#ideal relationship dynamic: thermodynamic equilibrium
Note
I've been a quiet fan of Nievan for a while and you wanting to talk about him on the latest dress art has me breaking my silence- since he ans Astarion are so touchy (which I love!) How does "sleeping" go. Are they tangled together? Do they move around a lot? Does Fern ever climb in with them and add to the mess??
Or just talk to us about him in general I love to hear about everyone's tavs (especially those paired with Astarion) 🖤🖤
(ty for the ask!! I love talking about them hehe)
Nievan likes to sleep as close as physically possible and also produces heat like he’s the sun itself (a perk of being half sun elf in my head). It took Astarion a while to get used to it, but I think eventually it got to a point he made it his personal mission to press himself as close as possible and refuse to disentangle himself. So Niev usually sleeps with Astarion’s cold ass nose pressed into his neck and come morning Astarion’s whole body has been warmed through, no blood drinking needed :>

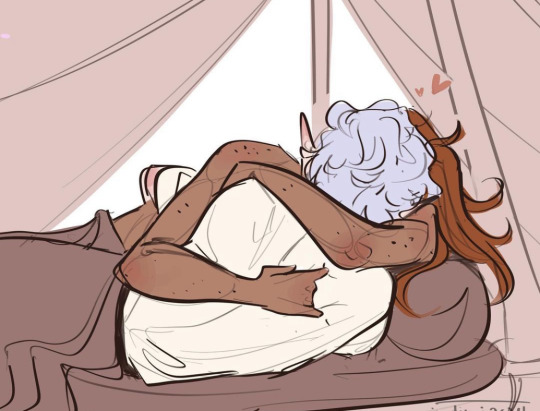
bonus: after Fern was born, they had to make some room because she really likes to kick in her sleep

:)
#bg3#astarion#tav#nievan ilthurin#ideal relationship dynamic: thermodynamic equilibrium#indimi asks#baldurs gate 3#baldur’s gate 3#baldurs gate fanart#bg3 tav#bg3 astarion#tav x astarion#fern ilthurin ancunin
82 notes
·
View notes
Text
Ideal Relationship Dynamic:
Autistic person who is mostly non speaking but doesn't mind being talked at
AND
Autistic person who is literally incapable of shutting the fuck up about their special interest for even a second.
It's like thermodynamic equilibrium but with talking.
#autistic#autism#relationship dynamics#platonic or romantic#either way it's cool#i want a relationship like this#a possum post™️
7 notes
·
View notes
Photo
(via Board Chemistry Question Paper - Resources & Key Concepts)
11th class Chemistry - Resources & Key Concepts
Chapter 1: Some Basic Concepts of Chemistry
➭ Matter, states of matter, and their properties
➭ Laws of Chemical Combination
➭ Dalton's atomic theory
➭ Mole concept and stoichiometry
➭ Chemical equations and balancing
Chapter 2: Structure of the Atom
➭ Subatomic particles: protons, neutrons, electrons
➭ Atomic number, mass number, isotopes
➭ Atomic models: Rutherford, Bohr, modern quantum mechanical model
➭ Electronic configuration and Aufbau principle
➭ Periodic trends: ionization enthalpy, electron affinity, atomic size
Chapter 3: Classification of Elements and Periodicity in Properties
➭ Modern periodic table and its organization
➭ Classification of elements into metals, non-metals, and metalloids
➭ Periodic trends in physical and chemical properties
Chapter 4: Chemical Bonding and Molecular Structure
➭ Ionic bonding: formation, characteristics, examples
➭ Covalent bonding: Lewis structure, octet rule, types of covalent bonds (single, double, triple)
➭ Coordinate covalent bonding
➭ Metallic bonding
➭ VSEPR theory and shapes of molecules
Chapter 5: States of Matter
➭ Gaseous state: kinetic theory of gases, ideal gas equation, gas laws
➭ Liquid state: properties of liquids, intermolecular forces (hydrogen bonding, dipole-dipole interactions, London dispersion forces)
➭ Solid state: crystalline and amorphous solids, types of crystals, unit cell
Chapter 6: Thermodynamics
➭ System, surroundings, types of systems (open, closed, isolated)
➭ The first law of thermodynamics: internal energy, work, heat
➭ Enthalpy (H) and its calculations
➭ Hess's law of constant heat summation
➭ Second law of thermodynamics: entropy (S), the spontaneity of reactions, Gibbs free energy (G)
Chapter 7: Equilibrium
➭ Reversible and irreversible reactions
➭ Chemical equilibrium: dynamic equilibrium, equilibrium constant (Kp andKc)
➭ Factors affecting equilibrium (concentration, temperature, pressure)
➭ Le Chatelier's principle
Chapter 8: Redox Reactions
➭ Oxidation and reduction, redox reactions
➭ Balancing redox reactions by oxidation number method
➭ Types of redox reactions (combination, decomposition, displacement)
➭ Electrochemical cells: galvanic and electrolytic cells
Chapter 9: The s-Block Elements (Li, Na, K, Mg, Ca, Sr, Ba)
➭ Electronic configuration trends
➭ Physical and chemical properties of each element
➭ Important compounds and their applications (e.g., sodium chloride, calcium carbonate)
➭ Diagonal relationships between Li and Mg, Be and Al
Chapter 10: The p-Block Elements (Group 13 to 17)
➭ Electronic configuration trends for each group
➭ Physical and chemical properties of each element group (e.g., Group 13 - Boron and Aluminum, Group 14 - Carbon and Silicon, Group 15 - Nitrogen and Phosphorus)
➭ Important compounds and their applications (e.g., boric acid, ammonia, sulfuric acid)
➭ Allotropy (e.g., carbon as diamond and graphite)
➭ Catenation (ability to form long chains)
Chapter 11: Organic Chemistry - I
➭ Basic concepts of organic chemistry (bonds, functional groups)
➭ Hydrocarbons: alkanes, alkenes, alkynes, aromatic hydrocarbons
➭ Isomerism (structural, geometrical, optical)
➭ Nomenclature of organic compounds (IUPAC system)
➭ Reactions of alkanes (substitution, combustion)
Chapter 12: Organic Chemistry - II
➭ Alcohols, phenols, ethers
➭ Aldehydes, ketones, carboxylic acids
➭ Amines, amides
➭ Organic compounds in everyday life (drugs, polymers, dyes)
Chapter 13: Polymers
➭ Classification of polymers (addition, condensation)
➭ Natural and synthetic polymers
➭ Important polymers and their properties (e.g., polyethylene, nylon, polyester)
➭ Biodegradable polymers and environmental concerns
Chapter 14: Environmental Chemistry
➭ Environmental pollution: types, sources, effects
➭ Air pollution, water pollution, soil pollution
➭ Strategies for pollution control and waste management
➭ Green chemistry and sustainable development
0 notes
Note
Perfect. I produce enough heat the even dulled senses could feel it and Lawrence is ice cold. We’ll balance each others heating issues
Thermodynamic equilibrium, the ideal relationship dynamic.
#Not Writing#BTD Chat#Lawrence Oleander#Asks#i don't even know if that's the right term#i spent most of my middle school science classes crying lmao
31 notes
·
View notes
Text
JEE Main Chemistry Syllabus
The National Test Agency (NTA) has released the official Chemistry syllabus of JEE Main 2020. Candidates are advised to check the JEE Main Chemistry syllabus to get an idea about the topics from where the questions are expected to appear in the exam. Engineering aspirants dreaming to crack JEE Main should ensure that they are familiar with the exam syllabus. JEE Main syllabus for Chemistry will be based on the topics covered in Class 10 and Class 12. The JEE Main syllabus for Chemistry gives the section wise break up of units, chapters, and topics. In the chemistry syllabus of JEE Main, there are three sections in total. Section A comprises of Physical Chemistry, Section B consists of topics on Inorganic Chemistry and Section C includes Organic Chemistry. Each section of JEE Main chemistry syllabus mentions in detail the topics which fall under each unit and chapter. Candidates can plan their preparation according to the syllabus for the chemistry of JEE Main 2020.
JEE Main Chemistry Syllabus:
The syllabus for Chemistry is divided into three sections - Physical Chemistry, Inorganic Chemistry, and Organic Chemistry.
Section A: Physical ChemistryUNIT 1: Some Basic concepts in ChemistryMatter and its nature, Dalton’s atomic theory; Concept of atom, molecule, element and compound; Physical quantities and their measurements in Chemistry, precision and accuracy, significant figures, S.I. Units, dimensional analysis; Laws of chemical combination; Atomic and molecular masses, mole concept, molar mass, percentage composition, empirical and molecular formulae; Chemical equations and stoichiometry.
UNIT 2: States of MatterClassification of matter into solid, liquid and gaseous states.Gaseous State:Measurable properties of gases; Gas laws - Boyle’s law, Charle’s law, Graham’s law of diffusion, Avogadro’s law, Dalton’s law of partial pressure; Concept of Absolute scale of temperature; Ideal gas equation, Kinetic theory of gases (only postulates); Concept of average, root mean square and most probable velocities; Real gases, deviation from Ideal behaviour, compressibility factor, van der Waals equation, liquefaction of gases, critical constants.Liquid State:Properties of liquids- Vapour pressure, viscosity and surface tension, and effect of temperature on them (qualitative treatment only).Solid State:Classification of solids: molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea); Bragg’s Law and its applications; Unit cell and lattices, packing in solids (fcc, bcc and hcp lattices), voids, calculations involving unit cell parameters, imperfection in solids; Electrical, magnetic and dielectric properties.
UNIT 3: Atomic Structure
Discovery of sub-atomic particles (electron, proton and neutron); Thomson and Rutherford atomic models and their limitations; Nature of electromagnetic radiation, photoelectric effect; Spectrum of hydrogen atom, Bohr model of hydrogen atom - its postulates, derivation of the relations for energy of the electron and radii of the different orbits, limitations of Bohr’s model; Dual nature of matter, de-Broglie’s relationship, Heisenberg uncertainty principle.
Elementary ideas of quantum mechanics, quantum mechanical model of atom, its important features, concept of atomic orbitals as one electron wave functions; Variation of ? and ?2 with r for 1s and 2s orbitals; various quantum numbers (principal, angular momentum and magnetic quantum numbers) and their significance; shapes of s, p and d - orbitals, electron spin and spin quantum number; Rules for filling electrons in orbitals - Aufbau principle, Pauli’s exclusion principle and Hund’s rule, electronic configuration of elements, extra stability of half-filled and completely filled orbitals.
UNIT 4: Chemical Bonding and Molecular StructureKossel - Lewis approach to chemical bond formation, the concept of ionic and covalent bonds.Ionic Bonding: Formation of ionic bonds, factors affecting the formation of ionic bonds; calculation of lattice enthalpy.Covalent Bonding: Concept of electronegativity, Fajan’s rule, dipole moment; Valence Shell Electron Pair Repulsion (VSEPR) theory and shapes of simple molecules.Quantum mechanical approach to covalent bonding: Valence bond theory - Its important features, the concept of hybridization involving s, p and d orbitals; Resonance.Molecular Orbital Theory - Its important features, LCAOs, types of molecular orbitals (bonding, antibonding), sigma and pi-bonds, molecular orbital electronic configurations of homonuclear diatomic molecules, the concept of bond order, bond length and bond energy.Elementary idea of metallic bonding. Hydrogen bonding and its applications.
UNIT 5: Chemical ThermodynamicsFundamentals of thermodynamics: System and surroundings, extensive and intensive properties, state functions, types of processes.First law of thermodynamics: Concept of work, heat internal energy and enthalpy, heat capacity, molar heat capacity; Hess’s law of constant heat summation; Enthalpies of bond dissociation, combustion, formation, atomization, sublimation, phase transition, hydration, ionization and solution.Second law of thermodynamics: Spontaneity of processes; ?S of the universe and ?G of the system as criteria for spontaneity, ?Go (Standard Gibbs energy change) and equilibrium constant.
UNIT 6: SolutionsDifferent methods for expressing concentration of solution - molality, molarity, mole fraction, percentage (by volume and mass both), vapour pressure of solutions and Raoult’s Law - Ideal and non-ideal solutions, vapour pressure - composition, plots for ideal and non-ideal solutions; Colligative properties of dilute solutions - relative lowering of vapour pressure, depression of freezing point, elevation of boiling point and osmotic pressure; Determination of molecular mass using colligative properties; Abnormal value of molar mass, van’t Hoff factor and its significance.
UNIT 7: EquilibriumMeaning of equilibrium, the concept of dynamic equilibrium.Equilibria involving physical processes: Solid-liquid, liquid - gas and solid - gas equilibria, Henry’s law, general characteristics of equilibrium involving physical processes.Equilibria involving chemical processes: Law of chemical equilibrium, equilibrium constants (Kp and Kc) and their significance, the significance of ?G and ?Go in chemical equilibria, factors affecting equilibrium concentration, pressure, temperature, effect of catalyst; Le Chatelier’s principle.Ionic equilibrium: Weak and strong electrolytes, ionization of electrolytes, various concepts of acids and bases (Arrhenius, Bronsted - Lowry and Lewis) and their ionization, acid-base equilibria (including multistage ionization) and ionization constants, ionization of water, pH scale, common ion effect, hydrolysis of salts and pH of their solutions, solubility of sparingly soluble salts and solubility products, buffer solutions.
UNIT 8: Redox Reactions and ElectrochemistryElectronic concepts of oxidation and reduction, redox reactions, oxidation number, rules for assigning oxidation number, balancing of redox reactions.Electrolytic and metallic conduction, conductance in electrolytic solutions, specific and molar conductivities and their variation with concentration: Kohlrausch’s law and its applications.Electrochemical cells - Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half - cell and cell reactions, emf of a Galvanic cell and its measurement; Nernst equation and its applications; Relationship between cell potential and Gibbs’ energy change; Dry cell and lead accumulator; Fuel cells; Corrosion and its prevention.
UNIT 9: Chemical KineticsRate of a chemical reaction, factors affecting the rate of reactions: concentration, temperature, pressure and catalyst; elementary and complex reactions, order and molecularity of reactions, rate law, rate constant and its units, differential and integral forms of zero and first order reactions, their characteristics and half-lives, effect of temperature on rate of reactions - Arrhenius theory, activation energy and its calculation, collision theory of bimolecular gaseous reactions (no derivation).
UNIT 10: Surface ChemistryAdsorption - Physisorption and chemisorption and their characteristics, factors affecting the adsorption of gases on solids - Freundlich and Langmuir adsorption isotherms, adsorption from solutions.Catalysis - Homogeneous and heterogeneous, activity and selectivity of solid catalysts, enzyme catalysis and its mechanism.Colloidal state - distinction among true solutions, colloids and suspensions, classification of colloids - lyophilic, lyophobic; multi molecular, macromolecular and associated colloids (micelles), preparation and properties of colloids - Tyndall effect, Brownian movement, electrophoresis, dialysis, coagulation and flocculation; Emulsions and their characteristics.
Section B: Inorganic ChemistryUNIT 11: Classification of Elements and Periodicity in PropertiesModem periodic law and present form of the periodic table, s, p, d and f block elements, periodic trends in properties of elements-atomic and ionic radii, ionization enthalpy, electron gain enthalpy, valence, oxidation states and chemical reactivity.
UNIT 12: General Principles and Process of Isolation of MetalsModes of occurrence of elements in nature, minerals, ores; steps involved in the extraction of metals - concentration, reduction (chemical. and electrolytic methods) and refining with special reference to the extraction of Al, Cu, Zn and Fe; Thermodynamic and electrochemical principles involved in the extraction of metals.
UNIT 13: HydrogenPosition of hydrogen in periodic table, isotopes, preparation, properties and uses of hydrogen; Physical and chemical properties of water and heavy water; Structure, preparation, reactions and uses of hydrogen peroxide; Classification of hydrides - ionic, covalent and interstitial; Hydrogen as a fuel.
UNIT 14: s Block Elements (Alkali and Alkaline Earth Metals)Group 1 and Group 2 ElementsGeneral introduction, electronic configuration and general trends in physical and chemical properties of elements, anomalous properties of the first element of each group, diagonal relationships.Preparation and properties of some important compounds - sodium carbonate, sodium chloride, sodium hydroxide and sodium hydrogen carbonate; Industrial uses of lime, limestone, Plaster of Paris and cement; Biological significance of Na, K, Mg and Ca.
UNIT 15: p Block ElementsGroup 13 to Group 18 ElementsGeneral Introduction: Electronic configuration and general trends in physical and chemical properties of elements across the periods and down the groups; unique behaviour of the first element in each group.Groupwise study of the p – block elementsGroup - 13Preparation, properties and uses of boron and aluminium; Structure, properties and uses of borax, boric acid, diborane, boron trifluoride, aluminium chloride and alums.Group - 14Tendency for catenation; Structure, properties and uses of allotropes and oxides of carbon, silicon tetrachloride, silicates, zeolites and silicones.Group - 15Properties and uses of nitrogen and phosphorus; Allotrophic forms of phosphorus; Preparation, properties, structure and uses of ammonia, nitric acid, phosphine and phosphorus halides, (PCl3, PCl5); Structures of oxides and oxoacids of nitrogen and phosphorus.Group - 16Preparation, properties, structures and uses of dioxygen and ozone; Allotropic forms of sulphur; Preparation, properties, structures and uses of sulphur dioxide, sulphuric acid (including its industrial preparation); Structures of oxoacids of sulphur.Group - 17Preparation, properties and uses of chlorine and hydrochloric acid; Trends in the acidic nature of hydrogen halides; Structures of Interhalogen compounds and oxides and oxoacids of halogens.Group - 18Occurrence and uses of noble gases; Structures of fluorides and oxides of xenon.
UNIT 16: d – and f – Block ElementsTransition ElementsGeneral introduction, electronic configuration, occurrence and characteristics, general trends in properties of the first row transition elements - physical properties, ionization enthalpy, oxidation states, atomic radii, colour, catalytic behaviour, magnetic properties, complex formation, interstitial compounds, alloy formation; Preparation, properties and uses of K2Cr2O7 and KMnO4.Inner Transition ElementsLanthanoids - Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction.Actinoids - Electronic configuration and oxidation states.
UNIT 17: Co-ordination CompoundsIntroduction to co-ordination compounds, Werner’s theory; ligands, coordination number, denticity, chelation; IUPAC nomenclature of mononuclear co-ordination compounds, isomerism; Bonding-Valence bond approach and basic ideas of Crystal field theory, colour and magnetic properties; Importance of co-ordination compounds (in qualitative analysis, extraction of metals and in biological systems).
UNIT 18: Environmental ChemistryEnvironmental pollution - Atmospheric, water and soil.Atmospheric pollution - Tropospheric and stratosphericTropospheric pollutants - Gaseous pollutants: Oxides of carbon, nitrogen and sulphur, hydrocarbons; their sources, harmful effects and prevention; Green house effect and Global warming; Acid rain; Particulate pollutants: Smoke, dust, smog, fumes, mist; their sources, harmful effects and prevention.Stratospheric pollution - Formation and breakdown of ozone, depletion of ozone layer - its mechanism and effects.Water Pollution - Major pollutants such as pathogens, organic wastes and chemical pollutants; their harmful effects and prevention.Soil pollution - Major pollutants such as: Pesticides (insecticides,. herbicides and fungicides), their harmful effects and prevention.Strategies to control environmental pollution.Section-C: Organic Chemistry
UNIT 19: Purification and Characterisation of Organic Compounds Purification - Crystallization, sublimation, distillation, differential extraction and chromatography - principles and their applications.Qualitative analysis - Detection of nitrogen, sulphur, phosphorus and halogens.Quantitative analysis (basic principles only) - Estimation of carbon, hydrogen, nitrogen, halogens, sulphur, phosphorus.Calculations of empirical formulae and molecular formulae; Numerical problems in organic quantitative analysis.
UNIT 20: Some Basic Principles of Organic Chemistry Tetravalency of carbon: Shapes of simple molecules - hybridization (s and p); Classification of organic compounds based on functional groups: - C = C - , - C ? C - and those containing halogens, oxygen, nitrogen and sulphur; Homologous series; Isomerism - structural and stereoisomerism.Nomenclature (Trivial and IUPAC)Covalent bond fission - Homolytic and heterolytic: free radicals, carbocations and carbanions; stability of carbocations and free radicals, electrophiles and nucleophiles.Electronic displacement in a covalent bond - Inductive effect, electromeric effect, resonance and hyperconjugation.Common types of organic reactions - Substitution, addition, elimination and rearrangement.
UNIT 21: HydrocarbonsClassification, isomerism, IUPAC nomenclature, general methods of preparation, properties and reactions.Alkanes - Conformations: Sawhorse and Newman projections (of ethane); Mechanism of halogenation of alkanes.Alkenes - Geometrical isomerism; Mechanism of electrophilic addition: addition of hydrogen, halogens, water, hydrogen halides (Markownikoff’s and peroxide effect); Ozonolysis, oxidation, and polymerization.Alkynes - Acidic character; Addition of hydrogen, halogens, water and hydrogen halides; Polymerization.Aromatic hydrocarbons - Nomenclature, benzene - structure and aromaticity; Mechanism of electrophilic substitution: halogenation, nitration, Friedel - Craft’s alkylation and acylation, directive influence of functional group in mono-substituted benzene.
UNIT 22: Organic Compounds Containing HalogensGeneral methods of preparation, properties and reactions; Nature of C-X bond; Mechanisms of substitution reactions.Uses; Environmental effects of chloroform, iodoform, freons and DDT.
UNIT 23: Organic Compounds Containing OxygenGeneral methods of preparation, properties, reactions and uses.Alcohols, Phenols and EthersAlcohols: Identification of primary, secondary and tertiary alcohols; mechanism of dehydration.Phenols: Acidic nature, electrophilic substitution reactions: halogenation, nitration and sulphonation, Reimer - Tiemann reaction.Ethers: Structure.Aldehyde and KetonesNature of carbonyl group; Nucleophilic addition to >C=O group, relative reactivities of aldehydes and ketones; Important reactions such as - Nucleophilic addition reactions (addition of HCN, NH3 and its derivatives), Grignard reagent; oxidation; reduction (Wolff Kishner and Clemmensen); acidity of ? - hydrogen, aldol condensation, Cannizzaro reaction, Haloform reaction; Chemical tests to distinguish between aldehydes and Ketones.Carboxylic Acids: Acidic strength and factors affecting it.
UNIT 24: Organic Compounds Containing NitrogenGeneral methods of preparation, properties, reactions and uses.Amines: Nomenclature, classification, structure, basic character and identification of primary, secondary and tertiary amines and their basic character.Diazonium Salts: Importance in synthetic organic chemistry.
UNIT 25: PolymersGeneral introduction and classification of polymers, general methods of polymerization - addition and condensation, copolymerization;Natural and synthetic rubber and vulcanization; some important polymers with emphasis on their monomers and uses - polyethene, nylon, polyester and bakelite.
UNIT 26: Bio MoleculesGeneral introduction and importance of biomolecules.Carbohydrates - Classification: aldoses and ketoses; monosaccharides (glucose and fructose), constituent monosaccharides of oligosacchorides (sucrose, lactose, maltose) and polysaccharides (starch, cellulose, glycogen).Proteins - Elementary Idea of amino acids, peptide bond, polypeptides; Proteins: primary, secondary, tertiary and quaternary structure (qualitative idea only), denaturation of proteins, enzymes.Vitamins - Classification and functions.Nucleic Acids - Chemical constitution of DNA and RNA. Biological functions of nucleic acids.
UNIT 27: Chemistry in Everyday LifeChemicals in medicines - Analgesics, tranquillizers, antiseptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines - their meaning and common examples.Chemicals in food - Preservatives, artificial sweetening agents - common examples.Cleansing agents - Soaps and detergents, cleansing action.
UNIT 28: Principles Related to Practical ChemistryDetection of extra elements (N, S, halogens) in organic compounds; Detection of the following functional groups: hydroxyl (alcoholic and phenolic), carbonyl (aldehyde and ketone), carboxyl and amino groups in organic compounds.Chemistry involved in the preparation of the following:Inorganic compounds: Mohr’s salt, potash alum.Organic compounds: Acetanilide, p-nitroacetanilide, aniline yellow, iodoform.Chemistry involved in the titrimetric exercises - Acids bases and the use of indicators, oxalic-acid vs KMnO4, Mohr’s salt vs KMnO4.Chemical principles involved in the qualitative salt analysis:Cations - Pb2+ , Cu2+, AI3+, Fe3+, Zn2+, Ni2+, Ca2+, Ba2+, Mg2+, NH4+.Anions- CO32-, S2-, SO42-, NO2-, NO3-, CI-, Br, I. (Insoluble salts excluded).Chemical principles involved in the following experiments:1. Enthalpy of solution of CuSO42. Enthalpy of neutralization of strong acid and strong base. .3. Preparation of lyophilic and lyophobic sols.4. Kinetic study of reaction of iodide ion with hydrogen peroxide at room temperature.
0 notes
Link
GET THIS BOOK
Author:
George W. Collins, II
Published in: W H Freeman & Co Release Year: 1989 ISBN: 978-0716-7-1993-9 Pages: 525 Edition: First Edition File Size: 9 MB File Type: pdf Language: English
(adsbygoogle = window.adsbygoogle || []).push({});
Description of The Fundamentals of Stellar Astrophysics
One may justifiability wonder why anyone would take the time to put a decade-old book on astrophysics on the WEB. Several events of the past few months have led me to believe that may well be some who wish to learn about the basics of stellar structure. Since the fundamentals of stellar astrophysics have changed little in the past decade and as The Fundamentals of Stellar Astrophysics book has been out of print for nearly that long, I felt that some may still find it useful for learning the basics.
The task was somewhat facilitated by my discovery of some old machine-readable disks that contained a version of the book including some of the corrections to the published version. With considerable help from Charles Knox, I was able to retrieve the information from the out-dated format and transfer the text to a contemporary word processor. However, the equations were lost in the process so that their inclusion in The Fundamentals of Stellar Astrophysics edition had to take another form.
This was accomplished by scanning the originals from the book and correcting those with errors in a variety of ways. This accounts for the fonts of the equations being somewhat at variance with that of the text. However, I believe that difference does not detract significantly from the understandability of the material. The most common form of correction was to simply re-set them with an equation editor embedded in the WORD processor. Equations look somewhat different from the others.
However, the ability to correct errors that arose in the published edition seemed to outweigh any visual inconvenience.
The reader will notice that all the recommended reading is to books published prior to 1987. Some of this is a result of a predilection of mine to cite initial references, but most of it is a result of my failure to update the references to contemporary times. There have been a number of books and many articles during the past decade or so which would greatly enlighten the reader, but to include them would be a major part of a new book and lies beyond the scope of this effort.
Content of The Fundamentals of Stellar Astrophysics
Part I Stellar Interiors
Chapter 1
Introduction and Fundamental Principles
1.1 Stationary or “Steady” Properties of matter
a Phase Space and Phase Density
b Macrostates and Microstates.
c Probability and Statistical Equilibrium
d Quantum Statistics
e Statistical Equilibrium for a Gas
f Thermodynamic Equilibrium – Strict and Local
1.2 Transport Phenomena
a. Boltzmann Transport Equation
b. Homogeneous Boltzmann Transport Equation
and Liouville’s Theorem
c. Moments of the Boltzmann Transport Equation
and Conservation Laws
1.3 Equation of State for the Ideal Gas and Degenerate
Matter
Problems
References and Supplemental Reading
Chapter 2
Basic Assumptions, Theorems, and Polytropes
2.1 Basic Assumptions
2.2 Integral Theorems from Hydrostatic Equilibrium
a Limits of State Variables
b β
Theorem and Effects of Radiation
Pressure
2.3 Homology Transformations
2.4 Polytropes
a Polytropic Change and the Lane-Emden
Equation
b Mass-Radius Relationship for Polytropes
c Homology Invariants
d Isothermal Sphere
e Fitting Polytropes Together
Problems
References and Supplemental Reading
Chapter 3
Sources and Sinks of Energy
3.1 "Energies" of Stars
a Gravitational Energy
b Rotational Energy
c Nuclear Energy
3.2 Time Scales
a Dynamical Time Scale
b Kelvin-Helmholtz (Thermal) Time Scale
c Nuclear (Evolutionary) Time Scale
3.3 Generation of Nuclear Energy
a General Properties of the Nucleus
b The Bohr Picture of Nuclear Reactions
c Nuclear Reaction Cross Sections
d Nuclear Reaction Rates
e Specific Nuclear Reactions
Problems
References and Supplemental Reading
Chapter 4
Flow of Energy through the Star and Construction of Stellar
Models
4.1 The Ionization, Abundances, and Opacity of
Stellar Material
a Ionization and the Mean Molecular Weight
b Opacity
4.2 Radiative Transport and the Radiative Temperature
Gradient
a Radiative Equilibrium
b Thermodynamic Equilibrium and Net Flux
c Photon Transport and the Radiative Gradient
d Conservation of Energy and the Luminosity
4.3 Convective Energy Transport
a Adiabatic Temperature Gradient
b Energy Carried by Convection
4.4 Energy Transport by Conduction
a Mean Free Path
b Heat Flow
4.5 Convective Stability
a Efficiency of Transport Mechanisms
b Schwarzschild Stability Criterion
4.6 Equations of Stellar Structure
4.7 Construction of a Model Stellar Interior
a Boundary Conditions
b Schwarzschild Variables and Method
c Henyey Relaxation Method for Construction of
Stellar Models
Problems
References and Supplemental Reading
Chapter 5
Theory of Stellar Evolution
5.1 The Ranges of Stellar Masses, Radii, and
Luminosity
5.2 Evolution onto the Main Sequence
a Problems concerning the Formation of
Stars
b Contraction out of the Interstellar Medium
c Contraction onto the Main Sequence
5.3 The Structure and Evolution of Main Sequence Stars
a Lower Main Sequence Stars
b Upper Main Sequence Stars
5.4 Post Main Sequence Evolution
a Evolution off the Lower Main Sequence
b Evolution away from the Upper Main Sequence
c The Effect of Mass-loss on the Evolution of Stars
5.5 Summary and Recapitulation
a Core Contraction - Envelope Expansion: Simple
Reasons
b Calculated Evolution of a 5 M⊙ star
Problems
References and Supplemental Reading
Chapter 6
Relativistic Stellar Structure
6.1 Field Equations of the General Theory of Relativity
6.2 Oppenheimer-Volkoff Equation of Hydrostatic
Equilibrium
a Schwarzschild Metric
b Gravitational Potential and Hydrostatic
Equilibrium
6.3 Equations of Relativistic Stellar Structure and
Their Solutions
a A Comparison of Structure Equations
b A Simple Model
c Neutron Star Structure
6.4 Relativistic Polytrope of Index 3
a Virial Theorem for Relativistic Stars
b Minimum Radius for White Dwarfs
c Minimum Radius for Super-massive Stars
6.5 Fate of Super-massive Stars
a Eddington Luminosity
b Equilibrium Mass-Radius Relation
c Limiting Masses for Super-massive Stars
Problems
References and Supplemental Reading
Chapter 7
Structure of Distorted Stars
7.1 Classical Distortion: The Structure Equations
a A Comparison of Structure Equations
b Structure Equations for Cylindrical Symmetry
7.2 Solution of Structure Equations for a Perturbing
Force
a Perturbed Equation of Hydrostatic Equilibrium
b Number of Perturbative Equations versus Number
of Unknowns
7.3 Von Zeipel's Theorem and Eddington-Sweet
Circulation Currents
a Von Zeipel's Theorem
b Eddington-Sweet Circulation Currents
7.4 Rotational Stability and Mixing
a Shear Instabilities
b Chemical Composition Gradient and Suppression
of Mixing
c Additional Types of Instabilities
Problems
References and Supplemental Reading
Chapter 8
Stellar Pulsation and Oscillation
8.1 Linear Adiabatic Radial Oscillations
a Stellar Oscillations and the Variational Virial
theorem
b Effect of Magnetic Fields and Rotation on Radial
Oscillations
c Stability and the Variational Virial Theorem
d Linear Adiabatic Wave Equation
8.2 Linear Nonadiabatic Radial Oscillations
a Adiabatic Exponents
b Nonadiabatic Effects and Pulsational Stability
c Constructing Pulsational Models
d Pulsational Behavior of Stars
8.3 Nonradial Oscillations
a Nature and Form of Oscillations
b Homogeneous Model and Classification of Modes
c Toroidal Oscillations
d Nonradial Oscillations and Stellar Structure
Problems
References and Supplemental Reading
Epilogue to Part I: Stellar Interiors
Part II Stellar Atmospheres
Chapter 9
The Flow of Radiation Through the Atmosphere
9.1 Basic Assumptions for the Stellar Atmosphere
a Breakdown of Strict Thermodynamic
Equilibrium 228
b Assumption of Local Thermodynamic
Equilibrium 229
c Continuum and Spectral Lines 230
d Additional Assumptions of Normal Stellar
Atmospheres 231
9.2 Equation of Radiative Transfer 233
a Specific Intensity and Its Relation to the Density
of Photons in Phase Space 233
b General Equation of Radiative Transfer
c "Creation" Rate and the Source Function
d Physical Meaning of the Source Function 240
e Special Forms of the Redistribution Function 241
9.3 Moments of the Radiation Field 243
a Mean Intensity 244
b Flux 244
c Radiation Pressure 245
9.4 Moments of the Equation of Radiative Transfer
a Radiative Equilibrium and Zeroth Moment of the
Equation of Radiative Transfer
b First Moment of the Equation of Radiative
Transfer and the Diffusion Approximation
c Eddington Approximation 249
Problems 251
Supplemental Reading 252
Chapter 10
Solution of the Equation of Radiative Transfer 253
10.1 Classical Solution to the Equation of Radiative Transfer
and Integral Equations for the Source Function 254
a Classical Solution of the Equation of Transfer for
the Plane-Parallel Atmosphere 254
b Schwarzschild-Milne Integral Equations 257
c Limb-darkening in a Stellar Atmosphere 260
10.2 Gray Atmosphere 263
a Solution of Schwarzschild-Milne Equations for
the Gray Atmosphere 265
b Solutions for the Gray Atmosphere Utilizing the
Eddington Approximation 266
c Solution by Discrete Ordinates: Wick-
Chandrasekhar Method 268
10.3 Nongray Radiative Transfer 274
a Solutions of the Nongray Integral Equation for the
Source Function 275
b Differential Equation Approach: The Feautrier
Method 276
10.4 Radiative Transport in a Spherical Atmosphere 279
a Equation of Radiative Transport in Spherical
Coordinates
b An Approach to Solution of the Spherical Radiative
Transfer Problem 283
Problems 287
References and Supplemental Reading 289
Chapter 11
Environment of the Radiation Field 291
11.1 Statistics of the Gas and the Equation of State 292
a Boltzmann Excitation Formula 292
b Saha Ionization Equilibrium Equation 293
11.2 Continuous Opacity 296
a Hydrogenlike Opacity 296
b Neutral Helium 297
c Quasi-atomic and Molecular States 297
d Important Sources of Continuous Opacity for
Main Sequence Stars 299
11.3 Einstein Coefficients and Stimulated Emission 300
a Relations among Einstein Coefficients 301
b Correction of the Mass Absorption Coefficient for
Stimulated Emission 302
11.4 Definitions and Origins of Mean Opacities 303
a Flux-Weighted (Chandrasekhar) Mean Opacity 304
b Rosseland Mean Opacity 304
c Planck Mean Opacity 306
11.5 Hydrostatic Equilibrium and the Stellar Atmosphere 307
Problems 308
References 309
Chapter 12
The Construction of a Model Stellar Atmosphere 310
12.1 Statement of the Basic Problem 310
12.2 Structure of the Atmosphere, Given the Radiation Field 312
a Choice of the Independent Variable of
Atmospheric Depth 314
b Assumption of Temperature Dependence with
Depth 314
c Solution of the Equation of Hydrostatic
Equilibrium 314
12.3 Calculation of the Radiation Field of the Atmosphere 316
12.4 Correction of the Temperature Distribution and Radiative
Equilibrium 318
a Lambda Iteration Scheme 318
b Avrett-Krook Temperature Correction Scheme 319
12.5 Recapitulation 325
Problems 326
References and Supplemental Reading 328
Chapter 13
Formation of Spectral Lines 330
13.1 Terms and Definitions Relating to Spectral Lines 331
a Residual Intensity, Residual Flux, and
Equivalent Width 331
b Selective (True) Absorption and Resonance
Scattering 333
c Equation of Radiative Transfer for Spectral
Line Radiation 335
13.2 Transfer of Line Radiation through the Atmosphere 336
a Schuster-Schwarzschild Model Atmosphere for
Scattering Lines 336
b Milne-Eddington Model Atmosphere for the
Formation of Spectral Lines 339
Problems 346
Supplemental Reading 347
Chapter 14
Shape of Spectral Lines 348
14.1 Relation between the Einstein, Mass Absorption, and
Atomic Absorption Coefficients 349
14.2 Natural or Radiation Broadening 350
a Classical Radiation Damping 351
b Quantum Mechanical Description of Radiation
Damping 354
c Ladenburg f-value 355
14.3 Doppler Broadening of Spectral Lines 357
a Microscopic Doppler Broadening 358
b Macroscopic Doppler Broadening 364
14.4 Collisional Broadening 369
a Impact Phase-Shift Theory 370
b Static (Statistical) Broadening Theory 378
14.5 Curve of Growth of the Equivalent Width 385
a Schuster-Schwarzschild Curve of Growth 385
b More Advanced Models for the Curve of Growth 389
c Uses of the Curve of Growth 390
Problems 392
References and Supplemental Reading 395
Chapter 15
Breakdown of Local Thermodynamic Equilibrium 398
15.1 Phenomena Which Produce Departures from Local
Thermodynamic Equilibrium 400
a Principle of Detailed Balancing 400
b Interlocking 401
c Collisional versus Photoionization 402
15.2 Rate Equations for Statistical Equilibrium 403
a Two-Level Atom 403
b Two-Level Atom plus Continuum 407
c Multilevel Atom 409
d Thermalization Length 410
15.3 Non-LTE Transfer of Radiation and the Redistribution
Function 411
a Complete Redistribution 412
b Hummer Redistribution Functions 413
15.4 Line Blanketing and Its Inclusion in the construction of
Model Stellar Atmospheres and Its Inclusion in the
Construction of Model Stellar Atmospheres 425
a Opacity Sampling 426
b Opacity Distribution Functions 427
Problems 429
References and Supplemental Reading 430
Chapter 16
Beyond the Normal Stellar Atmosphere 432
16.1 Illuminated Stellar Atmospheres 434
a Effects of Incident Radiation on the Atmospheric
Structure 434
b Effects of Incident Radiation on the Stellar Spectra 439
16.2 Transfer of Polarized Radiation 440
a Representation of a Beam of Polarized Light and
the Stokes Parameters 440
b Equations of Transfer for the Stokes 445
c Solution of the Equations of Radiative Transfer
for Polarized Light. 454
d Approximate Formulas for the Degree of
Emergent Polarization 457
e Implications of the Transfer of Polarization for
Stellar Atmospheres 465
16.3 Extended Atmospheres and the Formation of Stellar
Winds 469
a Interaction of the Radiation Field with the Stellar
Wind 470
b Flow of Radiation and the Stellar Wind 474
Problems 477
References and Supplemental Reading 478
Epilog 480
Index 483
Errata to the W. H. Freeman edition.
0 notes
Text
a more specific GRE study guide by topic
I. ANALYTICAL CHEMISTRY — 15%
Data Acquisition and Use of Statistics — Errors, statistical considerations
Solutions and Standardization — Concentration terms, primary standards
Homogeneous Equilibria — Acid-base, oxidation-reduction, complexometry
Heterogeneous Equilibria — Gravimetric analysis, solubility, precipitation titrations, chemical separations
Instrumental Methods — Electrochemical methods (electrode reactions; galvanic cells; current flow in cells/electrolysis; emf and free energy nernst equation; oxidation-reduction reactions), spectroscopic methods, chromatographic methods, thermal methods, calibration of instruments, determination of atomic weights
Environmental Applications
Radiochemical Methods — Detectors, applications
II. INORGANIC CHEMISTRY — 25%
General Chemistry — Periodic trends, oxidation states, nuclear chemistry; (properties of matter; elements, compounds and mixtures; laws of conservation of mass and definite proportion; dalton atomic theory; stoichiometry), atomic nucleus, isotopes, periodic law and table, variation of prperties with atomic structure
Nuclear chemistry — spontaneous radioactive decay, nuclear transformations, nuclear stability, nuclear fission and fusion
Chemical bonding — ionic and covalentbonds, valence bond theory, hybrid orbitals, multiple bonds, resonance, bond order, electronegativity, dipole moments, polar molecules, molecular structure, the valence shell electron, repulsion theory, oxidation and reduction
Ionic Substances — Lattice geometries, lattice energies, ionic radii and radius/ratio effects, ionic reactions
Covelalent Molecular Substances — Lewis diagrams, molecular point groups, VSEPR concept, valence bond description and hybridization, molecular orbital description, bond energies, covalent and van der Waals radii of the elements, intermolecular forces
Liquids and solids — crystalline solids, lattices, amorphis solids, phase diagrams, phase rule
Solution chemistry — electrolytes, density and formality, normality, molarity, molality, neutralizing reaction, balancing of redox equations
Gases — Boyle’s law, Charles’s law, Dalton’s Law of Partial Pressures, Gay-Lussac’s Laws, Avogadro’s law, the mole concept, the ideal gas law, the kinetic theory of gases, real gases, the van der waals equation
Metals and Semiconductors — Structure, band theory, physical and chemical consequences of band theory
Concepts of Acids and Bases — Brønsted-Lowry approaches, Lewis theory, solvent system approaches, arrhenius defintion of acids and bases
Chemistry of the Main Group Elements — Electronic structures, occurrences and recovery, physical and chemical properties of the elements and their compounds
Chemistry of the Transition Elements — Electronic structures, occurrences and recovery, physical and chemical properties of the elements and their compounds, coordination chemistry
Special Topics — Organometallic chemistry, catalysis, bioinorganic chemistry, applied solid-state chemistry, environmental chemistry, fundamental particles
III. ORGANIC CHEMISTRY — 30%
Structure, Bonding and Nomenclature — Lewis structures, orbital hybridization, configuration and stereochemical notation, conformational analysis, systematic IUPAC nomenclature, spectroscopy (IR and 1H and 13C NMR)
Functional Groups — Preparation, reactions, and interconversions of alkanes, alkenes, alkynes, dienes, alkyl halides, alcohols, ethers, epoxides, sulfides, thiols, aromatic compounds, aldehydes, ketones, carboxylic acids and their derivatives, amines
Reaction Mechanisms — Nucleophilic displacements and addition, nucleophilic aromatic substitution, electrophilic additions, electrophilic aromatic substitutions, eliminations, Diels-Alder and other cycloadditions
Reactive Intermediates — Chemistry and nature of carbocations, carbanions, free radicals, carbenes, benzynes, enols
Organometallics — Preparation and reactions of Grignard and organolithium reagents, lithium organocuprates, and other modern main group and transition metal reagents and catalysts
Special Topics — Resonance, molecular orbital theory, catalysis, acid-base theory, carbon acidity, aromaticity, antiaromaticity, macromolecules, lipids, amino acids, peptides, carbohydrates, nucleic acids, terpenes, asymmetric synthesis, orbital symmetry, polymers, isomerism (structural, stereoisomerism like cis/trans, optical isomerism), conformations of organic molecules, natural products (terpenes, steroids)
IV. PHYSICAL CHEMISTRY — 30%
Thermodynamics — temperature and state variables, heat and work equations of state, reversible/irreversible processes, Hess’s law, standard states, bond enregies, spontaneity of chemical reactions, entropy/enthalpy, free energy and equlibrium of them, standard entropies and relationship w free energies, First, second, and third laws, thermochemistry (heats of reaction), ideal and real gases and solutions, Gibbs and Helmholtz energy, chemical potential, chemical equilibriaum (equilibriua constant, equilibrium calculations, le chatelier’s principle, heterogenous and solution equilibria, ionization of water and pH scale, neutralization and titration: titration curves, equilibria in weak acids and bases, indicators, hydrolysis, buffers) phase equilibria (phase rules as well), colligative properties, statistical thermodynamics
Quantum Chemistry and Applications to Spectroscopy — Classical experiments (black body radiation, heat capacities, photoelectric effect, etc), principles of quantum mechanics, atomic and molecular structure and spectra, molecular spectroscopy, Bohr atom, wave nature of matter: the de broglie equation, wave function: uncertainty principle, Paul exclusion principle, hund’s rule, molecualr orbital theory
Dynamics — Experimental and theoretical chemical kinetics (rate law; order and molecularity of reactions; arrhenius equation, variation of reaction rates with temperature; opposing reactions, chain reactions, and competing reactions), solution and liquid dynamics, photochemistry
1 note
·
View note