#Bovine Respiratory Diseases
Explore tagged Tumblr posts
Text

Neurological disorders are conditions that affect the brain, spinal cord, or nervous system, leading to changes in brain function, behavior, and movement. These disorders can be caused by various factors, including genetic mutations, infections, trauma, and environmental toxins.
#wellsunmedicity#neurology#neurologist#best cardiologist in lucknow#best treatment for bovine respiratory disease#best hospital for respiratory diseases in gonda
0 notes
Text
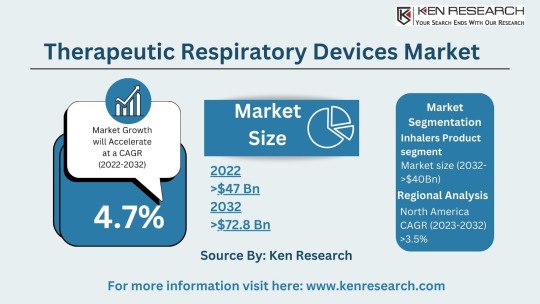
The $64.2 billion Respiratory Market & Its Future Trends, Segmentation and Forecast
The global respiratory market size reached a staggering USD 42.3 billion in 2023. This impressive figure highlights the significant need for respiratory devices and treatments to address a wide range of respiratory conditions. The market is projected to grow at a Compound Annual Growth Rate (CAGR) of approximately 7.4%, reaching an estimated USD 64.2 billion by 2030. This growth can be attributed to several factors:
Rising Prevalence of Chronic Respiratory Diseases: Conditions like asthma, chronic obstructive pulmonary disease (COPD), and sleep apnea are on the rise due to factors like air pollution, smoking, and an aging population.
Increased Life Expectancy: With an aging population, the demand for respiratory support devices for chronic conditions is expected to rise.
Technological Advancements: The development of innovative respiratory devices, such as portable nebulizers and advanced ventilators, offers improved treatment options.
Growing Focus on Homecare: The increasing emphasis on home-based care for respiratory patients fuels the demand for user-friendly respiratory devices.
Respiratory Market Segmentation: Catering to Diverse Needs
The respiratory market segmentation reflects the vast array of products and technologies available to address different respiratory needs:
By Application:
Therapeutic Respiratory Devices Market: This segment includes devices used for treatment, such as nebulizers, metered-dose inhalers (MDIs), and continuous positive airway pressure (CPAP) machines used for sleep apnea. The respiratory inhalers market is a significant sub-segment due to the widespread use of inhalers for asthma and COPD.
Anesthesia & Respiratory Devices: Specialized equipment used in surgical settings to deliver oxygen and maintain proper ventilation during anesthesia. The anesthesia and respiratory devices market caters to the specific needs of hospitals and surgical centers.
Respiratory Gas Analysis: This technology analyzes the composition of respiratory gases to assess lung function and identify potential respiratory issues.
By Device Type:
Respiratory Care Devices: This broad category encompasses various devices used for diagnosis, treatment, and monitoring of respiratory conditions. Examples include nebulizers, inhalers, ventilators, and CPAP (continuous positive airway pressure) machines.
Respiratory Monitoring Devices: These devices track vital signs such as blood oxygen levels and respiratory rate, allowing for continuous monitoring of patients with respiratory difficulties. The respiratory monitoring devices market is experiencing significant growth due to the increasing focus on patient safety and remote monitoring.
Respiratory Measurement Devices: These devices measure lung function and capacity, providing vital diagnostic information for respiratory conditions. The respiratory disease testing market relies heavily on these devices for accurate diagnosis.
Respiratory Protective Equipment (RPE): This equipment protects users from inhaling harmful substances, including masks and respirators. The respiratory protective equipment market is expected to witness growth due to rising concerns about air pollution and pandemics.
Take a look at: Forecasting the Respiratory Market, Size, Segmentation and Future Trends
Top Players in Respiratory Market: Breathing Innovation
Several established medical device manufacturers and specialty respiratory companies dominate the respiratory market:
Some of the top players in the respiratory market include:

Philips Healthcare
ResMed
Medtronic
GE Healthcare
Fisher & Paykel
Emerging Markets: A Rising Demand for Respiratory Solutions
Developing nations with growing populations and increasing healthcare expenditure present a significant opportunity. For instance, the bovine respiratory disease treatment market highlights the growing demand for respiratory solutions in the animal health sector.
Respiratory Market Trends: Shaping the Future of Respiratory Care
Exciting trends are shaping the respiratory market and transforming how we manage respiratory conditions:
Focus on Homecare Solutions: The emphasis on providing effective respiratory care solutions for patients in a home setting is driving innovation in portable and user-friendly devices.
Telemedicine Integration: Telehealth platforms allow remote monitoring and consultations with healthcare professionals, improving respiratory care management.
Connected Devices and Data Analytics: The integration of Internet of Things (IoT) technology allows for real-time data collection and analysis of respiratory parameters, leading to personalized treatment plans.
Emphasis on Early Detection and Prevention: The trend towards early detection and prevention of respiratory diseases through screening programs and lifestyle modifications is gaining momentum.
Challenges and Opportunities: Navigating the Respiratory Landscape
While the respiratory market offers promising opportunities, challenges also exist:
Challenges:
Cost Concerns: The high cost of some respiratory devices, particularly advanced equipment, can be a barrier to access for some patients.
Counterfeit Products: The presence of counterfeit respiratory products poses a safety risk and necessitates stringent quality control measures.
Compliance with Regulations: Navigating evolving regulatory requirements for medical devices can be complex and requires ongoing compliance efforts.
Opportunities:
Focus on Homecare: The trend towards homecare for respiratory patients creates a demand for portable and user-friendly respiratory devices.
Telemedicine Integration: Integrating respiratory monitoring devices with telemedicine platforms allows for remote patient monitoring and improved care coordination.
Emerging Technologies: The potential of new technologies like artificial intelligence and wearable devices can revolutionize respiratory care and diagnosis.
Respiratory Market Future Outlook: A Collaborative Approach
The respiratory market future outlook is promising, with a projected market size of USD 64.2 billion by 2030. And this suggests a market driven by innovation, collaboration, and a focus on improving patient outcomes. Here's what we can expect:
Collaboration between Medical Device Manufacturers and Healthcare Providers: Collaboration between these entities will be crucial for developing and implementing effective respiratory care solutions that address real-world clinical needs.
Increased Focus on Patient Education and Self-Management: Empowering patients with respiratory conditions to manage their health through education and user-friendly technology will be a key focus.
Conclusion:
The respiratory market plays a vital role in supporting lung health and improving the lives of millions suffering from respiratory illnesses. As the market continues to evolve, driven by innovation, collaboration, and a focus on patient-centric care, we can expect a future where managing respiratory conditions becomes more effective, accessible, and empowering for individuals and healthcare professionals alike.You can also read about: Future Forecast and Trends in the $35.58 Billion Respiratory Market
#Respiratory Market#Respiratory Industry#Respiratory Sector#Respiratory Market Size#Respiratory Market Segmentation#Respiratory Care Devices Market#Respiratory Devices Market#Therapeutic Respiratory Devices Market#Respiratory measurement devices market#respiratory gas analysis#anesthesia and respiratory devices market#respiratory disease testing market#bovine respiratory disease treatment market#respiratory inhalers market#respiratory monitoring devices market#respiratory protective equipment market#Top Players in Respiratory Market#Respiratory Market Trends#Respiratory Market Future Outlook
0 notes
Text
#Bovine Respiratory Disease Treatment Market#Bovine Respiratory Disease Treatment Market size#Bovine Respiratory Disease Treatment Market share#Bovine Respiratory Disease Treatment Market trends#Bovine Respiratory Disease Treatment Market analysis#Bovine Respiratory Disease Treatment Market forecast
0 notes
Text
Wrote a long one cos the in law family wanted him to take the flu shot, I said no.
"Dear Family, Friends, and Medical Professionals,
I am writing to share some thoughts and questions about vaccines, particularly in light of recent developments.
Do we believe that vaccines are the ultimate solution in medicine?
It is commonly known that influenza vaccines are reformulated each season due to the virus’s constant mutation, making it challenging to predict and protect against new strains accurately.
Is it true that these vaccines bypass the liver’s natural filtration system, potentially causing a shock to our bodies?
How should we classify these ingredients—as toxic or benign?
Here are just some vaccine ingredients, and these are being injected into your body and into your children’s bodies if you choose to vaccinate:
– Formaldehyde/Formalin – Highly toxic systemic poison and carcinogen.
– Betapropiolactone – Toxic chemical and carcinogen. May cause death or permanent injury after very short exposure to small quantities. Corrosive chemical.
– Hexadecyltrimethylammonium bromide – May cause damage to the liver, cardiovascular system, and central nervous system. May cause reproductive effects and birth defects.
– Aluminum hydroxide, aluminum phosphate, and aluminum salts – Neurotoxin. Carries risk for long-term brain inflammation/swelling, neurological disorders, autoimmune disease, Alzheimer’s, dementia, and autism. It penetrates the brain where it persists indefinitely.
– Thimerosal (mercury) – Neurotoxin. Induces cellular damage, reduces oxidation-reduction activity, cellular degeneration, and cell death. Linked to neurological disorders, Alzheimer’s, dementia, and autism.
– Polysorbate 80 & 20 – Trespasses the blood-brain barrier and carries with it aluminum, thimerosal, and viruses; allowing them to enter the brain.
– Glutaraldehyde – Toxic chemical used as a disinfectant for heat-sensitive medical equipment.
– Fetal Bovine Serum – Harvested from bovine (cow) fetuses taken from pregnant cows before slaughter.
– Human Diploid Fibroblast Cells – Aborted fetal cells. Foreign DNA has the ability to interact with our own.
– African Green Monkey Kidney Cells – Can carry the SV-40 cancer-causing virus that has already tainted about 30 million Americans.
– Acetone – Can cause kidney, liver, and nerve damage.
– E. Coli – Yes, you read that right.
– DNA from porcine (pig) Circovirus type-1
– Human embryonic lung cell cultures (from aborted fetuses)
You can view all of these ingredients on the CDC’s website. I encourage everyone to do their own research. Look up the MSDS on these chemicals. Read the thousands of peer-reviewed studies that have evaluated the biological consequences these chemicals can have on the body, especially when being injected.
Injecting foreign substances directly into the bloodstream—viruses, toxins, and proteins—has been linked to various diseases and disorders. These include conditions like atypical measles, cancer, leukemia, multiple sclerosis, and even SIDS (Sudden Infant Death Syndrome).
Conditions like Addison’s disease, anaphylactic shock, arthritis, asthma, asymptomatic COVID-19, Crohn’s disease, epilepsy, facial paralysis, fibromyalgia, fetal distress syndrome, foreign body embolism, genital herpes, hepatitis, hyperthyroidism, inflammatory bowel disease, jugular vein embolism, lung abscess, lupus, meningitis, MERS-CoV test positive, migraine-triggered seizures, multiple organ dysfunction syndrome, multiple sclerosis, multisystem inflammatory syndrome in children, pneumonia, stiff leg syndrome, stiff person syndrome, stillbirth, sudden heart attack, sudden respiratory failure, type 1 diabetes, uterine rupture, viral bronchitis—and much more.
This does not mean everyone will experience these reactions, but a significant number of test subjects have experienced one or more.
It is more than enough evidence to show that vaccine mandates are completely anti-scientific.
How can you make an informed decision if you do not have all the information?
We have also seen a shift where flu vaccines are now mRNA-based. But does a "vaccine" really prevent a virus or its recurrence as we expect it to?
The annual flu shot is, at best, a partial defense, aimed at last year’s strain. Does it truly help against the ever-mutating new flu, or is it just a temporary fix?
My concern is that this mindset—that a vaccine is a quick fix for everything—is flawed. The immune system may struggle to handle these types of agents, leading to breakthrough infections and potentially higher mortality rates.
For those who are vaccinated, I respect your choice. I simply ask for the same respect in return for my decision not to vaccinate. My reasons are personal and grounded in a belief that the government should not dictate my health choices and my family's.
Have you heard about Pfizer’s side effects?
Have you read the Pfizer documentation? Ask yourself if a drug with 32 pages of side effects is right for you.
The list of potential vaccine side effects released by Pfizer is alarming, ranging from autoimmune disorders to serious conditions like multiple organ dysfunction and sudden respiratory failure. Yet, this information was kept under wraps and only recently made public. Shouldn’t we be informed of the risks?
Do we even know the medium- or long-term effects of these vaccines?
Are they still in clinical trials? Is there a control group? What about Antibody-Dependent Enhancement (ADE) – has it been adequately tested? And why are ingredients like formaldehyde and mercury, known toxins, included in these vaccines?
Do you truly think this vaccine is 100% safe?
Transparency is crucial.
How can we make informed decisions if we are not given all the information?
We must ask ourselves, do we trust the pharmaceutical companies and their relationships with organizations like the CDC and FDA?
The FDA requested 75 years to release data on the Pfizer vaccine—why? Why did it take only 108 days to approve this vaccine, yet it supposedly requires decades to fully understand its effects?
Do you believe that SARS-CoV-2 has been isolated?
How well-informed are you about the CDC, FDA, pharmaceutical companies, and their donors? Do you think their qualifications are reliable?
These are important questions that deserve honest discussions. And, I believe it is crucial to acknowledge the existence of these alternative perspectives and engage in open discussions to gain a more comprehensive understanding.
Our health and freedom are at stake, and I urge everyone to think critically and seek out all the information before making decisions.
Thank you for taking the time to consider these points."
#vaccine questions#in laws family#we said no#stop using coercion#dont make me burn you#do your research people
11 notes
·
View notes
Text
In 1872, Cincinnati Ground To A Halt As The City’s Horses Succumbed To A Virus
It sounds like something out of a science fiction movie. For nearly three weeks in the autumn of 1872, Cincinnati was paralyzed by a virus with no known cure.
Humans were not susceptible to this virus. It only affected horses, but the entire operation of Cincinnati life and business depended primarily on horses. When the city’s horses were incapacitated, Cincinnati screeched into paralysis.
The strange episode began one evening in October when Dan Rice’s circus rolled into town. Four of the horses showed symptoms of some sort of respiratory illness and were taken to veterinarian George W. Bowler for treatment. Dr. Bowler readily identified the affliction as the “Canadian horse disease” that was then infesting the northern tier of states but doubted it would spread beyond his stable on Ninth Street.
Alas, Dr. Bowler’s optimism was unfounded and the next few days found cases throughout the downtown area. Journalists struggled to name the disease. “Epizooty” was a common label, but newspaper reports invoked “equine influenza” or “hippo-typhoid-laryngitis” or “epiglottic catarrh” or “epizootic influenza” and even “hipporhinorrheaeirthus”! Whatever they called it, the disease would hobble a city absolutely dependent on horse power to operate at all.
Josiah “Si” Keck, presiding at the Board of Aldermen, introduced a resolution to draft squads of men for duty at the city’s firehouses. With the horses out of commission, only manpower could replace horsepower to haul the heavy steam-powered fire engines of the day. Thankfully, only a few minor fires were reported during the height of the contagion.
According to the Cincinnati Enquirer [11 November 1872], other horse-dependent companies tried different alternatives:
“The United States Express Company has prepared to follow the example of the Eastern Companies. All of their horses, twenty-two in number, being completely disabled, they will at once substitute steers, and the streets of this city will show the curious spectacle of express wagons drawn by the propelling force of a farmer’s haycart.”
Historian Alvin F. Harlow, writing in the Bulletin of the Historical and Philosophical Society of Ohio [April 1951], noted that the bovine substitutes were simply not cut out for jobs readily accomplished by horses:
“The oxen, with great, wild, pathetic eyes, slobbering, swaying slowly through the streets, were a strange spectacle to city folk, and were followed by crowds of children for a day or two, until the novelty wore off. But as agencies of traction, they were a disappointment. Not all of them were well broken to the yoke; few men in town knew how to drive them, and as they are—with the possible exception of the tortoise and the two-toed sloth—the slowest walkers in the whole zoological category, they did not accomplish much in a day, according to city standards.”
Just think of an entire city operating on the capable talents of horses, now immobilized by an unseen microbe. Garbage piled up as the city’s sanitation wagons stood idle. “Garbage” back then meant kitchen and table scraps which, even in the chill of autumn, ripened malodorously in unattended cans. The situation was even worse at the city’s slaughterhouses. Even though the butchers had stopped working – there were no wagons available to deliver the slaughtered pork and beef – there were likewise no wagons to dispose of the offal and trimmings. The stench was indescribable.
Cincinnati’s streetcars were horsedrawn in 1872. It would be a decade before electrical trolleys debuted. The entire commuter system of the city shut down and the Cincinnati workforce, from C-suite executives to the lowliest laborers, had to hoof it. Harlow describes an exhausting scene:
“Towards dusk each evening the great trek homeward began, and from then until 9 P.M. the streets were thronged with business men, clerks, bookkeepers, warehouse and factory workers, trudging wearily. To reach their work again at 7 or 7:30 next morning, when most people's day began, soon proved too much for some of them, and they took to sleeping in their places of business; which in turn became less and less necessary, as those businesses were compelled to shut down for lack of transportation.”
Even funerals were affected. Teams of undertakers pulled hearses to the depot of the Cincinnati, Hamilton & Dayton railroad, whose tracks ran along the front of Spring Grove Cemetery. Mourners followed along on foot until the hearse was loaded on the train, then rode out for the burial. Other cemeteries put interments on hold for the duration.

The city faced the serious prospect of starvation. Food arrived in the city by rail and by river, but there were no carts to carry it from the wharf or the depot. Fresh vegetables rotted down by the river while families went hungry just a few blocks north. Farmers from the suburbs refused to bring their crops into Cincinnati for fear that their own draft animals would succumb to the dread epizooty.
As humans attempted to fill the horse’s role, every wheelbarrow in the city was drafted into use and some sold for astronomical sums. Even so, as noted by Harlow, human power had its very fragile limits:
“If the load was very heavy, as for instance, hogsheads of tobacco, massive machinery or an iron safe of a ton weight, ropes were also attached to each side of the wagon and passed over the shoulders of two files of straining men, while three or four others, their feet striving for toeholds in earth or cobbles, pushed against the wagon's tail until shoulder-bones threatened to wear through the flesh.”
Among the worst effects of the pandemic was the inability to dispose of dead horses. Horses died in Cincinnati at the rate of twenty or thirty a day at the height of the disease in November 1872, and there was nothing available to haul the carcasses out to the reduction plants, where they might be turned into soap fat or fertilizer. Alderman Si Keck, who owned one of these “stink factories,” found a partial solution by renting a small steam-powered truck from one of the city’s pork-packing plants but could still handle only a few of the equine corpses.
By the end of November, new cases and fatalities had diminished considerably. As December opened, the city was almost back to normal, with a new appreciation of the four-legged residents who truly powered our city.
Only one case of a human contracting the epizooty was recorded in 1872. Joseph Einstein was a well-known dealer when Cincinnati’s Fifth Street was the largest horse market in the United States. Einstein spent weeks, around the clock, nursing his stock and developed symptoms remarkably similar to those afflicting his horses. Several local doctors confirmed that he had somehow succumbed to the dread epizooty.
Just as mysteriously as it appeared, the epizooty vanished, and never visited Cincinnati to that degree ever again.

8 notes
·
View notes
Text
mRNA Vaccines for Livestock? - Questions For Corbett #097 - The Corbett Report
家畜用のmRNAワクチンは開発されているのでしょうか?もちろんです。では、これは何を意味するのでしょうか?いつものように、それは誰に聞くかによるのです。今週の「Questions For Corbett」で、第3世代ワクチンの悪いところ、悪いところ、腐敗したところ、そして食の未来について調べてみましょう。
SHOW NOTES
Bill Gates Vows To Pump mRNA Into Food Supply To ‘Force-Jab’ the Unvaccinated
Original video: Bill Gates and Penny Mordaunt launch the Global Academy of Agriculture and Food Security
Instagram post: someone's friend's neighbour's cows died from mRNA vaccines
Report: mRNA vaccines may be injected into livestock
Healthfeedback funded by Meta/TikTok/Google
Healthfeedback.org: Misrepresented 2018 clip of Bill Gates trigger inaccurate claims that mRNA COVID-19 vaccines for livestock could transfer to people through diet
Gates/Omidyar/US State Department-funding of International Fact-Checking Network
USA Today Fact check is an IFCN partner
USA Today Fact check: False claim about mandatory mRNA vaccines, deaths in Australian cattle
About AFP Fact Check
AFP Fact Check: Australian farmers not 'forced to inject livestock with deadly mRNA vaccines'
AFP Fact Check: mRNA vaccine cannot transfer through meat consumption
NSW fast-tracks mRNA FMD and Lumpy Skin Disease vaccines
Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines
NOVEL MRNA VACCINE TECHNOLOGY FOR PREVENTION OF BOVINE RESPIRATORY SYNCYTIAL VIRUS
The Future of Livestock Vaccines
Big Pharma pushes to get farm animals off antibiotics and on vaccines
Bayer Partners with BioNTech to Develop mRNA Vaccines, Drugs for Animal Health
mRNA Vaccines in Livestock and Companion Animals are here now.
SEQUIVITY
DNA vaccines in veterinary use
Veterinary biologics licensed in Canada
Paul Offitt: Can mRNA vaccines alter a person's DNA?
The Future of Vaccines
mRNA Vaccines: Disruptive Innovation in Vaccination
ビル・ゲイツ、ワクチン未接種者を「強制ジャブ」するためにmRNAを食品に注入することを誓う
オリジナル動画です。ビル・ゲイツとペニー・モーダントが「農業と食料安全保障のグローバル・アカデミー」を設立
Instagramの投稿:誰かの友達の近所の牛がmRNAワクチンで死亡した。
報告:mRNAワクチンを家畜に注射する可能性
ヘルスフィードバックはMeta/TikTok/Googleから出資を受けています。
Healthfeedback.org:家畜用mRNA COVID-19ワクチンが食事を通じて人に移行する可能性があるという不正確な主張を誘発するビル・ゲイツの2018年のクリップを誤報した。
ゲイツ/オミダイア/米国務省、国際ファクトチェック・ネットワークに資金提供
USA TodayのファクトチェックはIFCNのパートナーです。
USA Today ファクトチェック。mRNAワクチン義務化に関する虚偽の主張、オーストラリア牛の死亡例
AFPファクトチェックについて
AFPファクトチェック。オーストラリアの農家は「致命的なmRNAワクチンを家畜に注射することを強制された」わけではない
AFPファクトチェック:mRNAワクチンは肉食で移行できない
NSW州、FMDおよびLumpy Skin DiseaseのmRNAワクチンを早期開発
獣医学部における新規ワクチン技術。ヒト医療用ワクチンへのヘラルド
牛呼吸器シンシチアルウイルス予防のための新規MRNAワクチン技術
家畜用ワクチンの未来
大手製��会社は、家畜に抗生物質を与えず、ワクチンを投与するよう働きかけています。
バイエル、バイオエヌテック社と提携し、動物用mRNAワクチンおよび薬剤の開発を開始
家畜とコンパニオンアニマルにおけるmRNAワクチン「公式」はコチラから
シークイビティ
動物用DNAワクチン
カナダで認可された動物用生物製剤
Paul Offitt: mRNAワクチンは人のDNAを変えることができるのか?
ワクチンの未来
mRNA ワクチン。ワクチン接種における破壊的イノベーション
3 notes
·
View notes
Text
Pen-side test for bovine respiratory disease may save cattle industry millions, reduce antibiotic use
Sous-vide cooking inspired an idea that took promising technology out of the lab and into the barn. Researchers at Purdue University successfully developed an on-site bovine respiratory disease test that provides results within an hour. The team of researchers has been steadily advancing the point-of-care technology to address the disease, which is the most common and costly disease affecting…
0 notes
Text
Sniffin' Out the Bugs: How ELISA Kits Keep Animal Diseases in Check
The enzyme-linked immunosorbent assay (ELISA) is a widely used technique in diagnostics, particularly for identifying pathogens in animal disease testing. ELISA works by binding a known antigen or antibody to a solid-phase carrier, which then attracts specific antibodies or antigens in the sample. When an enzyme-labeled antibody is added, it reacts with a substrate to produce a color change. The depth of the color is directly proportional to the amount of antigen or antibody present, making ELISA a reliable and quick method for determining pathogen presence. The assay's ease of use and capability to process numerous samples simultaneously make it an invaluable tool in managing infectious disease outbreaks.
There are four main ELISA types: direct, indirect, sandwich, and competitive, each with unique strengths. In direct ELISA, the antigen is immobilized, and an enzyme-labeled antibody directly detects it. This method has fewer steps, minimizing potential error and cross-reactivity, though it requires costly enzyme labeling and doesn't amplify the detection signal, limiting sensitivity. Indirect ELISA, on the other hand, uses a secondary antibody that enhances the signal, improving sensitivity and flexibility. However, cross-reactivity is more likely, and the procedure takes longer than direct ELISA.
Sandwich ELISA uses a capture antibody bound to the plate to immobilize the target antigen, which can be detected by either direct or indirect ELISA methods. This setup makes it highly specific and suitable for complex samples without prior antigen purification, though it requires paired antibodies and isn't ideal for small molecules. Competitive ELISA, where antigen or antibody in the sample competes to bind labeled antibodies or antigens, offers robustness and reproducibility but involves a more cumbersome operation and is sensitive to sample matrix effects.
Given the spread of infectious animal diseases worldwide, high-throughput ELISA-based screening for animal pathogens is crucial to control outbreaks and protect the economy. BioVenic specializes in developing custom ELISA kits tailored to detect various animal pathogens, responding to the rising demand for affordable and reliable ELISA kits in the industry. With BioVenic's extensive selection, clients can choose kits for pathogens like avian influenza virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, and many more, ensuring comprehensive support for different livestock and poultry testing needs.
In developing ELISA kits, BioVenic considers factors like antigen capture and detection requirements, optimizing each kit for the target pathogen. This customization involves a series of precise steps to ensure quality and reliability. BioVenic's expertise and flexible approach make it a leader in ELISA kit development, helping veterinary and agricultural sectors respond swiftly to pathogen threats. Their streamlined, custom solutions underscore the role of ELISA in today's diagnostic landscape, providing dependable tools to meet growing global needs for pathogen monitoring and disease control.
0 notes
Link
0 notes
Text

Wellsun Medicity is Multi specialty hospital Healthcare service, premier medical hospitals and India's best Doctor who came together to drive their passion and commitment to providing quality healthcare,
#best treatment for prolonged cough#best treatment for bovine respiratory disease#best respiratory doctor in Gonda#best hospital for respiratory diseases in Gonda#best hospital for respiratory diseases#best pulmonologist doctor in gonda#best pulmonologist in gonda#best respiratory hospital in gonda
0 notes
Text
Coenzyme Q10 Detection Technology
In 1957, Prof. Grane of the Institute of Enzyme Research of the University of Wisconsin isolated a new quinone compound from the lipid extract of bovine heart mitochondria [1]. The compound is an orange-yellow crystal with a melting point of 48-49 ℃, capable of reversible oxygenation and reduction, and mainly involved in mitochondrial electron transfer. The compound is coded as Q-275 (Q is the initials of quinone, and 275 is the maximum absorption at 275 nm).
In 1958, American scholar Folkers and his team synthesized a series of coenzyme Q compounds, confirmed the structure of Q-275 and named it coenzyme Q10 [2]. In 1961, Mitchell, a British chemist, proposed the theory of "chemotaxis" in the study of energy conversion in living organisms and revealed the role of coenzyme Q10 in the energy conversion system of mitochondria [3], and was awarded the Nobel Prize in Chemistry in 1978. Since then, people have gradually recognized coenzyme Q10, and its applications have been widely and deeply studied.

Coenzyme Q10 (CoQ10), also known as ubiquinone, is chemically known as 2,3-dimethoxy-5-methyl 6-deca-isopentadienylbenzoquinone and consists of a benzoquinone ring and polyisoprene side chains. The number of isoprene units in the coenzyme Q series varies by species, with humans having 10 units. Coenzyme Q10 is available in both oxidized (ubiquinone, CoQ10, Ubiquinone) and reduced (ubiquinol, CoQ10H2, Ubiquinol) forms, and its chemical structure is shown in Figure 1.
Fig. 1 Chemical structures of oxidized (a) and reduced (b) forms of coenzyme Q10
Coenzyme Q10 is an important component of the mitochondrial respiratory chain, where it acts as an electron carrier and participates in electron transfer and ATP production. Furthermore, the cellular functions of coenzyme Q10 are multifaceted: it is present in all cell membranes, it limits the toxic effects of free radicals, it is a component of low-density lipoprotein (LDL), and it is involved in the aging process. Its deficiency is associated with a variety of diseases, such as mitochondrial disease, cardiovascular disease, age-related diseases, tumors, liver disease, kidney disease, etc. Panthenol is also a powerful antioxidant. Panthenol is also a powerful antioxidant, preventing lipid peroxidation in biological membranes [4].
With the deepening of the research on coenzyme Q10, the application of coenzyme Q10 is becoming more and more extensive. In addition to its use as a drug, it also has many applications in nutraceuticals, cosmetics and dietary supplements. Coenzyme Q10 is an endogenous substance, but its concentration in living organisms is very low. The analysis and determination of CoQ10 is important for the clinical diagnosis of diseases and the quality control of drugs and health products. In recent years, many analytical methods for the determination of coenzyme Q10 have been developed, which are summarized and discussed in this paper.
1 Coenzyme Q10 extraction and sample preparation
Coenzyme Q10 is insoluble in water and methanol at room temperature, slightly soluble in ethanol, soluble in acetone, 1-propanol, and soluble in organic solvents such as hexane and chloroform. Pharmaceuticals and dietary supplements such as tablets, capsules and softgels can be dissolved in ethanol, 1-propanol and other solvents, and analyzed by ultrasonication or filtration.
The isolation and enrichment of coenzyme Q10 from complex biological matrices is a laborious process.
Conventional liquid-liquid extraction is the most commonly used extraction method. This method is simple and has a large processing capacity, but has the disadvantage of high solvent consumption and some solvents can interfere with subsequent detection. Often the solvent is evaporated under N2 protection after extraction and redissolved in a mobile phase or other solvent. Whole blood samples were immediately dosed with the anticoagulants heparin or EDTA, and the plasma was centrifuged at low temperature and stored at -80°C. The plasma was then analyzed for the presence of coenzyme Q10 in the plasma. Coenzyme Q10 was extracted from plasma as follows [5]: Methanol was added to the plasma to precipitate the proteins, and the plasma was extracted with hexane. The mixture was rotated and shaken for 15 min, then centrifuged for 5 min, and the supernatant was extracted and the solvent was evaporated. The supernatant was dissolved in acetonitrile before analysis.
Coenzyme Q10 was extracted from animal heart tissue [6]. The extraction of coenzyme Q10 from animal heart tissue [6] was performed by precise weighing, transferring to homogenization tubes containing lysis medium A (containing garnet and zirconia beads), adding 1-propanol and the antioxidant 2,6-di-tert-butyl-4-methylphenol (BHT), shaking, centrifugation, and collection of the supernatant, which was analyzed immediately. The extraction of coenzyme Q10 from muscle tissue is most often done directly using muscle homogenate, or sometimes mitochondria are extracted from the tissue under ice-cold conditions, and then the mitochondrial suspension is diluted with 1-propanol, centrifuged, and the organic layer is extracted with ethanol and hexane [7]. The one-step extraction method is to use a suitable organic solvent to extract coenzyme Q10 while precipitating proteins. Yang et al. [8] studied the one-step precipitation of plasma proteins with different organic solvents (methanol, ethanol, acetonitrile, and acetone), and found that acetone was the best precipitant, and the extraction yield ranged from 71.00% to 93.07%, and was simpler than the operation of liquid-liquid extraction.
The solid phase extraction (SPE) technique can also be used for the extraction of coenzyme Q10. On-line SPE techniques are less time-consuming, less expensive, and reduce sample loss and contamination problems. The technique is usually automated using a programmable on/off valve [9]. However, protein precipitation is required prior to extraction.
Molecularly imprinted polymers (MIPs) are specialized molecular recognition techniques that have been developed in recent years. Molecularly imprinted polymers (MIPs) are formed by mixing template molecules with functional monomers, cross-linkers and initiators. After polymerization, the template molecules are removed and binding sites and cavities complementary to the templates in size, shape and function are formed [10], allowing selective recognition and adsorption of molecules structurally similar to the templates.
Contin et al. [10] synthesized MIP using coenzyme Q0 as the template, methacrylic acid as the functional monomer, acetonitrile as the pore-forming agent, ethylene glycol dimethylacrylate as the cross-linking agent, and benzoyl peroxide as the initiator. MIP was used as an adsorbent for solid-phase extraction of coenzyme Q10 from liver samples using dispersive solid-phase extraction. In addition, MIP synthesized in the same way could be used as the filling adsorbent for solid-phase extraction of coenzyme Q10 in urine. In addition, the MIP synthesized by the same method can also be used as the filling adsorbent of polypropylene columns for solid-phase extraction of coenzyme Q10 in urine, and the columns can be reused four times [11]. Compared with the traditional solid-phase extraction, MIP as a polymer adsorbent for solid-phase extraction has the advantages of simple synthesis, low cost, good stability, porous, and high selectivity for target molecules [11].
Sometimes it is necessary to maintain the original oxygenated and reduced state of coenzyme Q10 in the samples during the extraction process, which causes great difficulties due to the oxidizability of CoQ10H2. In this case, the temperature can be controlled at a low temperature of 4 ℃ during the extraction process [6,12], shortening the extraction time and using anhydrous extract will increase the stability of CoQ10H2 [13], and the use of HCl-acidified ethanol as a diluent can also prolong the stability of CoQ10H2 and prevent the auto-oxidation of CoQ10H2 [12]. BHT is an antioxidant often added in the extraction of plasma and tissue samples, which can prevent the oxidation of CoQ10H2 [6,12,14]. However, the addition of BHT to CoQ10H2 extracts from dietary supplements and pharmaceuticals was found to increase the oxidation of CoQ10H2 [13,15]. The difference in matrix composition between plasma samples and dietary supplements may be the main reason for the loss of antioxidant capacity of BHT [13].
Biological samples for coenzyme Q10 extraction include plasma, leukocytes or platelets, muscle, fibroblasts and urine [16]. Muscle biopsy is the best choice for studying coenzyme Q10 status in mitochondrial diseases, but it is very invasive; the correlation between the levels of coenzyme Q10 and tissues in plasma, blood cells and urine has been controversial, but the determination of coenzyme Q10 in these samples has an important role in therapeutic monitoring [16]. However, the determination of coenzyme Q10 in these samples is important for therapeutic monitoring [16].
The methods used to extract coenzyme Q10 from biological samples are summarized in Table 1.
Table 1 Extraction methods of Coenzyme Q10
Simple operation, large processing capacity, high solvent consumption, some solvents may interfere with the subsequent detection.
Plasma, animal heart, muscle homogenate, mitochondria
Online Solid Phase Extraction
Less time-consuming and costly, reducing sample loss and contamination.
plasma (medicine)
Molecular Blotting Techniques
Low cost, good stability, high selectivity for target molecules, and can be combined with solid phase extraction.
Animal liver, urine
2 The main assay for Coenzyme Q10
2.1 High Performance Liquid Chromatography (HPLC)
HPLC is currently the main analytical method for analyzing coenzyme Q10 in various matrices. The main detectors coupled with HPLC are ultraviolet (UV), tandem mass spectrometry (MS/MS), electrochemistry (ECD), fluorescence (FL), chemiluminescence (CL), etc. The separation effect of HPLC is good, and each detector has its own characteristics.
2.1.1 HPLC-UV
HPLC-UV is the most commonly used method for the determination of coenzyme Q10, and has become the national standard for drugs and health foods [17, 18]. It has been widely used for the determination of coenzyme Q10 in pharmaceuticals [15, 19-22], health foods or dietary supplements [15, 20], plasma [14, 23] and tissues [10]. Conventional C18 or C8 reversed-phase chromatographic columns can separate either one form of coenzyme Q10 (usually oxidized) or both oxidized and reduced forms.
Liposomes are a new type of pharmaceutical dosage form formed by the self-assembly of lipids (mainly phospholipids and cholesterol) with a bilayer structure similar to that of a cell membrane, which can encapsulate hydrophilic or hydrophobic drugs. Ruiz-Garcia et al. [21] prepared a small monolayer of liposomes encapsulating coenzyme Q10, phosphatidylserine, and fat-soluble vitamin C (6-o-palmitoyl-L-ascorbic acid) by thin-film hydration. The prepared samples were freeze-dried, solubilized in chloroform and determined by HPLC-DAD at two analytical wavelengths.
Clementino et al. [22] prepared lecithin/chitosan nanoparticles encapsulating simvastatin and coenzyme Q10. The chitosan-modified liposomes showed higher stability and narrower particle size distribution. The content of simvastatin, simvastatin hydroxylate and coenzyme Q10 was quantified by reversed-phase HPLC-UV method to account for possible degradation products. The encapsulation rate was determined and the in vitro release of the drugs was studied. According to the study, the serious side effects of statins, such as rhabdomyolysis, were associated with the decrease of coenzyme Q10, so the co-encapsulation of these two drugs is of great significance.
Coenzyme Q10, as a fat-soluble vitamin coenzyme, is often measured in conjunction with other fat-soluble vitamins. Franke et al. [14] analyzed 25 substances including 25-OH-vitamin D3, 25-OH-vitamin D2, retinol, tocopherols, carotenoids (including their stirrup isomers), and oxidized and reduced coenzyme Q10 in plasma on a fusion-nucleated 2.6 μm particle size C18 column in tandem with a C30 column, which is good at separating carotenoid isomers, and in conjunction with a six-pass valve. D2, retinol, tocopherols, carotenoids (including their stirrup isomers), and oxidized and reduced coenzyme Q10 in plasma. The switching of the six-way valve allows coenzyme Q10 to flow from the C18 column to the detector while the carotenoid isomers are eluted on the C30 column, avoiding the difficulty of separating these two substances on the same column. In addition, if a pressure-resistant UV-Vis detector is added between the C18 and C30 columns, it is possible to separate all substances without switching the six-way valve, but special software is required to control the two detectors and to acquire and process the data. It has also been reported that retinol, six carotenoids, two tocopherols, and coenzyme Q10 (10 fat-soluble vitamins) can be measured in human plasma using a MYC30 column, and the total amount of the oxidized form of coenzyme Q10 was measured by oxidizing coenzyme Q10H2 first with FeCl3 [23].
The HPLC-UV method is highly accurate and reproducible, with LOD generally on the order of μg-mL-1 and sometimes on the order of ng-mL-1 with highly sensitive detectors [14].
2.1.2 HPLC-MS/MS: HPLC-MS/MS has been developed rapidly and applied more and more widely. This method utilizes the high separation efficiency of HPLC for complex samples combined with the high sensitivity and high selectivity of mass spectrometry, which can detect low content samples under the background of complex matrix, and is widely used in the analysis and determination of target compounds in biological samples.
The main types of tandem mass spectrometry are triple quadrupole mass spectrometry [5,7,11,25,26], quadruple linear ion trap mass spectrometry [13,24], and hybrid quadruple orbit trap mass spectrometry [12], etc. Most of them use electrospray ionization, multiple reaction monitoring (MRM), and positive ionization mode. Due to the low sensitivity of [M + H]+ analysis of coenzyme Q10, ammonium adducts, i.e., [M + NH4]+, are often used to improve the sensitivity of the mass spectrometric response. By adding a certain amount of ammonium acetate to the mobile phase, [NH4]+ forms a stable five-membered chelated ammonium cation with coenzyme Q10 [8]. The formation of Li adducts has also been reported to greatly increase the sensitivity [24].
The electrostatic field orbitrap mass spectrometry (Otbitrap) is a new type of high-resolution mass spectrometry, which has the advantages of high resolution, high mass accuracy, and wide dynamic range, etc. Pandey et al. [12] applied HPLC-hybrid quadruple orbitrap mass spectrometry (Q-Orbitrap) to rapidly determine the redox state of coenzyme Q9 and coenzyme Q10. Two scanning modes, full MS/AIF and tSIM/data-dependent secondary scanning (tSIM/ddMS/MS), were compared, and it was found that full MS/AIF had higher signal sensitivity and good peak shape. During sample preparation, coenzyme Q9 and coenzyme Q10 were extracted with BHT-containing hexane to limit the oxidation of the reduced form, and the Kinetex C18 column, with fused-core SiO2 packing and smaller particle size (2.6 μm), was found to have higher column efficiency, better resolution, and good peak shape. Oxidized and reduced forms of coenzyme Q9 and coenzyme Q10 were analyzed in brain, heart, liver, adipose tissue, and muscle of healthy mice with a small amount of sample (<5 mg) and a very short analysis time (4 min). the LOD ranged from 0.01 to 0.49 ng mL-1 .
Due to the complexity of the biological sample matrix and the low concentration of coenzyme Q10, sample pretreatment is very important. Becerra et al. [11] analyzed coenzyme Q10 in human urine by molecularly imprinted polymer solid-phase extraction (MIP-SPE) coupled with HPLC-MS/MS. The pretreatment process concentrates the coenzyme Q10 by at least 5-fold. The high degree of sample purification reduces the ion suppression caused by the matrix effect of mass spectrometry. The analytical system does not interfere with protein or white blood cell elevations, which is important in cases of coenzyme Q10 deficiency with renal impairment.
The HPLC-MS/MS method uses a lot of internal standards, and the selection of suitable internal standards is also an effective way to eliminate matrix effects. Commonly used internal standards include coenzyme Q9 [5, 11, 25], coenzyme Q4 [12], and the isotopes of coenzyme Q10, coenzyme Q10-2 H6 [7] and coenzyme Q10-2 H9 [26], which are structurally similar to coenzyme Q10. Structural analogs of coenzyme Q10, such as coenzyme Q4 and coenzyme Q9, have many advantages. They are also endogenous ubiquinones and are present in human plasma at very low concentrations, or at least at levels that do not interfere with their use in analytes at the concentrations required for analysis, and therefore do not interfere with the quantification of analytes. In addition, it separates well from coenzyme Q10 [5]. A potential source of error in mass spectrometry is ion suppression, especially in electrospray ionization mass spectrometry, where the response signal of the analyte is altered and often suppressed if an interfering substance interferes with the ionization of the analyte on the surface of the droplet, or if there is competition. The use of an isotope internal standard is a good solution to the problem of ion suppression. By co-eluting the isotope internal standard with the analyte, the effects of various effects can cancel each other out, and the matrix effect can be minimized and the sample recovery can be better [7].
2.1.3 HPLC-ECD
Electrochemical detectors (ECDs) are widely used because of their high sensitivity, good selectivity and low price. Coenzyme Q10 can undergo a reversible redox reaction and can be detected by an ECD.
The commonly used detection methods are coulometric or voltammetric analysis. Different voltages are set according to the redox potentials of the substances to be measured. For oxidized coenzyme Q10, it is usually reduced to its reduced form first, and then oxidized as the original reduced coenzyme Q10 in the sample. This method can measure both oxidized and reduced coenzyme Q10 simultaneously.
Yubero et al. [27] used HPLC-ECD to determine coenzyme Q10 in urine and gave reference values for the pediatric population. An ESA Coulochem II electrochemical detector was used, and the cell voltages were -600 mV and +600 mV. The amount of coenzyme Q10 in urine fluctuated greatly at different times of the day, and the morning urine with the smallest fluctuation was chosen as the sample. The results were expressed as the amount of coenzyme Q10 per gram of particulate protein. The reference standards for children are: 2-10 years old: 24-109 nmol; 11-17 years old: 43-139 nmol. This assay provides a noninvasive method for assessing renal coenzyme Q10 status in patients with renal disease, but it is not currently available and requires up to 30 mL of urine per sample.
Schou-Pedersen et al. [6] determined reduced and oxidized coenzyme Q10 in canine plasma and cardiac tissue by HPLC-ECD and compared it with HPLC-MS/MS. The ECD was performed by fluid dynamic voltammetry using an RS6011 ultra-analytical cell at a voltage setting of 500 mV. A guard cell at -600 mV was used prior to the analytical cell to reduce oxidized coenzyme Q10 eluting from the column. Mass spectrometry was performed using a Waters Micromass Quattro micro API triple quadrupole mass spectrometer with multiple reaction monitoring (MRM) and the internal standard CoQ10-2 H9. Both methods used the same column with slightly different mobile phase ratios and additives. The results showed that CoQ10H2 was approximately 30% lower in the HPLC-MS/MS method than in the HPLC-ECD method, which may be due to differences in the calibration stock solutions or to accelerated oxidation during storage or analysis in the LC-MS/MS system. Therefore, the two methods are not interchangeable. In terms of sensitivity, the sensitivity of the two methods was comparable for coenzyme Q10H2, whereas the sensitivity of the HPLC-ECD method was higher for coenzyme Q10.
2.1.4 HPLC-FL and HPLC-CL
HPLC with a fluorescence (FL) detector is widely used for the determination of various substances in biological samples due to its high selectivity and sensitivity. Coenzyme Q10 is not a fluorescent substance and needs to be derivatized before determination. Nohara et al. [28] measured CoQ10 and CoQ10H2 in blood by post-column derivatization using HPLC using 2-cyanoacetamide and CoQ10 and CoQ10H2 heated under alkaline conditions to produce fluorescent products. The fluorescence emission and excitation wavelengths were 442 nm and 549 nm, respectively.
HPLC coupled with a chemiluminescence (CL) detector has also been reported for the determination of coenzyme Q10.Kishikawa et al. [29] used dithiothreitol (DTT) as a reductant to reduce quinone to semiquinone radicals, and semiquinone radicals converted dissolved oxygen to superoxide anion, which reacted with luminal to form CL.Accordingly, coenzyme Q10 was determined in plasma by HPLC-CL, and other components in plasma were not interfered with. Coenzyme Q10 in plasma was determined by HPLC-CL, and other components of plasma were not interfered.
Both methods require a reaction coil between the column and the detector, and require two or three pumps to mix the various reaction reagents with the coenzyme Q10-containing eluent after the column and then into the reaction coil, which is a cumbersome operation. In recent years, the literature in this area is relatively scarce.
2.2 Spectrophotometric and fluorescent methods
The Enzyme Labeler, also known as Microplate Reader, is an instrument for reading and analyzing the results of Enzyme Linked Immunosorbent Assay (ELISA) experiments. The basic principle of ELISA is similar to that of spectrophotometer or photoelectric colorimeter, using plastic microplates instead of cuvettes, usually 48-well, 96-well, or larger, with low reagent consumption, high speed, and good repeatability. Multifunctional enzyme labeling instrument often has a variety of detection functions such as absorbance, fluorescence, chemiluminescence, etc., in the medical and health inspection has been widely used.
Fukuda et al. [30] developed a rapid microtiter plate method for the determination of coenzyme Q10 using the redox cycle of quinone. Coenzyme Q10 was reduced to ubiquinone radical by NaBH4, and then the ubiquinone radical was oxidized to ubiquinone and superoxide anion radical by dissolved oxygen. The superoxide anion radical converts 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT) into a pink methanol dye. It has a strong absorbance at 510 nm, which increases with increasing concentrations of coenzyme Q10. The absorbance of Mazanine dye was measured quickly and easily by a microplate reader. As an application of this method, the content of coenzyme Q10 in cosmetics was successfully determined with an LOD of 14.8 nmol-L-1 . The proposed method can be used for the rapid high-throughput analysis of ubiquinone-containing products.
Fei et al. [31] developed a new method for the determination of coenzyme Q10 in serum and urine of Alzheimer's disease patients by fluorescence spectrophotometry, also using a microplate reader. The method is based on the fact that the chemical derivative between ethyl cyanoacetate (ECA) and coenzyme Q10 is fluorescent and can be detected by fluorescence spectrophotometer (FS-ECA) at λex/em = 450/515 nm. The results showed that serum and urine levels of coenzyme Q10 were significantly lower in Alzheimer's patients than in age-matched controls. This method has similar LOD and LOQ as the HPLC-UV method.
The FS-ECA method has some advantages over the HPLC-UV method, such as easier sample preparation, faster detection speed, and similar accuracy and specificity [31].
The important role of liposomes as a new drug dosage form for co-administration and targeted delivery was described in the literature [21,22], and liposomes can also be used as micro-containers to protect and concentrate reagents.Román-Pizarro et al. [32] prepared a new type of magnetic liposomes (MLs) containing hydrophobic magnetic gold nanoparticles (Fe3 O4 @ AuNPs) and the long-wavelength fluorophore cresyl violet for the determination of coenzyme Q10 in foodstuffs. AuNPs) and a long-wavelength fluorophore, cresyl violet, were used for the determination of coenzyme Q10 in food. First, the MLs were introduced into the flow-through system using a flow injection device and retained in front of the detector for 300 s by means of a solenoid device to achieve preconcentration. Then, a coenzyme Q10 solution containing the surfactant Triton X-100 was injected into the flow-through system. The surfactant caused the solubilization of the MLs and the release of cresyl violet, which was oxidized by coenzyme Q10, resulting in a decrease in the fluorescence signal. The concentration of coenzyme Q10 is directly proportional to the decrease in fluorescence signal. The LOD of this method is lower than that of the LC-UV method, but the equipment required is simpler and less expensive.
2.3 Electrochemical analysis
The redox properties of CoQ10/CoQ10H2 allow the determination of coenzyme Q10 by electrochemical analysis. The methods are generally voltammetric, such as cyclic voltammetry (CV) [33], differential pulse voltammetry (DPV) [34], square wave voltammetry (SWV) [35], etc. The samples can be pharmaceuticals, dietary supplements, animal and plant tissues, etc. The samples can also be used for the determination of CoQ10/CoQ10H2. Samples can be pharmaceuticals, dietary supplements, plant and animal tissues, etc.
Li et al. [34] investigated the electrochemical reduction mechanism of coenzyme Q10 at a silver electrode and developed a DPV method for the direct determination of coenzyme Q10 in plant and animal tissues. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) revealed that the reduction of coenzyme Q10 under anoxic conditions is a reversible one-electron, one-proton reduction and forms a stable semiquinone radical (coenzyme Q10H-), which is quenched by oxygen in an oxygen-filled environment. This is the reason why coenzyme Q10H2 is able to scavenge oxygen radicals due to its antioxidant function. Under the optimized experimental conditions, the DPV method can be used to determine coenzyme Q10 in complex samples, and it is rapid, sensitive, and highly selective, with an LOD of 3.33 × 10 -8 mol-L-1 .
Graphene, a single atom thick planar sheet composed of carbon atoms heterogeneously linked by sp2 in a honeycomb lattice, is a new type of sensor material [35]. Screen printing is a practical technique for manufacturing low-cost disposable sensors [35].
The new graphene sensor developed by this technology can be used for the determination of coenzyme Q10 and α-lipoic acid. The MnO2-modified screen-printed graphene electrode (MnO2/SPGE) has a larger capacitance and electrically active surface area, which facilitates electron transfer and significantly improves the oxidation performance of coenzyme Q10 and α-lipoic acid. The MnO2-modified screen-printed graphene electrode coupled with square wave dissolution voltammetry (SWV) can be used for the simultaneous determination of coenzyme Q10 and α-lipoic acid in dietary supplements with high sensitivity and practicality.
The electrochemical mechanism of coenzyme Q10 is complicated by the different electrodes and media. In a 1.1:1 methanol-ethanol solution, the electrochemical reaction of coenzyme Q10 at the glassy carbon electrode was controlled by adsorption, and the sensitivity of the determination could be improved by pre-enrichment [33]. In anaerobic ethanol solution, the cathodic process of coenzyme Q10 at the silver electrode was one-electron-single proton reduction [34], while the oxidation on MnO2/SPGE showed two-electron-single proton transfer [35]. Michalkiewicz [36] investigated the anodic oxidation of oxidized coenzyme Q10 in acetic acid solution using a glassy-carbon electrode and a carbon-fiber microelectrode coupled with voltammetry, respectively. The oxidized coenzyme Q10 in acetic acid solution was studied by
The results show clear oxidation peaks or waves in the potential range above 1.5 V. The presence of these signals cannot be linked to the well-known redox pair CoQ10/CoQ10H2, but may be attributed to the irreversible and diffusion-controlled two-electron oxidation of methoxy in coenzyme Q10 (formation of two additional quinone groups at the 2 and 3 positions of the ring). the total number of electrons involved in the CoQ10 anodic oxidation is much greater than two, suggesting that the oxidation also takes place in the unsaturated isopentadienyl side chain. The total number of electrons involved in CoQ10 anodic oxidation is much higher than two, suggesting that oxidation also occurs in the unsaturated isoprene side chain. The oxidation of the oxidized coenzyme CoQ10 has been rarely reported, is much less readily accessible than that of coenzyme Q10H2, and the mechanism of oxidation has yet to be demonstrated.
2.4 Other analytical methods
Supercritical fluids are substances whose temperature and pressure exceed the critical point, in a state of gas-liquid indistinguishability, with a density close to that of liquids, and a viscosity close to that of gases, and a high diffusion coefficient. Supercritical fluids with high diffusivity and low viscosity, very suitable for mobile phase. The supercritical fluid with more research and application is supercritical CO2. Supercritical fluid chromatography-tandem mass spectrometry (SFC-Ms/Ms) with supercritical CO2 as mobile phase can be used for the determination of coenzyme CoQ10 in rat plasma [8]. The method uses one-step acetone method to precipitate the protein and extract CoQ10 from the sample. Due to the low sensitivity of [M + H]+ of CoQ10 in mass spectrometry, methanol containing ammonium acetate was used as a post-column compensating solvent to provide [M + NH4]+ and improve the sensitivity. Supercritical CO2 is non-toxic, non-flammable, and relatively inexpensive, so it is widely used. Due to its non-polar nature, it is well suited for the analysis of fat-soluble compounds and can greatly reduce the use of organic solvents.
Other analytical methods include: high performance thin layer chromatography (HPTLC) [37], Fourier transform near infrared spectroscopy (FT-NIR) [38], nuclear magnetic resonance hydrogen spectroscopy (1 H- NMR) [39], etc. HPTLC is simple and rapid, but the sensitivity is not very high, and can be used for the analysis of coenzyme Q10 in raw materials and pharmaceutical preparations. FT-NIR does not require complex sample pretreatment, but requires a certain number of samples to establish a calibration model and obtain results through complex statistical analysis, and is generally used for the initial screening of the target analyte. 1 H- NMR also does not require complex sample pretreatment, can be both calibrated models, and obtained through complex statistical analysis, and is generally used for the initial screening of target analytes. FT-NIR does not require complex sample pretreatment, but requires a certain number of samples to establish a calibration model and obtain the results through complex statistical analysis, and is generally used for the initial screening of target analytes.1 H-NMR also does not require complex sample pretreatment, and can be used both qualitatively and quantitatively, with low sensitivity, and can be used for routine analysis of preparations. These methods can be used as a useful supplement to the quantitative analysis of coenzyme Q10.
3 Simultaneous determination of oxidized and reduced coenzyme Q10
In many methods, the total amount of coenzyme Q10 is determined by adding oxidizing agents such as FeCl3 to oxidize coenzyme Q10H2 to the oxidized form, and then the total amount of the oxidized form is determined. However, coenzyme Q10 coexists in both oxidized and reduced forms in biological matrices, and sometimes it is necessary to determine the two forms of coenzyme Q10 in biological samples, drugs and supplements separately. Commonly used methods include HPLC-UV [14, 15], HPLC-MS/MS [12, 13, 24, 25, 40], HPLC-ECD [6, 41], HPLC-FL [28], and so on.
Coenzyme Q10H2 standards are sometimes not readily available and can be obtained by reducing oxidized coenzyme Q10 with reducing agents such as NaBH4 [12-14,24,25,28,40,41]. The reduction of coenzyme Q10 at low concentrations may be incomplete, even if the amount of NaBH4 is 8800-fold excess [13], so the reduction process needs to be controlled at a certain concentration range. The reaction is usually carried out at low temperature and in the dark, and sometimes ED-TA is added to the solution after the reaction [12,13,15,25,41], which mainly binds to the metal ions that catalyze the oxidation process and acts as an antioxidant. Even if a standard of coenzyme Q10H2 is available, it may be partially oxidized and needs to be reduced before use [15], or the absorbance of the stock solution (ε = 4010) can be measured spectrophotometrically at 290 nm to determine the exact concentration [6].
Whether the quantitative results were expressed as the total amount of coenzyme Q10 or as the oxidized and reduced amounts, respectively, could affect the sample preparation. In order to maintain the reduced state of coenzyme Q10H2, in addition to the rapid operation at low temperature and the addition of antioxidants such as BHT during the preparation process, researchers have different opinions on whether the extracted solution should be re-dissolved by evaporating the solvent in the presence of N2. Some of them evaporate the solvent and re-dissolve it during sample pretreatment [12,14,15,24], while some scholars believe that there will be significant oxygenation of Coenzyme Q10H2 during solvent evaporation, so the extracted solution should be immediately dissolved in the presence of N2 [12,14,15,24].
Determination [6 ,13 ,25 ,28 ,40 ,41].
In fact, coenzyme Q10H2 is highly unstable during extraction and determination.Yamashita et al.[41] found that coenzyme Q10H2 was stable only at -78 °C, and the rate of oxidation of coenzyme Q10H2 to coenzyme Q10 increased with increasing storage time and temperature.Claessens et al.[25] found that significant oxidation of coenzyme Q10H2 to coenzyme Q10 occurred after 2 h in 1-propanol extracts of human plasma, so routine analysis was limited to 12 samples per batch in order to keep the total run time within 2 h. In addition, coenzyme Q10H2 is not stable at the same temperature as coenzyme Q1010. Claessens et al. [25] found that in 1-propanol extracts of human plasma, significant oxidation of coenzyme Q10H2 to coenzyme Q10 occurred after 2 h. Therefore, routine analyses were limited to 12 samples per batch in order to keep the total run time within 2 h. The results of this study are summarized below.
Due to the uncontrolled nature of oxidation, it has been suggested that almost all mitochondrial coenzyme Q10H2 is oxidized during sample pretreatment, and therefore quantification of total coenzyme Q10 in isolated mitochondria does not require ubiquinol oxidation [7]. Nevertheless, attempts have been made to control the rate of oxidation of coenzyme Q10H2 during analysis or to know the extent of oxidation. The choice of internal standards has helped to realize this desire. Structural analogs of coenzyme Q10 and coenzyme Q10H2, such as diethyl- or dibutyl-coenzyme Q10 [14] and dipropoxy-coenzyme Q10 [40], are sometimes used as internal standards in the analysis of coenzyme Q10 and coenzyme Q10H2. They are structurally very similar to CoQ10 and CoQ10H2 and exhibit the same chemical behavior as the analytes, especially with respect to artificial oxidation, which makes it possible to accurately back-calculate the original CoQ10H2/CoQ10 ratio [14].
The CoQ10H2/CoQ10 ratios in biological tissues varied, and Claessens et al. [25] showed that the plasma CoQ10H2/CoQ10 ratios in healthy volunteers without nutrient supplementation ranged from 22.3 to 64.4, with an average of 41.7, whereas Yamashita et al. [41] showed that the plasma CoQ10H2/CoQ10 ratio was about 95/5, suggesting that plasma coenzyme Q10 is mainly in the reduced form. These results indicate that plasma coenzyme Q10 exists mainly in the reduced form.
Changes in the CoQ10H2/CoQ10 ratio have important physiological significance and are associated with many functional disorders and diseases. Measurement of the CoQ10H2/CoQ10 ratio is useful in exploring the mechanisms of many diseases. Oxidative stress has been defined as a disturbance of the pro-oxidant-antioxidant balance in favor of the former, and is considered to be a causative factor in aging and degenerative diseases such as cardiac diseases, diabetes mellitus and cancer [41]. There is a consensus that the CoQ10H2/CoQ10 ratio is an important parameter in the assessment of oxygenation stress [24, 25, 41].
Another study showed that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TC-DD) damaged mouse liver in a dose-dependent manner. Tang et al. [40] investigated the mechanism, and found that exposure of mouse liver samples to TCDD resulted in a decrease in the total amount of coenzyme Q10, a decrease in the level of coenzyme Q10H2, and an increase in the CoQ10H2/total CoQ10 ratio. This may be due to the inhibition of succinate dehydrogenase in the electron transport chain. In addition, the decrease in the total amount of CoQ10 implies that CoQ10 is degraded by external environmental influences, which was confirmed by Temova Rakua et al [42]. They found that oxidized coenzyme Q10 was degraded during storage of dietary supplements and drugs containing coenzyme Q10, and that oxidized coenzyme Q10 was converted to reduced coenzyme Q10H2, especially in the presence of antioxidants such as vitamin C.
4 Summary
Coenzyme Q10 is an important electron carrier and antioxidant component of the mitochondrial respiratory chain and is widely found in human cells. Coenzyme Q10 deficiency may be associated with a variety of diseases. Although it is an endogenous substance, it can be used as a drug or dietary supplement to treat or ameliorate certain related diseases. Therefore, the selection of efficient isolation and analytical methods is of physiological and clinical importance. Liquid-liquid extraction is the most common method for the extraction of coenzyme Q10, while the extraction of reduced coenzyme Q10H2 requires temperature control and the addition of antioxidants. Solid-phase extraction and molecular blotting techniques have also been applied in the extraction of coenzyme Q10 from biological samples, which have greatly improved the extraction efficiency and detection sensitivity.
Coenzyme Q10 can be detected by a variety of methods, and currently the most commonly used method is HPLC. In clinical and pharmaceutical analysis, miniaturization of instrumentation by reducing column diameter and length and particle size is one of the major trends in improving separations [43]. HPLC-UV is easy to use as a standard method, has good stability, is not very sensitive, but is generally sensitive enough to meet the requirements for the simultaneous analysis of a variety of components.
HPLC-MS/MS has high sensitivity and good selectivity, and has unique advantages for the analysis of coenzyme Q10 in complex matrices, such as biological samples, but the operation of the instrument is complicated and the price is expensive. The electrochemical analysis method is simple, fast and sensitive, and has certain applications in the analysis of coenzyme Q10. The HPLC-ECD method is convenient for the simultaneous determination of oxidized and reduced Coenzyme Q10. Coenzyme Q10 co-exists in both oxidized and reduced forms in almost any sample. Some analytical methods are capable of determining both the total amount of coenzyme Q10 and the oxidized and reduced forms, while others can only determine the total amount, depending on the HPLC separation. The characteristics of the various methods, their determination formats and their applications in samples are shown in Table 2.
Table 2 Comparison of different analytical methods for Coenzyme Q10
Easy and fast to use, sometimes requires color development or derivatization, matrix may be interfering
Coenzyme Q10 is mainly present in reduced form in organisms, and the ratio of CoQ10H2/CoQ10 is clinically important, with greater bioavailability of CoQ10H2 in drugs and dietary supplements. Therefore, the simultaneous determination of oxidized and reduced coenzyme Q10 is a future development. The distribution of the two forms, their interconversion and their biological significance will be a focus of future research, which also brings opportunities and challenges to the study of extraction and analytical methods for both forms of coenzyme Q10.
In order to meet the clinical needs, coenzyme Q10 can be prepared by microbial fermentation [44] or chemical synthesis in addition to extraction from biological samples. Chemical synthesis is divided into total synthesis [45] and semi-synthesis [46], and the intermediate of semi-synthesis is ganiol. Microbial fermentation can be used for large-scale industrial production.
References:
[1] Crane FL, Hatefi Y, Lester RI, et al. Isolation of a quinone from beef heart mitochondria [J].Biochim.Biophys.Acta, 1957 , 25 ( 1 ): 220-221.
[2] Wolf DE , Hoffman CH, Trenner NR, et al. Coenzyme Q. I. Structural studies on the coenzyme Q group [J]. J. Am. Chem. Soc, 1958 , 80 (17) :4752.
[3] Mitchell P. Coupling of phosphorylation to electron and hydrogen trans- fer by a chemi-osmotic type of mechanism [J].Nature,1961 ,191 :144- 148.
[4] Pallotti F,Bergamini C ,Lamperti C ,et al. The roles of coenzyme Q in disease:direct and indirect involvement in cellular functions [J]. Int.J.Mol.Sci,2022 ,23(1) :128.
[5] Visconti GL, Mazzoleni L, Rusconi C ,et al. Determination by UPLC/ MS-MS of coenzyme Q10 (CoQ10) in plasma of healthy volunteers be- fore and after oral Determination by UPLC/ MS-MS of coenzyme Q10 (CoQ10) in plasma of healthy volunteers be- fore and after oral intake of food supplements containing CoQ10[J].
J. Anal. Bioanal. Tech, 2015 ,S13 :011.
[6] Schou-Pedersen AMV,Schemeth D,Lykkesfeldt J. Determination of re- duced and oxidized coenzyme Q10 in canine plasma and heart tissue by HPLC-ECD : a comparison with LC-MS/MS quantification [J].Antioxi- dants,2019 ,8(8) :253.
[7] Itkonen O,Suomalainen A,Turpeinen U. Mitochondrial coenzyme Q10 determination by isotope-dilution liquid chromatography - tandem mass spectrometry〔J〕.Clin.Chem,2013 ,59(8) :1260-1267.
[8] Yang R,Li Y,Liu C ,et al. An improvement of separation and response applying post-column compensation and one-step acetone protein pre cipitation for the determination of coenzyme Q10 in rat plasma by SFC- MS/MS [J].J. Chromatogr.B ,2016 ,1031 :221-226.
[9] Bompadrea S,Tulipanib S,Romandini S,et al. Improved HPLC col- umn-switching determination of coenzyme Q and Vitamin E in plasma [J]. 2008 ,32(1-4) :257-262.
[10] Contin M,Flor S,Martinefski M,et al. The use of coenzyme Q0 as a template in the development of a molecularly imprinted polymer for the selective recognition of coenzyme Q10[ J].Anal.Chim.Acta,2014 ,807 :67-74.
[11] Becerra CG,Baez F,Lucangioli S,et al. Miniaturized imprinted solid phase extraction to the selective analysis of Coenzyme Q10 in urine [J]. Chromatogr.B ,2019 ,1116 :24-29.
[12] Pandey R,Riley CL,Mills EM,et al. Highly sensitive and selective determination of redox states of coenzymes Q9 and Q10 in mice tis- sues. Application of orbitrap mass spectrometry [J].Anal.Chim.Acta, 2018 ,1011 :68-76.
[13] Vass A,Deák E ,Dernovics M. Quantification of the reduced form of coenzyme Q10 , ubiquinol, in dietary supplements with HPLC-ESI- MS/MS [J]. food Anal.Methods,2015 ,8(2) :452-458.
[14] Franke AA, Morrison CM, Custer LJ, et al. Simultaneous analysis of circulating 25-hydroxy-vitamin D3 ,25-hydroxy-vitamin D2 ,retinol, to- copherols,carotenoids,and oxidized and reduced coenzyme Q10 by high performance liquid chromatography with photo diode-array detec- tion using C18 and C30 columns alone or incombination [J].J. Chromatogr A,2013 ,1301 :1-9.
[15] Temova Rakua,Kristl A,Rokar R. Quantification of reduced and oxi- dized coenzyme Q10 in supplements and medicines by HPLC-UV [J].Anal.Methods,. 2020 ,12(20) :2580-2589.
[16] Yubero D,Allen G,Artuch R,et al. The value of coenzyme Q10 de- termination in mitochondrial patients [J].J.Clin.Med,2017 ,6(4) : 1-10.
[17] National Pharmacopoeia Commission, ed. Chinese Pharmacopoeia (Part II) [S]. Beijing: China Pharmaceutical Science and Technology Publishing House ,2020 :1457.
[18] GB/T 22252-2008. Determination of Coenzyme Q10 in Health Food [S].
[19] Grace AC,Prabha T,Jagadeeswaran M,et al. Analytical method develop- ment for simultaneous determination of ubidecarenone and vitamin E ace- tate in capsule dosage form by HPLC [J].Int.J.Pharm.Pharm.Sci, 2019 ,11(1) :79-84.
[20] Rakusa ZT,Srecnik E ,Roskar R. Novel HPLC-UV method for simul- taneous determination of fat-soluble vitamins and coenzyme Q10 in medicines and supplements [J].Acta.Chim.Slov,2017 ,64(3) :523 - 529.
[21] Ruiz-Garcia M, Perez-Lozano P, Mercade-Frutos D, et al. Development and validation of a new high-performance liquid chromatography method for the simultaneous quantification of coenzyme Q10 ,phos- phatidylserine, and vitamin C from a cutting-edge liposomal vehiculi- zation [J].ACS Omega,2019 , 4(22) :19710-19715.
[22] Clementino A,Sonvico F. Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvasta- tin ′s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles [J].J.Pharm.Biomed.Anal,2018 ,155 :33-41.
[23] Boulet L,Alex B ,Clavey N,et al. Simultaneous analysis of retinol,six carotenoids,two tocopherols,and coenzyme Q10 from human plasma by HPLC [J]. J. Chromatogr.B ,2020 ,1151 :122158.
[24] Kotnik D,Jazbec-Krizman P,Krizman M,et al. Rapid and sensitive HPLC-MS/MS method for quantitative determination of CoQ10 [J]. Research on Precision Instrument and Machinery ( RPIM) ,2013 ,2 (1) :6-13.
[25] Claessens AJ,Yeung CK,Risler LJ,et al. Rapid and sensitive analysis of reduced and oxidized coenzyme Q10 in human plasma by ultra per- formance liquid chromatography-tandem mass spectrometry and appli- cation to studies in healthy human subjects [J].Ann.Clin.Biochem, 2016 ,53(2) :265-273.
[26] Mathieu RE ,Riley CP. Quantitation of ubiquinone (coenzyme Q10) in serum/plasma using liquid chromatography electrospray tandem mass spectrometry (ESI-LC-MS/MS) [J].Methods.Mol.Biol,2016 , 1378 :61-69.
[27] Yubero D,Montero R,Ramos M,et al. Determination of urinary coen- zyme Q10 by HPLC with electrochemical detection:Reference values for a paediatric population [J].Biofactors,2015 ,41(6) :424-430.
[28] Nohara Y, Suzuki J, Kubo H. Determination of ubiquinone in blood by high-performance liquid chromatography with post-column fluorescence- cence derivatization using 2-cyanoacetamide[J].J. Fluoresc,2011 ,21 (6) :2093-2100.
[29] Kishikawa N,Ohkubo N,Ohyama K et al. Selective determination of ubiquinone in human plasma by HPLC with chemiluminescence reac- tion based on the redox cycle of quinine[ J].Anal.Bioanal.Chem,2011 ,400(2) :381-385.
[30] Fukuda M, Liu Q, Kishikawa N, et al. Development of ultrafast colori- metric microplate assay method for ubiquinone utilizing the redox cy- cle of the quinine [J].Microchem.J,2019 ,150(C) :104104.
[31] Fei X, Yu Y, Di Y, et al. A rapid and non-invasive fluorescence meth- od for quantifying coenzyme Q10 in blood and urine in clinical analy- sis [J]. Clin. Lab. Anal,2020 ,34(4) :e23130.
[32] Román-Pizarro V,Fernández-Romero JM,Gómez-Hens A. Automatic determination of coenzyme Q10 in food using cresyl violet encapsula- ted into magnetoliposomes [J].Food Chemistry,2017 ,221 :864-870.
LIU Yuhong,GUO Bin,TU Yifeng [33]. Determination of coenzyme Q10 by adsorption voltammetry[J]. Journal of Analytical Testing ,2021 ,40(8) :1224-1229.
[34] Li D,Deng W,Xu H,et al. Electrochemical investigation of coenzyme Q10 on silver electrode in ethanol aqueous solution and its determina- tion using differential pulse voltammetry〔J〕.J.Lab.Autom,2016 ,21 (4) :579-589.
[35] Charoenkitamorn K, Chaiyo S, Chailapakul O, et al. Low-cost and dis- posable sensors for the simultaneous determination of coenzyme Q10 and α- Lipoic acid using manganese (IV) oxide-modified screen-prin- ted graphene [J].Anal.Chim.Acta,2018 ,1004 :22-31.
[36] Michalkiewicz S. Anodic oxidation of oxidized forms of coenzymes Q10 and Q0 on carbon electrodes in acetic acid solutions [J].Bioel- ectrochemistry,2011 ,82(2) :103-111.
[37] Kulkarni MB ,Joshi AM,Patil RV.A novel HPTLC method for simul- taneous determination of co-enzyme Q10 and α-tocopherol in bulk and pharmaceutical formulation [J].Int.J.Pharm.Pharm.Sci,2018 , 10(10) :134-141.
[38] Rácz A,Vass A,Héberger K,et al. Quantitative determination of co- enzyme Q10 from dietary supplements by FT-NIR spectroscopy and statistical analysis [J].Anal.Bioanal.Chem, 2015 , 407 ( 10 ): 2887-2898.
[39] Monakhova YB ,Ruge I,Kuballa T,et,al. Rapid determination of co- enzyme Q10 in food supplements using 1H NMR spectroscopy.Int.J.Vitam.Nutr.Res,. 2013 ,83(1) ,67-72.
[40] Tang Z,Li S,Guan X,et al.Rapid assessment of the coenzyme Q10 redox state using ultrahigh performance liquid chromatography tandem mass spectrometry[J].Analyst,2014 ,139(21) :5600-5604.
[41] Yamashita S, Yamamoto Y. Simultaneous Detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress [J]. Anal. Biochem,1997 ,250(1) :66-73.
[42] Temova Rakua, Kristl A, Rokar R. Stability of reduced and oxidized coenzyme Q10 in finished products [J]. Antioxidants, 2021 , 10 (3) :360.
[43] Lucangioli S,Martinefski M,Tripodi V. Coenzyme Q10 analytical de- termination in biological matrices and pharmaceuticals [J]. Front.Biosci.Scholar,2016 ,8(2) :321-330.
[44] Fan JB ,Xu W,Xu Xi,et al. Production of Coenzyme Q10 by mi- crobes:an update [J].World J.Microbiol.Biotechnol,2022 ,38(11) :194.
[45] Nguyen T,Mac H,Pham P. Preparation of Key Intermediates for the Syntheses of Coenzyme Q10 and Derivatives by Cross-Metathesis Re- actions [J]. Molecules,2020 ,25 :488.
[46] Atla SR,Raja1 B ,Dontamsetti BR.A new method of synthesis of co- enzyme Q10 from isolated solanesol from tobacco waste [J]. Int.J.Pharm.Pharm.Sci,2014 ,6(8) :499-502.
#coenzymecoq10 #Coenzyme Q10 #Q10 #coq10
0 notes
Text
The Incredible Benefits of Boswellia Serrata Extract for Health and Wellness

Boswellia Serrata, a potent herb native to India, has long been revered in traditional medicine for its healing properties. Extracted from the resin of the Boswellia tree, this natural remedy has made its way into modern supplements, offering a variety of benefits for overall health and wellness. Whether you're looking to improve joint health, reduce inflammation, or support your body's response to chronic conditions, Boswellia Serrata Extract Benefits can be a game changer.
1. Natural Anti-Inflammatory Agent One of the most recognized benefits of Boswellia Serrata extract is its powerful anti-inflammatory properties. This makes it highly effective in reducing inflammation in the body, which is often linked to conditions such as arthritis, asthma, and inflammatory bowel disease (IBD). The active compounds in the extract, called boswellic acids, inhibit certain enzymes that cause inflammation. As a result, many individuals have experienced relief from pain and swelling, particularly those suffering from osteoarthritis and rheumatoid arthritis.
2. Joint Health Support As we age, joint pain and stiffness often become more prevalent, limiting mobility and reducing the quality of life. Boswellia Serrata extract has been shown to improve joint function and alleviate discomfort in individuals with joint-related issues. Regular supplementation with this extract can help enhance flexibility and ease movement, making it a natural option for those seeking relief from joint discomfort without relying on synthetic medications.
3. Improves Respiratory Health Boswellia Serrata extract is also known for its benefits in treating respiratory conditions, particularly asthma. Studies suggest that this herb can help reduce the production of leukotrienes, chemicals that cause constriction of bronchial muscles and make breathing difficult. For individuals with chronic asthma, Boswellia may offer significant relief by improving lung function and reducing the frequency of asthma attacks.
4. Supports Gut Health Chronic inflammation in the digestive system can lead to serious conditions such as IBD, Crohn’s disease, and ulcerative colitis. The anti-inflammatory properties of Boswellia Serrata extract can help reduce the inflammation that often accompanies these conditions, providing relief from abdominal pain, diarrhea, and other digestive issues. Some research also suggests that the extract can promote healing in the gut lining, improving long-term digestive health.
5. Boosts Skin Health Boswellia Serrata extract can work wonders for the skin as well. Due to its anti-inflammatory and antioxidant properties, it can help reduce redness, irritation, and even acne. Some skincare products now include Boswellia as a natural ingredient to promote healthy, glowing skin. Moreover, its ability to improve skin elasticity and hydration makes it a popular choice for those looking to reduce the appearance of fine lines and wrinkles.
Click here for more information:-
Botanical Extracts Supplier From India
Bovine Gelatin Powder
0 notes
Link
0 notes
Text
Chlortetracycline and Its Role in Preventing Bacterial Infections in Cattle
In the realm of livestock management, the prevention of bacterial infections is critical to maintaining the health and productivity of cattle. One of the key antibiotics used in this endeavor is Chlortetracycline, a broad-spectrum antimicrobial agent that has proven effective in combating a wide range of bacterial infections in cattle. As a vital tool in the agricultural industry, Chlortetracycline helps ensure that cattle remain healthy, thereby supporting the efficiency and sustainability of livestock production.
What is Chlortetracycline?
Chlortetracycline belongs to the tetracycline class of antibiotics, which are renowned for their effectiveness against both gram-positive and gram-negative bacteria. Discovered in the mid-20th century, Chlortetracycline was one of the first antibiotics introduced into veterinary medicine and remains a cornerstone in the prevention and treatment of bacterial infections in livestock, particularly cattle.
The Importance of Chlortetracycline in Cattle Health
Cattle, like other livestock, are susceptible to a variety of bacterial infections that can impact their health and reduce productivity. Infections such as bovine respiratory disease (BRD), foot rot, and enteric diseases are common concerns in the cattle industry. These infections can lead to decreased weight gain, poor feed efficiency, reduced milk production, and in severe cases, death.
Chlortetracycline is highly valued for its ability to prevent and treat these infections effectively. Its broad-spectrum activity allows it to target multiple bacterial pathogens simultaneously, making it a versatile solution for managing bacterial diseases in cattle.
Key Benefits of Using Chlortetracycline in Cattle
Prevention and Control of Bacterial Infections: Chlortetracycline is widely used as a preventative measure in cattle feed to control the occurrence of bacterial infections. By incorporating Chlortetracycline into the daily diet, cattle producers can reduce the incidence of infections, leading to healthier herds and improved overall performance.
Enhanced Growth and Productivity: By preventing infections that can impair growth and feed conversion, Chlortetracycline helps cattle achieve optimal weight gain and productivity. This is particularly important in beef production, where efficient growth is a key factor in profitability.
Improved Feed Efficiency: Healthy cattle convert feed into body mass more efficiently. By maintaining cattle health through the use of Chlortetracycline, producers can ensure better feed efficiency, leading to cost savings and improved returns on investment.
Safety and Efficacy: Chlortetracycline has a long history of safe and effective use in cattle. When used according to guidelines, it provides a reliable means of controlling bacterial infections without adverse effects on animal health or food safety.
Application and Dosage
Chlortetracycline is typically administered through feed or water, depending on the specific needs of the cattle and the management practices of the farm. The dosage varies based on factors such as the age, weight, and health status of the cattle, as well as the severity of the bacterial challenge.
It's essential to follow veterinary guidance and adhere to withdrawal periods before slaughter to ensure that antibiotic residues do not remain in the meat, maintaining the safety and quality of the final product.
Why Choose Swiss Chemie as Your Chlortetracycline Manufacturer?
Swiss Chemie is a leading manufacturer of Chlortetracycline, committed to providing high-quality veterinary antibiotics that meet the stringent requirements of the livestock industry. Our Chlortetracycline is produced under rigorous quality control standards, ensuring its purity, potency, and effectiveness.
At Swiss Chemie, we understand the critical role that antibiotics like Chlortetracycline play in maintaining cattle health and productivity. Our dedication to excellence in manufacturing ensures that our customers receive reliable and effective products that contribute to the success of their livestock operations.
Partner with Swiss Chemie
As a trusted Chlortetracycline manufacturer, Swiss Chemie is proud to support cattle producers in their efforts to maintain healthy, productive herds. Our expertise in veterinary antibiotics, combined with our commitment to quality, makes us the preferred choice for livestock professionals worldwide.
For more information about our Chlortetracycline and how it can benefit your cattle operation, please visit our website or contact us directly. We look forward to partnering with you to enhance the health and productivity of your livestock.
0 notes
Link
Cattle virus causes deaths in 3 week old kids. Read Bovine Rhinitis B Virus Variant as the Putative Cause of Bronchitis in Goat Kids
0 notes
Text
Comprehensive Guide to Common Cattle Diseases and Their Treatments
Introduction
Nepal, a country with a significant agricultural sector, heavily relies on cattle for its economy. Cattle are essential for plowing fields, providing milk and meat, and serving as a source of manure for fertilizing crops. However, cattle diseases substantially threaten livestock productivity and farmers’ livelihoods.
In Nepal, cattle diseases are influenced by various factors, including climate, farming practices, and the availability of veterinary services. Common cattle diseases in Nepal include Foot-and-Mouth Disease (FMD), Bovine Tuberculosis (BTB), Brucellosis, Mastitis, and Tick-borne diseases.
Cattle are integral to agriculture and the dairy industry and are susceptible to various diseases that can impact their health, productivity, and the economy. Understanding these diseases, their causes, symptoms, and preventive measures is crucial for effective cattle management.
FMD is a highly contagious viral illness among animals with cloven hoofs, causing fever, blisters, and lameness. BTB, caused by Mycobacterium bovis, leads to respiratory issues, weight loss, and reduced milk production. Brucellosis, a bacterial infection, can cause abortions, infertility, and decreased milk yield. Mastitis, a mammary gland inflammation, affects milk quality and quantity. Tick-borne diseases, such as Theileriosis and Babesiosis, result in anemia, fever, and reduced productivity.
Controlling these diseases in Nepal involves vaccination campaigns, improved farm management practices, and strengthening veterinary services. Education and awareness programs for farmers are crucial to enhance disease prevention and control measures.
What Are the Most Common Diseases in Cattle?
Like other livestock, cattle are susceptible to various diseases impacting their health and productivity. The most common diseases in cattle include:
Foot-and-mouth disease (FMD) is a virus that spreads quickly. It affects cloven-hoofed animals and causes fever, blisters in the mouth and feet, and lameness. FMD leads to severe economic losses due to decreased milk production, weight loss, and trade restrictions. The disease spreads rapidly through direct contact and contaminated objects.

Foot And Mouth Disease(Source: thelivestockproject)
Bovine Tuberculosis (BTB): Caused by Mycobacterium bovis, BTB affects the respiratory system, leading to chronic cough, weight loss, and reduced milk yield. Because it can spread to people, it is a zoonotic illness.
Brucellosis: An infection brought on by the bacteria Brucella species, brucellosis results in abortions, infertility, and reduced milk production. It is also a zoonotic disease, posing a risk to human health.
Mastitis is a mammary gland infection typically caused by bacterial infections. It affects milk quality and quantity, leading to economic losses for dairy farmers.
Tick-borne Diseases: Ticks transmit diseases such as Theileriosis, Babesiosis, and Anaplasmosis. These diseases cause fever, anemia, and decreased productivity.
Bovine Viral Diarrhea (BVD) is a viral disease that causes diarrhea, respiratory issues, and reproductive problems. It can lead to significant economic losses due to decreased milk production and increased mortality rates. The virus known as the Bovine Viral Diarrhea Virus (BVDV) causes BVD.
Read More: Top Cattle Farming Tips for Success
How Can Cattle Disease Be Spread?
Cattle diseases can spread through various mechanisms, including direct contact, indirect contact, vectors, and environmental factors. Understanding the types of transmission is essential to executing efficient disease control measures.
Direct Contact: Disease transmission through direct contact occurs when infected cattle come into contact with healthy animals. This can happen during grazing, housing, or transportation. For example, FMD spreads rapidly through contact with infected saliva, vesicle fluid, or excretions.
Indirect Contact: Indirect transmission occurs when cattle contact contaminated objects, such as feed, water, equipment, or clothing. Brucellosis can spread through contaminated feed or water sources, while mastitis can be transmitted through contaminated milking equipment.
Vectors: Vectors, such as ticks, flies, and mosquitoes, can transmit diseases between cattle. Tick-borne diseases like Theileriosis and Babesiosis are spread through tick bites, while flies can transmit diseases like pinkeye and anthrax.
Environmental Factors: Environmental factors, including soil, water, and air, can affect disease transmission. Bovine Tuberculosis can persist in the environment, and cattle can become infected by inhaling contaminated dust or ingesting contaminated water.
Wildlife: Wildlife can act as reservoirs for certain diseases, transmitting them to cattle. For instance, wildlife can carry and spread BTB to cattle grazing in shared areas.
How Do You Control Cattle Disease?
Controlling cattle diseases involves a combination of preventive measures, early detection, and effective treatment strategies. Implementing these measures can significantly reduce the incidence and impact of cattle diseases.
For More Details, Click Here
0 notes