#Medical Device Outsourcing Market share
Explore tagged Tumblr posts
Text
Medical Device Outsourcing Market to Hit $347.63 Billion by 2032
The global Medical Device Outsourcing Market was valued at USD 129.03 Billion in 2024 and it is estimated to garner USD 347.63 Billion by 2032 with a registered CAGR of 11.57% during the forecast period 2024 to 2032.
Are you looking for the Medical Device Outsourcing Market Research Report? You are at the right place. If you desire to find out more data about the report or want customization, Contact us. If you want any unique requirements, please allow us to customize and we will offer you the report as you want.
The global Medical Device Outsourcing Market can be segmented on the basis of product type, Applications, distribution channel, market value, volume, and region [North America, Europe, Asia Pacific, Latin America, Middle East, and Africa]. The Medical Device Outsourcing Industry 2024 report provides a comprehensive overview of critical elements of the industry including drivers, restraints, and management scenarios.
Download Sample PDF: @ https://www.vantagemarketresearch.com/medical-device-outsourcing-market-2383/request-sample
Top Players
Eurofins Scientific (Luxembourg), Integer Holdings Corporation (U.S.), Pace Analytical Services LLC (U.S.), Intertek Group PLC (UK), Plexus Corp. (U.S.), IQVIA Inc. (U.S.), North American Science Associates LLC (U.S.), Charles River Laboratories (U.S.)
Trending 2024: Medical Device Outsourcing Market Report Highlights:
A comprehensive assessment of the parent Industry
Development of key aspects of the business
A study of industry-wide market segments
Evaluation of market value and volume in past, present, and future years
Evaluation of market share
Tactical approaches of market leaders
Innovative strategies that help companies to improve their position in the market
You Can Buy This Report From Here: https://www.vantagemarketresearch.com/buy-now/medical-device-outsourcing-market-2383/0
Analysis Of The Top Companies, Product Types, and Applications In The Market Report:
This report provides sales, revenue growth rate, and verified information about the major players. Also includes a regional analysis and a labor cost analysis, tables, and figures. It also highlights characteristics such as technological growth. The product type segment is expected to continue to maintain its leading position in the future and capture a significant market share based on sales. This report provides analysis, discussion, forecast, and debate on key industry trends, market share estimates, Industry size, and other information. This report also discusses drivers, risks, and opportunities.
Global Medical Device Outsourcing Market report contains detailed data and analysis on the Medical Device Outsourcing Market drivers, restraints, and opportunities. Experts with market and industry knowledge as well as research experience from regional experts validate the report. The Medical Device Outsourcing Market report provides forecast, historical and current revenue for each industry, region, and end-user segment.
Regions Included
-North America [United States, Canada, Mexico]
-South America [Brazil, Argentina, Columbia, Chile, Peru]
-Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
-Middle East & Africa [GCC, North Africa, South Africa]
-Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
Global Medical Device Outsourcing Market report data will help you make more informed decisions. For example, in relation to prices, distribution channels are means of marketing or identifying opportunities to introduce a new product or service. These results will also help you make more informed decisions about your existing operations and activities.
Read Full Research Report with [TOC] @ https://www.vantagemarketresearch.com/industry-report/medical-device-outsourcing-market-2383
You Can Use The Medical Device Outsourcing Market Report To Answer The Following Questions:
What are the growth prospects of the Medical Device Outsourcing Market business?
Who are the key manufacturers in the Medical Device Outsourcing Market space?
What Forecast Period for Global Medical Device Outsourcing Industry Report?
What are the main segments of the global Medical Device Outsourcing Market?
What are the key metrics like opportunities and market drivers?
The Medical Device Outsourcing Market Insights
Product Development/Innovation: Detailed Information On Upcoming Technologies, R&D Activities, And Product Launches In The Market.
Competitive Assessment: In-Depth Assessment Of Market Strategies, Geographic And Business Segments Of Key Market Players.
Market Development: Comprehensive Information On Emerging Markets. This Report Analyzes The Market For Different Segments In Different Regions.
Market Diversification: Comprehensive Information On New Products, Untapped Regions, Latest Developments, And Investments In The Medical Device Outsourcing Market.
Check Out More Reports
Global Photovoltaic Inverter Market : Report Forecast by 2032
Global Industry 4.0 Market: Report Forecast by 2032
Global RF Over Fiber Market: Report Forecast by 2032
Global Trucking Market: Report Forecast by 2032
Global Anti-Vibration Mounts Market: Report Forecast by 2032
#Medical Device Outsourcing Market#Medical Device Outsourcing Market 2024#Global Medical Device Outsourcing Market#Medical Device Outsourcing Market outlook#Medical Device Outsourcing Market Trend#Medical Device Outsourcing Market Size & Share#Medical Device Outsourcing Market Forecast#Medical Device Outsourcing Market Demand#Medical Device Outsourcing Market sales & price
0 notes
Text
Intraoperative Neuromonitoring Market Analysis: Key Drivers, Opportunities, and Technological Advancements
The global intraoperative neuromonitoring market size is expected to reach USD 4.66 billion in 2030 and is anticipated to grow at a CAGR of 5.97% over the forecast period. This growth can be attributed to technological advancements, increasing awareness regarding the safety of the devices, and the growing geriatric population. The COVID-19 outbreak is expected to have a dual impact on the market. In March 2020, the American College of Surgeons (ACS) published a proposal to reduce, postpone, or cancel elective surgeries as COVID-19 cases grew quickly in the U.S., which is expected to hamper growth.
Moreover, according to a research study published in Elsevier journal (International Hemorrhagic Stroke Association; Jan 2021), up to 36% of hospitalized COVID-19 patients might exhibit neurological symptoms, and there were several cases related to ischemic & hemorrhagic infarction. In addition, another recent research study published in Neurology Online Journal in June 2021 proposed that the COVID-19 virus might be responsible for patients acquiring novel neurological diseases. Owing to the growth of new diseases, there is a higher risk of in-hospital mortality and a low discharge rate. These characteristics are expected to result in lucrative growth over the forecast period.
Furthermore, the market is expected to witness growth due to the increasing number & affordability of neurosurgeries and the growing emphasis on patient safety in surgeries. Hospitals are adopting Intraoperative Neuromonitoring (IONM) in a wide spectrum of surgeries, as it reduces the number of revision surgeries and results in fewer postoperative complications & cases of permanent impairment.
Intraoperative Neuromonitoring Market Report Highlights
By type, the insourced segment accounted for the largest share of 52.72% in 2024. This can be attributed to growing awareness among physicians as well as patients about the benefits of IONM, which results in the hospitals utilizing IONM during complex procedures. This is a key reason for the dominance of insourced IONM in the market
By region, North America accounted for the largest share of 49.35% in 2024. Favorable reimbursement scenarios along with technological advancements can be attributed to this dominance
The intraoperative neuromonitoring market in Europe is anticipated to grow fastest with a CAGR of 6.38% during the forecast period
Intraoperative Neuromonitoring Market Segmentation
Grand View Research has segmented the global intraoperative neuromonitoring market report on the basis of product, source, type, and region
Intraoperative Neuromonitoring Product Outlook (Revenue USD Million, 2018 - 2030)
Systems
Disposables
EMG Tubes And Electrodes
Sticker Electrode
Stimulating Probes
Intraoperative Neuromonitoring Source Outlook (Revenue USD Million, 2018 - 2030)
Insourced
Outsourced
Intraoperative Neuromonitoring Type Outlook (Revenue, USD Million, 2018 - 2030)
Intermittent Monitoring
Continuous Monitoring
Intraoperative Neuromonitoring Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
Norway
Denmark
Sweden
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
Key Players in Intraoperative Neuromonitoring Market
Natus Medical Incorporated
Accurate Monitoring
Neuromonitoring Technologies
Medtronic
NuVasive, Inc.
SpecialtyCare
Neurosoft
Ambu A/S
Brainlab AG (Dr. Langer Medical GmbH)
Welcony (Neurosign UK Ltd.)
Order a free sample PDF of the Intraoperative Neuromonitoring Market Intelligence Study, published by Grand View Research.
0 notes
Text
Regulatory Affairs Market Size To Reach USD 26.0 Billion By 2030

Regulatory Affairs Market Growth & Trends
The global regulatory affairs market size is expected to reach USD 26.0 billion by 2030, expanding at a CAGR of 8.2% from 2024 to 2030, according to a new report by Grand View Research, Inc. The factors expected to contribute to the growth of this market include changing regulatory requirements based on business activities and geographies, an increase in clinical trials & drug approvals along with accelerated regulatory approval, and technological advancement in regulatory software. Also, the evolution of personalized medicines, the increasing need for companies to focus on core business activities, and economic and competitive pressures are other factors that are contributing to the growth of the market.
The pharmaceutical and regulatory agencies joined forces to rapidly develop vaccines and medical products required to fight against the COVID-19 infection. The regulatory authorities take numerous precautions to ensure patient and personnel safety during a clinical trial, as well as data integrity and good laboratory practices are maintained. Growth in markets for biosimilars, orphan drugs, personalized medicines, companion diagnostics, and adaptive trial designs is projected to boost the demand for regulatory specialization in these areas. As companies venture into newer fields, the growing need to comply with regulations is boosting the demand for specialized service providers with expertise in regulatory affairs. Patent expiration of biologics, such as Simulect, Vectibix, Mircera, and Kineret, is increasing the demand and development of biosimilars, thereby contributing to the demand for regulatory services.
Several companies are actively involved in collaborations and new product development to gain leadership in the personalized medicine market, indicating a need for supportive regulatory affairs. For instance, in May 2020, Regeneron Pharmaceuticals, Inc. collaborated with Colorado Center for Personalized Medicine to design advancements in personalized medicine and human genetics.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/regulatory-affairs-market
Regulatory Affairs Market Report Highlights
The outsourcing segment dominated the market with a share of 58.16% in 2023. The segment's growth is mainly due to the growing focus of pharmaceutical and medical device companies on outsourcing their activities, which allows them to access specialized expertise and resources they may not have in-house.
The regulatory writing & publishing segment dominated the market, accounting for 36.41% of the global revenue in 2023.
The medical devices segment dominated the market with a share of 41.05% in 2023. The segment's growth can be attributed to factors such as constant technological advancements and the increasing focus of market players on outsourcing medical device activities so they can focus more on their core competencies.
The oncology segment dominated the market with a share of 32.77% in 2023. The growth of the segment is mainly due to the increasing prevalence of cancer, which requires effective and safe treatment options.
Asia Pacific dominated the market and accounted for a 37.82% share in 2023. The growth in the region is attributed to factors such as an enhanced regulatory landscape, an increasing number of clinical trials, a growing number of pharmaceutical companies, and the availability of skilled workers in the region.
Regulatory Affairs Market Segmentation
Grand View Research has segmented the global regulatory affairs market on the basis of services, categories, indications, stages, types, company size, End Use, and region:
Regulatory Affairs Type Outlook (Revenue, USD Billion, 2018 - 2030)
In-house
Outsourced
Regulatory Affairs Services Outlook (Revenue, USD Billion, 2018 - 2030)
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Other Services
Regulatory Affairs Categories Outlook (Revenue, USD Billion, 2018 - 2030)
Drugs
Biologics
Medical Devices
Regulatory Affairs Indication Outlook (Revenue, USD Billion, 2018 - 2030)
Oncology
Neurology
Cardiology
Immunology
Others
Regulatory Affairs Product Stage Outlook (Revenue, USD Billion, 2018 - 2030)
Preclinical
Clinical studies
PMA
Regulatory Affairs Company Size Outlook (Revenue, USD Billion, 2018 - 2030)
Small
Medium
Large
Regulatory Affairs End Use Outlook (Revenue, USD Billion, 2018 - 2030)
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
Regulatory Affairs Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
Europe
Asia Pacific
Latin America
MEA
List of Key Players of Regulatory Affairs Market
Accell Clinical Research, LLC
Genpact
Criterium, Inc.
ICON plc
iuvo BioScience, LLC.
WuXi AppTec
Medpace
Charles River Laboratories.
Laboratory Corporation of America Holdings
Parexel International (MA) Corporation
Freyr
AmerisourceBergen
NDA Group AB
Pharmexon
Qvigilance
BlueReg
Cambridge Regulatory Services
VCLS
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/regulatory-affairs-market
#Regulatory Affairs Market#Regulatory Affairs Market Size#Regulatory Affairs Market Share#Regulatory Affairs Market Trends
0 notes
Link
0 notes
Text
Life Sciences BPO Market: Key Trends and Growth Drivers
The global life sciences BPO market size is expected to reach USD 827.5 billion by 2030, registering a CAGR of 9.3% over the forecast years, according to a new report by Grand View Research, Inc. The rising costs of drug development; pre-clinical and clinical trials; and post-marketing surveillance; a rise in the patent cliff; a growing product pipeline; and regulatory constraints are some of the major factors driving the market. The pharmaceutical and biotech industries invest heavily in the R&D sector to continuously introduce new molecules, devices, and treatments. The different stages of drug development, such as drug discovery, pre-clinical studies, and clinical trials, require huge financial, technological, and human resources.
To cater to the growing needs of the industry, the outsourcing vendors are expanding their product and service offerings and they are targeting niche areas for long-term growth and profitability. Altogether, these trends are expected to boost the life sciences outsourcing market over the forecast period. During the COVID-19 pandemic, a significant number of outsourcing providers expanded their existing manufacturing and research facilities to meet the growing demand for COVID-19 vaccines and diagnostics. Even post-pandemic, CDMOs are practicing similar strategies to support the future demand for pharmaceuticals. For instance, in April 2022, Aenova Group developed a new facility for manufacturing highly potent drugs with an investment of EUR 10 million.
The adoption of such strategies by the market players is expected to be profitable for the market. There has been a rising demand to reduce the cost of manufacturing and development of drugs and medical devices. Outsourcing manufacturing, research, and marketing services provide pharmaceutical and medical device companies with cost- and time-saving benefits. This is expected to have a positive impact on the market. Over the years, mergers and acquisition deals between CROs and CDMOs have increased. The rising demand for clinical services and the growing need for specialized service providers to improve the focus on their core competencies are some of the factors that are expected to drive the incidence of M&A deals.
Gather more insights about the market drivers, restrains and growth of the Life Sciences BPO Market
Life Sciences BPOMarket Report Highlights
• The increasing number of M&A transactions has broadened the global reach and improved the capabilities of CROs and CDMOs to provide end-to-end services; a continuation of this trend is expected to benefit the market significantly
• COVID-19 incidence has decreased significantly as a result of a growing global vaccination campaign
• Owing to this, the CRO and CDMO are now refocusing on developing drugs for oncology and other diseases owing to their high burden
• For instance, in April 2022, Labcorp collaborated with Xcell Biosciences to support the company in developing cell and gene therapies for treating cancer, Parkinson’s, and other rare diseases
• Such initiatives by the CDMOs are likely to profit the market owing to the high effectiveness of gene therapy in treating cancer and other rare diseases
• The medical device segment is expected to register the fastest CAGR from 2023 to 2030 due to the complexities associated with medical device designing
• The strict regulatory framework for medical device approval globally has further contributed to the demand for medical device outsourcing services
• Asia Pacific held the largest revenue share in 2022 due to the presence of a significant number of CROs providing cost-effective BPO services
Life Sciences BPO Market Segmentation
Grand View Research has segmented the global life sciences BPO market based on service and region:
Life Sciences BPO Services Outlook (Revenue, USD Billion, 2018 - 2030)
• Pharmaceutical outsourcing
o Contract Manufacturing Market
o API
o Finished Dose Form
o Packaging
o Contract Research Organizations
o Drug Discovery
o Pre-clinical Studies
o Clinical Trial Studies
o Regulatory Services
o Pharmacovigilance
• Medical Devices Outsourcing
o Contract Manufacturing Market
o Electronic Manufacturing Services
o Finished Goods
o Raw Materials/ Components
o Contract Research Organizations
o Regulatory Consulting Services
o Product Design and Development Services
o Product Testing Services
o Product Implementation Services
o Product Upgrade Services
o Product Maintenance Services
• Contract sales and marketing outsourcing
• Others
Life Sciences BPO Regional Outlook (Revenue, USD Billion, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o Japan
o China
o India
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Life Sciences BPO Market Intelligence Study, published by Grand View Research.
#Life Sciences BPO Market#Life Sciences BPO Market Size#Life Sciences BPO Market Share#Life Sciences BPO Market Analysis#Life Sciences BPO Market Growth
0 notes
Link
0 notes
Text
Medical Device Testing Outsourcing: $3.5B in 2023 to $7.2B by 2033 (7.5% CAGR)
Medical Device Analytical Testing Outsourcing Market offers specialized services provided by third-party organizations to rigorously test and analyze medical devices. These services ensure compliance with regulatory standards, enhance product safety and efficacy, and support manufacturers in accelerating time-to-market while reducing operational costs.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS25883 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers
The market is experiencing robust growth, driven by:
Increasing Regulatory Scrutiny: Rising emphasis on product safety and efficacy has amplified the need for comprehensive testing.
Cost-Efficiency: Outsourcing helps manufacturers optimize resources and focus on core competencies.
Technological Advancements: The growing complexity of medical devices necessitates specialized and advanced testing capabilities.
Market Insights
The chemical characterization segment leads the market, accounting for 38% of the share in 2023, driven by stringent safety standards and the necessity for thorough material analysis.
Microbiology and sterility testing follow closely with a 32% share, underscoring the importance of ensuring device sterility and patient safety.
Biocompatibility testing holds a 30% share, reflecting the demand for evaluating device compatibility with biological systems.
In 2023, the global market recorded approximately 1.2 billion tests conducted, showcasing the growing reliance on outsourced testing services.
Regional Highlights
North America dominates the market due to advanced healthcare infrastructure and the high concentration of medical device manufacturers.
Europe follows, supported by a robust regulatory framework and a rising demand for innovative medical technologies.
The United States and Germany stand out as leading contributors, reflecting significant demand for outsourced testing services in these regions.
Market Segmentation
By Type: Chemical Testing, Physical Testing, Microbiological Testing, Biocompatibility Testing, Stability Testing, Electromagnetic Compatibility Testing, Packaging Testing By Product: Instruments, Reagents, Consumables By Services: Method Development, Method Validation, Extractables and Leachables Testing, Material Characterization, Batch Release Testing, Product Life Cycle Testing By Technology: Chromatography, Spectroscopy, Polymerase Chain Reaction (PCR), Flow Cytometry, Mass Spectrometry, Electrophoresis, Microscopy By Component: Sensors, Transducers, Microfluidics By Application: Cardiology Devices, Orthopedic Devices, In Vitro Diagnostic Devices, Ophthalmic Devices, Dental Devices, Drug Delivery Devices By Device: Wearable Devices, Portable Devices, Stationary Devices By Process: Preclinical Testing, Clinical Testing, Post-Market Surveillance By End User: Medical Device Manufacturers, Research Laboratories, Academic Institutions
Leading Market Players
Key players such as Eurofins Scientific, SGS SA, and Charles River Laboratories drive the market with their advanced technologies and robust testing capabilities, maintaining a competitive edge in this dynamic industry.
#MedicalDevices #AnalyticalTesting #OutsourcingServices #MedicalInnovation #HealthcareSafety #RegulatoryCompliance #BiocompatibilityTesting #ChemicalCharacterization #SterilityTesting #AdvancedTechnologies #GlobalHealthcare #PatientSafety #DeviceTesting #ResearchAndDevelopment #QualityAssurance
The Medical Device Analytical Testing Outsourcing Market is set to expand further as advancements in medical device technologies and stringent regulatory requirements fuel the demand for precise, specialized testing services.
0 notes
Text
Regulatory Affairs Outsourcing Market Size, Share, Trends, Opportunities, Key Drivers and Growth Prospectus
"Regulatory Affairs Outsourcing Market – Industry Trends and Forecast to 2030
Global Regulatory Affairs Outsourcing Market, By Services Outlook (Regulatory Consulting, Legal Representation, Regulatory Writing and Publishing, Product Registration, Clinical Trial Applications and Other Services), Size (Small, Medium and Large), Category (Drugs, Generics, Innovators, Biologics, Biotech, ATMPs, Medical devices, Therapeutic and Diagnostic), Indication (Oncology, Neurology, Cardiology, Immunology and Others), Stage (Preclinical, Clinical and PMA (Post Market Authorization), End-User (Medical Device Companies, Pharmaceutical Companies and Biotechnology Companies) – Industry Trends and Forecast to 2030.
Access Full 350 Pages PDF Report @
### Segments
- **Service Type** - Regulatory Writing and Publishing - Regulatory Consulting - Legal Representation - Clinical Trial Applications - **End-User** - Pharmaceutical Industry - Medical Device Industry - Biotechnology Industry - **Region** - North America - Europe - Asia-Pacific - Latin America - Middle East and Africa
Regulatory affairs outsourcing is segmented based on service type, end-user, and region. Service types include regulatory writing and publishing, regulatory consulting, legal representation, and clinical trial applications. End-users primarily consist of the pharmaceutical, medical device, and biotechnology industries. Geographically, the market is analyzed across regions such as North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
### Market Players
- **IQVIA** - **Charles River** - **Covance** - **Parexel** - **ICON** - **Kinapse** - **PRA Health Sciences** - **Intertek** - **WuXi AppTec** - **Medpace**
Key market players in the regulatory affairs outsourcing market include IQVIA, Charles River, Covance, Parexel, ICON, Kinapse, PRA Health Sciences, Intertek, WuXi AppTec, and Medpace. These companies offer a range of services in regulatory affairs outsourcing, catering to the diverse needs of the pharmaceutical, medical device, and biotechnology industries.
https://www.databridgemarketresearch.com/reports/global-regulatory-affairs-outsourcing-marketRegulatory affairs outsourcing is a crucial aspect of the pharmaceutical, medical device, and biotechnology industries, ensuring compliance with regulations and smooth approval processes for products. The service types offered by market players cater to specific needs within this niche sector. Regulatory writing and publishing services assist in creating documents required for submissions to regulatory authorities, while regulatory consulting provides strategic guidance on compliance matters. Legal representation services offer support in legal aspects of regulatory affairs, ensuring that companies navigate complex regulations effectively. Clinical trial applications are a vital service for organizations seeking approval for new drugs or medical devices, helping them adhere to regulatory guidelines throughout the trial process.
The end-users of regulatory affairs outsourcing services play a significant role in driving the market's growth and demand for specialized services. The pharmaceutical industry, characterized by stringent regulatory requirements and the need for timely approvals, relies heavily on outsourcing partners to streamline regulatory processes. Similarly, the medical device industry, with its focus on safety and efficacy standards, benefits from regulatory consulting and legal representation services that ensure adherence to industry regulations. The biotechnology sector, known for innovation and rapid development cycles, utilizes outsourcing services to navigate complex regulatory landscapes and accelerate product launches.
Geographically, the regulatory affairs outsourcing market is diversified across regions such as North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. Each region presents unique opportunities and challenges for market players, influenced by factors such as regulatory frameworks, market maturity, and technological advancements. North America, with its well-established pharmaceutical and biotechnology industries, represents a significant market for outsourcing services, driven by the need for compliance with FDA regulations. Europe, known for its stringent regulatory environment, offers opportunities for companies specializing in regulatory consulting and legal representation. The Asia-Pacific region, characterized by a growing pharmaceutical market and evolving regulatory landscape, presents opportunities for regulatory writing and publishing services.
Key market players in the regulatory affairs outsourcing sector, such as IQVIA, Charles River, Covance, and Parexel, play a pivotal role in shaping the industry dynamics**Global Regulatory Affairs Outsourcing Market, By Services Outlook**
- Regulatory Consulting - Legal Representation - Regulatory Writing and Publishing - Product Registration - Clinical Trial Applications - Other Services
The global regulatory affairs outsourcing market is a vital component of the pharmaceutical, medical device, and biotechnology industries, ensuring compliance with regulatory standards and facilitating the approval process for products. Market players offer a range of services catering to specific needs within this niche sector. Regulatory writing and publishing services aid in creating essential documents for submissions to regulatory authorities, while regulatory consulting provides strategic guidance on compliance matters. Legal representation services offer support in navigating the legal aspects of regulatory affairs effectively. Clinical trial applications are crucial for organizations seeking approval for new drugs or medical devices, assisting them in adhering to regulatory guidelines throughout the trial process.
**End-User Analysis**
The end-users of regulatory affairs outsourcing services, including the pharmaceutical, medical device, and biotechnology industries, are key drivers in the market's growth. The pharmaceutical sector, with its strict regulatory requirements and the imperative for timely approvals, heavily relies on outsourcing partners to streamline regulatory processes. Similarly, the medical device industry, focusing on safety and efficacy standards, benefits from regulatory consulting and legal representation services ensuring compliance with industry regulations. The biotechnology field, characterized by innovation and rapid development cycles, utilizes outsourcing services to navigate intricate regulatory frameworks and accelerate product launches efficiently.
**Regional Insights**
Geographically, the regulatory affairs outsourcing market is segmented across regions such as North America, Europe,
The Regulatory Affairs Outsourcing market research report displays a comprehensive study on production capacity, consumption, import and export for all the major regions across the globe. The target audience considered for this market study mainly consists of Key consulting companies & advisors, Large, medium, and small-sized enterprises, Venture capitalists, Value-added resellers (VARs), Third-party knowledge providers, Investment bankers, and Investors. This global market analysis report is the believable source for gaining the market research that will exponentially accelerate the business growth. The top notch Regulatory Affairs Outsourcing market report is the best option to acquire a professional in-depth study on the current state for the market.
Table of Contents: Regulatory Affairs Outsourcing Market
1 Introduction
2 Global Regulatory Affairs Outsourcing Market Segmentation
3 Executive Summary
4 Premium Insight
5 Market Overview
6 Regulatory Affairs Outsourcing Market, by Product Type
7 Regulatory Affairs Outsourcing Market, by Modality
8 Regulatory Affairs Outsourcing Market, by Type
9 Regulatory Affairs Outsourcing Market, by Mode
10 Regulatory Affairs Outsourcing Market, by End User
12 Regulatory Affairs Outsourcing Market, by Geography
12 Regulatory Affairs Outsourcing Market, Company Landscape
13 Swot Analysis
14 Company Profiles
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Recreational Cannabis Market Paediatric Gliomas Drugs Market Zinc Glycinates Market Restriction Endonucleases Products Market Food Grade Gases In Meat And Seafood Application Market Temperature Smart Roads Market Automotive Airbag Silicone Market Non Networked Sound Masking System Market Nitrile Butadiene Rubber Br Market Big Data As a Service Bdaas Market Robotically Assisted Surgical Devices Market Transactional Video Demand Market Rice Transplanter Market Bio Based Polyethylene Terephthalate Pet Packaging Market Bath Mats Market Synthetic Iron Oxide Pigments Market Polyvalent Anti Venom Market Managed Siem And Log Management Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
Empowering Device Innovation: The Key Role of Medical Device CROs in R&D
The global medical device contract research organization market size is expected to reach USD 14.0 billion by 2030, growing at a CAGR of 8.95% from 2024 to 2030, according to a new report by Grand View Research, Inc. The key market growth drivers include rising demand for time-saving, cost efficient, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a CRO with detailed expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis. This further supports the demand for medical device CRO services.
CROs also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to make the process fast and maintain quality. A significant number of CROs such as Iqvia, Inc., ICON Plc, Charles River, and others offering high-quality medical device-related research services are expected to improve medical device-related research collaborations in the coming years and thus boost the market growth.
Medical procedures are moving toward more laparoscopic and catheter-based technology. There has been a growing demand for minimally invasive procedures worldwide, as they are less risky compared to other surgical procedures. This is expected to boost the demand for advanced devices for screening and monitoring surgical procedures. Minimally invasive procedures are also enhancing the demand for new miniature technology. This is expected to improve the number of research in advanced less invasive medical devices and hence promote market growth.
Thus, growing complexities in medical devices, changing consumer preferences, and increasing demand for portable devices are among the factors expected to drive the need for an expert team for the development of medical devices, thereby increasing the demand for CROs in the market.
Medical Device Contract Research Organization Market Report Highlights
The clinical phase segment dominated the market in 2023. This can be attributed to the development of novel medical devices, which involves numerous clinical trials. Hence, medical device companies prefer appointing a CRO to provide clinical trial services with the required infrastructure, staff, and expertise or opening a separate laboratory
Based on service, clinical monitoring held the largest market share of 21.0% in 2023. Clinical trialsneed comprehensive monitoring for their success. Therefore, companies outsource clinical monitoring functions to CROs with staff expertise and updated training programs. These CRO conduct monitoring activities in accordance with a sponsor’s study protocol, standard operating procedures, FDA regulations, and Good Clinical Practice (GCP)
Based on device type, the diagnostic devices segment held a revenue share of 44.9% in 2023. The growing prevalence of diseases worldwide is supporting the demand for CRO activities for diagnostic devices. Besides, increasing prevalence of cardiovascular diseases, cancer, among other diseases drives the market growth
Asia Pacific dominated the market with a revenue share of 41.9% in 2023. The market’s strong growth is due to various factors such as improvements in the regulatory framework, higher cost savings, increasing complexity in devices, and a growing number of medical device research organizations in the region
Medical Device Contract Research Organization Market Segmentation
Grand View Research has segmented the global medical device contract research organization market based on phase, service, device type, and region:
Medical Device Contract Research Organization Phase Outlook (Revenue, USD Million, 2018 - 2030)
Preclinical
Clinical
Medical Device Contract Research Organization Service Outlook (Revenue, USD Million, 2018 - 2030)
Project Management/Clinical Supply Management
Data Management
Regulatory/Medical Affairs
Medical Writing
Clinical Monitoring
Quality Management/Assurance
Bio-statistics
Investigator Payments
Laboratory
Patient & Site Recruitment
Technology
Others
Medical Device Contract Research Organization Device Type Outlook (Revenue, USD Million, 2018 - 2030)
MedTech Devices
Diagnostic Devices
Handheld Devices
Others
Medical Device Contract Research Organization Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players
IQVIA, Inc.
Charles River Laboratories
ICON, plc
Syneos Health
Laboratory Corporation of America Holdings
WuXi AppTec
Medpace
Eurofins Scientific SE
Promedica International
Qserve
Order a free sample PDF of the Medical Device Contract Research Organization Market Intelligence Study, published by Grand View Research.
0 notes
Text
Electronic Design Automation Software Market Size And Share Report, 2030
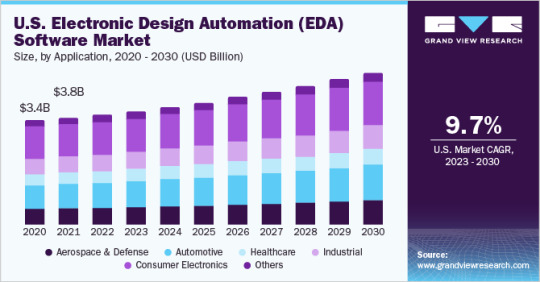
Electronic Design Automation Software Market Growth & Trends
The global electronic design automation software market size is anticipated to reach USD 22.21 billion by 2030, expanding at a CAGR of 9.1% from 2023 to 2030, according to a new study by Grand View Research Inc. The growing usage of advanced electronic components across different areas of healthcare, automotive, and consumer electronic sectors is driving Electronic Design Automation (EDA) software market growth. Apart from this, the proliferation of the Internet of Things (IoT) and connected devices further promotes EDA software market advancement.
Miniaturization of devices and chips is expected to create lucrative opportunities for EDA software. This software assists chipmakers in lowering the errors in Integrated Circuit (IC) and chips, thereby driving the electronic design automation software industry growth. The exponential growth of the integrated circuits market is driving the need for electronic design automation software. The software is increasingly being adopted for designing electronic systems such as printed circuit boards and integrated circuits, supporting the growth of the EDA market.
Furthermore, developments in technology, growing digitization, growth of the electronics manufacturing industry, and increasing adoption of advanced rendering & simulation tools by hardware designers are expected to positively influence the electronic design automation software industry growth over the forecast period. Furthermore, the increasing acceptance and awareness of smart fitness devices are factors enabling electronic design companies to invest in innovative design software tools for designing enhanced & compact products, thereby propelling the EDA software market growth over the forecast period.
Growing demand for EDA software is encouraging various market players to adopt various business strategies to achieve higher profitability in the market for electronic design automation software. Market players are unveiling new solutions by partnering with technology providers to improve their brand identity, propelling the EDA software market growth. For instance, in January 2022, Altium LLC announced a partnership with MacroFab, Inc., a U.S.-based cloud platform provider for electronic production, to launch “Altimade.” The new solution combines elastic manufacturing capabilities and real-time supply chain data, available on the Altium 365 electronic design platform. Altimade empowers customers to request instant quotes and place orders for manufacturing their PCB assembly without exiting the design environment.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/electronic-design-automation-eda-software-market
Electronic Design Automation Software Market Report Highlights
The services segment is expected to register a CAGR of 10.6% from 2023 to 2030. The segment growth can be attributed to the increasing proclivity of OEMs to outsource their design needs. Furthermore, electronic circuit boards are in high demand in the industry due to their increasing importance in a variety of vital electronic products such as mobile phones and tablets
The cloud segment is expected to register a CAGR of 10.8% from 2023 to 2030. Cloud-based deployment allows for faster distribution, less maintenance, lower costs, and more scalability
The healthcare segment is expected to register a CAGR of 11.5% from 2023 to 2030. The segment growth can be attributed to the benefits of EDA software in healthcare, such as lower costs, improved patient outcomes, faster design & development, enabled regulatory compliance of medical devices, and simulation and testing of medical equipment
The microprocessors & controllers segment is expected to register a CAGR of 9.5% from 2023 to 2030. The increased use of microprocessors in consumer devices such as smartphones, personal computers (PCs), and laptops is expected to drive global demand for the segment
Asia Pacific is anticipated to emerge as the fastest-growing region over the forecast period at a CAGR of 9.6%. A surge in demand for electronic devices together with the prevalence of key electronic component manufacturers in Greater China is expected to drive regional growth
Electronic Design Automation Software Market Segmentation
Grand View Research has segmented the global electronic design automation software market based on product, deployment, application, end-use, and region:
Electronic Design Automation (EDA) Software Product Outlook (Revenue, USD Billion, 2018 - 2030)
Computer-aided Engineering (CAE)
IC Physical Design and Verification
Printed Circuit Board and Multi-chip Module (PCB and MCM)
Semiconductor Intellectual Property (SIP)
Services
Electronic Design Automation (EDA) Software Deployment Outlook (Revenue, USD Billion, 2018 - 2030)
Cloud
On-premise
Electronic Design Automation (EDA) Software Application Outlook (Revenue, USD Billion, 2018 - 2030)
Aerospace and Defense
Automotive
Healthcare
Industrial
Consumer Electronics
Others
Electronic Design Automation (EDA) Software End-use Outlook (Revenue, USD Billion, 2018 - 2030)
Microprocessors & Controllers
Memory Management Unit (MMU)
Others
Electronic Design Automation (EDA) Software Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
List of Key Players in the Electronic Design Automation Software Market
Advanced Micro Devices, Inc.
Aldec, Inc.
Altair Engineering Inc.
Altium LLC
Autodesk, Inc.
ANSYS, Inc.
Cadence Design Systems, Inc.
eInfochips
EMA Design Automation, Inc.
Keysight Technologies
Microsemi
Synopsys, Inc.
Silvaco, Inc.
The MathWorks, Inc.
Vennsa Technologies
Zuken
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/electronic-design-automation-eda-software-market
#Electronic Design Automation Software Market#Electronic Design Automation Software Market Size#Electronic Design Automation Software Market Share
0 notes
Text
Medical Affairs Outsourcing Market Size, Share And Trends Analysis Report
The global medical affairs outsourcing market size is expected to reach USD 4.6 billion registering a CAGR of 13.6% from 2023 to 2030 according to a new report by Grand View Research, Inc. Transformation in the medical education and the rising number of orphan therapies for treatment of rare diseases are some of the key factors propelling the market growth. The pharmaceutical companies are looking for assistance to systematically position the therapeutic outcomes of these therapies in the market. During the year 2021, the Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) approved 50 new molecular entities, of which more than half (26) were orphan drugs. A growing number of orphan drug approvals is expected to drive the global market for medical affairs outsourcing over the forecast period.
Changes in the reimbursement scenario and pricing pressure are expected to increase the adoption of cost-saving measures by the pharmaceutical and medical device companies. This is anticipated to promote off-shoring of medical affairs outsourcing in countries like India and China. Pharmaceutical companies are outsourcing medical affairs services to get high-quality documents, control the R&D cost, and reduce investment costs required to train the medical affairs team. Furthermore, pharmaceutical and medical device companies are outsourcing their medical affairs services to diverge their business activities and manage product life cycles.
In addition, Contract Research Organizations (CROs) offer cost-efficient solutions and compliance with the health authority requirements, thereby propelling market growth. The bio/pharmaceutical outsourcing industry has seen a rise in R&D demand because of the COVID-19 pandemic. This increase in demand, as well as other impacts from the pandemic, such as rising demand for clinical trials and its outsourcing services, have caused some CROs to shift focus and adjust their operations. The pandemic has pushed more pharmaceutical companies and regulators to use new technologies for remote quality audits and inspections, thus driving positive changes in the pharma industry. Also, digitalization has for clinical trials that requires interactions with patient groups, and role in specialty care are expected to become integral to the medical affairs function and reduce the impact of pandemic.
Gather more insights about the market drivers, restrains and growth of the Medical Affairs Outsourcing Market
Medical Affairs Outsourcing market Report Highlights
• The medical writing & publishing segment led the market in 2022 and is expected to witness significant growth over the forecast period, owing to the increasing need for marketable written content to convey medical information to healthcare professionals and patients.
• North America dominated the global market with a share of 34.9% in 2022 owing to the presence of a large number of pharmaceutical and medical devices companies in the region.
• Moreover, the high cost of medical affairs services is a major challenge, which has encouraged various medical device companies in North America to outsource functions to third-party vendors in off-shore locations with a high level of expertise in the domain.
• However, Asia Pacific is anticipated to grow at the fastest CAGR over the forecast period, owing to cost-efficient service offering by the CROs in the region. In addition, improved regulatory framework and availability of a skilled workforce in APAC boosts the market growth.
Medical Affairs Outsourcing Market Segmentation
Grand View Research has segmented the global medical affairs outsourcing market report based on services, industry, and region
Medical Affairs Outsourcing Services Outlook (Revenue, USD Million, 2018 - 2030)
• Medical Writing & Publishing
• Medical Monitoring
• Medical Science Liaisons (MSLs)
• Medical Information
• Others
Medical Affairs Outsourcing Industry Outlook (Revenue, USD Million, 2018 - 2030)
• Pharmaceutical
• Biopharmaceutical
• Medical Devices
o Therapeutic Medical Devices
o Diagnostic Medical Devices
Medical Affairs Outsourcing Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o U.K.
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
o Rest of EU
• Asia Pacific
o Japan
o China
o India
o Australia
o Thailand
o South Korea
o Rest of APAC
• Latin America
o Brazil
o Mexico
o Argentina
o Rest of LATAM
• Middle East & Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
o Rest of MEA
Order a free sample PDF of the Medical Affairs Outsourcing Market Intelligence Study, published by Grand View Research.
#Medical Affairs Outsourcing Market#Medical Affairs Outsourcing Market Size#Medical Affairs Outsourcing Market Share#Medical Affairs Outsourcing Market Analysis#Medical Affairs Outsourcing Market Growth
0 notes
Text
Introduction to Veterinary Endoscopy: A Modern Approach to Animal Diagnostics
The global medical device outsourced manufacturing market size was USD 33.38 Billion in 2022 and is expected to register a revenue CAGR of 13.6% during the forecast period, according to the latest analysis by Emergen Research. Rising prevalence of chronic diseases such as diabetes and cardiovascular disease, rapid technological advancements in the product development of medical devices, and increased funding and investments toward early-stage medical technology-based startup companies are some of the key factors driving revenue growth of the medical device outsourced manufacturing market.
The global Medical Device Outsourced Manufacturing market report is a methodical research of the Medical Device Outsourced Manufacturing market done by extensive primary and secondary research. The fundamental purpose of the Medical Device Outsourced Manufacturing market report is to offer an accurate and strategic analysis of the Medical Device Outsourced Manufacturing business sphere.
Get Download Pdf Sample Copy of this Report@ https://www.emergenresearch.com/request-sample/1760
Competitive Terrain:
The global Medical Device Outsourced Manufacturing industry is highly consolidated owing to the presence of renowned companies operating across several international and local segments of the market. These players dominate the industry in terms of their strong geographical reach and a large number of production facilities. The companies are intensely competitive against one another and excel in their individual technological capabilities, as well as product development, innovation, and product pricing strategies.
The leading market contenders listed in the report are:
Eakin Surgical., IQVIA., SGS SOCIÉTÉ GÉNÉRALE DE SURVEILLANCE SA., Eurofins Scientific, Bio-Rad Laboratories, Inc., Intertek Group plc, Charles River Laboratories, WuXi AppTec, Nipro Corporation, and Johari Digital
Key market aspects studied in the report:
Market Scope: The report explains the scope of various commercial possibilities in the global Medical Device Outsourced Manufacturing market over the upcoming years. The estimated revenue build-up over the forecast years has been included in the report. The report analyzes the key market segments and sub-segments and provides deep insights into the market to assist readers with the formulation of lucrative strategies for business expansion.
Competitive Outlook: The leading companies operating in the Medical Device Outsourced Manufacturing market have been enumerated in this report. This section of the report lays emphasis on the geographical reach and production facilities of these companies. To get ahead of their rivals, the leading players are focusing more on offering products at competitive prices, according to our analysts.
Report Objective: The primary objective of this report is to provide the manufacturers, distributors, suppliers, and buyers engaged in this sector with access to a deeper and improved understanding of the global Medical Device Outsourced Manufacturing market.
Emergen Research is Offering Limited Time Discount (Grab a Copy at Discounted Price Now)@ https://www.emergenresearch.com/request-discount/1760
Market Segmentations of the Medical Device Outsourced Manufacturing Market
This market is segmented based on Types, Applications, and Regions. The growth of each segment provides accurate forecasts related to production and sales by Types and Applications, in terms of volume and value for the period between 2022 and 2030. This analysis can help readers looking to expand their business by targeting emerging and niche markets. Market share data is given on both global and regional levels. Regions covered in the report are North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Research analysts assess the market positions of the leading competitors and provide competitive analysis for each company. For this study, this report segments the global Medical Device Outsourced Manufacturing market on the basis of product, application, and region:
Segments Covered in this report are:
Device Type Outlook (Revenue, USD Billion; 2019-2032)
IVD Devices
Drug Delivery Devices
Diabetes Care Devices
Cardiovascular Devices
Diagnostic Imaging Devices
Surgical Devices
Others
Service Type Outlook (Revenue, USD Billion; 2019-2032)
Prototyping Development services
Pilot Production services
Device manufacturing services
Injection molding
CNC Machining and Laser Cutting & 3D Printing
Cleaning and Finishing
Others
Quality Management Services
Packaging Validation services
Inspection and Testing Services
Sterilization services
Others
Class of Medical Device Outlook (Revenue, USD Billion; 2019-2032)
Class I
Class II
Class III
Browse Full Report Description + Research Methodology + Table of Content + Infographics@ https://www.emergenresearch.com/industry-report/medical-device-outsourced-manufacturing-market
Major Geographies Analyzed in the Report:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
ToC of the report:
Chapter 1: Market overview and scope
Chapter 2: Market outlook
Chapter 3: Impact analysis of COVID-19 pandemic
Chapter 4: Competitive Landscape
Chapter 5: Drivers, Constraints, Opportunities, Limitations
Chapter 6: Key manufacturers of the industry
Chapter 7: Regional analysis
Chapter 8: Market segmentation based on type applications
Chapter 9: Current and Future Trends
Request Customization as per your specific requirement@ https://www.emergenresearch.com/request-for-customization/1760
About Us:
Emergen Research is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyse consumer behavior shifts across demographics, across industries, and help clients make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Emergen Research has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
Contact Us:
Eric Lee
Corporate Sales Specialist
Emergen Research | Web: www.emergenresearch.com
Direct Line: +1 (604) 757-9756
E-mail: [email protected]
Visit for More Insights: https://www.emergenresearch.com/insights
Explore Our Custom Intelligence services | Growth Consulting Services
Trending Titles: Geocell Market | Pancreatic Cancer Treatment Market
Latest Report: Ceramic Tiles Market | Life Science Analytics Market
0 notes
Text
Small Molecule Innovator CDMO Market - The Biggest Trends to watch out for 2024-2030
Small Molecule Innovator CDMO Industry Overview
The global small molecule innovator CDMO market size was estimated at USD 48.6 billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 6.21% from 2024 to 2030. Key drivers for this growth are increasing pharmaceutical R&D investment, growing demand for small molecules, and rising incidence of cancer & age-related disorders. Biological drugs are more expensive than small molecules. Hence, growing demand for cost-effective drugs is expected to further support market growth.
The COVID-19 pandemic significantly impacted on global economy in 2020 and caused an ongoing impact on various industries. However, the market for contract development and manufacturing organization (CDMO) witnessed a positive impact due to this pandemic. CDMOs played an important role in meeting the needs of pharmaceutical companies, biotech companies, and other end-users during this crisis. Overall, pandemic boosted market demand for small molecule innovator drugs. With the growing demand for outsourcing by pharma companies, heightened demand is observed in post-pandemic scenario.
Gather more insights about the market drivers, restrains and growth of the Small Molecule Innovator CDMO Market
Small Molecule Innovator CDMO Market Segmentation
Grand View Research has segmented the global small molecule innovator CDMO market based on product, stage type, customer type, therapeutic area, and region:
Small Molecule Innovator CDMO Product Outlook (Revenue, USD Million, 2018 - 2030)
Small Molecule API
Small Molecule Drug Product
Oral solid dose
Semi-Solid Dose
Liquid Dose
Others
Small Molecule Innovator CDMO Stage Type Outlook (Revenue, USD Million, 2018 - 2030)
Preclinical
Clinical
Phase I
Small
Medium
Large
Phase II
Small
Medium
Large
Phase III
Small
Medium
Large
Commercial
Small Molecule Innovator CDMO Customer Type Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceutical
Small
Medium
Large
Biotechnology
Small Molecule Innovator CDMO Therapeutic Area Outlook (Revenue, USD Million, 2018 - 2030)
Cardiovascular disease
Oncology
Respiratory disorders
Neurology
Metabolic disorders
Infectious disease
Others
Browse through Grand View Research's Medical Devices Industry Research Reports.
The global intrauterine devices marketsize was estimated at USD 6.25 billion in 2023 and is projected to grow at a CAGR of 3.66% from 2024 to 2030.
The global dual chamber prefilled syringes marketsize was valued at USD 167.3 million in 2023 and is projected to grow at a CAGR of 5.8% from 2024 to 2030.
Key Companies & Market Share Insights
Companies are undertaking various strategic initiatives to gain a competitive advantage. Key parameters affecting the competitive nature of the market include acquisition, geographic expansion, mergers, acquisitions, and product launches.
In September 2022, WuXi STA inaugurated a new sterile lipid nanoparticle (LNP) formulation development and manufacturing facility at its Wuxi city campus. The integrated drug product platform CRDMO provides a full range of services, including solid-state development, pre-formulation, and clinical to commercial drug product manufacturing.
In June 2022, Lonza inaugurated a new clinical phase development and manufacturing facility in its small molecules site in Bend, Oregon. It is dedicated to manufacturing bioavailability-enhancing spray-dried dispersion (SDD) finished dosage forms and drug product intermediates
List of Key Players of Small Molecule Innovator CDMO Market
Piramal Pharma Solutions
CordenPharma International
Wuxi AppTec
Cambrex Corporation
Recipharm AB
Pantheon (Thermo Fisher Scientific)
Lonza
Catalent Inc.
Siegfried Holding AG
Boehringer Ingelheim
Labcorp Drug Development
Order a free sample PDF of the Small Molecule Innovator CDMO Market Intelligence Study, published by Grand View Research.
0 notes
Text
4K UST Projectors Market 2024: Emerging Trends, Major Driving Factors, Business Growth Opportunities
4K UST Projectors Market provides in-depth analysis of the market state of 4K UST Projectors manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of 4K UST Projectors in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of 4K UST Projectors Market Report:
The report offers a comprehensive and broad perspective on the global 4K UST Projectors Market.
The market statistics represented in different 4K UST Projectors segments offers complete industry picture.
Market growth drivers, challenges affecting the development of 4K UST Projectors are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of 4K UST Projectors.
Major stakeholders, key companies 4K UST Projectors, investment feasibility and new market entrants study is offered.
Development scope of 4K UST Projectors in each market segment is covered in this report. The macro and micro-economic factors affecting the 4K UST Projectors Market
Advancement is elaborated in this report. The upstream and downstream components of 4K UST Projectors and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/4k-ust-projectors-market-100577
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Kids Retail Fitness MarketMarket Share
Passenger Boarding Bridge MarketMarket Growth Rate
Inhalation And Nasal Spray Generic Drugs MarketMarket Forecast
Global Medical Device Analytical Testing Outsourcing MarketMarket Size
Ultrasonic Level Sensors MarketMarket Growth
Vacuum Aluminum Plating Machine MarketMarket Analysis
Tricalcium Phosphate MarketMarket Size
Global Flow Cytometer Reagents MarketMarket Share
Global Camera Module MarketMarket Growth
Intranasal Drug Delivery Devices MarketMarket
Shared Mobility MarketMarket Share
Aminoethylethanolamine MarketMarket Growth Rate
Automotive CMOS Image Sensor MarketMarket Forecast
Global Biopesticides MarketMarket Size
Dermal Fillers and Botulinum Toxin MarketMarket Growth
Nitrile Butadiene Rubber (NBR) Powder MarketMarket Analysis
Automotive Vehicle To Everything (V2X) Communications MarketMarket Size
Global Hepatitis Drugs MarketMarket Share
Global Music Festival MarketMarket Growth
Mobile Payments MarketMarket
Iot Monetization MarketMarket Share
Digital Light Processing DLP Projector MarketMarket Growth Rate
Medical Device Contract Manufacturing MarketMarket Forecast
Global Semiconductor Packaging And Assembly Equipment MarketMarket Size
Home Meal Replacement Industry MarketMarket Growth
Grinding Media MarketMarket Analysis
Pad Printing Supplies MarketMarket Size
Global Sewing Thread MarketMarket Share
Global Ground Support Equipment (GSE) for OEM MarketMarket Growth
Surface-Enhanced Raman Spectroscopy (SERS) Substrate MarketMarket
Dog DNA Test MarketMarket Share
Carbonyl Iron Powder and Ultra Fine Iron Powder MarketMarket Growth Rate
Indocyanine Green MarketMarket Forecast
Global Toilet Bowls MarketMarket Size
Preimplantation Genetic Testing MarketMarket Growth
Dairy Packaging MarketMarket Analysis
Double-Sheet Control Sensor MarketMarket Size
Global LDI Exposure Machine MarketMarket Share
Global Ski Slope Material MarketMarket Growth
Polyolefin Plastomer (POP) MarketMarket
0 notes
Text
South East Asia Testing, Inspection & Certification Market - Forecast (2024-2030)
South East Asia Testing, Inspection & Certification (TIC) Market Overview
The South East Asia TIC Market size is estimated to reach US$4.7 billion by 2030, growing at CAGR 4.82% during the forecast period 2024-2030. The growth of South East Asia TIC Market is majorly driven by increasing need for food testing and rising safety regulations and standards for the enhancement of medical device safety measure. Moreover, the rapid growth of consumer electronics industry coupled with the increasing demand for electronics will also trigger the growth of the testing, inspection & certification market in South East Asia. The manufacturer of electronic products needs to comply with various governmental standards to ensure quality testing and certification through RoHS testing, Electromagnetic compatibility (EMC) testing, GS mark certification, CPSR and so on, which in turn drives the market growth of TIC (Testing, Inspection & Certification) services. Furthermore, the demand for TIC services which includes failure & damage analysis, various component testing, e-mobility & battery testing and others in automotive industry is also a major factor that can transform the South East Asia TIC industry outlook in the long run.
The South East Asia Testing, Inspection, and Certification (TIC) market is undergoing significant transformation driven by multiple converging trends. The region's rapid industrialization and economic growth are increasing the demand for robust TIC services to ensure compliance with international standards and regulations. Advancements in digital technologies, such as blockchain, IoT, and AI, are revolutionizing TIC processes, enhancing the accuracy and efficiency of inspections and certifications. The growing emphasis on quality and safety in sectors like food and beverages, pharmaceuticals, and consumer goods is fueling the need for stringent testing and certification protocols. Additionally, the expansion of the manufacturing sector, particularly in automotive and electronics, is driving the demand for specialized TIC services to maintain quality control and meet export requirements. The rise of e-commerce is also contributing to the market growth, as online retailers seek to ensure product safety and authenticity. Environmental sustainability and regulatory compliance are becoming increasingly important, prompting companies to adopt comprehensive TIC services to meet green standards and reduce environmental impact. These trends collectively are enhancing the significance of TIC services in ensuring product quality, safety, and compliance across various industries in South East Asia.
South East Asia TIC Report Coverage
The “South East Asia TIC Market Report – Forecast (2024-2030)” by IndustryARC, covers an in-depth analysis of the following segments in the South East Asia TIC Market.
By Type: Outsourcing, In-house
By Types of Services: Testing Services, Inspection Services, Certification Services
By End Users: Agriculture, Automotive, Food, Consumers (textile, cosmetic, toys, apparel, furniture, stationary, hand tools), Medical & Life Science, Marine, Manufacturing, Building & Infrastructure, Industrial Equipment, Retail, Rail, E-Commerce, Meteorology, Others.
By Geography: Indonesia, Malaysia, Sinagpore, Philliphines, Thailand, Vietnam, Others (Myanmar, Laos, Cambodia, Brunei, Timor-Leste)
Request Sample
Key Takeaways
Testing Services held the major market share in 2023 owing to rising implementations of integrated testing solutions for wide range of end users including automation and control systems, battery storage, F&B processing, communication protocol and so on.
E-Commerce segment is analysed to grow at the fastest rate during the forecast period 2024-2030 owing to increasing internet and mobile phone usage, high penetration of IoT as well as improved e-payment methods& logistics.
Indonesia held the highest market share in 2023 Vietnam is analysed to grow at the fastest rate during the forecast period 2024-2030 owing to rapid growth of manufacturing sector and rising investments on railway infrastructure.
The increasing demand towards food testing and rising safety regulations and standards imposed by global as well as regional government bodies for the enhancement of medical device safety measure are analysed to significantly drive the market growth of South East Asia TIC market during the forecast period 2023-2030.
South East Asia TIC Market Segment Analysis- by Type of Services
Testing Services held the major market share in 2023 with a market size of $1.7 Billion and is estimated to reach $2.3 Billion by 2030 with a CAGR of around 4.51% during the forecast period 2024-2030. The growth is mainly attributed to rising implementations of integrated testing solutions for wide range of end users including automation and control systems, battery storage, F&B processing, communication protocol, maritime equipment material, oil & gas structure and components and others to increase productivity and customer satisfaction. In September 2022, Intertek announced the launch of their new Vegan Foods Certification. The Intertek Vegan Certification is designed to determine the suitability of food products for vegan and plant-based consumers. These factors are set to influence the growth of global South East Asia industry in the long run.
Inquiry Before Buying
South East Asia TIC Market Segment Analysis- by End User
E-Commerce segment is analysed to grow at the fastest rate of 9.84% during the forecast period 2024-2030. The growth of the E-Commerce in South East Asia is mainly driven by increasing internet and mobile phone usage, high penetration of IoT as well as improved e-payment methods& logistics. According to an article by Inside Monkey, E-commerce growth in Southeast Asia stands out at an 20.6% expansion in 2022, with sales reaching $89.67 billion from $74.36 billion in 2021 and is expected to pass $100 billion by 2023. Ecommerce sites or mobile applications need to undergo different tests including functionality, usability, security, performance, database and mobile application. TIC services judge the authenticity of the websites by testing the design, specifications, functionalities and various features to check their sanity and to ensure the protection level of the sites against any potential threats. Such benefits of these services drive the growth of TIC services market in this region. Moreover, the E-commerce sector in this region witnessing increasing cases of fraud and cyber-attacks which enhances the demand for testing, inspection and certification services in this industry. In Southeast Asia, digital payment methods are becoming increasingly accepted by most businesses and services today. According to a recent Kaspersky research titled “Mapping a secure path for the future of digital payments in APAC” published in April 2022, e-payment are the top encountered threat for most Southeast Asia countries including Indonesia (40%), Malaysia (45%), The Philippines (42%), Singapore (32%), and Vietnam (38%). Thus, significant rise in number of e-payment frauds can cater to the demand for testing, inspection & certification services within the industry.
South East Asia TIC Market Segment Analysis- by Geography
Indonesia held the highest market share of 20.9% in 2023. The economy of Indonesia is majorly driven by exporting of crude oil and natural gas and holds well established manufacturing industry, agriculture, mining and others. According to U.S Energy Information Administration, the production of petroleum and other liquids totalled 887,000 barrels per day in Indonesia in 2022, making it the seventh-largest exporter of liquefied natural gas, thus creating significant opportunities for the growth of the TIC Market. The rising number of railway infrastructure projects can be considered vital in fueling the market demand for quality testing, electromagnetic testing, and related inspection services for rail components used in the construction process within the country. In January 2022, the Indonesian Government announced the construction of the multibillion-dollar railway project with an estimated cost of $7.9 billion. It will involve the deployment of signaling systems, rolling stocks, and many related components. Such projects are bound to drive the need for railway operators or authorities to meet up with the required regulatory compliance, which in turn, can be considered vital in transforming the South East Asia TIC industry outlook in the long run.
Schedule a Call
South East Asia TIC Market Drivers
Rising Demand Towards Food Testing or Inspection due to Increasing Import/Export activities, Agricultural or Food Contamination, Food Safety Violation and Others is Surging the Demand for Testing, Inspection & Certification Services:
The increasing demand towards food testing owing to rising import/export activities, agricultural or food contamination, food safety violation and others is one of the major growth drivers boosting the adoption rate of testing, inspection & certification services in South East Asia. Food industry is considered as a highly regulated industry due to stringent regulatory compliance ensuring food quality assurance and safety for the consumers. The levels of product recalls owing to the presence of harmful ingredients or contamination or the undeclared presence of an allergen or not an approved additives are rising within the Southeast Asian nations. Moreover, the levels of agricultural trade (both export and import) are successively increasing owing to the population boost in various countries under scope, which will support the growth of the TIC market. As per World Bank 2021, the population of Philippines grew by 1.3% in the period of 2019-20. According to International Trade Administration July 2022 update, agricultural imports in Indonesia reached over $24 billion in 2021, owing to high demand for rice, wheat, soybeans, fresh fruits, dairy beef and various feed ingredients. The following marks an increase by $5 billion or 26% from 2020 import values. Furthermore, nearly 57.8% of the total import value regarding the agricultural products in the Indonesia market was dominated by five suppliers including China, Australia, U.S, Brazil as well as India. Such increase in imports can boost the adoption of food testing services which in turn can influence the market growth.
The Safety Regulations and Standards imposed by Global as well as regional government bodies for the enhancement of medical device safety measure is accelerating the growth of South East Asia TIC Market:
A major driver in the South East Asia TIC market is the implementation of stringent regulatory requirements across various industries. Governments in the region are increasingly adopting and enforcing rigorous standards to protect consumer safety, ensure product quality, and safeguard the environment. This regulatory landscape compels companies to adhere to strict testing, inspection, and certification protocols to gain market access and maintain competitiveness. The pharmaceutical and food industries, in particular, face heightened scrutiny, necessitating comprehensive TIC services to comply with health and safety standards. The automotive and electronics sectors also require extensive TIC processes to meet international export standards. The continuous update and tightening of regulations drive the demand for specialized and advanced TIC services, fostering market growth as companies strive to align with evolving compliance requirements.
The rising safety regulations and standards imposed by global as well as regional government bodies for the enhancement of medical device safety measure is creating demand for the TIC services which in turn triggers the growth of this market. The demand for Class III medical devices such as High-frequency ventilators, blood sampling monitors, oxygen supply units and so on have been growing overtime majorly amidst the pandemic as these devices are used to maintain or protect human life. Since these devices require licensed FDA approvals before distribution across countries, the market growth of TIC services is impacted significantly. Medical device testing is critical to the entire medical device development lifecycle to ensure the safety of patients and device users. In January 2021 the Philippines Food and Drug Administration (FDA) issued circular n° 2021-001 on the product standards to which Medical Devices must comply for notification or registration. The circular was issued so that local Manufacturers, Importers and/or Distributors must comply to obtain a certificate of Medical Device notification (CMDN) or a certificate of Medical Device registration (CMDR). In August 2021, FDA had released a list of Class-A 1242 product categories in its circular 2021-017, in order to support and clarify regulatory requirements for medical manufacturers looking for market access within Philippines. Under this, medical device manufacturers of Class A products specified under the ASEAN MDD, need to mandatorily obtain a Certificate of Medical Device Notification, (CMDN), before manufacturing, distribution, importing, selling, or advertising the medical devices within the country. Thus, rise in regulations and standards can boost the demand of testing, inspection & certification services among the medical device manufacturers.
South East Asia TIC Market Challenges
Lower Level of Digital Adoption by the Key Players coupled with the Continued Impact of Bottlenecks in Trade Flows across South East Asia region owing to Shortage of Cargo Container are Limiting the Market Growth:
Low level of digital adoption coupled with continued impact of bottlenecks in trade flows acts as a major challenge restraining the market growth of TIC market in South East Asia. According to Rothchild & Co report published in May 2021, the testing-inspection-certification digital maturity substantially lags behind the other various end-user industries which shall pose problems in the long-run. Some of the common industries against which the TIC lacked under digital technology is energy, financial institutions, industrial goods, insurance, and telecommunications. Additionally, Shortage of shipping cargos, shipment cancellations, growing freight rates along with many others have emerged as some of the prime factors adversely impacting domestic manufacturing operations across various Southeast Asian countries owing to dependency on raw material imports within the country. According to Westports Holdings Berhad report, Malaysia faces a container throughput slipping 1% year on year to 10.4 million TEUs (20ft equivalent units) in 2021. Container throughput at Westports was down 10% y-o-y in 1Q2022, reaching 2.39 million TEUs versus 2.66 million TEUs handled in 1Q2021 which leads to supply chain disruption. These factors are limiting the demand for South East Asia TIC which in turn hampers the market growth.
Buy Now
South East Asia TIC Industry Outlook
Product launches, acquisitions and R&D activities are key strategies adopted by players in the South East Asia TIC Market. The top 10 companies in the South East Asia TIC market are:
SGS SA
Bureau Veritas
Intertek
DNV GL
TUV SUD AG
ALS GLOBAL
DEKRA SE
Eurofins
Cast Laboratories PTE LTD
Singapore Laboratory Services PTE LTD
Recent Developments
In December 2022- Intertek, a leading Total Quality Assurance provider to industries, announced the launch of Intertek Green R&D, an innovative integrated solution that ensures the sustainability, quality and safety attributes of a product are maintained.
In June 2022- DNV launched the MyISRS digital self-assessment tool. The service is estimated to aid organizations to run an online independent high-level survey for quality assessing applications. Some of the key industries in South East Asia that can benefit from the service include oil and gas, chemicals, utilities, power generation, telecommunication, pharmaceutics, transport, food, and beverage, and maritime.
#south east asia testing#inspection & certification market Size#inspection & certification market Trends#inspection & certification market Growth#inspection & certification market Forecast#inspection & certification market Revenue#inspection & certification market Vendors#inspection & certification market Share#inspection & certification market Industry
0 notes
Text
Fill Finish Manufacturing Market is Estimated to Witness High Growth Owing to Increasing Demand

The fill finish manufacturing market involves various downstream processing methods in the production of pharmaceutical products and medical devices which includes pre-filling inspection and labeling. The fill finish processes encompass liquid filling as well as lyophilization, assembly, labeling and packaging of vials, syringes, and cartridges. It ensures optimal product quality, safety and integrity for final use. The fill finish manufacturing plays a crucial role in bringing biologics and vaccines to patients requiring strict adherence to regulations. The need for personalized medicine and shortage of vaccines during the COVID-19 pandemic has also propelled the demand in recent times. The fill finish manufacturing market is estimated to be valued at USD 16.41 Bn in 2024 and is expected to reach USD 30.36 Bn by 2031, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2024 to 2031.
Key Takeaways Key players operating in the fill finish manufacturing market are Asymchem Inc., Syntegon Technology GmbH, I.M.A. INDUSTRIA MACCHINE AUTOMATICHE S.P.A, West Pharmaceutical Services, Inc., Gerresheimer AG, AptarGroup, Inc., Dätwyler Holding Inc., Stevanato Group, OPTIMA, SGD Pharma, Nipro Corporation, Bausch Advanced Technology Group, and Berry Global Inc. The key opportunities in the market include increased outsourcing activities by pharmaceutical companies and advanced technologies providing flexibility, connectivity and efficiency. Emerging economies in Asia Pacific and Latin America present lucrative growth prospects owing to rising healthcare expenditures and increasing biologics production. The Fill Finish Manufacturing Market Share is witnessing increased global expansion strategies by key market players through mergers, acquisitions and partnerships. This allows companies to broaden their service offerings and geographic footprints to tap high growth markets. Market drivers The major market driver is the rising demand for biologics and vaccines. Biologics have revolutionized the treatment of complex diseases but require specialized fill finish facilities owing to their sensitivity. Furthermore, inadequate vaccine supplies during the pandemic underscored the need to boost local manufacturing capacities through technology transfers. This is expected to drive greater outsourcing of fill finish activities to specialized contract service providers globally.
PEST Analysis Political: Regulations regarding pharmaceutical packaging have become increasingly stringent over the years, requiring compliance. This drives demand for technologically advanced fill and finish manufacturing solutions. Economic: With the economy recovering post-COVID and healthcare expenditure rising worldwide, the Fill Finish Manufacturing Market Challenges And Opportunities inindustry is benefitting from higher outsourcing and greater uptake of advanced machinery. Social: An aging global population is driving higher demand for medicines and therapeutics. Further, greater health awareness is boosting pharmacy visits and medication consumption. Technological: Advanced technologies like automation, Internet of Things connectivity, and digital manufacturing are being integrated to enhance production efficiency, throughput, quality control, and regulatory compliance documentation in fill and finish plants. Geographical regions of concentration The North American and European fill and finish manufacturing markets currently account for the largest share of the global market value, driven by strong domestic pharmaceutical industries and stringent quality and regulatory standards in countries like the US, Germany, France, and UK. Proximity to key pharmaceutical markets and customers provides an inherent advantage to manufacturers located in these regions. Fastest growing region The Asia Pacific region, led by China and India, is poised to witness the fastest growth in the fill and finish manufacturing market over the forecast period. This can be attributed to rising generic and biologics manufacturing in the region coupled with increasing localization requirements. Additionally, lower operating costs and a rapidly expanding local pharmaceutical customer base are encouraging global players to set up or expand operations in Asia Pacific.
PEST Analysis Political: Regulations regarding pharmaceutical packaging have become increasingly stringent over the years, requiring compliance. This drives demand for technologically advanced fill and finish manufacturing solutions. Economic: With the economy recovering post-COVID and healthcare expenditure rising worldwide, the fill and finish manufacturing industry is benefitting from higher outsourcing and greater uptake of advanced machinery. Social: An aging global population is driving higher demand for medicines and therapeutics. Further, greater health awareness is boosting pharmacy visits and medication consumption. Technological: Advanced technologies like automation, Internet of Things connectivity, and digital manufacturing are being integrated to enhance production efficiency, throughput, quality control, and regulatory compliance documentation in fill and finish plants. The North American and European fill and finish manufacturing markets currently account for the largest share of the global market value, driven by strong domestic pharmaceutical industries and stringent quality and regulatory standards in countries like the US, Germany, France, and UK. Proximity to key pharmaceutical markets and customers provides an inherent advantage to manufacturers located in these regions. The Asia Pacific region, led by China and India, is poised to witness the fastest growth in the fill and finish manufacturing market over the forecast period. This can be attributed to rising generic and biologics manufacturing in the region coupled with increasing localization requirements. Additionally, lower operating costs and a rapidly expanding local pharmaceutical customer base are encouraging global players to set up or expand operations in Asia Pacific.
Get more insights on Fill Finish Manufacturing Market
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Coherent Market Insights#Fill Finish Manufacturing Market#Fill Finish Manufacturing#Pharmaceutical Manufacturing#Biopharmaceuticals#Drug Production#Sterile Filling#Aseptic Processing#Vial Filling#Syringe Filling
0 notes