#2024Daily
Text

Niels Henrik David Bohr (7 October 1885 – 18 November 1962)
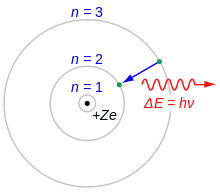
A Danish physicist, Niels Henrik David Bohr is perhaps most well known for his work on the structure of the atom and the resulting Bohr model. Bohr built off of Ernest Rutherford's previous atomic model, proposing that electrons orbited the nucleus at discrete energy levels and were able to jump between levels with the addition or expulsion of energy. Although more accurate models exist today, the Bohr model remains a useful tool for understanding atomic structure. Bohr was eventually awarded the 1922 Nobel prize in physics for his work. He also predicted the existence of hafnium (named for Copenhagen) and worked extensively in the field of quantum mechanics.
Though he spent most of his life in Denmark, Bohr and his family were forced to flee the country when the Nazis took over during World War II as his mother was Jewish. As a result, he spent the final years of the war in Britain and the United States, working on the Manhattan project and was an early proponent of international cooperation regarding nuclear weapons and nuclear power.
Element 107, bohrium, is named in his honor.
Sources/Further Reading: (Images source - Wikipedia) (Biography.com) (LiveScience) (Nobel Prize) (PBS)
33 notes
·
View notes
Text

Measuring Time: Atomic Clocks
The most accurate clocks that exist today are atomic clocks, measuring the flow of time based on the resonant frequency of atoms and molecules. This is possible because electrons associated with atoms exist at distinct energy levels, and the transitions between these energy levels can be determined, leading to a consistent resonance when probed. While there are several types of atomic clocks, with different setups and configurations, they all rely on a single substance.
Cesium clocks are the most common, and it is the resonant frequency of cesium (also written caesium) that is used for the current definition of a second. Some atomic clocks also use rubidium, or hydrogen. Less common and more recent is strontium, as well as aluminum, yttrium, and mercury. Historically, ammonia was actually used in the first atomic clock in 1949 before the first cesium clock was built in 1955.
Sources/Further Reading: (Image source - Wikipedia) (Time and Date) (Popular Mechanics) (NASA) (NIST)
81 notes
·
View notes
Text
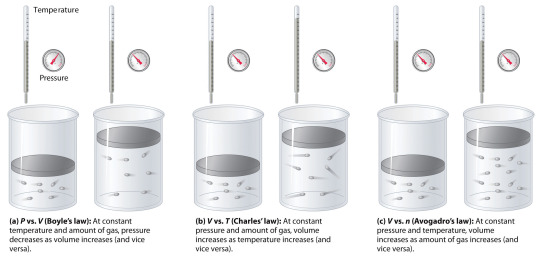
Gas Laws
A gas law is, simply put, a physical law that describes the behavior of gases. There are multiple gas laws, including the following four laws that are more well known than others:
Boyle's Law relates the pressure and volume of a gas. Published by Robert Boyle in 1662 it states that "the volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature". One mathematical representation of this law is P1 x V1 = P2 x V2.
Charles' Law was published in 1787 by Jacques Charles. It relates temperature and volume and states that "volume of a given fixed mass of a dry gas is directly proportional to its absolute temperature at a constant pressure". One mathematical representation of this law is V1/T1 = V2/T2
Gay-Lussac’s Law was published by Joseph Louis Gay-Lussac in 1808 and relates pressure and temperature, stating: "The pressure exerted by a given mass and constant volume of an ideal gas on the sides of its container is directly proportional to its absolute temperature." One mathematical representation of this law is P1/T1 = P2/T2.
Finally, Avogadro's Law relates the amount and volume of a gas. It was published in 1811 by Amedeo Avogadro and states that the "volume occupied by an ideal gas at a constant temperature is directly proportional to the number of molecules of the gas present in the container." One mathematical representation of this law is V1/n1 = V2/n2.
Sources/Further reading: (Image source - LibreTexts) (NIH) (Jove) (Wikipedia)
*P - pressure; V - volume; T - temperature; n - amount of a substance
55 notes
·
View notes
Text

Case Study: Liberty Ships Failure
In the 1940s, the US built over 2700 vessels referred to as the Liberty Ships. While it was far from the majority (most of the sunken Liberty ships were a result of German attacks), several of the Liberty ships suffered sudden, catastrophic failures that would go on to change the field of failure analysis. Examples include that of the Schenectady, pictured above cracked in half. These ship failures are perhaps some of the best well known examples of materials failure, often taught (or at least mentioned) in introductory classes.
While there were a number of mechanisms that led to the failure of the ships, one of the most cited causes is the ductile to brittle transition temperature (DBTT). Once the ships entered colder waters the structure of the steel changed and fracture occurred. The DBTT was actually discovered as a result of these failures by metallurgist Constance Tipper, of Cambridge. As expected, the thousands of Liberty ships had a significant impact during World War II, but, more unexpected, they had a significant impact on physical metallurgy.
Sources/Further Reading: (Image source - 2016 article) (2015 article) (University of Cambridge)
115 notes
·
View notes
Text

Laboratory Safety: The Hierarchy of Controls
Though many people may think of safety glasses or lab coats as the safest way to protect oneself in a laboratory setting, personal protective equipment (PPE) is actually considered the last and least effective step. The goal with safety in laboratories is almost always to make the use of PPE unnecessary or redundant - with the caveat and acknowledgement that sometimes, that just isn't possible.
Nevertheless, let's take a look at the steps that come before PPE:
Elimination of the hazard. Examples might be restricting the use of extension cords (possible trip hazard); doing work at ground level (e.g., eliminating the use of a ladder and bringing the work to the floor); or updating old or faulty equipment that might be more dangerous than modern versions.
Substitution of the hazard. Similar to elimination, examples include replacing chemicals with less dangerous versions that can perform the same task, or using less electricity or temperature.
Engineering controls serve to isolate people from hazards. Examples include chemical fume hoods, an interlock system for a laser setup, or remote controls to operate equipment from a distance.
Administrative controls are the rules and regulations that govern a laboratory space. They can include working on a buddy system, additional safety training, mandating rest breaks, putting up warning signs and labels, and developing standard operating procedures.
Sources/Further Reading*: (Image source - CDC) (OSHA) (OSHA hierarchy of controls) (SafetyCulture) (Lab Manager)
*Note: If you are looking for lab safety resources, any university with working laboratories should have guidelines available. Just keep in mind that as the rules get more specific, some may be location dependent (i.e., based on the local laws of the state, province, country, etc.).
86 notes
·
View notes
Text

General Lab Safety: Equipment Maintenance
Proper maintenance of equipment is important to the safety of any environment, not just in the laboratory. Maintenance goes beyond simply making sure a piece of equipment is functioning - it also includes calibrating, cleaning, and inspecting the equipment and surrounding space, as well as the laboratory overall, and ensuring that the necessary consumables are available. These steps - and proper usage of the equipment in the first place - will extend the lifetime of the equipment.
Sources/Further Reading: (VWR) (InterFocus blog) (NIH) (University of Washington)
Image source.
67 notes
·
View notes
Text
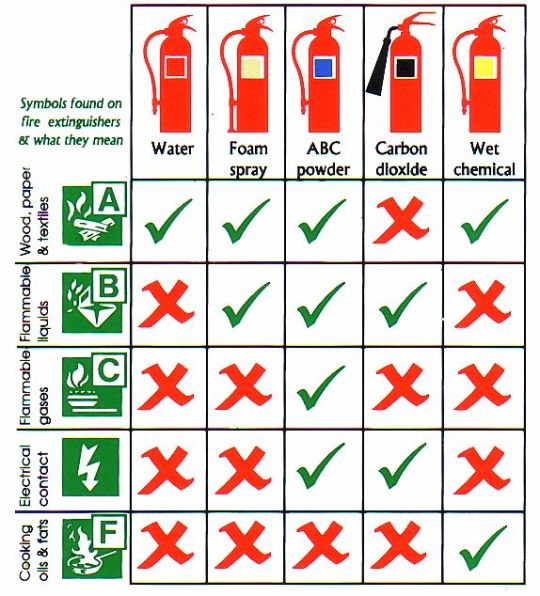
Safety Equipment: Fire Extinguishers
Not all fire extinguishers are the same and fire safety training is typically required to learn of the different varieties and fire extinguishing media before even attempting to use one in a laboratory setting. Generally speaking, fires are sorted by the media that is burning, and fire extinguishers are rated based on the classes they can extinguish:
Class A, globally, typically represents ordinary combustibles (paper, wood, etc.)
Class B typically represents flammable liquids. In the United States, it also represents flammable gases.
Class C outside the United States represents flammable gases. Within the United States it represents fires from electrical equipment.
Class D, globally, typically represents flammable metals.
Class E is typically only used in Australia and Asia to also represent electrical equipment fires.
Class F (known as class K in the United States) represents cooking fires, such as cooking oil or grease fires.
Some organizations/countries distinguish the varieties by color, while others are less specific. Symbols are typically used alongside class categories to distinguish which fires an extinguisher is capable of putting out. Extinguishers are typically only used on smaller fires that can be put out quickly.
It is important to note that, though organizational standards vary, seldom is using a fire extinguisher required. If a researcher feels in any way uncertain of their capabilities, or the size of the fire, they should not attempt to fight the fire - so long as other protocols (evacuation, pulling the fire alarm, contacting authorities, etc.) are followed.
Sources/Further Reading: (Image source - IFSEC Insider) (LSM) (Lab Manager) (University of Wisconsin-Madison) (Wikipedia)
60 notes
·
View notes
Text
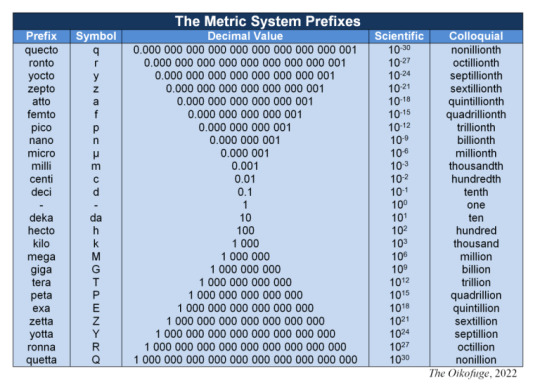
SI Prefixes
In addition to the units and derived units defined by the SI system of units, there are official SI prefixes used to increase or decrease the value of said unit, each multiplying by a value of ten to the power of a whole number. The first three prefixes from unity, in either direction, multiply by ten to the power of plus or minus one, two, or three, but from there the powers increase values of plus or minus three. As of this writing in early 2024 the highest and lowest prefixes respectively are 10 to the power of 30 and -30.
To distinguish between the positive and negative power prefixes, all of the positive power prefixes except the first three (deka, hecto, and kilo - for historic reasons) have capital letters as their symbols. The negative power prefixes use lowercase. Prefixes are added to units to form new words - hyphens and spaces are not used. Similarly, symbols for the prefixes are added directly to symbols for units. For example: a femtosecond, symbolized as fs; or a terabyte as TB.
Eight prefixes closest to unity were first adopted in 1795, two of which (myria and myrio) later fell out of use. These were officially recognized by the governing body in 1889. In 1960, myria and myrio were declared obsolete and the next three prefixes were added in either direction (mega, giga, and tera; and micro, nano, and pico). In 1964, the prefix chart was unbalanced with the addition of femto and atto among the negative powers, rectified in 1975 with the addition of peta and exa. Four prefixes were added again in 1991, with the final four only added in 2022.
Sources/Further Reading: (List from governing body) (Image source) (Wikipedia) (NIST) (Pronunciation and etymologies) (2022 article) (SI Brochure from governing body)
89 notes
·
View notes
Text
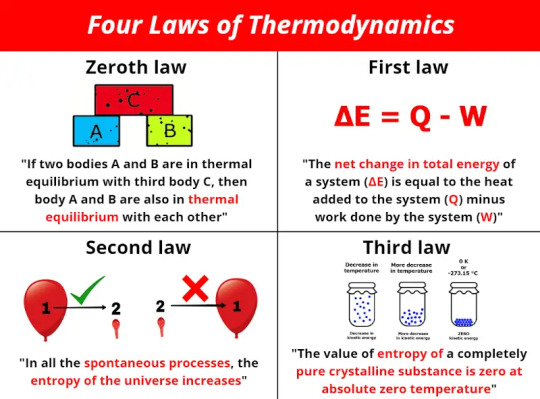
Thermodynamics
Thermodynamics is defined as a branch of physics that deals with energy, primarily heat, temperature, and the work of a system. More specifically, it deals with the relationships between these values and others, including entropy and the conversion of one form of energy to another. Only large scale, relatively speaking, system responses are defined as thermodynamics - the overall trend or reaction a system exhibits. There are four laws of thermodynamics, defined in the image above.
Sources/Further Reading: (Image source - LinkedIn) (Wikipedia) (NASA) (Harvard Lecture) (Livescience.com)
90 notes
·
View notes
Text

PPE: Lab Coats
Lab coats serve as torso, arm, and general body protection and, as with other forms of personal protective equipment, there are several varieties depending on the hazards one expects to encounter. The primary hazards lab coats are designed for are chemical hazards and temperature/fire hazards. Chemical resistant lab coats are typically white in color, made from a cotton-polymer blend. Fire-resistant lab coats are typically blue in color to distinguish them from their counterparts. For lower risks, pure cotton is used, treated for fire resistance. For higher risk situations, lab coats are typically made from Nomex.
Sources/Further Reading: (Image source - UMass) (University of Arizona) (UCSF) (NIH)
39 notes
·
View notes
Text

Cleanrooms
Cleanrooms are isolated rooms or chambers designed to limit airborne particles as well as separate hazardous particles from more everyday spaces. They are used in a variety of fields, including life sciences, but in materials science they are largely used in the electronics industry. Cleanrooms are rated and classified based on the size and quantity of particles that they remove from the air. Because people carry and create airborne particles themselves, significant PPE is required before entering a cleanroom.
Sources/Further Reading: (Image source - Wikipedia) (Clestra) (Clean Air Technology) (Mecart) (Colandis)
73 notes
·
View notes
Text
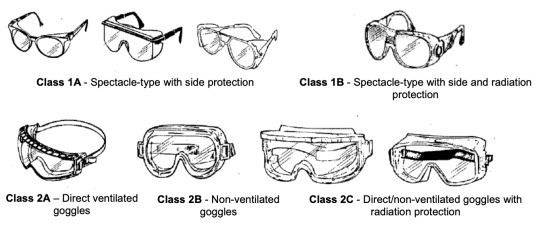
PPE: Eye Protection
There are four general types of eye protection common in laboratories. The first is safety glasses. These glasses typically have side protection are designed to protect primarily against impacts, though they do offer minor splash protection. The second type is safety goggles. Goggles cover more of the face and have their own categories, including vented and non-vented. Safety goggles are typically primarily protection against chemicals and chemical splashes, though they can offer minor impact protection. Third is face shields. These cover the entire face, providing greater splash protection. Finally, full-face respirators can also be considered eye protection, typically protecting against both chemicals and impacts.
Other forms of eye PPE exists, including welder's gear and varieties of the above categories where the lenses are tinted to protect against hot work, bright lights, or lasers. It is common in many organization's safety regulations to require eye protection to be put on before entering a laboratory or hazardous environment (or immediately after entering).
Sources/Further Reading: (Image source - University of Toronto Book Chapter) (UCSF) (True PPE) (3M) (University of Washington)
40 notes
·
View notes
Text
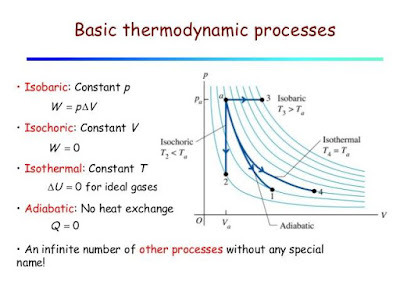
Thermodynamic Processes: Isobaric, Isochoric, Isothermal, and Adiabatic
A process in thermodynamics is the method by which the initial state of a system changes to the final state of a system. Technically speaking, there are countless types of thermodynamic processes to define each possible method in existence, most of which are unnamed. Of the specific named thermodynamic processes, there are four basic options.
Isobaric, isochoric, and isothermal (and, indeed, any other processes with the prefix iso-) are all processes in which a single variable is kept constant. For isobaric, pressure is constant, for isochoric, volume is constant, and for isothermal, temperature is constant. The fourth option, adiabatic processes, are processes in which there is no exchange of heat.
Sources/Further Reading: (Image source - Mechanics Tips blog) (University of Illinois lecture slides) (University of Central Florida) (Wikipedia) (Law of Thermodynamics Info)
51 notes
·
View notes
Text
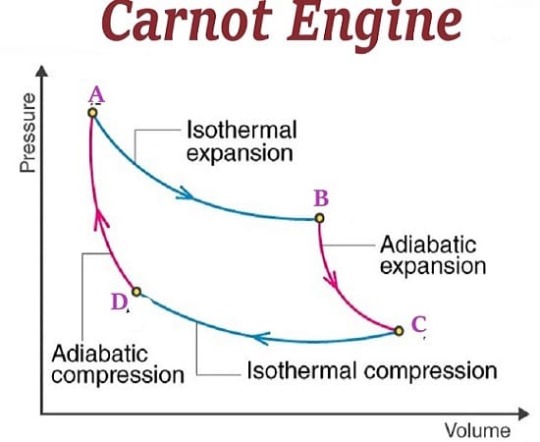
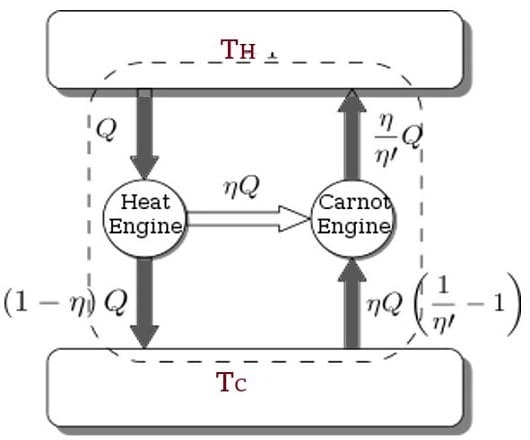
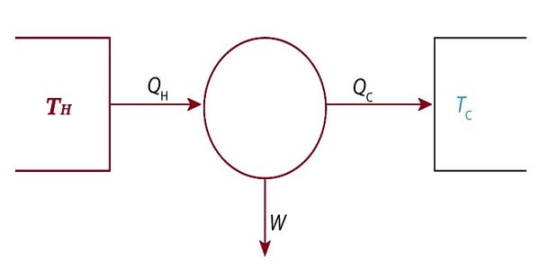
The Carnot Engine
The Carnot cycle is a specific thermodynamic cycle with four parts first designed by Nicolas Leonard Sadi Carnot. The Carnot engine, theorized by the same man, is a theoretical heat engine that operates on the Carnot cycle. It is an idealized, reversible process operating between two temperatures that represents the highest possible efficiency of a heat engine (between those two temperatures) - any irreversible process will have lower efficiency, any other reversible process will have equal efficiency. The second law of thermodynamics can be stated with the Carnot engine in mind, and mathematical study of this cycle ultimately lead to the discovery of the concept of entropy over thirty years later.
Sources/Further Reading: (Images source - Mechanical Boost) (LibreTexts) (Wikipedia)
29 notes
·
View notes
Text


Case Study: Bay Bridge Bolt Failure
Also known as the San Francisco-Oakland Bay Bridge, the east section of the Bay Bridge collapsed in 1989 as a result of earthquakes in the area. The decision was eventually made to replace the entire bridge with a more earthquake resistant structure, with the western portion begining construction in 2004. In 2013 the eastern section of the new bridge was completed. However, construction did not go as planned. When pre-tension was applied, before finalization of construction, 32 of 96 A354BD bolts fractured within two weeks.
The bolts that failed were placed in 2008 as part of the shear keys meant to increase seismic resistance, meaning they did not contribute to the structure of the bridge. After the failure, all 96 bolts in the shear keys were considered suspect and alternative anchors were devised. However, the A354BD bolts were used in other places of construction so a detailed metallurgical analysis was conducted to ensure the bolts throughout the bridge would not fail as well. The failure was ultimately determined to be a result of hydrogen embrittlement. It was also eventually concluded that this was a result of the environment the rods were subjected to (seawater), not the manufacturing process, and the bolts used elsewhere throughout the bridge were allowed to remain supplemental corrosion protection measures.
Sources/Further Reading: (Images source - Structure Magazine) (TBPOC Report) (TWI Presentation)
30 notes
·
View notes
Text

Hydrogen Embrittlement
When hydrogen diffuses into certain metals, it can reduce the ductility of the material in a process known as hydrogen embrittlement, hydrogen-assisted cracking, or hydrogen-induced cracking. This is most common in steels and other ferrous alloys (stainless steel is less susceptible), but can also occur in titanium, nickel, and cobalt alongside their alloys. The hydrogen itself is not enough to cause failure, but coupled with an external stress can lead to cracking.
Sources/Further Reading: (Image source - Wikipedia) (TWI) (2020 article) (2018 article)
33 notes
·
View notes