#c2c12
Explore tagged Tumblr posts
Text
Biomolecules, Vol. 14, Pages 1098: Inhibition of MAT2A Impairs Skeletal Muscle Repair Function
The regenerative capacity of muscle, which primarily relies on anabolic processes, diminishes with age, thereby reducing the effectiveness of therapeutic interventions aimed at treating age-related muscle atrophy. In this study, we observed a decline in the expression of methionine adenosine transferase 2A (MAT2A), which synthesizes S-adenosylmethionine (SAM), in the muscle tissues of both aged humans and mice. Considering MAT2A’s critical role in anabolism, we hypothesized that its reduced expression contributes to the impaired regenerative capacity of aging skeletal muscle. Mimicking this age-related reduction in the MAT2A level, either by reducing gene expression or inhibiting enzymatic activity, led to inhibiting their differentiation into myotubes. In vivo, inhibiting MAT2A activity aggravated BaCl2-induced skeletal muscle damage and decreased the number of satellite cells, whereas supplementation with SAM improved these effects. #RNA-sequencing analysis further revealed that the Fas cell surface death receptor (Fas) gene was upregulated in Mat2a-knockdown C2C12 cells. Suppressing MAT2A expression or activity elevated Fas protein levels and increased the proportion of apoptotic cells. Additionally, inhibition of MAT2A expression or activity increased p53 expression. In conclusion, our findings demonstrated that impaired MAT2A expression or activity compromised the regeneration and repair capabilities of skeletal muscle, partially through p53–Fas-mediated apoptosis. https://www.mdpi.com/2218-273X/14/9/1098?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Link
Keywords: core shell nanomedicine nanoparticles C2C12 silica
0 notes
Text
protocol: freezing C2C12 skeletal muscle cells
1. apply 2mL trypsin to each 10cm plate and incubate 1 minute RT under cell culture hood, 2 minutes in 38°C incubator. 2. collect all cultures into one plate and pipet repeatedly against plate surface to ensure single-cell cultures only. Place all cells in trypsin in large centrifuge tube and add 2-fold DMEM. Centrifuge for 3 minutes at 1000 rpm. 3. suction out media and re-suspend cells in [1.8mL DMEM, 0.5mL DMSO]. Place in cell culture freezing container with 250mL isopropanol and freeze in -80°C fridge.
1 note
·
View note
Text
3D bioprinting of collagen to rebuild components of the human heart
For biofabrication, the goal is to engineer tissue scaffolds to treat diseases for which there are limited options, such as end-stage organ failure. Three-dimensional (3D) bioprinting has achieved important milestones including microphysiological devices (1), patterned tissues (2), perfusable vascular-like networks (3–5), and implantable scaffolds (6). However, direct printing of living cells and soft biomaterials such as extracellular matrix (ECM) proteins has proved difficult (7). A key obstacle is the problem of supporting these soft and dynamic biological materials during the printing process to achieve the resolution and fidelity required to recreate complex 3D structure and function. Recently, Dvir and colleagues 3D-printed a decellularized ECM hydrogel into a heart-like model and showed that human cardiomyocytes and endothelial cells could be integrated into the print and were present as spherical nonaligned cells after 1 day in culture (8). However, no further structural or functional analysis was performed.
We report the ability to directly 3D-bioprint collagen with precise control of composition and microstructure to engineer tissue components of the human heart at multiple length scales. Collagen is an ideal material for biofabrication because of its critical role in the ECM, where it provides mechanical strength, enables structural organization of cell and tissue compartments, and serves as a depot for cell adhesion and signaling molecules (9). However, it is difficult to 3D-bioprint complex scaffolds using collagen in its native unmodified form because gelation is typically achieved using thermally driven self-assembly, which is difficult to control. Researchers have used approaches including chemically modifying collagen into an ultraviolet (UV)–cross-linkable form (10), adjusting pH, temperature, and collagen concentration to control gelation and print fidelity (11, 12), and/or denaturing it into gelatin (13) to make it thermoreversible. However, these hydrogels are typically soft and tend to sag, and they are difficult to print with high fidelity beyond a few layers in height. Instead, we developed an approach that uses rapid pH change to drive collagen self-assembly within a buffered support material, enabling us to (i) use chemically unmodified collagen as a bio-ink, (ii) enhance mechanical properties by using high collagen concentrations of 12 to 24 mg/ml, and (iii) create complex structural and functional tissue architectures. To accomplish this, we developed a substantially improved second generation of the freeform reversible embedding of suspended hydrogels (FRESH v2.0) 3D-bioprinting technique used in combination with our custom-designed open-source hardware platforms (fig. S1) (14, 15). FRESH works by extruding bio-inks within a thermoreversible support bath composed of a gelatin microparticle slurry that provides support during printing and is subsequently melted away at 37°C (Fig. 1, A and B, and movie S1) (16).

The original version of the FRESH support bath, termed FRESH v1.0, consisted of irregularly shaped microparticles with a mean diameter of ~65 μm created by mechanical blending of a large gelatin block (Fig. 1C) (16). In FRESH v2.0, we developed a coacervation approach to generate gelatin microparticles with (i) uniform spherical morphology (Fig. 1D), (ii) reduced polydispersity (Fig. 1E), (iii) decreased particle diameter of ~25 μm (Fig. 1F), and (iv) tunable storage modulus and yield stress (Fig. 1G and fig. S2). FRESH v2.0 improves resolution with the ability to precisely generate collagen filaments and accurately reproduce complex G-code, as shown with a window-frame calibration print (Fig. 1H). Using FRESH v1.0, the smallest collagen filament reliably printed was ~250 μm in mean diameter, with highly variable morphology due to the relatively large and polydisperse gelatin microparticles (Fig. 1I). In contrast, FRESH v2.0 improves the resolution by an order of magnitude, with collagen filaments reliably printed from 200 μm down to 20 μm in diameter (Fig. 1, I and J). Filament morphology from solid-like to highly porous was controlled by tuning the collagen gelation rate using salt concentration and buffering capacity of the gelatin support bath (fig. S3). A pH 7.4 support bath with 50 mM HEPES was the optimal balance between individual strand resolution and strand-to-strand adhesion and was versatile, enabling FRESH printing of multiple bio-inks with orthogonal gelation mechanisms including collagen-based inks, alginate, fibrinogen, and methacrylated hyaluronic acid in the same print by adding CaCl2, thrombin, and UV light exposure (fig. S4) (15).
We first focused on FRESH-printing a simplified model of a small coronary artery–scale linear tube from collagen type I for perfusion with a custom-designed pulsatile perfusion system (Fig. 2A and fig. S5). The linear tube had an inner diameter of 1.4 mm (fig. S6A) and a wall thickness of ~300 μm (fig. S6B), and was patent and manifold as determined by dextran perfusion (fig. S6, C to E, and movie S2) (15). C2C12 cells within a collagen gel were cast around the printed collagen tube to evaluate the ability to support a volumetric tissue. The static nonperfused controls showed minimal compaction over 5 days (Fig. 2B), and a cross section revealed dead cells throughout the interior volume with a layer of viable cells only at the surface (Fig. 2C). In contrast, after active perfusion for 5 days, C2C12 cells compacted the collagen gel around the collagen tube (Fig. 2D), demonstrating viability and active remodeling of the gel through cell-driven compaction. The cross section showed cells alive throughout the entire volume (Fig. 2E), and quantitative analysis using LIVE/DEAD staining confirmed high viability within the perfused vascular construct (Fig. 2F). Others have 3D-bioprinted vasculature by casting cell-laden hydrogels around fugitive filaments, which become the vessel lumens (4, 5). In comparison, we directly print collagen to form the walls of a functional vascular channel, serving as the foundation for engineering more complex architectures.

Engineering smaller-scale vasculature, especially on the order of capillaries (5 to 10 μm in diameter), has been a challenge for extrusion-based 3D bioprinting because this is far below common needle diameters. However, at this length scale, endothelial and perivascular cells can self-assemble vascular networks through angiogenesis (17). We reasoned that the gelatin microparticles in the FRESH v2.0 support bath could be incorporated into the 3D-bioprinted collagen to create a porous microstructure, specifically because pores on the order of 30 μm in diameter have been shown to promote cell infiltration and microvascularization (18). FRESH v2.0��printed constructs contained micropores ~25 μm in diameter resulting from the melting and removal of the gelatin microparticles purposely entrapped during the printing process (Fig. 2G and movie S3). Collagen disks 5 mm thick and 10 mm in diameter were cast in a mold or printed and implanted in an in vivo murine subcutaneous vascularization model (Fig. 2, H and I, and fig. S7, A and B) to observe cellular infiltration. After implantation for 3 and 7 days, collagen disks were extracted and assessed for gross morphology, cellularization, and collagen structure (fig. S7, C to E). The solid-cast collagen showed minimal cell infiltration (Fig. 2J), whereas the printed collagen had extensive cell infiltration and collagen remodeling (Fig. 2K). Quantitative analysis revealed that cells infiltrated throughout the printed collagen disk within 3 days (Fig. 2L and fig. S8) and that the number of cells in the constructs was significantly greater for the printed collagen at 3 and 7 days compared to cast control [N = 6, P < 0.0001, two-way analysis of variance (ANOVA)] (15).
To promote vascularization, we incorporated fibronectin and the proangiogenic molecule recombinant vascular endothelial growth factor (VEGF) into our collagen bio-ink (19). Collagen disks that were FRESH-printed with VEGF and extracted after 10 days in vivo showed enhanced vascularization relative to cast controls (Fig. 2, M and N). By histology, the addition of VEGF to the cast collagen increased cell infiltration without promoting microvascularization (Fig. 2O and fig. S9). In contrast, the addition of VEGF to the printed collagen resulted in widespread vascularization, with CD31-positive vessels and red blood cells visible within the lumens (Fig. 2P). Tail vein injection of fluorescent lectin confirmed an extensive host-derived vascular network with vessels ranging from 8 to 50 μm in diameter throughout the printed collagen disk (Fig. 2Q, fig. S10, and movie S4). Multiphoton microscopy enabled deeper imaging into the printed constructs and showed vessels containing red blood cells at depths of at least 200 μm (Fig. 2R and movie S5).
We next FRESH-printed a model of the left ventricle of the heart using human stem cell–derived cardiomyocytes. We used a dual-material printing strategy with collagen bio-ink as the structural component in combination with a high-density cell bio-ink (Fig. 3A) (15). A test print design (fig. S11A) verified that the collagen pH was neutralized quickly enough to maintain ~96% post-printing viability by LIVE/DEAD staining (fig. S11B). The ventricle was designed as an ellipsoidal shell (Fig. 3B) with inner and outer walls of collagen and a central core region containing human embryonic stem cell–derived cardiomyocytes (hESC-CMs) and 2% cardiac fibroblasts (fig. S11, C to H). Ventricles were printed and cultured for up to 28 days, during which the collagen inner and outer walls provided sufficient structural integrity to maintain their intended geometry (Fig. 3C). After 4 days, the ventricles visibly contracted, and after 7 days they became synchronous with a dense layer of interconnected and striated hESC-CMs, as confirmed by immunofluorescent staining of sarcomeric α-actinin–positive myofibrils (fig. S11, I to K). Calcium imaging revealed contracting hESC-CMs throughout the entire printed ventricles, with directional wave propagation in the direction of the printed hESC-CMs observed from the side (Fig. 3, D and E) and top (Fig. 3, F and G) during spontaneous contractions in multiple ventricles (N = 3) (movie S6). Point stimulation enabled visualization of anisotropic calcium wave propagation with longitudinal conduction velocity of ~2 cm/s and a longitudinal-to-transverse anisotropy ratio of ~1.5 (Fig. 3, H and I). The ventricles had a baseline spontaneous beat rate of ~0.5 Hz and could be captured and paced at 1 and 2 Hz by means of field stimulation (Fig. 3J). We imaged the ventricles top-down to quantify motion of the inner and outer walls (Fig. 3K). Wall thickening is a hallmark of normal ventricular contraction. The printed ventricle expanded both inward and outward during a contraction, as determined by particle tracking to map the deformation field (Fig. 3L). The decrease in cross-sectional area of the interior chamber during peak systole showed a maximum of ~5% at 1-Hz pacing (N = 4) (Fig. 3M and movie S6). We also observed electrophysiologic behavior associated with arrhythmogenic disease states, including multiple propagating waves (fig. S12, A and B) and pinned rotors (fig. S12, C and D).

To demonstrate the mechanical integrity and function of collagen constructs at adult human scale, we printed a tri-leaflet heart valve 28 mm in diameter (Fig. 4A). We first prototyped the valve using alginate, a material previously used to build valve models (20), and then printed a collagen valve and improved the mechanical properties by adapting published fixation protocols for decellularized porcine heart valves (fig. S13A) (15, 21). The collagen valve had well-separated leaflets, was robust enough to be handled in air (Fig. 4, B and C, and movie S7), and was imaged by micro–computed tomography (μCT) (Fig. 4, D and E, and movie S8). Print fidelity was quantified using gauging to overlay the μCT data on the 3D model (fig. S13B), showing average overprinting of +0.55 mm and underprinting of –0.80 mm (Fig. 4F and fig. S13, C and D). Mechanical function was demonstrated by mounting the valve in a flow system with a pulsatile pump to simulate physiologic pressures, and we observed cyclical opening and closing of the valve leaflets (Fig. 4G and movie S7). We quantified flow through the valves (Fig. 4H) and demonstrated <15% regurgitation (Fig. 4I) with a maximum area opening of 19.5% (Fig. 4G). Additionally, the maximum transvalvular pressure was greater than 40 mmHg for the collagen and alginate valves (Fig. 4J), exceeding standard physiologic pressures for the tricuspid and pulmonary valves but less than the aortic and mitral valves (22). Further, human umbilical vein endothelial cells (HUVECs) cultured on unfixed collagen leaflets formed a confluent monolayer (fig. S13E).

A magnetic resonance imaging (MRI)–derived computer-aided design (CAD) model of an adult human heart was created, complete with internal structures such as valves, trabeculae, large veins, and arteries, but lacking smaller-scale vessels. To address this, we developed a computational method that uses the coronary arteries as the template to generate multiscale vasculature (fig. S14 and movie S9). We created a space-filling branching network based on a 3D Voronoi lattice, where vessels further from the left coronary arteries (red to blue) have a denser network and smaller diameters, down to ~100 μm (Fig. 4K). A subregion of the generated vasculature containing the left anterior descending artery (LAD) was selected, rendered, and printed from collagen at adult human scale (Fig. 4, L to N). Patency of large vessels was demonstrated by perfusing the multiscale vasculature through the root of the LAD (Fig. 4O). We confirmed the patency of vessels ~100 μm in diameter by optically clearing and reperfusing the multiscale vasculature (Fig. 4P, fig. S14, N to P, and movie S9).
Finally, to demonstrate organ-scale FRESH v2.0 printing capabilities and the potential to engineer larger scaffolds, we printed a neonatal-scale human heart from collagen (Fig. 4, Q and R, and fig. S15, A to C). To highlight the microscale internal structure, we printed half the heart (Fig. 4S). Structures such as trabeculae were printed from collagen with the same architecture as defined in the G-code file (Fig. 4, T and U). The square-lattice infill pattern within the ventricular walls was similarly well defined (Fig. 4, V and W). We used μCT imaging to confirm reproduction of all the anatomical structures contained within the 3D model of the heart, including the atrial and ventricular chambers, trabeculae, and pulmonary and aortic valves (fig. S15, D to I, and movie S10).
We have used the human heart for proof of concept; however, FRESH v2.0 printing of collagen is a platform that can build advanced tissue scaffolds for a wide range of organ systems. There are still many challenges to overcome, such as generating the billions of cells required to 3D-bioprint large tissues, achieving manufacturing scale, and creating a regulatory process for clinical translation (23). Although the 3D bioprinting of a fully functional organ is yet to be achieved, we now have the ability to build constructs that start to recapitulate the structural, mechanical, and biological properties of native tissues.
4 notes
·
View notes
Text
Pulsed electromagnetic field applications: A corporate perspective
The three devices, Physio-Stim, Spinal-Stim and Cervical-Stim were each designed and developed specifically for osteogenesis stimulation. Each device incorporates a specific set of triangular shaped PEMF signals with the particular set of signals taking advantage of having its polarization and depolarization within the positive magnetic discipline vary as alerts inside each the bad and effective phase have been located to be much less effective. While PEMF indicators can be different thru transformations of their pulse period, burst period, amplitude, and variety of pulses/burst, the unique parameters for these three units had been chosen primarily based on preliminary preclinical research mixed with engineering issues such as battery existence and system portability. In vitro signalling pathways Pulsed electromagnetic field (PEMF) is a technology that has been used in the treatment of a variety of diseases, with the potential to treat more than 300 human conditions without side effects. PEMF therapy is comprised of either low-intensity, rhythmic Bio-field Systems (BFS) and/or high-intensity, pulsed electromagnetic fields placed on specific locations within the body. The study reported by Patterson et al also looked at the effect of PEMF therapy on bone marrow-derived mesenchymal stem cells (MSCs). The investigators found that both biofield stimulation and pulsed electromagnetic field exposure increased MSC proliferation with parathyroid hormone (PTH)-activated protein kinase signaling, which was similar to observations related to sustained PTH treatment Osteotomies/fracture repair Ibiwoye et al mentioned that bone was once preserved in a critical-sized osteotomy uncovered three hours day by day to PEMF (Physio-Stim) for 10 weeks. Specifically, bilateral, mid-diaphyseal fibular osteotomies have been carried out in aged rats that carried out a nonunion popularity inside 3–4 weeks which used to be accompanied by using PEMF exposure.Unilateral PEMF exposure preserved the fibulae bone mass as measured by microcomputed tomography (micro-CT). Osteoporosis Osteoporosis prevention in an early rodent model. The ovariectomized rats were subjected to daily PEMF exposure for 3 hours over 3 days after 3 days of ovariectomy and followed for 6 weeks, 12 weeks, 18 weeks, and 24 weeks. Other groups were treated with bisphosphonates instead (alendronate; 3 subcutaneous injections / week; 10 μg / kg body weight). Micro-CT showed significantly more trabecular bone remaining at the L4 vertebrae in the PEMF group relative to sham (30% more) but other groups had similar preservation with significantly more bone than both alendronate alone and alendronate+PEMF-treated groups. Tenogenic and myogenic experiments In vitro differentiation and proliferation The effects of pulsed electromagnetic field (PEMF) on myocyte proliferation and differentiation have been studied in vitro using C2C12 murine myoblasts. PEMF exposure enhanced gene expression of growth factors in both human rotator cuff tenocytes (COL1, TGFβ-1, PDGFβ, BMP12 and TIMP4) and myocytes (MyoD). The implications from these results may be the potential use of PEMF as a non-operative treatment to improve clinical outcomes following rotator cuff repair. In vivo tendon healing Pulsed electromagnetic field (PEMF) exposure is used to stimulate healing in tendon repair. This study aimed to investigate the effects of PEMF on tendon-to-bone healing after rotator cuff repair in rats, and the underlying mechanisms involved. The rats were treated with a PEMF at different frequencies (3.85–40 kHz) or durations (1 hours/day, 3 hours/day, or 6 hours/day). Tendon properties were assessed before surgery and at 4 weeks postoperatively. The results revealed that early improvements in tendon modulus was only found for PEMFs at lower fundamental frequencies (for all exposure durations). In addition, PEMFs with varying fundamental frequencies led to greater improvements in tendon modulus at higher fundamental frequencies
0 notes
Video
CAS 1370003-76-1 YK11 YK-11 SARMS Raw PowderCapsule Hallucinate For Reduce Fat For Muscle Gainning Bodybuilding Muscle Building
Best Price Bodybuilding muscles Sarms YK11 Powder Cas1370003-76-1
YK11 attaches itself to the AR (androgen receptor), but only inducts methods that lead to the traditional side effects of androgens such as growth of body hair and prostate and enhanced aggression – to a restricted degree.
Most SARMS have quite limited androgenic side effects, but frequently only quite few anabolic effects when likened .
Kanno tested C2C12 muscle cells and not lab animals or humans. It has been discovered that muscle cells produce more anabolic factors if exposed to 500 nmol (nanomoles) YK11 than if you expose the same muscle cells to 500 nmol DHT.
YK11 induces muscle cells to make more follistatin (more than DHT does) – a strong myostatin inhibitor. YK11 works through the androgen receptor,with that said, YK11 can be as good as in terms of muscle
Product NameYK11
Other NameYK-11
Purity98%min
CAS No.1370003-76-1
M.W.624.776
M.F.C27H36N4O5S.CH4O3S
AppearanceWhite powder
The dosage of YK-11: 10 mg per day is a good start, and it is recommended to use the dosage separately, that is, take 5 mg in the morning and 5 mg in the evening. This SARM has a short half-life, so take it twice a day for best results. Of course, I have seen someone use the highest dose of 25 mg per day, and he also has a significant improvement in muscle mass.
Weeks 1~4: 5 mg in the morning and 5 mg in the evening.
Weeks 5-8: Increase the daily dose to 15mg, 5mg in the morning, 5mg for pre-exercise, and 5mg at night.
Week 8-12 (optional): Increase the dose again to 20 mg per day. Take 5 mg in the morning, 10 mg before exercise, and 5 mg in the evening.
Is YK-11 suitable for muscle gain (bulking) or fat loss (cutting)?
One of the reasons why this SARM is popular is that it is very versatile and can be used for both purposes, but it is recommended to use a lower dose during fat loss, because your goal is to keep as much as possible while reducing fat. More muscle mass. If you are gaining muscle, you can evaluate your tolerance. If you feel comfortable, you can increase it to 20 mg per day. At the same time, many users also like to use it in combination with LGD 4033
0 notes
Text
IJMS, Vol. 24, Pages 15318: miR-103-3p Regulates the Proliferation and Differentiation of C2C12 Myoblasts by Targeting BTG2
Skeletal muscle, a vital and intricate organ, plays a pivotal role in maintaining overall body metabolism, facilitating movement, and supporting normal daily activities. An accumulating body of evidence suggests that #microRNA (#miRNA) holds a crucial role in orchestrating skeletal muscle growth. Therefore, the primary aim of this study was to investigate the influence of miR-103-3p on myogenesis. In our study, the overexpression of miR-103-3p was found to stimulate proliferation while suppressing differentiation in C2C12 myoblasts. Conversely, the inhibition of miR-103-3p expression yielded contrasting effects. Through bioinformatics analysis, potential binding sites of miR-103-3p with the 3’UTR region of BTG anti-proliferative factor 2 (BTG2) were predicted. Subsequently, dual luciferase assays conclusively demonstrated BTG2 as the direct target gene of miR-103-3p. Further investigation into the role of BTG2 in C2C12 myoblasts unveiled that its overexpression impeded proliferation and encouraged differentiation in these cells. Notably, co-transfection experiments showcased that the overexpression of BTG2 could counteract the effects induced by miR-103-3p. In summary, our findings elucidate that miR-103-3p promotes proliferation while inhibiting differentiation in C2C12 myoblasts by targeting BTG2. https://www.mdpi.com/1422-0067/24/20/15318?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
РЕЗЮМЕ Study on effects of AgSiO2 core shell nanoparticles on biocompatibility appraisal of myoblasts
РЕЗЮМЕ. Мета цього дослідження полягала у перевірці взаємодії наночастинок Ag-SiO2 зі структурою «ядро/оболонка» (CSNs) з клітинами C2C12. У цій статті ми повідомляємо про синтез та класифікацію нових CSNs. Було приділено увагу підготовці CSNs з метою перевірки їхньої біосумісності/цитотоксичного впливу на клітини м’язів C2C12, та перевірено ризики для здоров’я людини, пов’язані з CSNs. Використовувані CSNs синтезували за допомогою ефективного зольгель методу з використанням нітрату срібла і тетраетоксисилану в якості основних компонентів. Фізикохімічне дослідження CSNs проводили за допомогою рентгеноструктурного аналізу, оптичної спектроскопії та трансмісі��ної електронної мікроскопії. З метою дослідження біосумісності/цитотоксичності in vitro клітини C2C12 вирощували у середовищі in vitro, а потім піддавали впливу різних концентрацій CSNs. Аналіз життєздатності клітин C2C12 проводили з використанням Kit-8. Аутентифікацію результатів проводили за допомогою конфокальної мікроскопії, морфологію клітин C2C12 вивчали з використанням фазовоконтрастної мікроскопії. In vitro дослідження біологічного впливу CSNs виявило, що життєздатність клітин у культурі залежить від режиму дозування (0–20 мкг/мл). Загалом результати дослідження продемонстрували, що синтетичні CSNs здійснюють вплив на функціонування клітин C2C12 в залежності від часу та режиму, що залежить від абсорбції.
Ключові слова: структура «ядро/оболонка», біомедичний, наночастинки, C2C12, кремній
0 notes
Text
Metformin shares common mechanism with nearly every Anesthesia drug: AMPK links Consciousness with Jumping Genes & the Creation of Human Life

By ISAF Headquarters Public Affairs Office (originally posted to Flickr as 100410-F-7713A-002) [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons; By Anatomist90 [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], from Wikimedia Commons
A recently published study in the journal PLoS One in May of 2018 demonstrated that the anesthetic drug propofol significantly increased intracellular calcium (Ca2+) levels, induced a burst of reactive oxygen species (ROS), and activated the master metabolic regulator AMPK in C2C12 cells [18]. Similar results were also obtained in a recent study published in April of 2018, wherein propofol also increased intracellular Ca2+ levels and activated AMPK in HeLa cells [105]. AMPK is an evolutionarily conserved protein that increases lifespan and healthspan in several model organisms [34]. Activation of AMPK is also the primary mechanism of action of the anti-diabetic drug metformin, a compound that has displayed wide-raging efficacy in multiple disparate disease states, including cancer, dementia, depression, frailty-related diseases, and cardiovascular diseases [34,106]. Interestingly, propofol is considered one of the most popular and widely-used intravenous anesthetic drugs in modern medicine to induce and maintain general anesthesia in humans [107]. Curiously, a recent study published in the journal Current Biology in June of 2018 by researchers from the University of Michigan demonstrated that the compound carbachol reversed anesthesia induced by the inhaled anesthetic sevoflurane and restored wake-like behavior and level of consciousness in rats [27]. Carbachol is a compound that binds to and stimulates acetylcholine receptors in the brain but also activates AMPK in human cells, similar to both metformin and propofol [27,108].
Each of these studies substantiates several novel proposals in a recently published paper I authored in June of 2018 in which I proposed for the first time that cellular stress-induced AMPK activation links consciousness and accelerated emergence from anesthesia with paradoxical excitation, hippocampal long-term potentiation (essential for learning and memory), alleviation of accelerated cellular aging in Hutchinson-Gilford progeria syndrome, oocyte activation and the sperm acrosome reaction (prerequisites for human life creation), and transposable element (i.e. “jumping genes”)-mediated promotion of learning, memory, and the creation of human life [1-6].
As further explained below, nearly every neurotransmitter that plays a critical role in promoting wakefulness, arousal, and consciousness activates AMPK (glutamate, acetylcholine, orexin-A, histamine, norepinephrine, dopamine, and serotonin) [7-17]. Several drugs that are commonly used to induce and maintain general anesthesia also activate AMPK in low doses (propofol, sevoflurane, isoflurane, ketamine, dexmedetomidine, and midazolam) [18-23]. Also, several compounds that have recently been shown to promote accelerated emergence from anesthesia also activate AMPK (carbachol, orexin-A, histamine, dopamine, dopamine D1 receptor agonists, nicotine, caffeine, and forskolin) [9-11,13,24-33].
AMPK, an evolutionarily conserved kinase that is activated by the induction of cellular stress (i.e. increases in intracellular reactive oxygen species [ROS], calcium [Ca2+], and/or an AMP(ADP)/ATP ratio increase), increases lifespan and healthspan in several model organisms (yeast, worms, flies, mice, etc.) [34]. In my prior publication, I first proposed that cellular stress-induced AMPK activation is critical for facilitation of hippocampal long-term potentiation (LTP), considered a cellular correlate for learning and memory [5]. Indeed, AMPK has been found localized in hippocampal CA1 pyramidal neurons and glutamate, NMDA, potassium chloride, and high frequency stimulation have been shown to induce AMPK activation in cortical and hippocampal neurons [7,35,36]. Although an increase in Ca2+ levels is critical for neuronal activation and LTP induction, inhibition of ROS significantly inhibits hippocampal CA1 LTP, indicating that cellular stress-induced AMPK activation may play a pivotal role in neuronal excitation [37-40].
In my most recent publication, I noted that forskolin activates both AMPK and the transposable element syncytin-1 (necessary for human placental formation), increases human oocyte fertilization rates when combined with the AMPK activator cilostamide, and promotes chemically-induced LTP in hippocampal slices [6,26,41-44]. Transposable elements (TEs) are found in human oocytes, human sperm, and in human neural progenitor cells within the hippocampus [45-48]. TEs are also activated and can be induced to transpose or “jump” from one genomic location to another by increases in Ca2+ or ROS [49-51]. Exercise was shown to enhance LINE-1 (L1) retrotransposition (a TE of the retrotransposon class) in the dentate gyrus of the hippocampus in mice and L1 expression and retrotransposition in the adult mouse hippocampus was reported to enable long-term memory formation [52,53]. Because forskolin and caffeine, both of which activate AMPK, have recently been shown to promote accelerated emergence from anesthesia in rats and caffeine activates both mouse oocytes (models for human oocytes) and TEs, I proposed that cellular stress-induced AMPK activation may represent a common mechanism linking consciousness with learning, memory, and the creation of human life [25,26,33,54,55].
A primary cellular target of hypnotic agents (e.g. propofol) used for the induction and maintenance of general anesthesia is the GABAA receptor [66]. The GABAA receptor is located throughout the brain (cortex, thalamus, brain stem, and striatum) and binding of propofol post-synaptically to GABAA receptors enhances neural inhibition by the primary inhibitory neurotransmitter GABA, contributing to a loss of consciousness [66]. Interestingly, the GABAA receptor antagonist bicuculline, which reverses propofol anesthesia, activates AMPK in mouse cortical neurons via Ca2+ influx and flumazenil (a GABAA receptor antagonist) induces preconditioning by increasing the levels of ROS [56-58]. Basheer et al. as well as researchers from the University of Pennsylvania showed that AMPK is activated during extended periods of wakefulness but is inhibited during sleep in the basal forebrain and cerebral cortex of rats and mice [59,60]. Decreases in AMPK activation during sleep were also associated with increases in ATP, which would decrease AMPK activation as increases in the AMP(ADP)/ATP ratio activates AMPK [34,59]. Creatine, which also activates AMPK, decreased total sleep time, NREM sleep, and NREM delta activity significantly in rats [61,62]. Combined use of the anesthetic agents ketamine and xylazine in rats also led to an ATP increase that positively and significantly correlated with EEG delta activity [63]. However, the sedative and α2-receptor agonist clonidine activates AMPK in mice and xylazine, an analog of clonidine, activates AMPK in the rat cerebral cortex, hippocampus, thalamus, and cerebellum, provocatively indicating that low-dose anesthetic administration may actually promote wakefulness, arousal, and consciousness through activation of AMPK [64,65].
Low dose anesthetic-induced AMPK activation may also explain the phenomenon of paradoxical excitation. Curiously, low doses of nearly every anesthetic drug have been shown to induce paradoxical excitation [66]. As the name implies, before inducing unconsciousness, general anesthetic administration may result in a temporary increase in neuronal excitation, characterized by an increase in beta activity on the electroencephalogram (EEG) and eccentric body movements [66,109]. Because AMPK is activated by cellular stress induction (ROS, Ca2+, AMP(ADP)/ATP ratio increase) and because ROS and Ca2+ increases are critical for activation of pyramidal neurons, it is likely that many anesthetics induce rapid neuronal activation and paradoxical excitation in low doses by promoting cellular stress-induced AMPK activation [34,37-40]. Indeed, propofol, one of the most commonly-used anesthetics to induce and maintain general anesthesia, activates AMPK via an increase in ROS and Ca2+, promotes hippocampal neural stem cell differentiation, and promotes neuronal viability [67-69]. Sevoflurane, a commonly-used inhaled anesthetic, activates AMPK via an increase in ROS, increases Ca2+ levels in mouse brain cells, and enhances memory in rats at low doses [70-72]. Ketamine also activates Ca2+ channels in rat cortical neurons, increases ROS levels in the brain of rats, enhances hippocampal CA1 LTP in rats, and also functions as an antidepressant by activating AMPK in the rat hippocampus in vivo [73-76]. Prominent beta activity on the EEG has also been observed just before return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane (similar to paradoxical excitation), suggesting that the decrease of an anesthetic to a low, stimulatory level after removal of anesthesia may explain the increase in beta activity just before return of consciousness as well as during paradoxical excitation [6,66,77]. Hence, low dose anesthetic-induced AMPK activation may potentially accelerate emergence from anesthesia as well as promote beneficial arousal in disorders of consciousness (e.g. minimally conscious state, persistent vegetative state, coma, etc.) [6].
As noted above, nearly every neurotransmitter that plays a critical role in promoting wakefulness, arousal, and consciousness activates AMPK (glutamate, acetylcholine, orexin, histamine, norepinephrine, dopamine, and serotonin) and commonly used drugs that induce and maintain general anesthesia also activate AMPK in low doses (propofol, sevoflurane, isoflurane, ketamine, dexmedetomidine, and midazolam) [7-23]. Compounds that have recently been shown to accelerate emergence from anesthesia also activate AMPK (carbachol, orexin-A, histamine, dopamine, dopamine D1 receptor agonists, nicotine, caffeine, and forskolin) [9-11,13,24-33]. Additionally, a recent study by Hambrecht-Wiedbusch et al. strikingly demonstrated that although sub-anesthetic doses of ketamine increased anesthetic depth and induced burst suppression during isoflurane anesthesia, ketamine paradoxically accelerated recovery of consciousness in rats [78]. Such evidence supports the notion that while larger doses of anesthetics are effective at inducing loss of consciousness, low-dose anesthetic administration may facilitate rapid, cellular stress-induced neuronal activation that is mediated by AMPK activation [6].
Although they do not have a nervous system, plants produce nearly every neurotransmitter that promotes wakefulness, arousal, and consciousness in humans, including glutamate, acetylcholine, histamine, norepinephrine, dopamine, and serotonin [79-82]. The production of these neurotransmitters in plants is often associated with the induction of cellular stress (i.e. via wounding, osmotic stress, etc.) and partly serves as a defense mechanism [79-82]. Fungal infection of certain rice cultivars for example increases the production of serotonin, which suppresses leaf damage and reduces biotic stress [83]. ROS and Ca2+ also play critical roles in the production of secondary metabolites, compounds that plants produce partly for the purpose of self defense [84,85]. Interestingly, several abiotic stressors including nutrient deficiency, salt, osmotic, oxidative, and ER stress activates autophagy in Arabidopsis in a SnRK1-dependent manner. SnRK1 is the plant ortholog of AMPK [86]. Such evidence suggests that a mechanism of cellular stress-induced AMPK activation by neurotransmitters may have been evolutionarily conserved to promote neuronal activation in the human brain.
Indeed, the well-studied AMPK activator metformin activates AMPK in hippocampal neurons in vivo and enhances neurogenesis in the subventricular zone and the subgranular zone of the dentate gyrus, indicating that metformin may enhance brain repair and recovery of consciousness in disorders of consciousness [24,87,88]. Metformin also alleviates accelerated cellular aging defects and activates AMPK in Hutchinson-Gilford progeria syndrome (HGPS), a genetic disorder characterized by an accelerated aging phenotype caused by faulty splicing of the LMNA gene that also occurs in normal human cells at low levels [1,89,90]. Interestingly, temsirolimus (an analog of the macrolide rapamycin), alleviates accelerated aging defects in HGPS cells but increases the levels of ROS in both normal and HGPS cells within the first hour of treatment [91]. Metformin also activates the telomere-lengthening enzyme telomerase (which is derived from a transposable element) in an AMPK-dependent manner [92]. Cellular stress and AMPK activation also promotes oocyte maturation (precedes and is critical for oocyte activation), the acrosome reaction in human sperm (necessary for oocyte penetration and fertilization), and human placental development [26,93-95]. Forskolin and caffeine also induce the acrosome reaction in human sperm [96,110].
Lastly, increases in ROS, Ca2+, and AMPK activation are also critical for T cell activation and hence latent HIV-1 reactivation, a method currently pursued by HIV-1 cure researchers to reactivate dormant HIV-1 residing in T cells to facilitate virus detection and destruction by the immune system (called the “shock and kill” approach) [5,97-101]. Strikingly, forskolin reactivates latent HIV-1 in human U1 cells, a myelo-monocytic cell line used as a model for HIV-1 latency [102]. Early data has also demonstrated that metformin destabilized the latent HIV-1 reservoir in patients chronically infected with HIV-1 and significantly reduced cellular markers positively associated with T cells latently infected with HIV-1 [103,104]. Such evidence provides a compelling indication that cellular stress-induced AMPK activation links transposable elements and alleviation of accelerated cellular aging with potential HIV-1 eradication, consciousness, and the creation of human life, all hypotheses that I originally proposed [1-6].
https://www.linkedin.com/pulse/metformin-shares-common-mechanism-nearly-every-drug-ampk-finley/


References
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Finley J. Reactivation of latently infected HIV-1 viral reservoirs and correction of aberrant alternative splicing in the LMNA gene via AMPK activation: Common mechanism of action linking HIV-1 latency and Hutchinson-Gilford progeria syndrome. Med Hypotheses. 2015 Sep;85(3):320-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Finley J. Facilitation of hippocampal long-term potentiation and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking learning, memory, and the potential eradication of HIV-1. Med Hypotheses. 2018 Jul;116:61-73.
Finley J. Transposable elements, placental development, and oocyte activation: Cellular stress and AMPK links jumping genes with the creation of human life. Med Hypotheses. 2018.
Terunuma M, Vargas KJ, Wilkins ME, et al. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U.S.A. 2010;107(31):13918–23.
Zhao M, Sun L, Yu XJ, et al. Acetylcholine mediates AMPK-dependent autophagic cytoprotection in H9c2 cells during hypoxia/reoxygenation injury. Cell Physiol Biochem. 2013;32(3):601-13.
Merlin J, Evans BA, Csikasz RI, Bengtsson T, Summers RJ, Hutchinson DS. The M3-muscarinic acetylcholine receptor stimulates glucose uptake in L6 skeletal muscle cells by a CaMKK-AMPK-dependent mechanism. Cell Signal. 2010 Jul;22(7):1104-13.
Wu WN, Wu PF, Zhou J, et al. Orexin-A activates hypothalamic AMP-activated protein kinase signaling through a Ca²+-dependent mechanism involving voltage-gated L-type calcium channel. Mol Pharmacol. 2013 Dec;84(6):876-87.
Thors B, Halldórsson H, Thorgeirsson G. eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1. Biochim Biophys Acta. 2011 Feb;1813(2):322-31.
Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005 Nov;48(11):2386-95.
Bone NB, Liu Z, Pittet JF, Zmijewski JW. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J Leukoc Biol. 2017 Feb;101(2):357-365.
Laporta J, Peters TL, Merriman KE, Vezina CM, Hernandez LL. Serotonin (5-HT) affects expression of liver metabolic enzymes and mammary gland glucose transporters during the transition from pregnancy to lactation. PLoS One. 2013;8(2):e57847.
Jiang X, Lu W, Shen X, et al. Repurposing sertraline sensitizes non-small cell lung cancer cells to erlotinib by inducing autophagy. JCI Insight. 2018 Jun 7;3(11). pii: 98921.
Sun D, Zhu L, Zhao Y, et al. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018 Apr;51(2):e12402.
Jeong J, Park M, Yoon JS, et al. Requirement of AMPK activation for neuronal metabolic-enhancing effects of antidepressant paroxetine. Neuroreport. 2015 May 6;26(7):424-8.
Chen X, Li LY, Jiang JL, et al. Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line. PLoS One. 2018 May 24;13(5):e0197934.
Lamberts RR, Onderwater G, Hamdani N, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009 Sep 15;120(11 Suppl):S10-5.
Rao Z, Pan X, Zhang H, et al. Isoflurane Preconditioning Alleviated Murine Liver Ischemia and Reperfusion Injury by Restoring AMPK/mTOR-Mediated Autophagy. Anesth Analg. 2017 Oct;125(4):1355-1363.
Xu SX, Zhou ZQ, Li XM, Ji MH, Zhang GF, Yang JJ. The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav Brain Res. 2013 Sep 15;253:305-9.
Sun Y, Jiang C, Jiang J, Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin Exp Pharmacol Physiol. 2017 Sep;44(9):946-953.
Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J. 2007 Feb 1;401(3):735-41.
Brynildsen JK, Lee BG, Perron IJ, Jin S, Kim SF, Blendy JA. Activation of AMPK by metformin improves withdrawal signs precipitated by nicotine withdrawal. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4282-4287.
Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E286-92.
Egawa M, Kamata H, Kushiyama A, et al. Long-term forskolin stimulation induces AMPK activation and thereby enhances tight junction formation in human placental trophoblast BeWo cells. Placenta 2008;29(12):1003–8.
Pal D, Dean JG, Liu T, et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol. 2018 Jun 12. pii: S0960-9822(18)30627-4.
Zhang LN1, Li ZJ, Tong L, et al. Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth Analg. 2012 Oct;115(4):789-96.
Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009 Oct;111(4):725-33.
Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012 May;116(5):998-1005.
Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013 Jan;118(1):30-9.
Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007 Aug;107(2):264-72.
Wang Q, Fong R, Mason P, Fox AP, Xie Z. Caffeine accelerates recovery from general anesthesia. J Neurophysiol. 2014 Mar;111(6):1331-40.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 2012;11(2):230–41.
Potter WB, O'Riordan KJ, Barnett D, et al. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 2010 Feb 1;5(2):e8996.
Yu DF, Shen ZC, Wu PF, et al. HFS-triggered AMPK activation phosphorylates GSK3β and induces E-LTP in rat hippocampus in vivo. CNS Neurosci. Ther.2016;22(6):525–31.
Volianskis A, France G, Jensen MS, et al. Long-term potentiation and the role of Nmethyl-D-aspartate receptors. Brain Res. 2015;24(1621):5–16.
Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996 Feb 15;16(4):1324–36.
Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J. Neurophysiol. 1998;80(1):452–7.
Thiels E, Urban NN, Gonzalez-Burgos GR, et al. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2000;20(20):7631–9.
Kudo Y, Boyd CA, Sargent IL, Redman CW. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in preeclampsia. Biochim Biophys Acta 2003;1638(1):63–71.
Shu YM, Zeng HT, Ren Z, et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reprod 2008;23(3):504–13.
Chung YW, Ahmad F, Tang Y, et al. White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci Rep 2017;13(7):40445.
Otmakhov N, Khibnik L, Otmakhova N, et al. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol 2004;91(5):1955–62.
Bjerregaard B, Lemmen JG, Petersen MR, et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet 2014;31(5):533–9.
Georgiou I, Noutsopoulos D, Dimitriadou E, et al. Retrotransposon RNA expression and evidence for retrotransposition events in human oocytes. Hum Mol Genet 2009;18(7):1221–8.
Lazaros L, Kitsou C, Kostoulas C, et al. Retrotransposon expression and incorporation of cloned human and mouse retroelements in human spermatozoa. Fertil Steril 2017;107(3):821–30.
Coufal NG, Garcia-Perez JL, Peng GE, et al. L1 retrotransposition in human neural progenitor cells. Nature 2009;460(7259):1127–31.
Rodland KD, Muldoon LL, Lenormand P, Magun BE. Modulation of RNA expression by intracellular calcium. Existence of a threshold calcium concentration for induction of VL30 RNA by epidermal growth factor, endothelin, and protein kinase C. J Biol Chem 1990;265(19):11000–7.
Markopoulos G, Noutsopoulos D, Mantziou S, et al. Arsenic induces VL30 retrotransposition: the involvement of oxidative stress and heat-shock protein 70. Toxicol Sci. 2013 Aug;134(2):312-22.
Giorgi G, Marcantonio P, Del Re B. LINE-1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell Tissue Res 2011;346(3):383–91.
Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus 2009;19(10):1002–7.
Bachiller S, Del-Pozo-Martín Y, Carrión ÁM. L1 retrotransposition alters the hippocampal genomic landscape enabling memory formation. Brain Behav Immun 2017;64:65–70.
Scott L, Smith S. Human sperm motility-enhancing agents have detrimental effects on mouse oocytes and embryos. Fertil Steril. 1995 Jan;63(1):166-75.
Liu C, Chen Y, Li S, et al. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes. 2013 Oct;47(2):219-27.
Kenney JW, Sorokina O, Genheden M, Sorokin A, Armstrong JD, Proud CG. Dynamics of elongation factor 2 kinase regulation in cortical neurons in response to synaptic activity. J Neurosci. 2015 Feb 18;35(7):3034-47.
Irifune M, Sugimura M, Takarada T, et al. Propofol anaesthesia in mice is potentiated by muscimol and reversed by bicuculline. Br J Anaesth. 1999 Oct;83(4):665-7.
Zhang Q, Yao Z. Flumazenil preconditions cardiomyocytes via oxygen radicals and K(ATP) channels. Am J Physiol Heart Circ Physiol. 2000 Oct;279(4):H1858-63.
Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010 Jun 30;30(26):9007-16.
Nikonova EV, Naidoo N, Zhang L, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010 Jul;33(7):889-900.
Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol. 2004 Mar 1;555(Pt 2):409-21.
Dworak M, Kim T, Mccarley RW, Basheer R. Creatine supplementation reduces sleep need and homeostatic sleep pressure in rats. J Sleep Res. 2017 Jun;26(3):377-385.
Dworak M, McCarley RW, Kim T, Basheer R. Delta oscillations induced by ketamine increase energy levels in sleep-wake related brain regions. Neuroscience. 2011 Dec 1;197:72-9.
Kim SS, Park SH, Lee JR, Jung JS, Suh HW. The activation of α2-adrenergic receptor in the spinal cord lowers sepsis-induced mortality. Korean J Physiol Pharmacol. 2017 Sep;21(5):495-507.
Shi XX, Yin BS, Yang P, et al. Xylazine Activates Adenosine Monophosphate-Activated Protein Kinase Pathway in the Central Nervous System of Rats. PLoS One. 2016 Apr 6;11(4):e0153169.
Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010;363(27):2638–50.
Chen X, Li LY, Jiang JL, et al. Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line. PLoS One. 2018 May 24;13(5):e0197934.
Sall JW, Stratmann G, Leong J, Woodward E, Bickler PE. Propofol at clinically relevant concentrations increases neuronal differentiation but is not toxic to hippocampal neural precursor cells in vitro. Anesthesiology. 2012 Nov;117(5):1080-90.
Wu GJ, Chen WF, Hung HC, et al. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: new insights into neuroprotection. Brain Res. 2011 Apr 12;1384:42-50.
Lamberts RR, Onderwater G, Hamdani N, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009 Sep 15;120(11 Suppl):S10-5.
Pinheiro AC, Gomez RS, Guatimosim C, Silva JH, Prado MA, Gomez MV. The effect of sevoflurane on intracellular calcium concentration from cholinergic cells. Brain Res Bull. 2006 Mar 31;69(2):147-52.
Alkire MT, Nathan SV, McReynolds JR. Memory enhancing effect of low-dose sevoflurane does not occur in basolateral amygdala-lesioned rats. Anesthesiology. 2005 Dec;103(6):1167-73.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014 Oct 31;18(1). pii: pyu033.
de Oliveira L, Spiazzi CM, Bortolin T, et al. Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Aug 31;33(6):1003-8.
Widman AJ, Stewart AE, Erb EM, Gardner E, McMahon LL. Intravascular Ketamine Increases Theta-Burst but Not High Frequency Tetanus Induced LTP at CA3-CA1 Synapses Within Three Hours and Devoid of an Increase in Spine Density. Front Synaptic Neurosci. 2018 May 30;10:8.
Xu SX, Zhou ZQ, Li XM, Ji MH, Zhang GF, Yang JJ. The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav Brain Res. 2013 Sep 15;253:305-9.
Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth 2001;87(3):421–8.
Hambrecht-Wiedbusch VS, Li D, Mashour GA. Paradoxical Emergence: Administration of Subanesthetic Ketamine during Isoflurane Anesthesia Induces Burst Suppression but Accelerates Recovery. Anesthesiology. 2017 Mar;126(3):482-494.
Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Science Volume 172, Issue 3, March 2007, Pages 433-440.
Roshchina V.V. (2010) Evolutionary Considerations of Neurotransmitters in Microbial, Plant, and Animal Cells. In: Lyte M., Freestone P. (eds) Microbial Endocrinology. Springer, New York, NY.
Murch S.J. (2006) Neurotransmitters, Neuroregulators and Neurotoxins in Plants. In: Baluška F., Mancuso S., Volkmann D. (eds) Communication in Plants. Springer, Berlin, Heidelberg.
Skopelitis DS, Paranychianakis NV, Paschalidis KA, et al. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell. 2006 Oct;18(10):2767-81.
Hayashi K, Fujita Y, Ashizawa T, Suzuki F, Nagamura Y, Hayano-Saito Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016 Jan;85(1):46-56.
Jacobo-Velázquez DA, González-Agüero M, Cisneros-Zevallos L. Cross-talk between signaling pathways: the link between plant secondary metabolite production and wounding stress response. Sci Rep. 2015 Feb 25;5:8608.
Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 2000 Aug;12(8):1425-40.
Soto-Burgos J, Bassham DC. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One. 2017 Aug 4;12(8):e0182591.
Dadwal P, Mahmud N, Sinai L, et al. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Reports. 2015 Aug 11;5(2):166-73.
Ahmed S, Mahmood Z, Javed A, et al. Effect of metformin on adult hippocampal neurogenesis: comparison with donepezil and links to cognition. J Mol Neurosci 2017;62(1):88–98.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis 2016;10(2):16026.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol 2017;26(10):889–95.
Gabriel D, Gordon LB, Djabali K. Temsirolimus partially rescues the Hutchinson-Gilford progeria cellular phenotype. PLoS One 2016;11(12):e0168988.
Karnewar S, Neeli PK, Panuganti D, et al. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: relevance in age-associated vascular dysfunction. Biochim Biophys Acta 2018;1864(4 Pt A):1115–28.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod 2006;74(3):585–92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl 2017;19(6):707–14.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl 1998;19(5):585–94.
De Jonge CJ, Han HL, Lawrie H, Mack SR, Zaneveld LJ. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second messenger pathway. J Exp Zool 1991;258(1):113–25.
Dahabieh MS, Battivelli E, Verdin E. Understanding HIV latency: the road to an HIV cure. Annu Rev Med 2015;66:407–21.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013;38(2):225–36.
Rao E, Zhang Y, Zhu G, et al. Deficiency of AMPK in CD8+ T cells suppresses their anti-tumor function by inducing protein phosphatase-mediated cell death. Oncotarget 2015;6(10):7944–58.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 2008;4(5):495–504.
Chowdhury MI, Koyanagi Y, Horiuchi S, et al. cAMP stimulates human immunodeficiency virus (HIV-1) from latently infected cells of monocyte-macrophage lineage: synergism with TNF-alpha. Virology 1993;194(1):345–9.
Chew GM, Chow DC, Souza SA, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. J Virus Eradication 2017;3(Suppl. 1):6–19.
Chew GM, Chow DC, Souza SA, et al. http://viruseradication.com/abstract-details.php?abstract_id=1188, last accessed June 28, 2018.
Chen X, Li K, Zhao G. Propofol Inhibits HeLa Cells by Impairing Autophagic Flux via AMP-Activated Protein Kinase (AMPK) Activation and Endoplasmic Reticulum Stress Regulated by Calcium. Med Sci Monit. 2018 Apr 18;24:2339-2349.
Wang CP, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J. Diabetes Complications 2017;31(4):679–86.
Chidambaran V, Costandi A, D'Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015 Jul;29(7):543-63.
Olianas MC, Dedoni S, Onali P. Involvement of store-operated Ca(2+) entry in activation of AMP-activated protein kinase and stimulation of glucose uptake by M3 muscarinic acetylcholine receptors in human neuroblastoma cells. Biochim Biophys Acta. 2014 Dec;1843(12):3004-17.
McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci 2008;28(50):13488–504.
Tesarik J, Mendoza C, Carreras A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reactions in human sperm. Fertil Steril. 1992 Dec;58(6):1185-90.
1 note
·
View note
Text
In vitro Assessment of Biofield Energy Treated DMEM on Thermogenesis Using Myoblasts Cell Line (C2C12)- Juniper Publishers

Abstract
Mitochondrial dysfunction lead to various serious disorders, which are considered as one of the important components related with the aging, such as type-2 diabetes and Alzheimer’s disease. The aim of the present study was to examine the effect of Consciousness Energy Healing based DMEM medium on murine myoblasts (C2C12) cells to evaluate the mitochondrial mass content using 10-N-nonyl acridine orange (NAO) dye assay. The test item (DMEM medium) was divided into two parts, one part received Consciousness Energy Healing Treatment by a renowned Biofield Energy Healer, Dahryn Trivedi and was labeled as the Biofield Energy Treated DMEM group, while the other part did not receive any kind of Treatment and denoted as the untreated DMEM group. The level of mitochondrial mass content was assessed using 10-N-nonyl acridine orange (NAO) dye method. Cell viability of the test items using MTT assay showed 72.32% and 125.32% viable cells in the untreated DMEM and Biofield Energy Treated DMEM groups, respectively suggested a safe and nontoxic profile of the test items. Besides, the mitochondrial mass content in terms of Fluorescence Unit (FU) was significantly (p≤0.05) increased by 81.78% in the Biofield Energy Treated DMEM group compared to the untreated DMEM group. Overall, the experimental data suggested that the Consciousness Energy Healing Based DMEM showed a significant improvement of mitochondrial mass content and results in better thermogenesis with respect to naive DMEM. Thus, an increased level of NAO dye accumulation in muscle cells indicated increased mitochondrial mass content and hence, better thermogenesis. In the present study, results demonstrated that an increased mitochondrial mass content in the cells when treated with The Trivedi Effect®. This indicates that the test sample has the potential to improve thermogenesis, which can be used against various metabolic diseases, such as insulin resistance, type-2 diabetes, and cardiovascular diseases, etc.
Keywords: Biofield energy; The Trivedi Effect®; Thermogenesis; Mitochondrial biogenesis; Metabolic disorders; Murine myoblast cell; DMEM
Abbreviations: CAM: Complementary and Alternative Medicine, NCCAM: National Center for Complementary and Alternative Medicine; DMEM: Dulbecco’s Modified Eagle’s Medium; FBS: Fetal Bovine Serum
Introduction
Mitochondria (also known as power generator of the cell) produce most of the vital energy required for the cellular function through oxidative phosphorylation involved in electron transport and ATP synthesis. They produce ATP through the process of cellular respiration mainly aerobic respiration, which requires oxygen. Number and amount of mitochondria in a cell be governed by the energy requirement of the cell [1]. For example, the muscle cells have found comparatively more number of mitochondria since, they need to produce energy to move the body. On the other hand, red blood cells carry oxygen to other cells, do not need to produce energy as compared with the muscle cells. Mitochondria are the powerhouse in the cell, which produce energy from basic components. They undergo fusion, fission, transport, and degradation, all of the process is vital to maintain a healthy mitochondrial population [1]. However, the mitochondrial biogenesis process is involved an increased and controlled mitochondrial mass with number that helped to produce greater production of ATP as a response to greater energy expenditure [2]. Physiologic, metabolic, and pathologic changes along with morphological and functional adaptability are the vital factor to regulate the process of mitochondrial biogenesis. In addition, proteins and transcription factors, upstream regulatory proteins and secondary mechanisms are also involved in the biogenesis process, which also stabilizes the new mitochondrial DNA [3].
Mitochondrial biogenesis regulates and control various therapeutic interference in wide number of diseases such as metabolic syndrome, neurodegenerative disorders, sarcopenia, cardiac pathophysiology and physiological processes like aging [4]. Nonyl-acridine orange (NAO) is a non-fluorescent dye that converts into fluorescent dye in the presence of oxidative species [5]. NAO assay is one of the gold standard assays to detect the mitochondrial mass alteration, which is a metachromatic dye that binds to cardiolipin, an inner mitochondrial membrane lipid, regardless of the energetic state of the cell. Therefore, mitochondrial mass of the cells could be estimated by studying accumulation of the fluorescent dye in the mitochondria. Furthermore, an alternative therapies such as nuclear gene was reported to regulate total mitochondrial mass in response to mitochondrial dysfunction [6]. In order to improve the mitochondrial mass content via thermogenesis process, some alternative treatment approach without any associated side-effect is needed.
Biofield Energy Healing is a categorized as one of the Complementary and Alternative Medicine (CAM) accepted worldwide for the various treatment. Biofield Energy Therapy was accepted by National Center for Complementary and Alternative Medicine (NCCAM). Biofield Energy Healing is one of the emerging frontier in medicine, which has been increased in order to promote wellness by uncovering the root cause of diseases with universal solutions. CAM therapies have shown various significant clinical benefits. Over the past few decades, many energy healing practices have been reported a significant outcomes in various clinical and non-clinical fields. The effects of the CAM therapies have great potential, which include external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems both In vitro and in vivo [7]. Every living organisms possess some kind of unique energy known as Biofield Energy, which is infinite, para-dimensional and electromagnetic field surrounding the human body. Biofield (Putative Energy Fields) based Energy Healing Therapies have been reported to have significant outcomes against various disease conditions. Biofield Energy Healing Treatment (The Trivedi Effect®) contain a putative bioenergy, which is channeled by a renowned practitioner from a distance. Biofield Energy Healing as a CAM showed a significant results in biological studies [8]. However, the National Center for Complementary and Alternative Medicine (NCCAM), well-defined Biofield Therapies in the subcategory of Energy Therapies [9]. The Trivedi Effect®- Consciousness Energy Healing Treatment has been reported with significant revolution in the physicochemical properties of metals, chemicals, ceramics and polymers [10- 12], improved agricultural crop yield, productivity, and quality [13,14], transformed antimicrobial characteristics [15–17], biotechnology [18,19], improved bioavailability [20–22], skin health [23, 24], nutraceuticals [25,26], cancer research [27,28], bone health [29–31], human health and wellness. On the basis of outstanding benefits of Biofield Energy Treatment, the present study was aimed to evaluate the impact of the Biofield Energy Treatment (The Trivedi Effect®) on DMEM as test sample to alter the mitochondrial mass content on thermogenesis using NAO dye staining using standard in vitro assay in murine myoblasts (C2C12) cells.
Material and Methods
Chemicals and reagents
Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Life Technology, USA. Antibiotics solution (penicillin-streptomycin) were procured from HiMedia, India, and ethylenediaminetetraacetic acid (EDTA) was purchased from Sigma, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Cell culture
C2C12 (murine myoblasts) was used as a test system in the present study. The C2C12 cell line was maintained in DMEM growth medium for routine culture supplemented with 10% FBS. Growth conditions were maintained at 37°C, 5% CO2, and 95% humidity and subcultured by trypsinisation followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Before initiation of the experiment, cells were incubated in DMEM+2% horse serum (HS) for 3 days to allow the cells to differentiate into myotubes.
Experimental design
The experimental groups consisted of group 1 (G-I) with untreated DMEM and group 2 (G-II) included the Biofield Energy Treated DMEM.
Consciousness energy healing treatment strategies
The test item, DMEM was divided into two parts. One part of the test item was treated with the Biofield Energy by a renowned Biofield Energy Healer, Dahryn Trivedi remotely for ~5 minutes and coded as the Biofield Energy Treated DMEM, while the second part did not receive any sort of treatment and denoted as the untreated DMEM group. Biofield Energy Healer was located in the USA, while the test items were located in the research laboratory of Dabur Research Foundation, New Delhi, India. This Biofield Energy Treatment was administered through Healer’s unique Energy Transmission process to the test sample under laboratory conditions. Dahryn Trivedi in this study never visited the laboratory in person, nor had any contact with the test item (DMEM). Further, the untreated DMEM group was treated with a “sham” healer for comparative purposes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study.
Assessment of cell viability using MTT assay
The cell viability was performed by MTT assay in C2C12 cell line. The cells were counted and plated in 96-well plates at the density corresponding to 10 X 103 cells/well/180μL of cell growth medium (DMEM+2% HS). The above cells were incubated overnight under growth conditions and allowed the cell recovery and exponential growth, which were subjected to serum stripping or starvation. The cells were treated with the Untreated and Biofield Energy Treated test item. The cells in the above plate(s) were incubated for 24 to 72 hours in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. Following incubation, the plates were taken out and 20μL of 5 mg/mL of MTT solution were added to all the wells followed by additional incubation for 3 hours at 37°C. The supernatant was aspirated and 150μL of DMSO was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540nm using Synergy HT microplate reader, Bio Tek, USA [29]. The percentage cytotoxicity at each tested concentrations of the test items were calculated using the following equation (1):
%cytotoxicity = (1− X / R)*100………..(1)
Where, X = Absorbance of the Biofield Treated cells; R = Absorbance of untreated cells
The percentage cell viability corresponding to each treatment was obtained using the following equation (2):
% Cell Viability = (100- % Cytotoxicity)………(2)
The concentrations exhibiting ≥70% cell viability was considered as non-cytotoxic.
Assessment of mitochondrial content
For the assessment of mitochondrial mass, the cells were counted using an hemocytometer and plated at 4500 cells/well in dark walled 96-well plates in DMEM supplemented with 2% HS. The cells were incubated overnight under standard growth conditions to allow the cell recovery and exponential growth, which were treated by the test items in different groups followed by incubation with the test items for 72 hours. After incubation with the test items, mitochondrial content was determined by 10-N-nonyl acridine orange (NAO) dye. 50nM dye was added to each well and the cells were incubated for 30 minutes at 37°C and 5%CO2. After 30 minutes of incubation, media was discarded and cells were washed with phosphate buffer saline (PBS). 150μL of PBS was added to each well and fluorescence was read at 485/20 excitation, 528/20 emission filter using synergy HT microplate reader. The percentage increase in mitochondrial content was calculated using following equation
Where, FU denotes Fluorescence unit
Statistical analysis
All the values were represented as Mean ± SEM (standard error of mean) of three independent experiments. The statistical analysis was performed using Sigma Plot statistical software (v11.0). For two groups comparison student’s t-test was used. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
Cell viability using MTT assay
Cell viability data of the untreated and Biofield Energy Treated DMEM groups in C2C12 cells using MTT assay is shown in Figure 1. The percentage of cell viability in the untreated DMEM group was 72.32%, while it was 125.32% in the Biofield Energy Treated DMEM group (Figure 1). Overall, data suggest that all the test samples were found safe against the tested C2C12 cells, which were used for the estimation of mitochondrial mass content, which indicate extend of thermogenesis.
Extend of mitochondrial mass content in C2C12 cells
Mitochondrial mass content or biogenesis showed a significant effects against various metabolic diseases. The increased cell capacity to control and maintain the cell metabolism, signal transduction, and regulation of mitochondrial ROS production [2]. Alteration or decrease in the mitochondrial biogenesis are related with the mitochondrial dysfunction and mitochondrial oxidative stress, which leads to various diseases [32]. Mitochondrial mass content results in the improved production of ATP as a response to greater energy expenditure [33]. Various factors such as physical exercise, nutritional factors, etc. reported to have an improved mitochondrial mass content, which results in greater glucose uptake by muscles, along with an increased metabolic enzymes level for glycolysis, oxidative phosphorylation and ultimately a greater mitochondrial metabolic capacity [34]. Aging process leads to decrease level of mitochondrial mass content and results in various diseases such as enhanced aging, insulin resistance, type-2 diabetes, cardiovascular diseases, obesity, etc. [35]. The experiment was conducted to study the influence of Biofield Energy Healing Treatment on mitochondrial content in C2C12 cells using NAO dye assay. The results of mitochondrial mass content in terms of increase number of fluorescence unit (FU) among different groups in C2C12 cells using NAO dye assay are presented in Figure 2. The untreated DMEM group showed 272.3 ± 12.14 FU. Besides, the Biofield Energy Treated DMEM group showed 81.78% increase the level of mitochondrial mass in terms of FU, compared to the untreated DMEM group (Figure 2).
Thus, the data suggested that the Consciousness Energy Blessed DMEM showed a significant improvement of thermogenesis, which results in mitochondrial mass content. This phenomenon can be significantly used against various metabolic diseases, such as insulin resistance, type 2 diabetes, and cardiovascular diseases. Overall, the Biofield Energy Healing Treatment (The Trivedi Effect®) has the significant capacity to improve the overall Quality of life with an improved thermogenesis and mitochondrial content.
Conclusion
MTT data showed a significant cell viability with ranges from 72.32% to 125.32% in different test item groups, which indicated that the test items were safe and non-toxic in nature. Correspondingly, The Trivedi Effect® showed a significant change in mitochondrial mass among different groups. The Biofield Energy Treated DMEM group demonstrated significant increase in the mitochondrial mass by 81.78% as compared to the untreated DMEM group. Thus, Biofield Energy Healing based DMEM can be significantly used to improve the energy level, endurance, body energy, which can be utilized against many diseases such aging, diabetes, cancer, depression, hypertension, cardiovascular disease, aging, and physical strength. The Biofield Energy Treated (The Trivedi Effect®) DMEM were found to have a significant impact on thermogenesis, which might significantly improve the mitochondrial content in muscle cells. Therefore, the Consciousness Energy Healing based DMEM might be a suitable alternative media for cell growth. It can be useful for the management of various disorders such as Lupus, Systemic Lupus Erythematosus, Fibromyalgia, Addison Disease, Hashimoto Thyroiditis, Celiac Disease (gluten-sensitive enteropathy), Multiple Sclerosis, Dermatomyositis, Graves’ Disease, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Scleroderma, Psoriasis, Rheumatoid Arthritis, Reactive Arthritis, Diabetes, Sjogren Syndrome, Crohn’s Disease, Vasculitis, Vitiligo, Chronic Fatigue Syndrome and Alopecia Areata, as well as inflammatory disorders such as Irritable Bowel Syndrome (IBS), Asthma, Ulcerative Colitis, Alzheimer’s Disease, Parkinson’s Disease, Atherosclerosis, Dermatitis, Hepatitis, and Diverticulitis. Further, the Biofield Energy Healing Treatment can also be used in the prevention of immune-mediated tissue damage in cases of organ transplants (for example heart transplants, kidney transplants and liver transplants), for anti-aging, stress prevention and management, and in the improvement of overall health and Quality of Life.
https://juniperpublishers.com/ijcsmb/IJCSMB.MS.ID.555661.php
For more Open access journals please visit our site: Juniper Publishers
For more articles please click on Journal of Cell Science & Molecular Biology
0 notes
Text
IJMS, Vol. 24, Pages 8906: miR-100-5p Regulates Skeletal Muscle Myogenesis through the Trib2/mTOR/S6K Signaling Pathway
Micro#RNAs (#miRNAs) are endogenous small non-coding #RNAs that play crucial regulatory roles in many biological processes, including the growth and development of skeletal muscle. #miRNA-100-5p is often associated with tumor cell proliferation and migration. This study aimed to uncover the regulatory mechanism of #miRNA-100-5p in myogenesis. In our study, we found that the #miRNA-100-5p expression level was significantly higher in muscle tissue than in other tissues in pigs. Functionally, this study shows that miR-100-5p overexpression significantly promotes the proliferation and inhibits the differentiation of C2C12 myoblasts, whereas miR-100-5p inhibition results in the opposite effects. Bioinformatic analysis predicted that Trib2 has potential binding sites for miR-100-5p at the 3′UTR region. A dual-luciferase assay, qRT-qPCR, and Western blot confirmed that Trib2 is a target gene of miR-100-5p. We further explored the function of Trib2 in myogenesis and found that Trib2 knockdown markedly facilitated proliferation but suppressed the differentiation of C2C12 myoblasts, which is contrary to the effects of miR-100-5p. In addition, co-transfection experiments demonstrated that Trib2 knockdown could attenuate the effects of miR-100-5p inhibition on C2C12 myoblasts differentiation. In terms of the molecular mechanism, miR-100-5p suppressed C2C12 myoblasts differentiation by inactivating the mTOR/S6K signaling pathway. Taken together, our study results indicate that miR-100-5p regulates skeletal muscle myogenesis through the Trib2/mTOR/S6K signaling pathway. https://www.mdpi.com/1422-0067/24/10/8906?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Compound from Vietnamese herb shows promise in fight against sarcopenia
Gynostemma longipes is little used in herbal products in the West. The herb is called commonly called “That diep dom,” used in the Vietnamese herbal medicine tradition in tonics for treatment of diabetes and health strengthening.
A leaf extract from a related species, Gynostemma pentaphyllum, forms the basis of ActivAMP, an extract made by Gencor Pacific that is featured in a wide range of commonly sold dietary supplements marketed for muscle health and vitality.
Sarcopenia's prevelance
Sarcopenia is a rapidly expanding area of anti aging research. The term has been used in a somewhat amorphous fashion to basically refer to muscle wasting and weakness among older people that impairs quality of life and can raise the risk of things like falls and fractures.
Recently, there has been an attempt to define this condition in a more clinically relevant fashion. “It was recognized that the key element was a loss of muscle strength (dynapenia) rather than a loss of muscle mass. This has led to a change in the definition of sarcopenia to include strength (grip strength) or function (walking speed or distance),” said the authors of a 2014 paper on the subject.
Those authors put the prevalence of sarcopenia at about 5% to 10% of people over 65 years of age. According to recent statistics, more than 600 million people, or more than 8% of the world’s population, are now 65 years old or older. By 2050, that number is expected to grow to 1.6 billion, or as much as 15% of the world’s population. So as many as 60 million people may be exhibiting signs of sarcopenia today, with as many as 160 million in 30 years.
Eight new compounds isolated
In the Gynostemma longipes paper, researchers associated with institutions in South Korea, Vietnam and China isolated and characterized eight new 12,23-dione dammarane triterpenoids, as well as one known gypentonoside A from Gynostemma longipes and tested them in a mouse muscle cell line.
They were looking for the effects of the compounds on AMP-activated protein kinase (AMPK), an essential sensor and regulator of glucose, lipid and energy metabolism throughout the body and one that plays a vital role in muscle cell regeneration. The researchers chose the particular muscle cell line for its relevance to the development of sarcopenia.
“Regeneration of damaged skeletal muscles depends on satellite cells (known as quiescent muscle precursor cells), which play an important role in the proliferation and differentiation of myoblasts to form or repair muscle fibers. Recently, several studies have demonstrated that decreased proliferation of myoblasts and cytotoxicity can reduce the number of muscle fibers. Therefore, mouse C2C12 cells, also known as myoblastic cells, were chosen in this research because they are a valid model to study muscle cell proliferation,” they wrote.
Could common backbone of compounds be the deciding factor?
The researchers found significant effects on the AMPK pathway for seven of the eight newly isolated compounds. But only one of these is found in significant concentration in the plant, which the researchers referred to simply as compound 1. But the fact that almost all of the closely related compounds showed some activity led them to speculate that the backbone of these compounds is the important structural element.
“Considering the structures and activities of all nine isolates, the core structure of the dammarane skeleton might contribute mainly to the AMPK activation effect,” they wrote.
“A considerable amount of active compound 1 (over 2.08% per dried raw plant) in G. longipes suggested that it may be a promising candidate for development of functional food or botanical drug. These results also indicated that new dammarane-type compounds are promising candidates for muscle proliferation via activation of AMPK signaling pathways and could be further studied and developed as therapeutics for geriatric diseases,” they concluded.
Source: Scientific Reports “12,23-Dione dammarane triterpenes from Gynostemma longipes and their muscle cell proliferation activities via activation of the AMPK pathway” Published online 2019 Feb 4. doi: 10.1038/s41598-018-37808-9 Authors: Ha TKQ, Pham HTT, Cho HM, et al.
Source: https://www.nutraingredients-usa.com/Article/2019/02/12/Compound-from-Vietnamese-herb-shows-promise-in-fight-against-sarcopenia
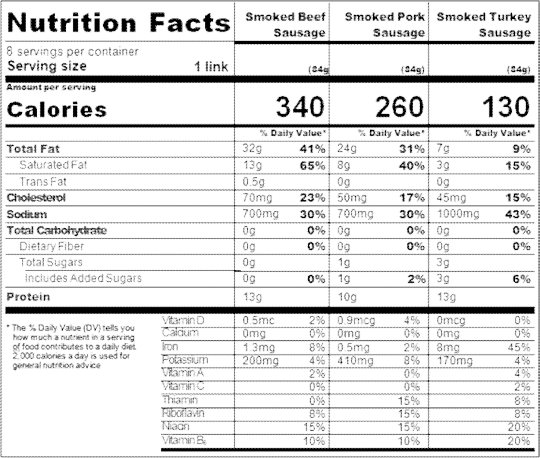
0 notes
Text
4.4.17 Second day of Spring Quarter
I woke up at 6:30, packed my things, and went to practice. I biked for 65 minutes as I watched spongebob and listened to music. Then I went to weights. After, I went to my first class, mechanical cell signaling, which is a bioengineeeing/biology upper division. I get to grow and experiment with stem cells, I am excited. I learned about components in the extracellular matrix and cell passaging lab procedures. My next class was ethics in technology. I like my professors personality, she uses a lot of internet memes in her PowerPoint. We learned about common ethical issues and broke into groups to come up with controversial topics in science/technology. Then I went to my mechanical cell signaling lab. The first part of the 3 hour lab was learning more about the cells that we are using, and we are using C2C12 cells, which are mouse muscle stem cells. Then we went to learn how to do cell passaging. I worked on the bioengineering research lab last summer, so I've already done that many times so that was review for me. Then I went to the gym, and biked for 30 minutes as I watched sponbebob. Next, I went to the cafeteria to get food before heading to the library. I worked on my physics and ethics homework as I ate dinner. When I finished, I drew, went back to my dorm, showered, and went to sleep.
2 notes
·
View notes
Text
GENE EXPRESSION
DNA is the genetic material of all organisms. This DNA material is divided up into functional unit called genes, and each gene provides instructions for the cell’s function. The central dogma describes transcription and translation, wherein the information in genes flows into proteins. In transcription, and RNA copy of the DNA segment is synthesized, whereas in translation, RNA is translated into protein.
Cancer results from a gene that is not normally expressed in a cell but may be expressed at high levels due to mutations or alterations in gene regulation. Alterations in histone acetylation, increased translational control, and protein modification are observed in cancer cells. Cancer can be described as a disease of altered gene expression since there are many proteins that are activated which affect the overall activity of the cell. This disease can also be detected by changes in epigenetic regulation, transcription, RNA stability, protein translation, and post-translational control. However, these changes do not occur all at once (Lumen, 2020).
Cancer Cachexia develops a wide range of dysfunctions from insulin and IGF-1 resistance to induction of mitochondrial uncoupling proteins and fat tissue browning which results to uncontrollable increased energy expenditure and heat generation. Tumor cells also actively promote tissue wasting by secreting specific factors such as microRNAs (Porporato, 2016). Moreover, AMPK activity is disrupted during cancer cachexia progression. Thus, it can contribute to altering skeletal muscle metabolism and gene expression. There is chronically elevated muscle AMPK activity during cachexia, which coincides with the suppression of mTORC1 signaling and disrupted mitochondrial quality control mechanisms. While AMPK activation by systemic IL-6 also corresponds with mTORC1 suppression, AMPK inhibition rescues IL-6-induced suppression of mTORC1 signaling in C2C12 myotubes (Hardee, Montalvo, & Carson, 2017).
miRNAs also play a major role in the regulation of the transcription of several genes involved with skeletal muscles. miRNA-486 decreases FoxO1 protein expression and promotes FoxO1 phosphorylation to suppress E3 ubiquitin ligases (Camargo, 2015). Moreover, skeletal muscle protein synthesis in cachexia reduces when dsRNA-dependent protein kinase (PKR) is activated by the tumor-secreted proteolysis-inducing factor (PIF), which is a potential inhibitor. This happens because of phosphorylation of eukaryotic initiation factor (eIF) 2, which interferes with the activity of eIF2B to promote translation initiation (Durham, Dillon, &. Sheffield-Moore, 2009). The inhibition of protein synthesis in muscle also reduces both RNA content or its protein synthesizing capacity and RNA activity such as the amount of protein synthesized by RNA. Levels of myosin follows the decrease in total protein, while actin levels remained constant (Bhogal, Lorite, & Tisdale, 2006).
References:
Photo retrieved from: https://images.app.goo.gl/hcZ1UXPxYPAnC17a7
Bhogal, Lorite, M., & Tisdale, M. (2006). Changes in Nucleic Acid and Protein Levels in Atrophying Skeletal Muscle in Cancer Cachexia. ANTICANCER RESEARCH, 26(6), 4149–4154. Retrieved from http://ar.iiarjournals.org/content/26/6B/4149.long
Camargo, R. (2015). Cancer Cachexia and MicroRNAs. Inflammation in Cachexia. doi: https://doi.org/10.1155/2015/367561
Cancer and Gene Regulation. 2020. Lumen Candela. Retrieved from https://courses.lumenlearning.com/boundless-biology/chapter/cancer-and-gene-regulation/
Durham, W. J., Dillon, E. L., & Sheffield-Moore, M. (2009). Inflammatory burden and amino acid metabolism in cancer cachexia. Current opinion in clinical nutrition and metabolic care, 12(1), 72–77. https://doi.org/10.1097/MCO.0b013e32831cef61
Hardee, J. P., Montalvo, R. N., & Carson, J. A. (2017). Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal Muscle Oxidative Metabolism. Oxidative Medicine and Cellular Longevity, 2017, 1–14. doi: 10.1155/2017/8018197
Porporato, P. E. (2016). Understanding cachexia as a cancer metabolism syndrome. Oncogenesis, 5(2). doi: 10.1038/oncsis.2016.3
0 notes
Text
Honokiol ameliorates oxidative stress-induced DNA damage and apoptosis of c2c12 myoblasts by ROS generation and mitochondrial pathway.
PMID: Anim Cells Syst (Seoul). 2020 ;24(1):60-68. Epub 2019 Dec 28. PMID: 32158617 Abstract Title: Honokiol ameliorates oxidative stress-induced DNA damage and apoptosis of c2c12 myoblasts by ROS generation and mitochondrial pathway. Abstract: Honokiol is one of the main active components of, and has been demonstrated to have multiple pharmacological activities against a variety of diseases. Recently, this phenolic compound is known to have antioxidant activity, but its mechanism of action remains unclear. The purpose of the current study was to evaluate the preventive effects of honokiol against oxidative stress-induced DNA damage and apoptosis in C2C12 myoblasts. The present study found that honokiol inhibited hydrogen peroxide (HO)-induced DNA damage and mitochondrial dysfunction, while reducing reactive oxygen species (ROS) formation. The inhibitory effect of honokiol on HO-induced apoptosis was associated with the up-regulation of Bcl-2 and down-regulation of Bax, thus reducing the Bax/Bcl-2 ratio that in turn protected the activation of caspase-9 and -3, and inhibition of poly (ADP-ribose) polymerase cleavage, which was associated with the blocking of cytochromerelease to the cytoplasm. Collectively, these results demonstrate that honokiol defends C2C12 myoblasts against HO-induced DNA damage and apoptosis, at least in part, by preventing mitochondrial-dependent pathway through scavenging excessive ROS.
read more
0 notes
Text
MiR-501-3p Forms a Feedback Loop with FOS, MDFI, and MyoD to Regulate C2C12 Myogenesis.
http://dlvr.it/R6yF0Y
0 notes