#Biopharmaceutical CMO Market share
Explore tagged Tumblr posts
Text
Comprehensive Outlook on Biopharmaceutical CMO Market Growth & Trends 2024-2032
The global Biopharmaceutical CMO Market Revenue is on an accelerated growth trajectory, fueled by the increasing demand for contract manufacturing services in the biopharmaceutical industry. As drug manufacturers seek innovative solutions to keep up with evolving market demands, the role of contract manufacturing organizations (CMOs) has become indispensable. These organizations support the pharmaceutical industry by providing specialized services, enabling companies to meet high-quality standards, manage costs efficiently, and expedite time-to-market for new therapies.

In recent years, CMOs have expanded their capabilities to offer a range of services, from drug discovery and development to manufacturing and regulatory support. This expansion has proven critical, particularly as biopharmaceutical companies increasingly outsource complex manufacturing processes to CMOs. As research and development (R&D) costs continue to rise, these partnerships allow companies to remain competitive by accessing state-of-the-art technology and expertise without extensive in-house investments. Moreover, this trend is driven by the increasing complexity of drug formulations and the growing regulatory requirements in biopharmaceutical manufacturing.
According to the SNS Insider report, the biopharmaceutical CMO market is set to witness considerable growth in the coming years. This growth is largely attributed to rising chronic diseases, a growing focus on biologics, and the advancements in biotechnology that are reshaping the drug development landscape. These developments have led to an uptick in demand for specialized manufacturing and regulatory support services, allowing CMOs to carve out a pivotal role in the biopharmaceutical sector.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/2938
Key Drivers of Market Growth
Rising Demand for Biologics: With an increasing number of biologics and biosimilars entering the market, the demand for contract manufacturing services is on the rise. Biologics are complex and require specialized manufacturing processes, which many pharmaceutical companies choose to outsource to CMOs that possess the required expertise.
Advancements in Technology: The rapid technological advancements in the biopharmaceutical sector, including single-use technologies, continuous manufacturing, and high-throughput screening, are enabling CMOs to improve production efficiency and product quality. This has positioned them as essential partners for companies looking to scale up their operations.
Growing Focus on R&D and Innovation: As companies look to develop breakthrough therapies, the demand for R&D outsourcing has also grown. CMOs are expanding their services to include early-stage R&D, providing companies with access to specialized facilities and a streamlined approach to drug development, making it possible to bring innovative drugs to market faster.
Opportunities and Future Outlook
The growth of the biopharmaceutical CMO market is further supported by the increase in government funding for life sciences research and the rising number of clinical trials globally. Furthermore, as CMOs continue to invest in cutting-edge technologies, the industry is expected to see advancements in manufacturing capabilities, providing a strong foundation for market expansion.
The competitive landscape of the biopharmaceutical CMO market is also evolving, with leading companies focusing on strategic partnerships, mergers, and acquisitions to expand their service offerings. These strategies enable CMOs to strengthen their market presence, broaden their expertise, and provide end-to-end solutions to biopharmaceutical companies. As a result, the CMO market is well-positioned to capitalize on the biopharmaceutical industry’s robust growth trajectory.
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us: Akash Anand – Head of Business Development & Strategy [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
#Biopharmaceutical CMO Market#Biopharmaceutical CMO Market Size#Biopharmaceutical CMO Market Share#Biopharmaceutical CMO Market Growth#Market Research
0 notes
Text
Biopharmaceutical CMO And CRO Market Is Estimated To Witness High Growth Owing To Increasing Demand For Outsourcing

Biopharmaceutical CMOs and CROs provide services like clinical trial management, data management, medical writing, clinical monitoring to biopharmaceutical and biotechnology companies. They help reduce development costs and improve productivity. With rise in complex drug development projects, outsourcing non-core functions have become essential.
The global biopharmaceutical CMO and CRO market is estimated to be valued at US$ 41.71 Mn in 2024 and is expected to exhibit a CAGR of 5.0% over the forecast period 2024 to 2031, as highlighted in a new report published by Coherent Market Insights. Market Dynamics: One of the key drivers for the growth of the biopharmaceutical CMO and CRO market is the increasing demand for outsourcing from biopharmaceutical companies. Due to high costs associated with drug development and pricing pressures, biopharma companies are increasingly outsourcing non-core activities like clinical trials to CROs and manufacturing to CMOs. This allows them to focus on drug discovery and commercialization while reducing fixed costs. Additionally, biopharmaceutical CMOs and CROs have capabilities across diverse therapeutic areas and expertise in handling complex projects involving novel biologics, vaccines, and cell and gene therapies. Their specialized expertise and economies of scale help accelerate development timelines and bring down costs for biopharma sponsors. SWOT Analysis Strength: The biopharmaceutical CMO and CRO market has experienced strong growth in recent years due to the increasing demand for biologics. CMOs provide services to drug makers from drug development to commercial manufacturing which helps biopharma companies focus on drug discovery. CROs help biopharma companies by outsourcing various research functions like preclinical and clinical trials which reduces operational costs. Weakness: CMOs and CROs face significant compliance and regulatory challenges to meet stringent manufacturing standards and quality controls set by regulatory agencies. Any issues with quality can damage reputation and business. Outsourcing clinical research also risks loss of control and intellectual property exposure. Opportunity: The growth of the biologics market and rising demand for personalized medicines is driving significant opportunity for CMOs and CROs. Emerging biotech companies also lack manufacturing capabilities and rely on CMOs. CROs can tap into the market for clinical trials outsourcing. Asia Pacific region offers lower costs that can boost outsourcing. Threats: Changing regulations and trade policies can disrupt operations and supply chains. Increasing M&A activity among CMOs and CROs leads to consolidation reducing client choice. Intense competition limits pricing power and pressure to diversify service offerings. Key Takeaways The global biopharmaceutical CMO and CRO market size is expected to witness high growth driven by the expanding biologics industry. The market size is projected to reach US$ 41.71 Mn in 2024 from US$ 31.13 Mn in 2021, registering a CAGR of 5.0% during the forecast period. Regional analysis:
North America currently dominates due to presence of major pharmaceutical players and global centers for drug development. However, Asia Pacific is expected to grow at the fastest pace due to rising scientific talent, lower costs and growing pharma industry in China and India. Countries are attracting CMOs and CROs through industry-friendly policies to boost local manufacturing and clinical research. Key players:
Key players operating in the biopharmaceutical CMO and CRO market are Albemarle Corporation, Greenchemicals S.r.l., ICL, Jordan Bromine Company, Kingboard Holdings Limited, LANXESS, Novel Chem, Shandong Brother Sci. & Tech. Co., Ltd., Shandong Futong Chemical Co., Ltd. and Tianjin Changlu Hangu Saltern Co., Ltd. The market has flourished due to strategic collaborations between biopharma firms and major global players. Market leaders are investing in expansions using advanced technologies to strengthen capabilities across various stages of drug development. Get more insights on this topic: https://www.newsstatix.com/biopharmaceutical-cmo-and-cro-market-industry-insights-trends-biopharmaceutical-cmo-and-cro-market/
#Biopharmaceutical CMO And CRO#Biopharmaceutical CMO And CRO Market#Biopharmaceutical CMO And CRO Market size#Biopharmaceutical CMO And CRO Market share#Biopharmaceutical CMO And CRO Market demand#Biopharmaceutical CMO And CRO Market analysis
0 notes
Text
Biopharmaceutical CMO Market SWOT analysis, Growth, Share, Size and Demand outlook by 2031 | Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd.

Global Biopharmaceutical CMO Market report from Global Insight Services is the single authoritative source of intelligence on Biopharmaceutical CMO Market . The report will provide you with analysis of impact of latest market disruptions such as Russia-Ukraine war and Covid-19 on the market. Report provides qualitative analysis of the market using various frameworks such as Porters’ and PESTLE analysis. Report includes in-depth segmentation and market size data by categories, product types, applications, and geographies. Report also includes comprehensive analysis of key issues, trends and drivers, restraints and challenges, competitive landscape, as well as recent events such as M&A activities in the market.
A Contract Manufacturing Organization (CMO), also known as a Biopharmaceutical CMO, is a company that provides manufacturing and other services to the pharmaceutical and biotechnology industries. CMOs are an important part of the pharmaceutical supply chain, and they play a vital role in bringing new drugs and therapies to market.
Get Free Sample Copy of This Report –https://www.globalinsightservices.com/request-sample/GIS21593/
Key Trends
The key trends in Biopharmaceutical CMO technology are:
1. The use of biotechnology to develop new drugs and therapies.
2. The use of cell culture and fermentation technologies to produce biopharmaceuticals.
3. The use of monoclonal antibodies and other protein-based drugs.
4. The use of nucleic acid-based drugs and gene therapy.
Key Drivers
The biopharmaceutical CMO market is driven by the increasing demand for biopharmaceuticals, the need for specialized manufacturing facilities, and the increasing number of biopharmaceutical companies. The biopharmaceutical industry is growing at a rapid pace, and the number of biopharmaceutical companies is increasing. This is resulting in an increased demand for CMOs. CMOs are specialized manufacturing facilities that are required for the production of biopharmaceuticals. They are required to meet the stringent quality standards set by the FDA. The increasing number of biopharmaceutical companies is resulting in an increased demand for CMOs.
The biopharmaceutical CMO market is facing a number of key restraints and challenges. Firstly, the market is highly competitive and there are a large number of players operating in the space. This makes it difficult for new entrants to gain a foothold in the market. Secondly, the market is capital intensive and requires significant investment in research and development. This is a major barrier for small and medium sized companies. Thirdly, the regulatory environment is constantly changing and this makes it difficult for companies to keep up with the latest regulations. Finally, the market is reliant on a small number of key customers and this makes it difficult to diversify revenue streams.
Market Segments
The biopharmaceutical CMO market report is bifurcated on the basis of product, source, service, and region. On the basis of product, it is segmented into biologics and biosimilars. Based on source, it is analyzed across mammalian and non-mammalian. By service, it is categorized into contract manufacturing, process development, packaging, and others. Region-wise, it is studied across North America, Europe, Asia-Pacific, and rest of the World.
Key Player
The biopharmaceutical CMO market report includes players such as Toyobo Co., Ltd., Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd., JRS Pharma, Biomeva GmbH, and ProBioGen AG.
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Excel data pack included with all report purchases
Robust and transparent research methodology
Ground breaking research and market player-centric solutions for the upcoming decade according to the present market scenario
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1–833–761–1700Website:https://www.globalinsightservices.com/
#Biopharmaceutical CMO Market#Biopharmaceutical CMO Market share#Biopharmaceutical CMO Market size#Biopharmaceutical CMO Market trend#Biopharmaceutical CMO Market growth#Biopharmaceutical CMO Market demand
0 notes
Text
India Single-use Bioprocessing Probes And Sensors Market To Reach $191.0 Million By 2030
The India single-use bioprocessing probes and sensors market is anticipated to reach USD 191.0 million by 2030 and is anticipated to grow at a CAGR of 12.61% during the forecast period from 2024 to 2030, according to a new report by Grand View Research, Inc. The increasing demand for biopharmaceuticals and the growing popularity of disposable systems in preclinical trials are key factors driving the growth of the single-use bioprocessing probes and sensors market in India. The need for faster and more efficient drug development processes and the rising demand for personalized medicines contribute to the increased implementation of single-use bioprocessing systems.
The implementation of single-use technology (SUT) in biomanufacturing processes offers advantages such as reduced risk of cross-contamination and ensuring product purity and integrity, which is crucial for the growing domestic biosimilars and biologics market. It enhances operational efficiency, leading to shorter turnaround times and increased productivity - a key benefit for Indian manufacturers to scale and meet the rising domestic and global demand for biopharmaceuticals.
Furthermore, the commercial advantages of single-use sensors, such as streamlined operations, enhanced flexibility, and improved regulatory compliance and product quality, make them an attractive option for Indian biomanufacturers. The adoption of these technologies can help Indian companies lower their capital expenditure and achieve faster turnaround times.

Request a free sample copy or view report summary: India Single-use Bioprocessing Probes And Sensors Market Report
India Single-use Bioprocessing Probes And Sensors Market Report Highlights
The pH sensors type segment held the largest revenue share of 20.24% in 2023 and is expected to grow at the fastest CAGR over the forecast period. The growing demand for precise process monitoring, driven by the expanding biopharmaceutical industry, drives the adoption of single-use pH sensors. The oxygen sensors segment is expected to register a significant CAGR over the forecast period.
The upstream segment dominated the segment with a market share of 73.49% in 2023 and is anticipated to grow at the fastest CAGR over the forecast period. It is driven by the increasing demand for biopharmaceuticals and the need for efficient and cost-effective manufacturing processes.
The biopharmaceutical & pharmaceutical companies dominated the segment with a market share of 41.69% in 2023. The consistent introduction of new and innovative single-use bioprocessing probes and sensors is a key driver behind the growth of this market segment.
India Single-use Bioprocessing Probes And Sensors Market Segmentation
Grand View Research has segmented the India single-use bioprocessing probes and sensors market based on type, workflow, and end use:
India Single-use Bioprocessing Probes And Sensors Type Outlook (Revenue, USD Million, 2018 - 2030)
pH Sensor
Oxygen Sensors
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
India Single-use Bioprocessing Probes And Sensors Workflow Outlook (Revenue, USD Million, 2018 - 2030)
Upstream
pH Sensor
Oxygen Sensors
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
Downstream
pH Sensor
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
India Single-use Bioprocessing Probes And Sensors End-use Outlook (Revenue, USD Million, 2018 - 2030)
Biopharmaceutical & Pharmaceutical Companies
CROs & CMOs
Academic & Research Institutes
Others
List of Key Players in theIndia Single-use Bioprocessing Probes And Sensors Market
Thermo Fisher Scientific
Sartorius AG
PreSens Precision Sensing GmbH
Hamilton Company
Mettler-Toledo India Private Limited
PARKER HANNIFIN CORP
Danaher
Saint-Gobain
0 notes
Text
Healthcare Contract Development And Manufacturing Organization Market To Reach USD 471.0 Billion By 2030

Healthcare Contract Development and Manufacturing Organization Market Growth & Trends
The global healthcare contract development and manufacturing organization market size is expected to reach USD 471.0 billion by 2030, growing at a CAGR of 9.68% from 2024 to 2030, according to a new report by Grand View Research, Inc. The market is expected to show lucrative growth due to rising outsourcing trends and increasing R&D expenditure.
Healthcare contract development and manufacturing organization (CDMO) provide outsourcing services to various pharmaceutical industries on a contract basis. An increase in outsourcing by pharmaceutical companies, expansions in the pharmaceutical industry, and the support of CDMOs in reducing operational and capital expenses are some of the major factors anticipated to propel the market growth in the forecast period.
In addition, growing requirement among pharmaceutical and medical device companies to follow stringent timelines has increased the demand for outsourcing development and manufacturing activities to CDMOs. Further, due to the increasing demand for medical devices in emerging countries, various companies are shifting their focus on research and development activities for medical device contract development and manufacturing. Over the past 10 years, several pharmaceutical companies have turned to CMOs, CROs, and CDMOs to assist in pre-formulation and development & manufacturing of their novel innovations. Outsourcing is a high growth market, and most spending is focused on early development.
Around 75% of new drug pipelines come from small- and midsized biopharmaceutical companies. These companies have high profit margins, which make them easy targets for healthcare providers who are trying to reduce costs. Thus, instead of investing in establishing their own infrastructure, it is profitable for these companies to outsource services to third-party organizations that have expertise and the required equipment. Several pharmaceutical companies are seeking outsourced services for optimizing the development of their molecules. Furthermore, a large number of CDMO collaborations, expansions, mergers & acquisitions, and other strategic initiatives undertaken by market players operating in the country are anticipated to boost the market. For instance, In March 2023, Remedium Bio, a U.S.-based biotechnology company, entered into a collaboration agreement with Exothera, a Belgium-based CDMO, to scale up the production of Remedium’s lead gene therapy drug candidate, AAV2-FGF18 in the treatment of osteoarthritis. Similarly, in January 2024, Enzene Biosciences, a subsidiary of Alkem Labs announced the manufacturing site in the U.S. Such innovations are anticipated to drive the market.
However, increasing logistic costs, serialization issues faced by healthcare organizations, and the threat of infringement of Intellectual Property (IP) rights are anticipated to restrain the market growth for healthcare contract development & manufacturing organization over the forecast period.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/healthcare-contract-development-manufacturing-organization-market
Healthcare Contract Development and Manufacturing Organization Market Report Highlights
Based on services, the contract manufacturing segment dominated the market with a revenue share of 73.50% in 2023 due to increase in the outsourcing of manufacturing services by pharmaceutical and medical device companies.In addition, cost-effectiveness, and the increasing number of CMOs are some of the key factors that are positively affecting the market growth
By small molecule contract development sub-segment, the preclinical/segment is expected to register the highest CAGR of 9.53% in the forecast period due to the rising pipeline of novel therapeutics
North America dominated the healthcare contract development and manufacturing organization industry with the largest revenue share of 40.91%in 2023. High shares of the region are majorly due to the presence of a large number of actively functioning CROs and CMOs in the region, especially across the U.S.
Healthcare Contract Development and Manufacturing Organization Market Segmentation
For this study, Grand View Research has segmented the healthcare contract development and manufacturing organization market based on services and region:
Healthcare CDMO Services Outlook (Revenue, USD Billion, 2018 - 2030)
Contract Development
Small Molecule
Preclinical
Bioanalysis and DMPK Studies
Toxicology Testing
Other Preclinical Services
Clinical
Phase I
Phase II
Phase III
Phase IV
Laboratory Services
Bioanalytical Services
Analytical Services
Large Molecule
Cell Line development
Process Development
Upstream
Microbial
Mammalian
Others
Downstream
MABs
Recombinant Proteins
Others
Others
Contract Manufacturing
Small Molecule
Large Molecule
MABs
Recombinant Proteins
Others
High Potency API
Finished Dose Formulations
Solid Dose Formulation
Liquid Dose Formulation
Injectable Dose Formulation
Medical Devices
Class I
Class II
Class III
Healthcare CDMO Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
U.S.
Canada
Europe
Germany
UK
France
Italy
Spain
Netherlands
Belgium
Denmark
Norway
Sweden
Asia Pacific
China
India
Japan
Australia
South Korea
Malaysia
New Zealand
Singapore
Philippines
Thailand
Latin America
Brazil
Mexico
Argentina
Colombia
Chile
Middle East & Africa
South Africa
Saudi Arabia
UAE
Israel
Kuwait
List of Key Players in the Healthcare CDMO Market
Catalent Inc.
Lonza
Recipharm AB
Siegfried Holding AG
Thermo Fisher Scientific, Inc.
Labcorp Drug Development
Jabil Inc
Syngene International Limited
IQVIA Inc.
Almac Group
Ajinomoto Bio-Pharma
Adare Pharma Solutions
Alcami Corporation
Vetter Pharma International
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/healthcare-contract-development-manufacturing-organization-market
#Healthcare CDMO#Healthcare CDMO Size#Healthcare CDMO Share#Healthcare CDMO Trends#Healthcare CDMO Growth
0 notes
Text
Biopharmaceuticals Contract Manufacturing Market Poised for Steady Growth in the Future
Biopharmaceuticals Contract Manufacturing Industry Overview
The global biopharmaceuticals contract manufacturing market was valued at USD 17.0 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 5.2% during the forecast period.
The success of the biopharmaceutical market can be majorly attributed to the contract manufacturers. Reduction in the overall investment required to bring a new drug product to market, providing access to expensive technologies, quick entry of products into the markets, and greater flexibility are some advantages offered by contract manufacturing organizations (CMOs), which have prompted the companies to outsource their biopharmaceutical manufacturing.
Due to the disruptions from COVID-19, many companies have had to bring on new contract manufacturers or ingredient suppliers due to increasing demands. Furthermore, with a perpetual expansion of the biopharmaceutical industry, the companies are facing production issues, such as lack of expertise and sophisticated equipment, while practicing in-house manufacturing. The maturity of biotechnology and the availability of external funding have resulted in the growth in a number of early-stage bio/pharma companies. These companies are recognized as core customers of CMOs, as these organizations lack the capabilities for the development of robust manufacturing operations.
Gather more insights about the market drivers, restrains and growth of the Biopharmaceuticals Contract Manufacturing Market
In order to fulfill the growing demand in the market, key players are going for capacity expansion. For instance, in 2020, Thermo Fisher Scientific announced an investment of USD 180.0 million for the construction of a new commercial manufacturing site in Plainville, Mass for viral vector development and manufacturing services. Also, in 2019, Boehringer Ingelheim invested USD 84 million for expansion in Mexico for increasing production. The customers and CMOs are engaged in standardizing agreement terms for making contract negotiations easy. This is to address the issues pertaining to the regulatory landscape and complexity of service delivery. IP rights, warranty & liabilities, prices & timelines are major issues cited by CMOs and clients which are making negotiations innately difficult.
Breakthrough technological advancements and innovations in bioprocessing have played a pivotal role in the progress of contract service providers by overcoming the manufacturing issues such as high production costs and the need for changeover with every batch. Single-use bioprocessing systems are one of the most significant innovations as it helps in reducing the overall production and scale-up costs. Furthermore, the fast turnaround offered by single-use products while limiting allied activities, such as changeover and cleaning validation, has supported the growth of CMOs to a major extent.
Mergers, acquisitions, and joint ventures are one of the common trends observed across the industry, as it helps CMOs offer integrated bioprocessing services to their clients, which, in turn, makes them a more reliable option for a rapid product launch for commercial use. However, large firms consider outsourcing perilous due to loss of strategic control and limited management oversight. As a result, large pharma companies opt to maintain their manufacturing operations in-house. This is expected to challenge the growth of CMOs to a certain extent.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global intrauterine devices market size was estimated at USD 6.25 billion in 2023 and is projected to grow at a CAGR of 3.66% from 2024 to 2030.
• The global dual chamber prefilled syringes market size was valued at USD 167.3 million in 2023 and is projected to grow at a CAGR of 5.8% from 2024 to 2030.
Key Companies and Market Share Insights
The market service providers are focused on expanding their manufacturing capabilities as well as establishing new services to meet the growing demand of biopharmaceutical companies. Along with small players, these entities are also engaged in a partnership with established biopharma companies.
All the major biopharmaceutical firms have a wide-ranging product pipeline and are investing in developing new products. For instance, more than 60,000 clinical trials were registered globally in 2018. It is challenging for biopharmaceutical players to carry out regulatory-compliant manufacturing processes solely depending on in-house capacities. This, in turn, is driving the demand for biopharmaceutical contract manufacturing of potential drug candidates all across the globe. Some prominent key players in the global biopharmaceuticals contract manufacturing market include:
· Boehringer Ingelheim GmbH
· Lonza
· Inno Biologics Sdn Bhd
· Rentschler Biotechnologie GmbH
· JRS PHARMA
· AGC Biologics
· ProBioGen
· FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
· Toyobo Co. Ltd.
· Samsung BioLogics
· Thermo Fisher Scientific, Inc.
· Binex Co., Ltd.
· WuXi Biologics
· AbbVie, Inc.
· Novartis AG
· ADMA Biologics, Inc.
· Catalent, Inc
· Cambrex Corporation
· Pfizer Inc.
· Siegfried Holding AG
Order a free sample PDF of the Biopharmaceuticals Contract Manufacturing Market Intelligence Study, published by Grand View Research.
0 notes
Text
Advanced Metering Infrastructure (AMI) Market provides in-depth analysis of the market state of Advanced Metering Infrastructure (AMI) manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Advanced Metering Infrastructure (AMI) in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Advanced Metering Infrastructure (AMI) Market Report:
The report offers a comprehensive and broad perspective on the global Advanced Metering Infrastructure (AMI) Market.
The market statistics represented in different Advanced Metering Infrastructure (AMI) segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Advanced Metering Infrastructure (AMI) are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Advanced Metering Infrastructure (AMI).
Major stakeholders, key companies Advanced Metering Infrastructure (AMI), investment feasibility and new market entrants study is offered.
Development scope of Advanced Metering Infrastructure (AMI) in each market segment is covered in this report. The macro and micro-economic factors affecting the Advanced Metering Infrastructure (AMI) Market
Advancement is elaborated in this report. The upstream and downstream components of Advanced Metering Infrastructure (AMI) and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/advanced-metering-infrastructure-ami-market-100555
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Vein Finder MarketMarket Growth
Automobile Tire MarketMarket Analysis
Automotive LED Lighting MarketMarket Size
Global Peripheral Nerve Stimulators MarketMarket Share
Global Quartz Stone MarketMarket Growth
Specialty Pulp and Paper Chemicals MarketMarket
5G Security MarketMarket Share
Residential Air Purifiers MarketMarket Growth Rate
Sandalwood Oil MarketMarket Forecast
Global Analog Cheese MarketMarket Size
DNS, DHCP and IPAM (DDI) MarketMarket Growth
3D Display MarketMarket Analysis
Veterinary Imaging MarketMarket Size
Global Sports Hospitality MarketMarket Share
Global Robotic Vacuum Cleaner MarketMarket Growth
Welded Wire Mesh Panel MarketMarket
E-Signature MarketMarket Share
Drug-Eluting Balloon MarketMarket Growth Rate
Flow Switches MarketMarket Forecast
Global Organic PVC Stabilizers MarketMarket Size
4K UST Projectors MarketMarket Growth
Personalized Beauty Products MarketMarket Analysis
Skid Steer Loaders MarketMarket Size
Global Active Pharmaceutical Ingredient MarketMarket Share
Global Ready to Eat Rice MarketMarket Growth
Smart Water Cooler MarketMarket
Food Safety HACCP Software MarketMarket Share
Location-Based Services (LBS) and Real-Time Location Systems (RTLS) MarketMarket Growth Rate
Bacterial Vaginosis Drug MarketMarket Forecast
Global Stolen Vehicle Recovery System MarketMarket Size
Luxury Safari Tourism MarketMarket Growth
Telemedicine MarketMarket Analysis
Air Dryer MarketMarket Size
Global Biopharmaceutical CMO and CRO MarketMarket Share
Global Blue-green Algae Fertilizer MarketMarket Growth
Bus Handrail MarketMarket
Cricket Farming MarketMarket Share
Parallel Drive Actuator Market Market Growth Rate
Reflow Oven for PCB and Semiconductor MarketMarket Forecast
0 notes
Text
Downstream Processing: Market Size and Growth Potential

The Downstream Processing Market Size was valued at USD 35.89 billion in 2023, and is expected to reach USD 109.78 billion by 2031 and grow at a CAGR of 15% over the forecast period 2024-2031.The downstream processing market is witnessing significant growth, driven by the increasing demand for biopharmaceutical products and the rising adoption of advanced technologies in biotechnology. This market segment encompasses the critical stages of purification and recovery of biosynthetic products, such as antibodies, hormones, and vaccines, from natural sources like plant and animal tissues or fermentation broths. With advancements in chromatography, filtration, and centrifugation techniques, the efficiency and yield of downstream processing have improved, reducing costs and production time.
Get Sample Of This Report @ https://www.snsinsider.com/sample-request/2618
Market Scope & Overview
The market research report displays the market shares of the leading companies as well as the competitive landscape of the major industry rivals. The report thoroughly investigates the Downstream Processing Market . The market estimations and predictions in the research study are based on the opinions of internal subject matter experts, extensive secondary research, and primary interviews. Market research aids in the evaluation of numerous essential variables, such as product performance, market share growth, and investments in emerging markets, to mention a few.
The most recent report will provide you with information on recent trends, opportunities, and components that may have an impact on future growth, as well as a comprehensive overview of the worldwide Downstream Processing Market . This research provides a high-level overview of the market and its profitable opportunities. In addition to market size, the report looks into market drivers, challenges, and opportunities.
Market Segmentation Analysis
By Technique
Purification
Solid-liquid Separation
Clarification/Concentration
By Product
Chromatography Columns and Resins
Filters
Membrane Adsorbers
Single-use Products
By Application
Monoclonal Antibody Production
Vaccine Production
Insulin Production
Immunoglobulin Production
Erythropoietin Production
By End User
Biopharmaceutical Manufacturers
Contract Manufacturing Organizations (CMOs)
COVID-19 Impact Analysis
The impact studies on COVID-19 will aid market participants in building pandemic preparedness strategies. The research looks into the demand and supply side effects on the target market. Primary and secondary research, as well as private databases and a paid data source, were all included in this study report. This study examines the impact of COVID-19 on the domestic and global Downstream Processing Market places.
Regional Outlook
The Downstream Processing Market research report explores the effects of COVID-19 on a wide range of geographical markets, including North America, Latin America, Asia Pacific, Europe, and the Middle East and Africa.
Competitive Analysis
The Downstream Processing Market research analysis comprehensively examines the consequences of COVID-19 on numerous geographical markets, including North America, Latin America, Asia Pacific, Europe, and the Middle East and Africa.
Key Reasons to Purchase Downstream Processing Market Report
Market projections and estimates consider the many political, social, and economic elements that will influence market growth, as well as the industry's current position.
The market report investigates global market structure, segmentation, growth rates, and revenue share comparisons.
Conclusion
The research report analyses the global market and performs research on consumption, value, year-over-year growth, and future development plans to provide a comprehensive representation of the Downstream Processing Market .
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
AI in Cancer Diagnosis Market Growth
Blood Group Typing Market Growth
DNA Synthesis Market Growth
Active Pharmaceutical Ingredient Market Growth
Lymphoma Treatment Market Growth
0 notes
Text
Biotechnology Contract Manufacturing Market Projections: Global Industry Analysis and Forecast (2023-2032)

The Biotechnology Contract Manufacturing Market is projected to witness significant growth, with its value expected to surge from USD 19,149.43 million in 2024 to USD 36,105.98 million by 2032, reflecting a robust compound annual growth rate of 8.25%.
The biotechnology contract manufacturing market is experiencing robust growth, driven by the increasing outsourcing trends in the biopharmaceutical industry, where companies seek to leverage external expertise and infrastructure to streamline production processes and reduce costs. Contract manufacturing organizations (CMOs) offer a range of services, from cell line development and process optimization to large-scale production and regulatory support. This market expansion is fueled by the rising demand for biopharmaceuticals, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, which require sophisticated manufacturing capabilities and stringent regulatory compliance. As the complexity of biologic drugs increases, biopharmaceutical companies increasingly rely on CMOs to provide the necessary technological expertise, advanced facilities, and scalability.
North America dominates the biotechnology contract manufacturing market due to its well-established biopharmaceutical industry, robust research and development activities, and the presence of leading CMOs. The United States, in particular, benefits from a strong focus on innovation and substantial investment in biotechnology, which drives the demand for contract manufacturing services. Europe also holds a significant share of the market, supported by a strong regulatory framework, advanced healthcare infrastructure, and increasing investment in biotech research. The region's focus on personalized medicine and biosimilars further propels the demand for contract manufacturing services.
The study on the biotechnology contract manufacturing market reveals several key findings that underscore the market's dynamics, growth drivers, challenges, and future prospects.
1. Robust Market Growth
The biotechnology contract manufacturing market is experiencing substantial growth, driven by the increasing trend of outsourcing manufacturing processes in the biopharmaceutical industry. This growth is attributed to the rising demand for biopharmaceuticals, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, which require sophisticated manufacturing capabilities and stringent regulatory compliance.
2. North America Leads the Market
North America dominates the biotechnology contract manufacturing market, primarily due to its well-established biopharmaceutical industry, robust research and development activities, and the presence of leading CMOs. The United States is at the forefront, benefiting from substantial investments in biotechnology and a strong focus on innovation.
3. Significant Market Share in Europe
Europe holds a significant share of the market, supported by a strong regulatory framework, advanced healthcare infrastructure, and increasing investment in biotech research. The region's focus on personalized medicine and biosimilars further propels the demand for contract manufacturing services.
4. Rapid Growth in Asia-Pacific
The Asia-Pacific region is witnessing rapid growth in the biotechnology contract manufacturing market. Countries like China, India, and South Korea are emerging as key players, offering cost advantages and large-scale manufacturing capabilities. Government initiatives to develop biotechnological infrastructure and improve regulatory standards support market growth.
5. Increasing Demand for Biopharmaceuticals
The rising incidence of chronic diseases and the increasing adoption of biologics in treatment protocols are driving the demand for contract manufacturing services. Biopharmaceutical companies are increasingly outsourcing manufacturing to CMOs to leverage their expertise and advanced facilities, ensuring high-quality and scalable production.
6. Technological Advancements
Ongoing advancements in biotechnology and manufacturing technologies are critical drivers of market growth. CMOs are investing in state-of-the-art facilities and adopting innovative technologies such as single-use systems and continuous manufacturing to enhance efficiency and meet the evolving demands of their clients.
7. Operational Challenges
The market faces several challenges, including high operational costs, stringent regulatory requirements, and complex manufacturing processes. Ensuring consistent product quality and managing supply chain complexities are critical issues that CMOs must address to maintain their competitive edge.
8. Strategic Collaborations and Partnerships
Strategic collaborations and partnerships are becoming increasingly important in the biotechnology contract manufacturing market. These alliances help CMOs expand their service offerings, enhance their technological capabilities, and meet the growing demands of biopharmaceutical clients.
9. Focus on Personalized Medicine
The growing focus on personalized medicine is driving the demand for specialized manufacturing services. CMOs are adapting to this trend by developing capabilities to produce small batches of personalized therapies, which require highly specialized and flexible manufacturing processes.
Key Player:
Lonza (Switzerland)
Samsung Biologics (South Korea)
Thermo Fisher Scientific, Inc. (US)
Catalent, Inc. (US)
JSR Corporation (Japan)
WuXi Biologics (China)
AbbVie, Inc. (US)
Boehringer Ingelheim International GmbH (Germany)
Eurofins Scientific (Luxembourg)
GenScript Biotech Corporation (US)
More About Report- https://www.credenceresearch.com/report/biotechnology-contract-manufacturing-market
The biotechnology contract manufacturing market is influenced by several trending factors that shape its growth trajectory and operational dynamics. These factors highlight the evolving landscape of biopharmaceutical production and the strategic adjustments being made by Contract Manufacturing Organizations (CMOs) to stay competitive and meet industry demands.
1. Increasing Demand for Biologics
The demand for biologics, including monoclonal antibodies, vaccines, recombinant proteins, and cell and gene therapies, is on the rise. Biologics are becoming integral to modern therapeutic regimens due to their specificity and efficacy in treating complex diseases such as cancer, autoimmune disorders, and genetic conditions. This surge drives the need for specialized manufacturing capabilities offered by CMOs.
2. Advancements in Biomanufacturing Technologies
Technological innovations are transforming biomanufacturing processes. The adoption of single-use systems, continuous manufacturing, and automation enhances production efficiency, reduces contamination risks, and allows for greater flexibility in manufacturing operations. CMOs are increasingly investing in these advanced technologies to offer cutting-edge services and maintain a competitive edge.
3. Outsourcing Trends
Biopharmaceutical companies are increasingly outsourcing manufacturing to CMOs to focus on core competencies such as research and development, and to reduce capital expenditure on manufacturing infrastructure. Outsourcing also provides access to specialized expertise and advanced facilities, which are crucial for the production of complex biologics.
4. Regulatory Stringency and Compliance
The regulatory landscape for biopharmaceutical manufacturing is becoming more stringent, with regulatory bodies like the FDA and EMA enforcing rigorous quality standards. CMOs are responding by enhancing their compliance frameworks, investing in quality assurance, and adopting Good Manufacturing Practices (GMP) to ensure product safety and efficacy.
5. Personalized Medicine
The shift towards personalized medicine, where treatments are tailored to individual patient profiles, is driving demand for flexible and scalable manufacturing solutions. CMOs are developing capabilities to produce small, customized batches of therapies, which requires highly specialized manufacturing processes and sophisticated quality control measures.
6. Collaborations and Partnerships
Strategic collaborations between biopharmaceutical companies and CMOs are becoming more common. These partnerships enable the sharing of knowledge, resources, and technology, fostering innovation and expanding service offerings. Collaborations also help in risk-sharing and accelerating the time-to-market for new therapies.
7. Emerging Markets
The biotechnology contract manufacturing market is expanding rapidly in emerging markets, particularly in Asia-Pacific. Countries like China, India, and South Korea are investing heavily in biotechnology infrastructure and capabilities. These regions offer cost advantages and large-scale manufacturing capacities, attracting biopharmaceutical companies looking to optimize production costs.
8. Focus on Sustainable Manufacturing
Sustainability is gaining prominence in the biopharmaceutical industry. CMOs are adopting eco-friendly manufacturing practices, reducing waste, and improving energy efficiency. Sustainable manufacturing not only meets regulatory requirements but also aligns with the growing emphasis on corporate social responsibility and environmental stewardship.
9. Mergers and Acquisitions
The market is witnessing a wave of mergers and acquisitions, driven by the need for consolidation and expansion of service portfolios. Larger CMOs are acquiring smaller, specialized companies to enhance their capabilities and enter new markets. This trend is leading to the creation of integrated service providers that can offer end-to-end solutions.
10. Innovation in Cell and Gene Therapy Manufacturing
The rapid development of cell and gene therapies is creating new opportunities for CMOs. Manufacturing these advanced therapies requires specialized expertise and infrastructure. CMOs are investing in the necessary technologies and processes to support the production of these innovative treatments, which are expected to play a significant role in future therapeutics.
Segments:
Based on Service:
Manufacturing
Formulation and Fill-Finish
Packaging and Labeling
Other services
Based on Type:
Biologic Drug Substance Manufacturing
Biologic Drug Product Manufacturing
Based on Source:
Mammalian Expression Systems
Non-Mammalian Expression Systems
Based on Molecule:
Monoclonal Antibodies
Cell Therapy & Gene Therapy
Antibody-Drug Conjugates (ADCs)
Vaccines
Therapeutic Peptides & Proteins
Other Molecule Types
Browse the full report – https://www.credenceresearch.com/report/biotechnology-contract-manufacturing-market
Browse Our Blog: https://www.linkedin.com/pulse/biotechnology-contract-manufacturing-market-forecast-aedif
Contact Us:
Phone: +91 6232 49 3207
Email: [email protected]
Website: https://www.credenceresearch.com
0 notes
Text
Global CMO/CDMO Market Share: A Competitive Landscape Analysis
The global CMO/CDMO market revenue is experiencing significant growth, with the market size valued at USD 20.9 billion in 2023. Projections indicate the market will reach USD 51 billion by 2032, growing at a compound annual growth rate (CAGR) of 10.4% over the forecast period from 2024 to 2032.
The CMO/CDMO market plays a critical role in the pharmaceutical and biotechnology industries by offering outsourced services for the development and manufacturing of drugs, biologics, and other healthcare products. With increasing demand for pharmaceutical production efficiency and cost reduction, companies are increasingly turning to CMOs and CDMOs to support their drug development pipelines and manufacturing processes.
Key Market Drivers
Growing Biopharmaceutical and Pharmaceutical Demand: The rise of biopharmaceuticals, biologics, and personalized medicine has significantly increased the need for outsourced services in drug development and manufacturing. CMOs and CDMOs are essential in enabling biopharma companies to accelerate the commercialization of new drugs and biologics while reducing operational costs. The complexity of biologics, cell therapies, and gene therapies has further driven demand for CDMO expertise in these specialized areas.
Cost Efficiency and Focus on Core Competencies: The outsourcing of manufacturing and development services to CMOs and CDMOs allows pharmaceutical companies to focus on their core competencies, such as research and innovation. CMOs/CDMOs offer economies of scale, regulatory expertise, and advanced manufacturing facilities, helping companies reduce costs and time-to-market for new therapies. This trend is especially prevalent among small- and mid-sized biopharma companies that lack in-house capabilities for large-scale production.
Increasing Investment in Research and Development: Investment in research and development (R&D) is at an all-time high, particularly in the fields of oncology, immunology, and rare diseases. The surge in clinical trials and new drug approvals is driving demand for specialized CMO/CDMO services, from clinical-stage development to full-scale manufacturing. As pharmaceutical companies seek to streamline R&D processes, outsourcing to CDMOs has become an attractive solution to navigate complex production processes and regulatory requirements.
Rising Demand for Biologics and Biosimilars: The growing market for biologics and biosimilars is a key driver for CDMO services. The complexity and high manufacturing costs associated with biologics necessitate advanced production technologies, which CDMOs are equipped to provide. The growing acceptance and adoption of biosimilars, driven by cost savings and increasing healthcare needs, are further fueling the demand for contract manufacturing services.
Increasing Focus on Cell and Gene Therapy: The advancement of cell and gene therapies, including CAR-T therapies and gene editing technologies, is expanding the scope of CMO/CDMO services. These therapies require specialized production facilities and regulatory expertise, which CMOs and CDMOs are increasingly investing in to support the growing market for these innovative treatments.
Get Free Sample Report: https://www.snsinsider.com/sample-request/1289
Challenges and Opportunities
While the market presents substantial growth opportunities, challenges such as regulatory complexities, high manufacturing costs, and the need for skilled labor can present obstacles. Additionally, capacity constraints and lead times for large-scale biologics manufacturing may pose hurdles for CMOs and CDMOs in meeting the rising demand.
However, these challenges also present opportunities for investment in cutting-edge manufacturing technologies such as single-use bioreactors, continuous manufacturing, and automation. CMOs and CDMOs that invest in advanced capabilities will be well-positioned to capture market share, particularly in high-growth areas like biologics, cell therapies, and gene therapies.
Regional Insights
North America currently dominates the CMO/CDMO market, with significant investments in pharmaceutical research, strong healthcare infrastructure, and the presence of leading biopharmaceutical companies. Europe also holds a substantial market share, driven by its robust regulatory environment and focus on innovation in the life sciences sector.
The Asia-Pacific region is expected to experience the highest growth during the forecast period, bolstered by increasing pharmaceutical and biotech R&D activities, cost-effective manufacturing, and growing demand for innovative therapies in countries like China, India, and Japan. The region's favorable government policies and expanding healthcare infrastructure further support this growth.
Future Outlook
The global CMO/CDMO market is positioned for dynamic growth, driven by increasing demand for outsourced pharmaceutical services, the rise of biologics and biosimilars, and the rapid development of cell and gene therapies. As companies seek greater efficiency and specialization, outsourcing to CMOs and CDMOs will continue to be a strategic imperative in the pharmaceutical and biotech industries.
With a projected CAGR of 10.4% from 2024 to 2032, the CMO/CDMO market is set to expand significantly, from USD 20.9 billion in 2023 to an estimated USD 51 billion by 2032. The evolving landscape of drug development and manufacturing will further cement CMOs and CDMOs as key enablers of innovation and growth in the healthcare industry.
Other Trending Reports
Pancreatic Cancer Treatment Market Growth
gRNA Market Growth
Patient Portal Market Growth
Continuous Glucose Monitoring Market Growth
0 notes
Text
The Booming Bioreactors Market: Key Trends and Growth Drivers
The bioreactors market has witnessed significant growth over the past few years, driven by advancements in biopharmaceutical production, increasing research activities in biotechnology, and the rising demand for personalized medicine. Bioreactors, essential for cultivating cells or tissues in a controlled environment, are crucial in various applications, including pharmaceuticals, food and beverage production, and waste management. As the bioreactors market continues to expand, it is poised to revolutionize several industries by enhancing productivity and efficiency in bioprocesses.
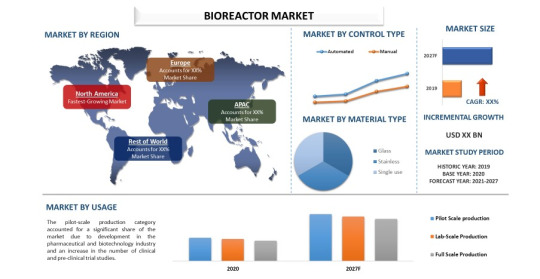
Market Drivers and Technological Advancements
One of the primary drivers of the bioreactors market is the burgeoning biopharmaceutical industry. With the global population's aging and the rise in chronic diseases, there is a growing demand for advanced therapeutics, including monoclonal antibodies, vaccines, and cell and gene therapies. Bioreactors play a pivotal role in the large-scale production of these biopharmaceuticals, ensuring high yield and consistent quality. The shift towards biologics, which are more complex and sensitive than traditional small-molecule drugs, necessitates sophisticated bioreactor systems capable of maintaining precise environmental conditions.
Technological advancements have significantly influenced the bioreactors market. Innovations such as single-use bioreactors (SUBs) have gained immense popularity due to their cost-effectiveness, reduced risk of contamination, and operational flexibility. Unlike traditional stainless-steel bioreactors, SUBs do not require extensive cleaning and sterilization processes, making them ideal for small-scale production and research purposes. Moreover, advancements in automation and digitalization have enabled real-time monitoring and control of bioprocesses, enhancing efficiency and reducing human error.
For a comprehensive analysis of the market drivers https://univdatos.com/report/bioreactors-market/
Market Segmentation and Applications
The bioreactors market can be segmented based on type, usage, scale, and end-user. Types of bioreactors include single-use bioreactors and stainless-steel bioreactors. Single-use bioreactors are witnessing higher adoption rates due to their advantages in terms of cost, scalability, and reduced contamination risk. However, stainless-steel bioreactors remain prevalent in large-scale commercial production due to their durability and suitability for high-volume manufacturing.
Usage segmentation includes microbial and cell culture bioreactors. Microbial bioreactors are primarily used for the production of antibiotics, enzymes, and other microbial products, while cell culture bioreactors are crucial for producing biopharmaceuticals, vaccines, and therapeutic proteins. The growing focus on cell and gene therapies has further fueled the demand for cell culture bioreactors, as these therapies require precise and controlled culturing environments.
In terms of scale, bioreactors are categorized into lab-scale, pilot-scale, and industrial-scale. Lab-scale bioreactors are essential for research and development activities, pilot-scale bioreactors for process development and optimization, and industrial-scale bioreactors for large-scale production. The increasing investment in R&D by biopharmaceutical companies and research institutions is driving the demand for lab-scale and pilot-scale bioreactors.
End-users of bioreactors include biopharmaceutical companies, academic and research institutions, and contract research and manufacturing organizations (CROs and CMOs). Biopharmaceutical companies hold the largest market share due to their extensive use of bioreactors in drug development and production. CROs and CMOs are also significant contributors to the market, offering bioreactor-based services to various industries.
Regional Insights and Growth Opportunities
Geographically, the bioreactors market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America dominates the market, driven by the presence of major biopharmaceutical companies, advanced healthcare infrastructure, and significant R&D investments. Europe follows closely, with a strong focus on biotechnology and pharmaceutical research.
The Asia-Pacific region is expected to witness the highest growth rate during the forecast period. Factors such as increasing healthcare expenditure, a growing biopharmaceutical industry, and favorable government initiatives to promote biotechnology research contribute to this growth. Countries like China and India are emerging as key markets due to their expanding biotechnological capabilities and large patient populations.
For a sample report, visit https://univdatos.com/get-a-free-sample-form-php/?product_id=22012
Challenges and Future Outlook
Despite the promising growth prospects, the bioreactors market faces several challenges. High initial investment costs, the complexity of bioprocessing, and stringent regulatory requirements can impede market growth. Additionally, the ongoing COVID-19 pandemic has disrupted supply chains and delayed clinical trials, impacting the bioreactors market.
However, the long-term outlook for the bioreactors market remains positive. Continuous advancements in bioprocessing technologies, increasing adoption of single-use systems, and the growing focus on personalized medicine and biologics are expected to drive market growth. As industries adapt to evolving healthcare needs and technological innovations, the bioreactors market will continue to expand, offering enhanced solutions for biopharmaceutical production and other applications.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411x
Website -www.univdatos.com
0 notes
Text
Serum Free Media Market - Global Growth, Share, Trends, Demand and Analysis Report Forecast 2031

The global serum-free media market is on a trajectory of exponential growth, projected to reach a staggering US$3.6 billion by 2031, according to a recent market analysis conducted by [Your Company/Research Firm]. This forecast marks a significant surge from the estimated US$1.9 billion by the end of 2024, reflecting a remarkable compound annual growth rate (CAGR) of 9.56% during the period of 2024-2031.
For more information: https://www.fairfieldmarketresearch.com/report/serum-free-media-market
Advantages of Serum-Free Media
Serum-free media, known for their improved consistency and reduced contamination risk over traditional media, have emerged as the preferred choice across various industries including biopharmaceuticals, stem cell research, and diagnostic applications.
Key Growth Drivers
1. Biopharmaceutical Advancements
The rapid expansion of biopharmaceutical research and development is driving the adoption of serum-free media. These media offer reduced batch-to-batch variability and eliminate potential contaminants, making them indispensable in biopharmaceutical production processes.
2. Regulatory Compliance
Stringent regulations governing the safety and quality of pharmaceutical products are propelling the demand for serum-free media. Regions with strict regulatory frameworks like North America and Europe are witnessing significant uptake due to the emphasis on defined and animal-origin-free components in biopharmaceutical manufacturing.
3. Cost Effectiveness and Scalability
Serum-free media offer cost savings by reducing the need for expensive serum supplements and minimizing downstream processing steps. Additionally, these media formulations are more scalable and reproducible, driving their adoption across academic research institutions, contract research organizations, and biopharmaceutical companies.
Major Growth Barriers
1. Cost Constraints
The increased cost associated with serum-free media formulations can be a significant restraint for adoption, especially for smaller research laboratories or institutions with limited budgets.
2. Complexity of Formulation
Developing serum-free media with comparable or superior performance to serum-containing media presents challenges due to the intricate balance of nutrients, growth factors, and supplements required for cell culture.
3. Regulatory Challenges
Serum-free media development and usage may face regulatory hurdles, particularly in industries such as pharmaceuticals, where stringent regulations govern the use of cell culture systems for drug development and production.
Trends and Opportunities
1. Increasing Demand for Customized Formulations
The rising demand for serum-free media presents a significant opportunity for companies to develop and offer customized formulations tailored to specific cell types, applications, and research requirements.
2. Expansion of CMOs in Developing Regions
The adoption of serum-free media is not limited to developed regions but is also gaining traction in emerging markets where biopharmaceutical R&D activities are expanding rapidly.
Regional Frontrunners
1. North America
North America, particularly the US, dominates the serum-free media market due to its pioneering position as a biopharmaceutical hub and stringent regulatory environment.
2. Europe
Europe stands out as another prominent market for serum-free media, driven by stringent regulations governing the use of animal-derived components in biopharmaceutical production.
3. Asia Pacific
The Asia Pacific region, with its rapid industrialization and increasing investments in healthcare infrastructure, is witnessing significant growth in serum-free media demand, fueled by expanding biopharmaceutical markets.
Leaders in the Serum Free Media Market Space
Leading players in the serum-free media market include Thermo Fisher Scientific Inc., Merck KGaA, GE Healthcare, Lonza, Corning Incorporated, Irvine Scientific, STEMCELL Technologies, PAN Biotech, MP Biomedicals, LLC, PromoCell GmbH, and Sartorius AG.
0 notes
Text
Biopharmaceutical CMO Market Growth Opportunities and Competitive Landscape Report to 2033
Market Definition
A Contract Manufacturing Organization (CMO), also known as a Biopharmaceutical CMO, is a company that provides manufacturing and other services to the pharmaceutical and biotechnology industries. CMOs are an important part of the pharmaceutical supply chain, and they play a vital role in bringing new drugs and therapies to market.
CMOs specialize in the manufacture of active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs). They also provide a range of other services, such as analytical testing, formulation development, and packaging. CMOs are typically large, multinational companies with extensive experience in drug manufacturing.
Market Outlook
The key trends in Biopharmaceutical CMO technology are:
1. The use of biotechnology to develop new drugs and therapies.
2. The use of cell culture and fermentation technologies to produce biopharmaceuticals.
3. The use of monoclonal antibodies and other protein-based drugs.
4. The use of nucleic acid-based drugs and gene therapy.
The biopharmaceutical CMO market is driven by the increasing demand for biopharmaceuticals, the need for specialized manufacturing facilities, and the increasing number of biopharmaceutical companies. The biopharmaceutical industry is growing at a rapid pace, and the number of biopharmaceutical companies is increasing. This is resulting in an increased demand for CMOs. CMOs are specialized manufacturing facilities that are required for the production of biopharmaceuticals. They are required to meet the stringent quality standards set by the FDA. The increasing number of biopharmaceutical companies is resulting in an increased demand for CMOs.
The biopharmaceutical CMO market is facing a number of key restraints and challenges. Firstly, the market is highly competitive and there are a large number of players operating in the space. This makes it difficult for new entrants to gain a foothold in the market. Secondly, the market is capital intensive and requires significant investment in research and development. This is a major barrier for small and medium sized companies. Thirdly, the regulatory environment is constantly changing and this makes it difficult for companies to keep up with the latest regulations. Finally, the market is reliant on a small number of key customers and this makes it difficult to diversify revenue streams.
To Know More: https://www.globalinsightservices.com/reports/biopharmaceutical-cmo-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21593/
Market Segmentation
The biopharmaceutical CMO market report is bifurcated on the basis of product, source, service, and region. On the basis of product, it is segmented into biologics and biosimilars. Based on source, it is analyzed across mammalian and non-mammalian. By service, it is categorized into contract manufacturing, process development, packaging, and others. Region-wise, it is studied across North America, Europe, Asia-Pacific, and rest of the World.
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS21593/
Major Players
The biopharmaceutical CMO market report includes players such as Toyobo Co., Ltd., Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd., JRS Pharma, Biomeva GmbH, and ProBioGen AG.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS21593/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS21593/
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC 16192, Coastal Highway, Lewes DE 19958 E-mail: [email protected] Phone: +1-833-761-1700 Website: https://www.globalinsightservices.com/
0 notes
Text
Beyond Borders: The Global freeze drying market Offers Hope for Millions
The global freeze drying market is poised for significant growth, with a projected market value of US$2.8 billion by 2034. This signifies a robust CAGR (Compound Annual Growth Rate) of 8.6% from 2024, reflecting the increasing demand for this versatile preservation technique across various industries.
Freeze drying, also known as lyophilization, is a low-temperature dehydration process that removes water content from products while preserving their structure and quality. This technology plays a vital role in the food processing and pharmaceutical industries, offering numerous advantages over traditional drying methods.
Secure Your Sample Report Now: https://www.futuremarketinsights.com/reports/sample/rep-gb-15758
Key Takeaways from Market Study:
Dryers are the leading segment as a product and hold approximately 7% market share in 2021, owing to the customer preference for nutrition from whole foods being supported by the fact that freeze-drying retains nutritional content better than other drying techniques.
Industrial-scale of operations holds a market share of around 6% in 2021. Because of the preservation of quality, freeze-drying is thought to be the best method for dehydrating food on an industrial scale.
Biopharmaceutical companies are the top distribution channel in the worldwide freeze drying market, and this trend is anticipated to continue with a projected CAGR of 7% over the forecasted years.
North America is considered as the leading region with a value share of 8% in 2021, owing to the presence of established biopharmaceutical companies in the region.
“Shifting consumer values, the rapid expansion of the pharmaceutical and food processing industries, and rising consumption of preserved foods are driving the global freeze drying market,” says an analyst of Future Market Insights.
Market Competition:
The global market can be characterized as being extremely competitive and consolidated, with a few significant competitors controlling the industry. Additionally, they are investing heavily in R&D to create novel solutions and acquire a competitive edge. To obtain a competitive edge, numerous businesses are engaging in mergers and acquisitions and deploying cutting-edge technologies.
Key Companies Profiled:
Azbil Corporation
Zirbus Technology GmbH
HOF Sonderanlagenbau GmbH
Millrock Technology, Inc.
Cryotec.Fr
MechaTech Systems Ltd.
SP Industries, Inc.
Martin Christ Gefriertrocknungsanlagen GmbH
Cuddon Freeze Dry
Neologic Engineers Private Limited
Labconco Corporation
BÜCHI Labortechnik AG
Yamato Scientific America Inc.
Freeze Drying Systems Ltd.
Freeze Drying Systems Pvt. Ltd.
IMA – Industria Macchine Automatiche S.p.A.
GEA Group
Key developmental instances include:
Telstar, a division of the Azbil Group, in December 2020, developed a new line of GMP freeze-dryers with a modular design. The new series of lyophilizers sold under the Lyozeta brand is a standardised version of its top-of-the-line GMP customizable freeze-dryers built to function aseptically.
In order to add two HOF horizontal plate freeze-thaw machines to Sartorius’ product line and offer customers a full selection of relevant freeze-thaw equipment and consumables, a partnership between Sartorius and HOF Sonderanlagenbau was announced in December 2021.
Key Market Segments Covered in Freeze Drying Industry Research
By Product:
Dryer
Tray-style dryers
Rotary Freeze Dryers
Manifold Freeze Dryers
Accessories
Drying Chamber
Vacuum System
CIP (Clean-in-place) Systems
Other Accessories
By Scale of Operations:
Pilot-scale
Laboratory-scale
Industrial-scale
By Distribution Channel:
Biopharmaceutical Companies
Food and Beverage Companies
Academic and Research Institutes
CRO & CMO
Hospitals
Research Laboratories
1 note
·
View note
Text
The Role of Contract Manufacturing Organizations in Pharmaceutical Industry: Driving Innovation and Efficiency

In the fast-paced world of pharmaceuticals, efficiency, innovation, and quality are paramount. With increasing demands for new drugs, improved formulations, and cost-effective production, pharmaceutical companies are turning to Contract Manufacturing Organizations (CMOs) to meet these challenges head-on. In this blog post, we’ll explore the critical role that CMOs play in the pharmaceutical industry, how they drive innovation, and enhance efficiency.
Understanding Contract Manufacturing Organizations (CMOs)
CMOs are third-party manufacturers hired by pharmaceutical companies to produce drugs or drug components on a contract basis. These organizations offer a wide range of services, including formulation development, manufacturing, packaging, and regulatory support. By outsourcing these tasks to specialized CMOs, pharmaceutical companies can focus on their core competencies such as research, marketing, and distribution.
Driving Innovation through Collaboration
Collaboration between pharmaceutical companies and CMOs fosters innovation in several ways:
Expertise Exchange: CMOs often employ highly skilled professionals with expertise in various fields such as chemistry, biotechnology, and engineering. By working closely with pharmaceutical companies, CMOs share their knowledge and insights, contributing to the development of novel drug formulations and production techniques.
Access to Advanced Technologies: CMOs frequently invest in cutting-edge technologies and equipment to stay competitive. Pharmaceutical companies can leverage these resources without having to make substantial investments themselves, thereby accelerating the pace of innovation.
Flexible Manufacturing Solutions: CMOs offer flexible manufacturing solutions tailored to the specific needs of their clients. Whether it’s small-scale production for clinical trials or large-scale commercial manufacturing, CMOs can adapt quickly to changing requirements, enabling pharmaceutical companies to bring new drugs to market more efficiently.
Enhancing Efficiency and Cost-Effectiveness
In addition to driving innovation, CMOs also enhance efficiency and cost-effectiveness for pharmaceutical companies:
Optimized Supply Chain Management: CMOs streamline the supply chain by consolidating manufacturing processes and sourcing raw materials cost-effectively. This optimization reduces lead times, minimizes inventory holding costs, and ensures timely delivery of products to market.
Economies of Scale: CMOs often operate at a larger scale than individual pharmaceutical companies, allowing them to achieve economies of scale in production. By spreading fixed costs across multiple clients, CMOs can offer competitive pricing while maintaining high-quality standards.
Regulatory Compliance: Regulatory compliance is a complex and constantly evolving aspect of the pharmaceutical industry. CMOs specialize in navigating regulatory requirements and maintaining compliance with stringent quality standards such as Good Manufacturing Practices (GMP). By partnering with CMOs, pharmaceutical companies can mitigate regulatory risks and expedite the approval process for new drugs.
The Future of Contract Manufacturing in Pharmaceuticals
As the pharmaceutical industry continues to evolve, the role of CMOs is expected to become even more critical. Key trends shaping the future of contract manufacturing include:
Biologics and Biosimilars: With the growing demand for biologic drugs and biosimilars, CMOs specializing in biopharmaceutical manufacturing are in high demand. These CMOs offer specialized expertise in cell culture, fermentation, and purification techniques required for the production of complex biologics.
Personalized Medicine: The rise of personalized medicine requires flexible manufacturing capabilities to produce small batches of customized treatments. CMOs equipped with agile manufacturing platforms and advanced analytics are well-positioned to meet this demand.
Digital Transformation: Industry 4.0 technologies such as artificial intelligence, robotics, and data analytics are transforming the pharmaceutical manufacturing landscape. CMOs investing in digitalization initiatives can improve operational efficiency, quality control, and regulatory compliance.
In conclusion, Contract Manufacturing Organizations play a vital role in driving innovation, enhancing efficiency, and ensuring the timely delivery of high-quality pharmaceutical products to market. By leveraging the expertise and resources of CMOs, pharmaceutical companies can focus on their core objectives of improving patient outcomes and advancing medical science. As the industry continues to evolve, collaboration between pharmaceutical companies and CMOs will be essential to meeting the evolving needs of patients and healthcare providers worldwide.
0 notes