#Multiple Myeloma Market trends
Text
CELMoDs – A Worthy Successor to REVLIMID?

In the ever-evolving field of cancer treatment, the discovery of new therapies remains crucial for improving patient outcomes. For years, REVLIMID (lenalidomide) has been a cornerstone treatment in multiple myeloma, an incurable form of blood cancer. However, as resistance to this drug builds over time, the need for next-generation therapies has become urgent. Enter CELMoDs—Cereblon E3 Ligase Modulators, a new class of drugs showing promise as the successors to REVLIMID. But can they live up to the legacy?
What Are CELMoDs?
CELMoDs, short for Cereblon E3 Ligase Modulators, are a novel class of immunomodulatory drugs that target the same protein, cereblon, as REVLIMID. However, CELMoDs are designed to modulate this protein more effectively and induce the degradation of specific cancer-driving proteins. By doing so, they aim to enhance the immune response against cancer cells while minimizing side effects often seen with REVLIMID and its class of drugs. The Cereblon E3 ligase complex is critical for both the antitumor and immunomodulatory effects that make these drugs so potent.
How Do They Compare to REVLIMID?
Mechanism of Action: While REVLIMID acts as an immunomodulatory drug by targeting cereblon, CELMoDs are engineered to degrade disease-driving proteins more efficiently. This increased specificity in targeting may allow for better control over cancer cells while potentially reducing unwanted effects.
Enhanced Efficacy: Early trials indicate that CELMoDs could surpass REVLIMID in efficacy, especially in patients who have developed resistance to existing treatments. For instance, iberdomide, a leading CELMoD in clinical trials, has demonstrated potent activity in patients with relapsed/refractory multiple myeloma, many of whom had previously been treated with REVLIMID.
Overcoming Drug Resistance: One of the critical challenges with long-term REVLIMID use is the development of drug resistance. CELMoDs offer a potential solution to this issue by employing a more robust degradation mechanism, which may prevent or delay resistance.
Broader Patient Applicability: REVLIMID’s usage is sometimes limited by its side effects, particularly in patients who are frail or have underlying health conditions. CELMoDs could broaden the scope of treatment by offering similar or enhanced efficacy with potentially fewer side effects, making them accessible to a wider patient population.
Clinical Trial Landscape
The most promising CELMoD currently under clinical investigation is iberdomide. Initial data from Phase 1 and Phase 2 trials have been encouraging. Iberdomide is being studied both as a monotherapy and in combination with other myeloma drugs, including dexamethasone and proteasome inhibitors. In these trials, iberdomide showed significant responses even in patients who were heavily pretreated and refractory to lenalidomide. Other CELMoDs like CC-92480 are also in the pipeline, showing early indications of their potential in treating multiple myeloma.
The Future of Multiple Myeloma Treatment
While it is too early to declare CELMoDs a definitive successor to REVLIMID, their early clinical performance is promising. If ongoing trials confirm their efficacy and safety, CELMoDs could become the next-generation standard for multiple myeloma treatment, particularly for patients who have exhausted existing options. The ultimate goal is to enhance survival rates while improving the quality of life for those suffering from this devastating disease.
Conclusion: A Worthy Successor?
CELMoDs represent an exciting new frontier in multiple myeloma treatment. With their ability to more effectively degrade cancer-promoting proteins and overcome resistance, they stand poised to succeed REVLIMID as the next gold standard in therapy. While more data is needed to fully establish their long-term benefits, these drugs hold great promise for patients looking for new hope in the fight against cancer.
#Multiple myeloma#Multiple myeloma Market#Multiple myeloma Forecast#Multiple myeloma Companies#Multiple myeloma Drugs#Multiple myeloma Therapies#Multiple myeloma Epidemiology#Multiple myeloma Pipeline#Multiple myeloma Market Size#Multiple myeloma Market Trends
0 notes
Text
Multiple Myeloma Market Size Projection, Future Trends, Regional Outlook to 2032 | GlaxoSmithKline plc, AbbVie Inc., Novartis AG
Multiple Myeloma Market Size Projection, Future Trends, Regional Outlook to 2032 | GlaxoSmithKline plc, AbbVie Inc., Novartis AG
The Global Multiple Myeloma Market 2023-2032 report is a collection of details related to industry performance. Every aspect is studied in detail in the research report. The market analysis report provides comprehensive analysis of all the financial topics associated with the Multiple Myeloma industry. The growth pattern seen in the Multiple Myeloma industry performance over the time is studied…

View On WordPress
#Multiple Myeloma#Multiple Myeloma manufacturing companies#Multiple Myeloma market#Multiple Myeloma Market Size#Multiple Myeloma Market trends#Scope of Multiple Myeloma industry
0 notes
Link
0 notes
Text
Carfilzomib Market Overview and Regional Outlook Study 2024 – 2034
Carfilzomib Market Defination:
TheCarfilzomib Market refers to the economic and clinical landscape surrounding the pharmaceutical drug carfilzomib. Carfilzomib is a proteasome inhibitor used primarily in the treatment of multiple myeloma, a type of cancer affecting plasma cells in bone marrow. It functions by selectively inhibiting the proteasome, a complex protein-degrading machinery essential for cell function and survival. This inhibition leads to the accumulation of proteins within cancer cells, triggering cell death through apoptosis.
Print This Guide for Future Reference:https://wemarketresearch.com/reports/request-free-sample-pdf/carfilzomib-market/1493
Exploring the Carfilzomib Market: Advancements in Multiple Myeloma Treatment
In the realm of oncology, particularly in the treatment landscape of multiple myeloma, carfilzomib has emerged as a cornerstone therapy, offering new hope and improved outcomes for patients. This blog delves into the dynamic carfilzomib market, examining its impact, current trends, challenges, and future prospects.
Understanding Carfilzomib
Carfilzomib is a proteasome inhibitor approved for the treatment of relapsed or refractory multiple myeloma. It works by selectively and irreversibly binding to the 20S proteasome, disrupting protein degradation in cancer cells and inducing apoptosis. Approved by the FDA in 2012, carfilzomib has since been integrated into treatment protocols, often in combination with other agents like lenalidomide and dexamethasone.
Market Dynamics
Current Landscape: The Carfilzomib Market is driven by its efficacy in treating relapsed or refractory multiple myeloma, particularly in patients who have received prior therapies. Its mechanism of action and clinical benefits have positioned it as a valuable option in the treatment algorithm for multiple myeloma.
Treatment Advancements: Clinical studies have demonstrated that carfilzomib-based regimens prolong progression-free survival and overall survival compared to traditional therapies. Its approval marked a significant advancement in the management of multiple myeloma, offering a targeted approach to combating the disease.
Competitive Environment: Within the proteasome inhibitor class, carfilzomib competes with bortezomib and ixazomib, each offering unique profiles in terms of efficacy, safety, and administration convenience. Ongoing research aims to optimize carfilzomib’s use through novel combinations and sequencing strategies to maximize patient benefit.
Clinical Applications
Approved Indications: Carfilzomib is primarily indicated for use in combination with other agents for the treatment of relapsed or refractory multiple myeloma. Clinical trials are also exploring its potential in newly diagnosed patients and maintenance therapy settings, broadening its scope of application.
Future Directions: Research efforts are focused on expanding carfilzomib’s indications and understanding its synergies with emerging therapies such as immunomodulators, monoclonal antibodies, and cellular therapies like CAR-T cells. These endeavors aim to further improve treatment outcomes and offer personalized therapeutic approaches.
Carfilzomib Market Challenges and Opportunities
Challenges: Economic considerations remain a significant challenge in the adoption of carfilzomib, given its high cost as a biologic therapy. Managing treatment-related adverse events, such as cardiovascular complications and hematologic toxicities, also requires vigilant monitoring and proactive management strategies.
Opportunities: Advances in biomarker identification and personalized medicine offer opportunities to tailor carfilzomib-based therapies to individual patient profiles. Moreover, ongoing research into combination therapies and novel formulations aims to enhance efficacy while minimizing adverse effects, thereby improving patient adherence and outcomes.
Patient Impact and Healthcare Considerations
Patient Experience: For patients diagnosed with relapsed or refractory multiple myeloma, carfilzomib represents a crucial treatment option that can potentially extend survival and improve quality of life. Education and support programs play a vital role in helping patients manage treatment-related challenges and adhere to therapy.
Healthcare System Implications: Integrating carfilzomib into clinical practice requires healthcare providers to navigate complex treatment algorithms and ensure appropriate patient monitoring. Collaboration among multidisciplinary teams, including oncologists, hematologists, and supportive care specialists, is essential for optimizing patient care and outcomes.
Regulatory and Market Access
Regulatory Landscape: Regulatory approvals and reimbursement policies influence the accessibility of cCarfilzomib Market in different regions. Streamlining regulatory processes and demonstrating cost-effectiveness through real-world evidence are crucial for enhancing market access and patient affordability.
Market Expansion: As clinical data continues to evolve and new indications are explored, the carfilzomib market is poised for growth. Market expansion strategies should prioritize evidence-based medicine and stakeholder collaboration to drive adoption and improve patient access.
Conclusion
In conclusion, the carfilzomib market represents a significant advancement in the treatment of multiple myeloma, reflecting the transformative impact of targeted therapies in oncology. Its approval and integration into treatment protocols underscore a shift towards personalized medicine and multidisciplinary care approaches that optimize patient outcomes.
While challenges such as economic considerations and treatment-related adverse events persist, ongoing research and collaborative efforts among stakeholders are paving the way for continued innovation and improvement inCarfilzomib-Based Therapies. By addressing these challenges proactively, healthcare providers and pharmaceutical companies can ensure that carfilzomib realizes its full potential in improving the lives of patients battling multiple myeloma.
Stay informed and engaged with the latest developments in the carfilzomib market to contribute to advancements in oncology and patient-centered care.
0 notes
Text
Bortezomib Market Estimated to Witness High Growth Owing to Rising Adoption of Proteasome Inhibitors

The bortezomib market is primarily driven by high incidence and prevalence of multiple myeloma across the globe. Bortezomib, which is commonly sold under the brand name Velcade among others, is a proteasome inhibitor primarily used for the treatment of multiple myeloma and mantle cell lymphoma.
The global proteasome inhibitor drug market size is valued at approximately US$ 24.54 million in 2024 and is expected to register a CAGR of 4.7% over the forecast period of 2024-2031.
The introduction of novel proteasome inhibitors and their increasing adoption in the treatment of cancer are the major factors anticipated to propel market growth.
Key Takeaways
Key players operating in the bortezomib market are Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, and Chengdu Aslee Biopharmaceuticals.
The introduction of generic versions of Bortezomib Market Demand has led to increased adoption and lowered treatment costs. Moreover, ongoing clinical trials evaluating the efficacy of bortezomib in other cancer indications are expected to expand the eligible patient pool. Technological advancements in proteasome inhibitor development focused on overcoming resistance, reducing toxicity, and novel delivery systems are further anticipated to support market growth.
Market Drivers
The primary factors driving the growth of the global bortezomib market include rising prevalence of multiple myeloma globally, increasing adoption of proteasome inhibitors in treatment regimens, availability of generic versions, and ongoing clinical research evaluating the efficacy of bortezomib in other cancer indications. Additionally, improving healthcare infrastructure and expenditures in emerging economies will further support the market growth during the forecast period.
Current challenges in Bortezomib Market
The Bortezomib Market Size And Trends faces several challenges primarily due to the presence of alternative therapeutic options for treating multiple myeloma (MM). Some of the major challenges include increasing generic competition from drugs like ixazomib and daratumumab which are leading to lower sales of bortezomib drugs. Further, the patents of bortezomib drugs have expired in several regions making them available in generic forms at lower costs. This increasing availability of low-cost generics is a major challenge faced by innovator bortezomib drug companies. Additionally, the adverse side effects associated with bortezomib drugs like neuropathy and thrombocytopenia require close patient monitoring during treatment posing operational challenges. Stringent regulations for drug approval is another regulatory challenge for new market entrants.
SWOT Analysis
Strength: Well-established drug with proven efficacy and safety profile in treating MM. It was the first proteasome inhibitor approved and remains a standard of care.
Weakness: Patent expiry has led to availability of low-cost generics reducing sales of innovator brands. Further, it causes serious side effects like neuropathy requiring cautious use.
Opportunity: Emerging economies with growing cancer burden and healthcare spending present an opportunity. Combination therapies with other anti-MM drugs can boost its use further.
Threats: Increasing competition from newer oral proteasome inhibitors and monoclonal antibody based therapies poses pricing and market share threats. Stringent regulations for approval delays market entry of new players.
Geographical regions with high market concentration
In terms of value, North America accounts for the largest share of over 40% of the global bortezomib market led by the US. This is due to established healthcare infrastructure and higher adoption of innovative therapies. Europe is the second major regional market with a value share of over 30% supported by favourable reimbursement policies. The Asia Pacific region is projected to be the fastest growing market during the forecast period due to rising healthcare expenditure, growing cancer incidence and increasing demand for cancer treatments from middle-income countries like China and India.
Fastest growing geographical region
The Asia Pacific region is poised to exhibit the highest growth rate during the forecast period in the global bortezomib market. This is attributed to rising disposable incomes, growing awareness about cancer treatments, expansion of healthcare facilities and increasing private sector investment in pharmaceutical research in emerging economies like China and India. Large patient pools undergoing cancer treatment in Asia present lucrative opportunities for bortezomib drug makers looking to tap high future growth potential in this region.
Get More Insights On, Bortezomib Market
For More Insights Discover the Report In language that Resonates with you
French, German, Italian, Russian, Chinese, Korean
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Bortezomib Market Demand#Bortezomib Market Size#Bortezomib Market Trends#Bortezomib Market Forcast#Bortezomib#Bortezomib Market
0 notes
Text
Velcade Market Size, Share, Industry Trends, and Forecast 2032
Introduction
Velcade, also known by its generic name bortezomib, is a proteasome inhibitor used primarily in the treatment of multiple myeloma and mantle cell lymphoma. Since its approval, Velcade has significantly impacted the oncology market, offering a promising therapeutic option for patients with these malignancies. The global Velcade market is characterized by its dynamic nature, driven by advancements in oncology research, increasing incidence of hematologic cancers, and a growing focus on personalized medicine.
Market Size and Growth Dynamics
Velcade Market Size was estimated at 1.76 (USD Billion) in 2023. The Velcade Market Industry is expected to grow from 1.83(USD Billion) in 2024 to 2.5 (USD Billion) by 2032. The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). This growth is fueled by the rising prevalence of multiple myeloma and other related cancers, which are increasingly being diagnosed due to advances in diagnostic technologies. Furthermore, the aging population, which is more susceptible to cancer, is also contributing to the expanding market.
North America holds the largest market share, attributed to the region's advanced healthcare infrastructure, high adoption rates of new therapies, and a strong focus on research and development. Europe follows closely, driven by similar factors. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, spurred by increasing healthcare expenditures, improving access to cancer treatments, and rising awareness about hematologic cancers.
Market Share Analysis
Velcade, originally developed by Millennium Pharmaceuticals and marketed by Takeda Oncology, has maintained a dominant position in the proteasome inhibitor segment. Its efficacy, safety profile, and first-mover advantage have contributed to its strong market share. However, with the expiration of key patents, the market has seen the entry of generic versions, which has led to increased competition and a shift in market dynamics.
The introduction of generics has made the treatment more accessible, particularly in developing regions, but it has also put pressure on the market share of branded Velcade. Despite this, the brand continues to hold a significant share due to its established presence and the trust it has garnered among healthcare professionals.
Industry Trends
Several key trends are shaping the Velcade market:
Rise of Combination Therapies: The use of Velcade in combination with other drugs, such as lenalidomide and dexamethasone, is becoming increasingly common. These combination therapies have shown improved efficacy and are becoming a standard of care in multiple myeloma treatment protocols.
Focus on Personalized Medicine: With the growing emphasis on personalized medicine, there is a trend towards tailoring treatments based on individual patient profiles, which includes genetic makeup and disease characteristics. This approach is expected to drive demand for Velcade as part of personalized treatment regimens.
Increased Research and Development: The oncology sector continues to see significant investment in research and development, leading to the discovery of new therapeutic targets and treatment options. While this fosters innovation, it also intensifies competition as new drugs enter the market.
Patent Expirations and Generic Competition: The expiration of Velcade’s patents has opened the market to generic competition, leading to reduced prices and increased accessibility. This trend is expected to continue, particularly in cost-sensitive markets.
Expanding Indications: Research is ongoing to explore the potential of Velcade in treating other cancers and conditions beyond multiple myeloma and mantle cell lymphoma. If successful, these efforts could lead to new indications, further driving market growth.
Forecast Through 2032
The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). Key drivers of this growth include the increasing global cancer burden, advancements in cancer therapy, and the expansion of healthcare infrastructure in emerging markets.
However, challenges such as the rising cost of cancer treatment, the availability of alternative therapies, and regulatory hurdles may pose risks to market expansion. Additionally, the shift towards biosimilars and generics is likely to impact the market dynamics, particularly in terms of pricing and market share distribution.
The forecast period is also expected to witness a greater emphasis on real-world evidence and outcome-based reimbursement models, which could influence the adoption of Velcade and its competitors.
Conclusion
The Velcade market is poised for significant growth in the coming years, driven by a combination of factors including rising cancer incidence, advancements in treatment protocols, and the expanding reach of healthcare services. While challenges such as generic competition and pricing pressures exist, the overall outlook remains positive, with opportunities for growth in emerging markets and through the development of new therapeutic indications. As the oncology landscape continues to evolve, Velcade is expected to remain a key player, contributing to improved outcomes for patients worldwide.
0 notes
Text
0 notes
Text
Globalization and Market Expansion in Multiple Myeloma

The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.The Multiple Myeloma market is characterized by a dynamic interplay of research, treatment advancements, and patient-centric care initiatives. As one of the most prevalent hematologic cancers, it continuously draws attention from pharmaceutical innovators and healthcare providers alike. Recent years have witnessed a surge in targeted therapies, immunotherapies, and personalized medicine approaches tailored to combatting its complexities. This evolving landscape not only fosters competition among biopharmaceutical companies but also emphasizes the importance of early detection and multidisciplinary treatment strategies. With ongoing clinical trials promising novel therapeutic avenues, the Multiple Myeloma market remains poised for further breakthroughs in extending patient survival and enhancing quality of life.
The Multiple Myeloma Market research study for the term also includes a variety of business opportunities and growth potential. A business plan detailing market risks and constraints as well as the effects of various regulatory regimes is given to executives by the market research. This is carried out to assist companies in reaching their main goals and making better judgments.
Get Sample Copy Of This Report @ https://www.snsinsider.com/sample-request/3490
Market Segmentation
By Type
Chemotherapy
Monoclonal Antibody
Protease Inhibitors
Others
By Disease Type
Smoldering Multiple Myeloma
Active Multiple Myeloma
By End User
Clinics
Hospitals
Others
Regional Outlook
The geographical categories that make up the Multiple Myeloma Market each have their own revenue, market share, sales, and growth rates. Among the important geographical areas covered in the market analysis are Europe, Asia-Pacific, South America, North America, and the Middle East and Africa. Latin America is expected to have a small market share in value, while North America is forecast to maintain its global leadership position and have a significant market share in both volume and value.
COVID-19 Impact Analysis
In the first half of 2020, the COVID-19 virus started to spread over the world, infecting millions of people and forcing major nations to implement work stoppage and foot restrictions. Nearly every area of the economy has suffered, with the exception of medical goods and equipment for life support, including the Multiple Myeloma Market .
Competitive Landscape
The competitive analysis section of the global Multiple Myeloma Market offers details and insights on the participants. Among the details provided are information on competition, a market overview by business status, and revenue projections by region. These businesses use a variety of strategies, such as product launches, partnerships, alliances, technology advancements, and contracts, to boost market income.
Conclusion
The market research is supported by first-hand experience, qualitative and quantitative analysis by industry analysts, and comments from key market players and actors in the value chain. The study investigates parent industry trends, micro and macroeconomic data, governing factors, and market attractiveness on a segment-by-segment basis. The study also illustrates how different market factors might have a qualitative impact on market segmentation based on geography and Multiple Myeloma Market.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Flash Chromatography Market Size
Cystic Fibrosis Market Size
Cancer Biopsy Market Size
Glaucoma Therapeutics Market Size
Genomics Services Market Size
0 notes
Text
Minimal Residual Disease Testing Market Future Trends to Look Out | Bis Research
Minimal Residual Disease Testing is a sophisticated diagnostic technique used primarily in oncology to detect and quantify residual cancer cells that may remain in the body during or after treatment.
The Global Minimal Residual Disease Testing Market is a rapidly growing segment in the healthcare industry, driven by the increasing demand for accurate and sensitive methods to monitor and manage cancer patients.
The Minimal Residual Disease Testing market was valued at $1.67 billion in 2023 and is expected to reach $6.67 billion by 2033, growing at a CAGR of 14.81% between 2023 and 2033.
MRD Testing Overview
Minimal Residual DiseaseTesting refers to the detection and quantification of residual cancer cells that remain in a patient after treatment, which are below the detection threshold of conventional diagnostic methods. MRD testing is particularly significant in hematologic malignancies such as leukemia, lymphoma, and multiple myeloma, where even a small number of remaining cancer cells can lead to relapse.
Have a look at our sample page here !
Market Segmentation
By Technology
By Target Detection
By End Users
By Region
China dominated the Asia-Pacific Minimal Residual Disease Testing Market in 2022 with a share of 36.08%. Although the market is expected to remain in a strong growth phase due to the massively growing number of cancer cases and the rising health-related awareness among people in Asia-Pacific, a significant barrier to the increasing adoption is an uneven economic balance among countries within the region.
Importance of Minimal Residual Disease Testing Market
Assessing Treatment Response
Predicting Relapse
Tailoring Therapy
Key Factors
The Minimal Residual Disease Testing Market has experienced significant growth in recent years, driven by several key factors like
advancements in technology
rising cancer burden,
clinical evidence supporting MRD monitoring
Key Players In the Minimal Residual Disease Testing Market includes
QIAGEN N.V.
Thermo Fisher Scientific Inc.
Sysmex Corporation
Mission Bio
OPKO Health
Bio-Rad Laboratories, Inc
ICON plc
Hoffmann-La Roche
and many others
Techniques used in Minimal Residual Disease Testing
Flow Cytometry - This technique uses fluorescent antibodies to identify cancer-specific markers on the surface of cells.
Polymerase Chain Reaction- PCR amplifies cancer-specific genetic sequences, allowing for the detection of one cancer cell among a million normal cells.
Next Generation Sequencing - NGS provides detailed genetic information by sequencing DNA or RNA at high depth, offering unparalleled sensitivity and the ability to identify clonal diversity and mutations.
Digital Droplet PCR- A more recent advancement, ddPCR partitions the sample into thousands of droplets and performs PCR on each droplet individually, providing high sensitivity and precise quantification.
Applications for Minimal Residual Disease Testing Market
Treatment Response Monitoring
Relapse Prediction
Treatment Decision-making
Prognostic Assessment
Clinical Trials and drug development
Minimal Residual Disease Testing Market Dynamics
Market Drivers
Advent of MRD and its Awareness among Consumers
Increasing Incidence of Cancer Cases Demanding MRD
Rise in administration of solid tumors
Expanding Medicare Coverage for MRD
Recent Developments in the Minimal Residual Disease Testing Market
•Quest Diagnostics acquired Haystack Oncology, expanding its oncology portfolio with the inclusion of advanced liquid biopsy technology. This addition aimed to enhance personalized cancer care by offering highly sensitive diagnostic capabilities.
Integrated DNA Technologies launched the Archer FUSIONPlex Core Solid Tumor Panel, a pioneering cancer research testing solution that has been enhanced and fine-tuned to include a broader range of single nucleotide variant (SNV) and indel coverage.
Visit our precision medicine page here!
Key Questions Answered
Q What is MRD ?
Minimal residual disease (MRD) testing is a supplementary approach to detect extremely low levels of blood cancer cells and solid tumors following the treatment of conditions such as acute and chronic leukemia, lymphoma, or multiple myeloma. MRD specifically pertains to the minute population of cancer cells that persist in the body despite achieving complete remission (CR) through chemotherapy or stem cell transplantation.
Q What kinds of New Strategies are adopted by the existing market players to strengthen their positions in the Industry ?
The global MRD market is currently witnessing several developments, primarily aimed at introducing new products and services. Major manufacturers of MRD products, along with the service providers, are actively undertaking significant business strategies to translate success in research and development into the commercial clinical setting.
Conclusion
In conclusion, Minimal Residual testing is a powerful tool in modern oncology, offering the potential to significantly improve patient outcomes through more precise and personalized treatment strategies.
0 notes
Text
Oncology Drugs Market Growth, Trends, Size, Share, Demand And Top Growing Companies 2031

In a landscape where the battle against cancer rages on, advancements in healthcare systems, public health measures, and novel pharmaceutical therapies have ushered in a new era of hope. According to the National Cancer Institute, the United States saw an estimated 1,806,590 new cancer cases and approximately 606,520 deaths due to the disease in 2020. However, over the past five decades, cancer survival rates have soared from 50% in 1970 to an impressive 70%, thanks to a trifecta of progress.
For more information: https://www.fairfieldmarketresearch.com/report/oncology-drugs-market
Unprecedented Growth Trajectory:
The global oncology therapy sales are forecasted to surpass US$ 300 billion by 2026, with oncology contributing 21.7% to total pharmaceutical sales. Fueling this growth are the top 10 pharmaceutical companies, which have declared oncology as their key focus area, driving multibillion-dollar M&A deals and strategic collaborations. Pfizer's acquisition of Array BioPharma for US$11 billion in 2019 and AbbVie's strategic partnership with Genmab for a bispecific antibody development deal worth US$3 billion are testament to this focus.
Diverse Indications Drive Demand:
While oncology represents over 20 different indications, a significant portion of revenue stems from just five of them: breast cancer, multiple myeloma, non-small-cell lung carcinoma (NSCLC), prostate cancer, and non-Hodgkin's lymphoma (NHL), which collectively accounted for approximately 65% of the market in 2020. Moreover, with breast, lung, and colorectal cancers expected to collectively account for ~50% of all new cancer diagnoses by 2026, the demand for innovative therapies continues to surge.
Disruptive Trends Reshape Landscape:
Innovation in oncology is accelerating, with disruptive technologies such as cell therapy, RNA therapy, viral vectors, and stem cell therapy gaining traction. Recent approvals of CAR-T cell therapies like Kymriah and Yescarta for acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) respectively signal a new frontier in cancer treatment. Precision medicine is also driving progress, with over 160 oncology biomarkers approved by 2019, paving the way for more targeted and effective therapies.
Impact of COVID-19:
Despite remarkable progress, oncology has been among the worst-hit therapeutic areas amid the COVID-19 pandemic. Decreased demand for physician-administered products, disruptions in cancer screenings, and a decline in new clinical trials have posed significant challenges. However, the industry remains resilient, adapting to the evolving landscape and ensuring continued innovation.
Immuno-Oncology Leads the Way:
Immuno-oncology sales are expected to soar to ~US$ 95 billion by 2026, with agents and protein kinase inhibitors comprising ~65% of sales. With over 550 active cell- and gene-therapy agents under clinical development, the future of cancer treatment looks promising. Investments in combination studies and the exploration of new mechanisms underscore the industry's commitment to advancing immuno-oncology therapies.Roche and Keytruda: Leading the Charge:
In a highly concentrated market where the top 10 companies capture over 75% of the market value, F. Hoffmann-La Roche AG (Roche) and Merck & Co. stand out as leaders. While Roche maintains its global leadership position, Merck's Keytruda is poised to become the world's top-selling oncology
0 notes
Text
T Cell Therapy Market Size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032
The T cell therapy market size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032 at a CAGR of 32.9%.
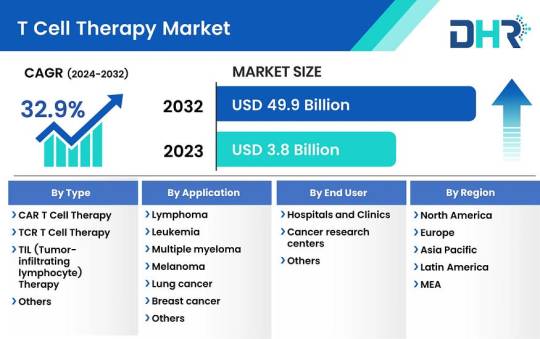
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/t-cell-therapy-market-3114
Top Companies are:
· Novartis AG
· Gilead Sciences, Inc.
· Bristol-Myers Squibb Company
· Adaptimmune Therapeutics plc
· Amgen Inc.
· Atara Biotherapeutics, Inc.
· Autolus Therapeutics plc
· bluebird bio, Inc.
· Cellectis S.A.
· Iovance Biotherapeutics, Inc.
· Kite Pharma (a Gilead Company)
· Tmunity Therapeutics, Inc.
Market Segmentations:
By Type-
CAR T cell therapy
TCR T cell therapy
TIL (Tumor-infiltrating lymphocyte) therapy
Others
By Application-
Lymphoma
Leukemia
Multiple myeloma
Melanoma
Lung cancer
Breast cancer
Colorectal cancer
Autoimmune disorders
Infectious diseases
Others
By End User-
Hospitals and Clinics
Cancer research centers
Others
Regional Analysis:
The dominance of the T Cell Therapy market in North America is underpinned by the presence of established biopharmaceutical firms, a robust clinical trial infrastructure, and a favorable regulatory landscape. Among North American countries, the United States stands out as the primary contributor, buoyed by the FDA’s approval of several CAR T cell therapies such as Kymriah, Yescarta, and Abecma for treating hematological malignancies. The U.S. National Library of Medicine’s database reveals an extensive presence of over 1,000 active clinical trials dedicated to evaluating T cell therapies, underscoring the region’s steadfast commitment to research and development in this field.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Multiple Myeloma: Understanding the Incurable B-Cell Cancer and Its Relentless Relapse Cycle

Multiple myeloma is a complex, incurable cancer that affects plasma cells, a type of B-cell in the immune system. Plasma cells are responsible for producing antibodies to fight infections, but in multiple myeloma, these cells become malignant and proliferate uncontrollably. The disease is characterized by cycles of remission and relapse, even after aggressive treatments. Despite significant advances in therapy, multiple myeloma remains incurable, with each relapse becoming progressively more difficult to treat.
The Biology of Multiple Myeloma
Multiple myeloma begins in the bone marrow, where plasma cells mutate and grow uncontrollably. This abnormal proliferation interferes with normal blood production, leading to anemia, weakened bones, and immune dysfunction. Myeloma cells also produce excessive amounts of monoclonal proteins, which can cause kidney damage and other organ complications. The result is a multisystem disorder that affects bone health, blood cell production, and overall immunity.
While treatment can often induce remission, myeloma cells persist, and the disease inevitably returns. This cycle of remission and relapse is a hallmark of the disease and a major challenge in treatment.
Why is Multiple Myeloma Incurable?
Unlike other cancers where remission may lead to a cure, multiple myeloma remains incurable due to its biological complexity. Cancerous plasma cells develop resistance to treatments over time, allowing them to survive and multiply even after aggressive therapy. One reason is that myeloma cells can evolve and adapt to various therapies, leading to drug resistance.
Even when treatments initially reduce the number of cancer cells, some remain dormant or undetected, only to reactivate later. This recurrence, known as minimal residual disease (MRD), is a significant barrier to curing the disease. As each relapse occurs, the cancer becomes more resistant to available treatments, making it more challenging to control.
The Vicious Cycle of Relapse
Relapse in multiple myeloma is inevitable for most patients, even after periods of remission. With each relapse, the disease becomes more aggressive and resistant to treatments that were previously effective. Patients may experience multiple remissions and relapses, requiring changes in treatment regimens. This cycle often leads to a shorter duration of remission and reduced quality of life over time.
Treatments like stem cell transplants, chemotherapy, and novel therapies can control the disease, but the cancer’s ability to adapt makes relapse almost certain. Each recurrence introduces new genetic mutations in the cancer cells, further complicating treatment options.
Innovations in Treatment: A Ray of Hope
Over the past decade, there have been breakthroughs in multiple myeloma treatment, though the disease remains incurable. Targeted therapies such as proteasome inhibitors (e.g., bortezomib), immunomodulatory drugs (e.g., lenalidomide), and monoclonal antibodies (e.g., daratumumab) have significantly improved outcomes, helping to extend patients' lives and improve their quality of life.
Newer therapies, such as CAR-T cell therapy, offer hope by reprogramming a patient's own immune cells to target and destroy myeloma cells. This innovative approach has shown promise in cases where multiple myeloma has relapsed multiple times and is resistant to other treatments. CAR-T cell therapy is a personalized treatment, but it is still in its early stages and may not yet provide a long-term cure.
Other promising avenues include bispecific T-cell engagers (BiTEs) and antibody-drug conjugates (ADCs), which combine the precision of targeting cancer cells with the potency of chemotherapy drugs, attacking myeloma cells directly.
The Future of Multiple Myeloma Research
While current treatments can prolong remission, the focus of ongoing research is to overcome the challenge of relapse. Scientists are working on understanding the underlying biology of drug resistance and developing more effective therapies. A deeper understanding of genetic mutations and molecular mechanisms of multiple myeloma could lead to more personalized treatment options and better disease management.
Researchers are also exploring combination therapies that target multiple pathways involved in the disease, hoping to prevent the cancer from adapting and recurring. The ultimate goal is to find a cure or at least prolong the periods of remission with fewer side effects, providing patients with a better quality of life.
Conclusion: A Persistent Battle Against a Relentless Disease
Multiple myeloma remains a challenging and incurable disease, defined by a cycle of relapse and remission. While significant strides have been made in treatment, the cancer’s ability to adapt and resist therapy means that patients face a lifetime of managing the disease. New therapies like CAR-T cells and targeted treatments provide hope for longer-lasting remissions, but the quest for a cure continues. Until then, the focus is on improving the quality of life for patients affected by multiple myeloma, controlling the disease for as long as possible, and pushing the boundaries of research to one day break the vicious cycle of relapse.
#Multiple myeloma#Multiple myeloma Market#Multiple myeloma Forecast#Multiple myeloma Companies#Multiple myeloma Drugs#Multiple myeloma Therapies#Multiple myeloma Epidemiology#Multiple myeloma Pipeline#Multiple myeloma Market Size#Multiple myeloma Market Trends
0 notes
Text
Multiple Myeloma Market Size Trends Growth Value Forecast to 2032 | Fresenius Kabi AG, Teva Pharmaceutical Industries Ltd, Mylan N.V
Multiple Myeloma Market Size Trends Growth Value Forecast to 2032 | Fresenius Kabi AG, Teva Pharmaceutical Industries Ltd, Mylan N.V
Global Multiple Myeloma Market Size, Status, and Forecast for the 2022-2032. Comprehensive analysis has been compiled to supply the foremost up-to-date data on key aspects of the market. This research report covers major aspects of the Multiple Myeloma industry, as well as drivers, restraints, PESTLE analysis, historical and current trends, regulative situations, and technological advancements.…

View On WordPress
#Multiple Myeloma#Multiple Myeloma market#Multiple Myeloma Market Size#Multiple Myeloma Market trends
0 notes
Text
Carfilzomib Market Statistics, Segment, Trends and Forecast to 2034

TheCarfilzomib Market: Trends, Opportunities, and Future Outlook
Introduction
In recent years, the pharmaceutical industry has seen significant advancements in the treatment of multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. Among these advancements, Carfilzomib has emerged as a critical player. This proteasome inhibitor, marketed under the brand name Kyprolis, has made substantial impacts in the management of multiple myeloma. In this blog, we will explore the current state of theCarfilzomib market, its growth drivers, opportunities, and future outlook.
Free Sample pdf copy:
What is Carfilzomib?
Carfilzomib is a next-generation proteasome inhibitor used in the treatment of multiple myeloma, especially in patients who have relapsed or are refractory to other therapies. Unlike its predecessor, bortezomib, Carfilzomib is known for its more selective action on the proteasome, potentially leading to fewer side effects and enhanced efficacy. Approved by the FDA in 2012, it has since become an integral part of combination therapies for multiple myeloma.
Carfilzomib Market Landscape
Growth Drivers
Rising Incidence of Multiple Myeloma: The increasing number of multiple myeloma cases globally is a significant driver for the Carfilzomib market. As the global population ages, the prevalence of multiple myeloma is expected to rise, leading to a higher demand for effective treatments.
Advancements in Treatment Protocols: Carfilzomib is often used in combination with other drugs like lenalidomide and dexamethasone, enhancing its effectiveness. This combination therapy approach has shown promising results, making Carfilzomib a preferred choice in advanced treatment regimens.
Increasing Awareness and Diagnosis: Improved diagnostic techniques and greater awareness about multiple myeloma have led to earlier detection and treatment. This trend is likely to boost the demand for Carfilzomib as part of first-line and subsequent lines of treatment.
Ongoing Clinical Trials: Continuous research and clinical trials exploring new indications and combination therapies for Carfilzomib are expanding its potential market. Studies focusing on different stages of multiple myeloma and other cancers could open new avenues for Carfilzomib use.
Carfilzomib Market Challenges
High Cost of Treatment: Carfilzomib is a high-cost drug, and its price can be a barrier to access, particularly in developing countries. The high cost of treatment may limit its market potential and lead to a preference for alternative therapies.
Side Effects and Resistance: Although Carfilzomib is well-tolerated, some patients may experience side effects such as cardiovascular issues or renal complications. Additionally, resistance to the drug can develop, leading to the need for alternative therapies.
Competition from Other Therapies: The multiple myeloma treatment landscape is competitive, with several other proteasome inhibitors and novel therapies in development. This competition can impact Carfilzomib’s market share and pricing strategies.
Market Opportunities
Expanding Indications: Research into expanding the use of Carfilzomib to other types of cancer or earlier stages of multiple myeloma could provide significant market opportunities. Successful clinical trials in these areas could lead to new indications and broaden its market.
Developing Markets: As healthcare infrastructure improves in developing regions, there ispotential for growth in these Carfilzomib Market Strategic partnerships and pricing strategies could enhance Carfilzomib’s reach in these areas.
Combination Therapies: Exploring new combination therapies and optimizing treatment regimens can improve patient outcomes and increase the demand for Carfilzomib. Collaborations with other pharmaceutical companies and research institutions could drive innovation in this space.
Carfilzomib Market Future Outlook
The Carfilzomib market is poised for continued growth driven by advancements in treatment protocols and increasing prevalence of multiple myeloma. However, challenges such as high treatment costs and competition from alternative therapies will require strategic planning and innovation.
The future of Carfilzomib will likely involve ongoing research and development to expand its therapeutic indications and improve patient outcomes. As the market evolves, stakeholders will need to navigate these dynamics to maximize the potential of Carfilzomib in the treatment of multiple myeloma and beyond.
Conclusion
Carfilzomib Market has made asignificant impact in the management of multiple myeloma, and its market prospects remain strong. With ongoing advancements in treatment protocols, increasing awareness, and expanding indications, Carfilzomib is set to continue playing a crucial role in cancer therapy. However, addressing market challenges and seizing emerging opportunities will be essential for stakeholders to fully realize the potential of this innovative drug.
#Carfilzomib Market Share#Carfilzomib Market Demand#Carfilzomib Market Scope#Carfilzomib Market Analysis#Carfilzomib Market Trend
0 notes
Text
Multiple Myeloma Market Trends & Forecasts 2032 | Drug Pipeline, FDA Approvals, and companies by DelveInsight | GSK, BMS, Bluebird bio, Oncopeptides AB, Secura Bio, Amgen, Takeda, Sanofi, Janssen
http://dlvr.it/T4Lddq
0 notes
Text
Multiple Myeloma Market Trends & Forecasts 2032 | Drug Pipeline, FDA Approvals, and companies by DelveInsight | GSK, BMS, Bluebird bio, Oncopeptides AB, Secura Bio, Amgen, Takeda, Sanofi, Janssen
http://dlvr.it/T4LddQ
0 notes