#Point-of-care Coagulation Testing Market
Explore tagged Tumblr posts
Text
Africa IVD Market - Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®, a premier global market research firm, has released its latest report titled, “Africa IVD Market Size, Share, Forecast & Trends Analysis by Offering, Technology (Immunoassay, PoC, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoCT), End User - Forecast to 2031.”
The report projects that the Africa in vitro diagnostics (IVD) market will reach $1.65 billion by 2031, growing at a CAGR of 3.1% from 2024 to 2031. This growth is driven by several factors, including:
Increasing prevalence of chronic and infectious diseases.
Significant investments from IVD market players in Africa.
Rising demand for Point-of-Care (POC) and rapid diagnostic solutions.
Growing geriatric population.
Government initiatives enhancing healthcare infrastructure.
Increased healthcare expenditure and R&D investments.
Download Sample Report Here : https://www.meticulousresearch.com/download-sample-report/cp_id=5415
Despite these opportunities, the market faces challenges such as high costs associated with advanced IVD products, variability in rapid test results, and stringent regulatory requirements for high and moderate-complexity tests.
Emerging Opportunities
The report highlights the growing awareness of early diagnosis, advancements in genomics and proteomics, and the rising adoption of personalized medicine as potential growth drivers for market stakeholders. However, uneven healthcare access and a shortage of trained professionals remain significant hurdles.
Check complete table of contents with list of table and figures: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Key Players in the Africa IVD Market
Leading companies in the Africa IVD market include:
Abbott Laboratories (U.S.)
Becton, Dickinson and Company (U.S.)
bioMérieux SA (France)
Danaher Corporation (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
QIAGEN N.V. (Netherlands)
Siemens Healthineers AG (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
Illumina, Inc. (U.S.)
Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China)
Market Insights and Future Outlook
The report segments the Africa IVD market by offering (reagents & kits, instruments, and software & services), technology (including immunoassay, molecular diagnostics, and others), application (focusing on infectious diseases, diabetes, oncology, etc.), diagnostic approach (laboratory testing, point-of-care testing, and OTC/self-testing), and end user (diagnostic laboratories, hospitals, home healthcare, etc.).
Reagents & Kits Segment: Expected to register the highest CAGR of 3.3% during the forecast period, driven by the increasing incidence of infectious diseases and rising test volumes.
Immunoassay Technologies: Anticipated to dominate with a 34.5% market share in 2024, favored for their efficiency and accuracy in diagnosing prevalent diseases like HIV and malaria.
Infectious Diseases Application: Projected to hold the largest market share due to the high prevalence of diseases such as COVID-19 and malaria.
Quick Buy : https://www.meticulousresearch.com/Checkout/47708335
Geographical Insights
The report provides an extensive analysis of key markets, including South Africa, Nigeria, Egypt, and more. Notably, South Africa is expected to exhibit the highest growth rate of 8.3% during the forecast period, fueled by increasing healthcare investments and improved access to diagnostic services.
Key Questions Addressed in the Report
What is the current revenue generated by IVD products in Africa?
What growth rate is expected for IVD product demand over the next 5-7 years?
What are the major factors impacting the Africa IVD market?
Which segments are experiencing significant traction?
What are the key geographical trends and opportunities?
For a deeper dive into these insights and more, access the full report : https://www.meticulousresearch.com/request-sample-report/cp_id=5415
Contact Information
For more details, please contact:
Meticulous Research® Email: [email protected] Sales Contact: +1-646-781-8004 Connect with us on LinkedIn.
0 notes
Text
In Vitro Diagnostics Market Growth Trends and Strategies 2024 - 2030
The global in vitro diagnostics (IVD) market size was estimated at USD 77.92 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 4.4% from 2024 to 2030.
The growth can be attributed to increasing adoption of IVD owing to a rise in the incidence of infectious and chronic diseases. The development of automated IVD systems for laboratories and hospitals to provide efficient, accurate, and error-free diagnoses is expected to fuel market growth. The rising number of IVD products being launched by key players is also fueling market growth. For instance, in November 2023, ARUP Laboratories received a CE mark from EU-IVDR for AAV5 DetectCDx, a companion diagnostic to select the eligibility of severe hemophilia A-affected patients for BioMarin’s new gene therapy, Roctavian.
Technological advancements in terms of accuracy, portability, and cost-effectiveness are expected to be one of the high-impact rendering drivers of this market. Introduction of novel and highly accurate clinical laboratory tests is boosting the adoption of novel IVD tests worldwide. In June 2023, Toray Industries, Inc. received marketing approval from Japan’s Ministry of Health, Labour and Welfare for its Toray APOA2-iTQ used to diagnose pancreatic cancer. Moreover, in March 2023, Abbott received U.S. FDA clearance for its novel laboratory Traumatic Brain Injury (TBI) blood test in the U.S. Increasing approvals of IVD tests for life-threatening diseases are expected to create new opportunities in the untapped market.
Gather more insights about the market drivers, restrains and growth of the In Vitro Diagnostics Market
In Vitro Diagnostics Market Report Highlights
• Molecular diagnostics is anticipated to grow at the fastest CAGR from 2024 to 2030 owing to the rising adoption and usage rate
• Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
• The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
• North America dominated the global market in 2023 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
• The global hepatitis diagnostic market size was valued at USD 3.82 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030.
• The global hematology diagnostics market size was valued at USD 7.54 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030.
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics (IVD) market report based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Revenue, USD Million, 2018 - 2030)
• Instruments
• Reagents
• Services
IVD Technology Outlook (Revenue, USD Million, 2018 - 2030)
• Immunoassay
o Instruments
o Reagents
o Services
• Hematology
o Instruments
o Reagents
o Services
• Clinical Chemistry
o Instruments
o Reagents
o Services
• Molecular Diagnostics
o Instruments
o Reagents
o Services
• Coagulation
o Instruments
o Reagents
o Services
• Microbiology
o Instruments
o Reagents
o Services
• Others
o Instruments
o Reagents
o Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
• Infectious Diseases
• Diabetes
• Oncology
• Cardiology
• Nephrology
• Autoimmune Diseases
• Drug Testing
• Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
• Point of Care
• Home-care
• Others
IVD End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals
• Laboratory
• Home-care
• Others
IVD Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Spain
o Italy
o Russia
o Denmark
o Sweden
o Norway
• Asia Pacific
o Japan
o China
o India
o South Korea
o Australia
o Thailand
o Singapore
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa (MEA)
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the In Vitro Diagnostics Market Intelligence Study, published by Grand View Research.
#In Vitro Diagnostics Market#In Vitro Diagnostics Market size#In Vitro Diagnostics Market share#In Vitro Diagnostics Market analysis#In Vitro Diagnostics Industry
0 notes
Text
Coagulation Markers Market By Product Type, By Manufacturers, By End-User And Market Trend Analysis Forecast 2033
The Coagulation Markers Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033).

Learn More On The Coagulation Markers Market: https://www.thebusinessresearchcompany.com/report/coagulation-markers-global-market-report
According to The Business Research Company’s Coagulation Markers Global Market Report 2024, The coagulation markers market size is expected to see strong growth in the next few years. It will grow to $1.52 billion in 2028 at a compound annual growth rate (CAGR) of 6.9%. The growth in the forecast period can be attributed to a growing incidence of chronic diseases, rising adoption of personalized medicine, a growing global population, rising healthcare expenditures, and expansion in emerging markets. Major trends in the forecast period include the integration of AI and machine learning, the development of novel biomarkers, enhanced point-of-care technologies, automation in laboratories, wearable health technologies, telehealth and remote monitoring, and sustainable diagnostic practices.
The rise in the prevalence of blood disorders is expected to propel the growth of the coagulation markers market going forward. Blood disorders refer to a wide range of conditions that affect the blood's ability to function correctly. The prevalence of blood disorders is growing due to aging populations, increased awareness, and improved diagnostic capabilities. Coagulation markers help diagnose and manage blood disorders by assessing clotting function, identifying abnormalities, and monitoring treatment effectiveness for conditions like hemophilia and thrombosis. For instance, in May 2024, according to the Leukaemia Foundation, an Australia-based non-profit organization, currently around 135,000 people in Australia are living with a blood cancer or blood disorder, with this number projected to exceed 275,000 by 2035. Therefore, an increasing prevalence of blood disorders is driving the coagulation marker market.
Get A Free Sample Of The Report (Includes Graphs And Tables): https://www.thebusinessresearchcompany.com/sample.aspx?id=17117&type=smp
The coagulation markers market covered in this report is segmented –
1) By Product: D-Dimer Assay, Fibrinogen Assay 2) By Sample Type: Plasma, Whole Blood Sample, Other Sample Types 3) By Technology: Fluorescence Immunoassay, Enzyme-Linked Immuno Sorbent Assay (ELISA), Rapid Test, Particle Enhanced Immunoturbidimetric Assay 4) By Application: Deep Venous Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Trauma, Other Applications 5) By End User: Hospital, Specialty Clinics, Academic And Research Institute, Diagnostics Laboratories
Major companies operating in the coagulation markers market are focusing on developing technologically innovative solutions, such as automated blood coagulation analyzers, to improve diagnostic accuracy, enhance efficiency, and provide faster results for better patient management. An automated blood coagulation analyzer is a medical device that automatically performs various tests to evaluate blood clotting function, providing rapid and accurate results for diagnosing and managing coagulation disorders. For instance, in July 2021, Sysmex Corporation, a Japan-based provider of in-vitro diagnostic solutions, announced the launch of its new automated blood coagulation analyzers named CN-6000 and CS-5100, designed to provide efficient and accurate coagulation testing in clinical laboratories. The CN-6000 is a fully automated coagulation analyzer with a maximum throughput of 240 tests per hour, featuring automatic sample pre-treatment, clot detection, and quality control management. The CS-5100, on the other hand, is a compact and versatile coagulation analyzer suitable for small to medium-sized laboratories, offering a throughput of up to 120 tests per hour and a user-friendly interface. Both analyzers support a wide range of coagulation assays and are designed to streamline coagulation testing workflows, improve turnaround times, and enhance patient care in clinical laboratories.
The coagulation markers market report table of contents includes:
1. Executive Summary
2. Coagulation Markers Market Characteristics
3. Coagulation Markers Market Trends And Strategies
4. Coagulation Markers Market - Macro Economic Scenario
5. Global Coagulation Markers Market Size and Growth ............
32. Global Coagulation Markers Market Competitive Benchmarking
33. Global Coagulation Markers Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Coagulation Markers Market
35. Coagulation Markers Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
0 notes
Text
0 notes
Text
Hemostasis/ Coagulation Analyzer Market Will Grow at Highest Pace Owing to Point-of-Care Diagnostics
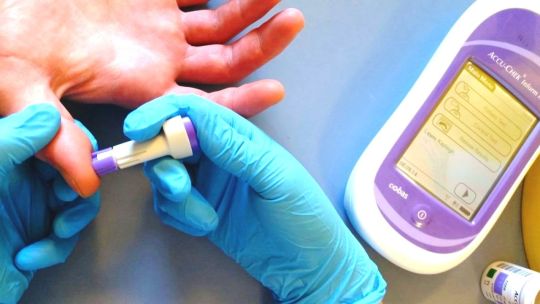
Hemostasis and coagulation analyzers are medical devices used to test coagulation factors in blood plasma. They help in monitoring blood coagulation disorders and management of anticoagulation therapies. Hemostasis and coagulation analyzers detect abnormalities related to hemostasis that may lead to excessive bleeding or thrombosis. Technological advancements have improved portability and results time of these analyzers enabling point-of-care diagnosis. The Global Hemostasis/ Coagulation Analyzer Market is estimated to be valued at US$ 6.47 Bn in 2024 and is expected to exhibit a CAGR of 5.8% over the forecast period 2024 to 2031. Key Takeaways Key players operating in the Hemostasis/ Coagulation Analyzer Market Growth are Horiba Medical, Siemens Healthineers AG, Agappe Diagnostics Ltd, NIHON KOHDEN CORPORATION, Sysmex Corporation, F. Hoffmann-La Roche Ltd, Stago Group, Maccura Biotechnology Co., Ltd., Thermo Fisher Scientific Inc., Haemonetics Corporation, Genrui Biotech Co., Ltd., BIOLABO S.A.S, Erba Mannheim, Sclavo Diagnostics International, and Helena Laboratories Corporation. The growing demand for point-of-care hemostasis testing is driving the market. Portable analyzers provide rapid results at the patient's bedside, ER, OR, and other locations to expedite clinical decision making. Technological advancements like dry chemistry methods, microfluidics, and automated sample-to-result technologies are reducing complex manual tasks. New devices have enhanced throughput, usability, and data management features for busy healthcare settings. Market Trends Point-of-care testing trend continues to grow for decentralized healthcare models. New devices offer miniaturized assaysoutside conventional laboratories directly at the site of patient care. Artificial intelligence and machine learning are being applied for tasks like automatic result interpretation, quality control, predictive maintenance, and remote diagnostics support. This helps maximize analyzer uptime for uninterrupted patient testing. Market Opportunities Emerging markets show strong growth potential driven by increasing access to healthcare and focus on non-communicable diseases. Local product assembly, innovative financingmodels, and regulatory approval strategies can help manufacturers tap opportunities. Integration of hemostasis testingonto general chemistry and coagulation analyzer platformsprovides a one-stop solution for routine and specialized testing. This consolidatestesting workflow while reducing costs per reportable result. Get More Insights On This Topic: Hemostasis/ Coagulation Analyzer Market
0 notes
Text
In Vitro Diagnostics Market To Reach $101.58 Billion By 2030
The global in vitro diagnostics market size is expected to reach USD 101.58 billion by 2030, according to a new report by Grand View Research, Inc. It is estimated to register a CAGR of 4.4% over the forecast period driven by the increasing geriatric population, COVID-19 pandemic, and technological advancements in diagnostics that are supporting its adoption. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be one of the high-impact rendering drivers. Technological advancements were further accelerated by the launch of COVID-19 IVD diagnostics and enhanced the adoption of instruments and consumables for technologies, such as PCR. Competitors in the market are increasingly adopting agreement and partnership strategies to maintain a constant flow of business for manufacturers & diagnostics for users.
These agreements are also a result of the harsh price containment strategies for government laboratories, which lowers the price in government settings. For instance, in April 2021, the Italian subsidiary of Seegene, Inc. received a USD 108.25 million tenders for public procurement for the supply of extraction reagents, as well as 7.15 million SARS-CoV-2 diagnostic tests. However, it increases the multiparty nature and complexity of the supply chain. The high prevalence of cancer and Cardiovascular Diseases (CVDs) globally is anticipated to drive diagnostic innovation to facilitate early diagnosis and meet the constantly evolving needs of consumers. Novel technologies, such as plasmonic PCR, are anticipated to commercially enter the market during the forecast period, influencing the business of existing products adversely.
Request a free sample copy or view the report summary: In Vitro Diagnostics Market Report
In Vitro Diagnostics Market Report Highlights
Molecular diagnostics is anticipated to grow at the fastest CAGR from 2024 to 2030 owing to the rising adoption and usage rate
Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
North America dominated the global market in 2023 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics (IVD) market report based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Revenue, USD Million, 2018 - 2030)
Instruments
Reagents
Services
IVD Technology Outlook (Revenue, USD Million, 2018 - 2030)
Immunoassay
Instruments
Reagents
Services
Hematology
Instruments
Reagents
Services
Clinical Chemistry
Instruments
Reagents
Services
Molecular Diagnostics
Instruments
Reagents
Services
Coagulation
Instruments
Reagents
Services
Microbiology
Instruments
Reagents
Services
Others
Instruments
Reagents
Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
Infectious Diseases
Diabetes
Oncology
Cardiology
Nephrology
Autoimmune Diseases
Drug Testing
Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
Point of Care
Home-care
Others
IVD End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Laboratory
Home-care
Others
IVD Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Spain
Italy
Russia
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Singapore
Latin America
Brazil
Mexico
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of In Vitro Diagnostics (IVD) Market
Abbott
bioMérieux SA
QuidelOrtho Corporation
Siemens Healthineers AG
Bio-Rad Laboratories, Inc.
Qiagen
Sysmex Corporation
Charles River Laboratories
Quest Diagnostics Incorporated
Agilent Technologies, Inc.
Danaher Corporation
BD
F. Hoffmann-La Roche Ltd.
0 notes
Text
Unveiling the Dynamics of the Global Hematology Testing Market

Introduction:
The field of hematology plays a crucial role in understanding and diagnosing various blood-related disorders. Hematology testing has evolved significantly over the years, with technological advancements paving the way for more accurate and efficient diagnostic methods. In this blog, we will delve into the dynamics of the Global Hematology Testing Market, exploring the key trends, challenges, and opportunities that define this rapidly growing sector.
Market Overview:
The Global Hematology Testing Market is witnessing unprecedented growth, driven by factors such as the rising prevalence of blood disorders, increasing geriatric population, and the continuous quest for innovative diagnostic solutions. Hematology testing encompasses a broad spectrum of tests, ranging from complete blood count (CBC) to more specialized tests like coagulation testing and blood typing.
Key Market Trends:
Automation and Integration: The demand for automated hematology analyzers is on the rise as laboratories seek to enhance efficiency and reduce turnaround times. Integrated systems that combine multiple testing functions are becoming increasingly popular, allowing for seamless workflow and improved accuracy.
Point-of-Care Testing (POCT): The shift towards decentralized testing is evident in the growing adoption of POCT devices. These compact, user-friendly instruments provide rapid results, enabling healthcare professionals to make quick decisions and initiate timely interventions.
Technological Advancements: The development of advanced technologies, including flow cytometry and molecular diagnostics, is revolutionizing hematology testing. These innovations not only improve the precision of diagnostics but also open doors to a more comprehensive understanding of blood-related disorders.
Experience Exponential Growth: Secure Your Sample Report and Dominate the Global Hematology Testing Market @ https://bisresearch.com/requestsample?id=778&type=download
Challenges in the Market:
Cost Constraints: The initial investment required for state-of-the-art hematology testing equipment can be a deterrent, especially for smaller healthcare facilities. The challenge lies in striking a balance between technological advancement and cost-effectiveness.
Regulatory Compliance: The hematology testing market is subject to stringent regulatory frameworks. Navigating these compliance requirements poses challenges for manufacturers and healthcare providers, necessitating a keen focus on quality control and assurance.
Opportunities on the Horizon:
Emerging Markets: Developing regions present untapped opportunities for growth in the hematology testing market. Increasing awareness, improving healthcare infrastructure, and rising disposable incomes contribute to the expansion of the market in these areas.
Personalized Medicine: The integration of hematology testing into the realm of personalized medicine is an exciting prospect. Tailoring treatment strategies based on individual patient profiles can significantly improve outcomes and redefine the landscape of blood disorder management.
Conclusion:
As the Global Hematology Testing Market continues to evolve, driven by technological advancements and a growing awareness of the importance of early and accurate diagnostics, the future holds immense promise. Overcoming challenges and leveraging emerging opportunities will be crucial for stakeholders in this dynamic and vital sector, ultimately contributing to better patient outcomes and a healthier global population.
#Global Hematology Testing Market#Global Hematology Testing Market Report#Global Hematology Testing Market Industry
0 notes
Text
Key Players in the POC Analyzers Market: A Company Spotlight
The Point-of-Care (POC) Analyzers Market is vibrant, with several key players contributing to the development and advancement of portable diagnostic solutions.
Buy the Full Report for Detailed Segments Insights into the POC Analyzers Market, Download a Free Report Sample
This company spotlight highlights some of the prominent players in this dynamic market:
Abbott Laboratories:
Abbott is a global healthcare company with a diverse portfolio, including a range of POC analyzers. Their offerings cover areas such as blood glucose monitoring, cardiac markers, infectious diseases, and molecular diagnostics. Abbott aims to provide rapid and accurate diagnostic solutions to improve patient outcomes.
Roche Diagnostics:
Roche Diagnostics, a division of the Swiss multinational Roche Group, is a leading player in the POC analyzers market. Their portfolio includes devices for testing blood gases, electrolytes, cardiac markers, and coagulation parameters. Roche focuses on delivering reliable and efficient solutions for decentralized testing.
Siemens Healthineers:
Siemens Healthineers is a global medical technology company known for its innovative diagnostic solutions. In the POC analyzers market, Siemens offers devices for testing blood gases, cardiac markers, and infectious diseases. Their emphasis on connectivity and data management contributes to streamlined healthcare workflows.
Danaher Corporation (Beckman Coulter):
Beckman Coulter, a subsidiary of Danaher Corporation, is a key player in the POC diagnostics space. Their POC analyzers cover a wide range of parameters, including chemistry, hematology, and immunoassays. Beckman Coulter focuses on delivering solutions that enhance clinical decision-making.
Sysmex Corporation:
Sysmex, a Japanese multinational corporation, is a prominent player in the global in-vitro diagnostics market. In the POC analyzers segment, Sysmex offers solutions for hematology testing, contributing to rapid and accurate blood analysis in various healthcare settings.
Quidel Corporation:
Quidel Corporation specializes in diagnostic testing solutions, including a range of POC analyzers. Their portfolio covers tests for infectious diseases, cardiac markers, and women's health. Quidel aims to provide rapid and accurate results to facilitate timely clinical decisions.
Nova Biomedical:
Nova Biomedical is a U.S.-based company known for its commitment to POC testing solutions. They offer analyzers for blood gas, electrolyte, and critical care testing. Nova Biomedical focuses on delivering high-performance POC analyzers for use in various healthcare settings.
Alere Inc. (Now part of Abbott):
Alere, acquired by Abbott, was a major player in POC diagnostics before the acquisition. Their portfolio included rapid tests and analyzers for infectious diseases, cardiac markers, and women's health. The integration with Abbott has strengthened the company's position in the POC market.
BioMérieux SA:
BioMérieux, a French multinational company, is a key player in the field of in-vitro diagnostics. In the POC analyzers market, BioMérieux focuses on solutions for infectious disease testing, including molecular diagnostics and immunoassays.
PTS Diagnostics:
PTS Diagnostics specializes in developing and manufacturing POC diagnostic products. Their portfolio includes analyzers for lipid panels, diabetes management, and cardiovascular risk assessment. PTS Diagnostics aims to provide cost-effective and user-friendly solutions for decentralized testing.
It's important to note that the POC analyzers market is dynamic, and the landscape may have evolved since my last knowledge update. Additionally, new players may have entered the market, and strategic partnerships or acquisitions may have occurred. For the latest information, it's advisable to refer to recent reports, company announcements, and industry publications.
0 notes
Text
Hemostasis/Coagulation Analyzer Market Top Winning Strategies and Industry Forecast 2023-2032
The field of hemostasis and coagulation diagnostics is undergoing a transformative phase, driven by rapid advancements in analyzer technology. This article delves into the current market insights, explores the revolutionary technologies shaping hemostasis analyzers, and provides a glimpse into the promising future prospects of this dynamic industry.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐑𝐞𝐩𝐨𝐫𝐭 : https://www.alliedmarketresearch.com/request-toc-and-sample/8012
Current Market Landscape:
The hemostasis/coagulation analyzer market is experiencing notable growth, fueled by an increasing prevalence of bleeding and clotting disorders worldwide. Diagnostic advancements are crucial in managing these conditions effectively, and the demand for more accurate, efficient, and rapid diagnostic tools has never been higher. The market is witnessing a surge in research and development activities, with a focus on delivering cutting-edge technologies that redefine the standards of hemostasis testing.
Revolutionary Technologies:
Point-of-Care Testing (POCT): The integration of point-of-care testing in hemostasis analysis is a game-changer. Portable analyzers that deliver rapid results at the patient's bedside are gaining traction, offering immediate insights for critical decision-making in emergency situations.
Automation and Robotics: Automation is streamlining hemostasis testing processes, reducing manual errors, and enhancing overall efficiency. Robotic systems are being employed to handle sample processing, allowing for increased throughput and consistency in results.
Enhanced Connectivity Solutions: The era of connectivity is influencing hemostasis analyzers, with the integration of cloud-based technologies and data analytics. This connectivity not only facilitates real-time monitoring but also opens avenues for telemedicine and remote diagnostics.
Multiplex Testing: Advancements in multiplexing technologies enable the simultaneous analysis of multiple coagulation parameters from a single sample. This not only saves time but also provides a comprehensive overview of a patient's coagulation profile.
Market Insights:
The COVID-19 pandemic has underscored the importance of hemostasis analyzers in managing critical conditions. The market has witnessed a notable uptick in demand for these analyzers, with a particular focus on their role in understanding the coagulation abnormalities associated with severe cases of the virus. This increased awareness and usage during the pandemic have positioned hemostasis analyzers as indispensable tools in modern healthcare.
𝐏𝐫𝐞-𝐛𝐨𝐨𝐤 𝐭𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭 𝐍𝐨𝐰 : https://www.alliedmarketresearch.com/hemostasis-coagulation-analyzer-market/purchase-options
Sysmex Corporation's Next-Gen Haemostasis Analyzers: Elevating Efficiency and Operability
Cutting-Edge Automation with CN-6000 and CN-3000: In December 2018, Sysmex Corporation made waves in the haemostasis industry with the launch of its next-generation automated blood coagulation analyzers, CN-6000 and CN-3000. These analyzers represent a significant leap forward in blood clotting technology, setting new standards for efficiency and operability. By introducing these advanced systems, Sysmex aims to cater to diverse customer demands effectively while substantially increasing operational efficiency in the haemostasis sector.
Sysmex CS-2500 System by Siemens Healthineers: Revolutionizing Mid-Volume Labs: In April 2018, Siemens Healthineers Laboratory Diagnostics unveiled the Sysmex CS-2500 System, a revolutionary addition to mid-volume labs. This cutting-edge system integrates proven PSI technologies, providing mid-volume labs with the capability to standardize test outcomes. The Sysmex CS-2500 not only brings advanced hemostasis technologies to local comparison labs but also seamlessly integrates into embedded distribution networks (IDNs). This launch marks a strategic move to enhance the accessibility and standardization of hemostasis testing, empowering laboratories with advanced capabilities for efficient and reliable diagnostics.
Meeting Varied Customer Demands and Increasing Industry Efficiency: Both product launches by Sysmex Corporation underscore a commitment to meeting diverse customer demands and driving efficiency in the haemostasis industry. The introduction of the CN-6000, CN-3000, and Sysmex CS-2500 represents a concerted effort to advance automation, improve operational workflows, and elevate the overall standards of blood coagulation analysis. As Sysmex continues to pioneer innovation in the field, these launches position the company as a frontrunner in shaping the future of haemostasis diagnostics, with a focus on precision, reliability, and enhanced customer satisfaction.
Future Prospects:
Looking ahead, the hemostasis/coagulation analyzer market is poised for significant growth. The integration of artificial intelligence (AI) and machine learning (ML) is anticipated to revolutionize result interpretation and enhance predictive analytics. Personalized medicine approaches, tailored to an individual's coagulation profile, are expected to become more prevalent, paving the way for more targeted and effective treatment strategies.
Strategic collaborations between diagnostic companies and research institutions are likely to accelerate innovation, bringing forth novel technologies that push the boundaries of hemostasis diagnostics. As the industry continues to evolve, accessibility to advanced hemostasis analyzers, especially in resource-limited settings, will be a key focus, ensuring that the benefits of these technological advancements reach diverse populations.
𝐈𝐧𝐭𝐞𝐫𝐞𝐬𝐭𝐞𝐝 𝐭𝐨 𝐏𝐫𝐨𝐜𝐮𝐫𝐞 𝐭𝐡𝐞 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐑𝐞𝐩𝐨𝐫𝐭? 𝐈𝐧𝐪𝐮𝐢𝐫𝐞 𝐁𝐞𝐟𝐨𝐫𝐞 𝐁𝐮𝐲𝐢𝐧𝐠 : https://www.alliedmarketresearch.com/purchase-enquiry/8012
Conclusion:
The advancements in hemostasis/coagulation analyzer technology are reshaping the landscape of diagnostic medicine. With a blend of innovative technologies, connectivity solutions, and a commitment to addressing emerging healthcare challenges, the future of hemostasis diagnostics holds promise for more precise, efficient, and patient-centric care. The journey towards these advancements signifies a transformative era in the diagnosis and management of coagulation disorders.
About Us
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
Pawan Kumar, the CEO of Allied Market Research, is leading the organization toward providing high-quality data and insights. We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
0 notes
Text
Meticulous Research® Unveils Comprehensive Analysis of the Africa IVD Market Forecasting Growth to $1.65 Billion by 2031
Meticulous Research®, a premier global market research firm, has released its latest report titled, “Africa IVD Market Size, Share, Forecast & Trends Analysis by Offering, Technology (Immunoassay, PoC, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoCT), End User - Forecast to 2031.”
The report projects that the Africa in vitro diagnostics (IVD) market will reach $1.65 billion by 2031, growing at a CAGR of 3.1% from 2024 to 2031. This growth is driven by several factors, including:
Increasing prevalence of chronic and infectious diseases.
Significant investments from IVD market players in Africa.
Rising demand for Point-of-Care (POC) and rapid diagnostic solutions.
Growing geriatric population.
Government initiatives enhancing healthcare infrastructure.
Increased healthcare expenditure and R&D investments.
Download Sample Report Here : https://www.meticulousresearch.com/download-sample-report/cp_id=5415
Despite these opportunities, the market faces challenges such as high costs associated with advanced IVD products, variability in rapid test results, and stringent regulatory requirements for high and moderate-complexity tests.
Emerging Opportunities
The report highlights the growing awareness of early diagnosis, advancements in genomics and proteomics, and the rising adoption of personalized medicine as potential growth drivers for market stakeholders. However, uneven healthcare access and a shortage of trained professionals remain significant hurdles.
Check complete table of contents with list of table and figures: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Key Players in the Africa IVD Market
Leading companies in the Africa IVD market include:
Abbott Laboratories (U.S.)
Becton, Dickinson and Company (U.S.)
bioMérieux SA (France)
Danaher Corporation (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
QIAGEN N.V. (Netherlands)
Siemens Healthineers AG (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
Illumina, Inc. (U.S.)
Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China)
Market Insights and Future Outlook
The report segments the Africa IVD market by offering (reagents & kits, instruments, and software & services), technology (including immunoassay, molecular diagnostics, and others), application (focusing on infectious diseases, diabetes, oncology, etc.), diagnostic approach (laboratory testing, point-of-care testing, and OTC/self-testing), and end user (diagnostic laboratories, hospitals, home healthcare, etc.).
Reagents & Kits Segment: Expected to register the highest CAGR of 3.3% during the forecast period, driven by the increasing incidence of infectious diseases and rising test volumes.
Immunoassay Technologies: Anticipated to dominate with a 34.5% market share in 2024, favored for their efficiency and accuracy in diagnosing prevalent diseases like HIV and malaria.
Infectious Diseases Application: Projected to hold the largest market share due to the high prevalence of diseases such as COVID-19 and malaria.
Quick Buy : https://www.meticulousresearch.com/Checkout/47708335
Geographical Insights
The report provides an extensive analysis of key markets, including South Africa, Nigeria, Egypt, and more. Notably, South Africa is expected to exhibit the highest growth rate of 8.3% during the forecast period, fueled by increasing healthcare investments and improved access to diagnostic services.
Key Questions Addressed in the Report
What is the current revenue generated by IVD products in Africa?
What growth rate is expected for IVD product demand over the next 5-7 years?
What are the major factors impacting the Africa IVD market?
Which segments are experiencing significant traction?
What are the key geographical trends and opportunities?
For a deeper dive into these insights and more, access the full report : https://www.meticulousresearch.com/request-sample-report/cp_id=5415
Contact Information
For more details, please contact:
Meticulous Research® Email: [email protected] Sales Contact: +1-646-781-8004 Connect with us on LinkedIn.
0 notes
Text
Emerging Technologies Driving the Blood Collection Tubes Market by 2030

The global blood collection tubes market is expected to grow US$ 3.20 billion by 2030, at a CAGR of 3.02%. The growth of the market is attributed to the increasing demand for blood diagnostic tests, growing prevalence of chronic diseases, and rising geriatric population.
Blood collection tubes are essential tools for collecting blood samples for diagnostic testing and other medical procedures. They are used to collect a variety of blood samples, including whole blood, serum, plasma, and blood gas. Blood collection tubes are available in a variety of materials, including glass, plastic, and PET.
Request A Sample Copy of This Research Report! https://absolutemarketresearch.com/Global-Blood-Collection-Tubes-Market/1977/request-sample
Samples of patient blood, urine, and serum are collected in sterilized glass or plastic blood collection tubes in order to diagnose blood-related illnesses. Blood collection tubes come in a variety of forms, such as sodium heparin tubes, vacutainer blood collection tubes, plasma separation tubes, serum separating tubes, and EDTA tubes.
In order to diagnose blood-related disorders, patient blood, urine, and serum samples are collected using sterilized plastic or glass blood collection tubes. Vacutainer blood collection tubes, plasma separation tubes, serum separating tubes, EDTA tubes, and sodium heparin tubes are only a few of the several varieties of blood collection tubes available.
Key Takeaways:
The global blood collection tubes market is expected to grow at a CAGR of 3.02% from 2023 to 2030, reaching a value of US$ 3.20 billion by 2030.
The increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population are key drivers of the market growth.
The serum separation tubes segment accounted for the largest market share in 2022 and is expected to continue to dominate the market during the forecast period.
North America is expected to remain the largest market for blood collection tubes throughout the forecast period, due to the high prevalence of chronic diseases and advanced healthcare infrastructure in the region.
Asia Pacific is expected to be the fastest-growing market during the forecast period, due to the increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population in the region.
Regional Outlook:
The global blood collection tubes market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is the largest market for blood collection tubes, followed by Europe and Asia Pacific. Asia Pacific is expected to be the fastest-growing market during the forecast period, due to the increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population in the region.
Key Players:
The key players in the global blood collection tubes market include:
BD
Terumo
Greiner Bio-One
Sarstedt
Vacutainer Systems
Jiangsu Yuyue Medical Equipment & Supplies Group Co., Ltd.
Nipro Corporation
Jiangsu Kanghui Medical Equipment Co., Ltd.
Zhejiang Kangda Medical Equipment Co., Ltd.
Hangzhou Singclean Medical Products Co., Ltd.
Sichuan Haimen Medical Equipment Co., Ltd.
Segmentation:
The global blood collection tubes market is segmented by type, material, and application.
By type, the market is segmented into:
Serum separation tubes
Coagulation tubes
Plasma collection tubes
Blood bank tubes
Microbiology tubes
Others
By material, the market is segmented into:
Plastic
Glass
Others
By application, the market is segmented into:
Hospitals
Diagnostic laboratories
Blood banks
Others
0 notes
Text
Blood Coagulation Testing Market - Future Growth Prospects for the Global Leaders | Credence Research

The latest market report published by Credence Research, Inc. “Global Blood Coagulation Testing Market: Growth, Future Prospects, and Competitive Analysis, 2023 – 2030. The global blood coagulation testing market has witnessed rapid growth in recent years and is expected to grow at a CAGR of 11.9% between 2023 and 2030. The market was valued at USD 3945.5 million in 2022 and is expected to reach USD 9699.35 million in 2030.
The Blood Coagulation Testing Market refers to the global healthcare industry segment that deals with the testing and analysis of blood coagulation or clotting processes in patients. Blood coagulation, also known as blood clotting, is a vital physiological process that prevents excessive bleeding when a blood vessel is injured. It involves a complex cascade of reactions that result in the formation of a blood clot.
Blood coagulation testing is essential for diagnosing and monitoring various medical conditions, such as bleeding disorders (e.g., hemophilia), thrombosis (formation of unwanted blood clots), and monitoring patients on anticoagulant medications (blood thinners). These tests help healthcare providers assess a patient's coagulation status and make informed decisions regarding treatment and management.
The Blood Coagulation Testing Market includes various diagnostic tests and equipment used to measure different aspects of blood coagulation, such as prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), and levels of specific clotting factors. This market encompasses a wide range of products and services, including coagulation analyzers, reagents, consumables, and software for data analysis.
The market is driven by factors such as the increasing prevalence of coagulation disorders, the aging population, and the rising demand for point-of-care testing devices. Additionally, advancements in technology and the development of novel anticoagulant drugs have contributed to the growth of this market.
Blood coagulation testing is crucial for patient care in various medical settings, including hospitals, clinics, and laboratories. It plays a pivotal role in ensuring that individuals with coagulation disorders receive the appropriate treatment and that those at risk of thrombosis are effectively managed. The Blood Coagulation Testing Market continues to evolve with ongoing research and innovation, aiming to improve the accuracy, speed, and convenience of coagulation testing methods.
Here are some key trends in the blood coagulation testing market:
Rise in Demand for Point-of-Care Testing: There has been a growing demand for point-of-care coagulation testing devices. These portable devices enable healthcare providers to perform quick and convenient tests at the patient's bedside, leading to faster diagnosis and treatment decisions.
Advancements in Technology: Technological advancements have led to the development of more accurate and efficient coagulation testing methods. This includes the use of automated analyzers, robotics, and improved reagent formulations.
Increasing Incidence of Hemostatic Disorders: The rising prevalence of hemostatic disorders, such as hemophilia and thrombophilia, has driven the demand for coagulation testing. Early diagnosis and monitoring of these conditions are crucial for patient management.
Growing Aging Population: The aging population is at a higher risk of developing coagulation disorders. As the global population continues to age, there is an increasing need for coagulation testing to assess and manage the health of elderly individuals.
The following are some of the top market players and their market shares:
Thermo Fisher Scientific Inc
Micropoint Biosciences
Medtronic
Helena Laboratories Corporation
F. Hoffmann-La Roche Ltd
Sysmex Corporation
Nihon Kohden Corporation
Abbott
Browse 247 pages report Blood Coagulation Testing Market By Test Type (Prothrombin Time (PT) and International Normalized Ratio (INR) Tests, Activated Partial Thromboplastin Time (aPTT) Tests, Thrombin Time (TT) Tests, Fibrinogen Tests, D-Dimer Tests, Platelet Function Tests, Others) By Technology (Optical Technology, Electrochemical Technology, Mechanical Technology, Immunological Technology, Other Technologies) – Growth, Future Prospects & Competitive Analysis, 2016 – 2030 - https://www.credenceresearch.com/report/blood-coagulation-testing-market
Blood Coagulation Testing Market Growth Factor Worldwide
The Blood Coagulation Testing Market has been experiencing substantial growth worldwide, driven by several key factors. One of the primary drivers is the increasing prevalence of bleeding disorders and cardiovascular diseases, which require accurate diagnosis and management of coagulation disorders. Moreover, the growing geriatric population susceptible to thrombotic events further contributes to market expansion.
The advancements in technology have significantly enhanced blood coagulation testing methods, allowing for quicker results and improved accuracy, thus boosting market demand. Additionally, continuous research and development activities by prominent market players are leading to innovative testing products with higher sensitivity and specificity. Furthermore, rising awareness about early disease detection among individuals coupled with expanding healthcare infrastructure across emerging economies foster market growth opportunities globally. With an escalating emphasis on preventive healthcare measures and personalized medicine approaches, the Blood Coagulation Testing Market is poised for remarkable progress in the future.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global Blood Coagulation Testing Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global Blood Coagulation Testing Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Complete Report- https://www.credenceresearch.com/report/blood-coagulation-testing-market
Visit our Website- https://www.credenceresearch.com
Related Reports- https://www.credenceresearch.com/report/compression-therapy-market
Browse Our Blog- https://www.linkedin.com/pulse/blood-coagulation-testing-market-key-players-growth-vanshika-shukla
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
0 notes
Text
Automated Blood Coagulation Analyzers Market Growth, Trend, and Prospects from 2023–2030

Automated Blood Coagulation Analyzers Market Growth,
The Automated Blood Coagulation Analyzers Market is expected to grow from USD 1.20 Billion in 2022 to USD 1.70 Billion by 2030, at a CAGR of 4.20% during the forecast period.
Get the Sample Report:https://www.reportprime.com/enquiry/sample-report/10612
Automated Blood Coagulation Analyzers Market Size
Automated Blood Coagulation Analyzers are medical devices used to diagnose and monitor blood clotting problems. The market research report identifies the market segment based on type, application, region, and market players. The type segment is further divided into fully automated and semi-automated devices. The application segment includes clinics, hospitals, research institutes, and others. The report covers the regions of North America, Asia Pacific, Middle East, Africa, Australia, and Europe. The major players mentioned in the report are Hycel, Tridema Engineering, Maccura Biotechnology Co, PZ Cormay, Wama Diagnostica, BPC BioSed, Caretium Medical Instruments, Grifols, HAEMONETICS, Roche, Medtronic, Instrumentation Laboratory, Technoclone, Rayto Life and Analytical Sciences, Accriva Diagnostics, URIT Medical Electronic, Helena Biosciences, Stago, ROBONIK, and Perlong Medical. The report also covers regulatory and legal factors specific to market conditions.
Automated Blood Coagulation Analyzers Market Key Players
Mazor Key Player is listed in the Automated Blood Coagulation Analyzers
Hycel
Tridema Engineering
Maccura Biotechnology Co
PZ Cormay
Wama Diagnostica
Buy Now & Get Exclusive Discount on this:https://www.reportprime.com/enquiry/request-discount/10612
Automated Blood Coagulation Analyzers Market Segment Analysis
The automated blood coagulation analyzers market is a rapidly growing and highly competitive segment of the clinical diagnostics industry. This market caters to the needs of hospitals, reference laboratories, and research institutions, which require reliable and efficient methods of measuring blood coagulation parameters. The market is expected to witness significant growth in the coming years due to several factors driving revenue growth.
One of the major factors contributing to the growth of the automated blood coagulation analyzers market is the increasing prevalence of chronic diseases such as cardiovascular diseases and cancer. The rising demand for accurate and timely diagnosis of blood coagulation disorders in these diseases is driving the adoption of advanced coagulation analyzers. In addition, the growing aging population and the increasing number of surgeries and trauma cases have also led to an increased demand for coagulation testing.
Another factor driving revenue growth is the increasing automation of laboratory processes. Automated blood coagulation analyzers are more efficient and have a higher level of accuracy compared to traditional manual methods. Additionally, the demand for point-of-care testing and decentralized testing is also on the rise, further driving the adoption of automated analyzers.
Some of the latest trends in the automated blood coagulation analyzers market include the integration of advanced technologies such as artificial intelligence and machine learning algorithms. This integration helps to improve the accuracy and efficiency of coagulation testing, enabling faster and more accurate diagnosis of blood coagulation disorders.
However, the market also faces some challenges, such as the high cost of automated blood coagulation analyzers, which may limit adoption in certain regions. Additionally, the lack of standardization in coagulation testing methods across different regions and laboratories can pose a challenge for the market.
The report’s main findings highlight the significant growth potential of the automated blood coagulation analyzers market in the coming years. The increasing prevalence of chronic diseases and the demand for more efficient and accurate laboratory processes are expected to drive revenue growth. In addition, the report recommends that companies focus on developing cost-effective analyzers that are easy to use and maintain, to expand market adoption.
In conclusion, the automated blood coagulation analyzers market is a highly competitive and growing segment of the clinical diagnostics industry. The market is being driven by factors such as the increasing prevalence of chronic diseases and the need for more efficient laboratory processes. The market also faces challenges such as the high cost of analyzers and the lack of standardization in testing methods. However, with the right strategies and product offerings, companies can successfully tap into this growing market.
This report covers impact on COVID-19 and Russia-Ukraine wars in detail.
Purchase This Report: https://www.reportprime.com/checkout?id=10612&price=3590
Automated Blood Coagulation Analyzers (by Application)
Clinics
Hospitals
Research Institutes
Others
Information is sourced from www.reportprime.com
0 notes
Text
Diagnostic Cartridge Field Diagnostic System Market Size, Growth with a CAGR of ~5% During 2023-2035 and Attain ~USD 12 Billion by 2035

Research Nester’s recent market research analysis on “Diagnostic Cartridge Field Diagnostic System Market: Global Demand Analysis & Opportunity Outlook 2035” delivers a detailed competitor’s analysis and a detailed overview of the global diagnostic cartridge field diagnostic system market in terms of market segmentation by type, end-user, and by region.
Increasing Importance of Point of Care Testing (POCT) to Promote Global Market Share of Diagnostic Cartridge Field Diagnostic System
The global diagnostic cartridge field diagnostic system market is estimated to grow majorly on account of the rising demand for point-of-care testing post-pandemic and amongst patients needing critical care. With the number of people with COVID-19 cases surging daily around the world, point-of-care testing (POCT) is gaining attention as a tool as it can be done rapidly and be performed by clinical personnel who are not trained in clinical laboratory sciences. Moreover, it enables more rapid, accurate, and timely clinical decision-making in the process of diagnosis, treatment choice, monitoring, and prognosis, as well as operational decision-making and resource utilization. Hence, with the growth in the utilization of POCT, the growth of the diagnostic cartridge field diagnostic system is also anticipated to prosper.
Request Free Sample Copy of this Report @ https://www.researchnester.com/sample-request-3936
Some of the major growth factors and challenges that are associated with the growth of the global diagnostic cartridge field diagnostic system market are:
Growth Drivers:
Growing Prevalence of Chronic and Acute Diseases
Expanding Diagnostic Sector
Challenges:
The immensely slower penetration of the diagnostic cartridge field diagnostic system in developing countries, and the challenge of quality control and result reporting faced by the market due to lack of accuracy owing to test error are some of the major factors anticipated to hamper the global market size of diagnostic cartridge field diagnostic system.
Request for customization @ https://www.researchnester.com/customized-reports-3936
By type, the global diagnostic cartridge field diagnostic system market is segmented into infectious diseases testing, cardiac markers testing, coagulation testing, electrolytes testing, and others. The cardiac marker testing segment is to garner the highest revenue by the end of 2035 by growing at a significant CAGR over the forecast period. The rise in the number of people suffering from myocardial infarction (MI), commonly known as a heart attack is estimated to propel the segment growth. Cardiac marker tests can predict the symptoms of the condition as they can identify blood chemicals associated with myocardial infarction.
Further, the hospital sub-segment amongst the end-user is garnered a notable share over the forecast period owing to the rapid patient footfall in hospitals and the increasing demand for handheld/ portable diagnostics devices that are preferred to perform a test at a patient’s bedside.
Access Our Report at:
By region, the Asia Pacific diagnostic cartridge field diagnostic system market is to generate the highest revenue by the end of 2035. The flourishing medical tourism industry in countries like India together with the surge in collaborations of corporates and multi-national companies for the upliftment of the diagnostic market is anticipated to pool investment in the diagnostic cartridge field diagnostic system market.
The North American diagnostic cartridge field diagnostic system market is anticipated to register the highest shareholding owing to the rapid adoption of advanced medical technology in the region especially in the healthcare sector.
Finally, the market in Europe is projected to hold a majority of the share by the end of 2035 owing to the widespread of chronic lifestyle diseases such as diabetes amongst the aging population.
This report also provides the existing competitive scenario of some of the key players of the global diagnostic cartridge field diagnostic system market which includes company profiling of Siemens Healthineers AG, F. Hoffmann-La Roche AG, Danaher Corporation, Bio-Rad Laboratories, Inc., Sysmex Corporation, Arkray Inc., Becton, Dickinson and Company, Binx Health Limited, Nova Biomedical Corporation, Abbott Laboratories, and others.
About Us
Research Nester is a leading service provider for strategic market research and consulting. We aim to provide unbiased, unparalleled market insights and industry analysis to help industries, conglomerates, and executives to take wise decisions for their future marketing strategy, expansion, investment, etc. We believe every business can expand to its new horizon, provided the right guidance at the right time is available through strategic minds. Our out of box thinking helps our clients to take wise decisions to avoid future uncertainties.
Contact for more Info:
AJ Daniel
Email: [email protected]
U.S. Phone: +1 646 586 9123
U.K. Phone: +44 203 608 5919
#Diagnostic Cartridge Field Diagnostic System Market#Diagnostic Cartridge Field Diagnostic System#Diagnostic Cartridge Field Diagnostic System Market size
0 notes