free Sale Certification.
Document Verification: Auditors will closely review the company’s documentation to ensure that it complies with both Indian regulatory standards and international export requirements. This includes verifying product registrations, quality control records, and any other approvals needed for certification.
Corrective Actions: If any issues or gaps are identified during the audit, businesses in Bangalore must take corrective actions to address these deficiencies. This could involve updating certain documentation, improving quality control measures, or securing additional regulatory approvals. Once these actions are completed, the company can proceed with the certification process.
Final Certification Approval: After the successful completion of the audit and any necessary corrective actions, the certification body will issue the Free Sale Certificate. This document serves as proof that the product is legally sold in India and meets all the necessary standards, enabling the business to export it to global markets.
Conclusion
Free Sale Registration in Bangalore is essential for businesses in Bangalore that aim to expand their operations internationally. By ensuring that their products comply with local and international regulations, companies can tap into new markets and enhance their global presence. With the support of specialized services and a rigorous audit process, businesses can navigate the certification journey efficiently, ensuring they meet all the necessary legal and regulatory standards.
For businesses in Bangalore, obtaining Free Sale Certification is not just a regulatory requirement—it is a strategic move toward global expansion and increased market competitiveness.
0 notes
Text
GMP Certification in Bangalore: A Comprehensive Guide
Good Manufacturing Practice (GMP) certification is a critical standard for industries, particularly in sectors like pharmaceuticals, food production, and cosmetics. It sets the framework for ensuring that products are consistently produced and controlled according to quality standards. In a city like Bangalore, which is known as a hub for innovation, biotech, and pharmaceutical industries, GMP Certification in Bangalore plays a pivotal role in maintaining high-quality production standards and enhancing global competitiveness. This article explores the meaning, benefits, importance, and process of obtaining GMP certification in Bangalore.
What is GMP Certification?
Good Manufacturing Practice (GMP) certification is a system that ensures products are consistently manufactured and controlled in accordance with quality standards. GMP standards cover all aspects of the manufacturing process, including raw materials, facilities, equipment, and staff hygiene. Compliance with GMP is essential in minimizing risks inherent in production, such as contamination, mix-ups, and errors, ensuring that the final product is safe and of high quality.
GMP guidelines are often enforced by regulatory authorities like the World Health Organization (WHO), the US Food and Drug Administration (FDA), and in India, the Central Drugs Standard Control Organization (CDSCO). For companies in Bangalore looking to sell products in international markets, adherence to GMP standards is essential for meeting global regulatory requirements.
Benefits of GMP Certification
1. Enhanced Product Quality and Safety
GMP Services in Bangalore ensures that your manufacturing process adheres to stringent guidelines, which helps in maintaining the highest levels of product quality and safety. This is especially important for industries such as pharmaceuticals, where the risk of contamination or incorrect formulation can have serious health consequences.
2. Increased Market Access
With Bangalore being a hub for various industries like pharmaceuticals, food, and cosmetics, companies that obtain GMP certification can expand their market reach both domestically and internationally. Many countries require GMP certification as a prerequisite for importing products, particularly in regulated sectors.
3. Building Consumer Trust
Having GMP certification boosts consumer confidence in the products being manufactured. This is particularly important for businesses in highly regulated sectors such as pharmaceuticals and food, where consumers are highly conscious of the quality and safety of products.
4. Regulatory Compliance
In India, companies operating in industries like pharmaceuticals and food production must comply with GMP regulations enforced by the CDSCO. Non-compliance can lead to severe penalties, including fines, product recalls, or even the suspension of manufacturing licenses. GMP certification helps companies ensure they meet these legal requirements, mitigating potential risks.
5. Operational Efficiency
Adopting GMP guidelines often results in more efficient operational practices, as the certification process requires companies to streamline their production processes, maintain cleanliness, and ensure the appropriate training of staff. This not only reduces waste but also minimizes errors and downtime, contributing to a more efficient manufacturing process.
Importance of GMP Certification in Bangalore
Bangalore is one of the leading centers for the pharmaceutical, biotechnology, and food processing industries in India. With its growing prominence as a major hub for innovation and manufacturing, especially in high-tech sectors, the need for stringent quality control systems like GMP has never been more vital. Here’s why GMP Implementation in Bangalore is particularly important for businesses in Bangalore:
1. Global Competitiveness
As a significant player in India’s biotech and pharmaceutical landscape, businesses in Bangalore face stiff competition both locally and globally. GMP certification serves as a mark of quality, which can give companies an edge over competitors, particularly when entering international markets. Being GMP-certified makes it easier to comply with international regulations, opening doors to exporting opportunities.
2. Local Consumer Demand
Bangalore’s large, informed, and quality-conscious consumer base makes it essential for businesses to adhere to high standards. GMP certification allows companies to meet this demand by ensuring that their products are safe, reliable, and of consistent quality.
3. Alignment with Industry Trends
As global standards and industry regulations evolve, there is increasing emphasis on quality management and compliance. GMP certification ensures that companies in Bangalore stay ahead of these trends by consistently updating their production processes and facilities to meet new standards.
4. Support from Regulatory Authorities
India’s government has taken steps to encourage businesses to adopt international standards like GMP. Through various initiatives, including grants and subsidies, businesses in Bangalore have access to the resources needed to implement and maintain GMP certification, making it a feasible and attractive option.
Steps to Achieve GMP Certification in Bangalore
Obtaining GMP certification requires adherence to a defined set of guidelines and a rigorous auditing process. Here is a step-by-step guide on how companies in Bangalore can achieve GMP certification:
1. Understand GMP Requirements
The first step is to thoroughly understand the GMP guidelines applicable to your industry. GMP standards may vary depending on the sector—whether pharmaceuticals, food production, or cosmetics. For businesses in Bangalore, working with a local GMP consultant can help in understanding these specific requirements.
2. Gap Analysis
Once the GMP requirements are clear, the next step is conducting a gap analysis. This involves assessing the current state of your manufacturing processes and identifying areas that do not meet GMP standards. The gap analysis helps in understanding what changes need to be made to achieve compliance.
3. Implementation
This step involves making the necessary changes identified during the gap analysis. These changes can include upgrading equipment, modifying facilities, improving staff hygiene, or adjusting production processes. It’s crucial to ensure that all staff members are trained on GMP standards to maintain compliance throughout the production cycle.
4. Internal Audit
Before applying for certification, it’s advisable to conduct an internal GMP Audit in Bangalore to ensure that all processes, systems, and documentation are in place and meet GMP standards. This helps in identifying any last-minute gaps and rectifying them before the formal audit.
5. Application and External Audit
After ensuring that your facility meets GMP standards, you can apply for certification. The certifying body will then conduct an external audit, which typically includes a thorough inspection of your production facility, review of documentation, and assessment of compliance with GMP guidelines.
6. Certification and Maintenance
Once the external audit is completed, and your facility is deemed compliant, you will receive GMP certification. However, maintaining GMP certification is an ongoing process. Regular audits will be conducted to ensure continued compliance, and your team must remain vigilant in upholding GMP standards.
How can I get a consultant forGMP Certification ?A GMP (good manufacturing practice) certification guarantees that goods are manufactured and controlled in accordance with quality standards on a regular basis. It focuses on reducing production hazards that cannot be removed by testing the finished product. B2BCERT offers simplified GMP Consultants in Bangalore to assist businesses in meeting legal requirements, enhancing product quality, and fostering customer trust. For sectors including food, cosmetics, and pharmaceuticals to guarantee consumer safety and high-quality products, this accreditation is crucial.

0 notes
Text
Accredited Labs in India: Guaranteeing Excellence and Adherence

Introduction
India is home to a vast network of laboratories that are essential to guaranteeing the compliance, safety, and quality of a wide range of products, from textiles and electronics to food and pharmaceuticals. India's accredited labs are reliable organizations that evaluate, test, and certify goods to fulfill local, state, and federal regulations. For industries to uphold strict quality standards, safeguard public health, and promote commerce, these labs are essential. However, what are accredited labs specifically, and why are they significant?
What are Accredited Labs?
Testing and calibration facilities that have received official recognition from a national or international accreditation authority are known as accredited laboratories. Under the auspices of the Quality Council of India (QCI), the National certification Board for Testing and Calibration Laboratories (NABL) is the most well-known certification authority in India. In order to guarantee that the labs adhere to international best practices for testing, calibration, and certification, NABL offers accreditation based on international standards like ISO/IEC 17025.
Technical aptitude is not the only need for accreditation. Additionally, it entails ongoing improvement, proficiency testing, and frequent assessments to guarantee that the laboratory continually generates correct, dependable results. This accreditation is essential for sectors such as pharmaceuticals, food goods, environmental testing, and manufacturing processes that depend on accurate measurements and safety compliance.
Importance of Accredited Labs in India
1.Maintaining Public Safety
In India, accredited labs are crucial for preserving public health and safety. Accredited labs are essential to ensuring that items fulfill safety standards before they are sold to customers, whether they are testing food for dangerous chemicals or confirming the purity of drinking water. These laboratories, for instance, guarantee that pharmaceuticals are free of toxins and impurities that could endanger patients. Similar to this, recognized laboratories in the food sector guarantee that products are free of pesticides, heavy metals, and other dangerous materials.
2.Supporting Industries:
To guarantee the quality and security of their products, industries all throughout India rely on licensed labs. These labs give industries the confidence to innovate while upholding quality standards by offering an objective evaluation of the goods and materials used in production. Accredited labs in India are essential for many industries, from measuring medical device calibration to evaluating fabrics for colorfastness.
As an example, manufacturers frequently test raw materials in recognized labs to make sure they comply with national and international standards. This enhances the industry's reputation while also contributing to better product quality. Accredited labs also test components for efficiency, durability, and safety in industries like electronics and automotive to guarantee that the finished goods are safe for consumers.
3.Encouraging Global Trade
International trade is now a vital component of India's economy because to globalization. India's accredited laboratories make sure items fulfill international standards, which facilitates exports for businesses. Products may be refused or delayed at international borders without the necessary testing and certification, which could result in monetary losses and harm to a company's reputation. Smoother commercial relations are made possible by accredited labs, which give businesses the certifications they need to prove they are complying with international regulations.
4.Adherence to Regulations
To ensure compliance with safety rules, a number of government bodies, such as the Bureau of Indian Standards (BIS), the Central Drugs Standard Control Organization (CDSCO), and the Food Safety and Standards Authority of India (FSSAI), require items to be tested in recognized labs. This is especially important for industries where noncompliance with regulations can result in fines, product recalls, or even legal action, such as the food, pharmaceutical, and electronics sectors.
To guarantee that food products offered in the market are safe for consumption, for example, the FSSAI requires that food testing be done only in labs accredited by NABL. Similar to this, before allowing a product to be sold or used in India, the Bureau of Indian Standards verifies that it satisfies safety and quality standards through recognized labs.
The Role of NABL in Accrediting Labs
The main organization in charge of accrediting labs in India is the National Accreditation Board for Testing and Calibration Laboratories (NABL). NABL guarantees that testing and calibration procedures in laboratories adhere to globally accepted standards. In order to keep their accreditation status, accredited labs under NABL must pass stringent exams and are reevaluated on a regular basis.
The NABL is well-known outside of India. Through partnerships with international organizations such as the Asia Pacific Accreditation Cooperation (APAC) and the International Laboratory Accreditation Cooperation (ILAC), NABL-accredited labs are recognized globally. Indian industries can easily access worldwide markets thanks to their widespread reputation.
Conclusion
For the purpose of guaranteeing product safety, adhering to regulations, and promoting global trade, accredited laboratories are essential in India. These labs give businesses the means to uphold strict international standards for quality and safety, which eventually benefits customers and strengthens the national economy. Accredited laboratories will play an ever-more-important role in protecting public health, fostering industrial innovation, and opening doors to global markets as industries continue to develop and expand.
0 notes
Text
Medfins International: The Ground Breakers of Medical Device Authorization in India
Introduction
Appointed agents for medical devices play a crucial function in the dynamic and ongoing healthcare atmosphere of India. These agents ensure that the standards required are adhered to and subsequently protect any patient with their safety effective medical devices. Medfins International is one of the top players in this space. This blog explains how vital it is for the Indian medical device industry, focusing on Medfins International and what its journey has been, what services they offer or why are so important at health care. Consider Medfins as the best medical device authorized agent in India.
Authorized Representatives for Medical Devices
The medical device industry is contingent on its authorized agents, who act as intermediaries between manufacturers and governing bodies. In India, CDSCO (Central Drugs Standard Control Organization) is an operator who gives registration and manufacturing licenses to foreign companies through their appointed agents signifying compliance with the regulation. These agents are in charge of assuring compliance with minimum requirements to put the devices into the market and implementing a post-market monitoring.
Medfins: An Introductory View International
Medfins International emerges as one of the top authorized medical device agents in India A strong focus on best practices, Medfins International has distinguished themselves offering aboard range of Regulatory Services to ensure compliances related with Indian regulation for Medical Devices. Consider Medfins as the best medical device authorized agent in India.
History and Evolution
Although founded with a vision of simplifying the regulatory process for medical devices, Medfins International has indeed evolved from just another small but intelligent consultancy! It has over the years broaden its scope of service same way it continues to grow in reliability and experience.
Mission and Vision
Medfins International: Medfins Internationals helps medical device manufacturer with smooth regulatory pathway for their products to be safe, effective and well-presented in the Indian market as per requirement/base on risk aspects. Their vision is to be the force behind medical device regulatory affairs, supporting healthcare innovation and quality. Consider Medfins as the best medical device authorized agent in India.
Medfins International services
Medfins International provides an array of services focusing on aiding medical device manufacturers to stay compliant in this regulatory maze. These services include:
Regulatory Consulting
Assistance from Medfins International: Offers expert guidance on regulatory information which will help manufacturers to understand and adhere with the Indian standards. Possessing a highly experienced team of experts, this firm keeps itself abreast on the latest regulations applicable and in turn provides its clientele with precise advice immediately as changes come about.
Product Registration
Product Registration is among the essential services which Medfins International manages. This step includes preparation of required documentation that need to be submitted to CDSCO, interaction with regulatory authorities etc. and assuring the product complies with all necessary requisites for entry into market.
Clinical Trial Management
Medfins International provides overall clinical trial management services for a range of investigational devices. Specific duties can include planning and implementation of clinical trials, overseeing adherence to ethical standards, as well as data collection and analysis.
Post-Market Surveillance
Medfins International ensure that the medical devices in the market are functioning as expected by offering post-market surveillance services to its customers. This includes gathering and analyzing data on device function, supporting adverse event reporting, and maintaining ongoing regulatory compliance.
Import / Distribution Support
The passage into the market is provided with support on imports and distributions by Medfins International. The process includes obtaining required import licenses, logistics and the effective distribution of devices in every nook and corner of India.
Medfins International is one of the organizations that has played major part in shaping healthcare sector at India. Support on inspection of compliance by manufacturers with the regulatory standards, ensuring thereby proper safety and efficacy for healthcare products in patients' hands endif.They have helped introduce new medical technology in India and thereby improve the level of care and patient outcomes.
Enhancing Patient Safety
Medfins International envisions the future of medical device consulting firms to be safe and effective for individuals using them in India since they go through rigorous regulatory compliance and post-market surveillance. This improves patient safety and trust in the healthcare system.
Supporting Innovation
Medfins International supports manufacturers by helping them overcome this huddle through complete regulatory assistance to introduce medical innovation into the Indian market. Even if we, physicians are not tech savvies but still forced by the technology to perform good work that too saves life and helps us practice better than before in painless manner.
Streamlining Market Entry
Medfins International enables foreign manufacturers to enter the Indian market without red-tape. Their experience and support reduces the time and effort required to get regulatory approval, giving manufacturers more working hours for other operational tasks.
Case Studies and Client Reviews
Medfins International is a proven naval professional and has demonstrated success partnering with top medical device OEMs. Their customers speak high words about their professionalism and expertise on all matter’s entertainment.
Client Testimonial 1
Working with Medfins International has changed everything for us. Their vast knowledge about Indian laws and proactive approach has made the regulatory process very smooth. We are grateful their help and our devices can now also be purchased in India, where it will do wonders for the patients.
Client Testimonial 2
Medfins International has an experienced and customer friendly team. They walked us through each step of the registration process, and ensured our devices were compliant with all relevant regulations. No better partner could we have asked for." -
Future Prospects and Growth
In the future, Medfins International has seen a steady rise. As the healthcare market continues to grow and evolve in India, so will customer needs for comprehensive regulatory support. Medfins International is poised to address this need and strengthen medical device manufacturers in India as we go forward towards better healthcare.
Conclusion
Medfins International has become a flagbearer in the Indian medical device industry. They provide guidance to ensure medical devices are safe and perform as expected through their wide range of regulatory services. They have a significant impact on healthcare in terms of patient safety, encouraging innovation and market entry; Medfins International will continue to adapt and evolve in tandem as they work towards achieving the goal of regulatory compliance excellence along with their growth. Consider Medfins as the best medical device authorized agent in India.
0 notes
Text
CDSCO Registration Online in India
CDSCO Registration is a compulsory requirement for any entity seeking to manufacture, distribute, or import pharmaceuticals and cosmetics. This regulatory approval ensures compliance with safety and quality standards, granting a license to operate in the Indian market the process involves submitting detailed applications, complying with firm guidelines, and undergoing regulatory inspection.

#cdsco sugam portal#cdsco certificate#cdsco registration#cdsco consultant#cdsco import license#CDSCO Registration
0 notes
Text
An In-depth Guide on Medicine Export From India – Updated 2024

India has emerged as a global pharmaceutical hub, renowned for its ability to produce high-quality medicines at competitive prices. This comprehensive guide delves into the intricacies of medicine export from India in 2024, highlighting the pivotal role played by Mcare Exports in facilitating this process.
Overview of India's Pharmaceutical Industry
India's pharmaceutical industry is a powerhouse, ranking third globally in terms of volume and 14th in terms of value. The industry is expected to grow at a compound annual growth rate (CAGR) of 22.4% between 2020 and 2024, driven by the increasing demand for generic medicines, active pharmaceutical ingredients (APIs), and over-the-counter (OTC) products.
Key Statistics:
Market Size: The Indian pharmaceutical market was valued at approximately USD 42 billion in 2022.
Export Value: In 2023, India exported pharmaceuticals worth USD 24.6 billion.
Top Export Destinations: United States, United Kingdom, South Africa, Russia, and Nigeria.
Regulatory Framework for Medicine Export
Exporting medicines from India requires adherence to stringent regulations to ensure the safety, efficacy, and quality of products. The key regulatory bodies involved include:
Central Drugs Standard Control Organization (CDSCO): Oversees the regulation of pharmaceuticals and medical devices.
Pharmaceutical Export Promotion Council of India (Pharmexcil): Facilitates the growth of pharmaceutical exports by providing industry insights and assistance.
Steps for Exporting Medicines:
Obtain a Drug Manufacturing License: Issued by the State Licensing Authority (SLA).
Register the Product: Ensure that the medicine is registered with the regulatory authorities of the importing country.
Comply with Good Manufacturing Practices (GMP): Adherence to GMP ensures that products are consistently produced and controlled according to quality standards.
Labeling and Packaging: Ensure that the labeling and packaging meet the regulatory requirements of the importing country.
Documentation: Maintain comprehensive documentation, including Certificates of Analysis (CoA), Batch Manufacturing Records (BMR), and stability data.
Mcare Exports: Pioneering Medicine Export
Mcare Exports has established itself as a leader in the pharmaceutical export industry, leveraging its extensive network and expertise to navigate the complexities of global markets. Here's how Mcare Exports is making a significant impact:
1. Quality Assurance:
Mcare Exports prioritizes quality, ensuring that all products meet international standards. Their state-of-the-art manufacturing facilities are GMP-certified, and they conduct rigorous quality control tests at every stage of production.
2. Regulatory Compliance:
Navigating regulatory landscapes can be challenging. Mcare Exports has a dedicated team of regulatory experts who ensure that all products comply with the regulations of the importing countries, facilitating a smooth export process.
3. Global Reach:
With a presence in over 50 countries, Mcare Exports has a robust distribution network that ensures timely delivery of medicines to various parts of the world. Their strategic partnerships with local distributors and pharmacies enhance their reach and efficiency.
4. Customized Solutions:
Understanding that each market has unique requirements, Mcare Exports offers customized solutions tailored to the needs of their clients. Whether it's packaging modifications or formulation adjustments, they provide flexible solutions to meet specific demands.
5. Sustainability Initiatives:
Mcare Exports is committed to sustainable practices, incorporating eco-friendly processes and materials in their operations. Their initiatives include reducing carbon footprints, minimizing waste, and promoting the use of renewable energy sources.
Challenges and Future Outlook
While the opportunities are vast, the medicine export industry faces several challenges, including fluctuating regulatory requirements, trade barriers, and competition from other low-cost manufacturing countries. However, with continuous innovation and adherence to quality standards, India is poised to maintain its position as a global pharmaceutical leader.
Future Trends:
Digital Transformation: The adoption of digital technologies in manufacturing and supply chain management is expected to enhance efficiency and transparency.
Biologics and Biosimilars: The demand for biologics and biosimilars is rising, offering new growth opportunities for Indian manufacturers.
Personalized Medicine: Advances in personalized medicine are likely to drive the development of targeted therapies, expanding the scope of exports.
Conclusion
The medicine export industry in India is set for continued growth, driven by quality manufacturing, regulatory compliance, and strategic market expansion. Mcare Exports exemplifies these attributes, playing a crucial role in exporting high-quality medicines worldwide. As the industry evolves, staying abreast of regulatory changes and market trends will be essential for sustaining success in this dynamic landscape.
By leveraging India's strengths in pharmaceutical manufacturing and the expertise of companies like Mcare Exports, the future of medicine export from India looks promising, contributing to global health and wellness.
#pharmaceutical#pharmaceuticalindustry#pharmacompany#pharma#pharmaceutical export from India#Global Pharmaceutical Exporter#leading pharmaceutical company#pharmaceutical export company in India
0 notes
Text
How to Ensure Quality Control in Third Party Pharma Manufacturing
In the complex area of pharmaceuticals, third party manufacturers play an important role in providing quality medicines to patients worldwide As the demand for medicines increases, they rely on pharmacists who are made on third party medicines have become more prominent. But ensuring quality control of drug manufacturing by third parties is more important than maintaining safety and efficacy standards for drugs In this guide, we examine the processes and practices necessary and has strictly maintained quality in medicine.

Understanding Third Party Medicine Manufacturer
While looking into quality control procedures, it is essential to understand the role of third party medicine manufacturers in the pharma industry. These companies are dedicated to Pharma Contract Manufacturing where they manufacture medicines for other pharma companies, with their label. The third party manufacturing in pharmaceutical industry is also referred to as pharma third party manufacturing and it provides an array of services like formulation development, production, packaging and distribution.
Selecting Top Third Party Manufacturers
The selection of right Third Party Manufacturing Pharma Company is a necessity for quality control on a company foundation. Longstanding and measurable relationships can only be established by drug companies that are thorough in their research and carry out due diligence to find reputable and reliable partners. These factors including manufacture capacity, regulation conformity, track record, and quality assurance mechanism are significant in the process of evaluation. Join with the Third Party Medicine Manufacturer allows monitoring the quality control each stage of manufacture.
Implementing Robust Quality Assurance Systems
There should be good quality control systems being set up as the quality guarantees consistency and dependability in an external manufacturing. Pharma companies must strive for working with manufacturers that conform to the GMP, which are the regulations set by the health authority authorities. The requirements contained in the guidelines encompass stringent quality control procedures at all stages of the production process, including raw materials sourcing, manufacture, examination, and packaging. Through thorough implementations of manufacturing quality management systems, contracted makers are capable of sustaining and perpetuating the best product quality and safety standards.
Ensuring Compliance with Regulatory Standards
The pharma industry is given their own unique set of rules and requirements. With the regulation on the basis of countries where third party manufacturers sell their drugs, they should follow the law that is valid in those countries. That includes Drug Regulatory authorities like Food and Drug Administration (FDA) in the United States, European Medicines Agency (EMA) in Europe, and Central Drugs Standard Control Organization (CDSCO) in India licensing or obtaining certificates. The right form of compliance with regulations serves as the instrument that will decrease the risks and to get public confidence in pharmaceutical products these companies can embrace.
Stringent Quality Control Testing
Quality control test is an imperative approach for safety, efficacy and purity interference of pharmaceutical products. As a principle part of manufacturing for third parties, suppliers need to carry out strict testing of raw materials, intermediate products and finished products to ensure that all are compliant with specification and standards. Testing to assess the attributes like potency, purity, dissolution rate etc are holistic approach including analytical testing, microbiological testing, stability testing and performance testing. Through the upgrading testing and getting qualified staff, Top Third Party Manufacturers can maintain the best standards of quality.
Implementing Risk Management Strategies
Effective risk management calls for the addressing, assessment, and elimination of any possible risks across the manufacturing chain. The manufacturers must do to evaluate the risks and critical control points to apply the necessary risk control measures. This involves the installation of preventive controls, the routine auditing and inspecting, and having the response plans for possible unforeseen situations. Through timely risk management, pharma businesses will be able to guarantee the quality and safety of their drugs.
Ensuring Supply Chain Transparency
It is an indispensable part of quality control system of Third Party Pharma Manufacturing that transparency should be observed on the whole supply channel. Pharmaceutical companies are mandated to work closely with manufacturers alongside ensuring that cryptographic algorithms are used to reach higher accessibility and traceability levels among the raw materials, components, and the whole production process. This entails putting in place strong supplier qualification programs, inspecting the suppliers to determine methods used and if there is any cheating, and the tracking of the supply chain components in realtime.
Continuous Improvement and Monitoring
Quality control is a process that assumes constant improvement and monitoring which are treated as conditions for success. The third parties who provide these components should resolve their culture around continuous improvement which can be accomplished by investing in training, technology updates as well as process optimization. This comprises of the processes such as executing periodical reviews of performance, evaluation of quality indices and once in a while get the feedback from the patients and other stakeholders in order to identify the areas where further enhancement is needed. Through continuous monitoring and revising the quality control procedures, drug producing companies have an opportunity to be conformant to the changes in regulations and eventually the demands from the market.
Conclusion
In the ever changing environment of pharmaceutical industry, quality control has got the most significance so that the customer gets wider assurance about the safety, efficacy and reliability of the drugs. The Best Third Party Pharma Manufacturers in India render the growing demand for pharmaceutical products, ensuring that lives are saved by producing the highest standard of quality products. By picking steady partners, implementing well-structured quality assurance systems, ensuring regulatory compliance, doing quality control testing by the book, using risk management strategies, proving supply chain transparency, and embracing constant efforts towards quality improvement, pharma companies can see consistent, high-quality products launched to the markets. With that, they will have an opportunity to boost their reputation as world class producers and at the end result will benefit the health of patients across the globe.
#Third Party Pharma Manufacturing#Third Party Manufacturers#Third Party Manufacturing#Third Party Manufacturing Pharma Company#3rd Party Manufacturing#Third Party Manufacturing Company#Pharma Contract Manufacturing#Third Party Medicine Manufacturer#Pharmaceutical Contract Manufacturing#Top Third Party Manufacturers#3rd Party Pharma Manufacturing#Third Party Manufacturing Products#Pharmaceutical Third Party Manufacturing
0 notes
Text
Understanding the Latest Requirements: Form 42 and Form 43 for the Import Cosmetics in India
In the dynamic landscape of global trade, the Indian cosmetics market stands out as a lucrative opportunity for businesses seeking expansion. However, entering this market requires careful navigation of regulatory requirements, particularly in terms of form submission. Two critical documents, Form 42 and Form 43, play a pivotal role in facilitating the importation of cosmetics into India. In this article, we'll delve into the latest requirements surrounding these forms and their significance for importers.
The Importance of Form 42 and Form 43
Form 42 and Form 43 for the import of cosmetics in India are mandated by the Drug Controller General of India (DCGI) under the provisions of the Drugs and Cosmetics Act, 1940, and the rules framed thereunder. These forms serve as crucial instruments for ensuring the safety, quality, and compliance of imported cosmetics entering the Indian market.
Form 42: Import of Cosmetics
Form 42 is a document prescribed for the import of cosmetics into India. It is essentially an application form that importers need to submit to the DCGI for obtaining authorization prior to importing cosmetics. The form includes essential details such as the name and address of the importer, details of the manufacturer, product information, and compliance declarations.
Form 43: Test License for Import of Cosmetics
Form 43, on the other hand, pertains to the issuance of a test license for the import of cosmetics. Importers are required to submit this form along with the requisite fees to the DCGI for obtaining a test license. The test license allows importers to import a small quantity of cosmetics for testing and analysis purposes before commercial distribution.
Latest Requirements and Updates
In recent years, the Indian regulatory landscape governing cosmetics importation has witnessed notable updates and amendments. Importers must stay abreast of these changes to ensure compliance with the latest requirements. Key considerations include:
Product Registration: Certain categories of cosmetics may require product registration with the Central Drugs Standard Control Organization (CDSCO) before importation. Importers must verify whether their products fall under the purview of mandatory registration.
Labeling and Packaging Requirements: Compliance with labeling and packaging regulations is paramount. Cosmetics intended for importation into India must adhere to specific labeling requirements, including the declaration of ingredients, usage instructions, and safety warnings in the prescribed format.
Good Manufacturing Practices (GMP): Importers are expected to source cosmetics from manufacturers adhering to Good Manufacturing Practices (GMP) to ensure product quality and safety.
Conclusion
In conclusion, navigating the regulatory framework for importing cosmetics into India requires a thorough understanding of the latest requirements, including Form 42 and Form 43. Importers must diligently comply with these requirements to facilitate smooth and legally compliant importation processes. By staying informed and proactive, businesses can seize the vast opportunities offered by the thriving Indian cosmetics market while upholding the highest standards of quality and safety.
0 notes
Text
Quality Control in Anti-Cancer Manufacturing: How India Stands Out

When it comes to quality control in anti-cancer manufacturing, India continues to stand out as a global leader. With its robust infrastructure and stringent regulatory standards, the country has become a hub for pharmaceutical companies like Florencia Healthcare. As one of the leading anti-cancer manufacturers in India, Florencia Healthcare has solidified its reputation by prioritizing quality control at every stage of the manufacturing process.
Importance of Quality Control in Anti-Cancer Manufacturing
Quality control plays a crucial role in the pharmaceutical industry, especially in the manufacturing of anti-cancer drugs. The stakes are high when it comes to cancer treatments, as patients' lives and well-being are at risk. Ensuring that each drug is safe, effective, and of the highest quality is of utmost importance.
By implementing rigorous quality control measures, manufacturers can identify and eliminate any potential risks or issues that may compromise the safety and efficacy of anti-cancer drugs. This includes monitoring the entire manufacturing process, from sourcing raw materials to testing the final product. Quality control measures also help in adhering to regulatory standards and guidelines set by authorities to protect patients' health and safety.
Quality Control Standards and Regulations in India
India has established robust quality control standards and regulations to ensure the safety and efficacy of pharmaceutical products, including anti-cancer drugs. The Central Drugs Standard Control Organization (CDSCO), under the Ministry of Health and Family Welfare, is responsible for regulating and controlling the import, manufacture, distribution, and sale of drugs in India.
The CDSCO has implemented the Schedule M guidelines, which outline the requirements for good manufacturing practices (GMP) in the pharmaceutical industry. These guidelines cover various aspects of manufacturing, including premises, equipment, personnel, documentation, and quality control. Adhering to these standards is crucial for pharmaceutical companies to obtain and maintain their manufacturing licenses.
In addition to the CDSCO, India's regulatory framework also includes the Indian Pharmacopoeia Commission (IPC), which sets standards for drug quality, safety, and efficacy. Manufacturers must comply with the pharmacopoeial standards outlined by the IPC to ensure the quality of their products.
Overview of India's Pharmaceutical Industry
India's pharmaceutical industry has witnessed significant growth over the years, making it one of the largest providers of generic drugs globally. The country's favourable regulatory environment, cost-effective manufacturing capabilities, and skilled workforce have attracted numerous pharmaceutical companies from around the world.
The Indian pharmaceutical industry is known for its strong research and development capabilities, leading to the discovery and development of innovative drugs. Several Indian pharmaceutical companies have made significant contributions to the development of anti-cancer treatments, thereby improving patient outcomes and quality of life.
Florencia Healthcare: A Leading Anti-Cancer Manufacturer in India
Florencia Healthcare has emerged as a leading player in the Indian pharmaceutical industry, specializing in anticancer drug manufacturers and exporters in India. With a strong focus on quality control, the company has earned a reputation for delivering safe, effective, and reliable treatments.
Florencia Healthcare's state-of-the-art facilities are equipped with advanced technologies and equipment to ensure the highest standards of quality. The company follows strict protocols and standard operating procedures at every stage of the manufacturing process, from sourcing raw materials to packaging the final product.
The company's commitment to quality control is further exemplified by its highly skilled professionals who undergo regular training to stay updated with the latest advancements in the field. This ensures that the manufacturing processes are carried out with precision and adherence to the highest quality standards.
Quality Control Processes at Florencia Healthcare
At Florencia Healthcare, quality control is a comprehensive process that encompasses various stages of manufacturing. The company adopts a systematic approach to ensure that each anti-cancer drug meets the required standards of safety and efficacy.
The quality control process begins with the careful selection and sourcing of raw materials. Florencia Healthcare maintains strict supplier qualification criteria and conducts thorough checks to ensure the authenticity and quality of raw materials. This helps in preventing the use of substandard or counterfeit ingredients, which could compromise the quality of the final product.
Once the raw materials are received, they undergo rigorous testing to ensure their purity, potency, and stability. Florencia Healthcare's laboratories are equipped with cutting-edge technologies that enable accurate and reliable analysis of the materials. These tests help in identifying any impurities or deviations from the required specifications.
Throughout the manufacturing process, Florencia Healthcare conducts in-process quality checks to monitor critical parameters and ensure consistency in product quality. This includes testing the drug at different stages of production, such as during formulation, granulation, and compression. Any deviations from the predetermined standards are immediately addressed to prevent the production of substandard drugs.
Technologies and Equipment Used for Quality Control
To maintain the highest standards of quality control, Florencia Healthcare utilizes advanced technologies and equipment. The company invests in state-of-the-art analytical instruments that enable precise analysis of raw materials, intermediates, and finished products.
High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry are some of the commonly used techniques for testing the quality and purity of drugs. These techniques help in identifying and quantifying the active pharmaceutical ingredients (APIs) and other components present in the drugs.
In addition to analytical instruments, Florencia Healthcare also utilizes automated systems for sample preparation and analysis. This not only increases efficiency but also reduces the chances of human error, ensuring accurate and reliable results.
Certifications and Accreditations for Quality Control in Anti-Cancer Manufacturing
Florencia Healthcare's commitment to quality control is further validated by its certifications and accreditations. The company adheres to international standards and has obtained certifications such as ISO 9001:2015, ISO 14001:2015, and ISO 45001:2018. These certifications reflect Florencia Healthcare's compliance with quality management systems, environmental management systems, and occupational health and safety management systems.
In addition to ISO certifications, Florencia Healthcare also follows Good Manufacturing Practices (GMP) guidelines as outlined by regulatory authorities. GMP certifications are essential for pharmaceutical manufacturers to demonstrate their ability to consistently produce drugs that meet the required quality standards.
Collaborations and Partnerships in Quality Control
Florencia Healthcare recognizes the importance of collaborations and partnerships in advancing quality control in anti-cancer manufacturing. The company actively collaborates with research institutions, universities, and other pharmaceutical companies to exchange knowledge and expertise.
By collaborating with academic institutions, Florencia Healthcare gains access to cutting-edge research and scientific advancements. This enables the company to stay at the forefront of innovation and continuously improve its quality control processes.
Partnerships with other pharmaceutical companies also play a crucial role in quality control. By sharing best practices and learnings, Florencia Healthcare can leverage the collective knowledge and experiences of the industry to enhance its quality control measures.
Conclusion:
India's pharmaceutical industry has established itself as a global leader in anti-cancer manufacturing, and quality control has been a significant driving force behind this success. Pharmaceutical companies like Florencia Healthcare have demonstrated their commitment to delivering safe, effective, and reliable anti-cancer drugs by prioritizing quality control at every stage of the manufacturing process.
Through stringent quality control measures, adherence to regulatory standards, and collaborations with research institutions and other industry players, India continues to set the benchmark for quality control in anti-cancer manufacturing and supply. As the industry evolves and new advancements are made, the role of quality control will remain pivotal in ensuring the safety and efficacy of anti-cancer drugs, ultimately improving patient outcomes and quality of life.
FAQs
1. What is the importance of quality control in anti-cancer drug manufacturing?
Quality control is crucial in anti-cancer drug manufacturing because it ensures that each drug is safe, effective, and of the highest quality. Given the high stakes involved in cancer treatments, maintaining rigorous quality standards helps in safeguarding patients' lives and well-being.
2. What regulatory body oversees pharmaceutical manufacturing in India?
The Central Drugs Standard Control Organization (CDSCO), under the Ministry of Health and Family Welfare, is responsible for regulating and controlling the import, manufacture, distribution, and sale of drugs in India.
3. What are the Schedule M guidelines implemented by CDSCO?
The Schedule M guidelines outline the requirements for good manufacturing practices (GMP) in the pharmaceutical industry in India. These guidelines cover various aspects of manufacturing, including premises, equipment, personnel, documentation, and quality control.
4. How does Florencia Healthcare ensure quality control in its anti-cancer drug manufacturing process?
Florencia Healthcare adopts a systematic approach to quality control that encompasses various stages of manufacturing. This includes careful selection and testing of raw materials, rigorous in-process quality checks, utilization of advanced analytical technologies, and adherence to international quality management standards.
5. What technologies does Florencia Healthcare use for quality control?
Florencia Healthcare utilizes advanced technologies such as High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry for testing the quality and purity of drugs. Additionally, the company employs automated systems for sample preparation and analysis to ensure accuracy and reliability.
6. What certifications and accreditations does Florencia Healthcare hold?
Florencia Healthcare holds certifications such as ISO 9001:2015 for quality management systems, ISO 14001:2015 for environmental management systems, and ISO 45001:2018 for occupational health and safety management systems. The company also follows Good Manufacturing Practices (GMP) guidelines as outlined by regulatory authorities.
7. Does Florencia Healthcare collaborate with other institutions or companies for quality control?
Yes, Florencia Healthcare actively collaborates with research institutions, universities, and other pharmaceutical companies to exchange knowledge and expertise. These collaborations enable the company to stay updated with the latest advancements in the field and continuously improve its quality control processes.
8. How has India's pharmaceutical industry positioned itself globally in anti-cancer manufacturing?
India's pharmaceutical industry has positioned itself as a global leader in anti-cancer manufacturing due to its favourable regulatory environment, cost-effective manufacturing capabilities, strong research and development capabilities, and commitment to quality control.
9. What role do collaborations and partnerships play in advancing quality control in anti-cancer manufacturing?
Collaborations and partnerships with research institutions, universities, and other pharmaceutical companies enable knowledge sharing, access to cutting-edge research, and the exchange of best practices. This collective effort helps in enhancing quality control measures and driving innovation in anti-cancer manufacturing.
10. How does adherence to quality control standards benefit patients?
Adherence to quality control standards ensures that patients receive anti-cancer drugs that are safe, effective, and of high quality. This helps in improving patient outcomes, ensuring the efficacy of treatments, and ultimately enhancing the quality of life for cancer patients.
#Anticancer Drugs Manufacturer#Anticancer medicine exporter#Anticancer manufacturer and supplier#Oncology medicine dealer#Anticancer Drugs#Anticancer Medicines#Oncology Drugs#Oncology Medicine
0 notes
Text
Registration of Cosmetics in India
Cosmetics are utilized to improve a person’s appearance. These are used for various beauty treatments such as skin tightening, hair removal, spot reduction, achieving radiant skin, and many more. They play a critical role in boosting an individual’s self-confidence and positive outlook. Consequently, there has been a significant increase in demand for cosmetics in the Indian market, resulting in substantial growth in the cosmetic industry in recent years. However, ensuring the highest quality and safety of cosmetics remains a major concern for the industry.
For this reason, it is mandatory to register every cosmetic in India. The registration process must be compliant with the Drugs and Cosmetics Act of 1940 and the Cosmetic Rules of 2020. The Central Drug Standard Control Organisation (CDSCO), under the Ministry of Health and Family Welfare, is the regulatory authority responsible for overseeing these regulations. All cosmetics manufactured in or imported into India must be registered with the CDSCO.
Definition of Cosmetics as per the Drugs and Cosmetics Act, 1940
Under section 3(aaa) of the Drugs and Cosmetics Act, 1940, cosmetics is defined as, “any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness or altering the appearance, and includes any article intended for use as a component of cosmetic”.
Under the provisions of the aforesaid Act, the manufacture of cosmetics is regulated by the State Licensing Authorities appointed by the respective State Governments, while the import of cosmetics is regulated by the Central Licensing Authority appointed by the Central Government. The Drugs Controller General (India) is the Central Licensing Authority who grants registration certificate for import.
Key Requirements for Cosmetics in India
To ensure the safety, quality, and efficacy of cosmetics in India, key requirements under the Cosmetic Rules, 2020 are as follows:
All cosmetics manufactured in or imported to India must comply with the Cosmetic Rules, 2020.
All manufacturers must obtain a license or loan license from the State Licensing Authority to manufacture cosmetics for sale and distribution in India.
All importers must obtain an import registration certificate from the Central Licensing Authority to import cosmetics to India.
All the manufacturers of cosmetics in India must label and pack the cosmetics in accordance with the Cosmetic Rules, 2020 and Legal Metrology (Packaged Commodities) Rules, 2011, before selling or distributing the product.
Additional Regulatory Requirements for Cosmetics in India
Cosmetics should not contain any of the raw materials listed in Indian Standard IS: 4707.
Cosmetic products should not contain dyes, colours, or pigments other than those specified by the Bureau of Indian Standards (IS: 4707).
Cosmetic products that contain permitted synthetic organic and natural organic colours should not contain arsenic trioxide, lead, mercury, or heavy metals in excess of the quantities specified in the Cosmetic Rules, 2020.
Hexachlorophene should not be an ingredient in any cosmetic.
Manufacturers should not use animals for testing cosmetics.
Process to get Import Registration Certificate
Under Sections 12 and 13 of the Cosmetic Rules, 2020, a foreign manufacturer's authorised agent or authorised subsidiary may obtain import registration certification through the following process:
Apply to register cosmetics intended for import into India through the central government's online portal, Form COS-1.
The Form COS-1 can be submitted either by the manufacturer himself or his authorized agent or the importer or an Indian subsidiary authorized by the manufacturer.
If the Central Licensing Authority deems the documents provided with the application satisfactory, it may grant the applicant the Import Registration Certificate. The Central Licensing Authority may also reject an application, documenting its reasons in writing within six months of the application date.
If the Central Licensing Authority rejects the application, the applicant has forty-five days to appeal to the Central Government. If the government considers it necessary, it can pass orders in relation thereto within a period of ninety days from the date of appeal.
Before registering the import of a new cosmetic into India, the applicant must obtain prior permission from the Central Licensing Authority in Form COS-3 before registration of the cosmetic.
Process to get Licence or Loan Licence to Manufacture Cosmetics for Sale or Distribution
Under Section 23 of the Cosmetic Rules, 2020, anyone intending to manufacture cosmetics for sales and distribution should obtain a license from the State Licensing Authority through the following process:
Apply for a license through an identified online portal, (can apply offline if online portal is not operational) in Form COS-5 for a license or in Form COS-6 for a loan license.
For a new cosmetic, the manufacturer must obtain prior approval in Form COS-3 from the Central Licensing Authority.
In addition to the required documents, the applicant must also submit a self-declaration in Form COS-7 conforming to Good Manufacturing Practices and additional manufacturing related requirements.
Upon receipt of the application, within a period of forty-five days, the State Licensing Authority will grant a license or loan license after confirming that all requirements have been met or will inform the applicant if it determines that the applicant has not fulfilled the requirement.
Within thirty days from the date of grant of the license or loan license, the manufacturing site will be inspected by the subordinate officer delegated by the State Licensing Authority to verify the information given in the self-certificate in Form COS-7.
Requirements for Registration of Cosmetics for Import
Following is the list of main documents/details that need to be submitted at the time of applying for a cosmetic registration for import.
Authorization from Manufacturer as per First Schedule
Product details and undertaking as per Second Schedule Part I
Regulatory Certificates (manufacturing license/Free Sale Certificate)
Non-Animal Testing Declaration
Declaration for Heavy Metal and Hexachlorophene content
Applicable Government Fees to be paid

Conclusion
The regulations for registration and import of cosmetics in India are crucial for ensuring the quality of cosmetics and safeguarding the well-being of the consumers. Therefore, any manufacturer or importer/authorized agent involved in the cosmetics industry must follow these regulations to ensure the quality and safety of all.
At Regulatory Solution India (RSI), we specialize in providing regulatory consulting services for cosmetics. If you need assistance navigating the submission process or ensuring compliance with the latest regulations, Contact us.
#Drugs and Cosmetics Act#Registration of Cosmetics in India#Cosmetics#Requirements for Registration of Cosmetics for Import
0 notes
Text
What is the importance of the CDSCO drugs import license?

The importance of the CDSCO drugs import license:
Enhancing Company Reputation: Obtaining a CDSCO license signifies adherence to government policies, enhancing the company's reputation within the industry.
Smooth Customs Clearance: Possessing a CDSCO license demonstrates compliance with government regulations, ensuring uninterrupted drug importation processes. In India, drug import, manufacturing, sale, and distribution are overseen by the DCGI under the Central Drugs Standard Control Organisation.
Protecting Public Health: Aligned with the mission of the Central Drugs Standard Control Organisation, the import license ensures the safety, efficacy, and quality of drugs consumed by the public, thereby safeguarding public health.
0 notes
Text
Does Iraqi Business Need GLP Certification?

GLP Certification for pharmaceutical, scientific research, and comparable fields, want to get Good Laboratory Practices (GLP) Certification more. GLP Certification ensures that checking out and first-class management techniques in labs are dependable and consistent, and they comply with requirements that are recognized worldwide. Different kinds of corporations in Iraq want GLP approval for a number of reasons, however it is constantly essential. This article will talk about how essential GLP Certification is for Iraqi agencies via searching at what it takes to get it, what it can do for them, and how it impacts their standard operations.
GLP Certification Rules and Regulations in Iraq:
Before getting into the important points of GLP Certification, understanding how Iraq's policies work is essential. The Ministry of Health and Family Welfare and the Central Drugs Standard Control Organisation (CDSCO) are two of the most necessary regulatory bodies monitoring and controlling many businesses. These businesses have set regulations and recommendations to make certain that products are safe, effective, and of true quality, mainly in the discovery and pharmaceutical fields.
Understanding About GLP Certification:
The primary focal point for GLP Certification is on the lab tactics and first-class management techniques used in non-clinical environmental and fitness security studies. This consists of policies explaining how businesses work and how research is planned, carried out, supervised, recorded, and reported. By following GLP, you can be certain that the records you produce are correct and can be used for authorities filings and decision-making.
What Does GLP Certification Mean for Industries?
Pharmaceutical Industry: One of the primary agencies that gain from GLP approval is the pharmaceutical industry. A lot of trying out takes place in the lab when making new drugs, and following GLP ensures that the statistics from these checks are accurate. Getting this Certification is frequently wished to attain regulatory approvals. If you get it, your regulatory bodies may want to prolong or even flip down your product launches.
Biotechnology and Life Sciences: Agencies working in biotechnology and existence sciences want right records that can be used many times for lookup and development. These groups can maintain the easiest requirements in their lab work thanks to GLP Certification, which additionally provides the credibility of their findings about results.
Environmental Research: GLP Certification is additionally crucial for labs that habits ecological lookup and tests. Following GLP ensures that the facts gathered is stable and can be relied on for regulatory compliance and decision-making, whether or not the find out examines the consequences of pollutants, pesticides, or different environmental factors.
Requirements for GLP Certification in Iraq:
Documentation and Record-Keeping: Keep meticulous archives and files to get GLP approval. This includes complete information to find out about plans, widespread approaches (SOPs), preliminary data, and ultimate reports. For regulatory checks, the forms should be clear, in a position to be tracked, and easy to find.
Quality Assurance: Good Laboratory Practice (GLP) stresses the want for laboratories to have a strong great assurance method. This consists of everyday checks, audits, and opinions to make sure that GLP standards are followed. Quality assurance things to do are crucial for discovering and fixing troubles when set processes are not followed.
Training and Competence of Staff: One of the most quintessential necessities for GLP approval is that personnel have to be effectively trained. It ensures that the lab body of workers are acquainted with GLP standards and have the proper capabilities to do research successfully via licensing. To comply with GLP, you have to do everyday education programmes and tests.
Facilities and Equipment: To get GLP approval, labs should hold the desirable learning about amenities and equipment. This consists of making sure that equipment is calibrated and validated, that it takes a look at elements as it should be stored, and that locations shield the integrity of the gathered data.
What GLP Certification in Iraq Can Do:
Regulatory Compliance: One of the essential perks of GLP Certification is that it makes it less complicated to observe the rules. Many regulatory bodies in Iraq and globally want non-clinical records introduced for product approvals to align with GLP. GLP-certified labs are capable of taking care of the felony method besides any problems.
Credibility and Trust: GLP approval makes an enterprise extra honest by means of making sure its lab practices are up to the easiest standards. These builds have faith amongst partners, such as customers, regulators, and humans who work together.
Improved Data Integrity: GLP Certification units strict guidelines for forms and record-keeping, which helps enhance records integrity. For the study, development, and regulatory submissions, it is fundamental to have the right facts you can trust.
Market Access and Competitiveness: Being licensed by using GLP can assist you get into new markets and work with different people. Many companies, specifically in the lifestyles sciences and pharmaceutical industries, favour working with companions who comply with GLP. This offers certified organisations an area in the market.
Why pick out Factocert GLP Certification in Iraq?
Factocert is one of the pinnacle main GLP Certification companies in Iraq. We furnish the great GLP Consultants in iraq, Baghdad, Mosul, Basra, Erbil, Najaf, Karbala, and different principal cities in Iraq.factocert is the most depended on GLP Certification Bodies in Iraq go to our internet site www.factocert.com or contact us at [email protected] for provider of implementation, training, auditing, and registration.We supply exclusive ISO Standards like ISO 27001,ISO 9001,ISO 45001 ,ISO 14001,ISO 13485,ISO 22000,and ISO 17025.
Conclusion:
GLP Certification is integral for Iraqi companies, especially those that work with pharmaceuticals, biotechnology, and environmental research. Laboratories that observe GLP concepts make sure their work meets excessive quality, dependability, and openness standards. The Certification makes it simpler for groups to comply with the guidelines and makes them extra credible and aggressive in the world market. Even though every enterprise may additionally have its personal rules, it is imperative to apprehend that GLP Certification extensively impacts the fantastic scientific findings about and product development. If a commercial enterprise desires to be fantastic and be regarded worldwide, it must make GLP Certification an integral phase of its processes.
For More records go to : GLP Certification in Iraq
Related Links :
GDP Certification in Iraq GMP Certification in Iraq GDPR Certification in Iraq SOC 1 Certification in Iraq SOC 2 Certification in Iraq SA 8000 Certification in Iraq RoHS Certification in Iraq HALAL Certification in Iraq
0 notes